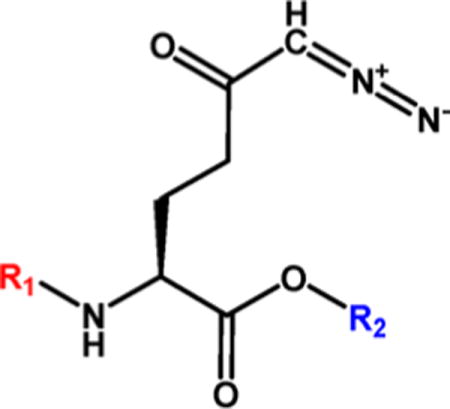
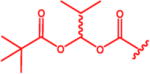
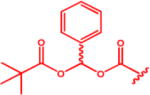
Abstract
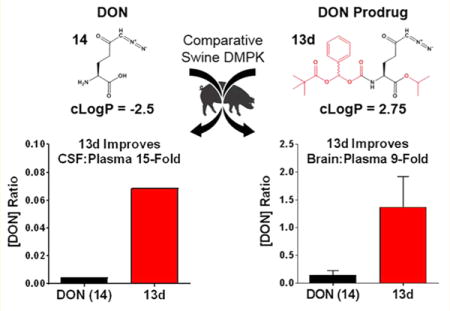
Aberrant excitatory neurotransmission associated with overproduction of glutamate has been implicated in the development of HIV-associated neurocognitive disorders (HAND). The glutamine antagonist 6-diazo-5-oxo-L-norleucine (DON, 14) attenuates glutamate synthesis in HIV-infected microglia/macrophages, offering therapeutic potential for HAND. We show that 14 prevents manifestation of spatial memory deficits in chimeric EcoHIV-infected mice, a model of HAND. 14 is not clinically available, however, because its development was hampered by peripheral toxicities. We describe the synthesis of several substituted N-(pivaloyloxy)alkoxy-carbonyl prodrugs of 14 designed to circulate inert in plasma and be taken up and biotransformed to 14 in the brain. The lead prodrug, isopropyl 6-diazo-5-oxo-2-(((phenyl(pivaloyloxy)methoxy)-carbonyl)amino)hexanoate (13d), was stable in swine and human plasma but liberated 14 in swine brain homogenate. When dosed systemically in swine, 13d provided a 15-fold enhanced CSF-to-plasma ratio and a 9-fold enhanced brain-to-plasma ratio relative to 14, opening a possible clinical path for the treatment of HAND.
Graphical abstract
INTRODUCTION
HIV-associated neurocognitive disorders (HAND) remain one of the central health issues in patients with chronic HIV infection1 despite viremic control by combined antiretroviral therapy (cART).2 While broad use of cART has markedly reduced the prevalence of HIV-associated dementia (HAD), it has had limited effects on milder forms of HAND which now represent the majority of new cases of HIV neurological disease.3–5
Although the mechanisms of HAND pathogenesis are unclear, accumulating evidence suggests that HAND is associated with aberrant glutamate metabolism in the central nervous system (CNS) culminating in elevated extracellular glutamate and disrupted excitatory neurotransmission.6,7 In cultured human macrophages and microglia, HIV infection induces elevated expression of the glutamate-synthesizing enzyme glutaminase which directly contributes to the toxic overproduction of glutamate.6,8–19 Activated microglia and macrophages also release viral proteins and pro-inflammatory cytokines6,7 that independently promote further glutamate synthesis and release20 and inhibit glutamate reuptake in astrocytes and neurons.6 These cumulative insults result in impaired glutamate-dependent synaptic function21–28 and cognition.25–28
Studies of HAND patients corroborate preclinical findings of disrupted glutamate metabolism in the CNS. Recent examination of cART-treated HIV patients revealed increased cerebrospinal fluid (CSF) glutamate in patients with HAND compared to those without neurocognitive impairment.29 Several magnetic resonance spectroscopy (MRS) studies have also shown dysregulated glutamate homeostasis in HIV patients that correlate with neurocognitive impairment including alterations in white and gray matter glutamate and glutamine.30–35 In addition, microarray analyses in post-mortem brain samples from HAND patients show frontal cortical alterations in the expression of genes critical for the regulation of glutamatergic signaling and glutamate-dependent synaptic plasticity, including glutaminase.18,36–38
Collectively, these studies suggest that interventions aimed at normalizing glutamate homeostasis offer therapeutic potential for HAND treatment. The glutamine antagonist 6-diazo-5-oxo-L-norleucine (DON, 14), which is known to inhibit glutaminase, has been shown to block glutamate over-production induced by HIV infection or immune challenge, and to mitigate excitotoxic neuronal damage both in vitro and in vivo,13,39–41 but has not yet been tested in an animal model of HAND.
In the present work, we tested the ability of 14 to prevent cognitive decline in EcoHIV-infected wild-type mice which serve as a model of HAND.42,43 EcoHIV is a chimeric HIV in which the coding region for envelope protein gp120 has been replaced with gp80 from murine leukemia virus, rendering EcoHIV capable of infecting conventional mice.42 EcoHIV infection results in expression of HIV viral proteins in relevant tissues including microglial cells in the brain43 and recapitulates many of the neuropathological features of HAND, bolstering the use of these mice as a model system for testing possible HAND therapeutics.42 We report that when 14 was administered to the mice prior to and during EcoHIV infection, it was able to completely prevent their cognitive decline.
Although exciting, the clinical utility of 14 is limited by its peripheral toxicities mediated primarily by the gastrointestinal (GI) system,44–46 which is known to be highly glutamine-utilizing. Recently, we described the synthesis of several N-(pivaloyloxy)alkoxy-carbonyl 14 prodrugs designed to enhance CNS penetration and brain delivery of 14 while reducing peripheral exposure and thus GI toxicity.47 Systemic administration in nonhuman primate of one such prodrug, compound 13b, with isopropyl ester on the carboxylate and N-(pivaloyloxy)ethoxy-carbonyl on the amine, resulted in about 10-fold enhancement in the CSF-to-plasma ratio of 14 exposure relative to administration of equimolar 14.47 These findings provided the first evidence that a prodrug strategy could significantly alter the tissue distribution of 14.
In the current study, we hypothesized that modification of the N-(pivaloyloxy)methoxy-carbonyl pro-moiety with additional steric bulk on the methylene bridge could slow peripheral bioconversion of 14 prodrugs and confer further improvement in CNS penetration, as has been shown for other prodrugs utilizing carboxylesterase-mediated hydrolyses.48–51 In addition to increasing steric hindrance and metabolic stability, our modification approach could also enhance lipophilicity.52,53 The new 14 prodrugs, compounds 13b–e, with addition of methyl, isopropyl, phenyl, and dimethyl groups to the N-(pivaloyloxy)methoxy-carbonyl pro-moiety, respectively, had substantially increased calculated partition coefficients (cLogP) versus 14 (1.50, 2.42, 2.75, and 1.81 vs –2.50, respectively). However, when evaluated in mouse plasma, all of the prodrugs were rapidly and completely metabolized to 14, precluding attempts to meaningfully differentiate their efficacy/toxicity from 14 in the EcoHIV murine model. Importantly, this conundrum is not uncommon in prodrug discovery, where increased metabolism in rodents is well documented and higher species are necessary to mimic human metabolism.54,55 Thus, these prodrugs were subsequently tested for stability in human plasma as well as swine, a model organism that closely recapitulates human drug metabolism and pharmacokinetics.56 Although the N-(pivaloyloxy)methoxy-carbonyl derivative of 14, compound 13a, was also labile in human and swine plasma, all of the substituted analogues, compounds 13b–e, were found to be metabolically stable. Furthermore, when incubated in homogenates of swine brain, the target tissue for 14 release, compounds 13b and 13d were readily converted. These compounds were then tested in vivo in swine. Relative to administration of equimolar 14, systemic administration of 13b and 13d resulted in lower 14 plasma exposure, higher CSF exposure, and a greater than 7- and 15-fold increased CSF-to-plasma ratio, respectively. Having shown the best profile, 13d and 14 were subsequently assessed in terminal swine studies in which brain levels were directly measured. 13d afforded a 9-fold enhancement in brain-to-plasma ratio relative to equimolar 14.
CHEMISTRY
As shown in Scheme 1, the intermediates 4a–c were synthesized by a three-step procedure starting from the appropriate 1-chloroalkyl carbonochloridate (1a–c), which was reacted with ethanthiol in the presence of triethylamine to yield corresponding O-(1-chloroalkyl)-S-ethyl carbonothioates (2a–c). These intermediates were transformed to 1-(((ethylthio)carbonyl)oxy)alkyl pivalate derivatives (3a–c) by reaction with pivalic acid in the presence of base (DIPEA). Oxidation of the thioester moiety by peracetic acid followed by reaction with N-hydroxysuccinimide57,58 provided 4a–c in a yield of 56–64% after column chromatography (total over three steps). The attempt to use the same methodology for phenyl analogue 13d in the last step resulted in a complex mixture, likely due to reactivity of the corresponding benzyl cation intermediate. The attempt to prepare the analogous intermediate with a phenyl was unsuccessful. Although it was not confirmed, this failure could be due to the oxidation of the benzoacetal group by peracetic acid in the third step.
Scheme 1. Synthesis of N-Hydroxysuccinimide Esters of Pivaloyloxy-alkoxycarbonates Intermediates 4a–ca.
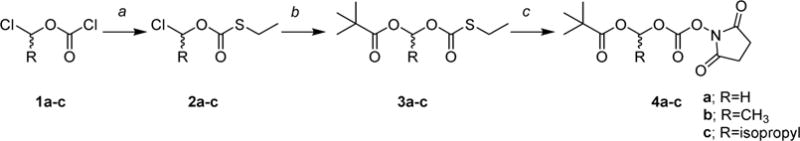
aReagents and conditions: (a) EtSH, Et3N, Et2O, 0 °C to rt, 17–25 h; (b) pivalic acid, DIPEA, 60–70 °C, 20–70 h; (c) N-hydroxysuccinimide, peracetic acid, DCM, 0 °C to rt, 3 h.
We thus chose to explore the para-nitrophenoxy derivative 8, which can be synthesized via a route devoid of an oxidant as shown in Scheme 2. First, the chloro(phenyl)methyl carbonochloridate (6) was prepared by reaction of benzaldehyde (5) with triphosgene in the presence of base (pyridine). Intermediate 6 was reacted with 4-nitrophenol to give the chloro(phenyl)methyl (4-nitrophenyl) carbonate (7), which was then subjected to reaction with freshly prepared mercury-(II) salt of pivalic acid to yield analogue 8. Pivalate 8 was then used to prepare 13d (Scheme 4). Despite several attempts with different pivalates (potassium, silver, palladium) we were not successful in eliminating the use of mercury in this step.
Scheme 2. Synthesis of 4-Nitrophenyl Esters of Pivaloyloxy-alkoxycarbonate Intermediate 8a.
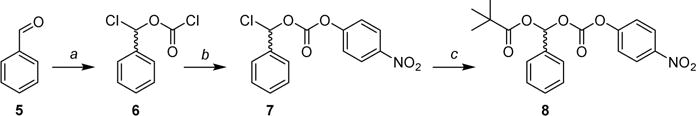
aReagents and conditions: (a) triphosgene, pyridine, Et2O, –20 °C to rt, 20 h; (b) 4-nitrophenol, pyridine, DCM, 0 °C to rt, 2 h; (c) Hg(OPiv)2, DCM, rt, 16 h.
Scheme 4. Synthesis of 14 Prodrugs 13a –ea.
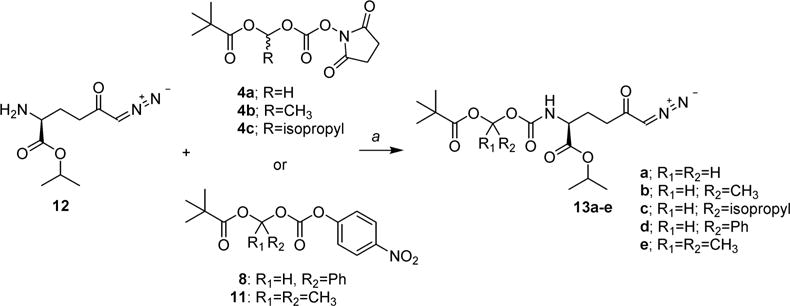
aReagents and conditions: (a) DCM or DMF, 0 °C to rt, 2–5 h.
As shown in Scheme 3, compound 11 was prepared in an analogous way. 4-Nitrophenyl prop-1-en-2-yl carbonate (9) was prepared from commercially available 2-propenyl chloroformate as previously reported59 and reacted with hydrogen chloride. The obtained 2-chloropropan-2-yl (4-nitrophenyl) (10) was then reacted with mercury(II) pivalate to yield the desired intermediate 11, which was then used to prepare analogue 13e.
Scheme 3. Synthesis of Intermediate 11a.
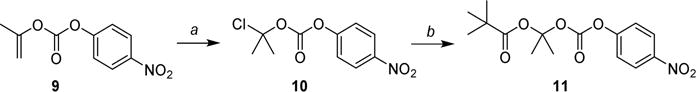
aReagents and conditions: (a) 4 M HCl, dioxane, rt, 21 h; (b) Hg(OPiv)2, DCM, rt, 20 h.
As shown in Scheme 4, the prodrugs 13a–e were prepared by reaction of 14 isopropyl ester (12) with 4a–c, 8, or 11, respectively, in dichloromethane (DCM) or dimethylformamide (DMF) at 0 °C or room temperature. Compound 12 was prepared as previously described in detail with yield and purity in agreement with the published data.47 The compounds 13a–e were obtained in 40–83% yield after purification by liquid chromatography (LC). To improve yields of 13d and 13e, an excess of compound 1247 and longer reaction time were necessary (see Experimental Section).
RESULTS AND DISCUSSION
Compound 14 Prevented Cognitive Decline, a Major Manifestation of HAND, in EcoHIV-Infected Mice
Similar to HAND patients,60 mice inoculated with EcoHIV exhibited impaired spatial learning and memory as measured by radial arm water maze (RAWM) 30 days postinfection (Figure 1). EcoHIV infection resulted in a significant increase in the number of errors (Figure 1A) and latency to escape (Figure 1B) onto a hidden platform in the maze. 14 treatment (1 mg/kg, ip, qod) beginning 1 day prior to EcoHIV or sham inoculation and continued throughout the infection period and RAWM testing, fully normalized cognitive performance as measured by both errors (main effect of treatment [F(3,140) = 261.8, p < 0.0001], trial [F(4,140) = 146.2, p < 0.0001], interaction [F(12,140) = 1.93, p = 0.0355]) and latency to escape (main effect of treatment [F(3,140) = 37.00, p < 0.0001], trial [F(4,140) = 56.03, p < 0.0001], interaction [F(12,140) = 1.832, p = 0.0484]). Neither EcoHIV infection nor 14 treatment affected latency to escape to a visible platform (Figure 1C; main effect of trial [F(3,112) = 34.44, p < 0.0001]), indicating no impairment in visual or motor function. Additionally, no mice exhibited overt signs of toxicity (i.e., diarrhea, weight loss). The EcoHIV-infected mice exhibited measurable viral loads in the spleen and brain (Figure 1D). 14 treatment actually caused an increase in peripheral viral load (t(14) = 2.58, p = 0.022), likely due to the well described 14-mediated inhibition of T cell activity/proliferation,61,62 which is known to be required for endogenous suppression of EcoHIV replication.43 Therefore, despite enabling a modest increase in EcoHIV replication, 14 still prevented cognitive decline, suggesting its mechanism of action to be secondary to the infection itself. These findings suggest that glutaminase inhibition through 14 delivery to the CNS prior to or during HIV infection may prevent the development of cognitive impairment in HAND patients.
Figure 1.
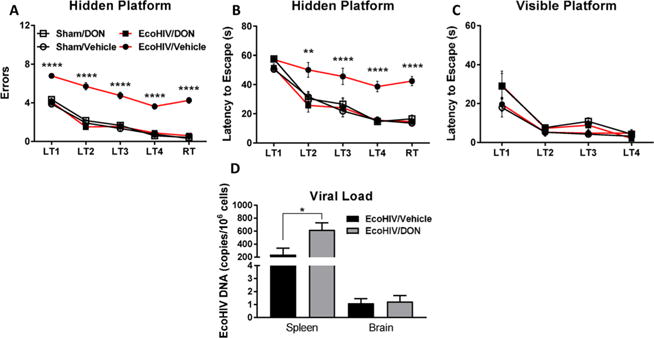
DON (14) prevented cognitive decline in the EcoHIV model of HAND. DON (14) treatment (1 mg/kg, ip) was begun prior to EcoHIV inoculation and continued every other day throughout the 30 day infection period and during radial arm water maze (RAWM) testing. 14 significantly attenuated spatial learning and memory deficits in the RAWM as measured by (A) number of errors across learning trials (LT) 1–4 and the retention trial (RT) and (B) latency to escape to a hidden platform relative to sham-inoculated control mice. 14 had no effect on (C) RAWM escape latency to a visible platform. 14 treatment also caused (D) a slight increase in EcoHIV viral load as measured by DNA copies in the spleen but had no effect on viral load in the brain. Behavioral comparison conducted by two-way ANOVA, posthoc comparison by Tukey’s test; ****p < 0.0001, EcoHIV/Veh vs Sham/Veh, EcoHIV/DON, and Sham/DON. Viral load comparison conducted by t test, *p < 0.05, n = 8/group.
N-(Pivaloyloxy)alkoxy-carbonyl Prodrugs of 14 Were Synthesized with Improved Lipophilicity
Despite robust efficacy in the EcoHIV model of HAND, the GI toxicity induced by 14 in humans has hampered its clinical development.44–46 Previous efforts by our group to develop more tolerable prodrugs of 14 yielded compound 13a (Table 1) through coverage of 14’s carboxylate and amine groups with isopropyl ester and a primary N-(pivaloyloxy)methoxy-carbonyl group, respectively.47 We rationalized that additional bulky, lipophilic substituents would increase cLogP and further improve CNS penetration. Thus, methyl-, isopropyl-, phenyl-, and dimethyl-analogues of 13a (compounds 13b–e) were synthesized as described above. cLogP for each of these prodrugs was incrementally and substantially increased relative to 14 (Table 1). We then initiated testing of their ex vivo metabolic stability.
Table 1.
Lipophilicity and Stability of N-(Pivaloyloxy)alkoxy-carbonyl Prodrugs of DON (14)
N-(Pivaloyloxy)alkoxy-carbonyl Prodrugs of 14 Were Completely Unstable in Mouse Plasma, Restricting Their Evaluation in Mouse Models
All prodrugs 13a–e were found to be completely metabolized during a 60 min incubation in mouse plasma (Table 1). A representative chromatogram for the rapid metabolism of 13d in mouse plasma is provided in Supporting Information, Figure S1, showing complete conversion to 14 within 10 min. The rapid metabolism of the 14 prodrugs precluded the examination of their efficacy and toxicity in mice as they would be immediately converted to 14. As mentioned above, enhanced metabolism in rodents is a common issue with prodrugs of various classes,54,55 necessitating the use of higher species, such as swine, that more closely model human metabolism.56
N-(Pivaloyloxy)alkoxy-carbonyl Prodrugs of 14 Were Stable in Human and Swine Plasma but Labile in Brain Homogenate
Consistent with previous reports of the metabolic lability of N-(pivaloyloxy)methoxy-carbonyl group-containing prodrugs,47–51,63 compound 13a was found to be rapidly metabolized during a 60 min incubation in human plasma (Table 1). In contrast, we found that modification of 13a with methyl-, isopropyl-, phenyl-, and dimethyl- substitutions (13b–e) resulted in a significant improvement in human plasma stability (Table 1). As our goal was to evaluate these prodrugs in an animal model which mimicked human metabolism, we next tested the stability of compounds 13b–e in swine plasma. We found that all of the prodrugs, similar to human plasma, were stable in swine plasma (Table 1). Given that the target compartment for 14 activity in HAND is the brain, 13b–e were then tested for metabolic lability in swine brain homogenate. Compounds 13c and 13d were found to be readily biotransformed in swine brain homogenate, whereas 13b was moderately labile and 13e was mostly stable (Figure 2). Swine brain homogenate half-lives were calculated for each compound and found to be significantly different from each other [F(3,8) = 31.42, p < 0.0001].
Figure 2.
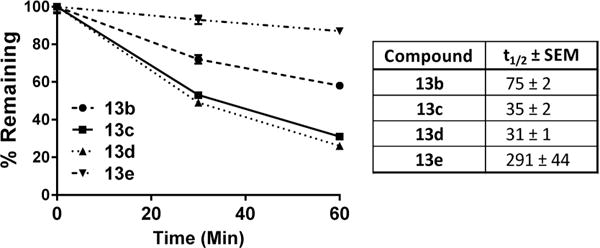
N-(Pivaloyloxy)alkoxy-carbonyl prodrugs of DON (14) showed differential rates of metabolism in swine brain homogenate. Compounds 13b–e (10 μM) were spiked into swine brain homogenate (1% w/v); concentrations of the prodrug remaining were measured by LC-MS/MS at 0, 30, or 60 min post incubation. The prodrugs were metabolized at varying rates, with 13c and 13d showing the highest lability. Data are depicted as mean ± SEM. Half-lives (t1/2) compared by one-way ANOVA, p < 0.0001, n = 3/group.
When Tested in Swine, Compounds 13b and 13d Resulted in Enhanced 14 CSF-to-Plasma Ratios; Compound 13d Showed an Optimal Profile
To determine if the ex vivo metabolism data translated in vivo, 14, 13b, and 13d were selected for pharmacokinetic evaluation in swine. Consistent with their observed metabolic stability, iv infusion of 13b and 13d (1.6 mg/kg 14 equivalent dose) resulted in 3–5-fold lower 14 plasma exposures relative to an equimolar dose of 14 (Figure 3A). Plasma AUC0–t for 14, 13b, and 13d were 29.9, 8.00, and 5.70 nmol·h/mL, respectively. The opposite trend occurred in CSF, where the prodrugs delivered substantially higher amounts of 14 to the CSF (Figure 3B; Table 2), resulting in significantly increased CSF-to-plasma ratios (Figure 3C; Table 3). The improvement over 14 in CNS penetration correlated with the lipophilicity of each prodrug. The cLogP for 13d was nearly twice that calculated for 13b (2.75 vs 1.50) and was associated with a doubling of the improvement in CSF-to-plasma ratio in vivo (approximately 15-fold increase for 13d vs 7-fold increase for 13b relative to equimolar 14). These experiments confirm that POM-based ester prodrugs substantially improve CNS delivery of 14 and support the hypothesis that iterative derivatization of this promoiety with sterically hindering, lipophilic residues promotes better CNS targeting. It should be noted that the 13b diastereomers were separable by standard column chromatography,47 whereas the 13d diastereomers were not readily separable by the same method and thus were administered as a mixture. This may not influence the clinical relevance of these findings, as human plasma stabilities of the 13b diastereomers were comparable (91% vs 89% remaining at 60 min for 13b-1 and 13b-2, respectively).47 However, as 13d moves toward clinical development, stereochemical purity will be prioritized.
Figure 3.
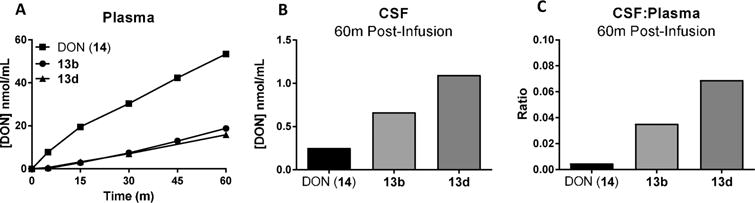
In vivo pharmacokinetics of DON following iv administration of DON (14), 13b, and 13d in swine plasma and CSF. DON (14, 1.6 mg/kg, iv) or an equivalent dose of either 13b or 13d were administered to swine. Plasma (0–60 min) and CSF (60 min) concentrations of 14 were evaluated via LC-MS/MS. Relative to 14, compounds 13b or 13d delivered (A) lower 14 plasma exposure and (B) higher 14 CSF concentrations, resulting in (C) more than 7-fold or 15-fold enhanced CSF:plasma ratio at 60 min postadministration, respectively.
Table 2.
Total and Free DON (14) Concentrations 60 min Post-Administration of 14 or 13d in Swine (1.6 mg/kg Equivalent, iv)
| brain (nmol/g)
|
plasma (nmol/mL)
|
CSF (nmol/mL)
|
|||
|---|---|---|---|---|---|
| total | free | total | free | total | |
| 14 | 5.15 ± 1.40 | 0.464 ± 0.126 | 41.2 ± 12.2 | 32.5 ± 9.64 | 0.252 |
| 13d | 5.90 ± 0.590 | 0.531 ± 0.053 | 4.88 ± 1.50 | 3.86 ± 1.19 | 1.10 |
Table 3.
Total and Free DON (14) Ratios 60 min Post-Administration of 14 or 13d in Swine (1.6 mg/kg Equivalent, iv)
| brain:plasma
|
CSF:plasma
|
CSF:brain
|
|||
|---|---|---|---|---|---|
| total:total | free:free | total:total | total:free | total:free | |
| 14 | 0.148 ± 0.078 | 0.017 ± 0.001 | 0.005 | 0.006 | 0.543 |
| 13d | 1.38 ± 0.544 | 0.157 ± 0.062 | 0.069 | 0.087 | 2.07 |
In Terminal Swine Studies, Compound 13d Resulted in Enhanced 14 Brain-to-Plasma Ratio
Because CSF is not always an accurate surrogate for brain concentrations, we conducted terminal studies in swine with the lead compound 13d where plasma and brain tissue were collected. At 60 min postinfusion of 13d (1.6 mg/kg 14 equivalent dose), 14 delivery to the plasma was substantially lower than equimolar 14 (Figure 4A) while brain levels were comparable (Figure 4B; Table 2). This resulted in a 9-fold enhancement in the brain-to-plasma ratio of 13d relative to equimolar 14 (Figure 4C), similar to its enhanced CSF-to-plasma ratio (Table 3). Given that the 14 CSF levels were significantly lower than that observed in brain tissues, we next conducted protein binding studies in an attempt to elucidate the reason for these differences. Plasma protein binding of 14 was low (Fb = 21%), consistent with previous reports.46 However, higher brain protein binding of 14 was observed (Fb= 91%), providing a possible explanation for the CSF and brain level discrepancies. On the basis of this value, free levels of 14 in the swine brain were calculated to be 0.531 nmol/g at 60 min postadministration of 13d, closer to observed levels of 14 delivered to the CSF by 13d at the same time point (~1.0 nmol/mL; Table 2).
Figure 4.
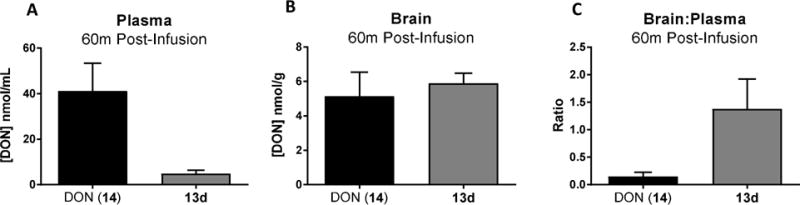
In vivo pharmacokinetics of DON following iv administration of DON (14) and 13d in swine plasma and brain. DON (14, 1.6 mg/kg, iv) or an equivalent dose of 13d was administered to swine. Swine were sacrificed, and plasma and brain (60 min) concentrations of 14 were evaluated via LC-MS/MS. Relative to 14, compound 13d delivered (A) lower 14 plasma concentrations and (B) comparable 14 brain concentrations, resulting in (C) a 9-fold enhanced brain:plasma ratio at 60 min postadministration. Data are depicted as mean ± SEM (n = 2/group). Free levels of 14 in the swine brain (Fb = 91%) were calculated to be 0.46 and 0.53 nmol/g following 14 and 13d administration, respectively.
CONCLUSION
14 was robustly efficacious in preventing cognitive decline in EcoHIV-infected mice used as a model of HAND. However, 14 is not suitable for clinical use due to its dose-limiting peripheral toxicities. To translate these findings to the clinic, we describe the synthesis of several substituted N-(pivaloyloxy)alkoxy-carbonyl prodrugs of 14 that improve CNS penetration. Rational design of 14 prodrugs by addition of lipophilic substituents to the N-(pivaloyloxy)alkoxy-carbonyl pro-moiety yielded sterically hindered compounds with increased metabolic stability in swine and human plasma, favorable conversion in brain homogenate, and substantially improved CNS delivery in vivo in swine, exemplified by compound 13d. Metabolic stability of these prodrugs in human plasma was very similar to that observed in swine, suggesting strong translational potential for this strategy. This approach may therefore enable the clinical use of brain glutaminase inhibition for the treatment of HAND and other neuropsychiatric disorders characterized by aberrant glutamate metabolism.
EXPERIMENTAL SECTION
The commercially available HPLC grade acetonitrile, catalysts, and reagent grade materials were used as received. TLC was performed on Silica gel 60 F254-coated aluminum sheets (Merck), and spots were detected by the solution of Ce(SO4)2.4H2O (1%) and H3P(Mo3O10)4 (2%) in sulfuric acid (10%). Flash chromatography was performed on Silica Gel 60 (0.040–0.063 mm, Fluka) or on Biotage KP-C18-HS or KP-Sil SNAP cartridges using the Isolera One HPFC system (Biotage, Inc.). All chemicals were purchased from Sigma-Aldrich or TCI and were used without further purification. The 1H NMR spectra were measured at 400.1 MHz and 13C NMR spectra at 100.8 MHz. For standardization of 1H NMR spectra, the internal signal of TMS (δ 0.0, CDCl3) or residual signals of CDCl3 (δ 7.26) were used. In the case of 13C NMR spectra, the residual signal of CDCl3 (δ 77.00) was used. The chemical shifts are given in δ scale; the coupling constants J are given in Hz. The ESI mass spectra were recorded using ZQ micromass mass spectrometer (Waters) equipped with an ESCi multimode ion source and controlled by MassLynx software. Alternatively, the low resolution ESI mass spectra were recorded using a quadrupole orthogonal acceleration time-of-flight tandem mass spectrometer (Q-Tof Micro, Waters) and high resolution ESI mass spectra using a hybrid FT mass spectrometer combining a linear ion trap MS and the Orbitrap mass analyzer (LTQ Orbitrap XL, Thermo Fisher Scientific). The conditions were optimized for suitable ionization in the ESI Orbitrap source (sheat gas flow rate 35 au, aux gas flow rate 10 au of nitrogen, source voltage 4.3 kV, capillary voltage 40 V, capillary temperature 275 °C, tube lens voltage 155 V). The samples were dissolved in methanol and applied by direct injection. The purity of all compounds subjected to biological testing was established using HPLC (Jasco Inc.) equipped with a Reprosil 100 C18, 5 μm, 250 mm × 4 mm column. The analysis was performed using a gradient of 2% CH3CN/98% H2O with 0.1% TFA → 100% CH3CN, with UV detection, λ = 210 nm. Purity of all compounds subjected to biological testing was over 95%. Optical rotations were measured in CHCl3 using an Autopol IV instrument (Rudolph Research Analytical). IR spectra were measured in CHCl3 on an FT-IR spectrometer.
((((2,5-Dioxopyrrolidin-1-yl)oxy)carbonyl)oxy)methyl Pivalate (4a)
Chloromethyl carbonochloridate 1a (2.00 g, 1.38 mL, 15.5 mmol) was dissolved in anhydrous Et2O (20 mL). The reaction mixture was cooled to 0 °C, and the mixture of Et3N (1.57 g, 2.16 mL, 15.5 mmol, 1 equiv) and EtSH (964 mg, 1.15 mL, 15.5 mmol, 1 equiv) in anhydrous Et2O (5 mL) was added dropwise over 5 min. The resulting mixture was then stirred overnight (25 h) at rt. Precipitate was filtered through a pad of Celite, and solvent was removed under reduced pressure. The crude O-(chloromethyl) S-ethyl carbonothioate 2a (2.4 g, colorless liquid) was used in the next step without purification. 2a (2.40 g, 15.5 mmol) was dissolved in pivalic acid (9.51 g, 93.1 mmol, 6 equiv), and freshly prepared salt of pivalic acid (4.76 g, 46.6 mmol, 3 equiv) and DIPEA (6.02 g, 8.10 mL, 46.6 mmol, 3 equiv) were added in few portions. The reaction mixture was heated to 60 °C for 22 h. EtOAc (100 mL) was added, and the organic phase was extracted with water (100 mL), satd NaHCO3 (3 × 100 mL), and satd NaCl (100 mL), dried over MgSO4, solvent was removed under reduced pressure, and the crude product 3a (3.30 g, 97%, light-yellow liquid) was used in the next step without purification. (((Ethylthio)-carbonyl)oxy)methyl pivalate 3a (3.20 g, 14.5 mmol) was dissolved in anhydrous DCM (40 mL), N-hydroxysuccinimide (3.34 g, 29.1 mmol, 2 equiv) was added, and the suspension was cooled to 0 °C. Peracetic acid (3.31 g (100%), 9.21 g (36%), 43.6 mmol, 3 equiv, 36% solution in acetic acid) was added dropwise over 15 min. The resulting mixture was stirred for 1 h at 0 °C and 2 h at rt. DCM (50 mL) was added, and the organic phase was washed with water (30 mL) and satd NaCl (30 mL), dried over MgSO4, solvent was removed under reduced pressure, and column chromatography of the residue (EtOAc/hexane 1:2, Rf 0.27) yielded 4a as a colorless solid (2.54 g, 64% over three steps). 1H NMR (400 MHz, CDCl3): δ 1.24 (9H, s), 2.84 (4H, s), 5.86 (2H, s). 13C NMR (101 MHz, CDCl3): δ 25.56 (2C), 26.86 (3C), 38.96, 83.67, 150.90, 168.34 (2C), 176.54. IR (CHCl3): 2979 m, 2939 w, 2876 w, 1823 s, 1796 vs, 1649 vs, 1481 m, 1463 m, 1456 w, 1431 m, 1398 w, 1371 m, 1367 m, 1280 m, 1199 vs, 1110 vs, 1047 m, 998 s, 986 s, 942 m, sh, 924 s, 853 w, cm−1. ESI MS: 296 ([M + Na]+). HR ESI MS: calcd for C11H15O7NNa 296.07407; found 296.07410.
((((2,5-Dioxopyrrolidin-1-yl)oxy)carbonyl)oxy)ethyl Pivalate (4b)
Chloroethyl carbonochloridate 1b (2.00 g, 1.51 mL, 14.1 mmol) was dissolved in anhydrous Et2O (20 mL). The reaction mixture was cooled to 0 °C, and the mixture of Et3N (1.43 g, 1.97 mL, 14.1 mmol, 1 equiv) and EtSH (876 mg, 1.02 mL, 14.1 mmol, 1 equiv) in anhydrous Et2O (5 mL) was added dropwise over 5 min. The resulting mixture was then stirred overnight (17 h) at rt. Precipitate was filtered through a pad of Celite, and solvent was removed under reduced pressure. The crude O-(1-chloroethyl) S-ethyl carbonothioate 2b (2.20 g, colorless liquid) was used in the next step without purification. 2b (2.20 g, 13.1 mmol) was dissolved in pivalic acid (8.02 g, 78.6 mmol, 6 equiv), and freshly prepared salt of pivalic acid (4.01 g, 39.3 mmol, 3 equiv) and DIPEA (5.08 g, 6.85 mL, 39.3 mmol, 3 equiv) was added in few portions. The reaction mixture was heated to 70 °C for 20 h. EtOAc (100 mL) was added, and the organic phase was extracted with water (100 mL), satd NaHCO3 (3 × 100 mL), and satd NaCl (100 mL), dried over MgSO4, solvent was removed under reduced pressure, and the crude product 3b (2.82 g, 92%, light-yellow liquid) was used in the next step without purification. (((Ethylthio)carbonyl)oxy)methyl pivalate 3b (2.82 g, 12.1 mmol) was dissolved in anhydrous DCM (40 mL), N-hydroxysuccinimide (2.77 g, 24.1 mmol, 2 equiv) was added, and the suspension was cooled to 0 °C. Peracetic acid (2.76 g (100%), 7.67 g (36%), 36.3 mmol, 3 equiv, 36% solution in acetic acid) was added dropwise over 15 min. The resulting mixture was stirred for 1 h at 0 °C and 2 h at rt. DCM (50 mL) was added, and the organic phase was washed with water (30 mL) and satd NaCl (30 mL), dried over MgSO4, solvent was removed under reduced pressure, and column chromatography of the residue (EtOAc/hexane 1:2, Rf 0.27) yielded 4b as a colorless oil (2.02 g, 50% over three steps). 1H NMR (400 MHz, CDCl3): δ 1.21 (9H, s), 1.60 (3H, d, J = 5.4), 2.83 (4H, s), 6.81 (1H, q, J = 5.4). 13C NMR (101 MHz, CDCl3): δ 19.43, 25.58 (2C), 6.88 (3C), 38.89, 93.83, 150.01, 168.43, 176.10. IR (CHCl3): 2978 m, 2939 w, 2875 w, 1821 s, 1795 s, 1748 vs, br, 1480 m, 1432 m, 1396 m, 1371 m, 1365 m, sh, 1298 w, sh, 1280 s, 1199 s, 1054 vs, 1028 s, 944 vw, sh, 935 w, sh, 811 w, cm−1. ESI MS: 310 ([M + Na]+). HR ESI MS: calcd for C12H17O7NNa 310.08972; found 310.08979.
1-((((2,5-Dioxopyrrolidin-1-yl)oxy)carbonyl)oxy)-2-methylpropyl Pivalate (4c)
1-Chloro-2-methylpropyl carbonochloridate 1c (2.00 g, 1.71 mL, 11.7 mmol) was dissolved in anhydrous Et2O (20 mL). The reaction mixture was cooled to 0 °C, and the mixture of Et3N (1.18 g, 1.63 mL, 11.7 mmol) and EtSH (727 mg, 866 μL, 11.7 mmol) in anhydrous Et2O (10 mL) was added dropwise over 10 min. The resulting mixture was stirred overnight (23 h) at rt. Precipitate was filtered through a pad of Celite, and solvent was removed under reduced pressure. The crude product O-(1-chloro-2-methylpropyl) S-ethyl carbonothioate 2c (2.20 g, 96%, colorless liquid) was used in the next step without purification. 2c (1.20 g, 6.10 mmol) was dissolved in pivalic acid (3.74 g, 4.20 mL, 36.6 mmol, 6 equiv), and freshly prepared salt of pivalic acid (1.87 g, 2.10 mL, 18.3 mmol, 3 equiv) and DIPEA (2.37 g, 3.19 mL, 18.3 mmol, 3 equiv) were added in few portions. The reaction mixture was heated to 60 °C for 70 h. EtOAc (100 mL) was added, and the organic phase was extracted with water (50 mL), satd NaHCO3 (3 × 50 mL), satd NaCl (50 mL), dried over MgSO4, solvent was removed under reduced pressure, and the crude product 1-(((ethylthio)carbonyl)oxy)-2-methylpropyl pivalate 3c (1.32 g, 83%, light-yellow liquid) was used in the next step without purification. 3c (1.28 g, 4.88 mmol) was dissolved in anhydrous DCM (13 mL), N-hydroxysuccinimide (1.12 g, 9.76 mmol, 2 equiv) was added, and the suspension was cooled to 0 °C. Peracetic acid (1.11 g (100%), 3.09 g (36%), 14.6 mmol, 3 equiv, 36% solution in acetic acid) was added dropwise over 10 min. The resulting mixture was stirred for 1 h at 0 °C and for 2 h at rt. DCM (40 mL) was added, and the organic phase was washed with water (20 mL) and satd NaCl (20 mL), dried over MgSO4, solvent was removed under reduced pressure, and column chromatography of the residue (EtOAc/hexane 3:5, Rf 0.26) yielded 4c as a light-yellow oil (863 mg, 56% over 3 steps). 1H NMR (400 MHz, CDCl3): δ 1.00 (6H, d, J = 6.9), 1.21 (9H, s), 2.08–2.19 (1H, m), 2.81 (4H, s), 6.55 (1H, d, J = 5.0). 13C NMR (101 MHz, CDCl3): δ 16.06, 16.38, 25.55, 26.89, 31.84, 39.05, 98.19, 150.37, 168.48, 176.14. IR (CHCl3): 2978 m, 2938 w, 2878 w, 1821 s, 1795 s, 1748 vs, br, 1481 m, 1463 w, 1432 m, 1396 w, 1373 m, 1366 m, sh, 1279 m, 1199 s, 1046 m, 998 m, sh, 987 m, 932 s cm−1. ESI MS: 338 ([M + Na]+). HR ESI MS: calcd for C14H21O7NNa 338.12102; found 338.12115.
Chloro(phenyl)methyl(4-nitrophenyl)carbonate (7)
Chloro-(phenyl)methyl carbonochloridate (6) was prepared from benzaldehyde (5) by a previously reported method.64 Compound 6 (900 mg, 4.39 mmol) was dissolved in anhydrous DCM (20 mL), 4-nitrophenol (611 mg, 4.39 mmol, 1 equiv) was added, and the mixture was cooled to 0 °C. Pyridine (347 mg, 355 μL, 4.39 mmol, 1 equiv) in anhydrous DCM (5 mL) was added dropwise over 5 min. Reaction mixture was stirred for 2 h at rt. DCM was evaporated, and the crude product was purified by column chromatography (DCM/hexane 1:1). Compound 7 was obtained as a colorless solid (520 mg, 39%). 1H NMR (400 MHz, CDCl3): δ 7.33 (1H, s), 7.41–7.50 (5H, m), 7.58–7.63 (2H, m), 8.28–8.34 (2H, m). 13C NMR (101 MHz, CDCl3): δ 87.37, 121.83 (2C), 125.59 (2C), 126.41 (2C), 129.07 (2C), 130.56, 136.35, 145.91, 150.50, 155.07. IR (CHCl3): 3119 w, 3088 w, 3071 vw, 3032 w, 1788 vs, 1772 s, sh, 1619 m, 1595 m, 1530 vs, 1492 s, 1456 m, 1349 vs, 1317 m, 1296 m, 1232 vs, sh, 1178 m, sh, 1165 m, 1111 m, 1105 w, sh, 1078 m, 1054 s, 1029 m, 1014 m, 1002 w, 978 s, 920 w, 872 s, 854 s, 830 vw, 708 s, 695 m, sh, 680 w, 626 vw, 618 vw, 530 vw, 495 w, 403 w cm−1. ESI MS: 329 ([M + Na]+). HR ESI MS: calcd for C14H10O5NClNa 330.01397; found 330.01367.
(((4-Nitrophenyloxy)carbonyl)oxy)(phenyl)methyl Pivalate (8)
Compound 7 (100 mg, 0.325 mmol) and mercury pivalate (157 mg, 0.390 mmol, 1.2 equiv) were dissolved in anhydrous DCM (6 mL). Reaction mixture was stirred under inert atmosphere at rt overnight (16 h). DCM (10 mL) was added, and reaction mixture was washed with satd NaHCO3 (10 mL) and brine (10 mL), the organic phase was dried over MgSO4, and DCM was evaporated. The product 8 (115 mg, 95%) was used in the next step without purification. 1H NMR (400 MHz, CDCl3): δ 1.28 (9H, s), 7.38–7.43 (2H, m), 7.44–7.50 (3H, m), 7.57–7.60 (1H, m), 7.61 (1H, s), 8.23–8.33 (2H, m). 13C NMR (101 MHz, CDCl3): δ 27.02 (3C), 39.11, 93.80, 121.86 (2C), 125.47 (2C), 126.84 (2C), 128.97 (2C), 130.48, 134.39, 145.69, 150.73, 155.32, 176.44. IR (CHCl3): 3118 w, 3087 w, 3072 w, 3031 m, 2980 m, 2875 w, 1775 vs, 1747 s, 1618 m, 1595 m, 1529 vs, 1493 s, 1480 m, 1459 m, 1399 m, 1365 m, 1349 vs, 1279 vs, 1248 vs, 1165 s, 1123 vs, 1112 s, sh, 1030 s, 1014 m, 1003 m, 970 s, br, 943 s, 918 m, 865 s, 860 s, 832 w, 697 s, 682 w, 633 w, 619 vw, 530 vw, 495 w, 403 vw cm−1. ESI MS: 396 ([M + Na]+). HR ESI MS: calcd for C19H19O7NNa 396.10537; found 396.10546.
2-(((4-Nitrophenyloxy)carbonyl)oxy)propan-2-yl Pivalate (11)
2-Chloropropan-2-yl (4-nitrophenyl) carbonate 1059 (300 mg, 1.16 mmol) was dissolved in anhydrous DCM (15 mL). Mercury pivalate (559 mg, 1.39 mmol, 1.2 equiv) was added, and the reaction mixture was stirred overnight (20 h) at rt under inert atmosphere. The solid precipitate (HgCl2) was removed by filtration, DCM (15 mL) was added, and organic phase was washed with satd NaHCO3 (15 mL) and satd NaCl (15 mL), dried over MgSO4, solvent was removed under reduced pressure, and the product 11 was obtained as a light-yellow oil (301 mg, 80%). 1H NMR (400 MHz, CDCl3): δ 1.20 (9H, s), 1.91 (6H, s), 7.34–7.40 (2H, m), 8.24–8.30 (2H, m). 13C NMR (101 MHz, CDCl3): δ 25.28 (2C), 27.02 (3C), 39.59, 107.71, 121.98 (2C), 125.40 (2C), 145.50, 149.07, 155.41, 175.97. IR (CHCl3): 3031 w, 2976 w, 2875 w, 1777 m, 1736 m, 1618 w, 1595 w, 1528 m-s, 1493 m, 1481 w, 1439 w, 1396 w, 1376 w, 1349 m, 1322 w, 1264 m, 1191 m, 1112 vs, 1094 s, sh, 1030 w, 980 w, 859 m, 682 vw, 491 vw cm−1. ESI MS: 348 ([M + Na]+). HR ESI MS: calcd for C15H19O7NNa 348.10537; found 348.10543.
Isopropyl 6-Diazo-5-oxo-2-((((pivaloyloxy)methoxy)-carbonyl)amino)hexanoate (13a)
Compound 4a (320 mg, 1.17 mmol) was suspended in anhydrous DCM (6 mL). The reaction mixture was cooled to 0 °C, and compound 1247 (250 mg, 1.17 mmol, 1 equiv) in anhydrous DCM (3 mL) was added dropwise. The mixture was stirred for 15 min at 0 °C and then 2 h at rt. Solvent was removed under reduced pressure, and column chromatography of the residue (EtOAc/hexane 1:2, Rf 0.21) yielded the desired compound 13a (175 mg, 40%) as yellow oil. 1H NMR (400 MHz, CDCl3): δ 1.19 (9H, s), 1.23 (3H, d, J = 6.2), 1.24 (3H, d, J = 6.2), 1.90–2.05 (1H, m), 2.14–2.25 (1H, m), 2.31–2.51 (2H, m), 4.28 (1H, td, J = 8.2, 4.7), 5.03 (1H, hept, J = 6.2), 5.27 (1H, bs), 5.65 (1H, d, J = 8.1), 5.69 (1H, d, J = 5.7), 5.73 (1H, d, J = 5.7). 13C NMR (101 MHz, CDCl3): δ 21.79, 21.81, 26.97, 36.34, 38.86, 53.63, 54.90, 69.75, 80.33, 154.40, 171.01, 177.51, 193.43. Optical rotation: [α]22D +13.0° (c 0.184, CHCl3). IR (CHCl3): 3424 m, 3354 w, br, 3116 w, 2984 s, 2937 m, 2875 s, 2110 vs, 1747 vs, 1730 vs, sh, 1642 s, 1512 s, 1481 m, 1466 m, 1453 m, 1377 s, 1282 s, 1182 m, 1145 s, 1105 s, 994 s, 942 m, cm−1. ESI MS: 394 ([M + Na]+). HR ESI MS: calcd for C16H25O7N3Na 394.15847; found 394.15855.
Isopropyl 6-Diazo-5-oxo-2-(((1-(pivaloyloxy)ethoxy)-carbonyl)amino)hexanoate (13b)
This compound was prepared according to a procedure previously described in detail.47 Yields and 1H NMR and 13C NMR spectra were in agreement with the published data.
Isopropyl 6-Diazo-2-(((2-methyl-1-(pivaloyloxy)propoxy)-carbonyl)amino)-5-oxohexanoate (13c)
Compound 4c (399 mg, 1.27 mmol, 0.9 equiv) was suspended in anhydrous DCM (7 mL). The reaction mixture was cooled to 0 °C, and compound 1247 (300 mg, 1.41 mmol, 1 equiv) in anhydrous DCM (3 mL) was added dropwise. The mixture was stirred for 15 min at 0 °C and then 2 h at rt. Solvent was removed under reduced pressure, and column chromatography of the residue (EtOAc/hexane 1:2, Rf 0.30) yielded 13c (285 mg, 54%) as yellow oil (mixture of two stereoisomers 1:1). 1H NMR (400 MHz, CDCl3, first stereoisomer): δ 0.94 (6H, d, J = 6.8), 1.16 (9H, s), 1.23 (6H, t, J = 6.3), 1.83–2.50 (4H, m), 4.22–4.31 (1H, m), 5.02 (1H, hept, J = 6.8), 5.29 (1H, bs), 5.48 (1H, d, J = 8.3), 6.52 (1H, d, J = 4.9). 13C NMR (101 MHz, CDCl3, first stereoisomer): δ 16.40, 16.54, 27.00, 28.05, 31.87, 36.29, 38.96, 53.38, 54.82, 69.64, 94.21, 154.28, 171.31, 176.56, 193.87. 1H NMR (400 MHz, CDCl3, second stereoisomer): δ 0.93 (6H, d, J = 6.8), 1.18 (9H, s), 1.22 (6H, t, J = 6.3), 1.83–2.50 (4H, m), 4.22–4.31 (1H, m), 5.00 (1H, sept, J = 6.8), 5.37 (1H, bs), 5.45 (1H, d, J = 8.3), 6.48 (1H, d, J = 4.9). 13C NMR (101 MHz, CDCl3, second stereoisomer): δ 16.37, 16.54, 26.98, 27.74, 31.91, 36.55, 38.92, 53.54, 54.82, 69.66, 93.87, 154.22, 171.17, 176.81, 193.58. Optical rotation: [α]22D +11.5° (c 0.261, CHCl3). IR (CHCl3): 3428 m, 3116 w, 2982 s, 2936 m, 2878 m, 2110 vs, 1741 vs, br, 1731 vs, sh, 1641 s, 1508 s, 1480 m, 1463 m, 1400 m, sh, 1385 s, sh, 1377 s, 1365 s, sh, 1281 s, 1231 s, 1183 m, 1146 s, 1105 s, 990 s, 941 m cm−1. ESI MS: 436 ([M + Na]+). HR ESI MS: calcd for C19H31O7N3Na 436.20542; found 436.20553.
Isopropyl 6-Diazo-5-oxo-2-(((phenyl(pivaloyloxy)methoxy)-carbonyl)amino)hexanoate (13d)
Compound 8 (817 mg, 2.19 mmol) was dissolved in anhydrous DMF (15 mL). Compound 1247 (700 mg, 3.18 mmol, 1.5 equiv) in anhydrous DMF (7 mL) was added dropwise. Reaction mixture was stirred at rt under inert atmosphere for 5 h. DMF was evaporated, and column chromatography of the residue (EtOAc/hexane 1:2, Rf 0.18) yielded product 13d (648 mg, 66%) as a light-yellow oil (mixture of two stereoisomers 1:1). 1H NMR (400 MHz, CDCl3): δ 1.17–1.31 (15H, m), 1.90–2.06 (1H, m), 2.12–2.31 (1H, m), 2.31–2.54 (2H, m), 4.27–4.36 (1H, m), 5.03 (1H, hept, J = 6.3), 5.29 (1H, bs), 5.59 (1H, d, J = 8.1), 7.36–7.42 (3H, m), 7.46–7.51 (2H, m), 7.61 (s, 1H). 13C NMR (101 MHz, CDCl3): δ 21.82 (2C), 26.99 (3C), 27.70, 36.35, 38.99, 53.55, 54.90, 69.79, 90.93, 126.57 (2C), 128.66 (2C), 129.64, 135.95, 153.82, 171.10, 176.33, 193.51. Optical rotation: [α]22D +12.5° (c 0.246, CHCl3). IR (CHCl3): 3425 w, 3116 w, 3098 vw, 3070 vw, 3029 m, 2984 m, 2937 m, 2875 w, 2110 s, 1735 vs, br, 1641 s, 1590 w, 1507 s, 1480 m, 1457 m, 1398 m, 1377 s, 1367 s, sh, 1366 s, sh, 1280 s, 1182 m, 1146 s, sh, 1133 s, 1105 s, 1085 m, 1057 s, 1027 s, 1003 m, 942 m, 918 w, 697 m, 619 vw cm−1. ESI MS: 470 ([M + Na]+). HR ESI MS: calcd for C22H29O7N3Na 470.18977; found 470.18985.
Isopropyl 6-Diazo-5-oxo-2-((((2-(pivaloyloxy)propan-2-yl)-oxy)carbonyl)amino)hexanoate (13e)
Compound 11 (936 mg, 2.88 mmol) was dissolved in anhydrous DMF (18 mL), and the reaction mixture was cooled to 0 °C. Compound 1247 (920 mg, 4.32 mmol, 1.5 equiv) in anhydrous DMF (6 mL) was added dropwise. Reaction mixture was stirred at 0 °C under inert atmosphere for 5 h. DMF was evaporated, and column chromatography of the residue (EtOAc/hexane 1:2, Rf 0.22) yielded product 13e (955 mg, 83%) as a light-yellow oil. 1H NMR (400 MHz, CDCl3): δ 1.19 (9H, s), 1.23 (3H, d, J = 6.3), 1.24 (3H, d, J = 6.3), 1.80 (3H, s), 1.83 (3H, s), 1.90–2.01 (1H, m), 2.14–2.27 (1H, m), 2.29–2.51 (2H, m), 4.24 (1H, dt, J = 8.3, 4.7), 5.05 (1H, hept, J = 6.3), 5.31 (1H, bs), 5.44 (1H, d, J = 8.2). 13C NMR (101 MHz, CDCl3): δ 21.80, 21.82, 25.76, 25.91, 27.07 (3C), 27.79, 36.46, 39.48, 53.26, 54.87, 69.59, 105.44, 153.16, 171.31, 176.21, 193.63. Optical rotation: [α]22D +12.8° (c 0.133, CHCl3). IR (CHCl3): 3430 w, 3116 w, 2984 m, 2936 m, 2874 m, 2110 s, 1732 vs, br, 1641 m, 1502 s, 1481 m, 1466 m, 1462 m, 1455 m, 1452 m, 1397 m, sh, 1384 s, 1374 s, 1365 s, sh, 1198 s, 1184 s, 1147 m, sh, 1128 s, 1112 s, 1105 s, 1045 m, 942 w cm−1. ESI MS: 329 ([M + Na]+). HR ESI MS: calcd for C18H29O7N3Na 422.18977; found 422.18982.
EcoHIV-Infected Mice
All mouse efficacy studies were conducted in full compliance with NIH guidelines and with the approval of the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai. Male C57BL/6J mice at 5 weeks old (The Jackson Laboratory, Bar Harbor, ME) were maintained on a 12 h light–dark cycle with ad libitum access to food and water. EcoHIV chimeric virus was generated by transfecting HEK293T cells with plasmid DNA containing the EcoHIV construct, specifically EcoNDK containing the V5C5 fragment of gp120.42 EcoHIV was then collected from the culture media, concentrated by centrifugation, and titered by p24 ELISA (Advanced Biosciences Laboratory, Rockville, MD). Each mouse was randomized to begin 14 (1 mg/kg, ip, qod) or saline treatment 1 day prior to inoculation with either EcoHIV (2 × 106 pg p24, ip) or sham inoculation with PBS (n = 8/group) as previously described.65 The dose of 14 was chosen based on several published efficacy studies in CNS models including multiple sclerosis,66 brain cancer,47 and sindbus virus62,67,68 as well as our lab’s mouse pharmacokinetic studies showing μM brain levels of 14 after 1 mg/kg systemic dosing.69 On day 30 postinoculation (p.i.), having allowed time for stable virus infection and emergence of cognitive deficits, all mice began behavioral training and testing.
Radial Arm Water Maze
The radial arm water maze (RAWM) test was administered in a pool of opaque water containing six swimming lanes and a hidden platform with visual cues essentially as described.70 The visual cues consisted of both two- and three-dimensional objects that were affixed to the side of the maze pool using clear packing tape. The two-dimensional cues were six different brightly colored 8½ × 11 sheets of paper each printed with a different black shape (square, triangle, star, etc.), and the three-dimensional cues were commonly found laboratory items (funnel, culture flask, pipet tip box, etc.). Briefly, RAWM consisted of four learning trials (LT) of 60 s and one post-training 60 s retention test (RT) administered after 30 min rest every day until test completion. Testing was considered complete when control mice reached asymptotic performance of one error or fewer in finding the hidden platform on trials LT4 and RT. Errors for the last 3 days of testing for all groups were then averaged and used for statistical analysis. The hidden platform was rotated randomly to a different arm each test day to ensure that mice used working memory to locate the platform. Each of the training trials began by placing a mouse randomly into one of the six swimming arms and allowing the mouse to swim for 60 s to find the hidden platform, during which time the number of errors (entering an arm without the platform and/or 20 s of immobility) and latency to locate the hidden platform were recorded. The retention test was performed in the same manner as the learning trials. The hidden platform tests were followed by measuring the latency it took for treated and control mice to find a visible platform in the same context, as a control for possible effects of treatment on animal vision, motivation, or ability to swim to the platform.
Viral Load
After completion of behavioral testing, all mice were sacrificed by carbon dioxide asphyxiation after brief isoflurane anesthesia. Spleen and brain were harvested, DNA was isolated, and viral load was measured as previously described.65,71–73 Briefly, total DNA was isolated from mouse tissues by sequentially homogenizing in Trizol, mixing with DNAzol, precipitating with ethanol, washing, and treating with NaOH. Quantitative PCR (qPCR) was then conducted to detect EcoHIV/NDK DNA from the gag gene region using Taqman chemistry with MGB probes with forward primer 5′-TGGGACCACAGGCTACACTAGA-3′, reverse primer 5′-CAGCCAAAACTCTTGCTTTATGG-3′, and probe 5′-TGATGACAGCATGCCAGGGAGTGG-3′ (ThermoFisher Scientific). A standard curve for quantitation of copy number was constructed using graded numbers of a plasmid containing the EcoNDK gag amplicon. Copy number was then normalized to cell count by amplification of murine gapdh (Mm99999915_g1). All qPCR amplification was performed in an Applied Biosystems 7500 instrument.
In Vitro Metabolic Stability
Metabolic stability of prodrugs were evaluated as previously described.47 Briefly, prodrugs (10 μM) were spiked in mouse, swine, and human plasma or swine brain homogenate and incubated in each matrix in an orbital shaker at 37 °C. Relative prodrug amounts at 0 and 60 min for plasma and 0, 30, and 60 min for brain were measured using liquid chromatography and tandem mass spectrometry (LC–MS/MS) by removing 100 μL aliquots of each mixture in triplicate and quenching each reaction by addition of three times the volume of ice-cold acetonitrile spiked with the internal standard (losartan 5 μM). The samples were vortexed for 30 s and centrifuged at 12000g for 10 min. Then 50 μL of each supernatant was diluted with 50 μL of water and transferred to a 250 μL polypropylene vial sealed with a Teflon cap. Prodrug detection was performed on a Thermo Scientific Accela UPLC system coupled to Accela open autosampler on an Agilent C18 (100 mm × 2.1 mm i.d.) UPLC column. The autosampler was temperature controlled and was operated at 10 °C. The mobile phase used for the chromatographic separation was composed of acetonitrile/water containing 0.1% formic acid and flow rate of 0.5 mL/min for 4.5 min using gradient elution. The column effluent was monitored using TSQ Vantage triple-quadrupole mass-spectrometric detector, equipped with an electrospray probe set in the positive ionization mode. Samples were introduced into the ionization source through a heated nebulized probe (350 °C). Relative prodrug amounts were measured from ratio of peak areas of analyte to IS; percent remaining was calculated by normalizing this value at 60 min to the value obtained at 0 min.
Protein Binding
The free 14 fraction (fu) in swine plasma and swine brain homogenate was determined using a previously described ultrafiltration method.74 Briefly, the 14 (10 μM) spiked plasma and/or brain homogenate was incubated at 37 °C for 30 min, following which the samples were loaded onto an ultrafiltration column tube (Corning Spin-X UF with a MW cut off of 30 kDa), whose base is impermeable to the plasma and brain proteins. The samples were centrifuged for 45 min at 37 °C and 4000 rpm (1800g). The concentrations were determined in both the filtrate and plasma before filtration, following extraction and quantified via LC-MS/MS. Fraction unbound was calculated from the equation Cu/Ct × 100, where Cu was the concentration in the ultrafiltrate and Ct was the concentration in the plasma or brain homogenate prior to filtration.
In Vivo Pharmacokinetics
Swine studies were conducted under a protocol approved by the Johns Hopkins Animal Care and Use Committee. Adult, female Göttingen × Yucutan miniature swine (Massachusetts General Hospital, MA) were housed in Johns Hopkins University facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in compliance with the Animal Welfare Act, Animal Welfare Regulations, and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. Animals were maintained on a 14 h light and 10 h dark schedule, and provided ad libitum water and a commercial miniswine diet (Teklad, Madison, WI) with environmental enrichment (fruit/vegetables) twice daily. Animals were individually housed while on study in order to monitor behavior and clinical health following drug administration. Whole blood for drug pharmacokinetic evaluation was collected from a dual lumen central venous catheter (CVC) implanted in the external jugular vein prior to study initiation. Animals were anesthetized with a combination of ketamine hydrochloride (20–30 mg/kg, im) and xylazine (2 mg/kg, im), intubated, and maintained under isoflurane (1–2%) inhalant anesthesia. A temporary peripheral saphenous vein catheter was placed in the hind limb to allow for anatomical separation of drug infusion and whole blood sampling via CVC. 14, 13b, or 13d were dissolved in a sterile saline solution containing 5% ethanol and 5% Tween 80 prior to iv infusion via saphenous vein catheter over 1 h (1 mL/min) for a final dose of 1.6 mg/kg or molar equivalent administered at 1 mL/kg (n = 1/dose). Blood samples (1 mL) were taken from CVC at predose, 5, 15, 30, 45, and 60 min. Plasma was separated by low speed centrifugation at 3000g for 10 min at 4 °C. CSF was obtained from the cisterna magna using a 3.5 in. × 22 gauge spinal needle (Becton Dickinson Health Care, Franklin Lakes, New Jersey, USA) at 60 min postdose. All samples were flash frozen upon harvest and stored at –80°C until bioanalysis.
In a separate study, the above dosing procedure was repeated except that the animals (n = 2/dose) were euthanized at 60 min postinfusion using Euthasol (Virbac Animal Health, Fort Worth, TX, USA) containing pentobarbital sodium and phenytoin sodium at a dose of 1 mL per 10 lbs of body weight and plasma and brain tissues were harvested. To obtain brain tissues, a horizontal incision connecting both orbits was extended toward the base of the ears and skin, and subcutaneous tissue over the swine skull were removed. A reciprocating saw was used to cut through the frontal aspect of the calvarium to create a window for intracranial access. Next, the dura mater was cut and a 2 cm × 2 cm sample of full thickness frontal cortex was obtained. All samples were flash frozen and stored at –80 °C until bioanalysis.
Bioanalysis
Quantitation of 14 in plasma, CSF, and brain homogenate by LC-MS/MS was performed as previously described.47 Briefly, 14 was extracted from plasma, CSF, and brain samples with methanol containing glutamate-d5 (10 μM ISTD) by vortexing followed by centrifugation 16000g for 5 min. Supernatants were aliquoted and dried at 45 °C for under vacuum for 1 h. Sodium bicarbonate buffer (0.2M, pH 9.0) and dabsyl chloride (10 mM) in acetone were added to each tube, mixed, and incubated for 15 min at 60 °C to derivatize. Samples were then injected and separated on an Agilent 1290 equipped with an Agilent Eclipse plus C18 RRHD 2.1 mm × 100 mm column over a 2.5 min gradient from 20 to 95% acetonitrile +0.1% formic acid and quantified on an Agilent 6520 QTOF mass spectrometer. Peak area ratio of the analyte to the internal standard was plotted against a 14 standard curve to yield 14 concentrations for each sample.
Pharmacokinetic and Statistical Analysis
Area under the curve (AUC) was calculated by log-linear trapezoidal rule to the end of sample collection by noncompartmental analysis module in WinNonlin (version 5.3, Certara, St. Louis, MO). cLogPs were calculated using ChemDraw Professional (PerkinElmer, Waltham, MA). The half-life (t1/2) of prodrug metabolic stability from brain homogenates was estimated using the first-order equation t1/2 = 0.693/Kel, where Kel (elimination rate constant) is the slope of linear regression from natural log percentage substrate remaining versus incubation time.75 For all studies, group means and standard errors were calculated and used for statistical comparisons where appropriate. Differences in radial arm water maze errors and latencies between groups were assessed by two-way ANOVA (treatment × trial) with posthoc Tukey test. Viral load in each tissue was compared between treatment groups by t test. Prodrug half-lives were compared by one-way ANOVA. For all tests, significance was defined as p < 0.05.
Supplementary Material
ABBREVIATIONS
- DON
6-diazo-5-oxo-L-norleucine
- HAND
HIV-associated neurocognitive disorders
- cART
combined antiretroviral therapy
- HAD
HIV-associated dementia
- CNS
central nervous system
- MRS
magnetic resonance spectroscopy
- CSF
cerebrospinal fluid
- GI
gastrointestinal
- cLogP
calculated partition coefficient
- DCM
dichloromethane
- DMF
dimethylformamide
- RAWM
radial arm water maze
- LT
learning trial
- RT
retention trial
- AUC
area under the curve
- POM
pivaloyloxymethyl
- ELISA
enzyme-linked immunosorbent assay
- pi
post-inoculation
- iv
intravenous
- ip
intraperitoneal
- qPCR
quantitative polymerase chain reaction
- CVC
central venous catheter
- LC
liquid chromotagraphy
- MS
mass spectrometry
- ESI
electrospray ionization
Footnotes
ORCID
Rana Rais: 0000-0003-4059-2453
Barbara S. Slusher: 0000-0001-9814-4157
Notes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.7b00966.
Representative chromatogram showing metabolism of 13d in mouse plasma (PDF)
Molecular formula strings with ex vivo and in vivo metabolism data (CSV)
References
- 1.Zink MC. Translational research models and novel adjunctive therapies for neuroAIDS. J Neuroimmune Pharmacol. 2007;2:14–19. doi: 10.1007/s11481-006-9062-y. [DOI] [PubMed] [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25:625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J NeuroVirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 6.Potter MC, Figuera-Losada M, Rojas C, Slusher BS. Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2013;8:594–607. doi: 10.1007/s11481-013-9442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vazquez-Santiago FJ, Noel RJ, Jr, Porter JT, Rivera-Amill V. Glutamate metabolism and HIV-associated neurocognitive disorders. J NeuroVirol. 2014;20:315–331. doi: 10.1007/s13365-014-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi A, Iijima K, Kano S, Ishizaka Y. Viral protein R of HIV type-1 induces retrotransposition and upregulates glutamate synthesis by the signal transducer and activator of transcription 1 signaling pathway. Microbiol Immunol. 2015;59:398–409. doi: 10.1111/1348-0421.12266. [DOI] [PubMed] [Google Scholar]
- 9.Erdmann N, Tian C, Huang Y, Zhao J, Herek S, Curthoys N, Zheng J. In vitro glutaminase regulation and mechanisms of glutamate generation in HIV-1-infected macrophage. J Neurochem. 2009;109:551–561. doi: 10.1111/j.1471-4159.2009.05989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdmann N, Zhao J, Lopez AL, Herek S, Curthoys N, Hexum TD, Tsukamoto T, Ferraris D, Zheng J. Glutamate production by HIV-1 infected human macrophage is blocked by the inhibition of glutaminase. J Neurochem. 2007;102:539–549. doi: 10.1111/j.1471-4159.2007.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erdmann NB, Whitney NP, Zheng J. Potentiation of excitotoxicity in HIV-1 associated dementia and the significance of glutaminase. Clin Neurosci Res. 2006;6:315–328. doi: 10.1016/j.cnr.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Zhao L, Jia B, Wu L, Li Y, Curthoys N, Zheng JC. Glutaminase dysregulation in HIV-1-infected human microglia mediates neurotoxicity: relevant to HIV-1-associated neurocognitive disorders. J Neurosci. 2011;31:15195–15204. doi: 10.1523/JNEUROSCI.2051-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas AG, O’Driscoll CM, Bressler J, Kaufmann W, Rojas CJ, Slusher BS. Small molecule glutaminase inhibitors block glutamate release from stimulated microglia. Biochem Biophys Res Commun. 2014;443:32–36. doi: 10.1016/j.bbrc.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian C, Erdmann N, Zhao J, Cao Z, Peng H, Zheng J. HIV-infected macrophages mediate neuronal apoptosis through mitochondrial glutaminase. J Neurochem. 2008;105:994–1005. doi: 10.1111/j.1471-4159.2007.05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian C, Sun L, Jia B, Ma K, Curthoys N, Ding J, Zheng J. Mitochondrial glutaminase release contributes to glutamate-mediated neurotoxicity during human immunodeficiency virus-1 infection. J Neuroimmune Pharmacol. 2012;7:619–628. doi: 10.1007/s11481-012-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu B, Huang Y, Braun AL, Tong Z, Zhao R, Li Y, Liu F, Zheng JC. Glutaminase-containing microvesicles from HIV-1-infected macrophages and immune-activated microglia induce neurotoxicity. Mol Neurodegener. 2015;10:61. doi: 10.1186/s13024-015-0058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, Qian G, Zheng JC. IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem. 2013;125:897–908. doi: 10.1111/jnc.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao L, Huang Y, Tian C, Taylor L, Curthoys N, Wang Y, Vernon H, Zheng J. Interferon-alpha regulates glutaminase 1 promoter through STAT1 phosphorylation: relevance to HIV-1 associated neurocognitive disorders. PLoS One. 2012;7:e32995. doi: 10.1371/journal.pone.0032995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Huang Y, Zheng J. STAT1 regulates human glutaminase 1 promoter activity through multiple binding sites in HIV-1 infected macrophages. PLoS One. 2013;8:e76581. doi: 10.1371/journal.pone.0076581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musante V, Summa M, Neri E, Puliti A, Godowicz TT, Severi P, Battaglia G, Raiteri M, Pittaluga A. The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cereb Cortex. 2010;20:1974–1984. doi: 10.1093/cercor/bhp274. [DOI] [PubMed] [Google Scholar]
- 21.Anderson ER, Boyle J, Zink WE, Persidsky Y, Gendelman HE, Xiong H. Hippocampal synaptic dysfunction in a murine model of human immunodeficiency virus type 1 encephalitis. Neuroscience. 2003;118:359–369. doi: 10.1016/s0306-4522(02)00925-9. [DOI] [PubMed] [Google Scholar]
- 22.Anderson ER, Gendelman HE, Xiong H. Memantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitis. J Neurosci. 2004;24:7194–7198. doi: 10.1523/JNEUROSCI.1933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behnisch T, Francesconi W, Sanna PP. HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1012:187–189. doi: 10.1016/j.brainres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 24.Dong J, Xiong H. Human immunodeficiency virus type 1 gp120 inhibits long-term potentiation via chemokine receptor CXCR4 in rat hippocampal slices. J Neurosci Res. 2006;83:489–496. doi: 10.1002/jnr.20745. [DOI] [PubMed] [Google Scholar]
- 25.Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keblesh JP, Dou H, Gendelman HE, Xiong H. 4-Aminopyridine improves spatial memory in a murine model of HIV-1 encephalitis. J Neuroimmune Pharmacol. 2009;4:317–327. doi: 10.1007/s11481-009-9161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li ST, Matsushita M, Moriwaki A, Saheki Y, Lu YF, Tomizawa K, Wu HY, Terada H, Matsui H. HIV-1 Tat inhibits long-term potentiation and attenuates spatial learning [corrected] Ann Neurol. 2004;55:362–371. doi: 10.1002/ana.10844. [DOI] [PubMed] [Google Scholar]
- 28.Tang H, Lu D, Pan R, Qin X, Xiong H, Dong J. Curcumin improves spatial memory impairment induced by human immunodeficiency virus type 1 glycoprotein 120 V3 loop peptide in rats. Life Sci. 2009;85:1–10. doi: 10.1016/j.lfs.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014;28:1579–1591. doi: 10.1097/QAD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bairwa D, Kumar V, Vyas S, Das BK, Srivastava AK, Pandey RM, Sharma SK, Jagannathan NR, Sinha S. Case control study: magnetic resonance spectroscopy of brain in HIV infected patients. BMC Neurol. 2016;16:99. doi: 10.1186/s12883-016-0628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010;32:1045–1053. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohamed MA, Barker PB, Skolasky RL, Selnes OA, Moxley RT, Pomper MG, Sacktor NC. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magn Reson Imaging. 2010;28:1251–1257. doi: 10.1016/j.mri.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sailasuta N, Shriner K, Ross B. Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients. NMR Biomed. 2009;22:326–331. doi: 10.1002/nbm.1329. [DOI] [PubMed] [Google Scholar]
- 34.Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, Jarvik JG, Miller EN, Singer EJ, Ellis RJ, Kolson DL, Simpson D, Nath A, Berger J, Shriver SL, Millar LL, Colquhoun D, Lenkinski R, Gonzalez RG, Lipton SA. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21:1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- 35.Stankoff B, Tourbah A, Suarez S, Turell E, Stievenart JL, Payan C, Coutellier A, Herson S, Baril L, Bricaire F, Calvez V, Cabanis EA, Lacomblez L, Lubetzki C. Clinical and spectroscopic improvement in HIV-associated cognitive impairment. Neurology. 2001;56:112–115. doi: 10.1212/wnl.56.1.112. [DOI] [PubMed] [Google Scholar]
- 36.Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, Masliah E, Commins DL, Brandt D, Grant I, Singer EJ, Levine AJ, Miller J, Winkler JM, Fox HS, Luxon BA, Morgello S. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One. 2012;7:e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH, Jr, Soukup VM. Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. J Neuroimmune Pharmacol. 2012;7:686–700. doi: 10.1007/s11481-012-9345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borjabad A, Morgello S, Chao W, Kim SY, Brooks AI, Murray J, Potash MJ, Volsky DJ. Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog. 2011;7:e1002213. doi: 10.1371/journal.ppat.1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conti F, Minelli A. Glutamate immunoreactivity in rat cerebral cortex is reversibly abolished by 6-diazo-5-oxo-L-norleucine (DON), an inhibitor of phosphate-activated glutaminase. J Histochem Cytochem. 1994;42:717–726. doi: 10.1177/42.6.7910617. [DOI] [PubMed] [Google Scholar]
- 40.Chung SH, Johnson MS. Studies on sound-induced epilepsy in mice. Proc R Soc London, Ser B. 1984;221:145–168. doi: 10.1098/rspb.1984.0028. [DOI] [PubMed] [Google Scholar]
- 41.Zhao J, Lopez AL, Erichsen D, Herek S, Cotter RL, Curthoys NP, Zheng J. Mitochondrial glutaminase enhances extracellular glutamate production in HIV-1-infected macrophages: linkage to HIV-1 associated dementia. J Neurochem. 2004;88:169–180. doi: 10.1046/j.1471-4159.2003.02146.x. [DOI] [PubMed] [Google Scholar]
- 42.Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, Belem P, Sharer L, Brooks AI, Volsky DJ. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci U S A. 2005;102:3760–3765. doi: 10.1073/pnas.0500649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He H, Sharer LR, Chao W, Gu CJ, Borjabad A, Hadas E, Kelschenbach J, Ichiyama K, Do M, Potash MJ, Volsky DJ. Enhanced human immunodeficiency virus Type 1 expression and neuropathogenesis in knockout mice lacking Type I interferon responses. J Neuropathol Exp Neurol. 2014;73:59–71. doi: 10.1097/NEN.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Earhart RH, Amato DJ, Chang AY, Borden EC, Shiraki M, Dowd ME, Comis RL, Davis TE, Smith TJ. Phase II trial of 6-diazo-5-oxo-L-norleucine versus aclacinomycin-A in advanced sarcomas and mesotheliomas. Invest New Drugs. 1990;8:113–119. doi: 10.1007/BF00216936. [DOI] [PubMed] [Google Scholar]
- 45.Magill GB, Myers WP, Reilly HC, Putnam RC, Magill JW, Sykes MP, Escher GC, Karnofsky DA, Burchenal JH. Pharmacological and initial therapeutic observations on 6-diazo-5-oxo-1-norleucine (DON) in human neoplastic disease. Cancer. 1957;10:1138–1150. doi: 10.1002/1097-0142(195711/12)10:6<1138::aid-cncr2820100608>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 46.Rahman A, Smith FP, Luc PT, Woolley PV. Phase I study and clinical pharmacology of 6-diazo-5-oxo-L-norleucine (DON) Invest New Drugs. 1985;3:369–374. doi: 10.1007/BF00170760. [DOI] [PubMed] [Google Scholar]
- 47.Rais R, Jancarik A, Tenora L, Nedelcovych M, Alt J, Englert J, Rojas C, Le A, Elgogary A, Tan J, Monincova L, Pate K, Adams R, Ferraris D, Powell J, Majer P, Slusher BS. Discovery of 6-Diazo-5-oxo-l-norleucine (DON) prodrugs with enhanced CSF delivery in monkeys: a potential treatment for glioblastoma. J Med Chem. 2016;59:8621–8633. doi: 10.1021/acs.jmedchem.6b01069. [DOI] [PubMed] [Google Scholar]
- 48.Rasheed A, Kumar CKA. Novel approaches on prodrug based drug design. Pharm Chem J. 2008;42:677–686. [Google Scholar]
- 49.Li F, Maag H, Alfredson T. Prodrugs of nucleoside analogues for improved oral absorption and tissue targeting. J Pharm Sci. 2008;97:1109–1134. doi: 10.1002/jps.21047. [DOI] [PubMed] [Google Scholar]
- 50.Fattash B, Karaman R. Chemical approaches used in prodrugs design. In: Karaman R, editor. Prodrugs Design – A New Era ; Pharmacology—Research, Safety Testing and Regulation. Nova Science Publishers Inc.; New York: 2014. pp. 103–138. [Google Scholar]
- 51.Liederer BM, Borchardt RT. Enzymes involved in the bioconversion of ester-based prodrugs. J Pharm Sci. 2006;95:1177–1195. doi: 10.1002/jps.20542. [DOI] [PubMed] [Google Scholar]
- 52.Rautio J, Laine K, Gynther M, Savolainen J. Prodrug approaches for CNS delivery. AAPS J. 2008;10:92–102. doi: 10.1208/s12248-008-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlieghe P, Khrestchatisky M. Medicinal chemistry based approaches and nanotechnology-based systems to improve CNS drug targeting and delivery. Med Res Rev. 2013;33:457–516. doi: 10.1002/med.21252. [DOI] [PubMed] [Google Scholar]
- 54.Fu J, Pacyniak E, Leed MGD, Sadgrove MP, Marson L, Jay M. Interspecies differences in the metabolism of a multiester prodrug by carboxylesterases. J Pharm Sci. 2016;105:989–995. doi: 10.1002/jps.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Gelder J, Shafiee M, De Clercq E, Penninckx F, Van den Mooter G, Kinget R, Augustijns P. Species-dependent and site-specific intestinal metabolism of ester prodrugs. Int J Pharm. 2000;205:93–100. doi: 10.1016/s0378-5173(00)00507-x. [DOI] [PubMed] [Google Scholar]
- 56.Suenderhauf C, Parrott N. A physiologically based pharmacokinetic model of the minipig: data compilation and model implementation. Pharm. Res. 2013;30:1–15. doi: 10.1007/s11095-012-0911-5. [DOI] [PubMed] [Google Scholar]
- 57.Gallop MA, Yao F, Ludwikow MJ, Phan T, Peng G. Acyloxyalkyl Carbamate Prodrugs, Methods of Synthesis and Use. WO Pat Appl. 2005;2005019163:A2. [Google Scholar]
- 58.Gallop MA, Xu F, Phan T, Dilip U, Peng G. Acyloxyalkyl Carbamate Prodrugs, Methods of Synthesis and Use. WO Pat Appl. 2008;2008033572:A1. [Google Scholar]
- 59.Gallop M, Cundy K, Zhou C, Yao F, Xiang JN, Ollman I, Qiu F. Prodrugs of GABA Analogs, Compositions and Uses Thereof. US Pat Appl. 2006;29361:A1. 2006/02. [Google Scholar]
- 60.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee CF, Lo YC, Cheng CH, Furtmuller GJ, Oh B, Andrade-Oliveira V, Thomas AG, Bowman CE, Slusher BS, Wolfgang MJ, Brandacher G, Powell JD. Preventing allograft rejection by targeting immune metabolism. Cell Rep. 2015;13:760–770. doi: 10.1016/j.celrep.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baxter VK, Glowinski R, Braxton AM, Potter MC, Slusher BS, Griffin DE. Glutamine antagonist-mediated immune suppression decreases pathology but delays virus clearance in mice during nonfatal alphavirus encephalomyelitis. Virology. 2017;508:134–149. doi: 10.1016/j.virol.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Annaert P, Tukker JJ, van GelderJ, Naesens L, de ClercqE, van DenMooter G, Kinget R, Augustijns P. In vitro, ex vivo, and in situ intestinal absorption characteristics of the antiviral ester prodrug adefovir dipivoxil. J Pharm Sci. 2000;89:1054–1062. doi: 10.1002/1520-6017(200008)89:8<1054::aid-jps10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 64.Masuda K, Naiki M. Method for Promoting the Synthesis of Collagen and Proteoglycan in Chondrocytes. US Pat Appl. 2013;64383:A1. 2013/01. [Google Scholar]
- 65.Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, Belem P, Sharer L, Brooks AI, Volsky DJ. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci U S A. 2005;102:3760–3765. doi: 10.1073/pnas.0500649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shijie J, Takeuchi H, Yawata I, Harada Y, Sonobe Y, Doi Y, Liang J, Hua L, Yasuoka S, Zhou Y, Noda M, Kawanokuchi J, Mizuno T, Suzumura A. Blockade of glutamate release from microglia attenuates experimental autoimmune encephalomyelitis in mice. Tohoku J Exp Med. 2009;217:87–92. doi: 10.1620/tjem.217.87. [DOI] [PubMed] [Google Scholar]
- 67.Manivannan S, Baxter VK, Schultz KL, Slusher BS, Griffin DE. Protective Effects of Glutamine Antagonist 6-Diazo-5-Oxo-l-Norleucine in Mice with Alphavirus Encephalomyelitis. J Virol. 2016;90:9251–9262. doi: 10.1128/JVI.01045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potter MC, Baxter VK, Mathey RW, Alt J, Rojas C, Griffin DE, Slusher BS. Neurological sequelae induced by alphavirus infection of the CNS are attenuated by treatment with the glutamine antagonist 6-diazo-5-oxo-l-norleucine. J NeuroVirol. 2015;21:159–173. doi: 10.1007/s13365-015-0314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alt J, Potter MC, Rojas C, Slusher BS. Bioanalysis of 6-diazo-5-oxo-l-norleucine in plasma and brain by ultra-performance liquid chromatography mass spectrometry. Biochem. 2015;474:28–34. doi: 10.1016/j.ab.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arendash GW, Gordon MN, Diamond DM, Austin LA, Hatcher JM, Jantzen P, DiCarlo G, Wilcock D, Morgan D. Behavioral assessment of Alzheimer’s transgenic mice following long-term Abeta vaccination: task specificity and correlations between Abeta deposition and spatial memory. DNA Cell Biol. 2001;20:737–744. doi: 10.1089/10445490152717604. [DOI] [PubMed] [Google Scholar]
- 71.Hadas E, Borjabad A, Chao W, Saini M, Ichiyama K, Potash MJ, Volsky DJ. Testing antiretroviral drug efficacy in conventional mice infected with chimeric HIV-1. AIDS. 2007;21:905–909. doi: 10.1097/QAD.0b013e3281574549. [DOI] [PubMed] [Google Scholar]
- 72.He H, Sharer LR, Chao W, Gu CJ, Borjabad A, Hadas E, Kelschenbach J, Ichiyama K, Do M, Potash MJ, Volsky DJ. Enhanced human immunodeficiency virus type 1 expression and neuropathogenesis in knockout mice lacking type I interferon responses. J Neuropathol Exp Neurol. 2014;73:59–71. doi: 10.1097/NEN.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelschenbach JL, Saini M, Hadas E, Gu C-J, Chao W, Bentsman G, Hong JP, Hanke T, Sharer LR, Potash MJ, Volsky DJ. Mice chronically infected with chimeric HIV resist peripheral and brain superinfection: A model of protective immunity to HIV. J Neuroimmune Pharmacol. 2012;7:380–387. doi: 10.1007/s11481-011-9316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rais R, Hoover R, Wozniak K, Rudek MA, Tsukamoto T, Alt J, Rojas C, Slusher BS. Reversible disulfide formation of the glutamate carboxypeptidase II inhibitor E2072 results in prolonged systemic exposures in vivo. Drug Metab. Dispos. 2012;40:2315–2323. doi: 10.1124/dmd.112.046821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baranczewski P, Stanczak A, Sundberg K, Svensson R, Wallin A, Jansson J, Garberg P, Postlind H. Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacol Rep. 2006;58:453–472. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.