Abstract
Nicotinamide adenine dinucleotide (NAD +) biosynthesis and its regulation have recently been attracting markedly increasing interest. Aging is marked by a systemic decrease in NAD + across multiple tissues. The dysfunction of NAD + biosynthesis plays a critical role in the pathophysiologies of multiple diseases, including age-associated metabolic disorders, neurodegenerative diseases, and mental disorders. As downstream effectors, NAD +-dependent enzymes, such as sirtuins, are involved in the progression of such disorders. These recent studies implicate NAD + biosynthesis as a potential target for preventing and treating age-associated diseases. Indeed, new studies have demonstrated the therapeutic potential of supplementing NAD + intermediates, such as nicotinamide mononucleotide and nicotinamide riboside, providing a proof of concept for the development of an effective anti-aging intervention.
Keywords: NAD+, Biosynthesis Aging
Introduction
In recent years, interest in nicotinamide adenine dinucleotide (NAD +) biology has significantly increased in many different fields of biomedical research. A number of new studies have revealed the importance of NAD + biosynthesis for the pathophysiologies of aging and aging-related diseases. This short review will highlight the recent progress in this new connection between NAD + biosynthesis, aging, and disease. In particular, we will focus on the role of NAD + in aging and longevity control, its effect on the function of NAD +-dependent enzymes such as sirtuins, and its relation to the development and progression of age-associated disorders. Finally, we will address the preventive and therapeutic potential of NAD + intermediates.
NAD + biosynthetic pathways
NAD + is an essential component of cellular processes necessary to support various metabolic functions 1– 5. The classic role of NAD + is a co-enzyme that catalyzes cellular redox reactions, becoming reduced to NADH, in many fundamental metabolic processes, such as glycolysis, fatty acid beta oxidation, or the tricarboxylic acid cycle 6– 8. In addition to playing these roles, NAD + has a critical role as the substrate of NAD +-consuming enzymes such as poly-ADP-ribose polymerases (PARPs), sirtuins, and CD38/157 ectoenzymes 9– 11. These NAD +-consuming enzymes have been known to mediate many fundamental cellular processes 5.
There are five major precursors and intermediates to synthesize NAD +: tryptophan, nicotinamide, nicotinic acid, nicotinamide riboside (NR), and nicotinamide mononucleotide (NMN). NAD + can be synthesized de novo by the conversion of the amino acid tryptophan through multiple enzymatic steps to nicotinic acid mononucleotide (NaMN) 12, 13. NaMN is converted to nicotinic acid dinucleotide (NaAD +) by NMN/NaMN adenylyltransferases (NMNATs) and then amidated to NAD + by NAD + synthetase.
In mammals, a major pathway of NAD + biosynthesis is the salvage pathway from nicotinamide ( Figure 1). Nicotinamide is converted to NMN, a key NAD + intermediate, by nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme in this pathway 12. NMNATs then convert NMN into NAD + 14, 15. NAMPT plays a critical role in regulating cellular NAD + levels 12, 13. On the other hand, nicotinic acid is converted to NaMN by nicotinic acid phosphoribosyltransferase (NPT) 12, 14, 15. NR needs to be converted to NMN by nicotinamide ribose kinases, NMRK1 and NMRK2 (also known as NRK1 and NRK2), which phosphorylate NR 16.
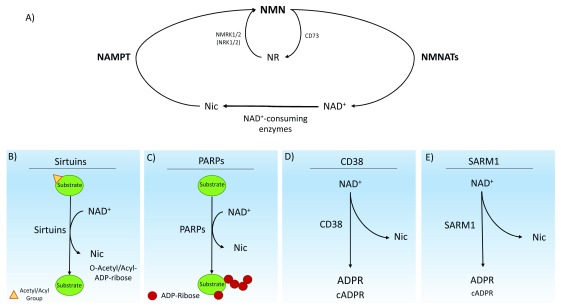
Figure 1. The major nicotinamide adenine dinucleotide (NAD +) biosynthetic pathway and NAD +-consuming enzymes in mammals.
( A) The NAD + biosynthetic pathway from the precursor nicotinamide (NIC). The pathway is mediated by nicotinamide phosphoribosyltransferase (NAMPT), which produces nicotinamide mononucleotide (NMN). NMN is immediately converted to NAD + by NMN adenylyltransferases (NMNATs). Multiple enzymes consume NAD +, producing nicotinamide and various products. NIC can be salvaged to begin the biosynthetic pathway again. NMRK1 and NMRK2 (also known as NRK1 and NRK2), as well as CD73, can produce NMN and NR. (B) Enzymatic activity of sirtuins. The most common enzymatic reaction performed by sirtuins is the deacetylation of acetylated substrate proteins. The resulting products from the consumption of NAD + are NIC and O-acetyl-ADP-ribose. Sirtuins can also catalyze several other deacylation reactions. ( C) Enzymatic activity of poly-ADP-ribose polymerases (PARPs). In response to DNA damage, PARPs synthesize poly-ADP-ribose chains on a variety of target proteins, including itself, to act as a signal for DNA repair enzymes. The reaction produces the ADP-ribose chains and NIC. ( D) Enzymatic activity of CD38. The CD38 ectoenzyme catalyzes the synthesis of ADP-ribose (ADPR) or cyclic ADPR (cADPR) from NAD +. ( E) Enzymatic activity of SARM1. A newly discovered class of NADase, SARM1, consumes axonal NAD + after injury, catalyzing the synthesis of ADPR and NIC as well as a small amount of cADPR.
Maintenance of adequate NAD + biosynthesis is paramount for cell survival and function. Derailment from normal NAD + homeostasis substantially affects not only the NAD +/NADH pool required for redox reactions but also activities of NAD +-dependent enzymes for crucial cellular functions.
Mediators of aging: NAD +-dependent enzymes
It is now becoming a consensus that NAD + levels decline at cellular, tissue/organ, and organismal levels during the course of aging 17. Activities of NAD +-consuming enzymes are affected by this NAD + decline, contributing to a broad range of age-associated pathophysiologies 5, 18.
Sirtuins are a family of NAD +-dependent deacetylases/deacylases which have central roles in translating NAD + changes to the regulation of many regulatory proteins for metabolism, DNA repair, stress response, chromatin remodeling, circadian rhythm, and other cellular processes. Through the mediation of such broad functions, sirtuins are evolutionarily conserved regulators for aging and longevity in diverse organisms 5, 18. Mammals have seven sirtuin family members, SIRT1–7, among which SIRT1 is the ortholog of silent information regulator 2 (Sir2) in budding yeast 11. The various sirtuin family members have a number of enzymatic functions and are localized to different subcellular compartments 19. Briefly, SIRT1 is localized mainly to the nucleus but is also present in the cytosol 20. SIRT2 is present mainly in the cytosol but can also be present in the nucleus 21. SIRT3–5 are localized in the mitochondrial compartment 22. SIRT6 is localized in the nucleus as well, and SIRT7 is localized in the nucleolus 23, 24. Sirtuins are classified as class III histone deacetylases dependent on NAD +. However, they target numerous non-histone proteins to alter their functions. Furthermore, sirtuins have other enzymatic activities, including demethylglutarylase and other lysine deacylase activities of SIRT4 25, demalonylase and desuccinylase activities of SIRT5 26, de-long chain fatty deacylase activity of SIRT6 27, and ADP-ribosyltransferase activity of SIRT4/SIRT6 28, 29. These various NAD +-dependent functions of sirtuins place them at a key position for the regulation of aging and longevity in diverse organisms 5, 18. For example, we have demonstrated that brain-specific SIRT1-overexpressing (BRASTO) transgenic mice are able to delay the process of aging and extend life span 30. Whole-body SIRT6-overexpressing male mice also show life span extension 31.
PARPs also consume NAD +, cleaving it into nicotinamide and ADP-ribose (ADPR) and producing a chain of ADPR. Among many PARP family members, PARP1 and 2 are major NAD + consumers in the nucleus, responding to DNA strand breaks and facilitating the DNA repair process 32. As NAD + is a common substrate between PARPs and SIRT1, there is a competition between their activities. PARP1/2 deletion is able to enhance the activity of SIRT1, resulting in the increases in mitochondrial content, fatty acid oxidation, and protection from diet-induced obesity 33. Whereas PARP1 deletion increases NAD + levels, PARP2 deletion increases Sirt1 expression through its function to bind to the promoter of the Sirt1 gene and repress its expression 33. During the course of aging, PARP activation, possibly due to constant DNA damage, appears to contribute to significant decreases in intracellular NAD +, exacerbating the decrease in SIRT1 activity 34.
CD38, one of the primary NADases in mammals, can modulate the NAD + levels as observed in CD38-deficient mice 35, 36. Although the activity of CD38 mainly generates ADPR and nicotinamide by hydrolysis of NAD +, it has a secondary role to mediate cellular signaling through the generation of cyclic ADPR (cADPR), a potent Ca 2+ inducer 10. The NADase activity of CD38 has been studied in depth 35– 37. CD38 can also degrade the NAD + precursors, NMN and NR, as well as NAD +, thus modulating cellular NAD + content 38, 39. It has been reported that CD38 protein levels increase in multiple tissues and organs over age, contributing to NAD + decline 40. Therefore, CD38-dependent modulation of NAD + can alter the activity of SIRT1 and other sirtuins, as well as other NAD +-consuming enzymes, and affect cellular signaling and metabolism 36, 37. Inhibiting CD38 can also promote NAD + levels and improve glucose and lipid metabolism 41.
A newly discovered class of NAD + hydrolases is sterile alpha and Toll/interleukin-1 receptor motif-containing 1 (SARM1) 42. SARM1 is central to the degeneration of axons after injury. Axonal injury is accompanied by a depletion of NAD +, and loss of SARM1 function delays axonal degeneration. It has been shown that the Toll/interleukin-1 receptor (TIR) domain of SARM1 is responsible for the NAD + hydrolase activity and promotes axonal degeneration 42. This discovery opens a new opportunity to develop the treatment of axonopathy, brain injury, and other neurodegenerative diseases.
NAD + decline as an important trigger for age-associated pathophysiologies
The decline in NAD + over age was originally recognized in mice overexpressing SIRT1 in pancreatic β cells (BESTO mice) 43. Young BESTO mice showed a significant improvement of glucose-stimulated insulin secretion. However, as they aged, this phenotype was completely lost. Interestingly, NMN supplementation was able to restore this phenotype in the aged BESTO mice and even improve glucose-stimulated insulin secretion in aged wild-type mice 44. Thus, NAD + decline over age was the cause for the loss of the BESTO phenotype. These findings suggest that the reduction of the NAD + pool with age is responsible for the age-associated impairment of glucose-stimulated insulin secretion. Since this report, a number of studies have also found that NAD + declines over age in worms, flies, and mice 5, 8, 17, 18. Particularly in mice, it has been shown that several different tissues and organs show decreases in NAD + levels over age, causing metabolic dysfunctions, cardiovascular diseases, neurodegenerative disorders, and cancer 17, 43– 45.
A significant cause for this age-associated NAD + decline is the decrease in NAMPT-mediated NAD + biosynthesis. It has been shown that the expression of Nampt at both mRNA and protein levels is reduced over age in a variety of tissues 45, 46. This age-associated decrease in Nampt expression causes a reduction in NAD + in those same tissues, affecting the activities of NAD +-dependent enzymes and redox reactions within the cell and leading to functional decline. Therefore, supplementation with NAD + intermediates, such as NMN and NR, can effectively restore the NAD + pool and cellular functions in aged animals.
Another cause for NAD + decline with age is the increase in NAD + consumption, and this is mainly due to the activation of PARPs 33. It has been reported that PARP1 activity increases, potentially due to the accumulation of DNA damage, so that more poly-ADP-ribose molecules are synthesized in aged tissues 33. This continuous PARP activation further depletes the NAD + pool and causes a reduction in the activity of SIRT1. Furthermore, ectopic PARP1 expression can cause multiple age-associated phenotypes 47. When PARP1 is knocked out, NAD + levels and SIRT1 activity significantly increase. Similar effects can be obtained by pharmacologically inhibiting PARP activity 33. The inhibition of PARP activity thus improves metabolic phenotypes through the activation of SIRT1. In contrast, it was recently reported that DNA damage repair decreases with age, along with a decrease in PARP1 activity 48. Interestingly, deleted in breast cancer 1 (DBC1) can bind to NAD + through its Nudix homology domain (NHD), which prevents it from binding to PARP1. As NAD + declines over age, DBC1 begins to bind to PARP1, reducing its DNA damage repair capacity 49. Therefore, it has been proposed that age-associated NAD + decline triggers the interaction between DBC1 and PARP1, contributing to the accumulation of DNA damage over age 49. Whether PARP1 is activated or inhibited over age could be cell type- or tissue-dependent, and further investigation will be required to clarify this contradiction. As mentioned above, the expression and activity of CD38 have been reported to increase with age 40. Indeed, CD38-deficient mice maintain NAD + levels, mitochondrial respiration, and metabolic functions with age 36. Therefore, CD38 might have a significant contribution to age-associated NAD + decline in certain tissues.
The combination of decreased NAD + biosynthesis and increased NAD + consumption exacerbates the depletion of NAD +, causing a variety of age-associated pathophysiologies 43– 45. Which one contributes further to the depletion of NAD + may be dependent on cell types and tissues. No matter what causes NAD + decline, it seems that major downstream mediators are sirtuins. The roles of sirtuins in the pathogenesis of age-associated diseases are summarized below.
Diabetes
SIRT1 is important for promoting glucose-stimulated insulin secretion in pancreatic β-cells 50, 51. Additionally, SIRT1 has a protective effect against insulin resistance in peripheral tissues, including adipose tissue, liver, and skeletal muscle 52. These findings suggest that SIRT1 is important for glucose homeostasis and the prevention of type 2 diabetes. Whole-body Sirt1-overexpressing transgenic mice, when fed a high-fat diet (HFD), have shown improvements in glucose tolerance through reduction of hepatic glucose production 52. Additionally, these mice do not show changes in body weight or composition. In the kidney of diabetic model mice, SIRT1 inhibits oxidative stress, which can lead to nephropathy, by induction of cyclooxygenase-2 (COX-2) expression 53. It has also been shown that administration of NMN ameliorates glucose intolerance in HFD-induced type 2 diabetic mice, enhances hepatic insulin sensitivity, and restores oxidative stress gene expression, and inflammatory responses, partly through the activation of SIRT1 45.
Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is characterized by steatosis of the liver and is linked with insulin resistance and metabolic syndrome. Studies have observed a reduction of sirtuins in NAFLD 54. SIRT1/3/5/6 are reported to be reduced in patients with NAFLD 54. This reduction is accompanied by an increase in lipogenic genes such as fatty acid synthase and SREBP-1. SIRT1 and SIRT3 have particularly been investigated in regard to NAFLD. SIRT1 expression is reduced by HFD 55. Overexpression of SIRT1 upregulates fatty acid oxidation pathways and downregulates lipogenic pathways, protecting the liver from steatosis. SIRT3 function is impaired in HFD, leading to hyperacetylation of target proteins in the mitochondria and impairing their activities 56– 58. SIRT3-deficient mice exacerbate these phenotypes, while overexpression can ameliorate NAFLD 59.
Atherosclerosis
SIRT1 has been shown to improve vascular function. SIRT1 is positioned to affect many pathways important for endothelial function 60– 63. SIRT1 suppresses the expression of inflammatory factors, including interleukin-6 (IL-6), monocyte chemoattractant protein 1 (MCP-1), intercellular adhesion molecule 1 (ICAM-1), matrix metalloproteinase 14 (MMP14), and vascular cell adhesion molecule 1 (VCAM-1) 64. Additionally, SIRT1 improves free fatty acid, triglyceride, total cholesterol, and blood glucose levels 65, 66. These protective effects of SIRT1 indicate that it acts as an anti-atherosclerosis agent. Consistent with these findings, NMN administration dramatically improves vascular function in aged mice, partly through the activation of SIRT1 67.
Alzheimer’s disease
Alzheimer’s disease (AD) is marked by multiple pathologies, including neuroinflammation, amyloid-beta plaques, mitochondrial damage, and increased oxidative stress 68, 69. Patients with AD have lowered expression of SIRT1 70, 71, which is recapitulated in the hippocampus of AD model mice 72– 75. SIRT1 activation is capable of reducing the amount of oligomerized amyloid beta through upregulating the production of alpha-secretase 68, 69. This is corroborated by mouse models overexpressing SIRT1 and amyloid precursor protein. Additionally, SIRT1 promotes neuronal function and survival in AD model mice. CA1-localized SIRT1 overexpression not only preserves learning and memory in AD mice but enhances cognitive function in non-AD model mice 76.
Retinal degeneration
Retinal degeneration is prominent in diseases such as macular degeneration and diabetic retinopathy. A recent study reported the importance of SIRT3 and SIRT5 in the survival of retinal photoreceptors 77. In particular, mitochondrial SIRT3 activity is sensitive to the reduction in NAD +. Decreases in retinal NAD + were detected in multiple retinal degenerative disorders, including age-associated dysfunction, diabetic retinopathy, and light-induced degeneration 77. Supplementation with the NAD + intermediate NMN was able to restore retinal function 77. These findings suggest a possible therapeutic treatment for a wide variety of diseases with photoreceptor degeneration.
Depression
Depression is a complex psychiatric disorder associated with a number of pathologies, including inflammation, synaptic dysfunction, metabolic syndrome, and cognitive deficit. Sirtuins have been shown to have a role in the development of depression 78. In the dentate gyrus region of the hippocampus, it has been shown that SIRT1 is decreased under conditions of chronic stress, which has been associated with depressive-like behaviors 79. Additionally, inhibition of SIRT1 by genetic or pharmacological methods has reproduced depressive behaviors. Activation of SIRT1 is able to lead to anti-depressive behaviors 79. However, it has been observed that SIRT1 regulates expression of monoamine oxidase A (MAO-A), which lowers serotonin and drives anxiety-like behaviors 80, indicating that a balance in SIRT1 expression/activity is important for mood disorders.
SIRT2 has also been reported in mood disorders. Hippocampal SIRT2 expression is decreased in chronic stress conditions 81. Pharmacological inhibition of SIRT2 recapitulates depressive behaviors. Adenovirus-mediated overexpression of SIRT2 produces anti-depressive behaviors, which were abolished when hippocampal neurogenesis was disrupted by X-irradiation 81.
Interventions to achieve “productive aging”
NAD + intermediates, NMN and NR, are promising candidates to restore NAD + levels in disease models and aged animals 17. A number of studies have shown that both NAD + intermediates are effective to prevent and treat age-associated pathophysiologies.
We have shown that supplementation of NMN, a key NAD + intermediate, is effective at ameliorating age-associated metabolic disorders and slowing the progression of a multitude of age-associated physiological phenotypes 45, 82. Briefly, in the 12-month NMN administration study, age-associated body weight gain was ameliorated, energy metabolism and physical activity were improved, and gene expression changes associated with age were reversed. This study demonstrates NMN as an effective anti-aging agent 82. Other recent studies have also reported that NMN administration restores a depleted NAD + pool and is able to improve multiple aspects of disease. In a mouse AD model, one study reported that NMN improved mitochondrial respiration, a hallmark in the progression of AD and other neurodegenerative disorders 83. NMN administration has also shown improvements of mouse cognitive behaviors in the context of AD as well as improving electrophysiological deficits detected on hippocampal slices 84, 85. These findings suggest that NMN could also be a promising therapeutic agent for the treatment of AD and other neurodegenerative disorders. Additionally, we have shown the importance of NAD + biosynthesis in neuronal function. NAMPT is critical for neural stem cell proliferation and self-renewal. With age, NAMPT and NAD + levels decrease in the hippocampus, along with a decrease in the neural stem cell pool 46. NMN administration is able to rescue the NAD + levels and enhance the neural stem cell pool 46.
NR, another NAD + intermediate, has also shown beneficial effects in age-associated disorders. In prediabetic and diabetic mice under an HFD, NR administration improves steatosis of the liver, glucose tolerance, and weight gain 86, 87. These findings also suggest that NR administration could be an effective therapeutic agent for age-associated metabolic disorders. With age, the regenerative capacity of muscle decreases as muscle stem cells enter senescence. This is concomitant with a decrease in NAD + and a reduction of the mitochondrial unfolded protein response (mtUPR) 88. When NR is given, the muscle stem cell self-renewal capacity is restored, and the mtUPR is activated, improving the mitochondrial stress response. Additionally, in this study, mice which started receiving NR supplementation at two years of age showed a significant, moderate extension of life span 88. Dietary supplementation of NR significantly improves NAD + levels in the cerebral cortex and ameliorates cognitive deterioration 89. Application of NR in the context of hippocampal slice electrophysiology ameliorates deficits in long-term potentiation in the CA1 region. In this model system, NR increases PGC-1α, which regulates β-secretase and decreases amyloid-beta peptide. Though not addressed, the role of NAD +-consuming enzymes could be central to these beneficial effects observed. It seems likely that NAD + depletion occurs in certain neurodegenerative diseases. Nuclear DNA damage has been suggested to be associated with neurodegenerative disorders 90. Thus, supplementation of NAD + intermediates, NMN and NR, would be effective agents to prevent and treat neurodegenerative disorders ( Table 1), and this is critical to achieve “productive aging”.
Table 1. Beneficial effects of supplementation of NAD + intermediates, such as nicotinamide mononucleotide and nicotinamide ribose.
| Phenotype | Normal
progression |
NAD
+ precursor
intervention |
Reference | |
|---|---|---|---|---|
| Aging | Body weight | ↑ | ↓ | 82 |
| Energy metabolism | ↓ | ↑ | 82 | |
| Mitochondrial function | ↓ | ↑ | 82 | |
| Insulin sensitivity | ↓ | ↑ | 17, 82 | |
| Diabetes | Insulin sensitivity | ↓ | ↑ | 17 |
| Glucose tolerance | ↓ | ↑ | 17, 86, 87 | |
| Oxidative stress
response |
↓ | ↑ | 86, 87 | |
| Liver steatosis | ↑ | ↓ | 86, 87 | |
| Weight gain | ↑ | ↓ | 86, 87 | |
| Alzheimer’s
disease |
Mitochondrial respiration | ↓ | ↑ | 83 |
| PGC1α | ↓ | ↑ | 89 | |
| Beta-secretase | ↑ | ↓ | 89 | |
| Cognitive behaviors | ↓ | ↑ | 84, 85, 89 | |
| Long-term potentiation | ↓ | ↑ | 84, 85 |
A more detailed summary is available in 17. NAD +, nicotinamide adenine dinucleotide.
Conclusions
It is now clear that systemic NAD + decline is one of the fundamental molecular events that regulate the process of aging and possibly limit organismal life span. NAD + biosynthesis particularly mediated by NAMPT and NAD + consumption by NAD +-consuming enzymes are in a delicate balance so that perturbations to either side can cause significant derailment of the system. If NAMPT-mediated NAD + biosynthesis is disturbed or if NAD + consumption is increased because of chronic DNA damage that elicits PARP activation, the intracellular NAD + pool is decreased, causing organismal functional decline. Different NAD +-consuming enzymes, such as sirtuins, PARPs, CD38, and SARM1, might be affected in a cell type- or tissue-dependent manner, and loss of NAD + homeostasis can lead to dysfunction of basic physiological systems throughout the body. We now have increasing bodies of evidence supporting that interventions using NAD + intermediates, such as NMN and NR, can bolster the system by restoring the available NAD + and mitigate physiological decline associated with aging. We are at an exciting point in time when we can effectively test the importance of NAD + for the prevention and treatment of aging and aging-related diseases in humans.
Acknowledgments
We apologize to those whose work we did not cite due to space limitation.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Eduardo N Chini, Signal Transduction Laboratory, Kogod Aging Center, Department of Anesthesiology, Oncology Research, GI Signaling Center, Mayo Clinic College of Medicine, Rochester, USA
Wendy Hanna-Rose, Department of Biochemistry & Molecular Biology, Pennsylvania State University, Pennsylvania, USA
Funding Statement
SI is supported by grants from the National Institute on Aging (AG037457 and AG047902), the American Federation for Aging Research, and the Tanaka Fund.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Belenky P, Bogan KL, Brenner C: NAD + metabolism in health and disease. Trends Biochem Sci. 2007;32(1):12–9. 10.1016/j.tibs.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 2. Cantó C, Menzies KJ, Auwerx J: NAD + Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22(1):31–53. 10.1016/j.cmet.2015.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Garten A, Schuster S, Penke M, et al. : Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11(9):535–46. 10.1038/nrendo.2015.117 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Yang Y, Sauve AA: NAD + metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta. 2016;1864(12):1787–800. 10.1016/j.bbapap.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Imai S, Guarente L: NAD + and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–71. 10.1016/j.tcb.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imai S, Yoshino J: The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes Metab. 2013;15(Suppl 3):26–33. 10.1111/dom.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verdin E: The many faces of sirtuins: Coupling of NAD metabolism, sirtuins and lifespan. Nat Med. 2014;20(1):25–7. 10.1038/nm.3447 [DOI] [PubMed] [Google Scholar]
- 8. Haigis MC, Sinclair DA: Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–95. 10.1146/annurev.pathol.4.110807.092250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chambon P, Weill JD, Mandel P: Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res Commun. 1963;11(1):39–43. 10.1016/0006-291X(63)90024-X [DOI] [PubMed] [Google Scholar]
- 10. De Flora A, Zocchi E, Guida L, et al. : Autocrine and paracrine calcium signaling by the CD38/NAD +/cyclic ADP-ribose system. Ann N Y Acad Sci. 2004;1028:176–91. 10.1196/annals.1322.021 [DOI] [PubMed] [Google Scholar]
- 11. Imai S, Armstrong CM, Kaeberlein M, et al. : Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. 10.1038/35001622 [DOI] [PubMed] [Google Scholar]
- 12. Revollo JR, Grimm AA, Imai S: The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279(49):50754–63. 10.1074/jbc.M408388200 [DOI] [PubMed] [Google Scholar]
- 13. Revollo JR, Körner A, Mills KF, et al. : Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6(5):363–75. 10.1016/j.cmet.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang T, Zhang X, Bheda P, et al. : Structure of Nampt/PBEF/visfatin, a mammalian NAD + biosynthetic enzyme. Nat Struct Mol Biol. 2006;13(7):661–2. 10.1038/nsmb1114 [DOI] [PubMed] [Google Scholar]
- 15. Bogan KL, Brenner C: Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD + precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–30. 10.1146/annurev.nutr.28.061807.155443 [DOI] [PubMed] [Google Scholar]
- 16. Bieganowski P, Brenner C: Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD + in fungi and humans. Cell. 2004;117(4):495–502. 10.1016/S0092-8674(04)00416-7 [DOI] [PubMed] [Google Scholar]
- 17. Yoshino J, Baur JA, Imai SI: NAD + Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2017; pii: S1550-4131(17)30670-8. 10.1016/j.cmet.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imai S, Guarente L: It takes two to tango: NAD + and sirtuins in aging/longevity control. NPJ Aging Mech Dis. 2016;2: 16017. 10.1038/npjamd.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bheda P, Jing H, Wolberger C, et al. : The Substrate Specificity of Sirtuins. Annu Rev Biochem. 2016;85:405–29. 10.1146/annurev-biochem-060815-014537 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Tanno M, Sakamoto J, Miura T, et al. : Nucleocytoplasmic shuttling of the NAD +-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282(9):6823–32. 10.1074/jbc.M609554200 [DOI] [PubMed] [Google Scholar]
- 21. Vaquero A, Scher MB, Lee DH, et al. : SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006;20(10):1256–61. 10.1101/gad.1412706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verdin E, Hirschey MD, Finley LW, et al. : Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35(12):669–75. 10.1016/j.tibs.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mostoslavsky R, Chua KF, Lombard DB, et al. : Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–29. 10.1016/j.cell.2005.11.044 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Ford E, Voit R, Liszt G, et al. : Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20(9):1075–80. 10.1101/gad.1399706 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Anderson KA, Huynh FK, Fisher-Wellman K, et al. : SIRT4 Is a Lysine Deacylase that Controls Leucine Metabolism and Insulin Secretion. Cell Metab. 2017;25(4):838–855.e15. 10.1016/j.cmet.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Du J, Zhou Y, Su X, et al. : Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334(6057):806–9. 10.1126/science.1207861 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Jiang H, Khan S, Wang Y, et al. : SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496(7443):110–3. 10.1038/nature12038 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Haigis MC, Mostoslavsky R, Haigis KM, et al. : SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126(5):941–54. 10.1016/j.cell.2006.06.057 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Liszt G, Ford E, Kurtev M, et al. : Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280(22):21313–20. 10.1074/jbc.M413296200 [DOI] [PubMed] [Google Scholar]
- 30. Satoh A, Brace CS, Rensing N, et al. : Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18(3):416–30. 10.1016/j.cmet.2013.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanfi Y, Naiman S, Amir G, et al. : The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–21. 10.1038/nature10815 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Morales J, Li L, Fattah FJ, et al. : Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit Rev Eukaryot Gene Expr. 2014;24(1):15–28. 10.1615/CritRevEukaryotGeneExpr.2013006875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bai P, Cantó C, Oudart H, et al. : PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13(4):461–8. 10.1016/j.cmet.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Braidy N, Guillemin GJ, Mansour H, et al. : Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6(4):e19194. 10.1371/journal.pone.0019194 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Chini EN: CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions. Curr Pharm Des. 2009;15(1):57–63. 10.2174/138161209787185788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aksoy P, White TA, Thompson M, et al. : Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345(4):1386–92. 10.1016/j.bbrc.2006.05.042 [DOI] [PubMed] [Google Scholar]
- 37. Barbosa MT, Soares SM, Novak CM, et al. : The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21(13):3629–39. 10.1096/fj.07-8290com [DOI] [PubMed] [Google Scholar]
- 38. Grozio A, Sociali G, Sturla L, et al. : CD73 protein as a source of extracellular precursors for sustained NAD + biosynthesis in FK866-treated tumor cells. J Biol Chem. 2013;288(36):25938–49. 10.1074/jbc.M113.470435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Preugschat F, Carter LH, Boros EE, et al. : A pre-steady state and steady state kinetic analysis of the N-ribosyl hydrolase activity of hCD157. Arch Biochem Biophys. 2014;564:156–63. 10.1016/j.abb.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 40. Camacho-Pereira J, Tarragó MG, Chini CCS, et al. : CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23(6):1127–39. 10.1016/j.cmet.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Escande C, Nin V, Price NL, et al. : Flavonoid apigenin is an inhibitor of the NAD + ase CD38: implications for cellular NAD + metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62(4):1084–93. 10.2337/db12-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Essuman K, Summers DW, Sasaki Y, et al. : The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD + Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron. 2017;93(6):1334–1343.e5. 10.1016/j.neuron.2017.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Ramsey KM, Mills KF, Satoh A, et al. : Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7(1):78–88. 10.1111/j.1474-9726.2007.00355.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mouchiroud L, Houtkooper RH, Moullan N, et al. : The NAD +/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154(2):430–41. 10.1016/j.cell.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Yoshino J, Mills KF, Yoon MJ, et al. : Nicotinamide mononucleotide, a key NAD + intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–36. 10.1016/j.cmet.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stein LR, Imai S: Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014;33(12):1321–40. 10.1002/embj.201386917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mangerich A, Herbach N, Hanf B, et al. : Inflammatory and age-related pathologies in mice with ectopic expression of human PARP-1. Mech Ageing Dev. 2010;131(6):389–404. 10.1016/j.mad.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 48. Grube K, Bürkle A: Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proc Natl Acad Sci U S A. 1992;89(24):11759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li J, Bonkowski MS, Moniot S, et al. : A conserved NAD + binding pocket that regulates protein-protein interactions during aging. Science. 2017;355(6331):1312–7. 10.1126/science.aad8242 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Bordone L, Motta MC, Picard F, et al. : Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4(2):e31. 10.1371/journal.pbio.0040031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moynihan KA, Grimm AA, Plueger MM, et al. : Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2(2):105–17. 10.1016/j.cmet.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 52. Pfluger PT, Herranz D, Velasco-Miguel S, et al. : Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105(28):9793–8. 10.1073/pnas.0802917105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. He W, Wang Y, Zhang MZ, et al. : Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010;120(4):1056–68. 10.1172/JCI41563 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Wu T, Liu YH, Fu YC, et al. : Direct evidence of sirtuin downregulation in the liver of non-alcoholic fatty liver disease patients. Ann Clin Lab Sci. 2014;44(4):410–8. [PubMed] [Google Scholar]
- 55. Deng XQ, Chen LL, Li NX: The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27(5):708–15. 10.1111/j.1478-3231.2007.01497.x [DOI] [PubMed] [Google Scholar]
- 56. Kendrick AA, Choudhury M, Rahman SM, et al. : Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J. 2011;433(3):505–14. 10.1042/BJ20100791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hirschey MD, Shimazu T, Goetzman E, et al. : SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–5. 10.1038/nature08778 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Choudhury M, Jonscher KR, Friedman JE: Reduced mitochondrial function in obesity-associated fatty liver: SIRT3 takes on the fat. Aging (Albany NY). 2011;3(2):175–8. 10.18632/aging.100289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nassir F, Arndt JJ, Ibdah JA: 233 Hepatic Overexpression of SIRT3 in Mice Heterozygous for Mitochondrial Trifunctional Protein Rescues Hepatic Steatosis and Improves Insulin Sensitivity. Gastroenterology. 2015;148(4):S–973. 10.1016/S0016-5085(15)33324-2 [DOI] [Google Scholar]
- 60. de Kreutzenberg SV, Ceolotto G, Papparella I, et al. : Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes. 2010;59(4):1006–15. 10.2337/db09-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Orimo M, Minamino T, Miyauchi H, et al. : Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009;29(6):889–94. 10.1161/ATVBAHA.109.185694 [DOI] [PubMed] [Google Scholar]
- 62. Ota H, Eto M, Kano MR, et al. : Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(9):1634–9. 10.1161/ATVBAHA.108.164368 [DOI] [PubMed] [Google Scholar]
- 63. Ota H, Akishita M, Eto M, et al. : Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43(5):571–9. 10.1016/j.yjmcc.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 64. Chen YX, Zhang M, Cai Y, et al. : The Sirt1 activator SRT1720 attenuates angiotensin II-induced atherosclerosis in apoE −/− mice through inhibiting vascular inflammatory response. Biochem Biophys Res Commun. 2015;465(4):732–8. 10.1016/j.bbrc.2015.08.066 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Brandes RP: Activating SIRT1: a new strategy to prevent atherosclerosis? Cardiovasc Res. 2008;80(2):163–4. 10.1093/cvr/cvn245 [DOI] [PubMed] [Google Scholar]
- 66. Yu W, Fu YC, Chen CJ, et al. : SIRT1: a novel target to prevent atherosclerosis. J Cell Biochem. 2009;108(1):10–3. 10.1002/jcb.22240 [DOI] [PubMed] [Google Scholar]
- 67. de Picciotto NE, Gano LB, Johnson LC, et al. : Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522–30. 10.1111/acel.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Qin W, Yang T, Ho L, et al. : Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281(31):21745–54. 10.1074/jbc.M602909200 [DOI] [PubMed] [Google Scholar]
- 69. Heneka MT, O'Banion MK, Terwel D, et al. : Neuroinflammatory processes in Alzheimer's disease. J Neural Transm (Vienna). 2010;117(8):919–47. 10.1007/s00702-010-0438-z [DOI] [PubMed] [Google Scholar]
- 70. Lutz MI, Milenkovic I, Regelsberger G, et al. : Distinct patterns of sirtuin expression during progression of Alzheimer's disease. Neuromolecular Med. 2014;16(2):405–14. 10.1007/s12017-014-8288-8 [DOI] [PubMed] [Google Scholar]
- 71. Julien C, Tremblay C, Emond V, et al. : Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68(1):48–58. 10.1097/NEN.0b013e3181922348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Marques SC, Lemos R, Ferreiro E, et al. : Epigenetic regulation of BACE1 in Alzheimer's disease patients and in transgenic mice. Neuroscience. 2012;220:256–66. 10.1016/j.neuroscience.2012.06.029 [DOI] [PubMed] [Google Scholar]
- 73. Torres-Lista V, Parrado-Fernández C, Alvarez-Montón I, et al. : Neophobia, NQO1 and SIRT1 as premorbid and prodromal indicators of AD in 3xTg-AD mice. Behav Brain Res. 2014;271:140–6. 10.1016/j.bbr.2014.04.055 [DOI] [PubMed] [Google Scholar]
- 74. Revilla S, Suñol C, García-Mesa Y, et al. : Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology. 2014;81:55–63. 10.1016/j.neuropharm.2014.01.037 [DOI] [PubMed] [Google Scholar]
- 75. Rodriguez-Ortiz CJ, Baglietto-Vargas D, Martinez-Coria H, et al. : Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J Alzheimers Dis. 2014;42(4):1229–38. 10.3233/JAD-140204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Corpas R, Revilla S, Ursulet S, et al. : SIRT1 Overexpression in Mouse Hippocampus Induces Cognitive Enhancement Through Proteostatic and Neurotrophic Mechanisms. Mol Neurobiol. 2017;54(7):5604–19. 10.1007/s12035-016-0087-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Lin JB, Kubota S, Ban N, et al. : NAMPT-Mediated NAD + Biosynthesis Is Essential for Vision In Mice. Cell Rep. 2016;17(1):69–85. 10.1016/j.celrep.2016.08.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Song J, Kim J: Role of Sirtuins in Linking Metabolic Syndrome with Depression. Front Cell Neurosci. 2016;10:86. 10.3389/fncel.2016.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Abe-Higuchi N, Uchida S, Yamagata H, et al. : Hippocampal Sirtuin 1 Signaling Mediates Depression-like Behavior. Biol Psychiatry. 2016;80(11):815–26. 10.1016/j.biopsych.2016.01.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Libert S, Pointer K, Bell EL, et al. : SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147(7):1459–72. 10.1016/j.cell.2011.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu R, Dang W, Du Y, et al. : SIRT2 is involved in the modulation of depressive behaviors. Sci Rep. 2015;5: 8415. 10.1038/srep08415 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Mills KF, Yoshida S, Stein LR, et al. : Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24(6):795–806. 10.1016/j.cmet.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Long AN, Owens K, Schlappal AE, et al. : Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer's disease-relevant murine model. BMC Neurol. 2015;15:19. 10.1186/s12883-015-0272-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Wang X, Hu X, Yang Y, et al. : Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016;1643:1–9. 10.1016/j.brainres.2016.04.060 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Yao Z, Yang W, Gao Z, et al. : Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neurosci Lett. 2017;647:133–40. 10.1016/j.neulet.2017.03.027 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Trammell SA, Weidemann BJ, Chadda A, et al. : Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci Rep. 2016;6: 26933. 10.1038/srep26933 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Trammell SA, Schmidt MS, Weidemann BJ, et al. : Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7: 12948. 10.1038/ncomms12948 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Zhang H, Ryu D, Wu Y, et al. : NAD + repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–43. 10.1126/science.aaf2693 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Gong B, Pan Y, Vempati P, et al. : Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer's mouse models. Neurobiol Aging. 2013;34(6):1581–8. 10.1016/j.neurobiolaging.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chow HM, Herrup K: Genomic integrity and the ageing brain. Nat Rev Neurosci. 2015;16(11):672–84. 10.1038/nrn4020 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation