Abstract
Adeno-associated viral (AAV) gene delivery to skeletal muscle is being explored for systemic delivery of therapeutic proteins. To better understand the signals that govern antibody formation against secreted transgene products in this approach, we administered an intramuscular dose of AAV1 vector expressing human coagulation factor IX (hFIX), which does not cause antibody formation against hFIX in C57BL/6 mice. Interestingly, co-administration of a TLR9 agonist (CpG-deoxyoligonucleotide, ODN) but not of lipopolysaccharide, caused a transient anti-hFIX response. ODN activated monocyte-derived dendritic cells and enhanced T follicular helper cell responses. While depletion of regulatory T cells (Tregs) also caused an antibody response, TLR9 activation combined with Treg depletion instead resulted in prolonged CD8+ T cell infiltration of transduced muscle. Thus, Tregs modulate the response to the TLR9 agonist. Further, Treg re-population eventually resolved humoral and cellular immune responses. Therefore, specific modes of TLR9 activation and Tregs orchestrate antibody formation in muscle gene transfer.
Keywords: Adeno-associated virus, regulatory T cell, factor IX, tolerance, antibody formation, CD8+ T cell, toll-like receptor, TLR9, gene therapy, muscle
1. Introduction
Gene delivery to skeletal muscle is not only being developed for the treatment of muscular disorders, but also for systemic delivery of therapeutic proteins and production of antibodies that protect against viral pathogens such as HIV. However, neutralization of systemic expression by antibody formation has emerged as a major hurdle for this approach [1–3].
Initiation of antigen-specific adaptive immune responses, mediated by B and T cells, depends on a variety of cellular and receptor-driven recognition events. For example, innate immune sensing of pathogen-associated molecular patterns (PAMPs), such as unmethylated DNA of viral or bacterial genomes, viral capsids, or bacterial cell wall components, induce pattern recognition receptors (PRRs) to provide potent activation signals to specific immunocytes. Particularly well-characterized PRRs are the toll-like receptors (TLRs), which upon recognition of specific ligands induce immunomodulatory cytokine expression by dendritic cells (DCs) and macrophages, which consequently promote the differentiation of T helper 1 (TH1) or TH17 cells [4]. By contrast, TLR signals that govern regulatory T cells (Tregs, which suppress immune responses), are less well understood.
Adeno-associated viral (AAV) gene transfer represents the most widely utilized in vivo approach to systemic delivery of proteins by expression in skeletal muscle. A limitation to this method is the potential for the development of a local immune response, resulting in B and T cell activation in draining lymph nodes [3, 5, 6]. Vector dose, serotype, delivery method, and intrinsic properties of the expressed antigen are among the factors that impact the immune response [5, 7–9]. Depending on the serotype and level of transgene expression in dendritic cells (DCs), direct or cross-presentation of transgene product-derived antigen by professional antigen presenting cells (APCs) contributes to activation of lymphocytes [6, 10, 11]. Intrinsic properties of the antigen and genetic factors of the host influence the extent of cytotoxic CD8+ T cell and B cell activation and subsequent antibody formation following gene delivery to the muscle. Recently, evidence was provided that activation of CD8+ T cells can be increased by antibody formation, thus illustrating interactions between cellular and humoral immune responses [9]. Conversely, endogenously induced or adoptively transferred CD4+CD25+FoxP3+ regulatory T cells (Tregs) can suppress immune responses in muscle-directed AAV gene transfer [12–15]. Orally induced tolerance, which may be mediated by non-FoxP3+ Treg, can also suppress responses to the transgene product in skeletal muscle [16].
CD8+ T cell responses to the transgene product are dependent on endosomal sensing of the AAV genome by TLR9, which rapidly induces type I interferon (IFN) and pro-inflammatory cytokine expression [17, 18]. TLR9 recognizes, in particular, unmethylated oligodeoxynucleotides (ODN) containing CpG motifs, which are typical for viral and bacterial DNA genomes. Importantly, elimination of CpG motifs in the AAV vector genome substantially reduces CD8+ T cell activation, thus avoiding elimination of transduced muscle fibers by cytotoxic T lymphocytes (CTL) [17]. On the other hand, antibody formation against the transgene product and the viral capsid occur largely independently of sensing of the viral genome by TLR9 following AAV gene transfer (although there is a modulatory effect on the ratio of Th1 vs Th2-dependent antibodies). Therefore, antibody formation is not overtly affected by CpG depletion [17, 19].
Here, we seek to elucidate a role for Tregs and for TLR activation in regulating the antibody response to a secreted protein in muscle-directed AAV gene transfer. AAV serotype-1 (AAV1) for muscle-directed expression of human coagulation factor IX (hFIX) in C57BL/6 mice was chosen as an experimental model for B cell unresponsiveness to a secreted transgene product. While intramuscular administration of AAV vector typically results in antibody responses against hFIX in mice, anti-hFIX formation is limited for this serotype/strain combination within a certain vector dose range. Therefore, this strategy allowed us to identify mechanisms that drive antibody formation. Interestingly, we revealed that ligands for TLR2 and TLR4, which are potent activators of DCs and of inflammatory responses, failed to induce antibody formation. Whereas, a TLR9 ligand that is known to enhance T follicular helper (Tfh) cell responses via activation of monocyte-derived DCs (moDCs) induced anti-hFIX formation [20]. This outcome suggests that only specific inflammatory signals can override the suppressive effects of Tregs and thus promote antibody formation against the transgene product. We also found that Treg depletion caused a potent anti-hFIX response. Surprisingly though, Treg depletion combined with TLR9 activation failed to induce anti-hFIX formation and instead resulted in a robust inflammatory CD8+ T cell response in the muscle. Hence, Treg are important modulators of the response to TLR9 signaling.
2. Materials and Methods
2.1 Viral vectors
Single-stranded AAV serotype 1 vector expressing human F9 cDNA under the control of the cytomegalovirus immediate early enhance/promoter (AAV1-CMV-FIX) was as described [7, 21]. Vector was produced by triple transfection of HEK-293 cells, purified by differential precipitation with polyethylene glycol followed by CsCl gradient centrifugation, filter sterilized, and quantified by silver staining and slot-blot hybridization as described elsewhere [22].
2.2 Animal experiments
Wild-type male C57BL/6 mice (4–6 weeks old) and B6.129(Cg)-Foxp3tm3(DTR/GFP)Ayr/J (DTRFoxp3gfp+) breeder pairs were obtained from Jackson Laboratory (Bar Harbor, ME). TLR9-deficient mice were as published [17]. Mice received intramuscular (IM) administration of vector at a dose of 1–2×1011 vector genomes per animal) by injection into the tibialis anterior (25 µl) and the quadriceps muscle (50 µl) of a single hind leg, using a Hamilton syringe. Plasma samples were obtained from blood collected from the retro-orbital plexus in heparinized capillary tubes. TLR agonists were delivered at 50 µg/mouse mixed with the vector formulation. These were the type B CpG oligodeoxynucleotide (ODN-1826, an agonist for murine TLR9); or an intermediate purity preparation of lipopolysaccharide (LPS) from P. gingivalis (LPS-PG) that activates TLR2 and TLR4; or a highly purified LPS from E. coli that activates TLR4 only (LPS-EC). To deplete Tregs in DTR-Foxp3gfp+ mice, mice received two IP injections of diphtheria toxin (DT) 50 µg/kg on days 2 and 5 after gene transfer. The animals were breed and housed under specific pathogen-free conditions at the University of Florida and treated under approved protocols of the Institutional Animal Care and Use Committee. Experiments, in which mice were injected with ODN1826 or DT, were performed at least twice.
2.3 Measurement of cell frequencies by flow cytometry
For CD4+CD25+Foxp3+ T cells, splenocytes were isolated using standard methods. Nuclear stain for transcription factor FoxP3 was performed with the BD Biosciences (San Jose, CA) kit. Briefly, splenocytes (1×106 per mouse) were stained with fluorescent conjugated antibodies, including anti-mouse CD4-pacific blue, anti-mouse CD25-PE and anti-mouse FoxP3-Alexa Fluor 647 according to staining protocol. For TfH cell staining, anti-CXCR5-BV421, anti-CD4-BV605, anti-PD-1-APC, anti-B220-FITC, and anti-Bcl6-PE were purchased from BD Bioscience (San Jose, CA). Fixable viability dye was purchased from eBioscience (San Diego, CA). For dendritic cell characterization, antiCD11b, anti-CD11c, and anti-CD64 were purchased from eBioscience (San Diego, CA). FACS analysis using LSR-II instrumentation from BD Biosciences (San Jose, CA) and the FCS Express software (DeNovo Software).
2.4 Analyses of mouse plasma samples and ELISpot
Levels of hFIX in plasma samples were determined by enzyme-linked immunosorbent assay (ELISA), and antibody concentrations specific to hFIX were measured by immunoglobulin subclass-specific immunocapture assay as published [12]. For ELISpot assay, splenocytes were harvested at 2 weeks after gene transfer and cultured. Splenocytes (300,000 per well) from individual mice were incubated in triplicate for 18 hours in an ELISpot plate coated with capture antibody for IFN-γ (R&D systems, Minneapolis, MN) with media alone, 10 µg/mL of recombinant hFIX or concanavalin A. A biotinylated secondary detection IFN-γ antibody (R&D systems, Minneapolis, MN) was added overnight at 4°C. Detection/Development utilized streptavidin-AP (R&D systems, Minneapolis, MN) in 100 µl of dilution buffer for 2 hours at room temperature, followed by color development with 100 µl of BCIP/NBT chromogen (R&D systems, Minneapolis, MN). IFN-γ producing cells were counted with the CTL-ImmunoSpotH S5 UV analyzer (Cellular Technology, Shaker Heights, OH).
2.5 Histochemical analysis
Mouse muscle tissue was snap-frozen in liquid isopentene as described [23]. Cryosections of muscle tissue were analyzed for the presence of hFIX and CD8 cellular infiltrate by immunofluorescence staining as previously reported [23, 24]. Tissue were blocked with 5% donkey serum (Sigma, St. Louis, MO), and stained with rat anti-CD8α (eBioscience, San Diego, CA) and goat anti-hFIX (Affinity Biologicals, Ontario, Canada). Secondary antibody donkey anti-rat Alexa Fluor 488 and donkey anti-goat Alexa Fluor 568 (Life Technologies, Eugene, OR) were used for detection. Stained sections were viewed with the Eclipse E800 microscope (Nikon, Tokyo, Japan).
2.6 Adoptive T-cell transfer
Spleens from C57BL/6 mice were harvested from animals 2 months after muscle gene transfer with AAV1-hFIX vector and from age matched naive animals into 2-MLC media (DMEM, 2% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, 10 mM HEPES, 0.1 mM non-essential amino acids, 10-6 M 2-mercaptoethanol, and antibiotics) at room temperature, homogenized, filtered through a 70-µm cell strainer, and centrifuged at 300 g for 10 min. Cells were subsequently incubated with ACK lysing buffer (BD Bioscience) for 5 min and washed twice with 2-MLC medium. Viable splenocytes were counted with a hemocytometer and trypan blue. The CD4+CD25+ T cell isolation kit (Miltenyi, Auburn, CA) was used to purify Tregs from bulk splenocytes by magnetic cell sorting as described [25]. Purified CD4+CD25+ cells were pooled for each experimental group of donor mice and delivered to naive C57BL/6 mice at 1×106 by tail vein injection. Recipient mice were immunized with 5µg hFIX in Complete Freund’s Adjuvant (CFA) 24 hours after adoptive transfer.
2.7 Statistics
Unless otherwise specified, results are reported as means ± SEM. Significant differences between groups were determined with unpaired Student’s t test, the Mann-Whitney U test, or two-way ANOVA with Bonferroni posttests, as appropriate. P values <0.05 were considered significant. Analyses were performed using Graph Pad Prism (San Diego, Calif., USA).
3. Results
3.1 Antibody formation against hFIX in muscle gene transfer is induced by TLR9 but not by TLR2 or TLR4 agonists
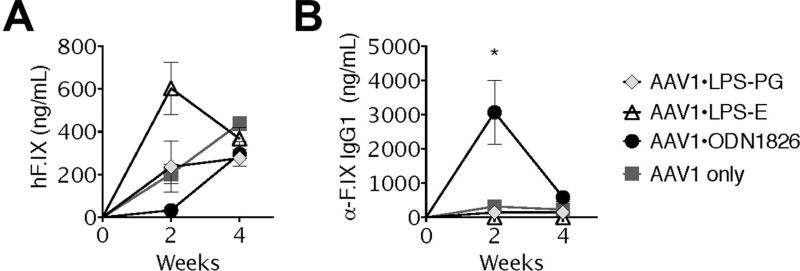
One of the first examples of long-term AAV-mediated transgene expression in an immune competent animal resulted from muscle-directed in vivo transfer of a LacZ gene, encoding a heterologous cytoplasmic protein of bacterial origin (β-galactosidase) [26]. However, it was subsequently noted that expression of secreted “non-self” proteins from skeletal muscle typically elicits an antibody response [6]. An exception to this has been shown with the expression of hFIX from an AAV1 vector within a narrow range of vector doses in C57BL/6 mice [12]. Intramuscular injection using 1–2×1011 vector genomes (vg) of AAV1-hFIX can achieve sustained expression of hFIX, with limited anti-hFIX response. Here, we tested 3 different TLR agonists for an adjuvant effect that may enhance anti-hFIX antibody formation. LPS-PG activates TLR2 and TLR4, while LPS-EC activates TLR4 only. ODN-1826 is a type B oligodeoxynucleotide (CpG-B) that contains an unmethylated phosphorothioate backbone with CpG motifs, which are known to activate TLR9 [27]. Therefore, to assess the role of TLR activation on the formation of neutralizing anti-transgene antibodies, cohorts of C57BL/6 mice were injected with a solution containing AAV1-hFIX alone (or mixed with one of the TLR agonists) into skeletal muscle. Analysis of plasma collected at 2 and 4 weeks after immunization revealed that only ODN-1826 induced anti-hFIX IgG1 formation, which was transient and correlated with an initial lack of systemic hFIX expression (Fig. 1A–B). Both LPS-PG and LPS-EC failed to induce IgG1 (or IgG2a) formation and thus did not affect systemic hFIX expression (Fig. 1A–B and data not shown). Notably, control mice injected with vector only expressed circulating hFIX protein in the absence of antibodies. Once the antibody response in ODN-1826 treated mice subsided, circulating hFIX antigen increased to a level similar to that of the control mice, suggesting that administration of the TLR9 agonist did not negatively effect gene transfer or transgene expression or induce an effective cellular immune response against transduced muscle.
Figure 1. Effects of TLR stimulation on systemic expression of and humoral immune responses to hFIX as a function of time after muscle-directed gene transfer.
Comparison of systemic hFIX antigen (A) and IgG1 anti-hFIX (B) levels in C57Bl/6 mice after intramuscular co-injection of 1011vg AAV1-hFIX and 50 µg of indicated TLR agonist. (Data represent averages +/−SEM for n=3–4 mice per cohort; *P<0.05 AAV1+TLR9 v. AAV1 only). Experiement was repeated at least twice.
3.2 Depletion of Tregs effects antibody formation against hFIX
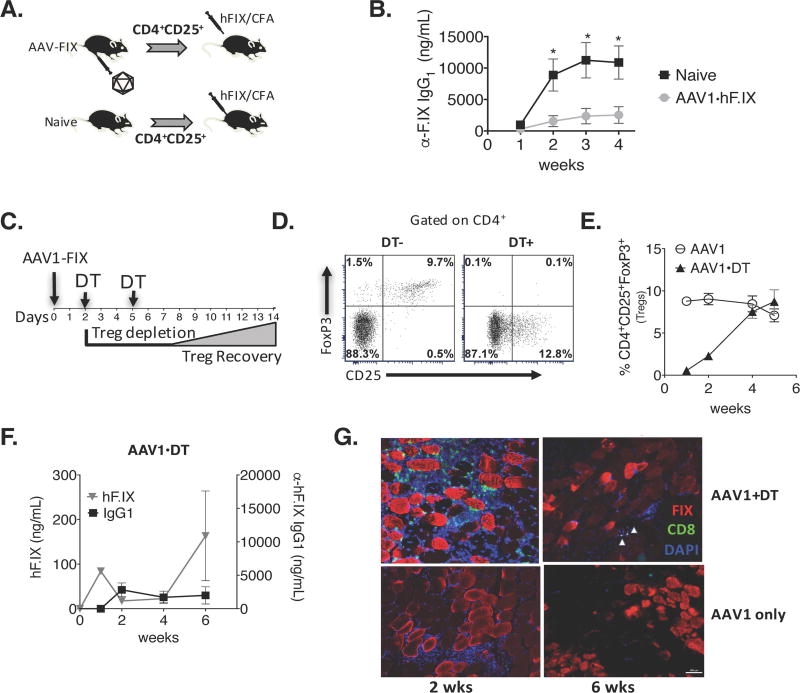
The induction of immune tolerance following AAV liver gene therapy is established by the expansion of antigen-specific FoxP3+ Tregs [12, 25]. It has been suggested that in the context of AAV1-hFIX muscle-directed gene therapy, tolerance to FIX was independent of Tregs and was not associated with Treg induction [28]. To test whether gene transfer induced Tregs that are able to suppress antibody formation against hFIX, we adoptively transferred CD4+CD25+ T cells from AAV1-hFIX transduced mice into syngeneic mice. These were subsequently challenged with hFIX protein emulsified in adjuvant (Fig. 2A). Mice that received CD4+CD25+ T cells from AAV1-hFIX immunized donor mice suppressed antibody formation against hFIX (Fig. 2B), indicating that Tregs indeed play a role in limiting anti-hFIX formation following gene transfer to muscle.
Figure 2. Effects of Treg depletion on systemic expression of and humoral immune responses to hFIX as a function of time after muscle-directed gene transfer.
(A) Schematic of experimental procedure for adoptive transfer of Tregs. (B) Plasma α-hFIX IgG1 levels in recipient mice after being challenged with hFIX protein emulsified in CFA. n=4 per group. (C) Timeline of Treg depletion in mice that were injected intramuscularly with AAV1-hFIX. To obtain an extended window of Treg depletion, mice received intraperitoneal DT on day 2 and 5. (D) Flow cytometry results from one representative experiment validating the robust depletion of CD4+CD25+FoxP3+ Tregs in peripheral blood 1 day after mice received a second dose of DT (1 week after gene transfer) compared to control mouse that received gene transfer but no DT. (E) Recovery of Tregs in peripheral blood as a function of time. (F) Temporal comparison of hFIX transgene levels and anti-hFIX antibody titers in mice that that were depleted of Tregs following muscle gene therapy. (G) Immunofluorescent labeling of muscle tissue revealed hFIX expression (red) and significant infiltration of CD8 cells (green) 2 weeks after AAV1-hFIX vector administration. Data represent averages +/−SEM for n=3–4 mice per cohort; performed up to 3 times. *P<0.05
Using transgenic DTR-Foxp3gfp+ mice that express diphtheria toxin receptor under the control of the FoxP3+ promoter allows the specific but transient depletion of FoxP3+ Tregs upon DT administration [29]. To further demonstrate the suppressive role that Tregs have in preventing induction of transgene product-directed antibody formation, we depleted FoxP3+ cells 2 and 5 days after AAV1-hFIX immunization. Flow cytometric analysis of PBMCs confirmed that FoxP3+ Tregs were indeed transiently depleted, but had recovered by 4 weeks (Fig. 2C–E). Mice that received gene transfer combined with Treg depletion showed an increase in activated non-Treg (CD4+CD25+FoxP3− cells, Fig. 2D). Moreover, there was an absence of systemic hFIX expression post AAV-hFIX gene transfer, which correlated with a potent anti-hFIX IgG1 response (Fig. 2F). To investigate whether, in addition to the loss of systemic expression due to antibody formation, there was also loss of local expression in transduced tissue, muscles were harvested from mice depleted of Tregs. Immunofluorescent labeling revealed that myocytes continued to express hFIX, although a moderate amount of CD8+ T cell infiltrates were seen in the muscle of Treg depleted mice at 2-weeks post injection, which was not the case in control mice transduced with vector without Treg depletion (Fig. 2G). By week 6, the Treg population recovered, and there was a noticeable decrease in CD8+ T cells (Fig. 2E & G). These results clearly indicate that FoxP3+ Tregs have a critical role in establishing immune tolerance that involves suppression of both cellular and humoral responses.
3.3 Transient antibody responses to hFIX are dependent on timing of TLR9 activation and Treg depletion
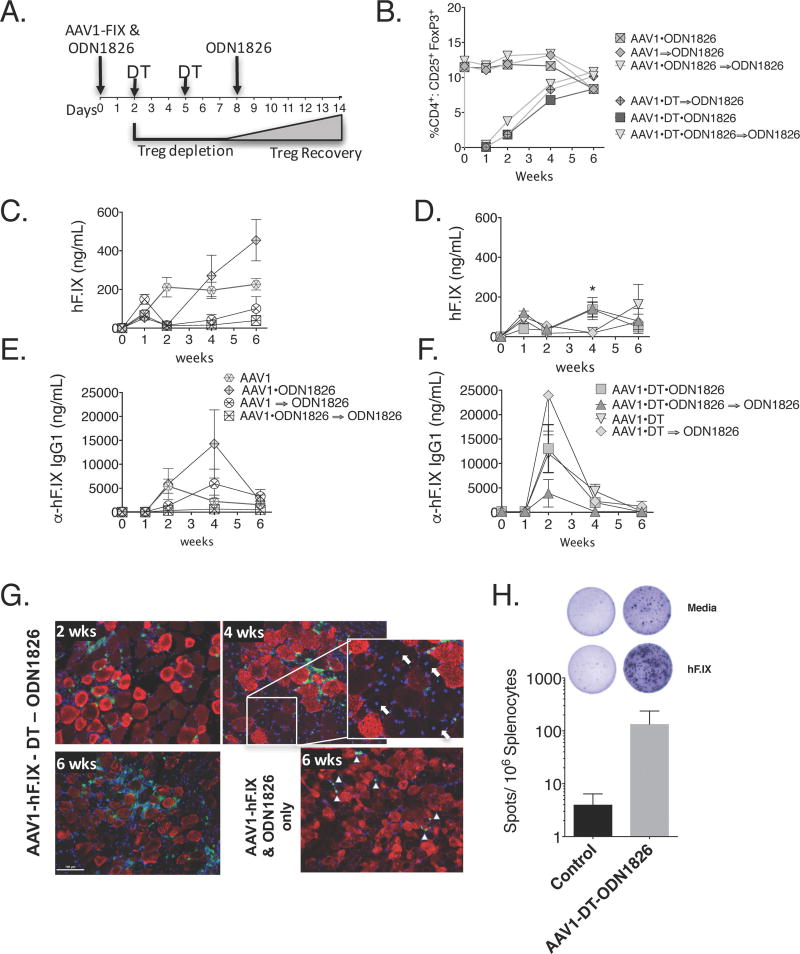
ODNs are rapidly degraded and cleared from the site of injection by nucleases. The tissue half-life of ODN with a phosphorothioate backbone is approximately 48 hours [30]. Since both Treg depletion and TLR9 activation independently broke tolerance and induced anti-hFIX formation, we sought to determine if the combination or timing of both could have a synergistic effect. First, we assessed if activation of TLR9 before, after, or before-and-after Treg depletion influenced recovery of Tregs (Fig. 3A). Flow cytometric analysis of PBMCs confirmed Tregs were indeed depleted in mice receiving DT. Furthermore, the analysis established that administration of CpG-ODN did not significantly influence the depletion or recovery of Tregs (Fig. 3B).
Figure 3. Combined effects of TLR9 stimulation and Treg depletion on systemic expression of and humoral immune responses after muscle-directed gene transfer.
(A) Timeline of experimental procedure for TLR9 stimulation and Treg depletion. The initial dose of the TLR9 agonist was combined with the vector and injected IM on day 0. Where indicated (⇒), the TLR9 agonist was injected in the same hind-leg on day 8. (B) Recovery of Tregs in peripheral blood as a function of time under various conditions. (C–F) Comparison of systemic hFIX antigen (C–D) and anti-hFIX IgG1 (E–F) levels in mice as a function of time after vector administration. (n=3–6 mice/group, *p=0.0178 AAV1-DT v. AAV1-DT-TLR9. (G) Immunofluorescent labeling of muscle tissue shows hFIX expression (red) and significant infiltration of CD8 cells (green) 2 weeks after AAV1-hFIX vector, TLR9 stimulation, and Treg depletion. (Arrows in insert point to centralized nuclei (blue) associated with new cell growth; ▲ indicate CD8+ (green) cells). (H) IFN-γ ELISpot showing a cellular immune response against hFIX in mice transduced with vector in the presence of ODN-1826 and Treg depletion, which was absent in mice treated with vector only (n=3–4/group; data are average ± SEM for epitope-stimulated minus mock-stimulated cultures).
We further compared the levels hFIX transgene and IgG1 antibody formation in AAV1-hFIX treated mice that were co-injected with CpG-ODN, to those that were injected 8 days after vector or both (Fig. 3C–F). All mice that received CpG-ODN, regardless of timing, responded with an initial decrease in detectable hFIX transgene level between week 1 and week 2 (Fig. 3C, D). Interestingly, mice that received both CpG-ODN doses or only the day 8 dose had a longer delay in transgene detection, compared to the mice that received only the first CpG-ODN injection (Fig. 3C, D). These responses directly correlated with a transient increase in anti-hFIX IgG1 titers. By week 6, as antibody levels returned to near baseline, circulating hFIX also began to rise.
Next, we evaluated the effect of combining Treg depletion and TLR9 activation had on hFIX expression and antibody formation. To our surprise, gene transfer combined with Treg depletion and TLR9 activation, at any time, failed to sustain anti-hFIX formation (Fig. 3E, F). A transient decrease in transgene at week 2, like that seen in CpG-ODN treated mice (Fig. 3C), was observed. At week 2, antibody titers peaked, which also correlated to low transgene product levels. By week 6, when the Treg compartment was restored, antibody titers were diminishing, and hFIX levels in circulation recovered (Fig. 3D, F).
Immunofluorescent staining revealed robust CD8+ T cell infiltration of transduced muscle in mice that received AAV1-hFIX/TLR9 agonist co-injection and Treg depletion (Fig. 3G), which correlated with an IFN-γ response to hFIX (Fig. 3H; ~30 SFU/million cells in 2 animals and a 10-fold higher response in a 3rd animal compared to <10 SFU/million cells in 4 animals that received AAV1-hFIX vector only).
Appearance of muscle fibers with central nuclei indicated new cell growth, suggesting a cytolytic immune response. While CD8+ T cells did not entirely eliminate transgene expression, this may explain the reduction in systemic hFIX expression despite the absence/low titer of antibody formation (Fig. 3G; 4 week insert). In contrast, vector transduced tissue that had received only TLR9 agonist, only Treg depletion, or neither had minimal CD8+ cells present at 6-weeks post injection. Thus, TLR9 activation in the absence of Treg caused a prolonged inflammatory T cell response.
3.4 Effect of TLR9 activation on Tfh responses
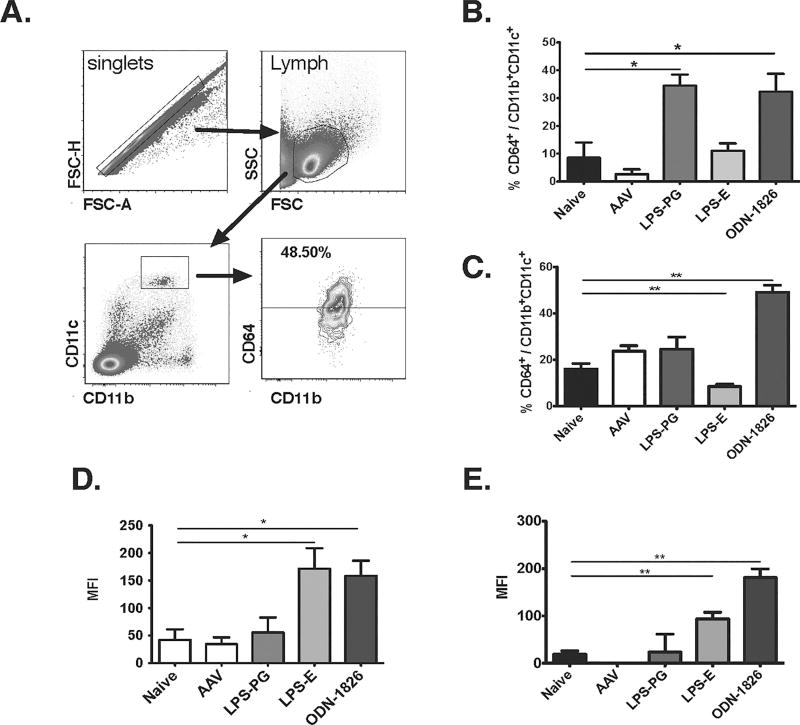
Tfh cells are a unique subset of CD4+ T cells promote the differentiation of activated, antigen-specific B cells into memory B cells as well as long-lived plasma cells [31]. Additionally, CpG-B ODNs have been described to enhance B cell responses by activating moDCs, which do not augment the overall activation of CD4+ T cells but specifically increase the Tfh response [32, 33]. To determine if moDC are being activated and contribute to the changes in antibody formation, we first determined the frequency of CD11b+CD11c+CD64+ moDC in mice that were immunized concomitantly with AAV1-hFIX and LPS-PG, LPS-EC, or ODN-1826 48 hours earlier. The results showed a significant increase in moDC in both draining lymph nodes and spleen following stimulation with ODN-1826 when compared to PBS (sham) and vector only cohorts (Fig. 4A–C). LPS-PG increased moDC frequencies in the lymph nodes only, while LPS-EC decreased the frequency of splenic moDC. LPS-EC and ODN-1826 (but neither vector alone of LPS-PG) up-regulated CD86 co-stimulatory molecules on CD11c+ DCs (Fig. 4D–E). In addition LPS-EC and ODN-1826 up-regulated CD40 and CD86 co-stimulatory molecules on plasmacytiod DCs, while LPS-PG did not (data not shown). Hence, TLR4 activation via LPS-EC or TLR9 activation via ODN-1826 results in activation of DCs after intramuscular injection, while only TLR9 activation activated moDCs and caused antibody formation against hFIX. In contrast, LPS-PG was not found to be an effective adjuvant at the dose and route tested.
Figure 4. TLR9 stimulation upregulates monocyte-derived dendritic cells (moDCs).
(A–B) C57Bl/6 mice were co-injected with vector and indicated TLR agonist. Twenty-four hours later splenocytes were isolated and analyzed by flow cytometry (A, gating scheme) for frequency of CD11b+CD11c+CD64+ moDC cells (n=3/group) in popliteal and inguinal lymph nodes (B) and spleen (C). Expression of the co-stimulatory molecule CD86 on CD11chi is shown as mean fluorescence intensity (MFI) for lymph nodes (D) and spleen (E). Data are average ± SEM, with *P<0.05 and **P<0.01.
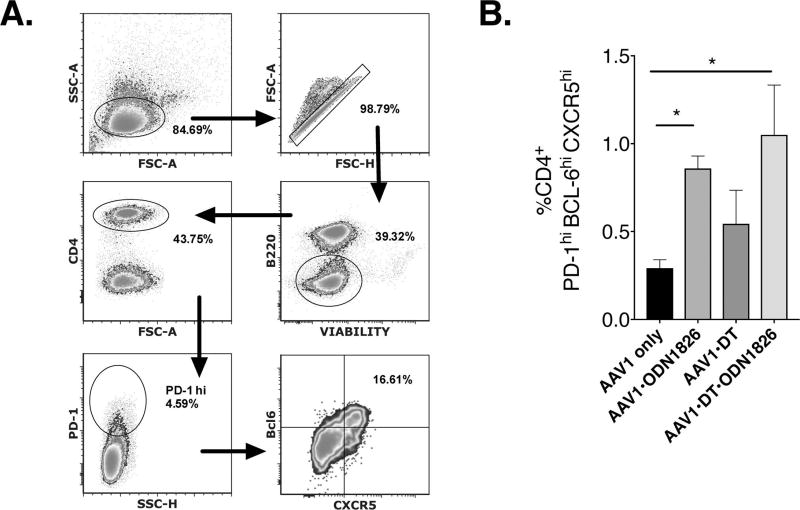
Next, we determined if CpG-ODN resulted in an increase in splenic Tfh frequency. Using the same scheme as above (Fig. 2E), mice were co-injected with AAV1-hFIX and ODN-1826, or not, and depleted of Tregs, or not. Spleens were harvested 14-days later and processed into cell suspensions and labeled with marker to identify the Tfh population. Analysis of the results revealed a significant difference in CD4+ T cells co-expressing PD-1, Bcl6 and CXCR5 between mice receiving only the vector and those co-injected with ODN-1826 (Fig 5A–B). Mice that were transduced with AAV1-hFIX and depleted of Tregs without addition of ODN-1826 failed to increase the frequency of CD4+PD-1hiBcl6hiCXCR5hi Tfh cells. In contrast, co-injection of vector and ODN-1826 significantly increased Tfh frequencies, which were highest when also depleting Tregs (Fig. 5B). These results are consistent with literature data that CpG-B ODNs activate moDCs, resulting in increased Tfh responses [20]. However, the enhanced Tfh response only led to an increase in antibody formation when TLR9 agonist was given in the presence of Tregs. In their absence, a prolonged inflammatory T cell response instead of antibody formation ensued. Thus, Tregs modulate the effect of TLR9 activation via CpG-B ODN.
Figure 5. TLR9 stimulation enhances Tfh responses.
C57Bl/6 mice were co-injected with vector and indicated TLR agonist. At 2-weeks post injection splenocytes were isolated and analyzed by flow cytometry (A, gating scheme) for frequency of CD4+PD-1hiBcl6hiCXCR5hi Tfh cells (B, n=5–6/group; data are are average ± SEM and represent %Tfh of total CD4+ T cells; *P<0.05).
4. Discussion
4.1 Only specific activation signals can heighten antibody formation
Our study demonstrates how the interplay between TLR9 signaling and Treg can shape the immune response to a secreted transgene product. We find that not any activation signal, but rather a specific signal that taps into a specific B cell-activating pathway, can induce antibody formation upon AAV-mediated muscle gene transfer. In this regard, an earlier study prematurely discounted signals that lead to DC activation based on the use of E. coli LPS [34]. Regardless, it was surprising that the 2 different types of lipopolysaccharide (LPS) that we tested, which both activate DCs and provide potent inflammatory signals, failed to induce antibody formation against hFIX. Therefore, TLR2 and TLR4 activation may not be a suitable signal to prompt antibody formation in skeletal muscle. The ability of P. gingivalis LPS preparations to activate TLR2 is likely be due to a lipoprotein contaminant rather than the LPS itself, as very high-purity preparations only activated TLR4 [35]. Furthermore, P. gingivalis and E. coli LPS both similarly activate DCs in vitro, but induce a different pattern of cytokine expression in the activated DCs [36]. Although TLR2 signaling in response to P. gingivalis LPS preparations has been shown to favor Th2 immunity [37], it failed to enhance antibody formation upon intramuscular injection in our experiments.
4.2 Specific modes of TLR9 activation can drive antibody responses
Among the TLR ligands tested here, CpG-ODN was uniquely capable of inducing antibody formation against FIX in the context of AAV muscle gene transfer. Type-B ODNs strongly activate TLR9 signaling, including in DCs, and thereby enhance both Th1 and B cell responses but induce little type I interferon. Th1 induction would explain the enhanced CD8+ T cell infiltration in the muscle, as Th1 cells augment CD8+ T cell proliferation and sensitize the target tissue through secretion of IFN-γ. The pathway by which Type-B ODNs enhance antibody formation has recently been uncovered [20, 38]. Antigen presentation and IL-6 production is specifically upregulated by TLR9 signaling in moDCs, thereby enhancing Tfh cell differentiation and promoting T cell-dependent antibody formation [20, 33]. Consequently, elimination of TLR9 from CD11chi cells abrogates the effect of the type-B ODN. In contrast, CpG-A and CpG-C ODNs fail to enhance antibody formation [20]. When we co-administered AAV1-hFIX vector and ODN-1826 in TLR9−/− mice, we found a non-significant increase in anti-hFIX formation by 2 weeks that was also much less robust compared to wild-type mice (on average 3- to 5-times lower), which nonetheless resulted in a reduction in hFIX antigen levels (Supplementary Figure 1). Therefore, we conclude that the effects of ODN-1826 are predominantly but not entirely due to TLR9 activation. Since these ODNs to a lesser extent also bind to TLR7 (an innate endosomal receptor for single-stranded RNA), future experiments should address whether TLR7 ligands may also induce antibody formation to AAV transgene products.
Tfh cells play a critical role in germinal centers, where they specialize in providing cognate help to B cells. Hence, we conclude that TLR9 activation via type-B ODN promotes antibody formation to secreted transgene products in muscle gene transfer through the moDC-Tfh axis. This mechanism is distinct from TLR9 immune responses resulting from TLR9 sensing of the AAV genome, which drives innate tissue inflammation and CD8+ T cell responses but has limited effects on antibody formation [17, 21, 39–41]. Therefore, IgG1 formation against hFIX upon muscle-directed AAV gene transfer is dependent on serotype and vector dose but occurs even in TLR9- and MyD88-deficient mice [39]. Consistent with prior studies using AAV1 serotype [7, 12, 42], mice formed only immunoglobulin of the IgG1 subclass against hFIX in the experiments presented here, which were increased by type-B ODN administration or Treg depletion. To summarize, although antibody formation against the AAV transgene product is largely independent of TLR9 sensing of the AAV genome (consistent with traditional thinking that TLR activation mostly drives Th1 responses), our new data show that TLR9 activation through alternative ligands can cause antibody responses in skeletal muscle through activation of moDCs and Tfh. Thus, infections or other events that cause immune signaling in skeletal muscle may enhance antibody formation if they tap into this pathway, while other inflammatory signals (such as LPS) may not affect antibody formation.
Activation of the moDC-Tfh axis by ODNs is not the only pathway for TLR9 activation to cause antibody formation. Another example is TLR9 activation in B cells by plasmid DNA containing CpG motifs [43]. In contrast, retroviral or lentiviral transgene expression in gene modified B cells is tolerogenic [43, 44]. Interestingly, TLR2 activation during retroviral infection can upregulate IL-10 expression, thereby enhancing the ability to induce tolerance to the transgene product expressed in the B cells [45].
4.3 Treg have a critical immune modulatory effect in muscle
The liver is an optimal target for induction of Tregs by AAV gene transfer [25, 46, 47], thereby promoting immune tolerance to the transgene product. Our new data illustrate that Treg induction also plays a role in limiting immune responses in muscle gene transfer. In the absence of Tregs, CD8+ T cell and antibody responses were induced, suggesting that Tregs promoted unresponsiveness in both T and B cell compartments of mice that received AAV1-hFIX vector. Similar to our previous findings in liver gene transfer [32], immune responses were downregulated after Treg were allowed to recover. Transgene expression, antibody titers, and CD8+ T cell infiltration returned to levels comparable to AAV1-hFIX transduced mice that had not undergone Treg depletion. This result is also reminiscent of data in α1-antitrypsin deficient patients who received AAV1 muscle gene transfer and developed CD8+ T cell responses to AAV capsid. These patients experienced prolonged inflammation at the site of gene transfer, including CD8+ T cell infiltration, which eventually resolved when Tregs started to dominate the response [48, 49]. Therefore, Tregs have the capacity to resolve T cell and antibody responses in muscle. However, in contrast to Tregs that infiltrate tissues, those Tregs that suppressed antibody formation in our experiments likely functioned in secondary lymphoid organs, such as draining lymph nodes and spleen, and may represent T follicular regulatory cells [50, 51]. This would explain how Tregs were able to modulate the response to activation of the moDC-Tfh pathways via TLR9 agonist. Although not specifically addressed in this study, published data on expression of secreted transgene products from AAV vectors would suggest that during the second month after gene transfer, thymic-derived Tregs likely emerged in addition to peripherally induced Tregs [24, 25, 52, 53].
In contrast to C57BL/6 mice, hemophilia B mice on other strain backgrounds (C3H/HeJ or BALB/c) form antibodies against FIX after muscle gene transfer with AAV1 vector. However, in these strains we were able to prevent antibody formation against FIX by Treg cell therapy or by in vivo Treg induction, further supporting that Tregs are capable of controlling immune responses to muscle gene transfer [14, 15, 54].
Unlike other strains [6], C57BL/6 mice normally fail to activate CD8+ T cell against hFIX upon AAV gene transfer to muscle [12]. Here, Treg depletion or TLR9 activation resulted in a modest CD8+ T cell infiltration of transduced muscle. A more potent CD8+ T cell response was evident in mice that received the TLR9 agonist and were also depleted of Tregs. However, in any scenario, hFIX expressing muscle fibers were not completely eliminated. This is consistent with literature data, demonstrating that CD8+ T cells against the transgene product encoded by a single-stranded AAV vector are not fully functional. This outcome is not Treg dependent but rather reflects expression of negative checkpoint regulators in activated CD8+ T cells [21]. In contrast, self-complementary AAV vectors induce more functional CD8+ T cell responses to the transgene product [39]. While we cannot formally rule out that the CD8+ T cell infiltrates we observed in C57BL/6 mice upon TLR9 activation combined with Treg depletion were also directed against AAV1 capsid. However, infiltrates in mice are typically against the transgene product rather than viral, regardless of Tregs [55]. Most likely, cross-presentation of input capsid by nonprofessional antigen presenting cells is limited in mice.
As mentioned before, the level of transgene expression (as determined by AAV serotype and vector dose) is a major factor that determines antibody formation against the hFIX transgene product in C57BL/6 mice. A prior study prematurely discounted the relevance of Tregs in limiting anti-hFIX formation [28], while our data clearly show that Tregs are required to maintain unresponsiveness. However, TLR9 activation via the type B CpG-ODN was able to override the suppressive effect of Tregs. Conversely, Tregs modulated the effects of TLR9 activation by CpG-ODN, which induced antibody formation in the presence of Tregs while causing a strong inflammatory response in the muscle in their absence. Surprisingly, even upon Treg depletion, the effects of TLR9 activation were transient, and tolerance was eventually established once the Tregs re-populated. Therefore, consistent with clinical data, Tregs aid in resolving immune responses in skeletal muscle in the context of AAV gene transfer. In conclusion, our results demonstrate the importance of the interplay of innate immune signals and immune regulation in shaping the adaptive immune response to AAV gene transfer in skeletal muscle, including antibody formation against the transgene product.
Supplementary Material
Highlights.
Treg suppressed antibody formation against factor IX in AAV muscle gene transfer
A TLR9 agonist specifically induced antibodies against the transgene product
Co-administration of vector and TLR9 agonist expanded and activated moDC and Tfh cells
In the absence of Treg, the TLR9 agonist induced the generation of CD8+ T cells instead of antibodies
Hence, Treg modulate the effect of TLR9 activation by CpG deoxyoligonucleotides
Acknowledgments
This work was supported by an ASPIRE award in hemophilia research to BEH from Pfizer Inc., and by NIH grants R01 AI51390 (to RWH) and R01 HL131093 (to RWH and CT).
Conflict of Interest. RWH received royalty payments from Spark Therapeutics for license of AAV gene transfer technology and serves on a scientific advisory board for Applied Genetic Technologies Corporation (AGTC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions. BEH, MC, GQP, ATM, and MB performed experiments. RWH, GQP, MB, and RWH designed experiments. RWH, GQP, MB, and BEH analyzed and interpreted data. RHW, CT, and BEH wrote the manuscript. RWH and BEH supervised the study.
References
- 1.Boisgerault F, Mingozzi F. The Skeletal Muscle Environment and Its Role in Immunity and Tolerance to AAV Vector-Mediated Gene Transfer. Current gene therapy. 2015;15:381–394. doi: 10.2174/1566523215666150630121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs SP, Desrosiers RC. Promise and problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies. Mol Ther Methods Clin Dev. 2016;3:16068. doi: 10.1038/mtm.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Cao O, Swalm B, Dobrzynski E, Mingozzi F, Herzog RW. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 2005;12:1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- 4.Mills KHG. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 5.Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, Bellinger DA, Couto LB, Nichols TC, High KA. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Human gene therapy. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Dobrzynski E, Schlachterman A, Cao O, Herzog RW. Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood. 2005;105:4226–4234. doi: 10.1182/blood-2004-03-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y, Mingozzi F, Xiao W, Couto LB, High KA. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arruda VR, Stedman HH, Haurigot V, Buchlis G, Baila S, Favaro P, Chen Y, Franck HG, Zhou S, Wright JF, Couto LB, Jiang H, Pierce GF, Bellinger DA, Mingozzi F, Nichols TC, High KA. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115:4678–4688. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpentier M, Lorain S, Chappert P, Lalfer M, Hardet R, Urbain D, Peccate C, Adriouch S, Garcia L, Davoust J, Gross DA. Intrinsic transgene immunogenicity gears CD8(+) T-cell priming after rAAV-mediated muscle gene transfer. Mol Ther. 2015;23:697–706. doi: 10.1038/mt.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarukhan A, Soudais C, Danos O, Jooss K. Factors influencing cross-presentation of non-self antigens expressed from recombinant adeno-associated virus vectors. J Gene Med. 2001;3:260–270. doi: 10.1002/jgm.175. [DOI] [PubMed] [Google Scholar]
- 11.Xu D, Walker CM. Continuous CD8(+) T-cell priming by dendritic cell cross-presentation of persistent antigen following adeno-associated virus-mediated gene delivery. J Virol. 2011;85:12083–12086. doi: 10.1128/JVI.05375-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman BE, Dobrzynski E, Wang L, Hirao L, Mingozzi F, Cao O, Herzog RW. Muscle as a target for supplementary factor IX gene transfer. Human gene therapy. 2007;18:603–613. doi: 10.1089/hum.2007.042. [DOI] [PubMed] [Google Scholar]
- 13.Doerfler PA, Todd AG, Clement N, Falk DJ, Nayak S, Herzog RW, Byrne BJ. Copackaged AAV9 Vectors Promote Simultaneous Immune Tolerance and Phenotypic Correction of Pompe Disease. Human gene therapy. 2016;27:43–59. doi: 10.1089/hum.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nayak S, Sarkar D, Perrin GQ, Moghimi B, Hoffman BE, Zhou S, Byrne BJ, Herzog RW. Prevention and Reversal of Antibody Responses Against Factor IX in Gene Therapy for Hemophilia B. Frontiers in microbiology. 2011;2:244. doi: 10.3389/fmicb.2011.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar D, Biswas M, Liao G, Seay HR, Perrin GQ, Markusic DM, Hoffman BE, Brusko TM, Terhorst C, Herzog RW. Ex Vivo Expanded Autologous Polyclonal Regulatory T Cells Suppress Inhibitor Formation in Hemophilia. Mol Ther Methods Clin Dev. 2014;1:14030. doi: 10.1038/mtm.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardet R, Chevalier B, Dupaty L, Naimi Y, Riou G, Drouot L, Jean L, Salvetti A, Boyer O, Adriouch S. Oral-tolerization Prevents Immune Responses and Improves Transgene Persistence Following Gene Transfer Mediated by Adeno-associated Viral Vector. Mol Ther. 2016;24:87–95. doi: 10.1038/mt.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martino AT, Suzuki M, Markusic DM, Zolotukhin I, Ryals RC, Moghimi B, Ertl HC, Muruve DA, Lee B, Herzog RW. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. The Journal of clinical investigation. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers GL, Herzog RW. Gene therapy for hemophilia. Front Biosci (Landmark Ed) 2015;20:556–603. doi: 10.2741/4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakarov S, Fazilleau N. Monocyte-derived dendritic cells promote T follicular helper cell differentiation. EMBO molecular medicine. 2014;6:590–603. doi: 10.1002/emmm.201403841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers GL, Martino AT, Zolotukhin I, Ertl HC, Herzog RW. Role of the vector genome and underlying factor IX mutation in immune responses to AAV gene therapy for hemophilia B. J Transl Med. 2014;12:25. doi: 10.1186/1479-5876-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayuso E, Mingozzi F, Montane J, Leon X, Anguela XM, Haurigot V, Edmonson SA, Africa L, Zhou S, High KA, Bosch F, Wright JF. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 2010;17:503–510. doi: 10.1038/gt.2009.157. [DOI] [PubMed] [Google Scholar]
- 23.Rogers GL, Hoffman BE. Optimal Immunofluorescent Staining for Human Factor IX and Infiltrating T Cells following Gene Therapy for Hemophilia B. Journal of genetic syndromes & gene therapy. 2012;S1:012. doi: 10.4172/2157-7412.s1-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman BE, Martino AT, Sack BK, Cao O, Liao G, Terhorst C, Herzog RW. Nonredundant roles of IL-10 and TGF-beta in suppression of immune responses to hepatic AAV-factor IX gene transfer. Mol Ther. 2011;19:1263–1272. doi: 10.1038/mt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, Herzog RW. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballas ZK, Krieg AM, Warren T, Rasmussen W, Davis HL, Waldschmidt M, Weiner GJ. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol. 2001;167:4878–4886. doi: 10.4049/jimmunol.167.9.4878. [DOI] [PubMed] [Google Scholar]
- 28.Kelly M, Bharadwaj AS, Tacke F, Chao H. Regulatory T cells and immune tolerance to coagulation factor IX in the context of intramuscular AAV1 gene transfer. Mol Ther. 2010;18:361–369. doi: 10.1038/mt.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 30.Mutwiri GK, Nichani AK, Babiuk S, Babiuk LA. Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides. J Control Release. 2004;97:1–17. doi: 10.1016/j.jconrel.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Ballesteros-Tato A, Randall TD. Priming of T follicular helper cells by dendritic cells. Immunol Cell Biol. 2014;92:22–27. doi: 10.1038/icb.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markusic DM, Hoffman BE, Perrin GQ, Nayak S, Wang X, LoDuca PA, High KA, Herzog RW. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO molecular medicine. 2013;5:1698–1709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, Lee LM, Coffman RL, Bachmann MF, DeFranco AL. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 2011;34:375–384. doi: 10.1016/j.immuni.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bharadwaj AS, Kelly M, Kim D, Chao H. Induction of immune tolerance to FIX by intramuscular AAV gene transfer is independent of the activation status of dendritic cells. Blood. 2010;115:500–509. doi: 10.1182/blood-2009-08-239509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa T, Asai Y, Makimura Y, Tamai R. Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A. Front Biosci. 2007;12:3795–3812. doi: 10.2741/2353. [DOI] [PubMed] [Google Scholar]
- 36.Netea MG, Van der Meer JW, Sutmuller RP, Adema GJ, Kullberg BJ. From the Th1/Th2 paradigm towards a Toll-like receptor/T-helper bias. Antimicrob Agents Chemother. 2005;49:3991–3996. doi: 10.1128/AAC.49.10.3991-3996.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 38.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 39.Rogers GL, Suzuki M, Zolotukhin I, Markusic DM, Morel LM, Lee B, Ertl HC, Herzog RW. Unique Roles of TLR9- and MyD88-Dependent and -Independent Pathways in Adaptive Immune Responses to AAV-Mediated Gene Transfer. J Innate Immun. 2015;7:302–314. doi: 10.1159/000369273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faust SM, Bell P, Cutler BJ, Ashley SN, Zhu Y, Rabinowitz JE, Wilson JM. CpG-depleted adeno-associated virus vectors evade immune detection. The Journal of clinical investigation. 2013;123:2994–3001. doi: 10.1172/JCI68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudres M, Cire S, Vasseur V, Brault L, Da Rocha S, Boisgerault F, Le Bec C, Gross DA, Blouin V, Ryffel B, Galy A. MyD88 signaling in B cells regulates the production of Th1-dependent antibodies to AAV. Mol Ther. 2012;20:1571–1581. doi: 10.1038/mt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers GL, Martino AT, Aslanidi GV, Jayandharan GR, Srivastava A, Herzog RW. Innate Immune Responses to AAV Vectors. Frontiers in microbiology. 2011;2:194. doi: 10.3389/fmicb.2011.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Moghimi B, Zolotukhin I, Morel LM, Cao O, Herzog RW. Immune tolerance induction to factor IX through B cell gene transfer: TLR9 signaling delineates between tolerogenic and immunogenic B cells. Mol Ther. 2014;22:1139–1150. doi: 10.1038/mt.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Herzog RW, Byrne BJ, Kumar SR, Zhou Q, Buchholz CJ, Biswas M. Immune modulatory cell therapy for hemophilia B based on CD20-targeted lentiviral gene transfer to primary B cells. Mol Ther Methods Clin Dev. 2017;5:76–82. doi: 10.1016/j.omtm.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahangarani RR, Janssens W, Carlier V, Vanderelst L, Vandendriessche T, Chuah M, Jacquemin M, Saint-Remy JM. Retroviral vectors induce epigenetic chromatin modifications and IL-10 production in transduced B cells via activation of toll-like receptor 2. Mol Ther. 2011;19:711–722. doi: 10.1038/mt.2010.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martino AT, Nayak S, Hoffman BE, Cooper M, Liao G, Markusic DM, Byrne BJ, Terhorst C, Herzog RW. Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PloS one. 2009;4:e6376. doi: 10.1371/journal.pone.0006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao O, Loduca PA, Herzog RW. Role of regulatory T cells in tolerance to coagulation factors. Journal of thrombosis and haemostasis : JTH. 2009;7(Suppl 1):88–91. doi: 10.1111/j.1538-7836.2009.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller C, Chulay JD, Trapnell BC, Humphries M, Carey B, Sandhaus RA, McElvaney NG, Messina L, Tang Q, Rouhani FN, Campbell-Thompson M, Fu AD, Yachnis A, Knop DR, Ye GJ, Brantly M, Calcedo R, Somanathan S, Richman LP, Vonderheide RH, Hulme MA, Brusko TM, Wilson JM, Flotte TR. Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. The Journal of clinical investigation. 2013;123:5310–5318. doi: 10.1172/JCI70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferreira V, Twisk J, Kwikkers K, Aronica E, Brisson D, Methot J, Petry H, Gaudet D. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Human gene therapy. 2014;25:180–188. doi: 10.1089/hum.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sage PT, Ron-Harel N, Juneja VR, Sen DR, Maleri S, Sungnak W, Kuchroo VK, Haining WN, Chevrier N, Haigis M, Sharpe AH. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol. 2016;17:1436–1446. doi: 10.1038/ni.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sage PT, Sharpe AH. T follicular regulatory cells. Immunol Rev. 2016;271:246–259. doi: 10.1111/imr.12411. [DOI] [PubMed] [Google Scholar]
- 52.Dobrzynski E, Mingozzi F, Liu YL, Bendo E, Cao O, Wang L, Herzog RW. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104:969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- 53.Perrin GQ, Zolotukhin I, Sherman A, Biswas M, de Jong YP, Terhorst C, Davidoff AM, Herzog RW. Dynamics of antigen presentation to transgene product-specific CD4+ T cells and of Treg induction upon hepatic AAV gene transfer, Molecular therapy. Methods & clinical development. 2016;3:16083. doi: 10.1038/mtm.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nayak S, Cao O, Hoffman BE, Cooper M, Zhou S, Atkinson MA, Herzog RW. Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. Journal of thrombosis and haemostasis : JTH. 2009;7:1523–1532. doi: 10.1111/j.1538-7836.2009.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siders WM, Shields J, Kaplan J, Lukason M, Woodworth L, Wadsworth S, Scaria A. Cytotoxic T lymphocyte responses to transgene product, not adeno-associated viral capsid protein, limit transgene expression in mice. Human gene therapy. 2009;20:11–20. doi: 10.1089/hum.2008.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.