Abstract
Proteolytic processing of the laminin-γ2 chain is a hallmark of basement membrane maturation in the skin. Integrin α3β1, a major receptor for epidermal adhesion to laminin-332, is critical for proper basement membrane organization during skin development and wound healing. Previously, we identified a role for α3β1 in promoting the processing of laminin-γ2 in cultured keratinocytes in vitro and in wound epidermis in vivo. In the current study we identify the Bmp1 gene, which encodes variants of the mammalian tolloid/bone morphogenetic protein-1 metalloproteases, as a critical regulator of α3β1-dependent laminin-γ2 processing, thereby expanding the role of this integrin in controlling the secretion by the epidermis of factors that modulate the tissue microenvironment. Since our previous studies identified another epidermal integrin, α9β1, as a suppressive regulator of α3β1-dependent wound angiogenesis, we investigated whether α9β1 has a similar cross-suppressive effect on ability of α3β1 to promote basement membrane organization. Here, we demonstrate that, rather than a cross-suppressive role, α9β1 has an opposing role in basement membrane assembly/maturation through reduced laminin-γ2 processing via mammalian tolloid/bone morphogenetic protein-1. Indeed, while α3β1 promotes this process during wound healing, α9β1 has an inhibitory role, suggesting that regulation of basement membrane assembly requires a complex interplay between these distinct epidermal integrins.
INTRODUCTION
The cutaneous basement membrane (BM) is a specialized extracellular matrix (ECM) that acts as a physical barrier between the epidermis and dermis of the skin. Additionally, the BM provides signals for intracellular pathways that modulate a range of keratinocyte functions including migration, survival, differentiation, and polarization. Keratinocyte-mediated changes to the BM, which may occur through ECM protein deposition or matrix proteolysis, can regulate many of the abovementioned keratinocyte functions.
Laminin-332 (LN-332), a major constituent of the epidermal BM, is made up of three chains designated α3, β3, and γ2 (Aumailley et al., 2005; Aumailley et al., 2003). In the mature BM of adult skin, proteolytic processing results in LN-332 which lacks the N-terminus of the laminin-γ2 (LNγ2) chain as well as the C-terminus of the α3 chain (Amano et al., 2000; Marinkovich et al., 1992; Sasaki et al., 2001; Tsubota et al., 2000). These processing events appear to modulate interactions of LN-332 with distinct ECM components, thereby regulating BM architecture (Aumailley et al., 2003). For example, the L4 module within the N-terminus of unprocessed LNγ2 is required for stable incorporation of LN-332 into nascent BM (Gagnoux-Palacios et al., 2001). The L4 module, notably, has binding sites for ECM components, such as nidogen-1 and fibulins-1 and -2, most of which are lost upon processing of the LNγ2 chain (Sasaki et al., 2001). This suggests that some preliminary ECM linkages necessary for stable LN-332 incorporation during BM assembly are dispensable in mature BM.
Integrins are transmembrane proteins consisting of an α and a β subunit, and function as the main receptors for cell adhesion to the ECM (Hynes, 1992). Abundant in basal keratinocytes of the epidermis, integrins α6β4 and α3β1 mediate adhesion to the underlying BM (Janes and Watt, 2006; Longmate and DiPersio, 2014; Margadant et al., 2010). LN-332 is the major adhesive ligand in the cutaneous BM for both α3β1 and α6β4 (Delwel et al., 1994; Nguyen et al., 2000), and inherited mutations in each of the individual LN-332 chains, as well as in the α3, α6 and β4 integrin subunits, lead to junctional forms of the human blistering skin disease, Epidermolysis Bullosa [reviewed in (Dang et al., 2008; McGrath, 2015; Pulkkinen and Uitto, 1999)]. In addition to their well known roles in cell adhesion, several epidermal integrins have been shown to regulate ECM proteolysis and assembly through their abilities to control the expression or functions of MMPs or other ECM-degrading extracellular proteases, and changes in these integrin functions are likely to contribute to the pathologies of chronic wounds, blistering skin diseases and certain skin cancers [reviewed in (Longmate and DiPersio, 2014)]. Indeed, we recently identified α3β1 as an important mediator of LNγ2 processing, both in vivo during wound healing and in cultured keratinocytes under high calcium conditions that promote differentiated function (Longmate et al., 2014). However, the extracellular protease(s) responsible for α3β1-dependent processing of LN-332 have not been identified previously.
While roles for distinct epidermal integrins during wound healing have been extensively investigated, understanding of how different integrins function cooperatively in keratinocytes to regulate wound healing has not been explored sufficiently (Longmate and DiPersio, 2014; Margadant et al., 2010). Our previous work has established a major role for α3β1 in epidermal keratinocytes in promoting the secretion of proteases and growth factors that modulate the tissue microenvironment in skin (DiPersio et al., 2000; Longmate et al., 2014; Missan et al., 2014; Mitchell et al., 2009). Recently, we identified epidermal α9β1 as a cross-suppressive regulator of α3β1-mediated crosstalk from keratinocytes to endothelial cells that promotes wound angiogenesis (Longmate et al., 2017). However, a cross-suppressive role for α9β1 in α3β1-mediated BM assembly has not been investigated previously. Here, we used mice with epidermis-specific deletion of α3β1, α9β1, or both α3β1 and α9β1, along with cultured mouse keratinocytes (MK cells) that express α3β1 or α9β1, separately or together, to determine how these two integrins coordinately regulate LN-332 processing and BM maturation. We demonstrate that α3β1 promotes LNγ2 processing at least in part through the upregulation of the Bmp1 gene, which encodes variants of the mammalian tolloid/bone morphogenetic protein-1 (mTLD/BMP-1) metalloproteases that have been shown previously to cleave LNγ2 in cultured keratinocytes and in skin (Amano et al., 2000; Muir et al., 2016; Veitch et al., 2003). Moreover, we show that α9β1 antagonizes both α3β1-dependent mTLD/BMP-1 expression and BM assembly/maturation. These findings indicate a complex interplay between these two integrins whereby α3β1 promotes, and coordinately, α9β1 inhibits mTLD/BMP-1 production and LNγ2 processing to effect timely BM assembly and maturation during wound healing.
RESULTS
Integrin α3β1 promotes mTLD/BMP-1-mediated LNγ2 processing in mouse keratinocytes
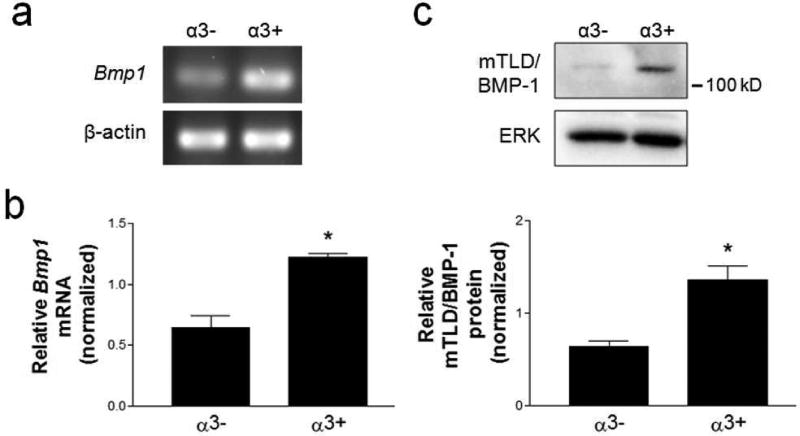
Previously, we reported that α3β1 is required for processing of the LNγ2 chain in cultured keratinocytes, as well as in the wound epidermis in vivo (Longmate et al., 2014). Since mTLD/BMP-1 proteases are known to process LNγ2 (Amano et al., 2000; Muir et al., 2016; Veitch et al., 2003), we first determined if α3β1 promotes mTLD/BMP-1 expression in keratinocytes. We compared mTLD/BMP-1 mRNA and protein levels between mouse keratinocyte (MK) cell lines that lack α3β1 (i.e., MKα3− cells, derived from an α3-null mouse), and the latter cells in which α3β1 expression was restored through stable transfection with human α3 (i.e., MKα3+ cells) (DiPersio et al., 2000; Iyer et al., 2005). Indeed, both mTLD/BMP-1 mRNA and protein were expressed at significantly greater levels in cells expressing α3β1, as determined by RT-PCR (Fig. 1a), qPCR (Fig. 1b), and western blot (Fig. 1c). Moreover, on a western blot the band with strongest reactivity to an antibody against mTLD/BMP-1 was consistent with the ~130 kD mTLD form of BMP-1 (Amano et al., 2000) (Fig. 1c).
Figure 1.
Integrin α3β1 promotes mTLD/BMP-1 expression in mouse keratinocytes. (a,b) mRNA or (c) whole cell lystates were prepared from α3-null MK cells (α3−), or the latter cells with restored α3 subunit expression (α3+). (a) Representative images from RT-PCR for Bmp1 gene products and β-actin. (b) qPCR analysis of relative Bmp1 mRNA, normalized to β-actin mRNA. (c) Representative immunoblots of whole MK cell lysates are shown for mTLD/BMP-1 and ERK. Graph shows quantification of relative mTLD/BMP-1 protein, normalized to ERK, for 3 independent experiments. Data are mean ± SEM.; two-tailed t-test, *P<0.05.
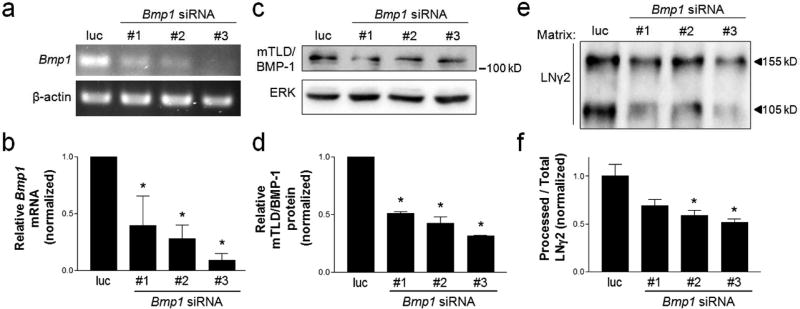
Next, we used RNA interference (RNAi) to determine whether mTLD/BMP-1 is required for LNγ2 processing in keratinocytes, which we previously showed was α3β1-dependent (Longmate et al., 2014). MKα3+ cells (i.e., that express α3β1) were transfected with three distinct siRNAs that target transcripts from the Bmp1 gene, or with a control siRNA that targets luciferase. Cells transfected with each mTLD/BMP-1-targeting siRNA displayed decreased mTLD/BMP-1 mRNA (Fig. 2a,b) and protein (Fig. 2c,d) compared to control transfected cells. Analysis of deposited matrix in each case revealed that suppression of mTLD/BMP-1 led to a reduction in the proportion of LNγ2 that was processed to the 105 kD form (Fig. 2e,f), demonstrating that this protease promotes efficient LNγ2 processing in MK cells. Together, these data demonstrate that integrin α3β1 promotes LNγ2 processing, at least in part, through regulation of Bmp1 gene expression.
Figure 2.
siRNA-mediated suppression of the Bmp1 gene reduces LNγ2 processing in α3β1-expressing keratinocytes. MKα3+ cells were treated with one of three distinct siRNAs that target Bmp1 gene transcripts (#1–3) or a luciferase-targeting siRNA as a control (luc). (a) Representative RT-PCR and (b) quantification of relative Bmp1 mRNA levels, normalized to β-actin mRNA. (c) Representative western blot of whole cell lysates and (d) quantification of relative mTLD/BMP-1 protein level, normalized to ERK protein. (e) Representative western blot of matrix preparations with anti-LNγ2. The unprocessed (155 kD) and processed (105 kD) forms of LNγ2 are indicated. (f) Quantification of processed LNγ2 as a proportion of total LNγ2, normalized to the daily mean to account for variability by day. All data are mean ± SEM; n=3; 1-way ANOVA; Dunnett’s multiple comparison, *P<0.05.
Integrins α3β1 and α9β1 have opposing roles in regulating LNγ2 processing and mTLD/BMP-1 expression
We recently identified a role for the epidermal integrin α9β1 in suppression of α3β1 functions that promote paracrine stimulation of endothelial cells and wound angiogenesis (Longmate et al., 2017). Although α9β1 is expressed in the epidermis of skin, it is lost upon culture of rodent and human keratinocytes (Choma et al., 2004; DiPersio et al., 2000; Singh et al., 2009). Therefore, to investigate whether α9β1 has a similar cross-suppressive effect on α3β1-dependent LNγ2 processing, we stably re-expressed α9 in both MKα3− and MKα3+ cells to generate a panel of MK variants that express α9β1 and α3β1 in various combinations, as we described previously (Longmate et al., 2017). Henceforth, these MK variants will be referred to as MKα3−/α9−, MKα3+/α9−, MKα3−/α9+, or MKα3+/α9+ to reflect their expression profiles of the α3 and α9 subunits (Longmate et al., 2017). We previously showed that these MK variants display appropriate and similar cell surface expression of α9β1 and α3β1 (Longmate et al., 2017). Although other groups have reported changes in other integrins in keratinocytes with natural or engineered deletion of the α3 subunit (Margadant et al., 2009; Pazzagli et al., 2017), we previously demonstrated that surface levels of other epidermal β1 integrins are not substantially altered in our MK model upon manipulated expression of α3, α9 or both together (Longmate et al., 2017).
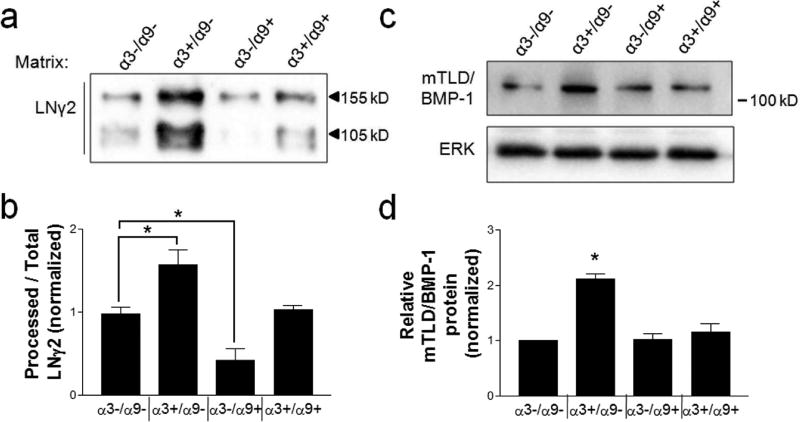
Consistent with our previous report that α3β1 promotes processing of the LNγ2 chain (Longmate et al., 2014), expression of α3 alone (i.e., in the absence of α9) promoted both LNγ2 deposition and the proportion that was proteolyzed to the 105 kD processed form (Fig. 3a, b, compare α3−/α9− with α3+/α9−). In contrast, expression of α9 alone led to reduced LNγ2 processing, indicating that α9β1 has an independent suppressive effect on this proteolytic event (Fig. 3a,b; compare α3−/α9− with α3−/α9+). Interestingly, co-expression of α9β1 and α3β1 in MK cells suppressed the level of LNγ2 processing to baseline levels seen in MK cells that express neither integrin (Fig. 3a,b, compare α3+/α9+ with α3−/α9−). Furthermore, co-expression of α9β1 and α3β1 abolished α3β1-dependent upregulation of cellular mTLD/BMP-1 (Fig. 3c,d; compare α3+/α9− with α3+/α9+). Mass spectrometric analysis of secreted mTLD/BMP-1 revealed a pattern of relative expression among the MK cell variants that closely resembled that observed for LNγ2 processing, wherein α3β1 and α9β1 had counter-balancing effects (compare Fig. S1 to Fig. 3b).
Figure 3.
α9β1 suppresses α3β1-dependent induction of LNγ2 processing and mTLD/BMP-1 expression. (a,b) ECM fractions or (c,d) whole cell lysates were collected from MK cell variants of the indicated integrin composition, which were cultured in high calcium to promote α3β1-dependent LNγ2 processing, as described (Longmate et al., 2014). (a) Representative immunoblots of matrix preparations using anti-LNγ2. The unprocessed (155 kD) and processed (105 kD) forms of LNγ2 are indicated. (b) Quantification of processed LNγ2 as a proportion of total LNγ2, normalized to the daily mean to account for variability by day. (c) Representative immunoblots for mTLD/BMP-1 and ERK. (d) Quantification of relative mTLD/BMP-1 protein, normalized to ERK. Data are mean ± SEM; n≥3; 1-way ANOVA; Newman-Keuls multiple comparison, *P<0.05.
Taken together, these observations are consistent with a cross-suppressive role for α9β1 over other α3β1 functions that we have reported (Longmate et al., 2017), and they identify mTLD/BMP-1 as a mediator of α3β1-dependent LNγ2 processing. Of note, cellular levels of two BM/matricellular proteins, fibulin-2 and LNγ2 itself, were similarly counter-regulated by integrins α9β1 and α3β1 (Fig. S2), indicating that this suppressive role of α9β1 extends to α3β1-dependent expression of other matrix/matricellular proteins that modulate the tissue microenvironment.
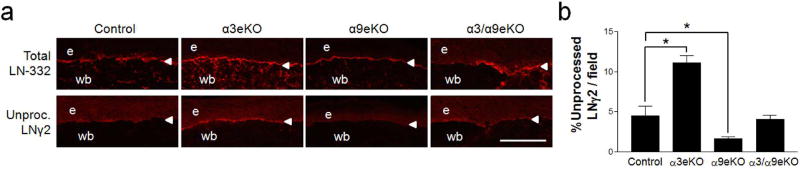
Epidermal deletion of integrin α9β1 promotes LNγ2 processing in vivo
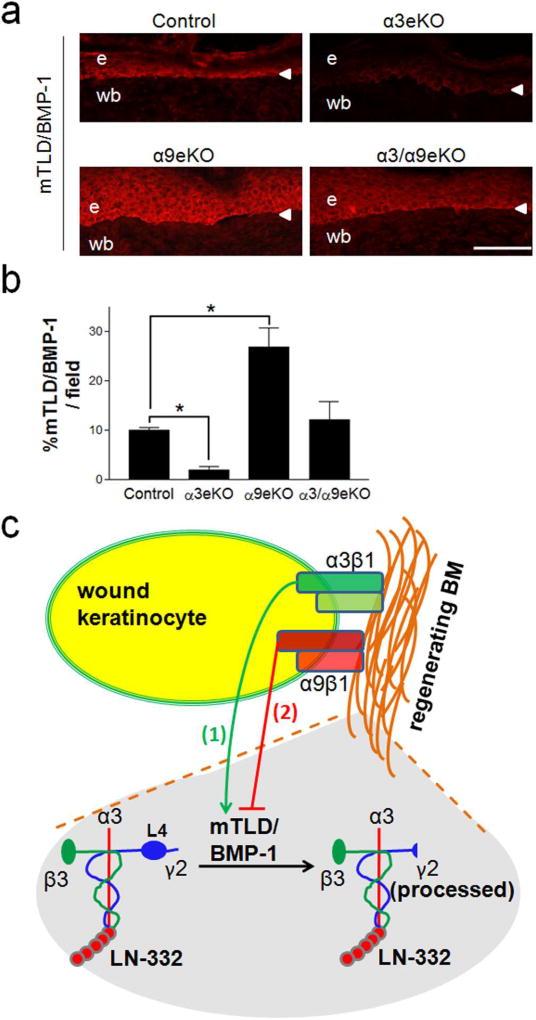
In order to determine whether α3β1 and α9β1 have similar counter-balancing effects on LNγ2 processing in vivo, we utilized mutant mice lacking α3β1 (α3eKO), α9β1 (α9eKO), or both integrins (α3/α9eKO) in epidermis (Longmate et al., 2017). We previously reported that α3eKO mice display a persistent accumulation of unprocessed LNγ2 in 10-day wounds, compared to control mice, indicating that α3β1 promotes LNγ2 processing in vivo (Longmate et al., 2014). As before, we utilized an antibody (anti-γ2L4m) directed against the globular L4 module of LNγ2 (depicted in Fig. 5c) to monitor its loss due to proteolysis during wound healing, as a readout for LNγ2 processing in vivo (i.e., positive staining indicates the presence of the unprocessed, precursor form of LN-332) (Longmate et al., 2014; Sasaki et al., 2001). As expected, wounds of α3eKO mice showed reduced LNγ2 processing, indicated by accumulation of unprocessed LNγ2. Levels of precursor LNγ2 in wounds from α3/α9eKO mice were comparable to that of wounds in control animals (Fig. 4a,b). In contrast, α9eKO mice showed reduced levels of unprocessed LNγ2 compared to controls (Fig. 4a,b), indicative of increased LNγ2 processing and consistent with the inhibitory role for α9β1 in LNγ2 processing that we observed in vitro (see Fig. 3).
Figure 5.
α3eKO wound epidermis displays reduced mTLD/BMP-1 levels while α9eKO wound epidermis displays increased mTLD/BMP-1 levels. (a) Representative cryosections of 10-day reepithelialized excisional wounds were prepared from control, α3eKO, α9eKO, or α3/α9eKO mice and immunostained with anti-BMP-1. e, epidermis; wb, wound bed; arrowhead, BMZ. (b) mTLD/BMP-1 level was quantified for each genotype as percent positive staining per field of wounded skin; scale bar = 100 µm; mean ± SEM; n ≥ 5 mice per genotype; 1-way ANOVA followed by Newman-Keuls multiple comparison, *P<0.05. (c) Model illustrating our findings that (1) α3β1 promotes and (2) α9β1 inhibits expression of mTLD/BMP-1, thereby regulating LNγ2 processing in regenerating BM. Note that the L4 module (detected by anti-γ2L4m) is present only in unprocessed LNγ2 (left).
Figure 4.
Epidermal deletion of integrin α9β1 promotes LNγ2 processing in vivo. Cryosections of 10-day, fully reepithelialized excisional wounds were prepared from control, α3eKO, α9eKO, or α3/α9eKO mice and stained by immunofluorescence with anti-LN-332 (to detect total LN-332) or anti-γ2L4m (to detect the L4 module of unprocessed LNγ2 chain). e, epidermis; wb, wound bed; arrowhead, BMZ. (a) Representative images are shown. (b) Unprocessed LNγ2 was quantified for each genotype as a percent of positive staining per field of wounded skin; scale bar = 100 µm; mean ± SEM.; n ≥ 4 mice per genotype; 1-way ANOVA followed by Newman-Keuls multiple comparison, *P<0.05.
mTLD/BMP-1 expression in post-wound epidermis is reduced in α3eKO mice but enhanced in α9eKO mice
Given that mTLD/BMP-1 mediated α3β1-dependent LNγ2 processing in vitro (Figs. 1 and 2), and wounds in mice that lack epidermal α3β1 displayed impaired LNγ2 processing (Fig. 4) (Longmate et al., 2014), we next assessed mTLD/BMP-1 expression in wound epidermis of α3eKO mice. Reepithelialized wounds from α3eKO mice displayed reduced levels of mTLD/BMP-1, compared with reepithelialized wounds of control mice which showed mTLD/BMP-1 expressed in the basal keratinocyte layer (Fig. 5a,b). Conversely, wounds of α9eKO mice showed elevated mTLD/BMP-1 staining compared to control wounds (Fig. 5a,b), correlating with more complete LNγ2 processing that we observed in these wounds (see Fig. 4). Interestingly, mTLD/BMP-1 staining in α9eKO wounds extended into the suprabasal layers of the epidermis (Fig. 5a), possibly reflecting a delay in epidermal differentiation that was previously reported in these mice (Singh et al., 2009). Of note, the BMP-1 antibody used in our immunohistochemistry studies is likely to have enhanced reactivity to the mTLD form of BMP-1 (see Materials and Methods). Overall, our data indicate opposing and balanced roles for integrins α3β1 and α9β1 in the regulation of BM maturation during wound healing, whereby α3β1 promotes mTLD/BMP-1 production and subsequent LNγ2 processing, while α9β1 coordinately tempers these processes (Fig. 5c).
DISCUSSION
Our previous findings have demonstrated that proteolytic processing of the LNγ2 chain, a hallmark of BM maturation, is impaired during adult wound healing in α3eKO mice, and in cultured α3-null keratinocytes (Longmate et al., 2014). Until now, the mechanism through which α3β1 promotes LNγ2 processing has been unclear. Our current findings suggest that α3β1 promotes this processing event at least partly through induction of the Bmp1 gene, which encodes mTLD/BMP-1, extracellular proteases that are known to mediate LNγ2 chain cleavage both in vitro and in vivo (Amano et al., 2000; Muir et al., 2016; Veitch et al., 2003). Previous work from our group has demonstrated that integrin α3β1 can regulate mRNA expression/stability of Cox-2 in breast cancer cells and MMP-9 in keratinocytes through alternative splicing and alternative polyadenylation, respectively (Missan et al., 2015; Subbaram et al., 2014). While we have not yet determined the mechanism of α3β1-dependent Bmp1 gene expression, we speculate that α3β1 may regulate mTLD/BMP-1 through similar posttranscriptional mechanisms. Indeed, several alternatively spliced transcripts of the Bmp1 gene have been identified, including those encoding BMP-1 or mTLD (Takahara et al., 1994), suggesting that the Bmp1 gene may be prone to such regulation.
While our findings indicate an important role for mTLD/BMP-1 in α3β1-mediated LNγ2 chain cleavage, it is important to note that we have not ruled out potentially important roles for other proteases. For example, MT1-MMP and MMP-2 are two other proteases known to mediate LNγ2 processing, as reviewed elsewhere (Tzu and Marinkovich, 2008). Moreover, differential processing of the LNα3 chain by plasmin has also been shown to influence keratinocyte behavior (Goldfinger et al., 1999; Goldfinger et al., 1998). The involvement of several distinct proteases, and the potential context- and species-specific differences in the relative importance of these proteases, indicate that LN-332 processing is nuanced and complex (Tzu and Marinkovich, 2008).
Studies in β1 integrin-deficient mice or embryoid bodies have revealed essential roles for β1 integrins, such as α3β1, in proper BM formation in embryonic or adult tissues (Aumailley et al., 2000; DiPersio et al., 1997; Henry and Campbell, 1998; Longmate et al., 2014), and mutations in the ITGA3 gene that encodes the α3 integrin subunit lead to compromised BM integrity and epidermal blistering in human patients with Junctional Epidermolysis Bullosa (Has et al., 2012; He et al., 2016; Nicolaou et al., 2012). Like α3β1, α9β1 is an epidermal integrin that is upregulated post-wounding (Singh et al., 2004). However, whether distinct epidermal integrins cooperate to mediate BM organization has not been investigated previously. Here, we speculated that coordinated activities of different integrins may be important for proper BM assembly. Like α3β1, integrin α9β1 is upregulated in the epidermis of healing wounds (Singh et al., 2004) and we recently reported that α9β1 promotes blood vessel regression and vascular normalization at later stages of wound healing through its ability to suppress pro-angiogenic functions of α3β1 (Longmate et al., 2017). Interestingly, our current findings indicate similarly opposing functions of α3β1 and α9β1 in BM maturation, where epidermal α3β1 promotes mTLD/BMP-1 expression and secretion and thus LNγ2 processing, while α9β1 inhibits these processes both in vitro and in vivo. We also found that addition of α9β1 to MK cells inhibited the ability of α3β1 to induce BM/matricellular proteins fibulin-2 and LNγ2, although in these cases we observed no statistically significant effects of α9β1 expression in the absence of α3β1. Together, these results indicate that in some cases α3β1 and α9β1 may also have independent and opposing effects on the same cellular function (e.g., LNγ2 processing), while in other cases α9β1 cross-suppresses α3β1-dependent function without having an obvious independent effect (e.g., expression of mTLD/BMP-1, fibulin-2 and LNγ2). In any case, counter-balancing roles for epidermal α3β1 and α9β1 appear critical for normal BM regeneration during wound healing, and for modification of the tissue microenvironment in general.
While integrin α3β1 has known roles in both stabilization and maturation of the cutaneous BM, these two processes appear separable. Indeed, previous findings from our lab indicate that α3β1 promotes BM stability in part through induction of fibulin-2, which was critical during adult wound-healing for epidermal adhesion since reepithelialized wounds of α3eKO mice blistered at the basement membrane zone (BMZ) (Longmate et al., 2014). However, accumulation of unprocessed LNγ2 was not observed at sites of epidermal blistering in neonatal α3eKO mice, and unprocessed LNγ2 was detected even after blisters resolved in wounds of adult α3eKO mice (Longmate et al., 2014), indicating that delayed LNγ2 processing was neither sufficient nor required to cause BM rupture that leads to blistering. Consistent with a clear separation between BM stability/epidermal adhesion and BM maturation, Bmp1-null mice die perinatally with defects in BM organization, but skin blistering was not reported (Burgeson and Christiano, 1997; Muir et al., 2016; Suzuki et al., 1996). In the current study, we observed that 10-day wounds of α3eKO mice have reduced mTLD/BMP-1 (Fig. 5) and display disorganized BM as evidenced by excess LN-332 staining beneath the BMZ (Fig. 4a, top panel), yet wound blistering resolves in these mice by 10 days (Longmate et al., 2014). We speculate that delayed LNγ2 processing might compensate for the blistering phenotype during wound healing in α3eKO mice, since the unprocessed LNγ2 short arm may allow for enhanced linkage to other ECM components that promote epidermal adhesion. Consistently, the unprocessed γ2 chain within LN-332 has been demonstrated to promote stronger cell adhesion rather than migration (Gagnoux-Palacios et al., 2001).
Although it is not clear why the N-terminus of LNγ2 is proteolytically removed, retention of the full γ2 chain and the accompanying ECM interactions is not required to maintain a stable BM, since this region is absent from processed LN-332 in mature cutaneous BM (Aumailley et al., 2003; Sasaki et al., 2001). Given that unprocessed LN-332 supports adhesion (Gagnoux-Palacios et al., 2001), while processed LN-332 may promote keratinocyte migration, we speculate that processed LNγ2 may poise the epidermis in a heightened “migration-ready” state to prepare skin for the next insult (Longmate et al., 2014). A complex coordination of α3β1 and α9β1 may be required to properly maintain a balance between adhesion/stabilization and maturation of wound epidermis.
Thus far, failure to understand how different keratinocyte integrins function cooperatively to regulate wound healing has hindered the development effective integrin-targeting therapies. While many more studies are required to fully understand how different integrins cooperate during the process of wound healing, it is intriguing to conjecture that “cooperating” integrins not only collaborate towards a common goal of efficient wound healing, but that counter-regulatory roles for certain integrins may be critically important to regulate the extent and timing of distinct aspects of wound healing. Overall, our current findings indicate that the opposing functions of integrins α3β1 and α9β1 in healing wounds are important for proper LN-332 processing and BM maturation. Moreover, they further support that the coordinated functions of α3β1 and α9β1 control the keratinocyte secretome and, consequently, modification of the tissue microenvironment.
MATERIALS & METHODS
MK cells
The derivation of a panel of immortalized MK cells that express integrins α3β1 and α9β1, separately or together, was described in detail previously (Longmate et al., 2017). MK cells were grown in keratinocyte growth medium consisting of Eagle's minimum essential medium (BioWhittaker, Walkersville, MD) supplemented with 4% fetal bovine serum (BioWhittaker) from which Ca2+ had been chelated, 0.05 mM CaCl2, 0.04 µg/ml hydrocortisone, 5 µg/ml insulin, 2×10−9 M T3, 10 units/ml interferon-γ (Sigma, St Louis, MO), 10 ng/ml epidermal growth factor, 100 units/ml penicillin, 100 µg/ml streptomycin, and L-glutmamine (Invitrogen, Waltham, MA). MK cells were maintained at 33°C, 8% CO2 on tissue-culture dishes coated with 30 µg/ml denatured rat tail collagen (BD Biosciences, Bedford, MA), as previously described (DiPersio et al., 2000).
RT-PCR and qPCR
Complete RNA was isolated using the RNeasy plus isolation kit (Qiagen Valencia, CA), and quality and quantity was confirmed by NanoDrop. cDNA was generated using iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA). qPCR for Bmp1 gene transcripts and β-actin were performed with iQ SYBR green Supermix (Bio-Rad) on a BioRad MyiQ PCR machine. The sequence and conditions for amplification of transcripts from the Bmp1 gene were as follows: forward primer 5’-GACTCACGGCGGACTCTAAG-3’; reverse primer, 5’-CACTGGTGGATGTCACCTTG-3’; 95°C, 3 minutes, 1 cycle; followed by 95°C for 30 sec; 53°C for 30 sec; 72°C for 1 minute; for 22 cycles; 72°C for 2 minutes, 1 cycle. The sequence and conditions for β-actin were as follows: forward primer, 5’-GCCAGGTCATCACTATTGG-3’; reverse primer, 5’-AGTAACAGTCCGCCTAGAAGC-3’; 94°C, 3 minutes, 1 cycle; followed by 94°C for 1 minute; 58°C for 1 minute 30 sec; 72°C for 1 minute 30 sec; for 18 cycles; 72°C for 2 minutes, 1 cycle.
Immunoblot
Whole cell lysates were prepared in non-reducing cell lysis buffer (Cell Signaling Technology, Beverly MA) and protein concentrations determined using the BCA Protein Assay kit (Pierce, Rockford, IL). Equal concentrations of protein were resolved by non-reducing 7% SDS/PAGE and assayed by immunoblot at the indicated dilutions of antibody: anti- mTLD/BMP-1 (1:200, ThermoFisher Scientific, Rockford, IL); anti-LNγ2 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA); anti-fibulin-2 (1:2000) (Pan et al., 1993); or anti-ERK (1:1000, Santa Cruz Biotechology, Santa Cruz, CA). The secondary antibodies used were horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1400, Cell Signaling, Danvers, MA) or donkey anti-goat IgG (1:1000, Santa Cruz Biotechnology), as appropriate. Chemiluminescence was performed using SuperSignal Kit (Pierce), then visualized using Bio-Rad ChemiDoc MP imaging system with Image Lab software (Bio-Rad, Hercules, CA).
siRNA transfection
Cells were plated in full MK medium onto non-coated tissue culture dishes 24 hours prior to transfection. Lipofectamine 2000 (Invitrogen) reagent was used to transfect 88nM of siRNA into MK cells. Three different Bmp1-targeting siRNA’s (Sigma-Aldrich, The Woodlands, TX) were as follows: siRNA #1- 5’-GCCAUAUCCAGUCCCAA-3’, siRNA #2-5’-CUCAAUCACCCAGACUGGA-3’, siRNA #3-5’-GCUAUAUUGUAUUCACCUA-3’. A luciferase-targeting siRNA was used as a control. Three days later, cells were either (1) isolated for immunoblot or PCR analyses (see above), or (2) lifted from the dish with 0.2% trypsin and re-plated onto non-coated tissue culture dishes for matrix preparation (see below).
Matrix Preparation
MK cells were seeded onto non-coated tissue culture dishes in MK media supplemented with 4 mM CaCl2 to promote matrix processing, as described (Amano et al., 2000). After three days of culture, cellular fractions were removed from dishes with 1mM EDTA and lysed for immunoblotting (see above). Matrix fractions were prepared as previously described (Longmate et al., 2014). Briefly, following cell removal, the deposited matrix was scraped into DOC buffer (2% sodium deoxycholate, 20 mM Tris-Cl pH 8.8, and 2 mM each of PMSF, EDTA, iodoacetic acid, N-ethylmaleimide), as described (Wierzbicka-Patynowski et al., 2004). The DOC-insoluble matrix fraction was solubilized in 4% SDS/reducing sample buffer, and equal volumes were assayed by immunoblot with anti-LNγ2 (1:200, Santa Cruz Biotechnology), followed by HRP-conjugated donkey anti-goat IgG (1:1000, Santa Cruz Biotechnology). Detection was performed as above (immunoblot).
Mass Spectrometry (MS) analysis of secreted protein
Duplicate samples of serum-free medium were conditioned for 24 hrs by MK cell variants then equal protein was analyzed by MS (Thermo Fisher Center for Multiplexed Proteomics, Harvard Medical School). MS spectra were searched using the SEQUEST algorithm against a mouse Uniprot composite database derived from the mouse proteome, and peptide spectral matches were filtered to <1% false discovery rate using the target-decoy strategy combined with linear discriminant analysis. There were 29 spectral counts for mTLD/BMP-1.
Mice
Epidermis-specific α3 knockout (α3eKO) mice, epidermis-specific α9 knockout (α9eKO) mice, and epidermis-specific α3/α9 double-knockout (α3/α9eKO) mice are homozygous for a floxed α3 allele (Itga3flx/flx) and/or α9 allele (Itga9flx/flx), and express a Cre recombinase transgene under control of the epidermal-specific keratin 14 promoter (K14-Cre), as described (Mitchell et al., 2009; Singh et al., 2009). PCR genotyping and confirmatory immunostaining for appropriate loss of integrin expression has been described (Mitchell et al., 2009; Singh et al., 2009). All mouse studies were approved by the Institutional Animal Care and Use Committee at Albany Medical College.
In vivo wounding and immunohistochemistry
Adult mice (6–10 weeks of age) were anaesthetized and shaved, and four full-thickness wounds were made on the back of each mouse using a sterile 4-mm biopsy punch, as described (Mitchell et al., 2009). After allowing wounds to heal for 10 days, mice were euthanized by CO2 narcosis and wounds were surgically excised. Wounds were frozen in OCT compound, and 10 µm sections were prepared for immunohistology (Albany Medical College Histology Services). For immunostaining, frozen sections were rehydrated in PBS with 0.2% Tween-20 for 10 minutes, blocked in 10% heat-inactivated goat serum and 5% milk in PBS for 1 hour, then stained with the following rabbit polyclonal antisera: anti-LN-332 (1:200; Abcam, Cambridge, MA); anti-LNγ2 L4m (1:1000) (Sasaki et al., 2001); or anti-BMP-1 (1:100; Abcam, Cambridge, MA). The anti-BMP-1 antibody used here was derived from a synthetic peptide based on the carboxyterminal end of the mTLD form of BMP-1, and is likely specific to mTLD. The secondary antibody used was Alexa Fluor 594 goat anti-rabbit IgG (1:250; Molecular Probes, Eugene, OR). Images were collected on a Nikon Eclipse 80i using a Spot camera (Diagnostic Instruments, Sterling Heights, MI).
Supplementary Material
Acknowledgments
We thank Christina Nickerson (AMC Histology Core) for assistance with tissue sectioning. Antibodies against LNγ2 L4m and fibulin-2 were provided previously by the laboratories of Dr. Rupert Timpl and Dr. Mon-Li Chu, respectively. This research was supported by an NIH grant from NIAMS to C. M. DiPersio and L. Van De Water (R01AR063778), as well as by an NIH pre-doctoral fellowship from NCI to W. M. Longmate (F31CA174198).
Abbreviations
- α3eKO
integrin α3 epidermal knockout
- α9eKO
integrin α9 epidermal knockout
- α3/α9eKO
integrin α3/α9 double epidermal knockout
- MK cells
mouse keratinocytes
- BM
basement membrane
- BMZ
basement membrane zone
- mTLD/BMP-1
mammalian tolloid/bone morphogenetic protein 1
- LNγ2
laminin-γ2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Amano S, Scott IC, Takahara K, Koch M, Champliaud MF, Gerecke DR, et al. Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain. J Biol Chem. 2000;275:22728–35. doi: 10.1074/jbc.M002345200. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Aumailley M, El Khal A, Knoss N, Tunggal L. Laminin 5 processing and its integration into the ECM. Matrix Biol. 2003;22:49–54. doi: 10.1016/s0945-053x(03)00013-1. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Pesch M, Tunggal L, Gaill F, Fassler R. Altered synthesis of laminin 1 and absence of basement membrane component deposition in (beta)1 integrin-deficient embryoid bodies. J Cell Sci. 2000;113(Pt 2):259–68. doi: 10.1242/jcs.113.2.259. [DOI] [PubMed] [Google Scholar]
- Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997;9:651–8. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- Choma DP, Pumiglia K, DiPersio CM. Integrin {alpha}3{beta}1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci. 2004;117:3947–59. doi: 10.1242/jcs.01251. [DOI] [PubMed] [Google Scholar]
- Dang N, Klingberg S, Rubin AI, Edwards M, Borelli S, Relic J, et al. Differential expression of pyloric atresia in junctional epidermolysis bullosa with ITGB4 mutations suggests that pyloric atresia is due to factors other than the mutations and not predictive of a poor outcome: three novel mutations and a review of the literature. Acta Derm Venereol. 2008;88:438–48. doi: 10.2340/00015555-0484. [DOI] [PubMed] [Google Scholar]
- Delwel GO, de Melker AA, Hogervorst F, Jaspars LH, Fles DL, Kuikman I, et al. Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–15. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. α3β1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–42. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Shao M, Di Costanzo L, Kreidberg JA, Hynes RO. Mouse keratinocytes immortalized with large T antigen acquire α3β1 integrin-dependent secretion of MMP-9/gelatinase B. Journal of Cell Science. 2000;113:2909–21. doi: 10.1242/jcs.113.16.2909. [DOI] [PubMed] [Google Scholar]
- Gagnoux-Palacios L, Allegra M, Spirito F, Pommeret O, Romero C, Ortonne JP, et al. The short arm of the laminin gamma2 chain plays a pivotal role in the incorporation of laminin 5 into the extracellular matrix and in cell adhesion. J Cell Biol. 2001;153:835–50. doi: 10.1083/jcb.153.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones JCR. The α3 laminin subunit, α6β4 and α3β1 integrin coordinately regulate wound healing in cultured epithelial cells in the skin. Journal of Cell Science. 1999;112:2615–29. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JCR. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–65. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Has C, Sparta G, Kiritsi D, Weibel L, Moeller A, Vega-Warner V, et al. Integrin alpha3 mutations with kidney, lung, and skin disease. N Engl J Med. 2012;366:1508–14. doi: 10.1056/NEJMoa1110813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Balasubramanian M, Humphreys N, Waruiru C, Brauner M, Kohlhase J, et al. Intronic ITGA3 Mutation Impacts Splicing Regulation and Causes Interstitial Lung Disease, Nephrotic Syndrome, and Epidermolysis Bullosa. J Invest Dermatol. 2016;136:1056–9. doi: 10.1016/j.jid.2015.11.031. [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–70. doi: 10.1016/s0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iyer V, Pumiglia K, DiPersio CM. Alpha3beta1 integrin regulates MMP-9 mRNA stability in immortalized keratinocytes: a novel mechanism of integrin-mediated MMP gene expression. J Cell Sci. 2005;118:1185–95. doi: 10.1242/jcs.01708. [DOI] [PubMed] [Google Scholar]
- Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6:175–83. doi: 10.1038/nrc1817. [DOI] [PubMed] [Google Scholar]
- Longmate WM, DiPersio CM. Integrin Regulation of Epidermal Functions in Wounds. Adv Wound Care (New Rochelle) 2014;3:229–46. doi: 10.1089/wound.2013.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmate WM, Lyons SP, Chittur SV, Pumiglia KM, Van De Water L, DiPersio CM. Suppression of integrin alpha3beta1 by alpha9beta1 in the epidermis controls the paracrine resolution of wound angiogenesis. J Cell Biol. 2017;216:1473–88. doi: 10.1083/jcb.201510042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmate WM, Monichan R, Chu ML, Tsuda T, Mahoney MG, DiPersio CM. Reduced fibulin-2 contributes to loss of basement membrane integrity and skin blistering in mice lacking integrin alpha3beta1 in the epidermis. J Invest Dermatol. 2014;134:1609–17. doi: 10.1038/jid.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C, Charafeddine RA, Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24:4133–52. doi: 10.1096/fj.09-151449. [DOI] [PubMed] [Google Scholar]
- Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, Sonnenberg A. Integrin {alpha}3{beta}1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122:278–88. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem. 1992;267:17900–6. [PubMed] [Google Scholar]
- McGrath JA. Recently Identified Forms of Epidermolysis Bullosa. Ann Dermatol. 2015;27:658–66. doi: 10.5021/ad.2015.27.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missan DS, Chittur SV, DiPersio CM. Regulation of fibulin-2 gene expression by integrin alpha3beta1 contributes to the invasive phenotype of transformed keratinocytes. J Invest Dermatol. 2014;134:2418–27. doi: 10.1038/jid.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missan DS, Mitchell K, Subbaram S, DiPersio CM. Integrin alpha3beta1 signaling through MEK/ERK determines alternative polyadenylation of the MMP-9 mRNA transcript in immortalized mouse keratinocytes. PLoS One. 2015;10:e0119539. doi: 10.1371/journal.pone.0119539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K, Szekeres C, Milano V, Svenson KB, Nilsen-Hamilton M, Kreidberg JA, et al. Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci. 2009;122:1778–87. doi: 10.1242/jcs.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir AM, Massoudi D, Nguyen N, Keene DR, Lee SJ, Birk DE, et al. BMP1-like proteinases are essential to the structure and wound healing of skin. Matrix Biol. 2016;56:114–31. doi: 10.1016/j.matbio.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BP, Ryan MC, Gil SG, Carter WG. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol. 2000;12:554–62. doi: 10.1016/s0955-0674(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Nicolaou N, Margadant C, Kevelam SH, Lilien MR, Oosterveld MJ, Kreft M, et al. Gain of glycosylation in integrin alpha3 causes lung disease and nephrotic syndrome. J Clin Invest. 2012;122:4375–87. doi: 10.1172/JCI64100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan TC, Sasaki T, Zhang RZ, Fassler R, Timpl R, Chu ML. Structure and expression of fibulin-2, a novel extracellular matrix protein with multiple EGF-like repeats and consensus motifs for calcium binding. J Cell Biol. 1993;123:1269–77. doi: 10.1083/jcb.123.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazzagli C, He Y, Busch H, Esser P, Kiritsi D, Gache Y, et al. Absence of the integrin alpha3 subunit induces an activated phenotype in human keratinocytes. J Invest Dermatol. 2017 doi: 10.1016/j.jid.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L, Uitto J. Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol. 1999;18:29–42. doi: 10.1016/s0945-053x(98)00005-5. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Gohring W, Mann K, Brakebusch C, Yamada Y, Fassler R, et al. Short arm region of laminin-5 gamma2 chain: structure, mechanism of processing and binding to heparin and proteins. J Mol Biol. 2001;314:751–63. doi: 10.1006/jmbi.2001.5176. [DOI] [PubMed] [Google Scholar]
- Singh P, Chen C, Pal-Ghosh S, Stepp MA, Sheppard D, Van De Water L. Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J Invest Dermatol. 2009;129:217–28. doi: 10.1038/jid.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Reimer CL, Peters JH, Stepp MA, Hynes RO, Van De Water L. The spatial and temporal expression patterns of integrin alpha9beta1 and one of its ligands, the EIIIA segment of fibronectin, in cutaneous wound healing. J Invest Dermatol. 2004;123:1176–81. doi: 10.1111/j.0022-202X.2004.23485.x. [DOI] [PubMed] [Google Scholar]
- Subbaram S, Lyons SP, Svenson KB, Hammond SL, McCabe LG, Chittur SV, et al. Integrin alpha3beta1 controls mRNA splicing that determines Cox-2 mRNA stability in breast cancer cells. J Cell Sci. 2014;127:1179–89. doi: 10.1242/jcs.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, et al. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development. 1996;122:3587–95. doi: 10.1242/dev.122.11.3587. [DOI] [PubMed] [Google Scholar]
- Takahara K, Lyons GE, Greenspan DS. Bone morphogenetic protein-1 and a mammalian tolloid homologue (mTld) are encoded by alternatively spliced transcripts which are differentially expressed in some tissues. J Biol Chem. 1994;269:32572–8. [PubMed] [Google Scholar]
- Tsubota Y, Mizushima H, Hirosaki T, Higashi S, Yasumitsu H, Miyazaki K. Isolation and activity of proteolytic fragment of laminin-5 alpha3 chain. Biochem Biophys Res Commun. 2000;278:614–20. doi: 10.1006/bbrc.2000.3851. [DOI] [PubMed] [Google Scholar]
- Tzu J, Marinkovich MP. Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol. 2008;40:199–214. doi: 10.1016/j.biocel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch DP, Nokelainen P, McGowan KA, Nguyen TT, Nguyen NE, Stephenson R, et al. Mammalian tolloid metalloproteinase, and not matrix metalloprotease 2 or membrane type 1 metalloprotease, processes laminin-5 in keratinocytes and skin. J Biol Chem. 2003;278:15661–8. doi: 10.1074/jbc.M210588200. [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Mao Y, Schwarzbauer JE. Analysis of fibronectin matrix assembly. Curr Protoc Cell Biol. 2004;(Unit 10):2. doi: 10.1002/0471143030.cb1012s25. Chapter 10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.