Abstract
Aims
This paper aims to report the rationale, design, and the specific methodology of an ongoing nested observational study that will determine the association of the metabolite and microbial composition of stool with fecal incontinence (FI).
Methods
Eligible cases are participants with FI enrolled in the Controlling Anal Incontinence in women by Performing Anal Exercises with Biofeedback or Loperamide (CAPABLe) trial, a Pelvic Floor Disorders Network trial across eight clinical centers in the United States. Women of similar age without FI in the last year served as controls. All subject collected stool samples at the baseline and 24-week visit at home using a standardized collection method. Samples were shipped to and stored at centralized laboratories.
Results
Specimen collection commenced December 2014 and was completed in May 2016. Fecal water and DNA has been extracted and is currently being analyzed by targeted metabolic profiling for stool metabolites and 16S rRNA gene sequencing for stool microbiota.
Conclusions
This article describes the rationale and design of a study that could provide a paradigm shift for the treatment of fecal incontinence in women.
INTRODUCTION
An estimated 9% of women in the United States suffer from fecal incontinence (FI) in the absence of a gastrointestinal (GI) malignancy.1 In these instances, FI etiology is poorly understood, and symptoms are typically attributed to obstetric injury. Prospective studies show that bowel symptoms related to gut motility and sensation such as diarrhea and fecal urgency are independent risk factors for FI, and more important than obstetric injury to the anal sphincter.2–4 Thus, there is a gap in knowledge regarding mechanisms underlying FI in women. Exploring factors that modulate gut sensation and motility, such as microbial stool metabolites, could provide insight into mechanisms underlying FI.
The human colon contains high densities of bacteria in concentrations of 1011 – 1012 cells/g of luminal contents belonging to more than 1000 species.5 Despite this wide diversity, in healthy adults, 80% of the fecal microbiota can be classified into three dominant phyla: Firmicutes, Bacteroidetes, and Actinobacteria. Genotypic sequencing studies based on 16S ribosomal RNA (16S rRNA)-encoding gene have demonstrated a core group of 18 species that are found in the stool of all individuals (Table 1).6 However, detection of microbial genes in a sample does not necessarily mean that these genes are functionally important. Metabolites produced by the bacteria provide a better insight into the functionality of the intestinal bacteria. Metabolomic analysis of fecal water has allowed the quantification of numerous metabolites in health and disease; a select list of metabolites that have been shown in human or animal studies to have a role in gut motility are listed in Table 2.7
Table 1.
Core group of 18 common bacterial species in human stool†
| Faecalibacterium prausnitzii SL3 3 |
| Roseburia intestinalis M50 1 |
| Bacteroides vulgatus ATCC 8482 |
| Bacteroides sp. 9_1_42FAA |
| Coprococcus comes SL7 1 |
| Ruminococcus sp SR1 5 |
| Bacteriodes xylanisolvens XB1A |
| Bacteroides sp. 2_1_7 |
| Bacteroides sp. 2_2_4 |
| Ruminococcus torques L2-14 |
| Bacteroides sp. D4 |
| Bacteroides dorei |
| Ruminococcus obeum A2-162 |
| Ruminococcus lactaris |
| Bacteroides capillosus |
| Bacteroides finegoldii |
| Clostridium sp M62 1 |
| Clostridium nexile |
Using 16S rRNA sequencing, 18 species listed above were identified in the stool specimens of all subjects (n=124), 57 in more ≥ 90% and 75 in ≥ 50% of individuals (From Qin et al, 2010).6
Table 2.
Microbial Metabolites in stool that have a putative role in gut motility†
| Short chain fatty acids (SCFA) | Luminal Gases | Secondary Bile Acids | Amino Acid Derivatives |
|---|---|---|---|
| Butyrate | Hydrogen sulfide (H2S) | Deoxycholic acid (DCA) | Tryptamine |
| Acetate | Hydrogen (H2) | Lithocholic acid (LCA) | |
| Propionate | Methane (CH4) | Ursodeoxycholic acid (UDCA) | |
| Lactate | IsoDCA | ||
| Succinate | IsoLCA | ||
| Valerate | |||
| Caproate | |||
| Isobutyrate | |||
| Isovalerate | |||
| Butyrate |
From Reigstad and Kashyap (2013)7
The neuro-hormonal mechanisms through which stool metabolites could alter gut motility, sensation, and stool consistency and therefore have a role in fecal incontinence are shown in Figure 1. For example, bacteria of the Clostridiales class and other bacteria ferment dietary fiber to produce butyrate and other a short chain fatty acids (SCFAs) such as acetate and propionate.7 In animal studies, butyrate has been implicated in enhanced neuronal excitability of the enteric nervous system and contractile responses of intestinal smooth muscle.8–9 Lower levels of butyrate colon transit time, and in humans, high luminal concentrations are associated with fecal urgency, disordered defecation, and altered stool consistency.10–11 Several additional metabolites that can diffuse through the mucus barrier of the gastrointestinal epithelium and affect neuronal excitability, muscle contractility, and epithelial permeability have been identified in stool samples. Bile acid metabolites, produced by intestinal microbiota, have a profound effect on intestinal motility.12 Tryptamine, a neuroactive bacterial metabolite resulting from the transformation of the aromatic amino acid tryptophan, mimics the effect of serotonin and increases gut contractility.7 In adults with irritable bowel syndrome, several studies have reported altered levels of multiple stool metabolites,13–14 and response to treatment has been shown to be mediated through a change in the level of stool metabolites acting on primary afferent neurons and enteric nervous system.15–16
Figure 1.
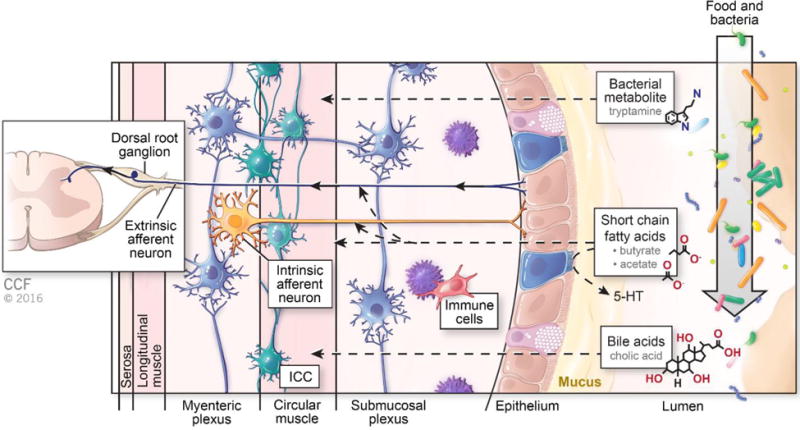
The presumed effects of stool metabolites on gut motility and sensation The complex effects of stool metabolites on gut motility and sensation are dependent on the diet and microbial make-up of the host. Food and bacteria enter the lumen of the bowel and produce bacterial metabolites. Short chain fatty acids such as butyrate and acetate affect GI motility through both direct effects on smooth muscle and production of mucosal serotonin (5-HT) (as outlined by the dashed black arrows). An additional action of butyrate is to activate both intrinsic and extrinsic afferent neurons in the dorsal root ganglion. Tryptamine, a bacterial metabolite, stimulates circular muscle of the bowel wall similar to serotonin. Bile acids also stimulate GI motility. Multiple cell types including enteric neurons (in blue), interstitial cells of Cajal (ICC, in teal) and immune cells such as macrophages (in red) also contribute to gut motility and sensation.
Thus we hypothesize that the stool metabolome and microbiome are associated with FI and may be implicated in response to therapy. Our primary aim is to compare the baseline stool metabolites between women with FI and unaffected similarly aged controls. Secondary aims include comparisons of microbial composition of stool at baseline, and to assess if the higher levels of metabolites predict response to FI therapy after 24 weeks. In this manuscript, we detail the specific methods used in our study, and in particular some of the unique methodologic challenges that must be considered for translational research conducted within a multi-institutional network.
METHODS
Study overview
Stool Metabolite and Microbiome (SMM)-Controlling Anal incontinence in women by Performing Anal exercises with Biofeedback or Loperamide (CAPABLe) is a prospective multi-center observational study that is nested within the CAPABLe randomized controlled trial investigating the efficacy of non-surgical treatments for fecal incontinence (NCT02008565).17 Institutional Review Board approval for SMM-CAPABLe was obtained from each of the eight participating sites in the Pelvic Floor Disorders Network (PFDN) sponsored by the Eunice Kennedy Shriver NICHD and the NIH Office of Research on Women’s Health. All participants provided written, informed consent. All women (cases and controls) participating in SMM-CAPABLe contributed a stool specimen at baseline and after 24 weeks. Stool samples are being analyzed for metabolites (including butyrate and other SCFAs, bile acids, and tryptamine) using targeted metabolite profiling of fecal water. The composition of microbiota in stool is being analyzed using 16S rRNA gene sequencing. The planned sample size for this study is 82 cases and 41 unaffected controls.
Participants
In CAPABLe, participants were randomized using a 2×2 factorial design to one of four treatments: loperamide, anorectal biofeedback, combined loperamide and anorectal biofeedback or oral placebo for loperamide. Cases for SMM-CAPABLe are women with FI who are participating in CAPABLe. Similarly aged controls without FI were recruited from each of the eight participating sites.
Eligibility
Selection of Cases
Cases consisted of women enrolled in the CAPABLe trial and willing to participate in SMM CAPABLe without any of the additional exclusion criteria specific to this study (Table 3). To be eligible to participate in CAPABLe, participants had to be age 18 and over with fecal incontinence and desiring treatment. Fecal incontinence was defined as any uncontrolled loss of liquid or solid fecal material that occurs at least monthly over the last 3 months that is bothersome enough to desire treatment.18–19 Enrollees age 50 to 75 years must have had negative colon cancer screening based on the US Preventative Task Force recommendation in 2008.
Table 3.
Exclusion criteria for Cases participating in SMM-CAPABLe
|
All participants of SMM-CAPABLe met the exclusion criteria for CAPABLe. Factors known to affect stool metabolites and microbial composition of stool were added such as HIV infection and treatment with antibiotics within the previous two weeks.20 Women did not collect a stool specimen if they had suffered a bout of infectious gastroenteritis within the previous month. Women on probiotics were not excluded, but this information was collected as a covariate for analysis.
Selection of Controls
Controls were women who are not affected by fecal incontinence defined strictly as a ‘never’ or ‘no’ response to all items of the St. Mark’s Vaizey score.21 All of the conditions listed in exclusion criteria for cases could be associated with abnormal stool metabolome and were therefore exclusion criteria for controls as well. Additional exclusion criteria for controls in SMM CAPABLe are listed in Table 4. Controls were selected to have an age distribution similar (within 5 years) to the cases.
Table 4.
Exclusion criteria for Controls participating in SMM-CAPABLe
|
Specimen collection and Mailing to Central Laboratory and Processing
Following usual consent procedures, participants were given kits to collect stool samples at home. Patients collected a stool sample: 1) prior to initiating baseline CAPABLe study procedures and 2) within one week of end of treatment visit at 24 weeks. Controls also collected two samples 24 weeks apart. At each time point, the participant shipped a portion of the stool sample to Colorado State University Proteomics and Metabolomics Facility (on frozen gel packs that maintain temp of −23°C) for metabolite analysis and another portion to University of Alabama at Birmingham Microbiome Resources in Cary-Blair medium (at room temperature) for microbiota analysis. Samples were shipped within 24 hours of collection using the provided packaging for overnight shipping. At each lab, the stool samples were frozen at −80° C until analysis.
Laboratory Procedures
Fecal water was extracted from frozen stool samples and targeted metabolite profiling was performed to measure metabolites in stool. A portion of fecal water has been stored for possible future analyses of additional metabolites for untargeted metabolite profiling. Detailed description of analytic methods for stool metabolites is included in Appendix 1. Microbial composition of stool will be measured using 16S rRNA gene sequencing. Detailed description of methods is in Appendix 2.
Clinical Variables
Several clinical variables that could potentially be associated with stool metabolites and microbiota were collected including fecal incontinence, diagnosis of irritable bowel syndrome, dietary fiber intake, and concurrent medications including probiotics.
Power Calculations
A review of prior published studies shows that the proportion of butyrate/total SCFA levels in stool samples ranged from 17.9% to 31% with standard deviations of 5% to 8%.13, 22 Our sample size calculation is conservatively based on a standard deviation of 8% for the proportion of butyrate/total SCFA levels. Data on levels of proportion of butyrate/total SCFA levels in stool samples in women with fecal incontinence are not available. In studies of irritable bowel syndrome, the relative proportion of butyrate in stool samples was 30% higher than controls.13 At an alpha of 0.05, a sample size of 82 participants and 41 controls will give us greater than 90% power to detect a difference of 5% in butyrate levels in stool samples between participants with FI and controls.
Data on the proportional abundance of Clostridiales in women with FI are not available. Jeffery et al (2012) reported that the proportional abundance of Clostridiales between subtypes of IBS differed by 0.6 standard deviations.14 Based on this assumption and a type I error threshold of 0.05, our planned enrollment of 82 participants with fecal incontinence and 41 controls will provide >90% power to detect a difference in proportional abundance of Clostridiales between women with FI and controls.
Analysis Plan
This study will examine a number of stool metabolites, with the primary outcome measure being butyrate level expressed as a percentage of total SCFA. Secondary outcomes are the proportions of other short chain fatty acids (SCFA), including acetate, propionate, lactate, succinate, valerate, caproate, isobutyrate, and isovalerate, also reported as a percentage of total SCFA. All SCFA including butyrate will also be reported as milligram/gram (mg/g) of stool. Other stool metabolites including bile acids (cholic, deoxycholic, chenodeoxycholic and lithocholic acids), 5-hydroxytryptamine (5-HT), tryptamine, and 5-HIAA will be reported as mg/g of stool. To determine the microbial composition of stool, sequences at a 97% sequence identity will be clustered into OTUs (Operational Taxonomic Units) using the GreenGenes taxonomic database and Quantitative Insights Into Microbial Ecology (QIIME) software. The proportion of OTUs at the phylum, class, order, family, and genus, and possibly the species taxonomic levels will be expressed as a proportion of the total number of OTUs.
Baseline proportional levels of all stool metabolites and microbiota genera/taxa will be summarized using mean, standard deviation, median, minimum and maximum. Pie charts to visualize the taxonomic levels at the phylum and the genus level for women with FI and controls will be constructed. The hypothesis for the primary objective comparing baseline proportional level of butyrate in women with FI and unaffected controls will be tested using probit analysis adjusted for age and dietary fiber intake (in grams). P values of < .05 will be considered statistically significant. Other metabolites and baseline proportional levels of Clostridiales and other genera/taxa will be compared between cases and controls using similar models. Comparisons will be conducted at the phylum, class, order and family taxonomic levels. Adjustments for multiple comparisons will be made using the false discovery rate method of Benjamini and Hochberg.23
To compare microbial diversity at baseline between women with FI and controls, first indices of alpha diversity (diversity within a sample) and beta diversity (differentiation between samples of same type) will be compared between women with FI and controls (gamma diversity) using different indices for richness (number of OTUs/species present in a sample) and evenness (relative abundance of different OTUs/species and their even distribution).24–25 The following metrics will be determined: observed species (measures unique OTUs in the sample), Chao (estimates the species richness), Shannon index (measures both richness and evenness with higher numbers indicating greater diversity), Simpson’s index (measures both richness and evenness, but less affected by the presence of rare species when compared to Shannon’s index), and phylogenetic distance (includes phylogenetic distance into the diversity calculation).
The dissimilarity in microbiota of women with FI and controls will be compared using UniFrac and statistical significance will be measured using PERMANOVA or similar statistical methods (e.g. PERMDISP which are a part of QWRAP).25 Unsupervised principal coordinates analysis (PCoA) will be used to visualize the clustering pattern of samples in women with FI and controls in order to identify potentially more homogenous subsets of microbiota samples within the cases and controls. Potential clusters will be compared to clinical covariates, demographic factors, and treatment groups.
The relationship between baseline proportional levels of butyrate and response to treatment (change in Vaizey score at 24 weeks) will be examined in the group of women with FI using linear models. Since the change in Vaizey score will be assessed at both 12 and 24 weeks in CAPABLe, the analysis will be based on a longitudinal model, with changes from baseline in Vaizey score at both 12 and 24 weeks as the dependent variable, and the independent variables including time, baseline proportional levels of butyrate, interactions between butyrate, treatment assignment, and time, and the following covariates known to affect butyrate levels: age, Rome III IBS clinical trial status, and dietary fiber intake.
RESULTS
Patient recruitment for the CAPABLe trial started in April 2014 and for SMM-CAPABLe in November 2014. Follow-up for both studies was completed in May, 2016. Analyses are ongoing.
DISCUSSION
The described methods and associated study results will provide insight into metabolomic and microbial factors that may be important in the mechanism and treatment of FI. The fundamental hypothesis of this study reflects that the interaction of the indigenous microbiome with substrates provided by dietary intake may result in the production of modifiable metabolic byproducts that modulate stool consistency and intrinsic bowel function. Our findings could provide new approaches for the treatment of fecal incontinence such as manipulation of gut microbiota through prebiotics, probiotics, or fecal microbiota transplantation.
The described methods have several unique strengths. Specimens are directly collected by patients and delivered in a standardized time sensitive, temperature regulated manner. A significant barrier to research in the area of FI is the significant stigma and embarrassment many patients experience in collecting stool specimens. By enabling patients to collect specimens in the privacy of their homes, we eliminated a significant barrier. Once obtained, these specimens were sent to centralized laboratories performing the analyses of stool metabolites and microbiota and were immediately processed and stored until batch analyzed using proven standardized protocols.
Women with extremes in stool consistency (i.e., watery diarrhea and hard, pebble like stools) were excluded allowing us to advance our understanding of stool microbiome and metabolome in women with stool of a more ‘normal’ consistency (Bristol scale types 2–6). Based on published NHANES data, women with loose, watery stool are at increased risk of FI, with an odds ratio of 2.8 (95% CI 1.9, 4.1).2 In a prospective study, diarrhea was associated with a 3.8 increased risk for the development of new onset FI.3 In that study, adults with >3 bowel movements a day were at 8 times increased risk for FI than adults with one or less bowel movements a day. Other studies have implicated constipation as an independent risk factor for FI in women with pelvic floor disorders.4 These studies suggest that factors that cause fecal urgency and alter the gut motility (either diarrhea or constipation) could predispose to the development of FI. By eliminating Bristol stool types 1 and 7 we reduce confounding from functional diarrhea, functional constipation, and varied conditions that could cause fecal incontinence such as severe irritable bowel syndrome, severe lactose intolerance, hepatic and pancreatic disorders. However, the information that is obtained from women with a normal spectrum of stool consistency could inform the treatment of these extreme types.
Importantly, this study has biologic plausibility and is an extension of previous work. Kamath et al reported that butyrate and other SCFAs stimulate intestinal motility and increase fecal urgency in healthy volunteers.10 In that study, intraluminal pressure was recorded in the ileocolic region in 18 human healthy volunteers after instillation of boluses of SCFAs, air, and saline. Instillation of SCFAs was associated with symptoms of cramps and an urge to defecate even at small volumes. The motility stimulated by the SCFAs was not associated with systemic release of gastrointestinal regulatory peptides suggesting that SCFAs are associated with the motor response of coloileal reflux of the enteric nervous system in humans. Repetitive instillation of larger doses of butyrate enemas is associated with decrease in visceral perception, likely due to overstimulation and subsequent desensitization of TRPV1 receptors.11 These findings suggest a ‘threshold effect’ for butyrate where levels above a certain ‘threshold level’ cause fecal urgency but very high levels of rectal butyrate reduce rectal sensation.26 Since both rectal hypersensitivity and lack of rectal sensation can cause fecal incontinence, these studies provide biologically plausible mechanisms through which elevated levels of fecal butyrate could potentially cause FI. We believe that it is important to study the role butyrate plays in the pathogenesis of FI and that it may represent an area for novel therapy. There is a gap in knowledge regarding the contributions and mechanisms underlying FI in women who have stools that are not in the extremes of spectrum of consistency, and this study will allow us to more fully understand the contribution of the microbiome and metabolome to this quality of life altering condition.
Our study is limited by the potential confounding effect of dietary factors, co-existent diseases, and drugs on stool metabolites and microbiota. Though short-term dietary interventions in healthy humans can lead to statistically significant and rapid alterations in the composition of the intestinal microbiota, the magnitude of the effect is modest relative to inter-subject variability in the intestinal microbiota.27 Pronounced effects on the human microbiota are induced by extreme changes in diet such as a change from a complete ‘animal-based diet’ to a complete ‘plant-based diet’.28 We have adopted several measures to assess and reduce variability among participants. A brief dietary fiber questionnaire will allow us to identify patients who have extremes of diet. We are excluding participants with conditions that could potentially affect stool microbial composition such as recent treatment with antibiotics and HIV infection. Finally, during analysis, we plan to assess ‘outliers’ of stool metabolites and microbiota for use of immunosuppressive therapies and/or probiotics and also measure the interaction between butyrate and treatment assignment, and control for the following covariates known to affect butyrate levels: age, Rome III IBS clinical trial status, and dietary fiber intake.
CONCLUSION
The methods described in this paper outline a plan for patient-centered translational research around FI. We anticipate that the description of these methods and the rationale for this approach will help other researchers interested in this debilitating, frequently undisclosed condition.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the contributions of Tiffany Weir, PhD., Colorado State University Proteomics and Metabolomics Facility, in preparing and storing stool specimens.
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (2U01HD41249, 2U10 HD41250, 2U10 HD41261, 2U10 HD41267, 1U10 HD54136, 1U10 HD54214, 1U10 HD54215, and 1U10 HD54241) and the National Institutes of Health Office of Research on Women’s Health. Dr. Kashyap’s effort was supported in part by NIH K08 DK 100638
Contributor Information
Lily A. Arya, Division of Urogynecology and Pelvic Reconstructive Surgery, Department of Obstetrics & Gynecology, University of Pennsylvania, Philadelphia, PA.
Holly E. Richter, Division of Urogynecology and Pelvic Reconstructive Surgery, Department of Obstetrics & Gynecology, University of Alabama at Birmingham, Birmingham, AL.
J. Eric Jelovsek, Center for Urogynecology and Reconstructive Pelvic Surgery, Obstetrics & Gynecology and Women’s Health Institute, Cleveland Clinic, Cleveland, OH.
Marie Gantz, Social, Statistical & Environmental Sciences, RTI International, Research Triangle Park, NC.
Sara Cichowski, Division of Female Pelvic Medicine and Reconstructive Surgery, Department of Obstetrics & Gynecology, University of New Mexico Health Sciences Center, Albuquerque, NM.
Halina Zyczynski, Women’s Center for Bladder and Pelvic Health, Division of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh Medical Center, Pittsburgh, PA.
Keisha Dyer, Division of Female Pelvic Medicine & Reconstructive Surgery, Department of Reproductive Medicine, UC San Diego Health System, San Diego, CA.
Nazema Siddiqui, Division of Urogynecology, Department of Obstetrics and Gynecology, Duke Medical Center, Durham, NC.
Cassandra Carberry, Division of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics & Gynecology, Alpert Medical School of Brown University, Providence, RI.
Corey Broeckling, Colorado State University Proteomics and Metabolomics Facility.
Casey Morrow, University of Alabama Microbiome Resources.
Purna Kashyap, Division of Gastroenterology, Department of Internal Medicine, The Mayo Clinic, Rochester, MN.
Susie Meikle, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD for the Pelvic Floor Disorders Network.
References
- 1.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, Spino C, Whitehead WE, Wu J, Brody DJ, Pelvic Floor Disorders Network Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311–6. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehead WE, Borrud L, Goode PS, Meikle S, Mueller ER, Tuteja A, Weidner A, Weinstein M, Ye W, Pelvic Floor Disorders Network Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512–7. 517.e1–2. doi: 10.1053/j.gastro.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rey E, Choung RS, Schleck CD, Zinsmeister AR, Locke GR, 3rd, Talley NJ. Onset and risk factors for fecal incontinence in a US community. Am J Gastroenterol. 2010;105:412–9. doi: 10.1038/ajg.2009.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559–66. doi: 10.1053/j.gastro.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamer HM, De Preter V, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol. 2012;302:G1–9. doi: 10.1152/ajpgi.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, MetaHIT Consortium. Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reigstad CS, Kashyap PC. Beyond phylotyping: understanding the impact of gut microbiota on host biology. Neurogastroenterol Motil. 2013;25:358–72. doi: 10.1111/nmo.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–82. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 9.Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA, Higginbottom SK, Million M, Tache Y, Pasricha PJ, Knight R, Farrugia G, Sonnenburg JL. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144:967–77. doi: 10.1053/j.gastro.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamath PS, Phillips SF, Zinsmeister AR. Short-chain fatty acids stimulate ileal motility in humans. Gastroenterology. 1988;95:1496–502. doi: 10.1016/s0016-5085(88)80068-4. [DOI] [PubMed] [Google Scholar]
- 11.Vanhoutvin SA, Troost FJ, Kilkens TO, Lindsey PJ, Hamer HM, Jonkers DM, Venema K, Brummer RJ. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. 2009;21:952–e76. doi: 10.1111/j.1365-2982.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 12.Ridlon JM, Hylemon PB. Identification and characterization of two bile acid coenzyme A transferases from Clostridium scindens, a bile acid 7α-dehydroxylating intestinal bacterium. J Lipid Res. 2012;53:66–76. doi: 10.1194/jlr.M020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23:280–6. doi: 10.1097/00005176-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 15.McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 2013;25:183–e88. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 16.Wu RY, Pasyk M, Wang B, Forsythe P, Bienenstock J, Mao YK, Sharma P, Stanisz AM, Kunze WA. Spatiotemporal maps reveal regional differences in the effects on gut motility for Lactobacillus reuteri and rhamnosus strains. Neurogastroenterol Motil. 2013;25:e205–14. doi: 10.1111/nmo.12072. [DOI] [PubMed] [Google Scholar]
- 17.Jelovsek EJ, Markland AD, Whitehead WE, Barber MD, Newman DK, Rogers RG, Dyer K, Visco A, Sung VW, Sutkin G, Meikle SF, Gantz MG, Pelvic Floor Disorders Network Controlling anal incontinence in women by performing anal exercises with biofeedback or loperamide (CAPABLe) trial: Design and methods. Contemp Clin Trials. 2015 doi: 10.1016/j.cct.2015.08.009. pii: S1551-7144(15)30067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothbarth J, Bemelman WA, Meijerink WJ, Stiggelbout AM, Zwinderman AH, Buyze-Westerweel ME, Delemarre JB. What is the impact of fecal incontinence on quality of life? Dis Colon Rectum. 2001;44:67–71. doi: 10.1007/BF02234823. [DOI] [PubMed] [Google Scholar]
- 19.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, Wexner SD, Bliss D, Lowry AC. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–32. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 20.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4554–61. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44:77–80. doi: 10.1136/gut.44.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One. 2013;8:e70803. doi: 10.1371/journal.pone.0070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 24.Jost L. Partitioning diversity into independent alpha and beta components. Ecology. 2007;88:2427–39. doi: 10.1890/06-1736.1. Erratum in: Ecology. 2009;90:3593. [DOI] [PubMed] [Google Scholar]
- 25.Lozupone C, Hamady M, Knight R. UniFrac–an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. 2006;7:371. doi: 10.1186/1471-2105-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kannampalli P, Shaker R, Sengupta JN. Colonic butyrate- algesic or analgesic? Neurogastroenterol Motil. 2011;11:975–9. doi: 10.1111/j.1365-2982.2011.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu GD, Lewis JD. Analysis of the human gut microbiome and association with disease. Clin Gastroenterol Hepatol. 2013;11:774–7. doi: 10.1016/j.cgh.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


