Abstract
Background & Aims
Dietary fructans exacerbate symptoms in some, but not all, adults with irritable bowel syndrome (IBS). We sought to determine whether fructans worsen symptoms in children with IBS and whether clinical and psychosocial factors, and/or gas production, can identify those who are fructan sensitive.
Methods
We performed a double-blind placebo-controlled (maltodextrin) cross-over trial of 23 children with IBS, based on pediatric Rome III criteria, from September 2014 through December 2016. At baseline, participants completed 1-week pain and stool diaries and a 3-day food record and psychosocial factors (depression, anxiety, and somatization) were measured. Subjects were randomly assigned to groups that were provided meals for 72 hrs containing either fructans or maltodextrin (0.5 g/kg; max 19 g). Following a washout period of 10 days or more, the subjects received the meal they were not given during the first study period (crossed over). Gastrointestinal symptoms and breath hydrogen and methane production were captured during each meal period. Fructan sensitivity was defined as an increase of 30% or more in abdominal pain frequency following fructan ingestion.
Results
Subjects had more mean episodes of abdominal pain/day during the fructan-containing diet (3.4 ± 2.6) vs the maltodextrin-containing diet (2.4 ± 1.7) (P<0.01), along with more severe bloating (P<0.05) and flatulence (P=0.01). Hydrogen (but not methane) production was greater while subjects were on the fructan-containing diet (617 ± 305 ppm·hr) than the maltodextrin-containing diet (136 ± 78 ppm·hr) (P<.001). Eighteen subjects (78.2%) had more frequent abdominal pain while on the fructan-containing diet and 12 (52.2%) qualified as fructan sensitive. We found no difference between fructan-sensitive and fructan-insensitive subjects in baseline abdominal pain or bowel movement characteristics, dietary intake, psychosocial parameters, IBS subtype, or gas production.
Conclusion
In a randomized controlled trial of children with IBS, we found fructans to exacerbate several symptoms. However, fructan sensitivity cannot be identified based on baseline gastrointestinal symptoms, dietary intake, psychosocial factors, or gas production. Clinicaltrials.gov no: NCT02842281.
Keywords: Fructooligosaccharide, FODMAP, inulin, intolerance
INTRODUCTION
Diet is an important clinical factor in both adults and children with irritable bowel syndrome (IBS). Dietary triggers worsen gastrointestinal symptoms by self-report in up to 84% and 93% of adults and children with IBS, respectively.1, 2 These may include fermentable oligosaccharide, disaccharide, monosaccharide, and polyol (FODMAP) carbohydrates. FODMAP carbohydrates can be malabsorbed leading to fermentation (with gas production) and increased luminal water content.3 As in adults, a low FODMAP diet has been shown to ameliorate symptoms in some, but not all children with IBS.4, 5 However, there is a paucity of data examining dietary interventions which restrict specific carbohydrates in children with IBS thus pointing toward the need for dietary intervention studies that evaluate the effect of different types of FODMAPs in this population.6
Fructans are a commonly ingested FODMAP carbohydrate in the Western diet, particularly in children.7 Fructans are oligosaccharides comprised primarily of fructosyl-fructose linkages which are unable to be hydrolyzed by human enzymes; therefore they arrive in the colon essentially intact.8, 9 These oligosaccharides have been shown to exacerbate symptoms in a subset of adults with IBS with the primary mechanism of action attributed to increased gas production via colonic fermentation with subsequent luminal distention.10, 11 However, predicting which subset of adults with IBS will have worsening symptoms when ingesting fructans (i.e., are fructan sensitive) remains elusive.
A comprehensive low FODMAP diet (which includes fructan restriction) can be difficult for patients to maintain and its long term effects are unknown given that it removes foods considered healthy (e.g., wheat) and reduces the abundance of organisms associated with health (e.g., Bifidobacteria).8, 12 Given that fructans are a common FODMAP carbohydrate ingested by children,7 we sought to determine whether fructans worsen symptoms in children with IBS and if so, to what extent. We also wished to evaluate whether clinical parameters and/or gas production could predict which children with IBS are fructan sensitive.
METHODS
Subjects
Children ages 7–18 years with pediatric Rome III IBS recruited from primary and tertiary care were included from September 2014 through December 2016.4 Children with potential organic etiologies were excluded as previously described.4 IBS subtype was assigned per Rome III criteria.4 Approval for the study was obtained from the Baylor College of Medicine Institutional Review Board. Parental consent and child assent were obtained. The trial was registered at clinicaltrials.gov (NCT02842281).
General design
Subjects completed a double blind, randomized, placebo-controlled crossover trial. Randomization was computer generated using www.randomization.com with blocks of 10 without stratification. Access to the scheme was only provided to the United States Department of Agriculture (USDA) Children’s Nutrition Research Center (CNRC) research dietitian. Subjects were provided low FODMAP foods to meet their nutritional needs during two 72-hour intervention periods (fructan or placebo) which were separated by a minimum 10-day washout period. During these 72 hours they ingested either fructans (Jarrow Formulas Inc., Inulin-FOS) 0.5 g/kg/day (up to 19 grams) or maltodextrin (Honeyville, Inc.) placebo at the same dosage; both provided in water divided over three meals. The dose of fructans was chosen as studies have demonstrated that 20–30 g/day fructans are well tolerated (particularly when given in divided doses) in healthy adults and a dose of 19 g was used in a previous adult IBS fructan challenge trial.10, 12 Inulin-FOS includes a mixture of short and long inulin-type fructans with an estimated average degree of polymerization of 14 (range: 4–60).13
Measures
The primary outcome measure (abdominal pain frequency) was assessed using a validated Pain and Stool Diary.4, 14 Subjects recorded the number of discrete abdominal pain episodes during each of three 8-hour time periods daily as previously described during a one-week baseline period and during each 72-hour dietary intervention period.15,16 In addition, during each diary time period subjects rated (using 0–10 Likert scale) several symptoms including: abdominal pain severity, bloating, flatulence, nausea, and fatigue. The modified Bristol stool form chart for children was used to characterize the form of each bowel movement during the same timeframe.17 Children were IBS-subtyped based on their baseline stool diary.18 Based on FDA and expert recommendations regarding clinically meaningful thresholds, fructan sensitivity was defined as a ≥30% higher number of abdominal pain episodes during the fructan intervention relative to the number of abdominal pain episodes during the maltodextrin intervention.19
A food record was kept for three days during the baseline period, and during each of the dietary intervention periods with analysis of total energy, total fat, total carbohydrate, dietary fiber, glucose, fructose, lactose, sucrose, and polyols as previously described.4 In addition subjects completed the Childhood Somatization Inventory20, and the Behavioral Assessment System for Children-2 (depression and anxiety scales).21
Subjects provided hourly breath samples to measure hydrogen and methane production during the final 8 hours of each dietary intervention.4 Gas chromatography (Quintron Instrument Company, Milwaukee, WI) was used to analyze the samples. Carbon dioxide analysis was used to correct the hydrogen and methane concentrations. Values (ppm) were plotted against time (8-hours) to calculate area under the curve for total hydrogen and methane gas production using the trapezoidal method (expressed as ppm*hr).22
Statistical Analyses
Based on our previous work evaluating the low FODMAP diet in children with IBS, we estimated that 23 children completing the trial would provide 90% power at a 2-sided α=.05 level to detect a clinically significant change of one pain episode per day between the two treatment periods (primary outcome – pain frequency).4 SPSS Statistics version 23 (IBM Corporation, Armonk, NY) was used for statistical analyses. Children completing the trial (therefore having data for both dietary interventions) were included in the final data analyses. Paired t test was used for intra-group comparisons during each dietary intervention. Independent t test or Mann-Whitney U testing (depending on the normality of the data) compared fructan sensitive vs. fructan insensitive participants. Data are presented as mean ± standard deviation. All the authors had access to the study data and have reviewed and approved the final manuscript.
RESULTS
Thirty-one children were enrolled of whom 23 completed both dietary interventions and were included for subsequent analyses (Supplementary Figure 1). Of the 8 children who did not complete the study, 5 (62.5%) dropped out during the one-week baseline period, and the remaining dropped out during dietary interventions due to non-compliance (e.g., did not complete the pain/stool diary during the intervention).
Baseline patient characteristics of the 23 subject completers are found in Table 1. Subjects had the following subtype delineations: 15 (65.2%) had IBS-constipation, 4 (17.4%) IBS-mixed, 2 (8.7%) IBS-diarrhea, and 2 (8.7%) IBS-undefined.
Table 1.
Baseline Demographic Characteristics, Gastrointestinal Symptoms, Stooling Characteristics, and Psychosocial Factors in Children with Irritable Bowel Syndrome Completing Both Dietary Interventions
| Baseline Characteristic | Study Cohort (n=23) |
|---|---|
| Age (years) | 12.4 ± 2.2 |
| Gender | 19 (82.6%) Female/4 (17.4%) Male |
| Race | 20 (87%) White/2 (8.7%) Mixed/1 (4.3%) African-American |
| Ethnicity | 12 (52.2%) Hispanic |
| Pain Frequency (episodes/day) | 2.7 ± 2.1 |
| Pain Severity (0–10) | 1.9 ± 1.3 |
| Bloating (0–10) | 0.9 ± 0.9 |
| Gas (0–10) | 1.4 ± 1.3 |
| Nausea (0–10) | 0.3 ± 0.4 |
| Fatigue (0–10) | 1.7 ± 1.8 |
| Bowel movements per day | 0.9 ± 0.5 |
| Mean Stool Type (1–5) a | 2.5 ± 0.7 |
| Somatization b | 30.8 ± 15.2 |
| Depression (T-score) c | 50.0 ± 9.9 |
| Anxiety (T-score) c | 56.5 ± 14.7 |
Data presented as number (percentage) or mean ± standard deviation
Measured using the modified Bristol Stool Scale for children
Measured using the Childhood Somatization Inventory
Measured using the Behavioral Assessment System for Children-2
Comparisons within the Entire Subject Cohort (Table 2)
Table 2.
Comparison of Symptoms, Gas Production, and Stooling Habits in children with irritable bowel syndrome (n=23) during 72-hour Fructan vs. Maltodextrin Interventions
| Symptom/Gas Production | Fructan | Maltodextrin | P-Value |
|---|---|---|---|
| Pain Frequency (episodes/day) | 3.4 ± 2.6 | 2.4 ± 1.7 | <0.01 |
| Pain Severity (0–10)# | 2.7 ± 1.9 | 2.3 ± 1.6 | 0.33 |
| Bloating (0–10)# | 1.9 ± 2.1 | 1.4 ± 1.6 | 0.04 |
| Gas (0–10)# | 2.7 ± 2.9 | 1.9 ± 2.4 | 0.01 |
| Nausea (0–10)# | 1.0 ± 1.5 | 0.8 ± 0.9 | 0.53 |
| Fatigue (0–10)# | 2.3 ± 2.0 | 1.8 ± 1.9 | 0.24 |
| Hydrogen (ppm*hr.) | 617 ± 305 | 136 ± 78 | <0.001 |
| Methane (ppm*hr.) | 149 ± 99 | 133 ± 161 | 0.43 |
| Bowel movements per day | 1.0 ± 0.6 | 0.8 ± 0.5 | 0.08 |
| Mean Stool Type (1–5) | 2.5 ± 0.7 | 2.6 ± 0.7 | 0.56 |
Two subjects with missing data for these items during both dietary intervention periods.
Pain frequency (primary outcome) and bloating were significantly greater during the fructan as compared with the placebo (maltodextrin) intervention. Hydrogen, but not methane excretion, also was significantly greater during the fructan (vs. maltodextrin) intervention. Bowel movement frequency and mean stool type did not differ between interventions. Neither hydrogen nor methane gas excretion after fructan ingestion correlated with the severity of any symptom (data not shown). Adverse events included worsening of skin eczema for one child during the trial which was not felt to be related to the dietary interventions.
Patterns of Response to the Dietary Interventions
Twelve (52.2%) subjects were fructan sensitive, 6 (26.1%) subjects had more frequent abdominal pain during fructan ingestion but did not meet the 30% increase threshold relative to the maltodextrin period for fructan sensitivity, and 5 (21.7%) subjects had a 30% increase in abdominal pain frequency with placebo relative to fructans. There were no differences (see Supplementary Figure 2) in clinical response during the first or second intervention phases when comparing the overall number of daily abdominal pain episodes (2.9 ± 1.9 vs. 2.9 ± 2.5, respectively).
Comparison of Fructan Sensitive vs. Fructan Insensitive Subjects at Baseline (Table 3)
Table 3.
Comparison by Fructan Sensitivity of Baseline Gastrointestinal Symptoms, Stooling Habits, and Psychosocial Distress in Children with Irritable Bowel Syndrome
| Baseline Characteristic | Fructan Sensitive (n=12) | Fructan Insensitive (n=11) |
|---|---|---|
| Pain Frequency (episodes/day) | 2.8 ± 2.4 | 2.6 ± 1.8 |
| Pain Severity (0–10) a | 1.6 ± 1.0 | 2.2 ± 1.5 |
| Bloating (0–10) b | 1.0 ± 1.0 | 0.8 ± 0.8 |
| Gas (0–10) b | 0.8 ± 0.8 | 2.0 ± 1.4 # |
| Nausea (0–10) b | 0.2 ± 0.2 | 0.3 ± 0.6 |
| Fatigue (0–10) b | 1.9 ± 1.8 | 1.5 ± 1.8 |
| Bowel movements per day | 0.9 ± 0.5 | 0.9 ± 0.4 |
| Mean Stool Type (1–5) | 2.5 ± 0.6 | 2.5 ± 0.7 |
| Somatization c | 31.1 ± 17.9 | 30.5 ± 12.6 |
| Depression (T-score) d | 49.3 ± 10.9 | 50.6 ± 9.4 |
| Anxiety (T-score) d | 55.5 ± 16.4 | 57.5 ± 13.4 |
P<0.05 comparison between fructan sensitive and fructan insensitive subjects
Data missing for one individual in fructan sensitive group
Data missing for one individual in each group
Measured using the Childhood Somatization Inventory
Measured using the Behavioral Assessment System for Children-2
Fructan sensitive subjects did not differ from fructan insensitive subjects with respect to demographics (age, gender, race, ethnicity). Fructan sensitive subjects had less gas at baseline compared with fructan insensitive subjects. Other baseline characteristics (pain frequency, pain severity, bloating, nausea, fatigue, stooling characteristics, IBS subtype, somatization, depression, anxiety) did not differ between groups. There were no differences in baseline dietary consumption between fructan sensitive and fructan insensitive subjects (Table 4).
Table 4.
Comparison of Baseline Dietary Intake (per day) in fructan sensitive (n=12) vs. fructan insensitive (n=11) children with irritable bowel syndrome.
| Dietary Component | Fructan Sensitive | Fructan Insensitive |
|---|---|---|
| Energy (kcal/kg) | 41.6 ± 12.0 | 38.1 ± 13.7 |
| Protein (g/kg) | 1.4 ± 0.3 | 1.4 ± 0.6 |
| Fat, (g/kg) | 1.7 ± 0.5 | 1.5 ± 0.6 |
| Carbohydrate, (g/kg) | 5.3 ± 1.8 | 4.8 ± 1.8 |
| Dietary Fiber, (g/kg) | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Glucose, (g/kg) | 0.5 ± 0.2 | 0.4 ± 0.2 |
| Fructose, (g/kg) | 0.5 ± 0.3 | 0.4 ± 0.2 |
| Lactose, (g/kg) | 0.2 ± 0.1 | 0.2 ± 0.2 |
| Sucrose, (g/kg) | 0.8 ± 0.5 | 0.7 ± 0.4 |
| Polyols, (g/kg) | 0.01 ± 0.1 | 0.01 ± 0.01 |
Fructan sensitive subjects did not differ from fructan insensitive subjects with respect to hydrogen or methane excretion during either the fructan or maltodextrin intervention (Figure 1).
Figure 1.
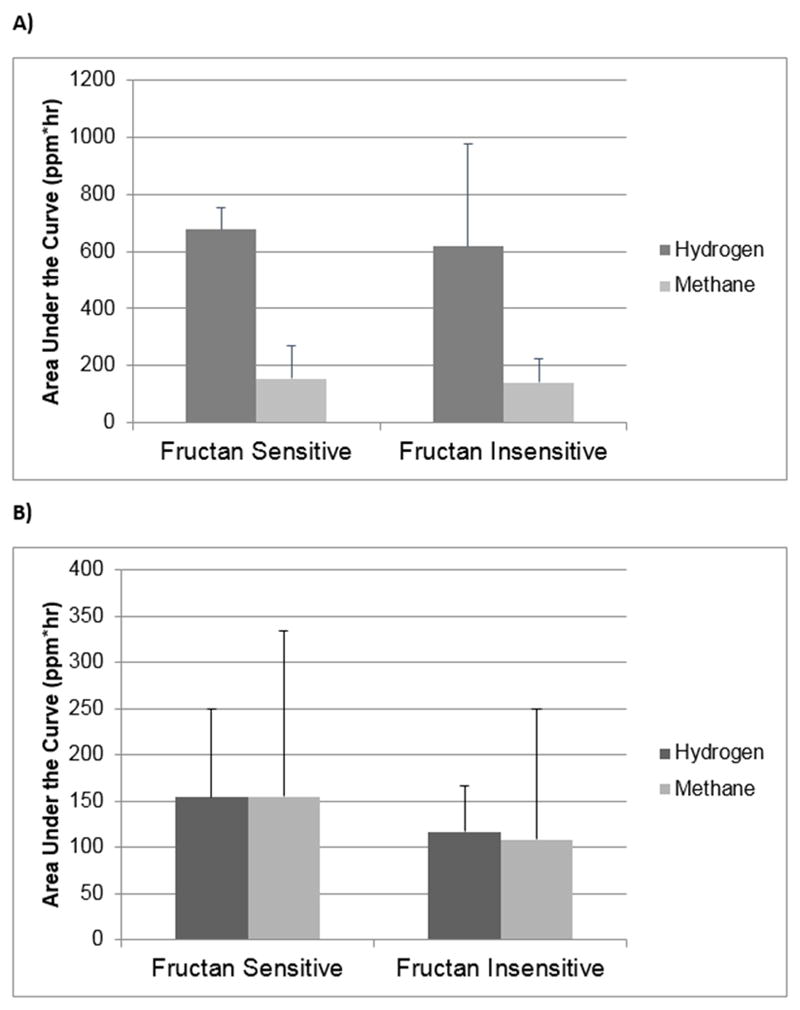
Hydrogen and Methane Gas Production in Children who were Fructan Sensitive vs. Fructan Insensitive Following A) Fructan Ingestion and B) Maltodextrin Ingestion. Data are presented as mean ± standard deviation.
DISCUSSION
To our knowledge this is the first study to prospectively evaluate fructan sensitivity in children with IBS. Within the context of a rigorous randomized, double blind, placebo (maltodextrin) controlled, crossover trial we report that as a group, children with IBS experience worse abdominal pain frequency, bloating, and gas in response to dietary fructans. As defined by FDA and expert recommendations, we identified that a subset of children with IBS who can be identified as fructan sensitive based on their change in abdominal pain frequency compared with baseline following fructan ingestion. Baseline diet, clinical parameters, and psychosocial characteristics were not associated with fructan sensitivity (Table 3). Similarly, hydrogen and methane excretion during fructan ingestion could not distinguish fructan sensitive vs. fructan insensitive children with IBS. These findings have potential implications with respect to personalized nutrition strategies for children with IBS as not all children are fructan sensitive and therefore may respond poorly or not at all to a low FODMAP diet. Indeed, 24–50% of adults and children do not respond to a low FODMAP diet; whether this is related to specifically to fructan insensitivity needs to be determined.23, 24
Given the results of previous breath hydrogen and magnetic resonance imaging studies, the mechanism by which fructans may exacerbate symptoms in those with IBS was previously attributed to luminal distention due to increased gas production.11 Adults with IBS (vs. controls) have been found to have increased hydrogen excretion when given fructans or other malabsorbed carbohydrates such as sorbitol, similar to our current findings (Table 2).25, 26 However, in our study, hydrogen and methane production increased to the same degree following fructan ingestion regardless of whether or not the subject had fructan sensitivity (Figure); therefore, gas production alone does not appear to be the sole mechanism by which fructans exacerbate IBS symptoms. These findings are supported by a recent study by Major et al. in adults with IBS given inulin (a fructan).27 During both breath testing and magnetic resonance imaging subjects had the same increase in both colonic luminal distention and breath hydrogen production in those meeting a predefined symptom threshold vs. those who did not meet the same symptom threshold.27 Given the above, additional factors beyond gas production are likely playing a role in determining fructan related symptoms in children with IBS.
While gas production is one byproduct of microbial carbohydrate fermentation, additional microbial carbohydrate fermentation metabolites may be produced which have direct biological effects.28 We have previously demonstrated that children with IBS who improve (vs. those who did not improve) on a one-week low FODMAP diet (which avoids fructans) have both a baseline different fecal microbiome composition and metabolite profile.23 McIntosh et al. demonstrated altered urine metabolite profiles in adults with IBS on a low vs. high FODMAP diet.29 Whether microbiome composition - which has been associated with low FODMAP diet efficacy in both children and adults with IBS4, 30 - or other microbial related factors are associated with childhood IBS fructan sensitivity remains to be determined.
A host-related factor which may be related to fructan sensitivity is visceral hypersensitivity. Visceral hypersensitivity was implicated in the previously mentioned study by Major et al. as peak colonic gas excretion correlated with peak symptom intensity in those with worsening symptoms in response to inulin ingestion.27 In parallel, both Yang et al. (via lactose challenge) and Le Neve et al. (via lactulose challenge) reported that adults with IBS with the most pronounced challenge-related symptoms had visceral hypersensitivity.31, 32 Though visceral hypersensitivity was not formally assessed in our study, we found that study subjects had an increase in symptoms (bloating and flatulence) following fructan ingestion which may be attributed, in part, to visceral hypersensitivity. Central sensitization, as occurs in visceral hypersensitivity, is induced by repeated or sustained nociceptor inputs.33 For example, headache frequency has been shown to correlate positively with pain thresholds.34 We speculate that the increased pain frequency triggered by fructan ingestion may contribute to visceral hypersensitivity and the associated symptoms experienced by the subjects (bloating and flatulence).
While we found an increase in abdominal pain frequency during fructan ingestion, we did not find a change in the severity of abdominal pain episodes. The length of the interventions (72 hours) was short in the setting of IBS (a chronic disorder); a longer intervention period may be required to affect pain severity. In addition, the relatively low level of baseline pain severity (1.9/10) likely blunted the ability to detect differences in pain severity between the two challenges. Future studies evaluating visceral sensitivity in children with IBS undergoing food challenges for longer time periods are needed.
We did not identify any significant changes in stool form or frequency when comparing the fructan vs. maltodextrin interventions. This may be due in large part to the fact that the majority of subjects in our cohort had the most common pediatric IBS sub-type: IBS with constipation.18 This is supported by previous FODMAP intervention studies (albeit of longer duration) in adults with IBS found that when given higher FODMAP content foods, only those with IBS with diarrhea had more bowel movements and looser stools.5 In addition it should be noted that our study used a validated pediatric stool scale measure which has fewer stool form categories than the standard Bristol Stool Form Scale; thereby potentially decreasing the ability of children to discern more subtle changes in stool form.17, 18
Our study has some limitations. Though we used abdominal pain frequency as our a priori primary outcome measure in this study, others have recommended the usage of abdominal pain intensity as a primary measure, particularly for pediatric IBS pharmacological intervention trials.19 However, we note that pediatric self-reported abdominal pain intensity (as compared to identification of the presence of pain) may not be reliable with low inter-rater agreement.35 Therefore, we chose abdominal pain frequency in order to provide an objective assessment of abdominal pain (which has a significant negative impact on children with IBS) within the context of short interventions, and to maintain consistency with our previous work evaluating the role of fermentable carbohydrates in children with IBS.4, 36 Not all enrolled subjects completed the trial. However, we note that the vast majority of dropouts occurred during the baseline period and therefore are unlikely to have significantly affected the dietary intervention results. Another limitation inherent in crossover design trials relates to a potential carryover effect; however, we note that the minimum 10-day washout period far exceeded the dietary intervention lengths thus allowing for a return to symptom baseline prior to the next challenge as recommended by experts in the field.37
One of the major strengths of this study relates to its prospective, double blind, randomized, placebo controlled design. This design follows expert recommendations with respect to dietary intervention trials in functional gastrointestinal disorders.38, 39 Further study strengths include the use of validated childhood pain and stooling measures, and psychosocial distress measures. Finally, subjects met Rome III pediatric IBS criteria which is likely to increase the generalizability of the study findings to other children meeting the same diagnostic criteria.
In conclusion, in children with IBS, dietary fructans exacerbate symptoms including abdominal pain frequency, bloating, and flatulence. However, not all children are fructan sensitive and commonly used clinical parameters are not predictive of those who are/are not fructan sensitive. Further studies are needed in children (and adults) with IBS to better define factors which contribute to sensitivity to fructans (and other dietary components).
Supplementary Material
Acknowledgments
Grant Support: Financial and/or intellectual support during the conduct of the study was provided by NIH K23 DK101688 (BPC) and NIH R01 NR05337 and NR013497, the Daffy’s Foundation, and the USDA/ARS under Cooperative Agreement No. 6250-51000-043 (RJS), and P30 DK56338 which funds the Texas Medical Center Digestive Disease Center.
Abbreviations
- FODMAP
fermentable oligosaccharides disaccharides monosaccharides and polyols
- FOS
fructooligosaccharides
- ppm
parts per million
- HC
healthy children
- IBS
irritable bowel syndrome
Footnotes
Disclosures: The authors disclose no conflicts.
Writing Assistance: None
Author Contributions: Study concept and design (BPC, ARM, RJS); acquisition of data (AV, AA, SO, AE); analysis and interpretation of data (BPC, AV, ARM, RJS); drafting of the manuscript (BPC); critical revision of the manuscript and approval of the final version (all authors).
Registry: Clinicaltrials.gov: NCT02842281
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chumpitazi BP, Weidler EM, Lu DY, et al. Self-Perceived Food Intolerances Are Common and Associated with Clinical Severity in Childhood Irritable Bowel Syndrome. Journal of the Academy of Nutrition and Dietetics. 2016;116(9):1458–64. doi: 10.1016/j.jand.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohn L, Storsrud S, Tornblom H, et al. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–41. doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):707–17. doi: 10.1038/ajg.2013.96. [DOI] [PubMed] [Google Scholar]
- 4.Chumpitazi BP, Cope JL, Hollister EB, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther. 2015;42(4):418–27. doi: 10.1111/apt.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halmos EP, Power VA, Shepherd SJ, et al. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology. 2014;146(1):67–75. e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Newlove-Delgado TV, Martin AE, Abbott RA, et al. Dietary interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev. 2017;3:CD010972. doi: 10.1002/14651858.CD010972.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moshfegh AJ, Friday JE, Goldman JP, et al. Presence of inulin and oligofructose in the diets of Americans. J Nutr. 1999;129(7 Suppl):1407S–11S. doi: 10.1093/jn/129.7.1407S. [DOI] [PubMed] [Google Scholar]
- 8.Roberfroid MB. Inulin-type fructans: functional food ingredients. J Nutr. 2007;137(11 Suppl):2493S–502S. doi: 10.1093/jn/137.11.2493S. [DOI] [PubMed] [Google Scholar]
- 9.Niness KR. Inulin and oligofructose: what are they? J Nutr. 1999;129(7 Suppl):1402S–6S. doi: 10.1093/jn/129.7.1402S. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd SJ, Parker FC, Muir JG, et al. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6(7):765–71. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 11.Murray K, Wilkinson-Smith V, Hoad C, et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109(1):110–9. doi: 10.1038/ajg.2013.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carabin IG, Flamm WG. Evaluation of safety of inulin and oligofructose as dietary fiber. Regul Toxicol Pharmacol. 1999;30(3):268–82. doi: 10.1006/rtph.1999.1349. [DOI] [PubMed] [Google Scholar]
- 13.Patterson JK, Yasuda K, Welch RM, et al. Supplemental dietary inulin of variable chain lengths alters intestinal bacterial populations in young pigs. J Nutr. 2010;140(12):2158–61. doi: 10.3945/jn.110.130302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chumpitazi BP, Weidler EM, Shulman RJ. Lactulose Breath Test Gas Production in Childhood IBS is Associated with Intestinal Transit and Bowel Movement Frequency. J Pediatr Gastroenterol Nutr. 2016 doi: 10.1097/MPG.0000000000001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman RJ, Eakin MN, Jarrett M, et al. Characteristics of pain and stooling in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. 2007;44(2):203–8. doi: 10.1097/01.mpg.0000243437.39710.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Baeyer CL, Spagrud LJ, McCormick JC, et al. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143(3):223–7. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Chumpitazi BP, Lane MM, Czyzewski DI, et al. Creation and initial evaluation of a Stool Form Scale for children. J Pediatr. 2010;157(4):594–7. doi: 10.1016/j.jpeds.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weidler EM, Self MM, Czyzewski DI, et al. Stooling Characteristics in Children With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2017;15(1):140–1. doi: 10.1016/j.cgh.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saps M, van Tilburg MA, Lavigne JV, et al. Recommendations for pharmacological clinical trials in children with irritable bowel syndrome: the Rome foundation pediatric subcommittee on clinical trials. Neurogastroenterol Motil. 2016;28(11):1619–31. doi: 10.1111/nmo.12896. [DOI] [PubMed] [Google Scholar]
- 20.Garber J, Walker LS, Zeman J. Somatization symptoms in a community sample of children and adolescents: Further validation of the Children’s Somatization Inventory. Psychol Assess. 1991;3:588–95. [Google Scholar]
- 21.Schurman JV, Danda CE, Friesen CA, et al. Variations in psychological profile among children with recurrent abdominal pain. J Clin Psychol Med Settings. 2008;15(3):241–51. doi: 10.1007/s10880-008-9120-0. [DOI] [PubMed] [Google Scholar]
- 22.Chumpitazi BP, Weidler EM, Shulman RJ. Lactulose Breath Test Gas Production in Childhood IBS Is Associated With Intestinal Transit and Bowel Movement Frequency. J Pediatr Gastroenterol Nutr. 2017;64(4):541–5. doi: 10.1097/MPG.0000000000001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chumpitazi BP, Hollister EB, Oezguen N, et al. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes. 2014;5(2):165–75. doi: 10.4161/gmic.27923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107(5):657–66. doi: 10.1038/ajg.2012.49. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Banares F, Esteve-Pardo M, de Leon R, et al. Sugar malabsorption in functional bowel disease: clinical implications. Am J Gastroenterol. 1993;88(12):2044–50. [PubMed] [Google Scholar]
- 26.Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25(8):1366–73. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 27.Major G, Pritchard S, Murray K, et al. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology. 2017;152(1):124–33. e2. doi: 10.1053/j.gastro.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 28.Rajilic-Stojanovic M, Jonkers DM, Salonen A, et al. Intestinal Microbiota And Diet in IBS: Causes, Consequences, or Epiphenomena? Am J Gastroenterol. 2015;110(2):278–87. doi: 10.1038/ajg.2014.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIntosh K, Reed DE, Schneider T, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66(7):1241–51. doi: 10.1136/gutjnl-2015-311339. [DOI] [PubMed] [Google Scholar]
- 30.Bennet SMP, Bohn L, Storsrud S, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. 2017 doi: 10.1136/gutjnl-2016-313128. [Epub] [DOI] [PubMed] [Google Scholar]
- 31.Le Neve B, Brazeilles R, Derrien M, et al. Lactulose Challenge Determines Visceral Sensitivity and Severity of Symptoms in Patients With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2016;14(2):226–33. doi: 10.1016/j.cgh.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Fox M, Cong Y, et al. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: the roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment Pharmacol Ther. 2014;39(3):302–11. doi: 10.1111/apt.12582. [DOI] [PubMed] [Google Scholar]
- 33.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The journal of pain: official journal of the American Pain Society. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchgreitz L, Lyngberg AC, Bendtsen L, et al. Increased pain sensitivity is not a risk factor but a consequence of frequent headache: a population-based follow-up study. Pain. 2008;137(3):623–30. doi: 10.1016/j.pain.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Lavigne JV, Saps M. Pain Measurement in Children with Functional Abdominal Pain. Curr Gastroenterol Rep. 2016;18(4):20. doi: 10.1007/s11894-016-0493-1. [DOI] [PubMed] [Google Scholar]
- 36.Varni JW, Shulman RJ, Self MM, et al. Symptom Profiles in Patients With Irritable Bowel Syndrome or Functional Abdominal Pain Compared With Healthy Controls. J Pediatr Gastroenterol Nutr. 2015;61(3):323–9. doi: 10.1097/MPG.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 37.Yao CK, Gibson PR, Shepherd SJ. Design of clinical trials evaluating dietary interventions in patients with functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):748–58. doi: 10.1038/ajg.2013.77. [DOI] [PubMed] [Google Scholar]
- 38.Staudacher HM, Irving PM, Lomer MCE, et al. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017:1–10. doi: 10.1017/S0029665117002816. [DOI] [PubMed] [Google Scholar]
- 39.Suarez F, Levitt M. Assessing food intolerance: don’t lose control. Gut. 1997;41(5):715–6. doi: 10.1136/gut.41.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


