Abstract
Telomere biology, a key component of the hallmarks of ageing, offers insight into dysregulation of normative ageing processes that accompany age-related diseases such as cancer. Telomere homeostasis is tightly linked to cellular metabolism, and in particular with mitochondrial physiology, which is also diminished during cellular senescence and normative physiological ageing. Inherent in the biochemistry of these processes is the role of magnesium, one of the main cellular ions and an essential cofactor in all reactions that use ATP. Magnesium plays an important role in many of the processes involved in regulating telomere structure, integrity and function. This review explores the mechanisms that maintain telomere structure and function, their influence on circadian rhythms and their impact on health and age-related disease. The pervasive role of magnesium in telomere homeostasis is also highlighted.
Keywords: telomeres, telomerase, TERT, mitochondria, oxidative stress, magnesium
1. Introduction
Ageing is not a simple passive degenerative process, but one regulated by distinct biochemical pathways. It has been classified by a number of common hallmarks that are shared across taxa. These can be sub-classified into primary, antagonistic, and integrative hallmarks, one of which is telomere attrition [1].
Telomeres are nucleo-protein complexes at the linear ends of vertebrate chromosomes containing (TTAGGG)n DNA repeats, which are essential for maintaining genomic integrity [2]. Telomere attrition is an integral part of the end replication problem [3,4] and acceleration of the rate of telomeric sequence loss is a feature of a plethora of non-communicable diseases [5]. Telomere shortening has been reported to accelerate in association with physical and psychological stress exposures [5]. Critical cellular telomeric shortening can trigger a persistent DNA damage response (DDR), that signals the cell to enter senescence, or undergo apoptosis, dependent upon the relative level of genomic damage the cell has accrued during its replicative lifespan [2].
Telomere shortening, however, is not a unidirectional process: stem cells, cancer cells, as well as some differentiated cells—including B and T lymphocytes—can counter telomere shortening and maintain the length of their telomeric DNA despite mitotic activity, typically through the action of the holoenzyme telomerase [6]. Approximately 90% of human cancers demonstrate up-regulation of telomerase activity [7], while most of the remaining 10% use an alternative lengthening of telomeres (ALT) mechanism [8]. This ability to preserve telomere function is necessary to confer replicative immortality, one of the hallmarks of cancer [9].
Telomere maintenance is a dynamic process, which is responsive to metabolic and endocrine mediators of cellular stress [10]. Factors that mitigate the effect of cellular stress, such as free radical scavengers and antioxidants, may thereby offer the possibility of slowing the rate of telomere attrition [11].
2. Magnesium Homeostasis and Disease
Magnesium (Mg2+) is one of the most abundant metal ions in cells and plays a variety of roles, including the stabilization of DNA, RNA, adenosine triphosphate (ATP), and other nucleotide triphosphates/dioxynucleotide triphosphates (NTPs/dNTPs) [12]. It participates in over 600 enzymatic reactions, including all those that involve ATP, and is essential for maintenance of DNA structure, cellular excitability and cellular health span [13,14,15]. Mg2+ deficiency has been implicated in many disease processes, including type 2 diabetes (T2DM) [16], Alzheimer’s disease [17], and cancer [18,19,20,21], while Mg2+ supplementation has been shown to be beneficial for blood pressure control, endothelial function, insulin sensitivity and T2DM [22,23,24]. In vitro, low [Mg2+] has also been shown to promote traits associated with senescence in endothelial cells, including slower cell growth and up-regulation of p21 (a cell cycle inhibitor) and IL-1α (a pro-inflammatory cytokine) [25]. However, cells appear to have a remarkable ability to preserve intracellular [Mg2+] in spite of a low extracellular concentration of this ion [26], or a dynamic metabolism [27]. On the other hand, intracellular [Mg2+] increases several fold during the log phase of cell growth, in spite of a constant ATP/ADP and extracellular [Mg2+], providing further evidence for the hypothesis that cellular [Mg2+] is actively regulated [27].
In the extracellular environment, Mg2+ is involved in inhibiting ectopic calcification, which is a key feature of accelerated physiological ageing [28,29,30]. Mg2+ has been shown to interfere with the growth of ectopic calcium phosphate crystals in blood vessels [30] and to delay the morphological shift from primary to secondary calciprotein particle formation [31]. In addition, Mg2+ may also contribute to the osteogenic transformation of vascular smooth muscle cells [30]. Mg2+ has been found to correlate negatively to both Fetuin-A and FGF23 in serum [32]. Fetuin-A is a major calcification inhibitor in the circulation [31,32], while FGF23 is an endocrine factor that can contrast calcification by inducing phosphate excretion [33,34].
Mg2+ transport across membranes is still poorly understood, but homeostasis at an organismal level appears to be maintained by modulating absorption in the intestine and reabsorption in the kidney [30,35]. In both cases, Mg2+ is imported apically by TRPM6 channels, transferred paracellularly across tight junctions with the contribution of claudin-16 and claudin-19, and exported basolaterally by the SLC41A1 Na/Mg exchanger [35,36]. In all cell types, Mg2+ is imported by TRPM7 channels and exported by the action of SLC41A1 [35,36]. Another important member of the SLC41 family is the Na/Mg exchanger SLC41A3, which is involved in transport across the mitochondrial membrane along with the Mrs2 channel [35,36,37].
3. Magnesium and Telomeres
Cytosolic Mg2+ concentration is approximately 1 mM [30], but significant reserves are present in bound form, mainly chelated by ATP, or within intracellular compartments, especially inside the mitochondria [38]. In a manner analogous to the regulation of calcium biochemistry, release or uptake of Mg2+ can cause large local shifts in [Mg2+], which in turn can regulate a variety of processes, including the regulation of flow of metabolites through glycolysis, the TCA cycle, oxidative phosphorylation, and ATP export from the mitochondria [38]. Critically, Mg2+ plays a role in telomere maintenance and the activity of telomerase [39]. These interactions are discussed in context in the following sections.
3.1. Telomere Attrition
The main driver of telomere attrition is DNA replication. However, the amount of telomeric sequence loss can vary dramatically, from the theoretical minimum of less than 10 base pairs (bp) lost per replication, to an average of 50–200 bp, up to about one thousand bp [40]. Reactive oxygen species (ROS) and oxidative stress produced by dysfunctional mitochondria can accelerate telomere attrition [40,41], while reducing ROS can mitigate this effect [11].
Telomeric sequences display a particular sensitivity to ROS-induced damage, as they are guanine-rich (G-rich) and have stacked G repeats. G has the lowest oxidation potential among the four DNA bases [42,43], and vulnerability to oxidative stress increases exponentially when G nucleotides are stacked [44]. The human telomeric DNA repeat stretch has been shown to be more sensitive to strand breakage, particularly at the G repeat, compared to non-telomeric sequences [45,46,47,48]. Additionally, DNA repair appears to be less efficient at telomeres in comparison to the rest of the genome [41]. The shelterin protein complex, which ‘caps’ telomeres, provides protection and impairs access to nucleases [49]. This prevents telomere ends from being recognised as DNA breaks, from being subjected to nuclease activity inherent in DNA repair processes, and from acting as a trigger for apoptosis [50]. In light of these considerations, it is thought that higher levels of ROS lead to an accumulation of unrepaired DNA damage at the telomeric region, which causes a larger DNA sequence loss during subsequent replication [40,51]. As such, the stacked G nucleotides integral to telomere structure may act as a redox-sensitive ‘alarm system’ that senses and signals the presence of oxidative damage before it can cause significant damage to the rest of the genome.
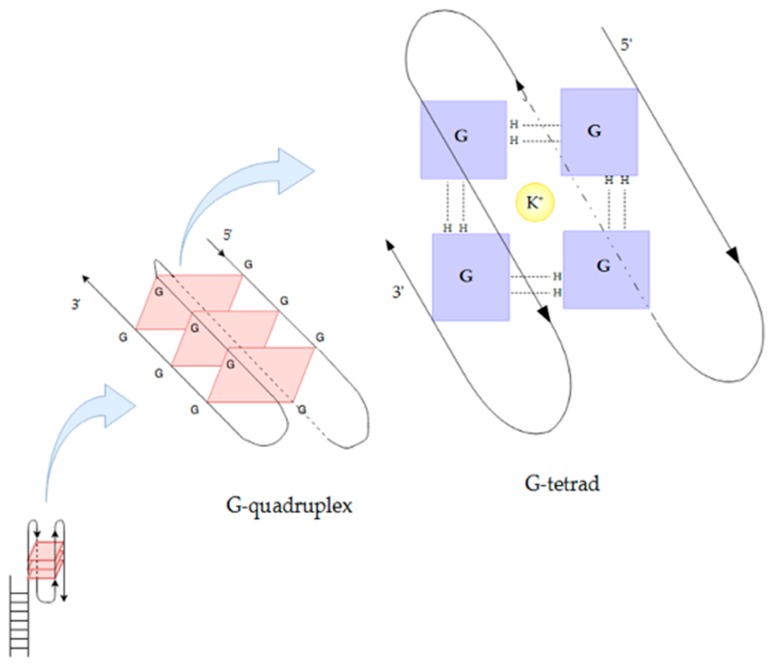
Secondary telomeric DNA structure is also important for telomere maintenance. In particular, it has been shown that the formation of G-quadruplexes prevents DNA repair machinery and telomerase from accessing the telomeric region [49,52]. G-quadruplexes can be formed by either a single DNA strand, or multiple DNA strands, when four G bases join by Hoogsteen hydrogen bonding to form a square planar structure called a G-tetrad, and two or more G-tetrads stack on top of each other in the presence of monovalent K+ and Na+ ions to form a G-quadruplex (Figure 1) [53]. G-quadruplexes may form stable nano-wires (G-wires) when guanine-rich DNA strands are annealed in the presence of suitable divalent cations [54]. Mg2+ can cause a configuration switch in G-quadruplex structures [55] and has been reported to stabilise G-wires [53,56]. G-wires are highly resistant to denaturation and have been reported to interfere with telomerase function [53,55,57]. In addition, some proteins appear to recognise specifically G-quadruplex structures, such as the GAR domain of the shelterin protein TRF2 [58].
Figure 1.
Schematic representation of an antiparallel G-quadruplex formed from a single DNA strand. This type of structure can form at telomere ends, protecting and stabilising the single strand overhang. The G-quadruplex is composed of several stacked G-tetrads, each of which is in turn formed by four guanines (G) joined by Hoogsteen hydrogen bonding and stabilised by a monovalent cation (K+ in this figure).
Mg2+ has been reported to enhance the stability of G-quadruplexes at oncogene promoter regions and prevent conversion to their pro-oncogene duplex form [59] and thus can act as an off switch in transcriptional regulation [60]. For instance, the promoter of the proto-oncogene c-Myc contains a parallel-stranded G-quadruplex that appears to bind Mg2+ most avidly [53,59]. Telomere attrition was found to be faster when cells were cultured in Mg2+-deficient conditions [26]. It has recently been reported that telomere G-quadruplexes may render DNA particularly sensitive to direct ultra-violet radiation (UV) damage and allow the oxidation of telomeric Gs even at wavelengths that are below the G ionization potential [61]. The implications of this observation for cancer neogenesis, progression and treatment remain to be explored. Recently, however, melanoma risk among individuals with light skin has been hypothesised to be mitigated by shortening of their telomeres through polygenic adaptation [62]. This may explain, in part, the shorter telomere lengths in Caucasians than in sub-Saharan Africans [63]. G quadruplex sensitivity to UV light may thus provide a mechanistic/structural basis for telomeres within the framework of such polygenic adaptation and cancer risk.
3.2. Magnesium and Telomeric Structure
Telomeric chromatin structure and integrity is impacted upon by Mg2+ biochemistry [39]. In particular, this can involve regulation and maintenance of higher order telomeric chromatin structure. More than 50% of telomeres localise to the lamina, a scaffold of intermediate filaments called lamins, that contributes to chromatin organisation, supports the mechanical stability of the nucleus, while also modulating signal transduction and transcription regulation [64,65]. The nuclear lamina comprises two lamin types, A and B, which are organised into distinct but overlapping networks that perform different functions within the nucleus [64]. Modulation of the lamin tail domain, which is recognised by lamin-binding proteins and provides an attachment site for chromatin and other structural proteins, has been demonstrated to be dependent on the presence of Mg2+ [64]. Lamin B1 has been shown to be sensitive to Mg2+ [64]. Down-regulation of lamin B1 is required for progression to full senescence, which triggers both local and global chromatin modifications [65]. Indeed, the decrease of lamin B1 can serve as a biomarker of senescence [66].
3.3. The Role of Telomeric Repeat-Containing RNA (TERRA)
Regulation of higher order telomeric structure is also a general feature of the epigenetic landscape and involves non-canonical epigenetic modifiers, such as non-coding RNAs (ncRNAs). An important player in this context is TERRA, a class of long ncRNA (lncRNA) transcribed from insulated promoters within telomeric and sub-telomeric regions that largely escape heterochromatinization. This class of RNA varies considerably in length, from approximately 100 bases to 9 kilobases (kb), and contains telomeric repeats [58]. The accumulation of mutations within the sequence allows TERRA to have, at the same time, specific binding in cis to the telomere of origin and generalised activity in trans on any chromosome [67].
While the picture of TERRA’s full role inside the cell is not yet clear, evidence has been accumulating on TERRA fulfilling a variety of functions related to telomere maintenance [58,67,68]. TERRA can interact with telomeric DNA to form hybrid DNA-RNA G-quadruplexes, interact with components of the shelterin complex, and bind telomerase [58]. TERRA is also thought to play a role in organising and expanding telomeric heterochromatin [69] and to help in telomere homeostasis. Indeed, TERRA appears to be expressed at a higher rate from short telomeres, to nucleate enzyme clusters by binding to multiple telomerase copies, and to recruit them to its telomere of origin [67].
3.4. Telomerase Activity in the Nucleus
Apart from its contribution to the maintenance of telomere structure and function, Mg2+ also has implications for the regulation of telomerase activity. The telomerase holoenzyme is formed by two essential components, the telomerase RNA that provides the template sequence for reverse transcription (TERC) and the catalytic protein component, telomerase reverse transcriptase (TERT), as well as by a number of accessory proteins [70]. It preferentially elongates short telomeres, because the stability of the shelterin complex is higher on longer telomeres [71]. This canonical function of telomerase requires Mg2+, which has therefore the potential to modulate its activity [6,39]. As with telomere shortening, elongation is also restricted to the S phase of the cell cycle, which is the only point at which active telomerase can access and elongate telomeres [71].
Interestingly, TERT is known to be subject to alternative splicing, with more than 20 known isoforms described in man, many of which lack a functional reverse transcriptase (RT) domain [72,73]. These isoforms have been hypothesised to act as antagonists and compete in binding TERC, and to be involved in some of the non-canonical functions of TERT which appear to be largely TERC-independent and even RT-independent [74]. These are discussed below.
3.5. Non-Canonical TERT Functions in the Nucleus
In the nucleus, TERT can act as a transcription factor (TF) and affect genes involved in energy metabolism, ‘stemness’, and proliferation, including the up-regulation of transfer RNA (tRNA) transcription, interaction with NF-κB and β-catenin to activate their respective pathways, and regulation of cyclin D1 expression [70]. Interestingly, β-catenin has, in turn, been shown to bind to the TERT promoter, suggesting a possible feed-forward loop that supports stemness and proliferation in wnt-dependent cancers [70].
Another emerging role for TERT is as an RNA-dependent RNA polymerase [73]. Human TERT (hTERT) has been shown in cancer cells to catalyse the creation of double-stranded RNA (dsRNA) from a non-coding RNA template, which is processed as a small interfering RNA (siRNA) and induces silencing, targeting in particular transcription from centromeres and transposons and possibly helping in the maintenance of heterochromatin [73].
3.6. Non-Canonical TERT Functions in the Mitochondria
TERT is known to be shuttled between the nucleus, cytoplasm, and mitochondrion [10]. The localisation of TERT within different cellular sub-compartments is a responsive process, which is regulated by growth factors, oxidative stress, caloric restriction [75], as well as antigen exposure in lymphocytes [76]. During increased oxidative stress, TERT is excluded from the nucleus and translocates to the mitochondria where it is reported to exert an antioxidant effect and promote mitochondrial health and cell survival [76]. Indeed, dampening of mitochondrial ROS production correlates with mitochondrial TERT accumulation [75]. The exact role of TERT in mitochondria is not yet clear, but several possibilities have been proposed. It has been shown to increase potential across the inner mitochondrial membrane, decrease ROS production, protect mitochondrial DNA (mtDNA) from damage, inhibit intrinsic apoptosis, and up-regulate activity and efficiency of the electron transport chain (ETC) [73,74].
TERT may also be tied to the general energy metabolism of the cell by interacting with the mammalian target of rapamycin (mTOR) pathway and the activities of the sirtuin family, key mediators in the mitochondrion-telomere-ribosome biogenesis (MTR) trinity and regulators of the cellular epigenetic landscape.
3.7. Telomeres and mTOR
The mammalian target of rapamycin complex 1 (mTORC1) pathway plays a crucial role in integrating signals from growth factor and nutrient availability sensors, and in regulating a variety of metabolic downstream pathways, including protein synthesis, cell cycle progression, energy metabolism, mitochondrial function, autophagy, and cell survival [77]. Intriguingly, hTERT has been shown to bind to and inhibit mTORC1, leading to increased autophagy [78] and possibly mimicking other effects of caloric restriction (CR). Conversely, mTOR activation of TERT has also been proposed [79].
Notably, mTOR demonstrates high sensitivity to Mg2+ oscillations [80] and it is therefore possible that Mg2+ acts as a regulator of the balance between anabolism and catabolism [81]. The observation that serum [Mg2+] increases in animals undergoing hibernation, or torpor, supports this hypothesis, because this increase cannot be due to nutrition and is therefore likely to be the result of extrusion from cells [81]. It is worth mentioning that depletion of cellular Mg2+ reserves, with likely repercussions on the metabolic balance, has been identified as both a cause and a consequence of ageing [26,39,82].
4. Mitochondrial Health, Ageing and Cancer
Mitochondrial homeostasis has been shown to deteriorate with age [83,84], and multiple mechanisms link it to telomere attrition [11]. An adequate concentration of mitochondrial Mg2+ ([Mg2+]mito) is essential for maintaining the integrity of the electron transport chain, as well as for controlling the flow of metabolites through glycolysis and the TCA cycle [38]. Consequently, [Mg2+]mito is a key determinant of cellular oxidative resilience, i.e., the ability to prevent or repair oxidative damage. This is pertinent, as mitochondrial dysfunction leads to increased ROS production, which in turn causes increased DNA damage and accelerated telomere attrition [11]. Furthermore, mitochondrial dysfunction induces specific nuclear gene expression through “retrograde signalling” [76].
On the other hand, telomere dysfunction activates p53 signalling, which represses the expression of PGC1-α/β, the master regulators of mitochondrial biogenesis [85]. In addition, a PGC1-α/β-independent pathway has been described, and its activity has been shown to decline during ageing along with changes in mitochondrial nicotinamide adenine dinucleotide (NAD+ levels [86]. This decline can be reversed if intramitochondrial NAD+ is increased by supplementation of its metabolic precursor nicotinamide mononucleotide (NMN) [86]. The decline in mitochondrial NAD+ appears to be due to an imbalance between nuclear and mitochondrial-encoded subunits, which is associated with an accumulation of HIF-1α, creating a pseudohypoxic state in spite of normal oxygen levels [86]. In mice, deletion of SIRT1 accelerates HIF-1α production, whereas raising NAD+ levels in old mice restores mitochondrial function to that of a young mouse in a SIRT1-dependent manner [86].
Interestingly, Mg2+ deficiency was shown to decrease resistance to hypoxia in vivo [87] and to impair the upregulation of HIF-1α caused by hypoxia in cell culture [88], while Mg2+ supplementation helps in reducing axon pathfinding errors caused by hypoxia [89] and has a neuroprotective effect in preterm birth cases [90]. It is worth noting that HIF-1α activation and pseudo-hypoxic reprogramming also occurs in cancer, shifting the cell towards a glycolytic phenotype [91].
It has been reported that an elevated concentration of TERT within the mitochondrion ([TERT]mito) may be associated with a prolonged period of reduced psychological stress and improved rest [92]. These observations are in keeping with the finding that telomere length has been shown to negatively correlate with increased cortisol responsiveness [93], anxiety and depression [94,95], lower socio-economic status [96], chronic low-grade inflammation, a higher frequency of adverse early life events [97,98], and adult daily life stressors [99]. For example, Alzheimer’s dementia (AD) and cardiovascular disease are characterised by mitochondrial dysfunction and increased ROS production [100,101], while also being associated with accelerated telomere shortening [102,103].
5. Telomere Length and Disease
Telomere attrition is a hallmark of ageing and age-related disease. However, it has been reported that individuals with telomeres that are longer than expected, given their age and gender, in healthy tissue (i.e., “constitutively” long telomeres) may have a higher risk for major cancers than individuals with “constitutively” shorter telomeres [104]. This is not intuitive, as established physiological stressors [105,106,107] have been reported to accelerate age-related telomere shortening. It is therefore of interest that the majority of cancerous tissues have been reported to display short telomere lengths in comparison to a previous disease-free measurement [104]. The reasons for this discrepancy remain unclear. One consideration that may be pertinent to this observation is the fact that telomerase appears to have a different activity in healthy and cancerous cells [71]. In health, telomerase targets short telomeres preferentially and extends them with high processivity, while in cancer cells telomerase appears to target telomeres at random and extend them with low processivity [71]. In combination with a significantly elevated rate of cell proliferation, this may explain why telomeres tend to be shorter in cancerous tissue, in spite of the presence of active telomerase. In addition, this leads to the preservation of average telomere length (TL), providing replicative immortality, while at the same time allowing the existence of critically short telomeres that act as a source of genomic instability (t-stumps) [71,108,109,110,111,112].
The impact of TL dynamics remains controversial in oncoscience, with equivocal findings highlighting an onco-protective role for long telomeres, while other studies indicate a tumour-promoting association for long telomeres [113,114]. A large number of meta-analyses have also reached conflicting conclusions, with some reporting short telomeres to be associated with increased cancer risk [115,116,117], and others no significant overall effect [118,119,120]. Interestingly, one meta-analysis found that, while shorter TL is associated with lower survival, a lower TL ratio of tumour versus healthy tissue was associated with better survival [121].
It is worth mentioning that these meta-analyses carry significant caveats: results were highly heterogeneous and often not statistically significant; in addition, correcting for a large number of potential confounding factors, including age, gender, stress, smoking status, duration of treatment, etc. was not possible. The difficulty in finding a clear link may reflect the fact that TL can play a variety of roles in different types and at different stages of cancer. Additionally, epigenetic and technical issues relating to telomere measurement may also confound many studies. TL, for example, is a highly heritable genetic trait that displays wide variation across the population. It is significantly influenced by parental age at the time of birth [122,123]. Issues relating to differing TL measurement methodology between labs also influence study outcomes and meta analyses and are elegantly covered by Martin-Ruiz et al. [124]. It is worth noting that most methods provide an average TL value; however, TL varies significantly between cells and even between chromosomes within the same cell, and a single uncapped telomere can trigger senescence [125,126,127]. In addition, many studies use white blood cell DNA; in such cases, reliability of the results is further limited by the fact that different white blood cell types have different average TL and their abundance in blood, both in relative and in absolute terms, can vary significantly depending on the subject and their immunological status [125,128,129]. These issues may, however, be resolved through the use of intrinsic and extrinsic epigenetic clocks [130,131,132] in future studies.
The role of Mg2+ in cancer prevention and genesis is also complex. Epidemiological studies have shown an inverse relationship between Mg2+ consumption and cancer prevalence [20,133], and in vitro studies have indicated that Mg2+ may be protective during the early reprogramming phase [134]. In contrast, Mg2+ supplementation to an established tumour has been shown to promote tumour growth [134,135,136]. Interestingly, a similar double-edged relationship, based on tumour progression, has been established for thiamine supplementation as well [137].
Any pro-growth effect of Mg2+ may be due to a direct DNA stabilization effect, but may also be attributable to the enhanced performance of the non-oxidative arm of the pentose phosphate pathway (PPP) through co-operative optimization of transketolase (TK) function by Mg2+ and thiamine [138] resulting in increased production of ribose-5-phosphate [139].
6. Telomeres, Metabolism, and Circadian Rhythms
The circadian rhythm relies on a transcription-translation feedback loop that regulates numerous metabolic processes during the day-night cycle and is reported to target 43% of the human protein-coding genes [140]. In addition, 56 of the top 100 most commonly prescribed medications directly target the products of circadian genes [140]. In mammals, clock pathways operate in individual cells, but are synchronised by the master clock in the suprachiasmatic nucleus (SCN) in the hypothalamus, which in turn is sensitive to light cues from the retina [141,142]. Circadian fluctuations are present in the sleep/wake cycle, body temperature, hormone production, cell proliferation and DNA repair [143].
TERT mRNA expression has also been reported to oscillate with the circadian rhythm [144]. In mice, deficiency of the clock gene CLOCK has been demonstrated to cause lower telomerase activity, loss of rhythmicity in TERT mRNA expression, and shorter telomeres [144]. Similarly, hospital physicians who work a regular day time pattern have regular and rhythmical circadian oscillation of telomerase activity, while physicians who work a shift pattern in the emergency department lose the normal circadian rhythms of telomerase activity [144]. This dysregulation of telomerase activity may contribute to accelerated ageing and be linked to the higher risk seen in shift workers for a variety of health conditions [145]. In addition, cellular senescence appears to impair circadian rhythms [146], so it’s tempting to speculate that the accumulation of senescent cells with age may contribute to the observed disruption of the circadian clock, creating a possible vicious cycle. Loss of circadian regulation is a feature of mammalian ageing [29].
Given its role in energy metabolism and redox homeostasis, it is perhaps not too surprising that Mg2+ flux across the cell membrane, especially in the SCN, was recently identified as a regulator of circadian metabolic oscillations, leading to speculation that [Mg2+] fluctuations may be a component of the cellular clock [80] and that Mg2+ deficiency may compromise sleep quality and circadian rhythm [147]. If so, this would indicate that Mg2+ is a key component of cellular ageing and thus physiological ageing. Furthermore, N-methyl-d-aspartate receptors (NMDARs) within the SCN, which orchestrate light-induced phase resetting of the circadian rhythm [148], are Mg2+-dependent [149]. An indirect effect of cellular Mg2+ homeostasis that may be mediated through hepatocyte activation of cortisone remains relatively unexplored from a circadian perspective [150,151]. The increased cortisol activity may cause further Mg2+ loss and aggravate the circadian disruption [152].
Taken together, these findings indicate that the circadian regulation of telomerase activity and Mg2+ homeostasis may play an important role in health, disease, and ageing [144,147]. In light of the twin role of telomerase and Mg2+ in telomere maintenance and oxidative resilience, circadian disruption could impair the homeostasis and synchronization between telomerase activity and localisation, local [Mg2+], DNA replication, and redox balance. This could in turn cause accelerated telomere attrition, increased ROS production, and faster ageing.
7. Conclusions
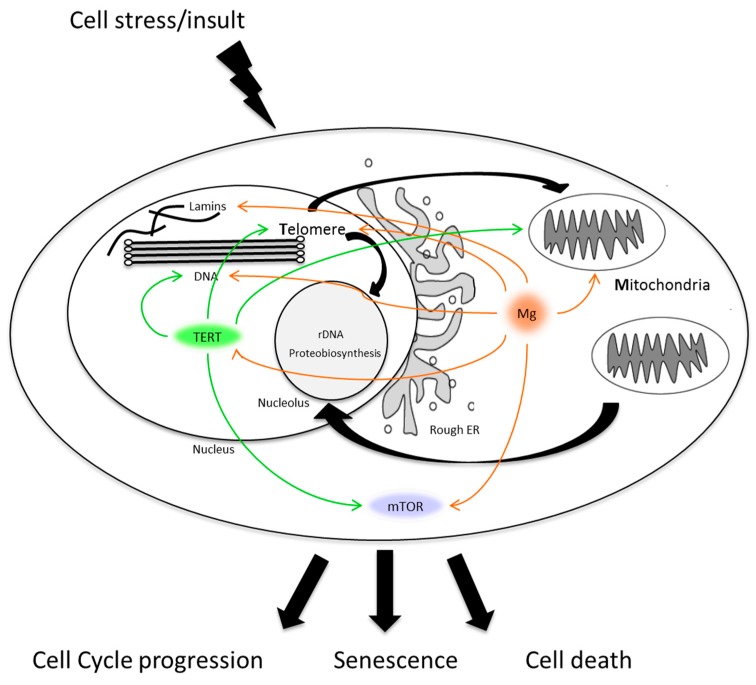
Telomere structure and function are tightly linked to the homeostatic regulation of cellular metabolism [153]. Cellular energy production, energy expenditure and protein biosynthesis are coupled under the aegis of the MTR (Figure 2) which integrates cellular responses to metabolic and environmental stressors. As a key ion involved in energy metabolism and DNA/RNA stabilisation, magnesium has a pervasive role as modulator of many of the pathways that make up the MTR trinity. This review has indicated the complexities of how such processes might act,either independently, additively or cumulatively in normative ageing and disease.
Figure 2.
A schematic of the interplay between mitochondria, telomere nucleo-protein complexes and proteo-biosynthesis regulation in response to cellular stress/damage. In response to the stress, the cell will decide how much fuel to burn and energy to expend to repair any damage accrued. If the cell can repair the damage it can progress through the cell cycle. Too much damage and the high risk of onconeogenesis induces cell death. Senescence, strictly in this simplified scenario, sees the cell carry accumulated, sub-lethal damage, as wear and tear, leaving the cell metabolically active, but physiologically non-contributory to the function of the tissue and organ in which it resides. The pervasive and overlapping roles that TERT (green) and magnesium (orange) play in targeting major components of the MRT trinity are highlighted. ER, mTOR/.
One important sequitur from the perspective of the MTR is that the use of TL solely as a cellular biomarker of ageing to study disease will yield equivocal findings based on disease context. In keeping with this, many studies using TL as a singular biomarker of ageing have often yielded equivocal results. This is particularly pertinent to the use of TL as a biomarker in cancer studies. Given the role that telomeres play in cellular homeostasis and the multi-faceted nature of the telomere nucleo-protein complex, a more complete understanding of the various pathways that link cellular metabolism and telomere homeostasis will help resolve any such equivocation. An examination of the non-canonical functions of TERT, for example, in the context of the MTR might be of particular interest in this regard.
Another avenue that deserves further study is the impact of disruption of circadian rhythms on the ability of cells to both co-ordinate maintenance of telomere nucleo-protein complexes and regulate ROS production, which may in turn affect cellular and tissue health span and the rate of physiological ageing.
Author Contributions
Paul G. Shiels, Donogh Maguire, Ognian Neytchev, Donald McMillan and Dinesh Talwar conceived and wrote the article. Paul G. Shiels, Donogh Maguire and Ognian Neytchev edited and drafted the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The Hallmarks of Aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Victorelli S., Passos J.F. Telomeres and Cell Senescence—Size Matters Not. EBioMedicine. 2017;21:14–20. doi: 10.1016/j.ebiom.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olovnikov A.M. Principle of marginotomy in template synthesis of polynucleotides. Dokl. Akad. Nauk SSSR. 1971;201:1496–1499. [PubMed] [Google Scholar]
- 4.Olovnikov A.M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 5.Ridout S.J., Ridout K.K., Kao H.-T., Carpenter L.L., Philip N.S., Tyrka A.R., Price L.H. Clinical Challenges in the Biopsychosocial Interface. Karger Publishers; Basel, Switzerland: 2015. Telomeres, Early-Life Stress and Mental Illness; pp. 92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackburn E.H., Collins K. Telomerase: An RNP Enzyme Synthesizes DNA. Cold Spring Harb. Perspect. Biol. 2011;3:a003558. doi: 10.1101/cshperspect.a003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T., Yuan X., Xu D. Cancer-Specific Telomerase Reverse Transcriptase (TERT) Promoter Mutations: Biological and Clinical Implications. Genes. 2016;7:38. doi: 10.3390/genes7070038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henson J.D., Neumann A.A., Yeager T.R., Reddel R.R. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21:598–610. doi: 10.1038/sj.onc.1205058. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Saretzki G. Extra-telomeric functions of human telomerase: Cancer, mitochondria and oxidative stress. Curr. Pharm. Des. 2014;20:6386–6403. doi: 10.2174/1381612820666140630095606. [DOI] [PubMed] [Google Scholar]
- 11.Passos J.F., Saretzki G., Von Zglinicki T. DNA damage in telomeres and mitochondria during cellular senescence: Is there a connection? Nucleic Acids Res. 2007;35:7505–7513. doi: 10.1093/nar/gkm893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilchova I., Klacanova K., Tatarkova Z., Kaplan P., Racay P. The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxid. Med. Cell. Longev. 2017;2017:1–8. doi: 10.1155/2017/6797460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P., Wang Z.-Y. Metal ions influx is a double edged sword for the pathogenesis of Alzheimer’s disease. Ageing Res. Rev. 2017;35:265–290. doi: 10.1016/j.arr.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Romani A.M.P. Interrelations between Essential Metal Ions and Human Diseases. Springer; Dordrecht, The Netherlands: 2013. Magnesium in Health and Disease; pp. 49–79. [DOI] [PubMed] [Google Scholar]
- 15.Jahnen-Dechent W., Ketteler M. Magnesium basics. Clin. Kidney J. 2012;5:i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertinato J., Wang K.C., Hayward S. Serum Magnesium Concentrations in the Canadian Population and Associations with Diabetes, Glycemic Regulation, and Insulin Resistance. Nutrients. 2017;9:296. doi: 10.3390/nu9030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veronese N., Zurlo A., Solmi M., Luchini C., Trevisan C., Bano G., Manzato E., Sergi G., Rylander R. Magnesium Status in Alzheimer’s Disease: A Systematic Review. Am. J. Alzheimers Dis. Other Dement. 2016;31:208–213. doi: 10.1177/1533317515602674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vormann J. Magnesium and Kidney Health—More on the “Forgotten Electrolyte”. Am. J. Nephrol. 2016;44:379–380. doi: 10.1159/000450863. [DOI] [PubMed] [Google Scholar]
- 19.Barbagallo M. Magnesium and type 2 diabetes. World J. Diabetes. 2015;6:1152. doi: 10.4239/wjd.v6.i10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Baaij J.H.F., Hoenderop J.G.J., Bindels R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 21.Kolisek M., Sponder G., Mastrototaro L., Smorodchenko A., Launay P., Vormann J., Schweigel-Röntgen M. Substitution p.A350V in Na+/Mg2+ Exchanger SLC41A1, Potentially Associated with Parkinson’s Disease, Is a Gain-of-Function Mutation. PLoS ONE. 2013;8:e71096. doi: 10.1371/journal.pone.0071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson S.C., Wolk A. Magnesium intake and risk of type 2 diabetes: A meta-analysis. J. Intern. Med. 2007;262:208–214. doi: 10.1111/j.1365-2796.2007.01840.x. [DOI] [PubMed] [Google Scholar]
- 23.Fang X., Wang K., Han D., He X., Wei J., Zhao L., Imam M.U., Ping Z., Li Y., Xu Y., et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016;14:210. doi: 10.1186/s12916-016-0742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaguchi Y., Hamano T., Isaka Y. Effects of Magnesium on the Phosphate Toxicity in Chronic Kidney Disease: Time for Intervention Studies. Nutrients. 2017;9:112. doi: 10.3390/nu9020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrè S., Mazur A., Maier J.A.M. Low-magnesium induces senescent features in cultured human endothelial cells. Magnes. Res. 2007;20:66–71. [PubMed] [Google Scholar]
- 26.Killilea D.W., Ames B.N. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc. Natl. Acad. Sci. USA. 2008;105:5768–5773. doi: 10.1073/pnas.0712401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf F.I., Torsello A., Fasanella S., Cittadini A. Cell physiology of magnesium. Mol. Asp. Med. 2003;24:11–26. doi: 10.1016/S0098-2997(02)00088-2. [DOI] [PubMed] [Google Scholar]
- 28.Kooman J.P., Kotanko P., Schols A.M.W.J., Shiels P.G., Stenvinkel P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014;10:732–742. doi: 10.1038/nrneph.2014.185. [DOI] [PubMed] [Google Scholar]
- 29.Shiels P.G., McGuinness D., Eriksson M., Kooman J.P., Stenvinkel P. The role of epigenetics in renal ageing. Nat. Rev. Nephrol. 2017;13:471–482. doi: 10.1038/nrneph.2017.78. [DOI] [PubMed] [Google Scholar]
- 30.Ter Braake A.D., Shanahan C.M., de Baaij J.H.F.F. Magnesium Counteracts Vascular Calcification: Passive Interference or Active Modulation? Arterioscler. Thromb. Vasc. Biol. 2017;37:1431–1445. doi: 10.1161/ATVBAHA.117.309182. [DOI] [PubMed] [Google Scholar]
- 31.Pasch A., Farese S., Graber S., Wald J., Richtering W., Floege J., Jahnen-Dechent W. Nanoparticle-Based Test Measures Overall Propensity for Calcification in Serum. J. Am. Soc. Nephrol. 2012;23:1744–1752. doi: 10.1681/ASN.2012030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada H., Kuro-O M., Ishikawa S.-E., Funazaki S., Kusaka I., Kakei M., Hara K. Daily variability in serum levels of calciprotein particles and their association with mineral metabolism parameters: A cross-sectional pilot study. Nephrology. 2017 doi: 10.1111/nep.12994. [DOI] [PubMed] [Google Scholar]
- 33.Kuro-o M. Klotho and aging. Biochim. Biophys. Acta Gen. Subj. 2009;1790:1049–1058. doi: 10.1016/j.bbagen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faul C., Amaral A.P., Oskouei B., Hu M.C., Sloan A., Isakova T., Gutiérrez O.M., Aguillon-Prada R., Lincoln J., Hare J.M., et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romani A.M.P. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011;512:1–23. doi: 10.1016/j.abb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahni J., Scharenberg A.M. The SLC41 family of MgtE-like magnesium transporters. Mol. Asp. Med. 2013;34:620–628. doi: 10.1016/j.mam.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mastrototaro L., Smorodchenko A., Aschenbach J.R., Kolisek M., Sponder G. Solute carrier 41A3 encodes for a mitochondrial Mg2+ efflux system. Sci. Rep. 2016;6:27999. doi: 10.1038/srep27999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanaka R., Tabata S., Shindo Y., Hotta K., Suzuki K., Soga T., Oka K. Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016;6:30027. doi: 10.1038/srep30027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowe W.J. Correcting magnesium deficiencies may prolong life. Clin. Interv. Aging. 2012;7:51–54. doi: 10.2147/CIA.S28768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/S0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 41.Sun L., Tan R., Xu J., LaFace J., Gao Y., Xiao Y., Attar M., Neumann C., Li G.-M., Su B., et al. Targeted DNA damage at individual telomeres disrupts their integrity and triggers cell death. Nucleic Acids Res. 2015;43:6334–6347. doi: 10.1093/nar/gkv598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neeley W.L., Essigmann J.M. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem. Res. Toxicol. 2006;19:491–505. doi: 10.1021/tx0600043. [DOI] [PubMed] [Google Scholar]
- 43.Burrows C.J., Muller J.G. Oxidative Nucleobase Modifications Leading to Strand Scission. Chem. Rev. 1998;98:1109–1152. doi: 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]
- 44.Viel A., Bruselles A., Meccia E., Fornasarig M., Quaia M., Canzonieri V., Policicchio E., Urso E.D., Agostini M., Genuardi M., et al. A Specific Mutational Signature Associated with DNA 8-Oxoguanine Persistence in MUTYH-defective Colorectal Cancer. EBioMedicine. 2017;20:39–49. doi: 10.1016/j.ebiom.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henle E.S., Han Z., Tang N., Rai P., Luo Y., Linn S. Sequence-specific DNA Cleavage by Fe2+-mediated Fenton Reactions Has Possible Biological Implications. J. Biol. Chem. 1999;274:962–971. doi: 10.1074/jbc.274.2.962. [DOI] [PubMed] [Google Scholar]
- 46.Oikawa S., Tada-Oikawa S., Kawanishi S. Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry. 2001;40:4763–4768. doi: 10.1021/bi002721g. [DOI] [PubMed] [Google Scholar]
- 47.Kawanishi S., Oikawa S. Mechanism of Telomere Shortening by Oxidative Stress. Ann. N. Y. Acad. Sci. 2004;1019:278–284. doi: 10.1196/annals.1297.047. [DOI] [PubMed] [Google Scholar]
- 48.Hewitt G., Jurk D., Marques F.D.M., Correia-Melo C., Hardy T., Gackowska A., Anderson R., Taschuk M., Mann J., Passos J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webb C.J., Wu Y., Zakian V.A. DNA Repair at Telomeres: Keeping the Ends Intact. Cold Spring Harb. Perspect. Biol. 2013;5:a012666. doi: 10.1101/cshperspect.a012666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palm W., de Lange T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 51.Saretzki G., Walter T., Atkinson S., Passos J.F., Bareth B., Keith W.N., Stewart R., Hoare S., Stojkovic M., Armstrong L., et al. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26:455–464. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- 52.Moye A.L., Porter K.C., Cohen S.B., Phan T., Zyner K.G., Sasaki N., Lovrecz G.O., Beck J.L., Bryan T.M. Telomeric G-quadruplexes are a substrate and site of localization for human telomerase. Nat. Commun. 2015;6:7643. doi: 10.1038/ncomms8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y., Luo H.Q., Li N.B. A highly sensitive resonance Rayleigh scattering method to discriminate a parallel-stranded G-quadruplex from DNA with other topologies and structures. Chem. Commun. 2013;49:6209. doi: 10.1039/c3cc42140f. [DOI] [PubMed] [Google Scholar]
- 54.Oliviero G., D’Errico S., Pinto B., Nici F., Dardano P., Rea I., De Stefano L., Mayol L., Piccialli G., Borbone N. Self-Assembly of G-Rich Oligonucleotides Incorporating a 3′-3′ Inversion of Polarity Site: A New Route Towards G-Wire DNA Nanostructures. ChemistryOpen. 2017;6:599–605. doi: 10.1002/open.201700024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhattacharyya D., Mirihana Arachchilage G., Basu S. Metal Cations in G-Quadruplex Folding and Stability. Front. Chem. 2016;4:1–14. doi: 10.3389/fchem.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kosman J., Juskowiak B. Hemin/G-quadruplex structure and activity alteration induced by magnesium cations. Int. J. Biol. Macromol. 2016;85:555–564. doi: 10.1016/j.ijbiomac.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 57.Zahler A.M., Williamson J.R., Cech T.R., Prescott D.M. Inhibition of telomerase by G-quartet DNA structures. Nature. 1991;350:718–720. doi: 10.1038/350718a0. [DOI] [PubMed] [Google Scholar]
- 58.Oliva-Rico D., Herrera L.A. Regulated expression of the lncRNA TERRA and its impact on telomere biology. Mech. Ageing Dev. 2017;167:16–23. doi: 10.1016/j.mad.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Yan Y.Y., Lin J., Ou T.M., Tan J.H., Li D., Gu L.Q., Huang Z.S. Selective recognition of oncogene promoter G-quadruplexes by Mg2+ Biochem. Biophys. Res. Commun. 2010;402:614–618. doi: 10.1016/j.bbrc.2010.10.065. [DOI] [PubMed] [Google Scholar]
- 60.Du Z., Zhao Y., Li N. Genome-wide colonization of gene regulatory elements by G4 DNA motifs. Nucleic Acids Res. 2009;37:6784–6798. doi: 10.1093/nar/gkp710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banyasz A., Martínez-Fernández L., Balty C., Perron M., Douki T., Improta R., Markovitsi D. Absorption of Low-Energy UV Radiation by Human Telomere G-Quadruplexes Generates Long-Lived Guanine Radical Cations. J. Am. Chem. Soc. 2017;139:10561–10568. doi: 10.1021/jacs.7b05931. [DOI] [PubMed] [Google Scholar]
- 62.Stone R.C., Horvath K., Kark J.D., Susser E., Tishkoff S.A., Aviv A. Telomere Length and the Cancer–Atherosclerosis Trade-Off. PLoS Genet. 2016;12:e1006144. doi: 10.1371/journal.pgen.1006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansen M.E.B., Hunt S.C., Stone R.C., Horvath K., Herbig U., Ranciaro A., Hirbo J., Beggs W., Reiner A.P., Wilson J.G., et al. Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. Hum. Mol. Genet. 2016;25:2324–2330. doi: 10.1093/hmg/ddw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ganesh S., Qin Z., Spagnol S.T., Biegler M.T., Coffey K.A., Kalinowski A., Buehler M.J., Dahl K.N. The tail domain of lamin B1 is more strongly modulated by divalent cations than lamin A. Nucleus. 2015;6:203–211. doi: 10.1080/19491034.2015.1031436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah P.P., Donahue G., Otte G.L., Capell B.C., Nelson D.M., Cao K., Aggarwala V., Cruickshanks H.A., Rai T.S., McBryan T., et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013;27:1787–1799. doi: 10.1101/gad.223834.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freund A., Laberge R.-M., Demaria M., Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell. 2012;23:2066–2075. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cusanelli E., Romero C., Chartrand P. Telomeric Noncoding RNA TERRA Is Induced by Telomere Shortening to Nucleate Telomerase Molecules at Short Telomeres. Mol. Cell. 2013;51:780–791. doi: 10.1016/j.molcel.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 68.Cusanelli E., Chartrand P. Telomeric repeat-containing RNA TERRA: A noncoding RNA connecting telomere biology to genome integrity. Front. Genet. 2015;6:1–9. doi: 10.3389/fgene.2015.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahama K., Takada A., Tada S., Shimizu M., Sayama K., Kurokawa R., Oyoshi T. Regulation of telomere length by G-quadruplex telomere DNA- and TERRA-binding protein TLS/FUS. Chem. Biol. 2013;20:341–350. doi: 10.1016/j.chembiol.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Ozturk M., Li Y., Tergaonkar V. Current Insights to Regulation and Role of Telomerase in Human Diseases. Antioxidants. 2017;6:17. doi: 10.3390/antiox6010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armstrong C.A., Tomita K. Fundamental mechanisms of telomerase action in yeasts and mammals: Understanding telomeres and telomerase in cancer cells. Open Biol. 2017;7:160338. doi: 10.1098/rsob.160338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong M.S., Wright W.E., Shay J.W. Alternative splicing regulation of telomerase: A new paradigm? Trends Genet. 2014;30:430–438. doi: 10.1016/j.tig.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maida Y., Masutomi K. Telomerase reverse transcriptase moonlights: Therapeutic targets beyond telomerase. Cancer Sci. 2015;106:1486–1492. doi: 10.1111/cas.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma N.K., Reyes A., Green P., Caron M.J., Bonini M.G., Gordon D.M., Holt I.J., Santos J.H. Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res. 2012;40:712–725. doi: 10.1093/nar/gkr758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miwa S., Czapiewski R., Wan T., Bell A., Hill K.N., von Zglinicki T., Saretzki G. Decreased mTOR signalling reduces mitochondrial ROS in brain via accumulation of the telomerase protein TERT within mitochondria. Aging. 2016;8:2551–2567. doi: 10.18632/aging.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saretzki G. Telomerase, mitochondria and oxidative stress. Exp. Gerontol. 2009;44:485–492. doi: 10.1016/j.exger.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 77.Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ali M., Devkota S., Roh J.-I., Lee J., Lee H.-W. Telomerase reverse transcriptase induces basal and amino acid starvation-induced autophagy through mTORC1. Biochem. Biophys. Res. Commun. 2016;478:1198–1204. doi: 10.1016/j.bbrc.2016.08.094. [DOI] [PubMed] [Google Scholar]
- 79.Sundin T., Hentosh P. InTERTesting association between telomerase, mTOR and phytochemicals. Expert Rev. Mol. Med. 2012;14:e8. doi: 10.1017/erm.2012.1. [DOI] [PubMed] [Google Scholar]
- 80.Feeney K.A., Hansen L.L., Putker M., Olivares-Yañez C., Day J., Eades L.J., Larrondo L.F., Hoyle N.P., O’Neill J.S., van Ooijen G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature. 2016;532:375–379. doi: 10.1038/nature17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Ooijen G., O’Neill J.S. Intracellular magnesium and the rhythms of life. Cell Cycle. 2016;15:2997–2998. doi: 10.1080/15384101.2016.1214030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Killilea D.W., Maier J.A.M. A connection between magnesium deficiency and aging: New insights from cellular studies. Magnes. Res. 2008;21:77–82. doi: 10.1684/mrh.2008.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lanza I.R., Nair K.S. Mitochondrial function as a determinant of life span. Pfluger Arch. 2010;459:277–289. doi: 10.1007/s00424-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wallace D.C. Mitochondrial DNA mutations in disease and aging. Environ. Mol. Mutagen. 2010;51:440–450. doi: 10.1002/em.20586. [DOI] [PubMed] [Google Scholar]
- 85.Sahin E., Colla S., Liesa M., Moslehi J., Müller F.L., Guo M., Cooper M., Kotton D., Fabian A.J., Walkey C., et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gomes A.P., Price N.L., Ling A.J.Y., Moslehi J.J., Montgomery M.K., Rajman L., White J.P., Teodoro J.S., Wrann C.D., Hubbard B.P., et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watanabe M., Shinohara A., Matsukawa T., Chiba M., Wu J., Iesaki T., Okada T. Chronic magnesium deficiency decreases tolerance to hypoxia/reoxygenation injury in mouse heart. Life Sci. 2011;88:658–663. doi: 10.1016/j.lfs.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 88.Torii S., Kobayashi K., Takahashi M., Katahira K., Goryo K., Matsushita N., Yasumoto K., Fujii-Kuriyama Y., Sogawa K. Magnesium Deficiency Causes Loss of Response to Intermittent Hypoxia in Paraganglion Cells. J. Biol. Chem. 2009;284:19077–19089. doi: 10.1074/jbc.M109.004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stevenson T.J., Trinh T., Kogelschatz C., Fujimoto E., Lush M.E., Piotrowski T., Brimley C.J., Bonkowsky J.L. Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium. PLoS Genet. 2012;8:e1002638. doi: 10.1371/journal.pgen.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doyle L.W., Crowther C.A., Middleton P., Marret S., Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. In: Doyle L.W., editor. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd.; Chichester, UK: 2009. [DOI] [PubMed] [Google Scholar]
- 91.Maxwell P.H., Pugh C.W., Ratcliffe P.J. Activation of the HIF pathway in cancer. Curr. Opin. Genet. Dev. 2001;11:293–299. doi: 10.1016/S0959-437X(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 92.Jacobs T.L., Epel E.S., Lin J., Blackburn E.H., Wolkowitz O.M., Bridwell D.A., Zanesco A.P., Aichele S.R., Sahdra B.K., MacLean K.A., et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36:664–681. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 93.Ramirez J., Elmofty M., Castillo E., DeRouen M., Shariff-Marco S., Allen L., Gomez S.L., Nápoles A.M., Márquez-Magaña L. Evaluation of cortisol and telomere length measurements in ethnically diverse women with breast cancer using culturally sensitive methods. J. Community Genet. 2017;8:75–86. doi: 10.1007/s12687-016-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X., Sundquist K., Hedelius A., Palmér K., Memon A.A., Sundquist J. Leukocyte telomere length and depression, anxiety and stress and adjustment disorders in primary health care patients. BMC Psychiatry. 2017;17:148. doi: 10.1186/s12888-017-1308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ridout K.K., Ridout S.J., Price L.H., Sen S., Tyrka A.R. Depression and telomere length: A meta-analysis. J. Affect. Disord. 2016;191:237–247. doi: 10.1016/j.jad.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McGuinness D., McGlynn L.M., Johnson P.C., MacIntyre A., Batty G.D., Burns H., Cavanagh J., Deans K.A., Ford I., McConnachie A., et al. Socio-economic status is associated with epigenetic differences in the pSoBid cohort. Int. J. Epidemiol. 2012;41:151–160. doi: 10.1093/ije/dyr215. [DOI] [PubMed] [Google Scholar]
- 97.Ridout K.K., Levandowski M., Ridout S.J., Gantz L., Goonan K., Palermo D., Price L.H., Tyrka A.R. Early life adversity and telomere length: A meta-analysis. Mol. Psychiatry. 2017 doi: 10.1038/mp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coimbra B.M., Carvalho C.M., Moretti P.N., Mello M.F., Belangero S.I. Stress-related telomere length in children: A systematic review. J. Psychiatr. Res. 2017;92:47–54. doi: 10.1016/j.jpsychires.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 99.Shiels P.G., McGlynn L.M., MacIntyre A., Johnson P.C.D., da Batty G.D., Burns H., Cavanagh J., Deans K.A., Ford I., McConnachie A., et al. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid Cohort. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dai D.-F., Chiao Y.A., Marcinek D.J., Szeto H.H., Rabinovitch P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bakunina N., Pariante C.M., Zunszain P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015 doi: 10.1111/imm.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cai Z., Yan L.-J., Ratka A. Telomere shortening and Alzheimer’s disease. Neuromol. Med. 2013;15:25–48. doi: 10.1007/s12017-012-8207-9. [DOI] [PubMed] [Google Scholar]
- 103.Fyhrquist F., Saijonmaa O. Telomere length and cardiovascular aging. Ann. Med. 2012;44:S138–S142. doi: 10.3109/07853890.2012.660497. [DOI] [PubMed] [Google Scholar]
- 104.Aviv A., Anderson J.J., Shay J.W. Mutations, Cancer and the Telomere Length Paradox. Trends Cancer. 2017;3:253–258. doi: 10.1016/j.trecan.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valdes A.M., Andrew T., Gardner J.P., Kimura M., Oelsner E., Cherkas L.F., Aviv A., Spector T.D. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 106.Astuti Y., Wardhana A., Watkins J., Wulaningsih W. PILAR Research Network Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Environ. Res. 2017;158:480–489. doi: 10.1016/j.envres.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Panich U., Sittithumcharee G., Rathviboon N., Jirawatnotai S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016;2016:1–14. doi: 10.1155/2016/7370642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu L., Blackburn E.H. Human cancer cells harbor T-stumps, a distinct class of extremely short telomeres. Mol. Cell. 2007;28:315–327. doi: 10.1016/j.molcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pai R.B., Balakrishna Pai S., Yang L., Joshi H.C. Abundance of a distinct cluster of telomere t-stumps in advanced breast cancer cell line. Oncol. Lett. 2010;1 doi: 10.3892/ol_00000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Knecht H., Bruderlein S., Wegener S., Lichtensztejn D., Lichtensztejn Z., Lemieux B., Moller P., Mai S. 3D nuclear organization of telomeres in the Hodgkin cell lines U-HO1 and U-HO1-PTPN1: PTPN1 expression prevents the formation of very short telomeres including “t-stumps”. BMC Cell Biol. 2010;11:99. doi: 10.1186/1471-2121-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Knecht H., Righolt C., Mai S. Genomic Instability: The Driving Force behind Refractory/Relapsing Hodgkin’s Lymphoma. Cancers. 2013;5:714–725. doi: 10.3390/cancers5020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao Y., Sfeir A.J., Zou Y., Buseman C.M., Chow T.T., Shay J.W., Wright W.E. Telomere Extension Occurs at Most Chromosome Ends and Is Uncoupled from Fill-In in Human Cancer Cells. Cell. 2009;138:463–475. doi: 10.1016/j.cell.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lynch S.M., Major J.M., Cawthon R., Weinstein S.J., Virtamo J., Lan Q., Rothman N., Albanes D., Stolzenberg-Solomon R.Z. A prospective analysis of telomere length and pancreatic cancer in the α-tocopherol β-carotene cancer (ATBC) prevention study. Int. J. Cancer. 2013;133:2672–2680. doi: 10.1002/ijc.28272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Julin B., Shui I., Heaphy C.M., Joshu C.E., Meeker A.K., Giovannucci E., De Vivo I., Platz E.A. Circulating leukocyte telomere length and risk of overall and aggressive prostate cancer. Br. J. Cancer. 2015;112:769–776. doi: 10.1038/bjc.2014.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma H., Zhou Z., Wei S., Liu Z., Pooley K.A., Dunning A.M., Svenson U., Roos G., Hosgood H.D., Shen M., et al. Shortened Telomere length is associated with increased risk of cancer: A meta-analysis. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wentzensen I.M., Mirabello L., Pfeiffer R.M., Savage S.A. The association of telomere length and cancer: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2011;20:1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang C., Chen X., Li L., Zhou Y., Wang C., Hou S. The Association between Telomere Length and Cancer Prognosis: Evidence from a Meta-Analysis. PLoS ONE. 2015;10:e0133174. doi: 10.1371/journal.pone.0133174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu X., Han W., Xue W., Zou Y., Xie C., Du J., Jin G. The association between telomere length and cancer risk in population studies. Sci. Rep. 2016;6:22243. doi: 10.1038/srep22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang X., Zhao Q., Zhu W., Liu T., Xie S.-H., Zhong L.-X., Cai Y.-Y., Li X.-N., Liang M., Chen W., et al. The Association of Telomere Length in Peripheral Blood Cells with Cancer Risk: A Systematic Review and Meta-analysis of Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2017;26:1381–1390. doi: 10.1158/1055-9965.EPI-16-0968. [DOI] [PubMed] [Google Scholar]
- 120.Adam R., Díez-González L., Ocaña A., Šeruga B., Amir E., Templeton A.J. Prognostic role of telomere length in malignancies: A meta-analysis and meta-regression. Exp. Mol. Pathol. 2017;102:455–474. doi: 10.1016/j.yexmp.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 121.Xu X., Qu K., Pang Q., Wang Z., Zhou Y., Liu C. Association between telomere length and survival in cancer patients: A meta-analysis and review of literature. Front. Med. 2016;10:191–203. doi: 10.1007/s11684-016-0450-2. [DOI] [PubMed] [Google Scholar]
- 122.Prescott J., Du M., Wong J.Y.Y., Han J., De Vivo I. Paternal age at birth is associated with offspring leukocyte telomere length in the nurses’ health study. Hum. Reprod. 2012;27:3622–3631. doi: 10.1093/humrep/des314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Broer L., Codd V., Nyholt D.R., Deelen J., Mangino M., Willemsen G., Albrecht E., Amin N., Beekman M., de Geus E.J.C., et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet. 2013;21:1163–1168. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martin-Ruiz C.M., Baird D., Roger L., Boukamp P., Krunic D., Cawthon R., Dokter M.M., van der Harst P., Bekaert S., de Meyer T., et al. Reproducibility of telomere length assessment: An international collaborative study. Int. J. Epidemiol. 2015;44:1673–1683. doi: 10.1093/ije/dyu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Montpetit A.J., Alhareeri A.A., Montpetit M., Starkweather A.R., Elmore L.W., Filler K., Mohanraj L., Burton C.W., Menzies V.S., Lyon D.E., et al. Montpetit Telomere Length: A Review of Methods for Measurement. Nurs. Res. 2015;63:289–299. doi: 10.1097/NNR.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hemann M.T., Strong M.A., Hao L.Y., Greider C.W. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/S0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 127.Abdallah P., Luciano P., Runge K.W., Lisby M., Géli V., Gilson E., Teixeira M.T. A two-step model for senescence triggered by a single critically short telomere. Nat. Cell Biol. 2009;11:988–993. doi: 10.1038/ncb1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin J., Cheon J., Brown R., Coccia M., Puterman E., Aschbacher K., Sinclair E., Epel E., Blackburn E.H. Systematic and Cell Type-Specific Telomere Length Changes in Subsets of Lymphocytes. J. Immunol. Res. 2016;2016:1–9. doi: 10.1155/2016/5371050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Blumenreich M.S. The White Blood Cell and Differential Count. Butterworths; Boston, MA, USA: 1990. [PubMed] [Google Scholar]
- 130.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Horvath S. Erratum to: DNA methylation age of human tissues and cell types. Genome Biol. 2015;16:96. doi: 10.1186/s13059-015-0649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Quach A., Levine M.E., Tanaka T., Lu A.T., Chen B.H., Ferrucci L., Ritz B., Bandinelli S., Neuhouser M.L., Beasley J.M., et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9:419–446. doi: 10.18632/aging.101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mahabir S., Wei Q., Barrera S.L., Dong Y.Q., Etzel C.J., Spitz M.R., Forman M.R. Dietary magnesium and DNA repair capacity as risk factors for lung cancer. Carcinogenesis. 2008;29:949–956. doi: 10.1093/carcin/bgn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wolf F.I., Trapani V. Magnesium and its transporters in cancer: A novel paradigm in tumour development. Clin. Sci. 2012;123:417–427. doi: 10.1042/CS20120086. [DOI] [PubMed] [Google Scholar]
- 135.Wolf F.I., Trapani V., Cittadini A., Maier J.A.M. Hypomagnesaemia in oncologic patients: To treat or not to treat? Magnes. Res. 2009;22:5–9. [PubMed] [Google Scholar]
- 136.Wolf F.I., Trapani V., Simonacci M., Boninsegna A., Mazur A., Maier J.A.M. Magnesium deficiency affects mammary epithelial cell proliferation: Involvement of oxidative stress. Nutr. Cancer. 2009;61:131–136. doi: 10.1080/01635580802376360. [DOI] [PubMed] [Google Scholar]
- 137.Zastre J.A., Sweet R.L., Hanberry B.S., Ye S. Linking vitamin B1 with cancer cell metabolism. Cancer Metab. 2013;1:16. doi: 10.1186/2049-3002-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peake R.W.A., Godber I.M., Maguire D. The effect of magnesium administration on erythrocyte transketolase activity in alcoholic patients treated with thiamine. Scott. Med. J. 2013;58:139–142. doi: 10.1177/0036933013496944. [DOI] [PubMed] [Google Scholar]
- 139.Patra K.C., Hay N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang R., Lahens N.F., Ballance H.I., Hughes M.E., Hogenesch J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2016;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paul K.N., Saafir T.B., Tosini G. The role of retinal photoreceptors in the regulation of circadian rhythms. Rev. Endocr. Metab. Disord. 2009;10:271–278. doi: 10.1007/s11154-009-9120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fu L., Kettner N.M. The Circadian Clock in Cancer Development and Therapy. Prog. Mol. Biol. Transl. Sci. 2013;119:221–282. doi: 10.1016/B978-0-12-396971-2.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen W.-D., Wen M.-S., Shie S.-S., Lo Y.-L., Wo H.-T., Wang C.-C., Hsieh I.-C., Lee T.-H., Wang C.-Y. The circadian rhythm controls telomeres and telomerase activity. Biochem. Biophys. Res. Commun. 2014;451:408–414. doi: 10.1016/j.bbrc.2014.07.138. [DOI] [PubMed] [Google Scholar]
- 145.Samulin Erdem J., Notø H.Ø., Skare Ø., Lie J.-A.S., Petersen-Øverleir M., Reszka E., Pepłońska B., Zienolddiny S. Mechanisms of breast cancer risk in shift workers: Association of telomere shortening with the duration and intensity of night work. Cancer Med. 2017;6:1988–1997. doi: 10.1002/cam4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kunieda T., Minamino T., Katsuno T., Tateno K., Nishi J., Miyauchi H., Orimo M., Okada S., Komuro I. Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circ. Res. 2006;98:532–539. doi: 10.1161/01.RES.0000204504.25798.a8. [DOI] [PubMed] [Google Scholar]
- 147.Uetani N., Hardy S., Gravel S.-P., Kiessling S., Pietrobon A., Wong N.N., Chénard V., Cermakian N., St-Pierre J., Tremblay M.L. PRL2 links magnesium flux and sex-dependent circadian metabolic rhythms. JCI Insight. 2017;2 doi: 10.1172/jci.insight.91722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Clark J.P., Kofuji P. Stoichiometry of N-methyl-D-aspartate receptors within the suprachiasmatic nucleus. J. Neurophysiol. 2010;103:3448–3464. doi: 10.1152/jn.01069.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Glasgow N.G., Siegler Retchless B., Johnson J.W. Molecular bases of NMDA receptor subtype-dependent properties. J. Physiol. 2014 doi: 10.1113/jphysiol.2014.273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Voma C. Low Hepatic Mg2+ Content promotes Liver dysmetabolism: Implications for the Metabolic Syndrome. J. Metab. Syndr. 2014;3 doi: 10.4172/2167-0943.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Voma C., Barfell A., Croniger C., Romani A. Reduced cellular Mg2+ content enhances hexose 6-phosphate dehydrogenase activity and expression in HepG2 and HL-60 cells. Arch. Biochem. Biophys. 2014;548:11–19. doi: 10.1016/j.abb.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Büttner M., Jezova D., Greene B., Konrad C., Kircher T., Murck H. Target-based biomarker selection—Mineralocorticoid receptor-related biomarkers and treatment outcome in major depression. J. Psychiatr. Res. 2015;66–67:24–37. doi: 10.1016/j.jpsychires.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 153.Shiels P.G., Davies R.W. Molecular Biology of the Neuron. Oxford University Press; Oxford, UK: 2004. Ageing and the death of neurones; pp. 439–468. [Google Scholar]