ABSTRACT
The contribution of environmental factors to pancreatic islet damage in type 1 diabetes remains poorly understood. In this study, we crossed mice susceptible to type 1 diabetes, where parental male (CD8+ T cells specific for IGRP206-214; NOD8.3) and female (NOD/ShiLt) mice were randomized to a diet either low or high in AGE content and maintained on this diet throughout pregnancy and lactation. After weaning, NOD8.3+ female offspring were identified and maintained on the same parental feeding regimen for until day 28 of life. A low AGE diet, from conception to early postnatal life, decreased circulating AGE concentrations in the female offspring when compared to a high AGE diet. Insulin, proinsulin and glucagon secretion were greater in islets isolated from offspring in the low AGE diet group, which was akin to age matched non-diabetic C57BL/6 mice. Pancreatic islet expression of Ins2 gene was also higher in offspring from the low AGE diet group. Islet expression of glucagon, AGEs and the AGE receptor RAGE, were each reduced in low AGE fed offspring. Islet immune cell infiltration was also decreased in offspring exposed to a low AGE diet. Within pancreatic lymph nodes and spleen, the proportions of CD4+ and CD8+ T cells did not differ between groups. There were no significant changes in body weight, fasting glucose or glycemic hormones. This study demonstrates that reducing exposure to dietary AGEs throughout gestation, lactation and early postnatal life may benefit pancreatic islet secretion and immune infiltration in the type 1 diabetic susceptible mouse strain, NOD8.3.
KEYWORDS: advanced glycation end products, dietary intervention, islet, insulin, insulitis, NOD8.3, Type 1 diabetes
Introduction
Type 1 diabetes is a chronic autoimmune disease which generally manifests in early life. Over the past three decades, the global incidence of type 1 diabetes has increased,1 particularly in individuals without any known high-risk genotypes and in infants.2,3 This suggests that environmental factors in utero4,5 or in early life may contribute to pancreatic islet autoimmunity and promote destruction of insulin-producing β-cells later in life.1 Indeed, autoantibodies to common pancreatic islet antigens are already measurable during the first two years of life in children who subsequently develop type 1 diabetes.6
A number of longitudinal studies in infants at risk of developing type 1 diabetes have provided insight into the contribution of environmental factors that could act as precipitating events.7–11 Nutritional factors have been of interest in these prospective birth cohorts for over a decade. For instance, the Trial to Reduce IDDM in the Genetically at-Risk (TRIGR) study in Finland11 and the Diabetes and Autoimmunity Study in the Young (DAISY) in Colorado,3 suggested that early or late exposure to certain foods may alter islet autoimmunity and increase the risk for type 1 diabetes. However, the multicenter German BABYDIAB study found only weak associations between infant feeding and the risk of developing type 1 diabetes,12 which may relate to variability in infant nutrition between the countries where individuals were recruited.13 Mirroring the pathogenesis seen in humans, the susceptibility to type 1 diabetes development in the non-obese diabetic (NOD/ShiLt) and transgenic NOD8.3 mouse can also be influenced by environmental factors.14–18
Protein modifications termed advanced glycation end products (AGEs) are environmental factors which, together with modifications in their receptor, RAGE, associate with risk for islet autoimmunity and type 1 diabetes development.19–21 AGEs are made endogenously22 and may be absorbed from dietary sources.23,24 Circulating levels of AGEs appear to correlate between mother and baby,25 suggesting maternal transmission of AGEs to the fetus. Previous studies have identified that decreasing dietary AGE intake can improve glucose homeostasis in individuals with type 2 diabetes26 and in healthy, but overweight, adults.27 In type 1 diabetes, dietary reduction in AGE intake across generations of NOD/ShiLt mice suppressed diabetes incidence and improved glucose tolerance,28 confirming previously described effects of AGEs on murine islet function,29,30 islet infiltration,29 and glucose homeostasis29,31,32 in the NOD/ShiLt mouse and the type 2 diabetic db/db mouse. Together, these data highlight the importance of excessive AGEs as risk factors that impact on metabolic health in diabetes.
RAGE, which is expressed on cells from the innate and adaptive immune system, including antigen presenting cells33,34 and T cells35–38 allows for interaction with a number of ligands, including AGEs.19 Manipulating AGE concentrations has been shown to affect macrophage proliferation in vitro,39 CD4+ T cell proportions and their ability to proliferate in response to β-cell antigens in adult NOD/ShiLt mice.28 These, and other studies reviewed elsewhere,19 emphasize interplay of RAGE and its ligands in immunology and autoimmunity that is yet to be fully appreciated.
In this study, we explored the influence of low and high dietary AGE exposure from conception to early postnatal life in islet function and immune cell infiltration in a transgenic mouse strain susceptible to accelerated type 1 diabetes development. We fed IGRP206–214 NOD8.3 male and NOD/ShiLt female mating pairs a diet that was either low or high in AGE content and after weaning, maintained the NOD8.3 female offspring on the same parental diet until sacrifice at day 28. We examined effects of both dietary exposures on glucose homeostasis, pancreatic histopathology, static ex vivo hormone secretion and immune cell subsets within lymphoid tissues in the NOD8.3 female offspring.
Results
Biochemical parameters
Offspring exposed to a low AGE (LAGE) diet, from conception until the study end at day 28 of life, had lower circulating AGE concentrations (carboxymethyllysine; CML) as compared to offspring exposed to a high AGE (HAGE) diet (p = 0.03; Table 1). At the study end, fasting plasma insulin, proinsulin, glucagon and blood glucose concentrations, as well as body weight were not different between groups (Table 1).
Table 1.
Biochemical parameters of NOD8.3+ female 28 day old pups after parental and weaning diets either high (HAGE) or low (LAGE) in AGE content.
| HAGE | LAGE | |
|---|---|---|
| Circulating AGE (CML; ng/mL) | 16.8 (17.8) | 0.0 (7.2)* |
| Fasted Blood Glucose (mmol/L) | 8.5 (3.7) | 8.0 (3.9) |
| Fasted Plasma Insulin (pmol/L) | 88.8 (95.2) | 102.0 (129.3) |
| Fasted Plasma Proinsulin (pmol/L) | 18.6 (23.3) | 21.6 (22.3) |
| Fasted Plasma Glucagon (pmol/L) | 3.08 (2.0) | 2.20 (5.99) |
| Body Weight (g) | 12.0 (4.0) | 13.0 (4.3) |
Median (Interquartile rage) shown.
P = 0.03 (Mann Whitney U test). n = 6–10 mice/group.
Exposure to low dietary AGEs from conception to early postnatal life increases islet hormone secretion ex vivo
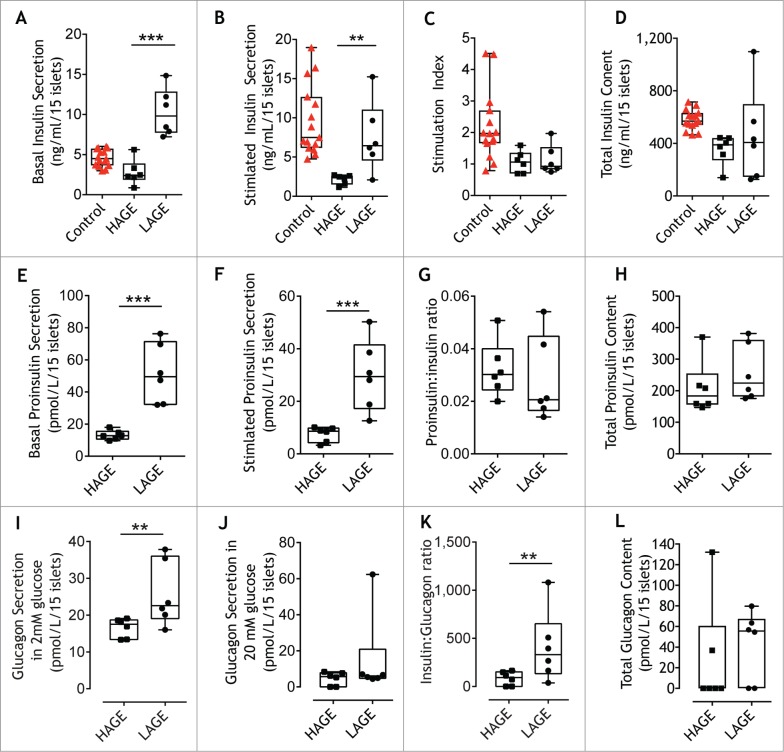
Type 1 diabetes is characteristically preceded by defects in insulin secretion.40–42 Pancreatic islets were isolated and exposed to various concentrations of glucose to stimulate insulin secretion and measure glucagon secretion. Insulin secretion from LAGE islets was 4-fold higher at both 2.8 mM (p = 0.002; Fig. 1A) and 20 mM (p = 0.02; Fig. 1B) glucose concentrations when compared to islets from HAGE offspring. The ratio of insulin released between stimulated and basal glucose concentrations (stimulation index) did not differ between treatment groups (Fig. 1C). At the end of the secretion assay, islets isolated from LAGE offspring had a more variable insulin content compared to that of HAGE offspring, but no significant differences were seen between groups (Fig. 1D). Stimulated insulin secretion and total insulin content from islets from NOD8.3 offspring exposed to a low AGE diet was more comparable to non-diabetes susceptible control mice than from NOD8.3 offspring exposed to a high AGE diet (Fig. 1B-D).
Figure 1.
Low AGE diets increase ex vivo islet hormone secretion. Insulin secretion during (A) resting (2.8 mM glucose) and (B) following stimulation (20 mM glucose) from islets isolated from 28 day old mice (6 replicates from n = 4 mice/group, black symbols) fed either a high (HAGE) or low (LAGE) AGE diet, or 28 day old C57BL/6 control mice (5 replicates from n = 3 mice; WT, red symbols). (C) Stimulation index calculated from insulin secreted during exposure to 2.8 mM and 20 mM glucose. (D) Insulin content after acid ethanol extraction. Proinsulin secretion in response to 2.8 mM glucose (E) and 20 mM glucose (F). (G) Proinsulin to insulin ratio at 20 mM glucose. (H) Proinsulin content after acid ethanol extraction. Glucagon secretion after 2.8 mM glucose (I) and 20 mM glucose (J). (K) Insulin to glucagon ratio at 20 mM glucose. (L) Glucagon content after acid ethanol extraction. The median (line), first and third quartile distributions (box), and minimum and maximum values (whiskers) are shown. ** p = 0.02, *** p = 0.002 Mann Whitney U Test.
Similar to that of insulin, proinsulin secretion from LAGE islets after 2.8 mM (Fig. 1E) and 20 mM (Fig. 1F) glucose concentrations showed a 4-fold increase compared to HAGE islets. While the majority of LAGE islets tended to have a reduced proinsulin/insulin ratio compared to HAGE islets, this effect was not significant (Fig. 1G). Islet proinsulin content after acid ethanol treatment did not differ between groups (Fig. 1H). Failure of the glucagon response to hypoglycemia,43 and insufficient glucagon suppression during hyperglycemia are observed in individuals with type 1 diabetes.44 Glucagon secretion was elevated during low glucose concentrations in islets isolated from LAGE offspring (p = 0.02; Fig. 1I) and reduced by 3.5-fold under high glucose concentrations (Fig. 1J). Although the overall decrease in glucagon secretion was ∼3-fold between glucose concentrations when islets were exposed to a HAGE diet (compare HAGE Fig. 1I and Fig. 1J), islets derived from LAGE fed mice had a greater insulin/glucagon ratio under high glucose conditions (p = 0.02; Fig. 1K). Islet glucagon content upon acid extraction showed no differences between groups (Fig. 1L).
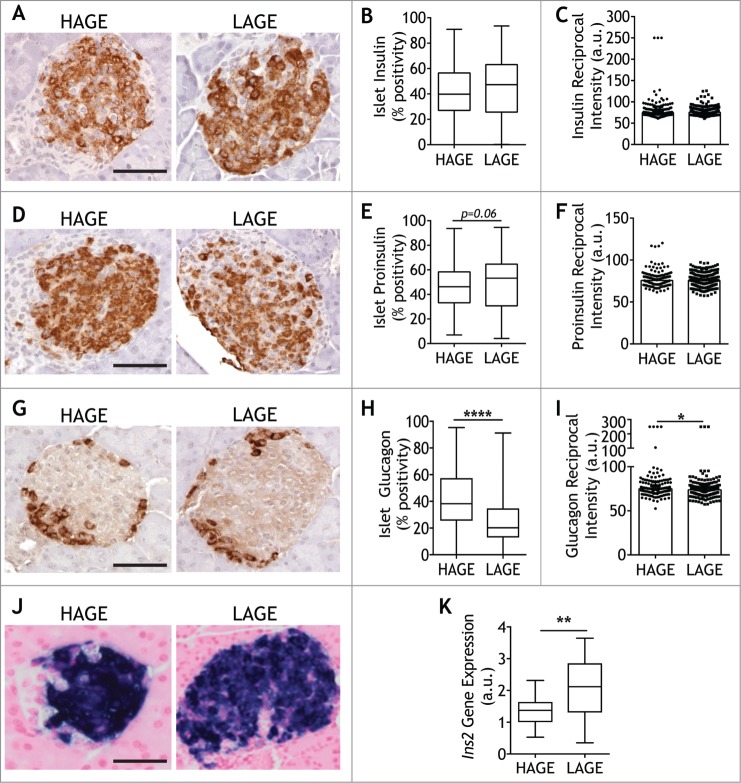
We next determined whether the expression of islet hormones differed between LAGE and HAGE offspring. Morphometric analyses of pancreatic islet immunohistochemical staining for insulin (Fig. 2A) revealed a similar pattern of staining in both groups (Fig. 2B). Determination of the reciprocal intensity45 of insulin staining within islets, which is directly proportional to the amount of diaminobenzidine (DAB) present, also showed no difference between offspring exposed to a low or high AGE diet (Fig. 2C). Proinsulin area in islets (Fig. 2D) tended to be higher in LAGE mice, although this did not reach statistical significance (P = 0.06; Fig. 2E). Consistent with insulin immunohistochemistry, no differences were observed in the intensity of proinsulin staining (Fig. 2F), resulting in an unchanged proinsulin to insulin ratio (data not shown). Glucagon localization (Fig. 2G) demonstrated more alpha cells in islets of offspring exposed to a high AGE diet (P < 0.0001; Fig. 2H) and greater glucagon intensity (P = 0.05; Fig. 2I) in comparison to LAGE offspring.
Figure 2.
Low AGE diets reduce alpha cell positivity and intensity Representative images of islets expressing insulin (A), proinsulin (D), and glucagon (G) were quantified in 28 day old NOD8.3 mice after exposure to a high (HAGE; left panel) or low (LAGE; right panel) AGE diet (n = 5–7/group). Chromogen detection (DAB+) was quantified using Visiopharm software and expressed as DAB+/islet area and as DAB reciprocal intensity from sections using antibodies against insulin (B, C), proinsulin (E, F) and glucagon (H, I) (n = 2 serial sections/mouse, 29 ± 15 islets/mouse). (J) Representative images of Ins2 gene expression by in situ hybridization (K) Quantification of Ins2 gene expression (4 pancreatic sections per mouse/n = 6 mice per group). Scale bar = 50 µm. Median is represented in quantification plots with single islets shown as either as circles (HAGE) or squares (LAGE). * p = 0.05, **** p < 0.0001 Mann Whitney U Test.
In situ hybridization (Fig. 2J) demonstrated increased expression of the insulin gene Ins2 in islets from LAGE offspring as compared with HAGE mice (Fig. 2K).
Exposure to low dietary AGEs from conception to early postnatal life reduced insulitis
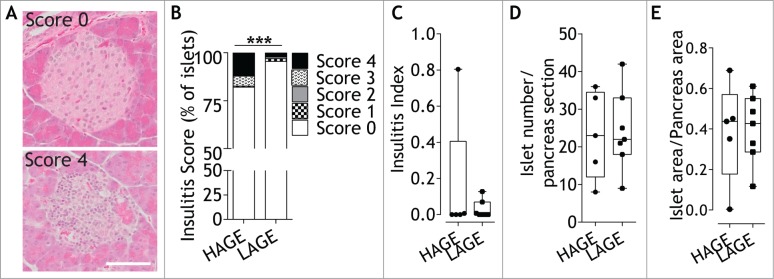
Immune infiltration into pancreatic islets (insulitis) was next examined since it is postulated as a pathological feature of type 1 diabetes development.40 Histopathology and chi-square test revealed that the severity of insulitis was significantly reduced in offspring exposed to a low AGE diet from conception (p = 0.0008; Fig. 3A, B), although there was no difference in the overall insulitis index (Fig. 3C). There were no differences in islet number (p = 0.51; Fig. 3D) or islet area (p = 0.98; Fig. 3E) between groups.
Figure 3.
Dietary modulation decreases insulitis without changing islet numbers or area. (A) Representative images of immune infiltration in hematoxylin and eosin stained pancreatic islets scored as either no infiltrate (score 0) or end stage insulitis (score 4) from 28 day old NOD8.3 mice fed either a high (HAGE) or low (LAGE) AGE diet. Scale bar represents 50 µm. (B) Islets were scored for the degree of insulitis described as (0) no infiltrate, (1) perivascular/periductal infiltrate, (2) <25% infiltrate, (3) <75% infiltrate, (4) end stage insulitis. n = 5–7/group. *** p = 0.0008 Chi-square test for trend. (C) Insulitis index calculated from insulitis score in (B). (D) Islet number per pancreatic section (n = 5–7/group, 23 ± 11 islets/mouse). (E) Insulin+ islet area per pancreatic area (n = 5–7/group, 37 ± 7 islets/mouse). The median (line), first and third quartile distributions (box), and minimum and maximum values (whiskers) and individual mice are shown as circles (HAGE) or squares (LAGE).
Low dietary AGE exposure from conception to early postnatal life increased the variance of CD4+ and CD8+ T cells and cDCs in local lymphoid tissues and proportions of pDCs in the spleen
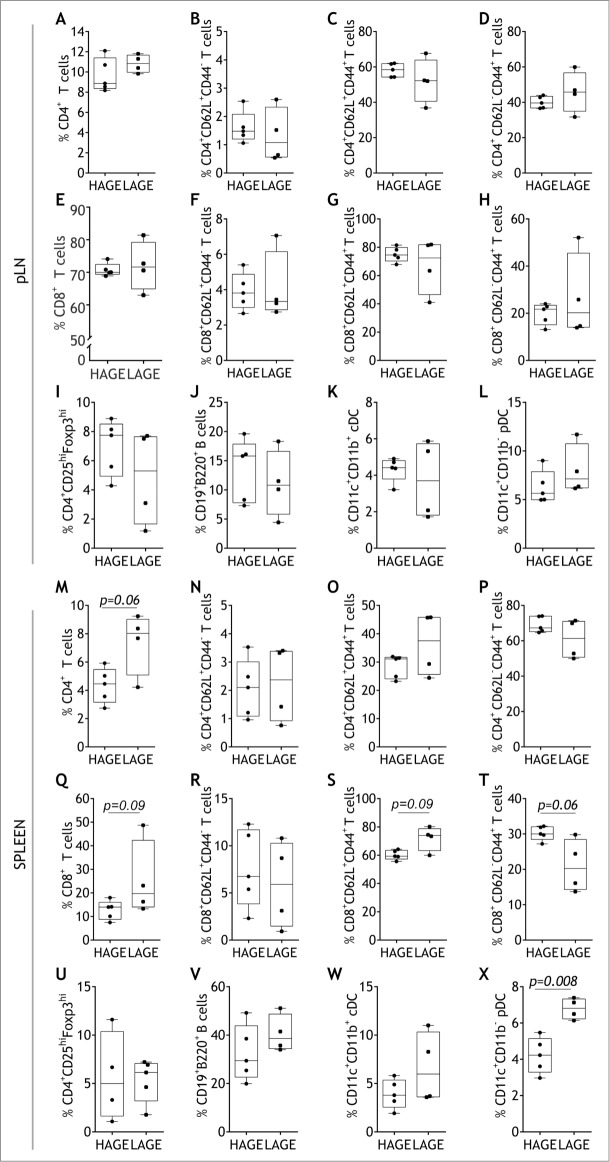
Given the reduction in pancreatic islet immune cell infiltration in LAGE offspring, we studied the proportion of T cells, B cells and dendritic cells in the pancreatic lymph nodes (pLN) and spleen. Despite an increase in variance in central and effector memory subsets (p = 0.04 and p = 0.03, respectively), there was no change in the proportion of total, naïve (CD62L+CD44−) or antigen experienced (CD62L+/−CD44+) CD4+ or CD8+ T cells in the pLN in LAGE offspring (Fig. 4A-H). Regulatory (CD4+CD25hi Foxp3hi) T cells (Tregs) were not affected by LAGE treatment (Fig. 4I). There was an increase in the variance of CD11c+CD11b+ conventional dendritic cells (cDCs) in the pLN of LAGE offspring (p = 0.05; Fig. 4K), but no proportional changes were observed in cDCs, or other antigen presenting subsets such as CD11c+CD11b− plasmacytoid dendritic cells (pDCs) or CD19+B220+ B cells (Fig. 4F, L).
Figure 4.
Low AGE diets elevate proportions of CD4+T cells and plasmacytoid dendritic cells within the spleen. Proportions of total CD4+ (A), naïve CD4+CD62L+CD44− (B), antigen experienced CD4+CD62L+CD44+ (C), antigen experienced CD4+CD62L−CD44+ (D), total CD8+ E), and naïve CD8+CD62L+CD44− (F), antigen experienced CD8+CD62L+CD44+ (G), antigen experienced CD8+CD62L−CD44+ (H), CD4+CD25hiFoxp3hi T regulatory (I), CD19+B220+ B (J), CD11c+CD11b+ conventional dendritic (cDC; K) and CD11c+CD11b− plasmacytoid dendritic (pDC; L) cells within the pancreatic lymph node (pLN). Proportions of total splenic CD4+ (M), naïve CD4+CD62L+CD44− (N), antigen experienced CD4+CD62L+CD44+ (O), antigen experienced CD4+CD62L−CD44+ (P), total CD8+ (Q), and naïve CD8+CD62L+CD44− (R), antigen experienced CD8+CD62L+CD44+ (S), antigen experienced CD8+CD62L−CD44+ (T) T cells, CD4+CD25hiFoxp3hi T regulatory (U), CD19+B220+ B (V), CD11c+CD11b+ cDC (W) and CD11c+CD11b− pDC (X) cells (n = 4–5/group). The median (line), first and third quartile distributions (box), and minimum and maximum values (whiskers) are shown. Trends are reported as italicized p values, Mann Whitney U Test.
Except for the increasing trend in total CD4+ T cells (p = 0.06; Fig. 4M), a low AGE environment did not promote an increase in the proportion of splenic CD4+ T cell naïve or memory subsets compared to a high AGE environment (Fig. 4N-P). A similar increasing trend was observed in the proportion (p = 0.09) and variance (p = 0.03) of total splenic CD8+ T cells in LAGE offspring compared to HAGE diet-fed offspring (Fig. 4Q). This shift corresponded with an increase in central memory T cells and a reduction effector memory T cells in LAGE mice, but these differences remained not significant (p = 0.09 and p = 0.06, respectively; Fig. 4S, T). While low AGE dietary exposure in utero did not promote changes in the Treg, B cell or cDC subsets within the spleen (Fig. 4U-W), higher proportions were observed in the pDC subset compared to high AGE diet-fed offspring (p = 0.008; Fig. 4X).
Exposure to low dietary AGEs from conception to early postnatal life altered islet expression of the AGE, CML, and AGE receptors
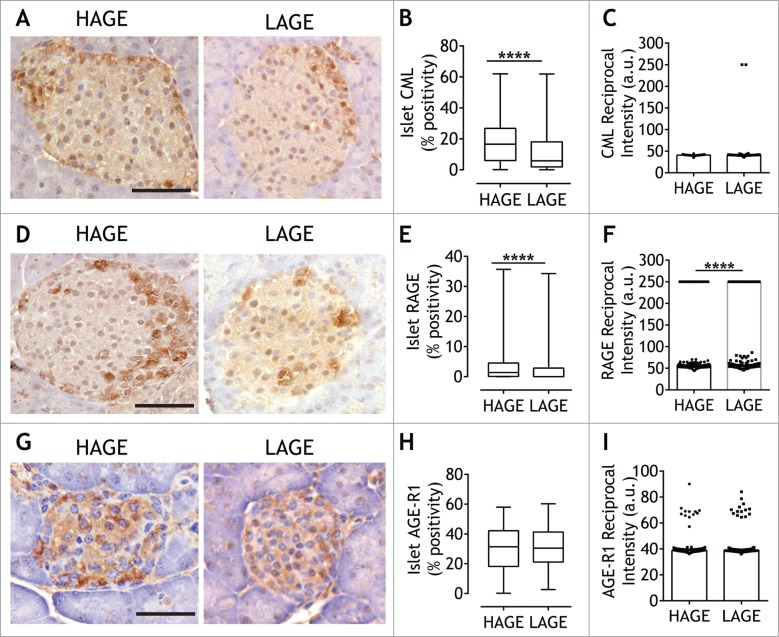
In agreement with circulating levels of the clinically relevant AGE protein modification, CML46 (Table 1), the area of islet CML positivity in LAGE offspring was 2.8-fold lower compared to HAGE (p < 0.0001; Fig. 5A, B), while the reciprocal intensity of CML did not differ between groups (Fig. 5C). A proportion of NOD8.3 islet cells also expressed one of the AGE cell surface receptors, RAGE (Fig. 5D). Although variable, there was less RAGE positivity in islets after exposure to a low AGE diet (p < 0.0001; Fig. 5E). Quantification of the reciprocal DAB intensity also revealed considerable variation, particularly in the mice exposed to a low AGE diet, however, in comparison to a high AGE diet, intensity was reduced (p < 0.0001; Fig. 5F). In pancreatic islets, the AGE clearance receptor, advanced glycation end product- receptor 1 (AGE-R1) (Fig. 5G) was more abundant than RAGE (compare Fig. 5H and Fig. 5E). Unlike anti-RAGE staining, however, low dietary AGEs did not affect AGE-R1 islet positivity (Fig. 5H) or stain intensity (Fig. 5I).
Figure 5.
Low AGE dietary exposure reduces pancreatic CML and RAGE expression. Representative images of pancreatic sections from 28 day old NOD8.3+ mice fed a high AGE diet (HAGE; left panel) or low AGE diet (LAGE; right panel) after chromogen detection (DAB) using antibodies against CML (A), RAGE (D) and AGE-R1 (G) (n = 5–7/group). Scale bar represents 50 µm. The percentage of DAB+/islet and DAB reciprocal intensity from CML (B, C), RAGE (E, F) and AGE-R1 (H, I) were quantified using Visiopharm software (n = 2 serials sections/mouse, 29 ± 15 islets/mouse). Median is represented in quantification plots with single islets shown as either as circles (HAGE) or squares (LAGE). ****p < 0.0001 Mann Whitney U Test.
Discussion
In a mouse model of accelerated type 1 diabetes, we demonstrate that prenatal and early postnatal exposure to low dietary AGEs increased glycemic hormone secretion and Ins2 gene expression, decreased islet immune cell infiltration, suggesting a reduced susceptibility to type 1 diabetes development. A low AGE diet from conception, pregnancy, and lactation (parental diet) through to early life (offspring post-weaning diet) resulted in lower circulating concentrations of the AGE, CML, and reduced pancreatic expression of glucagon, CML and RAGE.
Environmental influences such as the diet consumed by parents prior to conception during pregnancy and lactation and that consumed by the offspring in postnatal life, may predispose individuals to metabolic abnormalities47,48 and chronic diseases such as diabetes.49 Maternal diets and early infant feeding patterns have been associated with changes in type 1 diabetes-related autoantibodies in children. For example, lower consumption of vegetables by mothers, assessed by questionnaires immediately following birth, was associated with a higher risk of islet autoimmunity in children.50 The introduction of gluten to newborns at less than 3 months of age also increased the risk of developing islet autoantibodies.12 Similarly, more frequent consumption of cow's milk in childhood related to a greater chance of developing type 1 diabetes-associated autoantibodies.51 However, no longitudinal study to date has investigated whether beta cell function and autoantibody development are impacted by parental dietary AGE intake, maternal-infant AGE transmission (in utero and lactation), or early life AGE consumption.
In line with this paucity of data, we randomized mating pairs of mice to one of two identical diets, that differed only in their AGE content and maintained offspring on the respective diet post-weaning. To our knowledge, this is the first time that the transgenic NOD8.3 mouse model was used to examine the impact of parental dietary programming of susceptibility factors for type 1 diabetes development, such as pancreatic islet hormone secretion and infiltration of immune cells. Low AGE feeding increased insulin, proinsulin and glucagon secretion from islets isolated from 28 day-old mice in the context of decreased islet immune infiltration. Further, the expression of the Ins2 gene was also increased by exposure to a low AGE diet. This finding is supported by previous studies where chronic administration of high AGE diets to healthy rodents impaired insulin secretion, expression and led to islet infiltration.29,30 Other studies in pancreatic beta cell lines have demonstrated that chronic exposure to high AGE concentrations decreases insulin secretion, most likely via increases in oxidative stress/reactive oxygen species production,29,52–54 by limiting the effectiveness of anti-oxidant pathways and enzymes.29,30 AGEs have also been shown to impair insulin secretion in otherwise healthy isolated islets.29,30,54 Suppression in glucagon secretion was also shown following high glucose conditions in the LAGE offspring. One could speculate that, in the context of type 1 diabetes, a LAGE diet may therefore allow for more appropriate glucagon secretion given that glucagon secretion is physiologically a natural antagonist of insulin secretion. Indeed, we observed a higher insulin/glucagon ratio and lower alpha cell numbers in the islets of LAGE offspring in the context of higher insulin secretion as compared with the HAGE offspring. To our knowledge, the effects of AGEs on glucagon secretion are novel and have not been previously reported. We did not however, identify the mechanism responsible for the changes in glucagon and insulin secretion following a low AGE diet due to limited islet material. Although these increases in hormone secretion were not seen in the circulation, our findings are consistent with those from pre-diabetic individuals where fasting circulating insulin levels appear normal and abnormalities are only detected during stimulated secretion during glucose tolerance tests.55 Thus, future work should investigate the mechanism by which low AGE dietary exposure impacts glucagon secretion at different stage of diabetes including non-diabetic control mice.
In 28 day-old NOD8.3 mice, we observed that prenatal and early postnatal reductions in dietary AGEs did not result in statistically significant changes in the proportions of CD4+ T cells, CD8+ T cells, Tregs, B cells or cDCs in the pancreatic lymph node and the spleen. However, there was a trend towards increases in CD4+ splenic T cells. This increase did not appear to be due to an elevation in antigen-experienced, CD44+ T cells and may be a consequence of higher T cell turnover, reductions in other immune cell subsets or represent a desirable cytokine environment for T cell proliferation.56 Peppa et al., demonstrated that exposure to low dietary AGEs throughout prenatal development in the NOD/ShiLt mouse reduced pancreatic CD4+ T cell proportions which remained unresponsive to β-cell antigen stimulation.28 On the other hand, splenic lymphocytes, which mainly expressed IL-10, remained responsive to antigenic stimuli. The authors speculated that low AGE environments allowed for the selective recruitment of T helper 2 (Th2) cells in the spleen, inhibiting autoreactive T cell recruitment to the inflamed pancreas. While further data would be required to confirm these processes in the NOD8.3 model, it is conceivable that AGEs can influence T cell polarization and T regulatory (Treg) cell function.19 Previous reports suggest that naïve human CD4+ T cells increase RAGE expression57 and secretion of Th17 and Th1 cytokines, when exposed to AGEs.37 AGEs also appear to reduce Treg suppression in vitro57 and, in an animal model with impaired AGE clearance, high levels of circulating AGEs associated with reduced Treg numbers.58 In addition, we also observed an increase in the proportions of pDCs in the spleen after low AGE exposure. This has not been previously described. pDCs are key mediators in autoimmunity and are involved in regulating T-cell mediated responses.59 However, in the present study the function, proliferation or suppressive capacity of the T cell and antigen presenting cell subsets was not examined due to the limited material obtained from the four week old mice. These data however, do support previous studies suggesting that a low AGE environment may promote a more self-tolerant environment. However, whether an improvement in β-cell health and function causes a down-modulation of the immune system in early life represents an important research area to explore.
Transmission of AGEs between mother and baby occurs in utero and also during breastfeeding, and maternal AGE levels predict both insulin levels and insulin resistance in the 12-month-old infant.25 In the present study, we did not measure maternal or paternal AGE levels, but circulating concentrations of the AGE modification, CML, was reduced in offspring exposed to low dietary AGEs, which is consistent with previous dietary studies.31,32,60–62 CML was also lowered within the pancreas, which corresponded to a reduction in RAGE islet positivity. Indeed, in islet cell autoantibody positive children, elevations in circulating CML are an independent predictor of type 1 diabetes progression.20 In the NOD/ShiLt mouse, Peppa et al., demonstrated a loss of diabetes protection after pups fed a low AGE diet in utero were substituted with a high AGE diet after weaning.28 Hence, in future studies, it would be important to determine the relative contributions of maternal versus paternal dietary AGEs on infant susceptibility to type 1 diabetes, and disease progression, as well as the effect of AGE exposure in relation to cellular events including proliferation and apoptosis, during different critical periods of development (i.e. conception, prenatal, lactation and/or post-weaning).
Similar to our short-term dietary intervention, a previous study in the related NOD/ShiLt mouse, showed that 50 weeks of a low AGE diet did not result in changes in body weight or blood glucose concentrations in females.28 However, by 16 weeks of age, the F1 progeny prenatally exposed to a low AGE diet, had improved glucose homeostasis and less insulitis which translated to reduced diabetes incidence as compared to mice prenatally exposed to high AGEs.28 Since we aimed to determine the effects of perinatal dietary AGE intervention on pancreatic islet function and immune system in early life, we sacrificed mice at 28 days of age. Indeed, following NOD8.3 offspring to diabetes diagnosis, post-AGE dietary intervention, and characterizing their islet function, immunology and disease pathology overtime, would be worthwhile in future studies.
For the first time, the effect low and high dietary AGE content at mating, in utero and post-weaning have been examined in a model of accelerated type 1 diabetes, the NOD8.3 mouse. A low AGE diet elevated hormonal secretion, decreased immune cell infiltration into the pancreatic islets of offspring and resulted in elevated proportions of pDCs in the spleen, in early life. These findings support future studies, which should examine the role of both prenatal and early life exposure to dietary AGEs in increasing risk for the development of type 1 diabetes.
Materials and methods
Preclinical study design
This study adhered to the National Health and Medical Research Council of Australia (NHMRC) guidelines and the protocol was approved by the Alfred Medical, Research and Education Precinct Animal Ethics committee (AMREP AEC) and the University of Queensland Animal Ethics committee. Six-week old female NOD/ShiLt mice and six-week old male NOD8.3+ were purchased from Walter and ELISA Hall Institute, Australia. Three females (6-weeks old) were housed with one male (6-weeks old) and each box was randomized to a diet either low (LAGE) or high (HAGE) in AGE content. The groupings remained together for one week for mating. The NOD8.3 strain express the TCRαβ rearranged transgenes derived from the H-2Kd-restricted, beta cell reactive, CD8+ T cell clone NY8.3 specific for IGRP206–214.63 Female mice found to be pregnant were maintained on the same LAGE or HAGE regimen throughout pregnancy and lactation. Offspring were genotyped to identify female NOD8.3+ (n = 10 offspring/high or low AGE dietary group, 5 different litters). After weaning at day 21, these female NOD8.3+ offspring were maintained on the same diet as the parents until the end of the study at day 28 of life. Low AGE diets (unbaked AIN-93G (LAGE); Specialty Feeds) and high AGE diets (baked AIN-93G for 1 h at 165°C (HAGE); Specialty Feeds), contained the AGE Nε (carboxymethyl)lysine (CML), (LAGE; 20.09 nmol/mol lysine/100 mg CML vs HAGE; 101.9 nmol/mol lysine/100 mg CML), as previously reported.29
Islet isolation
Islets were isolated from the juvenile NOD8.3 female mice as previously described.29 Islets were also isolated from high AGE fed juvenile female C57Bl/6J mice as controls for insulin secretion. Briefly, the bile duct was cannulated and infused with 0.5 mg/ml ice-cold collagenase P (11213857001; Roche Biochemicals) in RPMI 1640 (11875119; Gibco) containing L-glutamine (25030081; Gibco), 100 U/mL penicillin, 100 µg/mL streptomycin (15140122; Gibco). The inflated pancreas was then excised and incubated in 10 ml of warm RPMI 1640 at 37°C for 20 min. Once the digestion was complete, the pancreas was disrupted by vigorous shaking, filtered through 500 µm mesh and washed three times in cold RPMI 1640 with centrifugation for 3 min, 1,000 rpm at 4°C. The pancreatic islets were separated from exocrine tissue by histopaque density gradient (10771; Sigma Aldrich). The hand-picked islets were rested overnight in RPMI 1640 medium containing L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin and 5.0 mM glucose with 10% (vol/vol) heat-inactivated foetal bovine, in a 37°C humidified atmosphere of 95% air: 5% CO2.
Static assessment of islet hormone secretion
Handpicked islets (n = 4 mice/group) were pooled and 15 islets were plated per well (n = 6 wells). Hormone secretion assays were performed using low (2.8 mM) and high (20 mM) glucose concentrations, as previously described.29 Briefly, isolated islets were washed once in Krebs-Ringer bicarbonate buffer (KRBB) with 2.8 mM glucose and pre-incubated for 30 min in 0.5 mL of the same buffer. This medium was collected and measured for basal levels of insulin and proinsulin secretion and stimulating levels of glucagon secretion. The islets were then incubated KRBB containing 20 mM glucose for another 30 min, in which the medium was collected and measured for glucose stimulated insulin and proinsulin secretion and the inhibition of glucagon secretion. After the test, islets were washed with PBS and lysed by acid ethanol to detect the total content of insulin, proinsulin and glucagon. Hormone concentrations in the incubation buffer and islet extract were measured using the mouse/rat insulin ELISA kit (EZRMI-13K; Linco Research), rat/mouse proinsulin ELISA kit (10-1232-01; Mercodia) and glucagon ELISA (10-1281-01; Mercodia). A stimulation index was calculated as the ratio of the insulin released during high glucose to the insulin release during low glucose treatment. The proinsulin to insulin ratio and insulin to glucagon ratio were calculated as the ratio of the proinsulin, insulin and glucagon release during high glucose conditions, respectively.
Biochemical assays
Fasting blood glucose was measured using a glucometer. Fasting plasma insulin (90080; Ultra-sensitive mouse insulin ELISA; Crystal Chem Inc.), plasma proinsulin (10-1232-01; Mercodia), plasma glucagon (10-1281-01; Mercodia) and fasting CML (STA-816; Oxiselect CML Competitive ELISA; Cell Biolabs Inc.) concentrations were determined using commercial ELISA kits according to the manufacturers' instructions.
Histological and immunohistochemical analysis
Pancreata were fixed in 10% neutral buffered formalin and embedded in paraffin. Serial sections (4 µm) were taken 100 µm apart. To determine the presence of islets sections (n = 2/mouse) were stained with hematoxylin and eosin using standard protocols. Insulitis of islets with ≥ 10 cells were semi-quantified using a previously published method.64 Islet area per pancreas area was quantified using Visiopharm and anti-insulin stained immunohistochemical images described below. To determine proteins of interest, further pancreatic serial sections (n = 2, 100 µm apart) were blocked with 3% hydrogen peroxide (H1009; Sigma) and 10% donkey serum (S30-100; Merck Millipore), and stained with either primary antibodies (rat anti-insulin (MAB1417; R&D Systems); mouse anti-proinsulin (MAB13361; R&D Systems); rabbit anti-glucagon (A0565; DAKO); goat anti-RAGE (5484; Merck Millipore); rabbit anti-AGE-R1 (sc25558; Santa Cruz Biotechnology); rabbit anti-CML (ab27684; Abcam)) or used as negative controls (in blocking buffer; 10% donkey serum) overnight at 4°C. The presence of antigen was determined using secondary biotin-labelled antibodies, followed by avidin labelling (VEPK6100; Vector Laboratories) and 3,3′-diaminobenzidine detection (DAB; VESK4100; Vector Laboratories). Sections were counterstained with hematoxylin. Sections were imaged using an Olympus VS-ASW Slide Scanner light microscope (Olympus Australia Pty Ltd; Notting Hill, VIC, Australia) at a total magnification of x200. Quantitation of DAB positivity within each islet was completed in serial sections using Visiopharm® image analysis software (Olympus Australia Pty Ltd). To determine the quantity of antigen present from DAB+ areas, the reciprocal DAB intensity was calculated, as previously described.45
In Situ Hybridisation for Islet Ins2 Gene Expression
Gene expression of Ins2 in pancreatic islet sections was quantified in 7 μm paraffin embedded sections as previously described (Biol Reprod. 2012 Aug 23;87(2):43). Briefly, a cDNA template for Ins2 was generated from pancreatic cDNA using PRIMERS, gel purified (Qiagen) and sequence verified (AGRF). Digoxigenin (DIG) labeled cRNA probes were synthesized in accordance with manufacturer's directions (Roche). In situ hybridizations on paraffin-embedded sections were done as previously described.65 A sense probe was used as a negative control. Quantification was performed using Visiopharm image software as per immunohistochemistry section above.
Flow cytometry
Pancreatic lymph nodes (pLN) and spleen were harvested from NOD8.3 mice and prepared as single-cell suspensions. Cell surface markers were assessed by staining the cells with pacific-blue conjugated anti-CD4 (558107), PerCP conjugated anti-CD8 (561092), to allophycocynin conjugated anti-CD44 (559250), fluorescein isothiocyanate conjugated anti-CD62 (561917), PECy7 conjugated anti-CD25 (552880), PECy7 conjugated CD19 (552854), APCCy7 conjugated anti-B220 (552094), PE conjugated anti-CD11b (553311), Pacific Orange-conjugated streptavidin against biotin-conjugated anti-CD11c (553800) (all antibodies 1:400; BD Bioscience). Intracellular staining with Foxp3 (560409; BD Biosciences) was performed according to the manufacturer's instructions. Flow cytometry was performed on FACSCanto II (BD Bioscience, San Jose, CA, USA) and data was analyzed with Flowjo analysis software (v10.0.8; BD Bioscience). Cells were gated on total lymphocytes and the plot was either gated on CD8+ and CD4+, CD19+ and B220+, or quadrant gates for CD11c and CD11b to determine CD11c+CD11b+ and CD11c+CD11b− sub-populations. The positive CD8 or CD4 cells were gated for additional activation markers CD44 and CD62L and Treg markers CD25 and Foxp3 (for the CD4+ population). Proportions were calculated as percentages of total live cells in each gated box, according to the parent gate.
Statistical analyses
Non-gaussian distributed data were analysed using a Mann-Whitney U test and are reported as median values with the interquartile range (IQR) and minimum and maximum values. Chi-squared test for trend was performed to analyze differences between insulitis frequencies. A value of p < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism (GraphPad Software v.6, San Diego, CA).
Funding Statement
This work was completed with support from the National Health and Medical Research Council of Australia (NHMRC; 1023664), the Victorian Government's Operational Infrastructure Support Program and the Mater Foundation. FYTY was the recipient of a conjoint NHMRC and Juvenile Diabetes Research Foundation Scholarship, LAG was supported by an Early Career Fellowship from the NHMRC and Heart Foundation (Australia; 1089763, 100519). AKF was supported by an Australian Postgraduate Award Scholarship. MTC has been supported by the Skip Martin Australian Diabetes Society Early Career Fellowship and the Australian and New Zealand Society of Nephrology Career Development Fellowship. JMF was supported by fellowships from the NHMRC (1004503, 1102935). SH was supported by a UQ Fellowship. AZ is supported by Kidney Health Australia (SCH17; 141516) and the Mater Research Foundation. DS holds an NHMRC project grant (APP1130255).
Abbreviations
- AGE
Advanced glycation end products
- AGE-R1
Advanced glycation end product receptor 1
- cDCs
Conventional dendritic cells
- CML
Nε (carboxymethyl)lysine
- GSIS
Glucose stimulated insulin secretion
- HAGE
High AGE diet
- IGRP
Islet-specific glucose-6-phosphatase catalytic subunit-related protein
- LAGE
Low AGE diet
- pDCs
Plasmacytoid dendritic cells
- pLN
Pancreatic lymph node
- RAGE
Receptor for advanced glycation end products
- TCR
T cell receptor
- Th
T helper cell
- Treg
T regulatory cell
Declaration of potential conflicts of interest
No potential conflicts of interest were disclosed
Acknowledgments
We would like to acknowledge the Baker IDI animal facility staff for the breeding and animal housing maintenance in this study. The authors would also like to acknowledge Mrs. Domenica McCarthy, Dr. David Vesey and Mr. Ali Ju for their technical assistance.
Statement of authorship
DJB and FYTY contributed to planning, performed experiments, analyzed and interpreted data and drafted the manuscript. DGS, SK, LAG, AZ and AKF performed experiments, analyzed data and reviewed the manuscript. RS, MTC, SH and PK contributed to planning experiments, interpretation of the data, and reviewed the manuscript. JMF is the guarantor of this work, designed and planned experiments, interpreted data and drafted/reviewed the manuscript.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, EURODIAB Study Group . Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: A multicentre prospective registration study. Lancet. 2009;373:2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 3.Catanzariti L, Faulks K, Moon L, Waters AM, Flack J, Craig ME. Australia's national trends in the incidence of Type 1 diabetes in 0-14-year-olds, 2000–2006. Diabet Med. 2009;26:596–601. doi: 10.1111/j.1464-5491.2009.02737.x. [DOI] [PubMed] [Google Scholar]
- 4.La Torre D, Seppänen-Laakso T, Larsson HE, Hyötyläinen T, Ivarsson SA, Lernmark Å, Oresic M, DiPiS Study Group . Decreased Cord-Blood Phospholipids in Young Age–at–Onset Type 1 Diabetes. Diabetes. 2013;62:3951–56. doi: 10.2337/db13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orešič M, Gopalacharyulu P, Mykkänen J, Lietzen N, Mäkinen M, Nygren H, Simell S, Simell V, Hyöty H, Veijola R, et al.. Cord Serum Lipidome in Prediction of Islet Autoimmunity and Type 1 Diabetes. Diabetes. 2013;62:3268–74. doi: 10.2337/db13-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, et al.. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–9. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frederiksen B, Kroehl M, Lamb MM, Seifert J, Barriga K, Eisenbarth GS, Rewers M, Norris JM. Infant Exposures and Development of Type 1 Diabetes Mellitus: The Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediatr. 2013;167:808–15. doi: 10.1001/jamapediatrics.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.T. S. Group The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Annals N York Acad Sci. 2008;1150:1–13. doi: 10.1196/annals.1447.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–8. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 10.Couper JJ, Steele C, Beresford S, Powell T, McCaul K, Pollard A, Gellert S, Tait B, Harrison LC, Colman PG. Lack of association between duration of breast-feeding or introduction of cow's milk and development of islet autoimmunity. Diabetes. 1999;48:2145–9. doi: 10.2337/diabetes.48.11.2145. [DOI] [PubMed] [Google Scholar]
- 11.Knip M, Virtanen SM, Seppä K, Ilonen J, Savilahti E, Vaarala O, Reunanen A, Teramo K, Hämäläinen AM, Paronen J, et al.. Dietary Intervention in Infancy and Later Signs of Beta-Cell Autoimmunity. N Eng J Med. 2010;363:1900–08. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes–associated autoantibodies. JAMA. 2003;290:1721–8. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 13.Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–48. doi: 10.1016/S0140-6736(16)30507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 15.Leiter EH. The Role of Environmental Factors in Modulating Insulin Dependent Diabetes In: de Vries RRP, Cohen IR, van Rood JJ, Editors, The Role of Micro-organisms in Non-infectious Diseases. London: Springer London; 1990. p. 39–55. [Google Scholar]
- 16.Virtanen SM, Knip M. Nutritional risk predictors of beta cell autoimmunity and type 1 diabetes at a young age. Am J Clin Nutr. 2003;78:1053–67. [DOI] [PubMed] [Google Scholar]
- 17.Hansen CHF, Krych L, Buschard K, Metzdorff SB, Nellemann C, Hansen LH, Nielsen DS, Frøkiær H, Skov S, Hansen AK. A Maternal Gluten-Free Diet Reduces Inflammation and Diabetes Incidence in the Offspring of NOD Mice. Diabetes. 2014;63:2821–32. doi: 10.2337/db13-1612. [DOI] [PubMed] [Google Scholar]
- 18.Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, McKenzie C, Kranich J, Oliveira AC, Rossello FJ, et al.. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol. 2017;18:552–62. [DOI] [PubMed] [Google Scholar]
- 19.Leung SS, Forbes JM, Borg DJ. Receptor for Advanced Glycation End Products (RAGE) in Type 1 Diabetes Pathogenesis. Curr Diab Rep. 2016;16:100. doi: 10.1007/s11892-016-0782-y. [DOI] [PubMed] [Google Scholar]
- 20.Beyan H, Riese H, Hawa MI, Beretta G, Davidson HW, Hutton JC, Burger H, Schlosser M, Snieder H, Boehm BO, et al.. Glycotoxin and autoantibodies are additive environmentally determined predictors of type 1 diabetes: A Twin and Population Study. Diabetes. 2012;61:1192–8. doi: 10.2337/db11-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salonen KM, Ryhanen SJ, Forbes JM, Harkonen T, Ilonen J, Laine AP, Groop PH, Knip M, Finnish Pediatric Diabetes Register . Circulating concentrations of soluble receptor for AGE are associated with age and AGER gene polymorphisms in children with newly diagnosed type 1 diabetes. Diabetes Care. 2014;37:1975–81. doi: 10.2337/dc13-3049. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9:233–45 doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 23.Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A. 1997;94:6474–79. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai W, He JC, Zhu L, Chen X, Zheng F, Striker GE, Vlassara H. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. 2008;173:327–36. doi: 10.2353/ajpath.2008.080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mericq V, Piccardo C, Cai W, Chen X, Zhu L, Striker GE, Vlassara H, Uribarri J. Maternally transmitted and food-derived glycotoxins: a factor preconditioning the young to diabetes? Diabetes Care. 2010;33:2232–7. doi: 10.2337/dc10-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uribarri J, Cai W, Ramdas M, Goodman S, Pyzik R, Chen X, Zhu L, Striker GE, Vlassara H. Restriction of Advanced Glycation End Products Improves Insulin Resistance in Human Type 2 Diabetes. Diabetes Care. 2011;34:1610–6. doi: 10.2337/dc11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Courten B, de Courten MP, Soldatos G, Dougherty SL, Straznicky N, Schlaich M, Sourris KC, Chand V, Scheijen JL, Kingwell BA, et al.. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: a double-blind, randomized, crossover trial. Am J Clin Nutr. 2016;103:1426–33 doi: 10.3945/ajcn.115.125427. [DOI] [PubMed] [Google Scholar]
- 28.Peppa M, He C, Hattori M, McEvoy R, Zheng F, Vlassara H. Fetal or neonatal low-glycotoxin environment prevents autoimmune Diabetes in NOD Mice. Diabetes. 2003;52:1441–8. doi: 10.2337/diabetes.52.6.1441. [DOI] [PubMed] [Google Scholar]
- 29.Coughlan MT, Yap FY, Tong DC, Andrikopoulos S, Gasser A, Thallas-Bonke V, Webster DE, Miyazaki J, Kay TW, Slattery RM, et al.. Advanced glycation end products are direct modulators of beta-cell function. Diabetes. 2011;60:2523–32. doi: 10.2337/db10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Z, Zhao C, Zhang XH, Zheng F, Cai W, Vlassara H, Ma ZA. Advanced Glycation End Products Inhibit Glucose-Stimulated Insulin Secretion through Nitric Oxide-Dependent Inhibition of Cytochrome c Oxidase and Adenosine Triphosphate Synthesis. Endocrinology. 2009;150:2569–76. doi: 10.1210/en.2008-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin Resistance and Type 2 Diabetes in High-Fat–Fed Mice Are Linked to High Glycotoxin Intake. Diabetes. 2005;54:2314–9. doi: 10.2337/diabetes.54.8.2314. [DOI] [PubMed] [Google Scholar]
- 32.Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci. 2012;109:15888–93. doi: 10.1073/pnas.1205847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura J, Uchigata Y, Yamamoto Y, Takeuchi M, Sakurai S, Watanabe T, Yonekura H, Yamagishi S, Makita Z, Sato A, et al.. AGE down-regulation of monocyte RAGE expression and its association with diabetic complications in type 1 diabetes. J Diabetes Complications. 2004;18:53–9. doi: 10.1016/S1056-8727(02)00281-7. [DOI] [PubMed] [Google Scholar]
- 34.Dumitriu IE, Baruah P, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol. 2005;35:2184–90. doi: 10.1002/eji.200526066. [DOI] [PubMed] [Google Scholar]
- 35.Akirav EM, Preston-Hurlburt P, Garyu J, Henegariu O, Clynes R, Schmidt AM, Herold KC. RAGE expression in human T cells: a link between environmental factors and adaptive immune responses. PLoS One. 2012;7:e34698. doi: 10.1371/journal.pone.0034698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moser B, Janik S, Schiefer AI, Mullauer L, Bekos C, Scharrer A, Mildner M, Rényi-Vámos F, Klepetko W, Ankersmit HJ. Expression of RAGE and HMGB1 in thymic epithelial tumors, thymic hyperplasia and regular thymic morphology. PLoS One. 2014;9:e94118. doi: 10.1371/journal.pone.0094118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Akirav EM, Chen W, Henegariu O, Moser B, Desai D, Shen JM, Webster JC, Andrews RC, Mjalli AM, et al.. RAGE ligation affects T cell activation and controls T cell differentiation. J Immunol. 2008;181:4272–8. doi: 10.4049/jimmunol.181.6.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moser B, Desai DD, Downie MP, Chen Y, Yan SF, Herold K, Schmidt AM, Clynes R. Receptor for advanced glycation end products expression on T cells contributes to antigen-specific cellular expansion in vivo. J Immunol. 2007;179:8051–8. doi: 10.4049/jimmunol.179.12.8051. [DOI] [PubMed] [Google Scholar]
- 39.Yui S, Sasaki T, Araki N, Horiuchi S, Yamazaki M. Induction of macrophage growth by advanced glycation end products of the Maillard reaction. J Immunol. 1994;152:1943–9. [PubMed] [Google Scholar]
- 40.Greenbaum CJ, Cuthbertson D, Krischer JP. Type 1 Diabetes Manifested Solely by 2-h Oral glucose tolerance test criteria. Diabetes. 2001;50:470–6. doi: 10.2337/diabetes.50.2.470. [DOI] [PubMed] [Google Scholar]
- 41.Kano Y, Kanatsuna T, Nakamura N, Kitagawa Y, Mori H, Kajiyama S, Nakano K, Kondo M. Defect of the First-Phase Insulin Secretion to Glucose Stimulation in the Perfused Pancreas of the Nonobese Diabetic (NOD) Mouse. Diabetes. 1986;35:486–90. doi: 10.2337/diab.35.4.486. [DOI] [PubMed] [Google Scholar]
- 42.Sherry NA, Tsai EB, Herold KC. Natural History of β-Cell Function in Type 1 Diabetes. Diabetes. 2005;54:S32–9. doi: 10.2337/diabetes.54.suppl_2.S32. [DOI] [PubMed] [Google Scholar]
- 43.Siafarikas A, Johnston RJ, Bulsara MK, O'Leary P, Jones TW, Davis EA. Early Loss of the Glucagon Response to Hypoglycemia in Adolescents With Type 1 Diabetes. Diabetes Care. 2012;35:1757–62. doi: 10.2337/dc11-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unger RH, Orci L. The role of glucagon in the endogenous hyperglycemia of diabetes mellitus. Annu Rev Med. 1977;28:119–30. doi: 10.1146/annurev.me.28.020177.001003. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen D. Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. Cancer InCytes. 2013;2:e. [Google Scholar]
- 46.Hwang JS, Shin CH, Yang SW. Clinical implications of Nε-(carboxymethyl)lysine, advanced glycation end product, in children and adolescents with type 1 diabetes. Diabetes Obes Metab. 2005;7:263–7. doi: 10.1111/j.1463-1326.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 47.Jahan-Mihan A, Rodriguez J, Christie C, Sadeghi M, Zerbe T. The Role of Maternal Dietary Proteins in Development of Metabolic Syndrome in Offspring. Nutrients. 2015;7:9185–217. doi: 10.3390/nu7115460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tzanetakou IP, Mikhailidis DP, Perrea DN. Nutrition During Pregnancy and the Effect of Carbohydrates on the Offspring's Metabolic Profile: In Search of the “Perfect Maternal Diet”. Open Cardiovasc Med J. 2011;5:103–9. doi: 10.2174/1874192401105010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerf ME. Parental high-fat programming of offspring development, health and beta-cells. Islets. 2011;3:118–20. doi: 10.4161/isl.3.3.15420. [DOI] [PubMed] [Google Scholar]
- 50.Brekke HK, Ludvigsson J. Daily vegetable intake during pregnancy negatively associated to islet autoimmunity in the offspring–the ABIS study. Pediatr Diabetes. 2010;11:244–50. doi: 10.1111/j.1399-5448.2009.00563.x. [DOI] [PubMed] [Google Scholar]
- 51.Virtanen SM, Hypponen E, Laara E, Vahasalo P, Kulmala P, Savola K, Räsänen L, Aro A, Knip M, Akerblom HK. Cow's milk consumption, disease-associated autoantibodies and type 1 diabetes mellitus: a follow-up study in siblings of diabetic children. Childhood Diabetes in Finland Study Group. Diabet Med. 1998;15:730–8. doi: 10.1002/(SICI)1096-9136(199809)15:9%3c730::AID-DIA646%3e3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 52.Lin N, Zhang H, Su Q. Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes Metab. 2012;38:250–7. doi: 10.1016/j.diabet.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Viviani GL, Puddu A, Sacchi G, Garuti A, Storace D, Durante A, Monacelli F, Odetti P. Glycated fetal calf serum affects the viability of an insulin-secreting cell line in vitro. Metabolism. 2008;57:163–9. doi: 10.1016/j.metabol.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Kong X, Wang GD, Ma MZ, Deng RY, Guo LQ, Zhang JX, Yang JR, Su Q. Sesamin Ameliorates advanced glycation end products-induced pancreatic β-Cell dysfunction and apoptosis. Nutrients. 2015;7:4689–704. doi: 10.3390/nu7064689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo SSS, Hawa M, Beer SF, Pyke DA, Leslie RDG. Altered Islet beta-cell function before the onset of Type-1 (Insulin-Dependent) diabetes-mellitus. Diabetologia. 1992;35:277–82. doi: 10.1007/BF00400930. [DOI] [PubMed] [Google Scholar]
- 56.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Han XQ, Gong ZJ, Xu SQ, Li X, Wang LK, Wu SM, Wu JH, Yang HF. Advanced glycation end products promote differentiation of CD4(+) T helper cells toward pro-inflammatory response. J Huazhong Univ Sci Technolog Med Sci. 2014;34:10–7. doi: 10.1007/s11596-014-1224-1. [DOI] [PubMed] [Google Scholar]
- 58.Pejnovic NN, Pantic JM, Jovanovic IP, Radosavljevic GD, Milovanovic MZ, Nikolic IG, Zdravkovic NS, Djukic AL, Arsenijevic NN, Lukic ML. Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes. 2013;62:1932–44. doi: 10.2337/db12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guery L, Hugues S. Tolerogenic and Activatory Plasmacytoid Dendritic Cells in Autoimmunity. Front Immunol. 2013;4:59. doi: 10.3389/fimmu.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–33. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lubitz I, Ricny J, Atrakchi-Baranes D, Shemesh C, Kravitz E, Liraz-Zaltsman S, Maksin-Matveev A, Cooper I, Leibowitz A, Uribarri J, et al.. High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Abeta deposition in an Alzheimer mouse model. Aging Cell. 2016;15:309–16. doi: 10.1111/acel.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hofmann SM, Dong HJ, Li Z, Cai W, Altomonte J, Thung SN, Zeng F, Fisher EA, Vlassara H. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes. 2002;51:2082–9. doi: 10.2337/diabetes.51.7.2082. [DOI] [PubMed] [Google Scholar]
- 63.Verdaguer J, Yoon JW, Anderson B, Averill N, Utsugi T, Park BJ, Santamaria P. Acceleration of spontaneous diabetes in TCR-beta-transgenic nonobese diabetic mice by beta-cell cytotoxic CD8+ T cells expressing identical endogenous TCR-alpha chains. J Immunol. 1996;157:4726–35. [PubMed] [Google Scholar]
- 64.Leiter EH. The NOD mouse: A model for insulin-dependent diabetes mellitus. Curr Protoc Immunol. ed: John Wiley & Sons, Inc., 1997;Suppl. 24:15.9.1–15.9.23. doi: 10.1002/0471142735.im1509s24. [DOI] [PubMed] [Google Scholar]
- 65.Dawson, PA, Rakoczy J, Simmons DG Placental, renal, and ileal sulfate transporter gene expression in mouse gestation.” Biol Reprod. 2012;87:43. [DOI] [PubMed] [Google Scholar]