Abstract
We sought to determine whether high-dose folinic acid improves verbal communication in children with non-syndromic autism spectrum disorder (ASD) and language impairment in a double-blind placebo control setting. Forty-eight children (mean age 7 years 4 months; 82% male) with ASD and language impairment were randomized to receive 12 weeks of high-dose folinic acid (2 mg kg−1 per day, maximum 50 mg per day; n=23) or placebo (n=25). Children were subtyped by glutathione and folate receptor-α autoantibody (FRAA) status. Improvement in verbal communication, as measured by a ability-appropriate standardized instrument, was significantly greater in participants receiving folinic acid as compared with those receiving placebo, resulting in an effect of 5.7 (1.0,10.4) standardized points with a medium-to-large effect size (Cohen’s d=0.70). FRAA status was predictive of response to treatment. For FRAA-positive participants, improvement in verbal communication was significantly greater in those receiving folinic acid as compared with those receiving placebo, resulting in an effect of 7.3 (1.4,13.2) standardized points with a large effect size (Cohen’s d=0.91), indicating that folinic acid treatment may be more efficacious in children with ASD who are FRAA positive. Improvements in subscales of the Vineland Adaptive Behavior Scale, the Aberrant Behavior Checklist, the Autism Symptom Questionnaire and the Behavioral Assessment System for Children were significantly greater in the folinic acid group as compared with the placebo group. There was no significant difference in adverse effects between treatment groups. Thus, in this small trial of children with non-syndromic ASD and language impairment, treatment with high-dose folinic acid for 12 weeks resulted in improvement in verbal communication as compared with placebo, particularly in those participants who were positive for FRAAs.
Introduction
Autism spectrum disorder (ASD) is a behaviorally defined disorder whose etiology remains poorly understood. Recent estimates suggest that up to 2% of children in the United States are affected by an ASD.1 Recent research has uncovered associated physiological abnormalities,2, 3 but high-quality clinical trials investigating biological targeted treatments remain limited.4, 5 Thus, the development and investigation of treatments that target underlying pathophysiological abnormalities and core and associated symptoms is urgently needed.4
Several abnormalities in the metabolism of folate, an essential water-soluble B vitamin, have been linked to ASD.6 ASD is associated with polymorphisms in folate-related pathway genes and disruptions in folate-related metabolism may be related to glutathione abnormalities associated with ASD (Supplementary Figure S1).7 Supplementation with folate during the prenatal and conception periods has been shown to lower the risk of ASD in offspring.8, 9, 10
Folate is primarily transported across the choroid plexus epithelium attached to the folate receptor α (FRα) using energy-dependent endocytosis (Supplementary Figure S1).11 Cerebral folate deficiency, a disorder in which folate concentrations are below normal in the cerebrospinal fluid (CSF) but not in the blood, was first described in six children with neurodevelopmental regression and neurological abnormalities. Treatment with folinic acid, a reduced form of folate, normalized CSF folate concentrations and significantly improved neurological symptoms.12 Further case descriptions demonstrated that many of the children with cerebral folate deficiency had ASD and that treatment with folinic acid improved the ASD symptoms as well as other neurological symptoms.5, 11, 13, 14, 15 Interestingly, individuals with Rett syndrome, a disorder closely related to ASD, have also been found to have cerebral folate deficiency.16, 17, 18
FRα dysfunction was first linked to FRα autoantibodies (FRAAs)11 with later reports also linking FRα dysfunction to mitochondrial disease.19, 20, 21, 22 An intriguing finding is that genetic mutations in the FOLR1 gene, which is the gene for the FRα, rarely accounts for cerebral folate deficiency.23 Two types of FRAAs, blocking and binding, impair folate transport24 and serum titers of the blocking FRAA have been correlated with CSF folate concentrations in independent studies.24, 25 The blocking FRAA directly interferes with the binding of folate to the FRα while the binding FRAA binds to the FRα and triggers an antibody-mediated immune reaction.26, 27
The presence of central folate disturbances in ASD is supported by several studies.
Of 93 children with ASD, 60 and 44% were positive for blocking and binding FRAAs, respectively.24 Another study which examined only blocking FRAAs in children with ASD confirmed this high prevalence.28 These rates are clearly higher than the 4–15% prevalence reported in healthy adults24 and the 3% prevalence reported in developmentally delayed non-autistic children.28 Interestingly, a recent animal study suggested that FRAAs can disrupt folate metabolism during gestation resulting in ASD-like behaviors in the offspring.29 More recently, up to 23% of children with ASD who underwent lumbar puncture were reported to have abnormally low CSF folate concentrations.30
The reduced folate carrier is a secondary mechanism which transports reduced folates, such as folinic acid, across the blood–brain barrier, although high serum concentrations are required since the reduced folate carrier has a lower affinity for folate (i.e., micromolar concentrations) than the FRα (i.e., nanomolar concentrations; Supplementary Figure S1A).11, 24 Case reports and series note that high-dose folinic acid markedly improves symptoms in children with ASD and low CSF folate concentrations.11, 24 In a controlled open-label study, we found that children with ASD who were positive for at least one FRAA experienced significant improvements in verbal communication, receptive and expressive language, attention, and stereotypical behavior with high-dose (2 mg kg−1 per day in two divided doses; maximum 50 mg per day) folinic acid treatment with very few adverse effects reported.24
To determine whether high-dose folinic acid can improve core and associated ASD symptoms, we conducted a single-site randomized double-blind placebo-controlled clinical trial. It was hypothesized that high-dose folinic acid would alleviate ASD symptoms, particularly in children with folate-related metabolic abnormalities. In addition, we sought to determine if biomarkers of disruptions in folate metabolism, such as the FRAA, could predict which children would respond to folinic acid treatment, so that invasive diagnostic procedures such as a lumbar puncture might be avoided.
Materials and methods
The study was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences (Little Rock, AR, USA). Parents of participants provided written informed consent.
Study design
This two-arm double-blind randomized placebo-controlled parallel study with a 1:1 allocation was performed at Arkansas Children’s Research Institute (Little Rock, AR, USA) from 4 June 2012 to 22 November 2013.
Participants who met inclusion and exclusion criteria were screened for language impairment. Preverbal (<25 functional words) children qualified as language impaired. Otherwise, the age-appropriate version of the Clinical Evaluation of Language Fundamentals (CELF) confirmed language impairment. Language impairment was defined as a core standardized score <85 if the preschool version was used or failure on the CELF screener if other versions were used.
Those confirmed to have language impairment were randomized to the folinic acid or placebo group and a fasting blood sample was obtained. Randomization was performed using a random number generator with a block size of four. The research pharmacists had exclusive access to the randomization allocation. After breakfast, the participants returned for language, developmental and behavioral assessments. Following these assessments the family was given the 12 weeks of the intervention and was instructed on its administration. Language, developmental and behavioral assessments were repeated after 12 weeks of treatment.
Parent and teacher questionnaires were requested at baseline and 6 and 12 weeks after starting treatment. Parents were asked to deliver baseline questionnaires to teachers or therapists. After the first visit, questionnaires were mailed to the parents and teachers at least 1 week prior to the target date of completion. Parents were asked to bring the completed teacher and parent 12-week questionnaires to the final assessment. Other questionnaires were returned in a preaddressed postage-paid envelope.
Intervention
The target dose of the intervention (INN: DL folinic acid calcium salt; USAN: leucovorin calcium) was 2 mg kg−1 per day (maximum 50 mg per day) in two equally divided doses with half of the target dose given during the first 2 weeks. Dye-free, milk-product-free, vegetarian capsules were provided in three strengths (5, 10 and 25 mg) by Lee Silsby Compounding Pharmacy (Cleveland Heights, OH, USA). Certificate of analysis was provided for each capsule strength by an independent analytical service (Eagle Analytical Services, Houston, TX, USA) for each batch of capsules produced. In all cases, potency was at least 99%.
To verify that folinic acid and placebo capsules were indistinguishable by sight and feel, 10 scientists, 10 medical staff and 10 parents of children with ASD not involved in the study were asked to sort eight small plastic numbered bags, each containing two same strength capsules, into two groups (placebo and folinic acid) of four based upon capsule similarity. No one was able to accurately sort these bags (Binomial P=0.04). Parents were instructed that capsules could be opened and the powder added to food or drink if swallowing the medication was difficult for the child. Both the placebo and folinic acid powder were odorless and tasteless. No parent or child commented on the odor or taste of the medication, providing further evidence of the tasteless and odorless nature of the treatment.
Parents were asked about missed doses and returned pill containers were examined for adherence which was calculated by the research pharmacy.
Inclusion and exclusion criteria
Participants were recruited from our research registry (48%), autism clinic (23%), community advertisement and social media (13%), word-of-mouth (10%) and physician referrals (2%). The ASD diagnosis was defined by one of the following: (i) a gold-standard diagnostic instrument such as the Autism Diagnostic Observation Schedule and/or Autism Diagnostic Interview-Revised; (ii) the state of Arkansas diagnostic standard, defined as agreement of a physician, psychologist and speech therapist; and/or (iii) Diagnostic Statistical Manual (DSM) diagnosis by a physician along with standardized validated questionnaires and diagnosis confirmation by the Principal Investigator. Reconfirmation of the diagnosis using the lifetime version of the Autism Diagnostic Interview-Revised by an independent research reliable rater was requested from all participants.
Inclusion criteria included: (i) age 3–14 years of age; (ii) documentation of language impairment; (iii) unchanged complementary, traditional, behavioral and education therapy 8 weeks prior to enrollment; and (iv) intention to maintain ongoing therapies constant throughout the trial. Exclusion criteria included: (i) antipsychotic medications; (ii) supplementation exceeding the recommended daily allowance; (iii) prematurity; (iv) uncontrolled gastroesophageal reflux; (v) history of liver or kidney disease; (vi) drugs known to affect folate metabolism (see Supplementary Material); (vii) profound sensory deficits; (viii) well-defined genetic syndromes; (ix) genetic mutations known to significantly affect folate-associated pathways; (x) brain malformations or damage found on magnetic resonance imaging; (xi) ongoing therapies that could interfere with the study; (xii) a clinical seizure within the last 6 months; and (xiii) moderate-to-severe irritability or self-abusive behavior on the aberrant behavior checklist.
Outcome measures
All primary and secondary outcomes were obtained at baseline and study end. Questionnaires were also requested 6 weeks after starting the intervention. Aside from the research pharmacists, study staff, participants, parents and teachers were blind to treatment allocation.
Primary outcome
Verbal communication was the primary outcome for several reasons. First, verbal communication improved in preliminary folinic acid treatment studies.24 Second, verbal communication in children with ASD is closely linked to parental quality of life.31 Third, the development of language and communication skills is associated with favorable outcomes.32, 33, 34
It should be acknowledged that communication impairment was considered a core feature of ASD up until the DSM-V, which has now combined communication and social symptoms into a social–communication symptom cluster. In the DSM-V language impairment is recognized as a significant comorbidity interrelated to the ASD diagnosis.
Verbal communication was assessed by an ability-appropriate instrument. Instruments used were the CELF-preschool-2, CELF-4 and the Preschool Language Scale-5 (PLS-5). The CELF is a standardized, well-validated instrument that assesses skills that are abnormal in ASD35 and has been used in studies focusing on verbal communication in ASD.36, 37 The PLS-5 is a standardized, well-validated instrument that measures subtle changes in verbal communication, particularly in preverbal children.38 The standardized summary score of each instrument (mean 100, standard deviation 15) was the primary outcome measure and ranges from 50 to 150 for the PLS-5 and 45 to 155 for the CELF.
The ability-appropriate instrument was selected using a structured algorithm. The goal was to select an instrument with an adequate dynamic range for assessing improvement in verbal communication. The assessment started with the most age-appropriate instrument.
If the child obtained a score at the floor, the next lower ability instrument was then used. This process was repeated until a score above the floor could be obtained. The score from the final instrument was the primary outcome measure at baseline and at trial end. If the child’s age exceeded the maximum age of the instrument’s standardization, the maximum standardized age was used. At trial end, all instruments used during the baseline assessment were repeated in the same order to simulate the same baseline assessment experience and to minimize a potential confounder of cognitive fatigue.
Studies have shown that early behavioral therapy improves verbal communication by one-standard deviation over 36 weeks.39, 40 Thus, a clinically meaningful change was defined as a 5-point increase in verbal communication in this 12-week study since the primary outcome has a 15 point standard deviation. Examining the standard error of participants in the current study suggests that an minimal clinically important difference is 2 points.
Secondary outcomes
Secondary outcome measures included the Ohio Autism Clinical Impression Scale (OACIS), Vineland Adaptive Behavior Scale 2nd Edition (VABS) Survey Interview Form and several questionnaires. Parents and teachers were asked to complete the Aberrant Behavior Checklist (ABC), Social Responsiveness Scale (SRS) and Behavioral Assessment System for Children 2nd Edition (BASC). Only parents were asked to complete the Autism Impact Measure (AIM) and Autism Symptoms Questionnaire (ASQ).
The OACIS is an observer-rated scale sensitive to clinically meaningful changes in ASD symptoms.41 It was first developed as the Ohio State University Autism Rating Scale42 and has been shown to have good inter-rater and cross-cultural reliability43 and has been successfully used in several ASD clinical trials.44, 45, 46, 47 Severity of each symptom was rated by the first author at baseline and at the final assessment by observing the entire assessment of verbal communication. In validation studies a 0.5-point change was considered clinically meaningful.43
The VABS is a reliable and valid measure of the ability to perform age-appropriate everyday skills though a 20–30 min structured interview with a caretaker.47 Standard scores from the communication, daily living, social and motor skills, and adaptive behavioral composite were analyzed. Standard scores have a mean of 100, standard deviation of 15 and range 20–160. Intervention studies in ASD have demonstrated a change of 6 points to be clinically meaningful.48
The ABC is a 58-item questionnaire47 that measures disruptive behaviors, including Irritability (15 items, range 0–45); Social Withdrawal (16 items, range 0–48); Stereotypy (7 items, range 0–21); Hyperactivity (16 items, range 0–48) and Inappropriate Speech (4 items, range 0–12). Each item is rated 0 to 3 with higher scores indicating greater severity. Multiple ASD clinical trials have used it and it has convergent and divergent validity.49 Interventional ASD studies suggest a 12-point decrease in the total score is clinically meaningful.45
The BASC ranges from 185 to 306 items and is validated in ASD.50 Each item is rated 0 to 3 with higher scores indicating greater severity. Standardized T-Scores (mean 50, standard deviation 10) range 20–120 for externalizing, internalizing and behavioral symptoms and 10–90 for adaptive skills.
The SRS is a 65-item questionnaire that measures social skills across five domains: Social Awareness (8 items, meaningful change 7.1), Social Cognition (12 items, meaningful change 5.8), Social Communication (22 items, meaningful change 4.2), Social Motivation (11 items, meaningful change 5.7), Autistic Mannerisms (12 items, meaningful change 5.5) and total (65 items). Each item is rated 0 to 3 with higher scores indicating greater severity. Standardized T-scores (mean 50, standard deviation 10) range 30–90.
The AIM, a 45-item parent-reported measure of the frequency and impact of core ASD symptoms during the past 2 weeks using two 5-point scales of increasing severity ranging from 1 to 5.51 The Frequency and Impact scores range 45–225.
The ASQ, a 34-item checklist (The Center for Autism and Related Disorders) that assesses social interaction (12 items, range 0–4), stereotyped behavior (7 items, range 0–4), communication symptoms (15 items, range 0–5) and total symptoms (34 items, range 0–13).52 Intervention ASD studies suggest a 1.1 point change as clinically meaningful.52
Biomarkers
Two folate-related biomarkers were investigated. FRAA titers, both blocking and binding, were analyzed.24 Plasma free reduced-to-oxidized glutathione redox ratio was determined.48 Folate-related vitamins and minerals were measured. Serum total folate and vitamin B12 were measured using MP Diagnostics SimulTRAC-SNB Radioassay Kit (Cat# 06B264806). Plasma zinc, whole blood copper and red blood cell magnesium were analyzed by Doctor’s Data.
Establishment and maintenance of assessment fidelity
Research staff was trained by a multispecialty team consisting of two licensed psychologists and a speech therapist prior to performing assessments. During the trial a research psychologist supervised research staff and provided feedback and retraining if necessary.
Adverse effects monitoring
Adverse events were monitored every 3 weeks using a modification of the Dosage Record Treatment Emergent Symptom Scale. Adverse events were considered related to the treatment if they started or worsened following the start of the trial. If adverse events were persistent or severe, the parents were offered the option of halving the dose or discontinuing the intervention. The dose could only be reduced once and was never increased if reduced.
Statistical analysis
An intention-to-treat analysis was used.53 Analyses used SAS version 9.3. To account for missing data multiple imputation was conducted.53, 54 An imputation of 20 was used55, 56 and sensitivity analysis was used to check for systematic bias.57
Mixed-effects regression models58 were used to estimate the effect and effect size of the treatment. The models included the effect of time and a random intercept to account for each individual’s symptom level. The models tested the a priori hypothesis that the change in the outcome measure was greater for the folinic acid group as compared with the placebo group. This interaction was tested specifically using a two-tailed t-test with a P<0.05. Since our previous study24 demonstrated a large effect size, this study was powered with a large effect size (Cohen’s d=0.80), which provided a 77% power with 24 participants per group.
Analyses were conducted on subgroups defined by biomarkers of abnormal folate metabolism. FRAAs were dichotomized as positive and negative and the glutathione redox ratio was dichotomized to relatively high (more normal; above the median of 8.30) and low (more abnormal; below the median of 8.30). Mixed-model regressions, similar to the one described above, were conducted on each subgroup separately since the study was not powered to investigate interaction with these biomarkers using the mixed model.
A responder analysis was conducted using backward elimination (P⩽0.05 to stay in model) logistic regression. Response was defined by a five standardized point increase in verbal communication since this defines a clinically meaningful change. Age, baseline language and baseline overall development (as indexed by the VABS Behavioral Composite Standardized Score) were entered as potential covariates. To investigate whether the biomarkers of abnormal folate metabolism were related to participant response, logistic regressions were conducted with an interaction between treatment group and each biomarker.
Secondary outcome measures were analyzed using the mixed-model regression. Because of the large number of secondary outcomes, correction for multiple comparisons was conducted using the false discovery rate.59
The total number of patients reporting each adverse event was compared across treatment groups using a Fisher exact test. Adverse events that were possibly, probably or definitely related to the treatments were analyzed.
Results
Participants
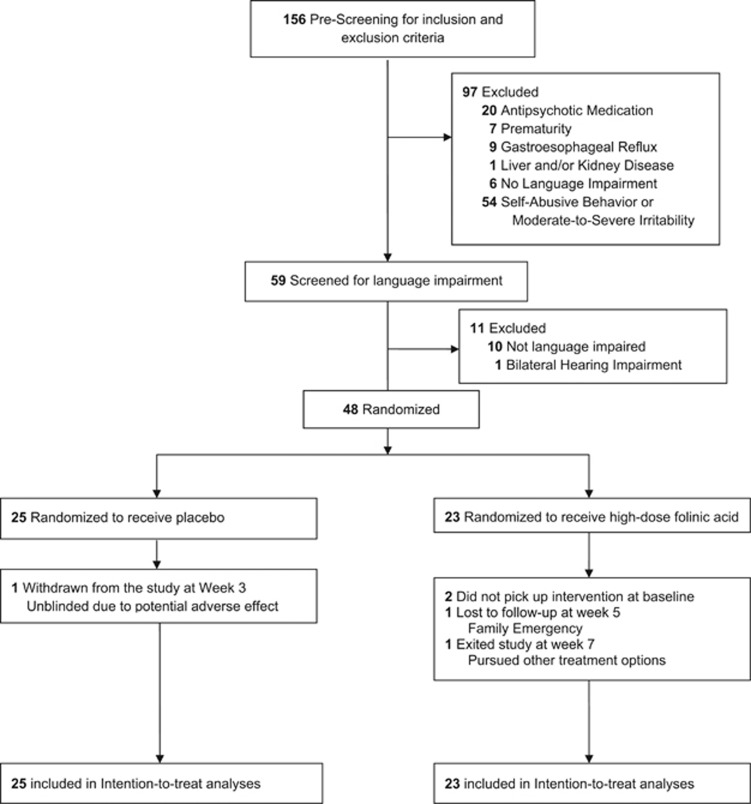
One hundred fifty-six participants were prescreened, with 59 found to potentially meet inclusion/exclusion criteria (Figure 1), of which 11 failed screening, 10 because of no language impairment and 1 because of congenital hearing impairment. Twenty-five participants were randomized to receive placebo and 23 were randomized to receive high-dose folinic acid (age range 3 years 4 months to 13 years 4 months).
Figure 1.
Flow diagram of participants through the trial.
Participant characteristics were similar across treatment groups except for multivitamins (Table 1). Baseline outcome measures were not significantly different across treatment groups except for verbal communication in FRAA-negative participants (F(1,14)=4.58, P=0.05; Tables 2A and 2B). All participants evaluated by an independent research reliable rater exceeded the diagnostic threshold for ASD. The mean number of missed doses per week was not significantly different across groups. Adherence was >90% for those who returned the bottles (20/25 placebo; 16/21 folinic acid).
Table 1. Demographic and clinical characteristics by treatment groupa.
| Variable | Folinic acid (n=23) | Placebo (n=25) |
|---|---|---|
| Age, mean (s.d.), years months | 7y 7 m (3y 6 m) | 7y 2 m (2y 10 m) |
| Males, N (%) | 18 (78) | 20 (80) |
| Number of missed doses | 0.2 (0.3) | 0.6 (1.3) |
| Vineland adaptive behavior composite, mean (s.d.) | 64.79 (7.25) | 65.84 (9.20) |
| Total therapy, minutes per week, mean (s.d.) | 339.52 (498.32) | 500.80 (591.56) |
| Total therapy, minutes per week, median (min; max) | 195 (0; 2070) | 300 (0; 2790) |
| Speech therapy, minutes per week, mean (s.d.) | 99.52 (63.26) | 220.00 (377.77) |
| Behavioral therapy, minutes per week, mean (s.d.) | 133.57 (409.75) | 214.80 (569.58) |
| Motor therapy, minutes per week, mean (s.d.) | 103.57 (133.31) | 137.60 (95.98) |
| Folate receptor autoantibody positive, N (%) | 13 (57) | 18 (72) |
| Blocking titer (pmol ml−1), mean (s.d.) | 0.08 (0.20) | 0.06 (0.15) |
| Blocking titer (pmol ml−1), median (min; max) | 0.00 (0.00, 0.86) | 0.00 (0.00; 0.55) |
| Binding titer (pmol ml−1), mean (s.d.) | 0.39 (0.74) | 0.61 (0.73) |
| Binding titer (pmol ml−1), median (min; max) | 0.00 (0.00; 2.46) | 0.38 (0.00; 2.46) |
| Glutathione redox ratio, mean (s.d.) | 9.21 (2.40) | 9.09 (1.72) |
| Folate (ng ml−1) (normal 5–21), mean (s.d.) | 17.15 (3.41) | 17.79 (2.42) |
| B12 (pg ml−1) (normal 200–900), mean (s.d.) | 859.79 (447.54) | 725.39 (368.06) |
| Zinc (mg dl−1) (normal 65–120), mean (s.d.) | 109.64 (31.64) | 99.00 (19.29) |
| Copper (μg dl−1) (normal 70–128), mean (s.d.) | 103.10 (12.56) | 109.19 (17.07) |
| Magnesium (μg g−1) (normal 39-59 l), mean (s.d.) | 42.15 (10.60) | 47.77 (11.50) |
| Language testing, N (%) | ||
| Preverbal at start of study | 8 (35) | 11 (44) |
| Preschool Language Scales | 3 (14) | 5 (20) |
| Clinical Evaluation of Language Fundamentals 2 | 14 (62) | 12 (48) |
| Clinical Evaluation of Language Fundamentals 4 | 6 (26) | 8 (32) |
| Diagnostic Ddcumentation, N (%) | ||
| Autism Diagnostic Observation Schedule | 12 (52) | 16 (64) |
| 3 Practitioner Agreement | 18 (78) | 21 (84) |
| Single practitioner with standardized questionnairesb | 2 (9) | 1 (4) |
| Autism Diagnostic Interview-Revised | ||
| Participated in confirmation testing, N(%) | 19 (83) | 21 (84) |
| Social Interaction Score, mean (s.d.) [range] | 20.50 (5.50) [10–27] | 23.80 (4.80) [11–30] |
| Communication Score: Verbal, mean (s.d.) [range] | 17.00 (3.92) [10–22] | 19.00 (5.16) [7–25] |
| Communication Score: Non-Verbal, mean (s.d.) [range] | 12.80 (2.68) [8–14] | 13.40 (0.52) [13–14] |
| Restricted & Repetitive Play Score, mean (s.d.) [range] | 4.78 (1.86) [2–9] | 6.40 (2.08) [2–12] |
| Summary Score, mean (s.d.) [range] | 4.22 (1.06) [2–5] | 4.25 (0.91) [3–5] |
| Medications (concurrent treatments), N (%) | ||
| Stimulant | 6 (26) | 6 (24) |
| Melatonin | 6 (26) | 5 (20) |
| Allergy/asthma medications | 4 (17) | 7 (28) |
| Gastrointestinal medications | 4 (17) | 5 (20) |
| Alpha-adrenergic agonists | 5 (22) | 3 (12) |
| Selective serotonin reuptake inhibitors | 3 (13) | 1 (4) |
| Antiepileptic medication | 2 (9) | 2 (8) |
| Antimicrobial medications | 3 (13) | 0 (0) |
| Immunomodulatory medications | 1 (4) | 0 (0) |
| Supplements (concurrent treatments), N (%) | ||
| Multivitamin | 3 (13) | 11 (44) |
| Minerals | 4 (17) | 3 (12) |
| Vitamin B12 | 2 (9) | 4 (16) |
| Other B vitamins | 2 (9) | 2 (8) |
| Fatty acids | 1 (4) | 3 (12) |
| Other antioxidants | 1 (4) | 2 (8) |
| Folate | 0 (0) | 3 (12) |
| Carnitine | 1 (4) | 1 (4) |
| Coenzyme Q10 | 1 (4) | 0 (0) |
| Amino acids | 1 (4) | 0 (0) |
| Other vitamins | 0 (0) | 1 (4) |
| Comorbid medical conditions, N(%) | ||
| Allergic disorders | 9 (39) | 12 (48) |
| Gastrointestinal disorders | 9 (39) | 10 (40) |
| Neurological disorders | 6 (26) | 10 (40) |
| Sleep disorders | 4 (17) | 8 (32) |
| Other psychiatric disorders | 6 (26) | 4 (16) |
| Immune abnormalityc | 8 (35) | 5 (20) |
| Mild congenital malformations | 1 (4) | 1 (4) |
| Genetic disordersd | 1 (4) | 0 (0) |
Mean values with standard deviation in parenthesis and range in brackets.
Baseline outcome measures outlined in Table 1 were not significantly different across treatment groups.
Standardized questionnaires included Social Responsiveness Scale, Social Communication Questionnaire and/or Autism Symptoms Questionnaire.
Immune abnormalities include chronic ear infection in 9 (19%) of participants (6 (26%) placebo, 3 (12%) folinic acid), urinary tract infections in 2 (4%) of participants (1 (4%) placebo, 1 (4%) folinic acid), chronic infections in 2 (4%) of participants (0 (0%) placebo, 2 (8%) folinic acid), immunological disorder in 1 (2%) of the participants (1 (4%) placebo, 0 (0%) folinic acid), adenotonsillar hypertrophy in 1 (2%) of the participants (1 (4%) placebo, 0 (0%) folinic acid) and PANDAS/PANS in in 1 (2%) of the participants (1 (4%) placebo, 0 (0%) folinic acid).
During the study a child was found to have a phosphatase and tensin homolog gene mutation.
Table 2A. Statistical analysis of primary outcome measure of verbal communication mixed model analysis (standardized score, 95% confidence interval shown).
| N |
Folinic acid |
Placebo |
Estimated effecta | Effect sizeb | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Baseline | 12 weeks | |||||
| Overall | 48 | 58.1 (53.9, 62.1) | 65.4 (60.6, 70.2) | 56.8 (51.5, 62.2) | 58.5 (51.9, 65.1) | 5.7 (1.0, 10.4) | 0.70 | 0.02 |
| Antibody status | ||||||||
| Negative | 17 | 57.9 (50.6, 65.2) | 64.5 (57.3, 71.7) | 48.0 (45.5, 50.5) | 52.1 (45.5, 58.8) | 2.5 (−5.9, 10.9) | 0.30 | 0.58 |
| Positive | 31 | 58.1 (52.9, 63.3) | 66.1 (59.0, 73.1) | 60.3 (53.5, 67.0) | 60.9 (52.2, 69.6) | 7.3 (1.4, 13.2) | 0.91 | 0.02 |
| Glutathione ratio | ||||||||
| High | 24 | 59.2 (53.6, 64.8) | 65.0 (58.4, 71.6) | 55.1 (47.1, 63.1) | 58.1 (50.0, 66.2) | 3.0 (−2.5, 8.5) | 0.46 | 0.30 |
| Low | 24 | 56.1 (49.6, 62.7) | 66.0 (58.2, 73.8) | 58.0 (50.5, 65.5) | 58.7 (28.7, 68.7) | 9.1 (0.9, 17.3) | 0.95 | 0.04 |
Estimated effect of folinic acid treatment with 95% confidence interval. Estimated effect is the difference in the outcome measures between the folinic acid and placebo group as estimated by the mixed-model regression.
Cohen’s d effect size is a measure of the strength of the effect of the folinic acid intervention. Higher values represent stronger effects. For the Cohen’s d, 0.25 is a small effect, 0.5 is a medium effect and 0.8 is a large effect.
Table 2B. Statistical analysis of primary outcome measure of verbal communication responder analysisa.
| Folinic acid treatmentb,c | Placebo treatmentb,d | % Difference responders | Unadjusted odds ratio and P-value | Adjusted odds ratio and P-value | Number needed to treat | |||
|---|---|---|---|---|---|---|---|---|
| Overall | 15 65% (46%, 84%) | 6 24% (7%, 41%) | 41% (13%, 63%) | 5.9 (1.7, 20.9) | 0.01 | 14.9 (2.1, 116.9) | 0.003 | 2.4 (1.6, 7.7) |
| Antibody status | ||||||||
| Negative | 5 50% (19%, 81%) | 2 29% (−4%, 62%) | 21% (−27%, 60%) | 2.5 (0.3, 19.5) | 0.32 | 3.6 (0.1, 95.6) | 0.45 | 4.7 (1.5, −4.1) |
| Positive | 10 77% (54%, 99%) | 4 22% (3%, 41%) | 55% (19%, 78%) | 11.7 (2.1, 64,0) | 0.005 | 67.4 (5.6, 999.9) | 0.01 | 1.8 (1.3, 5.2) |
| Glutathione ratio | ||||||||
| High | 9 64% (39%, 89%) | 3 30% (2%, 58%) | 34% (−4%, 72%) | 4.2 (0.73, 23.9) | 0.11 | 21.9 (1.9, 956.9) | 0.04 | 2.9 (1.4, −27.6) |
| Low | 6 67% (36%, 98%) | 3 20% (0%, 40%) | 47% (10%, 84%) | 8.0 (1.2, 52.2) | 0.03 | 10.2 (1.4, 140.6) | 0.04 | 2.1 (1.2, 10.2) |
Response is defined as an increase in five standardized points on the primary outcome, which was measured using the Preschool Language Scales-5, the Clinical Evaluation of Language Fundamentals-preschool-2 or Clinical Evaluation of Language Fundamentals 4.
Number of responders %responders (95% confidence interval)).
Overall N=23; antibody negative=10; antibody positive=13; glutathione high=14; glutathione low=9.
Overall N=25; antibody negative=7; antibody positive=18; glutathione high=10; glutathione low=15.
Primary outcome
Improvement in verbal communication was significantly greater for the participants on folinic acid as compared with participants on placebo with a medium-to-large effect size (Cohen’s d=0.70) (Table 2A).
Separate analyses were conducted for each biomarker of folate metabolism (Table 2A). In general, improvement in verbal communication was significantly greater in participants on folinic acid as compared with those on placebo for participants with abnormal folate metabolism (i.e., FRAA positive, low glutathione redox ratio). For participants with biomarkers indicating more normal folate metabolism (i.e., FRAA negative, high glutathione redox ratio) improvement in verbal communication was not significantly different between groups.
A responder analysis was also performed. Overall, there were significantly more responders in the folinic acid group as compared with those on placebo (χ2(1)=8.92, P=0.003; Table 2B). FRAAs predicted response to folinic acid (χ2(1)=4.92, P=0.03). For both analyses, greater baseline Adaptive Behavior Composite Score increased the likelihood of response (χ2(1)=6.92, P=0.009 and χ2(1)=7.74, P=0.005, respectively) but all other potential covariates were removed by backward elimination. Glutathione redox status was not significantly associated with treatment response.
Secondary outcomes
Table 3 outlines secondary outcomes, including the minimal clinically important difference. The Daily Living Skills on the VABS significantly improved in the folinic acid group as compared with the placebo group.
Table 3. Secondary outcome measures.
| Measurea | Baseline | 12-week assessment | |||||
|---|---|---|---|---|---|---|---|
| Folinic acid, Mean (CIb) | Placebo, Mean (CIb) | Folinic acid, Mean (CIb) | Placebo, Mean (CIb) | Estimated effectc | MCIDd | P-valuee | |
| Vineland Adaptive Behavior Scale (Standard Score): Higher Scores=Better Performance | |||||||
| Communication | 66.2 (62.0, 70.4) | 65.9 (60.8, 71.1) | 68.3 (63.5, 73.2) | 66.0 (59.8, 72.2) | 0.2 (0.4, −0.2) | 3.8 | 0.87 |
| Daily living | 64.6 (61.2, 68.0) | 68.1 (62.5, 73.7) | 69.2 (64.4, 74.0) | 66.3 (60.3, 72.3) | 0.5 (0.0, 1.0) | 2.8 | 0.05 |
| Social skills | 64.4 (60.8, 68.0) | 66.1 (61.9, 70.4) | 68.3 (64.2, 72.5) | 67.4 (61.7, 73.2) | 0.2 (−0.2, 0.6) | 3.0 | 0.29 |
| Motor skills | 78.9 (72.5, 85.3) | 78.5 (73.0, 84.0) | 81.7 (75.3, 88.2) | 80.6 (74.5, 86.7) | 0.1 (−0.1, 0.3) | 4.4 | 0.69 |
| Adaptive behavior | 64.8 (61.6, 68.0) | 65.8 (62.0, 69.7) | 67.7 (63.7, 71.7) | 65.8(60.8, 70.8) | 0.3 (0.7, −0.1) | 2.4 | 0.16 |
| Aberrant Behavior Checklist (Raw Score): Lower Scores=Less Behavioral Problems | |||||||
| Irritability | 13.4 (10.3,16.5) | 10.2 (7.5, 13.0) | 9.1 (7.1, 11.0) | 8.5 (5.7, 11.4) | −1.2 (−0.2, −2.2) | 1.8 | 0.04 |
| Lethargy | 13.1 (10.8,15.4) | 13.1 (9.9, 16.2) | 9.7 (7.2, 12.2) | 11.1 (7.7, 14.6) | −1.4 (−1.0, −1.9) | 1.0 | 0.02 |
| Stereotyped behavior | 6.1 (4.5, 7.7) | 7.3 (5.2, 9.5) | 3.7 (2.5, 5.0) | 7.1 (5.1, 9.1) | −0.9 (−0.4, −1.4) | 0.7 | 0.007 |
| Hyperactivity | 25.0 (21.8, 28.1) | 18.5 (13.5, 23.5) | 22.1 (17.5, 26.4) | 16.0(11.6, 20.4) | −1.8 (−0.6, −3.0) | 2.6 | 0.02 |
| Inappropriate speech | 5.2 (3.8,6.6) | 4.1 (2.6, 5.6) | 4.0 (3.0, 5.0) | 3.8 (2.6, 5.1) | −1.7 (−0.9, −2.5) | 0.7 | 0.004 |
| Total score | 62.7 (54.9,70.6) | 53.2 (43.2, 63.2) | 48.5 (40.9, 56.1) | 46.6 (35.9, 57.2) | −4.7 (−3.0, −6.4) | 0.02 | |
| The Ohio Autism Clinical Impression Scale (Severity): Lower Scores=Less Severity and Greater Improvement | |||||||
| Social | 4.3 (4.0, 4.6) | 4.4 (4.0, 4.8) | 3.8 (3.3, 4.4) | 4.0 (3.4, 4.5) | −0.1 (−0.3, 0.1) | 0.4 | 0.52 |
| Aberrant behavior | 3.8 (3.3, 4.2) | 3.6 (3.2, 4.0) | 3.4 (2.9, 3.8) | 3.5 (3.0, 4.0) | −0.3 (−0.6, 0.0) | 0.4 | 0.32 |
| Repetitive behavior | 3.2 (2.8, 3.5) | 2.8(2.3, 3.3) | 2.5 (2.2, 2.8) | 2.7 (2.2, 3.2) | −0.4 (−0.8, 0.0) | 0.4 | 0.13 |
| Verbal communication | 4.4 (3.9, 4.8) | 4.7(4.1, 5.3) | 3.8 (3.2, 4.3) | 4.3 (3.7, 5.0) | −0.2 (−0.6, 0.2) | 0.4 | 0.37 |
| Non-verbal communication | 3.6 (3.2, 4.0) | 3.8(3.3, 4.4) | 3.3 (2.8, 3.8) | 3.5 (3.0, 4.1) | −0.0 (−0.4, 0.4) | 0.4 | 0.63 |
| Hyperactivity | 4.1 (3.8, 4.4) | 3.7 (3.2, 4.1) | 3.8 (3.3, 4.3) | 3.8 (3.3, 4.3) | −0.3 (−0.9, 0.3) | 0.4 | 0.29 |
| Anxiety | 2.5 (2.0, 2.9) | 2.1 (1.5, 2.7) | 2.3 (1.7, 2.9) | 2.0 (1.4, 2.5) | −0.1 (−0.5, 0.3) | 0.4 | 0.52 |
| Sensory sensitivity | 1.6 (1.4, 1.9) | 1.2 (1.0, 1.5) | 1.4 (1.2, 1.7) | 1.4 (1.0, 1.8) | −0.3 (−0.6, 0.0) | 0.4 | 0.15 |
| Restricted interest | 2.3 (1.9, 2.8) | 2.6(2.3, 3.0) | 2.0 (1.6, 2.3) | 2.0 (1.7, 2.4) | −0.2 (−0.6, 0.2) | 0.4 | 0.87 |
| Autistic behavior | 4.4 (4.0, 4.8) | 4.6(4.2, 5.0) | 4.0 (3.5, 4.5) | 4.4 (2.9, 5.0) | −0.2 (−0.6, 0.2) | 0.4 | 0.37 |
| Autism Symptoms Questionnaires (Raw Score): Lower Scores=Less Autism Symptoms | |||||||
| Social | 3.5 (3.1, 3.9) | 3.6 (3.3, 3.9) | 3.3 (2.8, 3.8) | 3.4 (3.0, 3.8) | −0.1 (0.0, −0.2) | 0.11 | 0.10 |
| Communication | 4.7 (4.4, 5.0) | 4.6 (4.3, 4.8) | 4.7 (4.5, 4.9) | 4.7 (4.5, 4.9) | 0.0 (−0.1, 0.1) | 0.09 | 0.51 |
| Stereotypic behavior | 3.1 (2.8, 3.4) | 3.4 (3.1, 3.8) | 3.2 (2.8, 3.6) | 3.6 (3.3, 3,9) | −0.2 (−0.1, −0.3) | 0.12 | 0.02 |
| Total score | 11.3 (10.6,12.0) | 11.6(11.1,12.1) | 11.2 (10.5,11.9) | 11.7 (11.1,12.3) | −0.3 (−0.1, −0.5) | 0.04 | 0.02 |
| Social Responsiveness Scale (T-Scores): Lower Scores=Less Social Symptoms | |||||||
| Awareness | 79.2 (74.8, 83.7) | 76.9 (72.6, 81.3) | 78.7 (74.9, 82.4) | 75.0 (69.6, 80.4) | 0.3 (1.9, −1.3) | 7.1 | 0.52 |
| Cognition | 83.7 (80.5, 87.0) | 82.1 (78.7, 85.6) | 79.3 (76.5, 82.2) | 80.0 (76.3, 83.7) | 0.8 (−0.6, 2.2) | 5.8 | 0.37 |
| Communication | 82.5 (78.2, 86.8) | 84.3 (81.1, 87.6) | 80.1 (75.9, 84.4) | 77.7 (72.7, 82.7) | 1.5 (−0.1, 3.1) | 4.2 | 0.11 |
| Motivation | 75.5 (70.4, 80.6) | 78.9 (74.9, 82.6) | 70.3 (65.8, 74.9) | 73.0 (68.5, 77.6) | 0.2 (−0.8, 1.2) | 5.7 | 0.52 |
| Mannerisms | 83.4 (79.6, 87.2) | 84.2 (80.8, 87.7) | 80.5 (76.1, 85.0) | 79.8 (75.9, 83.8) | 0.9 (−0.9, 2.7) | 5.5 | 0.29 |
| Total | 84.9 (81.7, 88.1) | 85.8 (83.0, 88.6) | 82.2 (79.0, 85.4) | 80.7 (76.9, 84.6) | 0.8 (−0.8, 2.4) | 0.29 | |
| Autism Impact Measure (Raw Scores): Lower Scores=Less Impact of Autism on the Family | |||||||
| Frequency | 135 (126, 144) | 143 (137, 149) | 114 (105, 123) | 125 (113,136) | −2.2 (0.8, −5.2) | 0.16 | |
| Impact | 105 (94, 116) | 123 (111, 136) | 87 (76, 99) | 103 (89, 118) | 3.4 (7.0, −0.2) | 0.11 | |
| Behavioral Assessment System for Children (T-Scores): Lower problems scores and higher skills scores are better | |||||||
| Externalizing problems | 60.9 (57.6, 64.2) | 53.4 (49.8, 56.9) | 57.7 (54.8, 60.6) | 51.6 (48.1, 55.1) | −0.1 (−0.9, 0.7) | 3.7 | 0.52 |
| Internalizing problems | 49.3 (45.1, 53.5) | 48.6 (43.4, 53.7) | 43.1 (40.0, 46.2) | 45.5 (41.5, 49.4) | −1.1 (−0.2, −2.0) | 3.8 | 0.05 |
| Behavior problems | 71.1 (68.1, 74.1) | 66.0 (62.5, 69.6) | 65.3 (62.3, 68.2) | 61.2 (57.3, 65.0) | 0.1 (−0.9, 1.1) | 3.7 | 0.63 |
| Adaptive skills | 24.5 (22.0, 27.0) | 25.0 (22.2, 27.7) | 27.8 (25.3, 30.4) | 26.1 (22.4, 29.7) | 0.4 (0.0, 0.8) | 3.8 | 0.87 |
Adherence to completing questionnaires: folinic acid 50/60 (83%), placebo 64/73 (88%)
.
95% confidence interval
.
Estimated effect of folinic acid treatment with 95% confidence interval. Statistically significant effects (P⩽0.05) are bold. Estimated effect is the difference in the outcome measures between the folinic acid and placebo group as estimated by the mixed-model regression.
The Minimal Clinically Important Difference (MCID) was calculated for each index using the standard error of measurement method. Reliability and standard deviations from autistic samples were used for the calculation. The MCID is defined as smallest change in the outcome that a patient would identify as important.
P-values adjusted using false discovery rate.
Adherence on the parental questionnaires was not significantly different across treatment groups. Irritability, lethargy, stereotyped behavior, hyperactivity, inappropriate speech and total score on the ABC significantly improved in the folinic acid group as compared with the placebo group. Stereotypic behavior and total score significantly improved for the folinic acid group as compared with the placebo group on the ASQ. Internalizing problems significantly improved for the folinic acid group as compared with the placebo group on the BASC.
Teacher questionnaires were not analyzed since adherence was below 35%.
Adverse events
There were no serious adverse events in the folinic acid group. One child on placebo was unblinded and removed from the study because of a potential serious adverse event. Three placebo participants underwent dose reduction. There were no significant group differences between adverse event frequencies (Table 4).
Table 4. Incidence of adverse events by treatment group.
| Adverse event, N(%) | Folinic acid group (n=21)a | Placebo group (n=25) | Overall (n=46)a | Fisher P |
|---|---|---|---|---|
| Excitement or agitation | 6 (29%) | 10 (40%) | 16 (35%) | 0.53 |
| Insomnia | 6 (29%) | 10 (40%) | 16 (35%) | 0.53 |
| Increased motor activity | 6 (29%) | 9 (36%) | 15 (33%) | 0.75 |
| Restlessness | 3 (14%) | 7 (28%) | 10 (22%) | 0.31 |
| Aggression | 2 (10%) | 6 (24%) | 8 (17%) | 0.26 |
| Increased tantrums | 1 (5%) | 6 (24%) | 7 (15%) | 0.11 |
| Involuntary movements | 2 (10%) | 4 (16%) | 6 (13%) | 0.67 |
| Dry mouth, excessive thirst | 3 (14%) | 1 (4%) | 4 (9%) | 0.32 |
| Decreased appetite | 2 (10%) | 2 (8%) | 4 (9%) | 1.00 |
| Depression | 1 (5%) | 3 (12%) | 4 (9%) | 0.61 |
| Gastroesophageal reflux | 1 (5%) | 3 (12%) | 4 (9%) | 0.61 |
| Emotional lability | 1 (5%) | 3 (12%) | 4 (9%) | 0.61 |
| Constipation | 2 (10%) | 1 (4%) | 3 (7%) | 0.59 |
| Nasal congestion | 0 (0%) | 3 (12%) | 3 (7%) | 0.24 |
| Confusion | 1 (5%) | 1 (4%) | 2 (4%) | 1.00 |
| Stiffness | 1 (5%) | 1 (4%) | 2 (4%) | 1.00 |
| Diarrhea | 1 (5%) | 1 (4%) | 2 (4%) | 1.00 |
| Weight gain | 2 (10%) | 0 (0%) | 2 (4%) | 0.20 |
| Headache | 2 (10%) | 0 (0%) | 2 (4%) | 0.20 |
| Weight loss | 1 (5%) | 0 (0%) | 1 (2%) | 0.46 |
| Drowsiness | 0 (0%) | 1 (4%) | 1 (2%) | 1.00 |
| Sweating | 0 (0%) | 1 (4%) | 1 (2%) | 1.00 |
| Decreased motor activity | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Tremors | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Blurred vision | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Increased salivation | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Nausea/vomiting | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Dizziness | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Rash | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Any adverse effect | 12 (57%) | 17 (68%) | 29 (63%) | 0.55 |
Two participants in the folinic acid group did not pick up the intervention, so they never had a chance to report any adverse effects and were not included in the adverse effect frequency calculations.
Discussion
This study found an improvement in an important core ASD symptom, verbal communication, in non-syndromic ASD children receiving high-dose folinic acid vs placebo, particularly in those participants who were positive for FRAAs. Improvement in a number of secondary outcomes was observed as well, with no significant adverse events. The effect of folinic acid is consistent with the therapeutic effect of early behavioral interventions.39, 40
Folinic acid may have positive effects on metabolism through multiple pathways (Supplementary Figure S1). First, folinic acid can normalize folate-dependent one-carbon metabolism.60 Second, unlike folic acid, the common oxidized synthetic form of folate, folinic acid can readily enter the folate cycle without being reduced by dihydrofolate reductase.7 Third, folinic acid can cross the blood–brain barrier using the reduced folate carrier when the FRα is blocked by FRAAs or is non-functional due to mitochondrial dysfunction or genetic mutations.24, 27
This study suggests that FRAAs predict response to high-dose folinic acid treatment. This is consistent with the notion that children with ASD and FRAAs may represent a distinct subgroup.61 Other factors such as genetic polymorphisms in folate-related genes or mitochondrial dysfunction may be important in determining treatment response but were not examined in this study. When methylcobalamin was combined with folinic acid, improvement in communication as well as glutathione redox status was found.48 Indeed, future studies will be needed to define factors that predict response to treatment, investigate optimal dosing and help understand whether other compounds could work synergistically with folinic acid.
This study had limitations. First, the small sample size may have resulted in the imbalance in baseline language scores for one subgroup and limited the sensitivity of the analyses to detect some treatment effects. Second, the single-site design only provides limited generalization of these results. Third, although no adverse events were identified, safety of this treatment requires further study since many folate studies utilize lower doses and a healthy population. Fourth, further studies will be needed to determine the optimal folinic acid dose. The oral bioavailability of folate is strongly influenced by the enteric microbiome62 but there is strong evidence that the enteric microbiome is altered in children with ASD.63
Only two drugs have been approved by the United States Food and Drug Administration for the treatment of ASD, both antipsychotic drugs indicated for associated, not core, ASD symptoms. Within 12 weeks these medications can detrimentally affect lipid, cholesterol and glucose metabolism and result in marked body weight gain64 and can increase the risk of developing type 2 diabetes.65 Thus, well-tolerated medications that target pathophysiological processes and core symptoms associated with ASD are sorely needed.
Folinic acid is among other recently described treatments that target metabolic abnormalities and core symptoms associated with ASD.45, 47, 48, 66, 67, 68, 69, 70 This study also supports the notion that measurement of FRAAs prior to a trial of folinic acid may be helpful for predicting response. In our previous study we offered folinic acid treatment to patients positive for FRAAs without obtaining CSF folate concentration measurement.24 We continue to believe this is a reasonable alternative to a diagnostic lumbar puncture but should be accompanied by close follow-up with an experienced physician. Since ASD is likely a lifelong disorder the long-term adverse effect of any treatment is of concern. As folinic acid may become increasingly used to treat ASD in the future, short-term and long-term adverse effects should be studied in more detail to ensure safety.
Conclusions
In this small trial of children with non-syndromic ASD and language impairment, treatment with high-dose folinic acid for 12 weeks resulted in improvement in measures of verbal communication as compared with placebo. These findings should be considered preliminary until treatment is assessed in larger multicenter studies with longer duration.
Acknowledgments
This research was supported, in part, by Lee Silsby Compounding Pharmacy (to REF), Autism Speaks (#8202 to EVQ), Brenen Hornstein Autism Research and Education Foundation (to REF), Fraternal Order of Eagles (to REF), the Autism Research Institute (to REF) and the Jane Botsford Johnson Foundation (to SJJ). This trial is registered on clinicaltrials.gov as NCT01602016.
Disclaimer
None of the sponsors were involved with the design or conduct of the study, collection, management, analysis or interpretation of the data; or preparation, review, approval of the manuscript or decision to submit the manuscript for publication REF, JS and LD had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study has not been published previously.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
EVQ and JMS are inventors in a patent for the detecting of the autoantibodies described in this study (US patent 7,846,672 B2) issued to the Research Foundation of the State University of New York. REF and EVQ are members of the Scientific Advisory Board to Illiad Neurosciences, Inc. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Zablotsky B, Black LI, Maenner MJ, Schieve LA, Blumberg SJ. Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 National Health Interview Survey. Natl Health Stat Rep 2015; 1–20. [PubMed]
- Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry 2012; 17: 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol 2014; 5: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Rossignol DA. Identification and treatment of pathophysiological comorbidities of autism spectrum disorder to achieve optimal outcomes. Clin Med Insights Pediatr 2016; 10: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Rossignol DA. Treatments for biomedical abnormalities associated with autism spectrum disorder. Front Pediatr 2014; 2: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahabzadeh A, McDougle CJ. Maternal folic acid supplementation and risk of autism. JAMA 2013; 309: 2208. [DOI] [PubMed] [Google Scholar]
- Frye RE, James SJ. Metabolic pathology of autism in relation to redox metabolism. Biomark Med 2014; 8: 321–330. [DOI] [PubMed] [Google Scholar]
- Suren P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013; 309: 570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenweg-de Graaff J, Ghassabian A, Jaddoe VW, Tiemeier H, Roza SJ. Folate concentrations during pregnancy and autistic traits in the offspring. The Generation R Study. Eur J Publ Health 2015; 25: 431–433. [DOI] [PubMed] [Google Scholar]
- Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr 2012; 96: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers VT, Rothenberg SP, Sequeira JM, Opladen T, Blau N, Quadros EV et al. Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. N Engl J Med 2005; 352: 1985–1991. [DOI] [PubMed] [Google Scholar]
- Ramaekers VT, Hausler M, Opladen T, Heimann G, Blau N. Psychomotor retardation, spastic paraplegia, cerebellar ataxia and dyskinesia associated with low 5-methyltetrahydrofolate in cerebrospinal fluid: a novel neurometabolic condition responding to folinic acid substitution. Neuropediatrics 2002; 33: 301–308. [DOI] [PubMed] [Google Scholar]
- Moretti P, Peters SU, Del Gaudio D, Sahoo T, Hyland K, Bottiglieri T et al. Brief report: autistic symptoms, developmental regression, mental retardation, epilepsy, and dyskinesias in CNS folate deficiency. J Autism Dev Disord 2008; 38: 1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Sahoo T, Hyland K, Bottiglieri T, Peters S, del Gaudio D et al. Cerebral folate deficiency with developmental delay, autism, and response to folinic acid. Neurology 2005; 64: 1088–1090. [DOI] [PubMed] [Google Scholar]
- Ramaekers VT, Blau N, Sequeira JM, Nassogne MC, Quadros EV. Folate receptor autoimmunity and cerebral folate deficiency in low-functioning autism with neurological deficits. Neuropediatrics 2007; 38: 276–281. [DOI] [PubMed] [Google Scholar]
- Ramaekers VT, Hansen SI, Holm J, Opladen T, Senderek J, Hausler M et al. Reduced folate transport to the CNS in female Rett patients. Neurology 2003; 61: 506–515. [DOI] [PubMed] [Google Scholar]
- Ramaekers VT, Sequeira JM, Artuch R, Blau N, Temudo T, Ormazabal A et al. Folate receptor autoantibodies and spinal fluid 5-methyltetrahydrofolate deficiency in Rett syndrome. Neuropediatrics 2007; 38: 179–183. [DOI] [PubMed] [Google Scholar]
- Perez-Duenas B, Ormazabal A, Toma C, Torrico B, Cormand B, Serrano M et al. Cerebral folate deficiency syndromes in childhood: clinical, analytical, and etiologic aspects. Archiv Neurol 2011; 68: 615–621. [DOI] [PubMed] [Google Scholar]
- Frye RE. Metabolic and mitochondrial disorders associated with epilepsy in children with autism spectrum disorder. Epilepsy Behav 2015; 47: 147–157. [DOI] [PubMed] [Google Scholar]
- Garcia-Cazorla A, Quadros EV, Nascimento A, Garcia-Silva MT, Briones P, Montoya J et al. Mitochondrial diseases associated with cerebral folate deficiency. Neurology 2008; 70: 1360–1362. [DOI] [PubMed] [Google Scholar]
- Hasselmann O, Blau N, Ramaekers VT, Quadros EV, Sequeira JM, Weissert M. Cerebral folate deficiency and CNS inflammatory markers in Alpers disease. Mol Genet Metab 2010; 99: 58–61. [DOI] [PubMed] [Google Scholar]
- Serrano M, Garcia-Silva MT, Martin-Hernandez E, O'Callaghan Mdel M, Quijada P, Martinez-Aragon A et al. Kearns-Sayre syndrome: cerebral folate deficiency, MRI findings and new cerebrospinal fluid biochemical features. Mitochondrion 2010; 10: 429–432. [DOI] [PubMed] [Google Scholar]
- Grapp M, Just IA, Linnankivi T, Wolf P, Lucke T, Hausler M et al. Molecular characterization of folate receptor 1 mutations delineates cerebral folate transport deficiency. Brain 2012; 135(Pt 7): 2022–2031. [DOI] [PubMed] [Google Scholar]
- Frye RE, Sequeira JM, Quadros EV, James SJ, Rossignol DA. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry 2013; 18: 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers VT, Sequeira JM, Blau N, Quadros EV. A milk-free diet downregulates folate receptor autoimmunity in cerebral folate deficiency syndrome. Dev Med Child Neurol 2008; 50: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira JM, Ramaekers VT, Quadros EV. The diagnostic utility of folate receptor autoantibodies in blood. Clin Chem Lab Med 2013; 51: 545–554. [DOI] [PubMed] [Google Scholar]
- Desai A, Sequeira JM, Quadros EV. The metabolic basis for developmental disorders due to defective folate transport. Biochimie 2016; 126: 31–42. [DOI] [PubMed] [Google Scholar]
- Ramaekers VT, Quadros EV, Sequeira JM. Role of folate receptor autoantibodies in infantile autism. Mol Psychiatry 2013; 18: 270–271. [DOI] [PubMed] [Google Scholar]
- Sequeira JM, Desai A, Berrocal-Zaragoza MI, Murphy MM, Fernandez-Ballart JD, Quadros EV. Exposure to folate receptor alpha antibodies during gestation and weaning leads to severe behavioral deficits in rats: a pilot study. PLoS One 2016; 11: e0152249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoffner J, Trommer B, Thurm A, Farmer C, Langley WA III, Soskey L et al. CSF concentrations of 5-methyltetrahydrofolate in a cohort of young children with autism. Neurology 2016; 86: 2258–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilford JM, Payakachat N, Kovacs E, Pyne JM, Brouwer W, Nick TG et al. Preference-based health-related quality-of-life outcomes in children with autism spectrum disorders: a comparison of generic instruments. Pharmacoeconomics 2012; 30: 661–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaddes NM, Tutkunkardas MD, Sari O, Aydin A, Kozanoglu P. Characteristics of children who lost the diagnosis of autism: a sample from Istanbul, Turkey. Autism Res Treat 2014; 2014: 472120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Rogers S, Cooper J, Landa R, Lord C, Paul R et al. Defining spoken language benchmarks and selecting measures of expressive language development for young children with autism spectrum disorders. J Speech Lang Hear Res 2009; 52: 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster R, Qiu S, Lopez K, Lord C. Predicting outcomes of children referred for autism using the MacArthur-Bates Communicative Development Inventory. J Speech Lang Hear Res 2007; 50: 667–681. [DOI] [PubMed] [Google Scholar]
- Condouris K, Meyer E, Tager-Flusberg H. The relationship between standardized measures of language and measures of spontaneous speech in children with autism. Am J Speech Lang Pathol 2003; 12: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Khan SY, Blaskey L, Chow VY, Rey M, Gaetz W et al. Neuromagnetic oscillations predict evoked-response latency delays and core language deficits in autism spectrum disorders. J Autism Dev Disord 2015; 45: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Van Oudenhove L, Lagae L et al. Structural and functional underconnectivity as a negative predictor for language in autism. Hum Brain Mapp 2014; 35: 3602–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volden J, Smith IM, Szatmari P, Bryson S, Fombonne E, Mirenda P et al. Using the preschool language scale, fourth edition to characterize language in preschoolers with autism spectrum disorders. Am J Speech Lang Pathol 2011; 20: 200–208. [DOI] [PubMed] [Google Scholar]
- Schreibman L, Stahmer AC. A randomized trial comparison of the effects of verbal and pictorial naturalistic communication strategies on spoken language for young children with autism. J Autism Dev Disord 2014; 44: 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby AM, Guthrie W, Woods J, Schatschneider C, Holland RD, Morgan L et al. Parent-implemented social intervention for toddlers with autism: an RCT. Pediatrics 2014; 134: 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butter E, Mulick J. The Ohio Autism Clinical Impressions Scale (OACIS). Institute CsR: Columbus, OH, 2006. [Google Scholar]
- Psychopharmacology TORUoPOSU Autism Rating Scale. OSU Research Unit on Pediatric Psychopharmacology: Columbus, OH, 2005. [Google Scholar]
- Choque Olsson N, Bolte S. Brief report: "Quick and (not so) dirty" assessment of change in autism: cross-cultural reliability of the developmental disabilities CGAS and the OSU autism CGI. J Autism Dev Disord 2014; 44: 1773–1778. [DOI] [PubMed] [Google Scholar]
- Wink LK, Early M, Schaefer T, Pottenger A, Horn P, McDougle CJ et al. Body mass index change in autism spectrum disorders: comparison of treatment with risperidone and aripiprazole. J Child Adolesc Psychopharmacol 2014; 24: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, Talalay P et al. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci USA 2014; 111: 15550–15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LE, Aman MG, Hollway J, Hurt E, Bates B, Li X et al. Placebo-controlled pilot trial of mecamylamine for treatment of autism spectrum disorders. J Child Adolesc Psychopharmacol 2012; 22: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, DeLatorre R, Taylor HB, Slattery J, Melnyk S, Chowdhury N et al. Metabolic effects of sapropterin treatment in autism spectrum disorder: a preliminary study. Transl Psychiatry 2013; 3: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O et al. Effectiveness of methylcobalamin and folinic acid treatment on adaptive behavior in children with autistic disorder is related to glutathione redox status. Autism Res Treat 2013; 2013: 609705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaat AJ, Lecavalier L, Aman MG. Validity of the aberrant behavior checklist in children with autism spectrum disorder. J Autism Dev Disord 2014; 44: 1103–1116. [DOI] [PubMed] [Google Scholar]
- Turygin NC, Matson JL, Adams H, Belva B. The effect of DSM-5 criteria on externalizing, internalizing, behavioral and adaptive symptoms in children diagnosed with autism. Dev Neurorehabil 2013; 16: 277–282. [DOI] [PubMed] [Google Scholar]
- Kanne SM, Mazurek MO, Sikora D, Bellando J, Branum-Martin L, Handen B et al. The Autism Impact Measure (AIM): initial development of a new tool for treatment outcome measurement. J Autism Dev Disord 2014; 44: 168–179. [DOI] [PubMed] [Google Scholar]
- Frye RE, Tippett M, Delhey L, Slattery J. Test-retest reliability and validity of the Autism Symptoms Questionnaire. N Am J Med Sci 2015; 8: 149–153. [Google Scholar]
- Detry MA, Lewis RJ. The intention-to-treat principle: how to assess the true effect of choosing a medical treatment. JAMA 2014; 312: 85–86. [DOI] [PubMed] [Google Scholar]
- White IR, Carpenter J, Horton NJ. Including all individuals is not enough: lessons for intention-to-treat analysis. Clin Trials 2012; 9: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YC. Multiple Imputation for Missing Data: Concepts and New Development (SAS Version 9.0). SAS Institute Inc.: Rockville, MD, 2011. [Google Scholar]
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci 2007; 8: 206–213. [DOI] [PubMed] [Google Scholar]
- Matts JP, Launer CA, Nelson ET, Miller C, Dain B. A graphical assessment of the potential impact of losses to follow-up on the validity of study results. The Terry Beirn Community Programs for Clinical Research on AIDS. Stat Med 1997; 16: 1943–1954. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982; 38: 963–974. [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B 1995; 57: 289–300. [Google Scholar]
- Boarman DM, Baram J, Allegra CJ. Mechanism of leucovorin reversal of methotrexate cytotoxicity in human MCF-7 breast cancer cells. Biochem Pharmacol 1990; 40: 2651–2660. [DOI] [PubMed] [Google Scholar]
- Frye RE, Delhey L, Slattery J, Tippett M, Wynne R, Rose S et al. Blocking and binding folate receptor alpha autoantibodies identify novel autism spectrum disorder subgroups. Front Neurosci 2016; 10: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M et al. Human gut microbiome viewed across age and geography. Nature 2012; 486: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Slattery J, MacFabe DF, Allen-Vercoe E, Parker W, Rodakis J et al. Approaches to studying and manipulating the enteric microbiome to improve autism symptoms. Microb Ecol Health Dis 2015; 26: 26878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 2009; 302: 1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo WV, Cooper WO, Stein CM, Olfson M, Graham D, Daugherty J et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry 2013; 70: 1067–1075. [DOI] [PubMed] [Google Scholar]
- Klaiman C, Huffman L, Masaki L, Elliott GR. Tetrahydrobiopterin as a treatment for autism spectrum disorders: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol 2013; 23: 320–328. [DOI] [PubMed] [Google Scholar]
- Lemonnier E, Degrez C, Phelep M, Tyzio R, Josse F, Grandgeorge M et al. A randomised controlled trial of bumetanide in the treatment of autism in children. Transl Psychiatry 2012; 2: e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui K, Sato A, Imataka G. Mitochondrial dysfunction and its relationship with mTOR signaling and oxidative damage in autism spectrum disorders. Mini Rev Med Chem 2015; 15: 373–389. [DOI] [PubMed] [Google Scholar]
- Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014; 83: 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naviaux JC, Schuchbauer MA, Li K, Wang L, Risbrough VB, Powell SB et al. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Transl Psychiatry 2014; 4: e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.