Abstract
Background
Approximately 30% of patients with schizophrenia show an inadequate response to antipsychotics, termed treatment resistance. Neuroimaging studies may help elucidate the underlying neurobiological reasons that certain patients show inadequate treatment response, and help identify them earlier. In addition, studies examining the effect of clozapine on the brain may help identify which aspects of clozapine make it uniquely effective in treatment resistance.
Method
We performed a systematic search of PubMed between January 1980 and April 2015 in order to identify all neuroimaging studies that had examined treatment resistant patients, or longitudinally studied the effects of clozapine treatment.
Findings
The search identified 330 papers, of which 60 met inclusion criteria. Replicated differences in treatment resistant relative to responsive patients include reductions in gray matter and perfusion of frontotemporal regions and increases in white matter and basal ganglia perfusion. Clozapine treatment has been shown to lead to reductions in caudate nuclei volumes in three separate studies.
Interpretation
The available evidence supports the possibility that some of the neurobiological changes observed in resistant schizophrenia lie along a continuum with non resistant schizophrenia; while other differences may be more categorical in nature. There is, however, limited replication and in order for neuroimaging findings to be clinically translatable, future studies need to provide clear a priori hypotheses and test these rigorously.
Funding
This study was funded by Medical Research Council-UK (no. MC-A656-5QD30), Maudsley Charity (no. 666) and Wellcome Trust (no. 094849/Z/10/Z) grants to Dr Howes and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Contributors
OH and EM conceived of the study and designed the literature search. RM and EM reviewed abstracts, selected studies for inclusion and extracted data. EM, RM and OH wrote the manuscript.
Introduction
Schizophrenia is a severe mental illness characterised by psychotic (positive), negative and cognitive symptoms, and has a prevalence of about 1%.1 Antipsychotic medication has revolutionised the treatment of schizophrenia.2 However, 20-30% of patients show limited response to anti-psychotic medication.3 Due to persistent symptoms such patients stay longer in hospital care, and have increased treatment costs in comparison to patients who have responded.4 Furthermore, the prognosis is worse the longer their symptoms do not show improvement.5
Careful studies in the late 1980s and early 1990s demonstrated that less than 5% of patients who had not responded to two different first-line antipsychotics showed a response to a further antipsychotic, with the exception of clozapine.6 This has subsequently been confirmed in further clinical trials and naturalistic studies.7
It has thus become clear that there is a group of patients whose illness does not respond to first-line treatment, and this has been termed treatment resistant schizophrenia (TRS).8 Studies of drug occupancy at D2/3 receptors have found comparable levels of D2 receptor occupancy in responders and non-responders, indicating that a failure to obtain adequate drug levels in the brain does not explain non-response.9
These findings raise two questions. First, what is different about the underlying neurobiology in these patients that means antipsychotic drugs, other than clozapine, have little benefit? And second, what is it about clozapine that makes it uniquely effective in these patients? Answering these questions is critical to developing new treatments for refractory schizophrenia. A further clinical need is the early identification of patients with TRS to allow them to start appropriate treatment without delay.10 Treatment guidelines recommend that patients should receive clozapine if they have not responded to two adequate antipsychotic trials.11 However in clinical practice there is generally a long delay before patients start clozapine.12 A biomarker that enabled the early identification of treatment resistance, potentially at first presentation, could obviate the current requirement for empirical trials of different antipsychotics.
The purpose of this paper is therefore to review the neuroimaging evidence regarding treatment resistant schizophrenia, and consider the implications for developing new treatments and biomarkers for treatment resistance.
Methods
The search was conducted within PubMed looking for studies published between January 1980 to April 2015. In addition to the online database search results, reference lists of reviews and papers identified by the search were reviewed for additional studies.
The following key words were used as a search strategy:
(treatment resistant OR treatment refractory OR drug resistant)
AND
(schizophrenia OR psychosis)
AND
(magnetic resonance imaging OR MRI OR functional magnetic resonance imaging OR fMRI OR positron emission tomography OR PET OR magnetic resonance spectroscopy OR MRS OR EEG OR electroencephalography OR magnetoencephalography OR MEG OR event related potential OR ERP OR voxel based morphometry OR VBM OR diffusor tensor imaging OR DTI OR SPECT or SPECT or CT)
Studies were selected by two independent reviewers (EM & RM). To qualify for inclusion, studies must have been published in peer-reviewed journals as an original research paper in English language. We included all studies that recruited treatment-resistant patients and used in vivo brain imaging modalities. We also included longitudinal studies reporting neuroimaging findings pre and post clozapine treatment in patients with resistant schizophrenia (studies solely examining clozapine receptor occupancy, were not included).
The data extracted from each paper were: sample size, criteria for definition of treatment-resistance, brain imaging modality, medication status, and diagnostic criteria for the schizophrenia diagnosis. Where possible effect sizes for the contrasts of interest were calculated, measured by Cohens’s d for differences between means.
Results
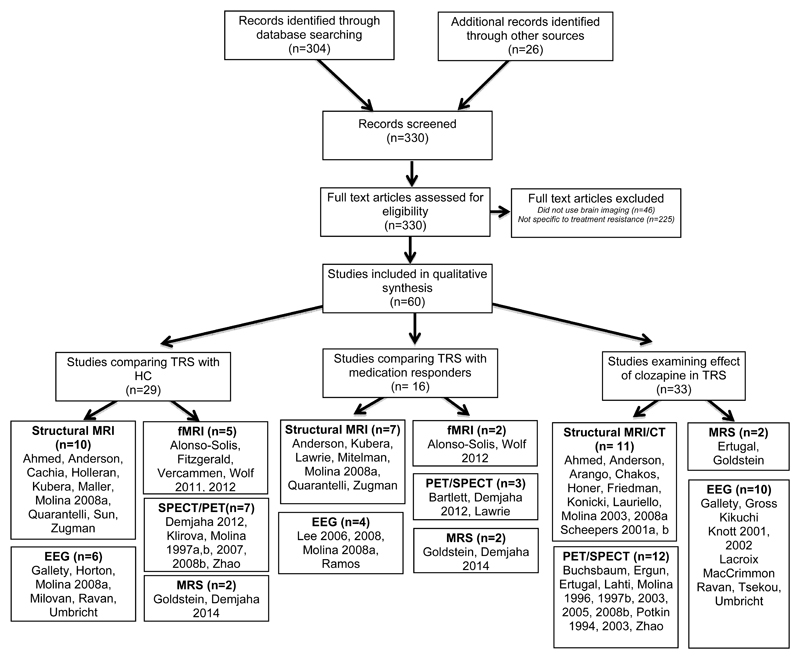
The search with the terms outlined above as well as reference list review identified 330 papers, of which 60 met the inclusion criteria (see Fig. 1). 14 of the studies defined treatment resistance according to the criteria of Kane et al.13 The remainder used a range of definitions, while eight studies did not specify any criteria (see Table 1).
Figure 1. Flow diagram of study selection and study characteristics.
Some studies use multiple imaging techniques and examine multiple populations – hence are represented more than once.
Table 1. Study characteristics.
1H-MRS – proton magnetic resonance spectroscopy; AP –antipsychotic; AVH – Auditory verbal hallucinations; BPRS – Brief Psychiatric Rating Scale; CNR – clozapine non responder; CPZ equiv – Chlorpromazine equivalents; DSM – Diagnostic and Statistical Manual of Mental Disorders; EEG – electroencephalogram; HC- healthy controls; MDD – major depressive disorder; MRI – Magnetic Resonance Imaging; PET – Positron Emission Tomography; Individuals with schizophrenia without auditory hallucinations; R – antipsychotic responders; Scz- unspecified whether responder/resistant; SPECT- single-photon emission computed tomography; TR – treatment resistant; TR-AVH, treatment resistant auditory verbal hallucinations.
| Authors | Year | Sample | Resistance criteria | Modality | Medication at time of scan | Diagnostic Criteria |
|---|---|---|---|---|---|---|
| Ahmed et al.17 | 2015 | 33 TR, 31 HC | Failed ≥2 Aps (≥1 atypical). Prolonged positive or negative symptoms of ≥moderate severity | MRI – Structural | Pre and post clozapine | DSM-IV-TR |
| Alonso-Solis et al.23 | 2015 | 19 TR-AVH, 14 R, 20 HC | Daily AVH AND failed ≥2 Aps (at dose equiv ≥600mg clozapine/day) | fMRI – resting state | Typical/Atypical Aps | DSM-IV-TR |
| Anderson et al.15 | 2015 | 15 CNR, 19 TR, 18 R, 20 HC | Lack of significant response despite trials (adequate dose and≥6 wk duration) of ≥2 Aps | MRI- structural | Atypical Aps (including clozapine) | DSM-IV-TR |
| Arango et al.51 | 2003 | 45 TR | Residual positive (≥8 BPRS psychotic) or negative symptoms (≥20 SANS) despite ≥2 6wk AP trials. Prospective trial fluphenazine 20mg/day – subjects with >30% improvement excluded. | MRI – Structural | Clozapine or Haloperidol | DSM-III-R |
| Bartlett et al.42 | 1998 | 7 TR, 7 R | Unmedicated BPRS ≥ 50 or medicated BPRS ≥ 42 AND no worsening when unmedicated. Prospective 4-wk AP trial for patients with no records | FDG-PET (haloperidol challenge) | Not specified | DSM-III-R |
| Buchsbaum et al.57 | 1992 | 12 Scz | Not specified | FDG-PET | Pre/post clozapine/thioxene | Not specified |
| Cachia et al.99 | 2008 | 30 TR-AVH, 28 HC | Kane et al (1988) | MRI-Structural | Typical /atypical Aps | DSM-IV |
| Chakos et al.54 | 1995 | 8 clozapine, 7 typical Aps | Not specified | MRI- Structural | Clozapine and typical Aps | Not specified |
| Demjaha et al.43 | 2012 | 12 TR, 12 R, 12 HC | Conley et al (2001) | FDOPA-PET | Non clozapine Aps | DSM-IV |
| Demjaha et al.34 | 2014 | 6 TR, 8 R, 10 HC | Conley et al (2001) | 1H-MRS | Typical and atypical Aps | DSM-IV |
| Ergun et al.62 | 2010 | 20 TR | Treatment refractory or AP intolerant | 99mTc-HMPAO SPECT | Pre and post clozapine | DSM-IV |
| Ertugrul et al.61 | 2009 | 22 TR | On clozapine due to treatment resistance or intolerance to previous Aps | 99mTc-HMPAO SPECT/ 1H-MRS | Typical and atypical Aps | DSM-IV |
| Fitzgerald et al.26 | 2007 | 3 TR, 4HC | Persistent severe refractory hallucinations that had not responded to ≥2 adequate courses of Aps | fMRI (word generation task) | Clozapine, amisulpride, sertraline, valproate, diazepam | Not specified |
| Friedman et al.50 | 1991 | 34 TR | Failure to respond to ≥2 different class Aps (each for ≥6 weeks, ≥ 800mg CPZ equiv). ≥4 on BPRS positive items | CT Scan | Clozapine | RDC |
| Galletly et al.36 | 2005 | 15 TR, 14 HC | Not specified | EEG | Pre and post clozapine | DSM-IV |
| Goldstein et al.35 | 2015 | 11 CNR, 16 TR, 15 R, 11 HC | NICE (2002), RANZCP (2005) | 1H-MRS | Atypical Aps including clozapine | DSM-IV |
| Gross et al.67 | 2004 | 16 TR | Kane et al (1988) | EEG | Risperidone or olanzapine | SCID + chart review |
| Holleran et al.22 | 2014 | 19 TR, 19 HC | Failure to respond to ≥2 Aps (≥1 atypical), prolonged moderate/severe positive or negative symptoms. | MRI- DTI | Atypical Aps, antidepressants | DSM-IV |
| Honer et al.48 | 1995 | 42 TR (inc 3 Schizoaffective) | Poor response to adequate AP dose for ≥6 months. May et al. (1988) scale. | CT scan | Antipsychotic class not specified | DSM-III-R |
| Hoptman et al.72 | 2005 | 49 TR | Kane et al (1988) | MRI-Structural | Typical and atypical Aps (including clozapine) | SCID + chart review |
| Horton et al.37 | 2011 | 21 TR, 19 HC | Not specified | EEG | Clozapine | DSM-IV and SCID |
| Kikuchi et al.100 | 2014 | 26 TR | Poor tolerance or poor response despite ≥2 Aps (≥1 atypical), ≥ 4 weeks and ≥600mg CPZ equiv. | EEG | Pre and post clozapine treatment | Not specified |
| Klirova et al28 | 2013 | 15 TR-AVH, 19HC | Non response to both typical and atypical Aps + ≥5 episodes AVH per day in the last month | FDG PET | Aps, Antidepressants, anticonvulsants | DSM-IV |
| Knott et al.68 | 2001 | 17 TR, 17 HC | Kane et al (1988) | EEG | Not specified | DSM-III-R |
| Knott et al.66 | 2002 | 17 TR | Kane et al (1988) | EEG | Pre/post clozapine | DSM-III-R |
| Konicki et al.49 | 2001 | TR 26 | Kane et al (1988) | CT scan | clozapine | DSM-III-R |
| Kubera et al.19 | 2014 | 10 TR-AVH, 10 nAVH, 14 HC | Persistent AVH despite ≥2 AP trials (adequate dose, ≥6 wks) | MRI Structural | Clozapine and other Aps | DSM-IV |
| Lacroix et al.101 | 1995 | 10 TR, 10 NR | 35% or more and a 30% or less reduction, respectively, on the Brief Psychiatric Rating Scale (BPRS) | EEG | Not specified | DSM-IV |
| Lahti et al.63,97 | 2003, 2004 | 6 partially responsive, 10 HV | Not specified | 15O-PET | Pre/post clozapine | DSM-III-R |
| Lauriello et al.53 | 1998 | 21 TR | Treatment intolerant or inadequate response. | MRI-Structural | Typical Aps | DSM-III-R |
| Lawrie et al.40 | 1995 | 20 TR, 20 R | May et al (1988) | MRI-Structural/ SPECT | Not specified | DSM-IV |
| Lee at al.46,47 | 2006, 2008 | 25 TR-AVH, 23 nAVH | Persistent AVH for ≥2yrs | EEG | Conventional neuroleptics | DSM-IV |
| Maller et al.18 | 2012 | 52 TR, 182 MDD, 76 HC | Not specified | MRI-Structural | Not specified | DSM-IV |
| MacCrimmon et al.69 | 2012 | 64 TR | Kane et al (1988) | EEG | Pre/post clozapine, (+ other psychotropics) | DSM-IV |
| Milovan et al.39 | 2004 | 13TR, 13 HC | Kane et al (1988) | EEG | Not specified | DSM-IV |
| Mitelman et al.41 | 2005 | 13 TR, 24 R, 27 HC | Keefe et al (1987) | MRI-Structural | Not specified | DSM-IV |
| Molina et al.59 | 1996 | 24 TR | Lack of adequate response to ≥2 chemically different Aps, ≥800mg CPZ equiv | 99mTc-HMPAO SPECT | Pre/post clozapine | DSM-IV |
| Molina et al.29 | 1997a | 36 TR, 28 HC | Kane et al (1988) | 99mTc-HMPAO SPECT | Not specified | DSM-IV-R |
| Molina et al.30 | 1997b | 39 TR (includes Molina et al. 1996 sample), 28 HC | Lack of response to ≥2 dissimilar Aps (≥800mg CPZ equiv), each one for ≥2 months over last year. | 99mTc-HMPAO SPECT | Pre/post clozapine | DSM-IV |
| Molina et al.102 | 2003 | 25 TR | Lack of response to ≥2 different Aps for ≥6 weeks in past 12 mths, dose ≥800mg CPZ equiv. Significant positive or disorganisation residual symptoms | MRI-structural FDG PET |
Pre/post clozapine | DSM-III-R |
| Molina et al.33,103 | 2005, 2007 | 23 TR, 17NN, 18HC | Lack of adequate response to ≥2 Aps for ≥4 weeks in preceding 12 months, dose ≥800mg CPZ equiv. All had haloperidol for ≥4wks before scan | FDG PET | Pre/post clozapine | DSM-IV |
| Molina et al.14 | 2008a | 30 TR, 19 R and 44 HC | Kane et al (1988) | MRI-structural, EEG | Haloperidol prior to first MRI, then olanzapine or clozapine | DSM-IV |
| Molina et al.31 | 2008b | 10 TR, 10 HC | A poor response during the previous year to haloperidol or risperidone followed by lack of response to 4 week trial of risperidone | 99mTc-HMPAO SPECT | Pre/post clozapine | DSM-IV |
| Potkin et al.58 | 1994 | 18 Scz | Not specified | FDG PET | Pre/post clozapine | Not specified |
| Potkin et al.64 | 2003 | 15 TR | Not specified | FDG PET | Not specified | DSM-IV |
| Quarantelli et al.16 | 2014 | 20 (TR + CNR), 15 R, 16 HC | <20 % improvement AND total > 45 on BPRS AND ≥4 in ≥2 BPRS psychotic items AND ≥2 yrs poor functioning despite 6-8 weeks with ≥2 Aps and good adherence. | MRI –structural | Typical and atypical Aps (including clozapine) | DSM-IV-TR |
| Ramos et al.44 | 2001 | 10 TR, 10 R | Keefe et al (1990) and Brenner & Merlo (1995) criteria | EEG | Not specified | DSM-IV |
| Ravan et al.98 | 2015 | 47 TR, 66 HC | Kane et al (1988) criteria | EEG (auditory evoked) | Pre/Post clozapine | DSM-IV |
| Scheepers et al.55 | 2001a | 26 TR | No response (CGI≥4) to ≥1 typical AP for ≥ 4 weeks OR severe EPSEs or TD | MRI-Structural | Pre/post clozapine | DSM-IV |
| Scheepers et al.56 | 2001 b | 28 TR | No response (CGI≥4) to ≥1 typical AP for ≥ 4 weeks OR severe EPSEs or TD | MRI-Structural | Pre/post clozapine | DSM-IV |
| Sun et al.21 | 2009 | 42 TR, 42 MDD, 30 HC | Kane et al (1988) criteria | MRI-Structural | Clozapine | DSM-III-R |
| Tsekou et al.104 | 2015 | 7 TR | Bremner et al (1990) criteria AND ≥70 on PANSS, and schizophrenia diagnosis for≥2 years | Sleep EEG | Pre/post clozapine | DSM-III-R |
| Umbricht et al.38 | 1998 | 11 TR, 6 R, 13 HC | Partially refractory –≥4 on any of the 4 BPRS positive symptom items | EEG | Clozapine and haloperidol | DSM-III-R |
| Vercammen et al.24 | 2010 | 27 TR-AVH, 27 HC | Daily AVH, ≥2 adequate AP trials | MRI-Resting state | Aps and benzodiazepines | DSM-IV |
| Wolf et al.25,27 | 2011, 2012 | 10 TR-AVH, 10 R, 14 HC | Kane et al (1988) | MRI-Resting state | Clozapine | DSM-IV |
| Zhao et al.32 | 2006 | 21 TR, 40 HC | Andreason’s negative symptom profile | SPECT | Medication free, follow up on clozapine | DSM-IV |
| Zugman et al.20 | 2013 | 61 TR, 67 R, 80 HC | Kane et al (1988) | MRI-Structural | Typical and atypical Aps (including clozapine) | DSM-IV |
Studies comparing treatment-resistant patients with healthy control groups
29 studies, comprising 680 patients and 714 controls, compared treatment-resistant patients with healthy volunteers (see Table 2).
Table 2. Treatment resistant versus healthy control studies.
ACC – anterior cingulate cortex; CC - corpus callosum; CNR – clozapine non responder; Cr – creatinine; CSF – cerebrospinal fluid; FC- functional connectivity; Glx – (glutamate + glutamine); GM – Gray matter; ICV – Intracranial volume; IFG – inferior frontal gyrus; ILF –inferior longitudinal fasciculus; ITG – inferior temporal gyrus; LIC - limb of the internal capsule; MMN - mismatch negativity; MTG - middle temporal gyrus; ns – not statistically significant; OFC - orbitofrontal cortex; pIPL- posterior inferior parietal lobule; SLF – superior longitudinal fasiculus; SMA –supplementary motor area; SMG – supramarginal gyrus; STG –superior temporal gyrus; TPC – temporoparietal cortex; TPJ – temporoparietal junction; UF- uncinate fasciulus; rsMRI – resting state MRI; VMPFC – ventromedial prefrontal cortex; WM- white matter
| Authors | Year | Modality | Effect of interest | Effect size (d) |
|---|---|---|---|---|
| Ahmed et al. | 2015 | Structural MRI | Raw brain volumes | |
| ↓ GM volume in TR | 0.33 (ns) | |||
| ↓ WM volume in TR | 0.32 (ns) | |||
| ↑CSF volume in TR | 0.19 (ns) | |||
| Anderson et al. | 2015 | Structural MRI | Normalised brain volumes | |
| ↓GM in TR and CNR | GM: TR – 1.23 | |||
| GM: CNR – 1.90 | ||||
| ↓ WM in TR and CNR | WM: TR – 0.63 | |||
| WM: CNR – 0.99 | ||||
| ↑ CSF volume in TR and CNR | CSF: TR – 0.23 (ns) | |||
| CSF: CNR – 0.80 | ||||
| ↓GM volume bilaterally across STG, MTG, Heschl’s gyrus, central and parietal operculum, post-central gyrus, insula, VMPFC, ACC in CNR. | ||||
| ↓GM volume in the right central operculum and right ITG in TR | ||||
| Cachia et al. | 2008 | Structural MRI | ↓ Cortical folding in TR: | |
| Left Frontal (middle) | 0.75 | |||
| Left Temporal (superior) | 0.61 | |||
| Left Sylvius (diagonal branch) | 0.56 | |||
| Right temporal (superior) | 0.83 | |||
| Hoptman et al. | 2005 | Structural MRI | Larger left OFC GM volumes and bilateral OFC WM volumes were associated with greater aggression | |
| Kubera et al. | 2014 | Structural MRI | ↓GM in TR in predominantly of lateral prefrontal, temporal and parietal regions. | |
| Maller et al. | 2012 | Structural MRI | Raw brain volumes | |
| ↓Gray matter volume in TR | 0.56 | |||
| ↓White matter volume in TR | 0.66 | |||
| ↓CSF volume in TR | 0.39 | |||
| ↓Hippocampus (tail) in TR (normalised by ICV): | ||||
| Right tail | 1.71 | |||
| Left tail | 1.20 | |||
| Molina et al. | 2008a | Structural MRI | Normalised brain volumes | |
| ↓ GM volume in TR | Frontal: 1.59 | |||
| Parietal:0.87 (ns) | ||||
| Occipital: 1.40 | ||||
| Temporal: 0.75 (ns) | ||||
| ↑ WM volume in TR | Frontal: 1.00 | |||
| Parietal: 1.42 | ||||
| Occipital: 1.85 | ||||
| Quaranatelli et al. | 2014 | Structural MRI | ↓global GM (normalised volumes) in TR. ↓GM at left post central gyrus and dorsolateral superior frontal gyrus; right rolandic operculum, inferior frontal gyrus, insula and amygdala; and bilateral precentral and middle frontal gyrus. | |
| Sun et al. | 2009 | Structural MRI | ↑ Total normalised CC volume in TR | 1.9 |
| ↑ CC3 volume in TR | 1 | |||
| ↑ CC4 volume in TR | 1 | |||
| ↑ CC5 volume in TR | 0.4 | |||
| Zugman et al. | 2013 | Structural MRI | ↓GM in TR in in left: orbitofrontal, middle temporal, fusiform, caudal middle frontal, STG, lingual areas; and right: precentral, pars triangularis, middle temporal and lateral occipital areas. | |
| Holleran et al. | 2014 | Structural MRI – DTI | ↓ Fractional anisotropy in TR in: genu, body, and splenum of CC; Temporal ILF, SLF, external capsule, temporal UF, posterior LIC, left anterior LIC, fornix, cerebellar peduncles and corticospinal tract. | |
| ↑Radial diffusivity in TR in voxels in genu, body and splenum of CC, right ILF, posterior LIC, external capsule. | ||||
| Alonso-Solis et al. | 2015 | rsMRI | ↑FC in TR between pIPL and occipital fusiform gyrus, ligual gyrus and L occipital pole. | |
| Vercammen et al. | 2010 | rsMRI | ↓ FC in TR-AVH between the Left TPJ and Right IFG. Severity of hallucinations correlated with ↓coupling between the left TPJ and: bilateral anterior cingulate and amygdala. | |
| Wolf et al. | 2011 | rsMRI | Speech-related network: ↓connectivity in bilateral temporal and ↓connectivity in L. anterior cingulate displayed by TR | |
| Attention network: ↑connectivity in R MFG in TR | ||||
| Executive function network: ↓connectivity in L. precuneus, R.MFG, SFG in TR | ||||
| Wolf et al. | 2012 | MRI – Arterial spin labelling | ↑rCBF in TR in the left IFG, the left ACC, the SMA) in a cluster including the left MTG and STG, the left insula, the right MTG and the right SMG, extending to the right TPC | |
| Fitzgerald et al. | 2007 | fMRI (word generation) | ↓activation in TR in medial frontal regions and greater activation in left caudal precentral gyrus. | |
| Demjaha et al. | 2012 | [18F]-DOPA PET | Healthy volunteers and TRS show no differences in striatal dopamine synthesis capacity | |
| Klirova et al. | 2013 | ↑perfusion in TR in lentiform nucleus, thalamus, postcentral gyrus, left parahippocampal gyrus and right superior frontal gyrus. In left acoustic-linguistic cortex ↑ found in MTG and TPJ. | ||
| Molina et al. | 1997a | 99mTc-HMPAO SPECT | ↓perfusion in TR | |
| Right posterior temporal | 1.52 | |||
| Left ventral prefrontal | 0.63 | |||
| Left dorsolateral | 1.56 | |||
| ↑perfusion for TR in right basal ganglia | -1.22 | |||
| Molina et al. | 1997 b | 99mTc-HMPAO SPECT | Right basal ganglia perfusion ↓in HC, ↑in CNR and ↑↑in TR. | |
| Thalamus and left basal ganglia perfusion similar between TR and HC, CNR however shows ↓perfusion in these regions. | ||||
| TR and CNR show ↓perfusion compared to HC in left lower prefrontal dorsolateral cortex | ||||
| TR shows ↑perfusion in upper dorsolateral cortex compared to HC or UTC | ||||
| Molina et al. | 2007 | FDG-PET | Clozapine treated TRS show ↓activity in, Dorsloateral cortex, OFC, ACC, insular cortex and head of caudate nuclei. | |
| Molina et al. | 2008b | 99mTc-HMPAO SPECT | Risperidone treated TR showed ↓ activity in the medial prefrontal, middle cingulate and insula. TR showed ↑perfusion in brainstem and hippocampus, and a small part of left posterior occipital and temporal region | |
| Zhao et al. | 2004 | 99mTc-ECD SPECT | ↓rCBF at rest and ↓percentage increase during Wisconsin card sorting test in TR | |
| Left Frontal Lobe | 1.48 | |||
| Right Frontal Lobe | 1.40 | |||
| Left temporal Lobe | 1.31 | |||
| Right Temporal Lobe | 1.48 | |||
| Demjaha et al. | 2014 | 1H-MRS | TR show increased ACC glutamate concentrations compared to HC | 1.45 |
| Goldstein et al. | 2015 | 1H-MRS | TR clozapine responders have higher Glx/Cr than HC in the putamen (although this does not survive multiple comparisons correction) | 3.68 |
| Gallety et al. | 2005 | EEG | TR compared to HC (prior to clozapine) show ↓Midline N1, P300, parietal slow wave activity | |
| Horton et al. | 2011 | EEG | Frequency deviant conditions: | |
| ↓MMN latencies TR | 0.93 | |||
| ↓MMN amplitude for TR | 1.19 | |||
| Duration deviant conditions | ||||
| No difference in MMN latency | 0.59 | |||
| ↓MMN amplitude for TR | 3.14 | |||
| Milovan et al. | 2004 | EEG | ↑MMN amplitude in TR | |
| midline electrode | 0.98 | |||
| lateral electrode | 0.89 | |||
| Molina et al. | 2008a | EEG | ↓P300 amplitude in TR | 2.94 |
| Ravan et al. | 2015 | EEG | Machine learning investigation of EEG responses to auditory odd ball task able to classify HC and TRS with 81.4% accuracy | |
| Umbricht et al. | 1998 | EEG | ↓ MMN amplitudes in TR | 0.99 |
Ten structural studies were identified. Five reported overall gray matter volumes,14–18 and all but one17 reported significant reductions. Four studies reported specific regions of gray matter reduction in TRS.15,16,19,20 Over 25 separate areas of reduction were reported, with the left middle frontal, right precentral and right middle temporal gyri being most consistently implicated. Regarding white matter volumes, one study of haloperidol treated individuals reported overall increases in TRS,14 while a study of clozapine treated individuals,15 and a study where medication status was not specified,18 reported reductions. One study showed that resistant patients demonstrated enlargement in posterior sections of the corpus callosum, particularly the splenium.21 Complementing this finding, a diffusion tensor imaging study showed widespread disruptions to white matter tract integrity in TRS.22 This was especially apparent in the corpus callosum, and illness duration was negatively related to fractional anisotropy in the splenium.
Five studies used functional MRI (fMRI). Three resting state studies,23–25 and a study using a word generation task,26 have produced findings that while not necessarily incompatible are hard to draw coherent conclusions from as a whole. An arterial spin labeling (ASL) in individuals with resistant auditory hallucinations demonstrated increased cerebral blood flow in a variety of areas involved in speech processing.27
Seven studies used positron emission tomography (PET) or single photon emission computed tomography (SPECT). Six of these used radiotracers that allow the measurement cerebral metabolic rate (e.g. 18F-fluorodeoxyglucose (FDG)) or blood flow (Technetium-99m-exametazime (99mTc-HMPAO), Oxygen-15(15O), and technetium-99m-ethyl cysteinate diethylester (99mTc-ECD)), and all but one28 demonstrated a degree of hypofrontality in TRS. Three studies employing 99mTc-HMPAO SPECT demonstrated reduced perfusion of frontal areas in TRS.29–31 In one study,29 resistant patients also showed increased perfusion ratios in the basal ganglia (replicated in a second study30), while reduced perfusion of the right dorsolateral prefrontal cortex correlated with negative symptom severity. Another study used 99mTc-ECD SPECT, while participants performed the Wisconsin Card Sorting test. Individuals with TRS were had reduced rCBF in fronto-temporal regions at rest, and a reduced percentage increase during the task.32 A FDG PET study demonstrated reduced activity in cortical and subcortical regions in TRS.33 Another FDG PET study looking specifically at resistant hallucinations demonstrated increase metabolic activity in a range of language related areas.28
Two studies employed magnetic resonance spectroscopy (MRS). One showed increased glutamate concentrations in the anterior cingulate cortex of individuals with TRS;34 while another showed increased glutamate+glutamine concentrations in the putamen.35
Six studies used electroencephalography (EEG). The P300 is an event related EEG component that occurs when a stimulus deviates from a preceding sequence of standard stimuli and is thought to index information processing efficiency. Two studies showed significant decreases in P300 amplitude in patients compared to controls.14,36 Two studies used the mismatch negativity (MMN) component which is believed to index the integrity of the pre-attentive sensory network. These showed decreased amplitudes in resistant patients.37,38 One study of thirteen patients was not in agreement with the above findings, both in terms of MMN and P300 components.39 Medication status of patients was not specified raising the possibility that this could explain the discrepancy.
Studies comparing treatment-resistant with treatment-responsive patient groups
Sixteen studies compared treatment-resistant (298 patients) and treatment-responsive groups (264 patients) (see Table 3).
Table 3. Treatment resistant versus treatment responder studies.
ACC – anterior cingulate cortex; CNR – clozapine non responder; dMPFC – dorsomedial prefrontal cortex; MFG - Middle frontal gyrus; nAVH – individuals not experiencing auditory halluccinations PCC – posterior cingulate cortex. PCG – post central gyrus, RS – responders to non-clozapine antipsychotics. STG – superior temporal gyrus. Superior frontal gyrus. SMG – supramarginal gyrus. TG – temporal gyrus,TR – treatment resistant. vMPFC – ventromedial prefrontal cortex.
| Authors | Year | Modality | Effect of interest | Effect size |
|---|---|---|---|---|
| Anderson et al. | 2015 | Structural MRI | ↓ global GM in TR/CNR | 0.84 (TR vs R) |
| ↓GM in TR vs R: in TG, PCG, MFG, SFG, SMG gyrus and lateral occipital cortex | ||||
| ↓GM CNR vs R: in right parietal operculum and left cerebellum | ||||
| TR vs CNR: no significant differences | ||||
| Kubera et al. | 2014 | Structural MRI | ↓GM in TR-AVH compared to nAVH across a structural network involving predominantly medial frontal, orbitofrontal and superior temporal regions. | |
| Lawrie et al. | 1995 | Structural MRI | ↓Whole brain volume in TRS | 0.41 (ns) |
| ↓Right temporal lobe volume in TR | 0.46 (ns) | |||
| Mitelman et al. | 2005 | Structural MRI | ↓GM in posterior cingulate and retrosplenial cortices in TR | |
| Molina et al. | 2008a | Structural MRI and EEG | ↓GM at baseline in TR | Frontal: 0.87 |
| Occipital: 0.81 | ||||
| ↑ WM at baseline in TRS | Frontal: 1.13 | |||
| Parietal: 1.35 | ||||
| Occipital: 1.43 | ||||
| ↑ in GM longitudinally in TR compared to R | Frontal: 1.95 | |||
| Parietal: 2.11 | ||||
| Occipital: 1.81 | ||||
| ↓in WM longitudinally in TR compared to R | Frontal: 1.18 | |||
| Parietal: 1.65 | ||||
| Occipital: 1.22 | ||||
| Quaranatelli et al. | 2014 | Structural MRI | ↓ GM in TR at left PCG and SFG (dorsolateral); and bilateral middle frontal gyrus. | |
| Zugman et al. | 2013 | Structural MRI | ↓ GM in DLPFC in TR | |
| Alonso-Solis et al. | 2015 | MRI- Resting state |
↓ FC in TR between dMPFC: and central opercular cortex, insular cortex, precentral gyrus and STG; and between the temporal pole: and cerebellum. ↓ FC in TR between vMPFC: and paracingulate cortex, ACC, and subcallosal cortex; and between hippocampal formation and: PCC, and precuneus complex. |
|
| Wolf et al. | 2012 | MRI – Arterial spin labelling | ↑ rCBF in TR-AVH compared to nAVH in the left STG and right SMG, TPC. | |
| Bartlett et al. | 1998 | FDG PET (haloperidol challenge) | ↓ Whole brain metabolic rate in TR | 1.2 |
| ↓ Left DLPFC metabolic rate in TR | 1.05 | |||
| ↓ Right DLPFC metabolic rate in TR | 0.87 | |||
| ↓ Left Temporal Cortex metabolic rate in TR | 1.19 | |||
| Demjaha et al. | 2012 | F-DOPA PET | ↓ whole striatum dopamine synthesis capacity in TR | 1.11 |
| ↓ associative subdivion dopamine synthesis capacity in TR | 1.31 | |||
| ↓ limbic subdivision dopamine synthesis capacity in TR | 1.04 | |||
| sensorimotor subdivision (ns) | ||||
| Lawrie et al. | 1995 | 99mTc-HMPAO SPECT | No significant differences in perfusion between TR and R. | |
| Goldstein et al. | 2015 | 1H-MRS | TR shows ↑ and CNR shows ↑↑ Glx/CR in DLPFC | 3.99 (CNR vs R) |
| ↑ Glx/Cr in TR (clozapine responders) compared to R and CNR in putamen. | 3.31(TR vs R) | |||
| 4.00 (TR vs CNR) | ||||
| Demjaha et al. | 2014 | 1H-MRS | ↑ anterior cingulate glutamate in TR compared to R | 0.70 (ns) |
| Lee et al. | 2006 | EEG | ↑ Beta1 in TR | 0.61 |
| ↑ Beta2 in TR | 0.69 | |||
| gamma-beta2 and beta3 correlation in TRS but not RS in posterior and anterior electrodes | range of r=0.42-0.61 | |||
| Lee et al. | 2008 | EEG | ↑ gamma frequency in TR at D2 (i.e. more chaotic) in right frontal electrode Fp2 | 0.58 |
| ↓ beta frequency in TR at D2 (i.e. less chaotic) in left parietal electrode P3 | 0.7 | |||
| Molina et al. | 2008a | EEG | TR have ↓ P300 amplitude | 0.53 (ns) |
| Ramos et al. | 2001 | EEG | TR have ↓ temporal alpha2, ↓ temporal beta1, ↓ temporal beta2, ↑ occipital beta2 | |
| TRS have ↑ intrahemispheric correlation in Fp2-F4 | ||||
| TRS have↓ intrahemispheric correlation between F8-T4 | ||||
Seven studies used structural MRI.14–16,19,20,40,41 All demonstrated reduced gray matter in frontal areas in resistant compared to responsive patients (although this was not significant in two studies14,40). Two studies14,15 report increased white matter volumes in resistant patients, but only in one14 is this significant.
Two studies used fMRI. A rsMRI study demonstrated that resistant patients display greater functional connectivity between the dorsomedial prefrontal cortex and other frontotemporal areas, but reduced connectivity between the ventromedial prefrontal cortex and areas of the cingulate cortex.23 The ASL study described above demonstrated increased rCBF in the left superior temporal gyrus, right supramarginal gyrus and temporal polar cortex in patients with treatment resistant auditory hallucinations.27
Three studies used PET or SPECT. In contrast to the ASL study discussed above,27 in a 99mTc-HMPAO SPECT study, no differences in perfusion between groups were reported.40 One FDG PET study used a haloperidol challenge and found that this caused widespread metabolic decreases in resistant but not treatment-responsive patients.42 Demjaha et al.43 used F-DOPA PET to show increased striatal dopamine synthesis capacity in responsive compared to resistant patients.
A sub-sample of the Demjaha et al. study43 was investigated using 1H-MRS.34 As described above they found that resistant patients had significantly higher anterior cingulate cortex glutamate levels compared to healthy controls,34 while responsive patients had similar levels to controls. Goldstein et al.35 showed that compared to individuals who have responded to first line antipsychotics, treatment resistant patients who respond to clozapine show greater concentrations of glutamate+glutamine in the putamen, and reduced concentrations in the dorsolateral prefrontal cortex.
Four studies used EEG. One study found that treatment-resistant patients showed trend level P300 decreases compared to responsive patients.14 Another showed that treatment-resistant patients had a different connectivity pattern than treatment-responders, with a higher inter-hemispheric correlation between frontal electrodes.44 Gamma-beta correlations index a response to novel auditory stimuli.45 One study reported significant gamma and beta frequency increases in speech-related areas and a significant gamma-beta correlation in resistant but not responsive patients.46 A second study by the same group examined this effect in terms of dimensional complexity and found reduced neuronal synchronisation in the prefrontal cortex of resistant patients.47
Longitudinal studies examining the effects of clozapine in treatment-resistant patients, and studies investigating predictors of clozapine response
We identified 33 papers, comprising a total of 844 patients and 322 controls (see Table 4).
Table 4. Longitudinal studies of treatment resistant patients pre- and post-clozapine, and studies predicting clozapine response.
CR – clozapine responder; CNR – clozapine non responder; Cr - creatinine ; DLPFC- dorsolateral prefrontal cortex; LMFC – left medial frontal cortex; mPFC – medial prefrontal cortex; NAA- N-acetly aspartate; pts –patients; rCBF – regional cerebral blood flow; RMTC – right medial temporal cortex
| Authors | Year | Modality | Effect of interes | Effect size | |
|---|---|---|---|---|---|
| Ahmed et al. | 2015 | Structural MRI | ↓GM over 6-12 months greater in TR treated with clozapine than HC | ||
| Right prefrontal cortex | 1.06 | ||||
| Left prefrontal cortex | 1.02 | ||||
| Periventricular area | 1.85 | ||||
| ↓cortical thickness of LMFC and RMTC in CNR compared to CR. | 1.07 | ||||
| Anderson et al. | 2015 | Structural MRI | No significant structural differences between TR and CNR | ||
| Arango et al. | 2003 | Structural MRI | In clozapine treated patients, ↑pretreatment right prefrontal GM vol associated with ↑response whereas converse true in haloperidol treated patients. | ||
| Chakos et al. | 1995 | Structural MRI | Patients scanned at baseline and then again 55 wks post clozapine, showed 10% ↓in caudate nuclei vol, while those remaining on typical antipsychotics showed 8% ↑ | 0.94 (change within clozapine group) | |
| Honer et al. | 1995 | CT scan | ↓cortical sulcal spaces in clozapine responders compared to nonresponders | ||
| Friedman et al. | 1991 | CT scan | ++ responders have ↓prefrontal sulcal spaces than + responders who in turn have ↓than non-responders | ||
| Konicki et al. | 2001 | CT scan | ↓prefrontal sulcal spaces in clozapine responders compared to poor responders | 3.80 | |
| Lauriello et al. | 1998 | Structural MRI | No correlation between change in BPRS and sulcal CSF or GM volumes in PFC and frontal cortex. ↑sulcal CSF volumes in anterior superior temporal lobe were associated with clinical improvement. | ||
| Molina et al. | 2003 | Structural MRI | Improvement in positive symptoms related to temporal GM vol. Improvement in negative symptoms predicted by DLPFC vol. Improvement in disorganised dimension predicted by intracranial and hippocampal vol. | ||
| Molina et al. | 2008a | Structural MRI | TR showed longitudinal changes compared to HC over (40pprox…)28 months – ↑ GM in frontal, parietal and occipital regions; and ↓in WM in frontal, parietal and occipital regions | GM Frontal: 1.24 | |
| GM Parietal: 1.68 | |||||
| GM Occipital: 1.99 | |||||
| WM Frontal: 1.36 | |||||
| WM Parietal: 1.53 | |||||
| WM Occipital: 1.63 | |||||
| Scheepers et al. | 2001a, b | Structural MRI | Clozapine use led to significant ↓in caudate nucleus volume over 24 wks. This was not related to clinical response at 24 weeks but when patients followed up for 52 weeks the change in left caudate volume was significantly greater in responders compared to non-responders. | 0.23 (change in caudate over 24wks) | |
| 0.56 (responders vs non responders) | |||||
| Buchsbaum et al. | 1992 | FDG-PET | Clozapine ↑and thioxene↓ metabolic rates in the basal ganglia; these effects most marked on right side.Baseline metabolic rates predicted clinical medication response, with right inferior caudate metabolic rates differentiating clozapine and thiothixene responders | ||
| Ergun et al. | 2010 | 99mTc-HMPAO SPECT | After 8 wks of clozapine treatment, changes in blood flow seen in 12/20 pts, mostly in basal ganglia or frontal cortex. | ||
| Ertugrul et al. | 2009 | 99mTc-HMPAO SPECT | In CR perfusion ratio of Right and Left(sup and medial) frontal:caudate ↑with treatment.This change not seen in CNR. Change in perfusion ratio correlates with improvements in cognitive testing. | ||
| Response to clozapine predicted by baseline right frontal:thalamus perfusion. | 0.56 (CR vs CNR) | ||||
| Lahti et al. | 2003, 2004 | 15O-PET | Clozapine ↑ and haloperidol ↑↑rCBF in the striatum. Clozapine ↑rCBF to ACC, dorsolateral frontal cortex and occipital cortex more than haloperidol. Both drugs led to ↓rCBF in the hippocampus, ventrolateral frontal cortex and right middle temporal cortex. | ||
| Molina et al. | 2003 | Structural MRI, FDG-PET | Improvement of positive symptoms predicted by ↑temporal gray matter at baseline | ||
| Improvement of disorganised symptoms predicted by smaller intracranial and hippocampal volume | |||||
| Improvement of negative symptoms predicted by DLPFC volume and activity | |||||
| Molina et al. | 2005 | FDG-PET | 6 mths of Clozapine treatment leads to metabolic ↓in DLPFC, mPFC, basal ganglia and left inferior temporal cortex. Leads to metabolic ↑in occipital cortex. | ||
| ↓activity in basal ganglia correlates with improvemnetin negative symptoms.↓activity in motor area relates to ↓in disorganisation symptoms. ↑activity in primary visual area correlates with ↑in positive symptoms. | |||||
| Molina et al. | 1996, 1997b | 99mTc-HMPAO SPECT | Prior to clozapine CR showed ↑ perfusion in thalamus, basal ganglia, left lower and right upper DLPFC. CR subsequently showed↓in perfusion post clozapine in L basal ganglia and bilateral thalamus. | ||
| Post clozapine treatment responders showed perfusion decrease in thalamus, basal ganglia, and dorsolateral cortex. Non repsonders did not show significant changes in any perfusion values. | |||||
| Molina et al. | 2008b | 99mTc-HMPAO SPECT | Following 1 mth of clozapine pts no longer showed ↓activity in cingulate or insular regions although pts still showed ↓perfusion in MPFC. Hyperactivity in brainstem, temporolateral and occipital areas still present. | ||
| Potkin et al. | 1994 | FDG PET | Clozapine responders showed greater increase in perfusion following clozapine treatment in: medial occipital cortex and caudate head. A decrease was found in the posterior cortex and hippocampus. | ||
| Potkin et al. | 2003 | FDG PET | D1 2,2 homozygotes show widespread metabolic decreases following clozapine treatment and good clinical response – while this is not observed for D1 1,2 heterzygotes. Interestingly, heterozygotes showed worsening of symptoms which was associated with metabolic decreases in the left prefrontal cortex, bilateral temporal and an increase in right inferior temporal cortices | ||
| Zhao et al. | 2006 | 99mTc-ECD SPECT | Clozapine had no effect on rCBF either during resting state or during Wisconsin card sorting test, although behavioural performance in the task occured | ||
| Etrugal et al. | 2009 | 1H-MRS | Nearly significant increase in NAA/Cr ratio in Left DLPFC after clozapine treatment. | ||
| Goldstein et al. | 2015 | 1H-MRS | CR show greater Glx/Cr than CNR in putamen. | 4.00 | |
| Gallety et al. | 2005 | EEG | Clozapine treatment was associated with normalisation of P3 and late slow waves and partial normalisation of N1 amplitude. | ||
| Gross et al. | 2004 | EEG | Clozapine treatment associated with increase in theta power in midline which correlates with clinical improvement | ||
| Kikuchi et al. | 2014 | EEG | 39% of patients treated with clozapine developed EEG abnormalities. Individuals who developed abnormalities were more likely to be younger and have a shorter duration of illness. | ||
| Knott et al. | 2001 | EEG | Clozapine treatment decreases relative alpha power and mean beta/total spectrum frequency; and increases absolute total and delta/theta power. | ||
| Knott et al. | 2002 | EEG | Clozapine treatment normalises some of the inter- and intrahemispheric coherence abnormalities present at baseline | ||
| Lacroix et al. | 1995 | EEG | Clozapine treatment led to increases in theta and alpha bands | ||
| Low responders show a greater beta1 increase than high responders | |||||
| High responders show increased coherence between a wide variety of regions (centred on the right anterior-medial temporal region and in the theta band) that is not observed in low responders | |||||
| MacCrimmon et al. | 2012 | EEG | Baseline EEG compared with second EEG taken on average 1.4 years after starting clozapine. Clozapine augments power in delta and theta bands globally (particularly in frontal areas). Beta3 power reduced. Alpha shows a frontal increase and posterior decrease. | ||
| Ravan et al. | 2014 | EEG | CR EEG become indistinguishable from HV EEG following clozapine treatment, whereas CNR remain markedly different. | ||
| Tsekou et al. | 2015 | EEG | Stage 2 sleep increased with clozapine treatment, slow wave sleep reduced and REM increased. | ||
| Umbricht et al. | 1998 | EEG | Clozapine partially normalise P300 decreases but does not affect MMN. | ||
Eleven studies used structural neuroimaging. Three early studies used computed tomography (CT) scans in an attempt to identify predictors of clozapine response.48–50 These consistently found that responders to clozapine have smaller prefrontal sulcal spaces compared to poor responders. Later findings that larger prefrontal51,52 and temporal52 gray matter volumes are associated with a good response to clozapine are in keeping with the earlier CT studies. There have been some conflicting findings, one MRI study showed almost diametrically opposed results in that response was associated with larger sulcal spaces in the anterior superior temporal lobe. 53 However, this study included treatment intolerant as well as treatment resistant patients, and this difference in patient population may explain this discrepancy. Another study, did not find any direct significant contrasts between individuals who had responded well to clozapine and clozapine non-responders.15
Regarding the effects of clozapine treatment a longitudinal study demonstrated that over the course of a year patients started on clozapine showed a 10% reduction in caudate nuclei volume, while those remaining on typical antipsychotics showed an 8% increase.54 These findings were replicated by in a study showing that clozapine use led to caudate nuclei reductions over 2455 and 52 weeks.56 Furthermore, greater reductions in left caudate volume were seen in clozapine responders compared to non-responders. The findings of widespread reduced gray and increased white matter volumes in TRS reported by Molina et al. were attenuated during clozapine treatment.14 This is in contrast to a recent study showing gray matter losses in the prefrontal cortex were (non significantly) greater in patients treated with clozapine compared to healthy volunteers, although clozapine responders had less cortical thinning over the left medial frontal cortex and right middle temporal cortex compared to clozapine non-responders during this period.17
Twelve PET/SPECT studies were identified. Two early FDG PET studies demonstrated increased metabolic rates in the basal ganglia,57,58 and reduced rates in the frontal cortex58 following clozapine treatment. Two 99mTc-HMPAO studies, however, suggested that clozapine response was predicted by pre-treatment increased basal ganglia and frontal cortex perfusion, and that treatment reduced perfusion.30,59 These differences may be accounted for by the fact that at the time of scanning individuals in the FDG studies had been antipsychotic free for at least 14 days while in 99mTc-HMPAO studies individuals were taking antipsychotics, which have been shown to alter brain metabolism.60 A later 99mTc-HMPAO study showed that clozapine treatment led to increased perfusion of the frontal cortex, and that this predicted response; again scanning occurred in this study following one week wash out as opposed to during antipsychotic treatment.61
Some of the discrepancies’ between study findings may be accounted for by differences in participant medication status. However the likelihood that some of this heterogeneity is more intrinsic to the question under examination is well illustrated by two studies. One study reported individual patient findings, and described a number of patients showing reductions in perfusion, and others increases, in both the basal ganglia and frontal cortex following clozapine treatment.62 Second, a 15O-PET study showed that clozapine treatment led to increases in perfusion the dorsolateral part of the frontal cortex but decreases in the ventrolateral part.63
A FDG PET study suggested that response to clozapine is modulated by different alleles of the DRD1 gene that codes for D1 receptors.64 It was found that cortical metabolic decreases were associated with clinical improvement for patients with the DRD1 2,2 receptor genotype but not for patients with the heterozygous DRD1 1,2 genotype..
One study employed 1H-MRS to measure N-Acetylaspartic acid (NAA), a marker of neuronal integrity.61 It found lower NAA levels in the dorsolateral prefrontal cortex were associated with clinical improvement, while 8 weeks of clozapine increased NAA levels (though no correlation was found with clinical improvement). Another study suggested individuals on clozapine who show a good response have greater glutamate + glutamine levels in the putamen compared to those with a poor response.35
Ten EEG studies were identified. One study found that clozapine normalised P300 and slow wave components;36 while another showed clozapine partially normalised P300 decreases, but did not have any effect on the MMN38. These findings suggest that clozapine possibly affects attentive but not pre-attentive processing. Five studies used spectral analysis to assess effects of clozapine.65–69 Two early studies65,66 measured coherence and showed that resistant patients display interhemispheric and intrahemispheric dysconnectivity over anterior brain regions that clozapine partially normalised. These changes in coherence were also related to improvement in negative symptoms. Three studies demonstrated the widespread effects of clozapine on spectral power, indicating both increases in fast wave and slow wave power. 67–69
Discussion
The Neurobiology of treatment resistance
Two main schools of thought exist regarding the neurobiology of TRS. One, which can be characterised as the continuum hypothesis, posits that the same pathophysiological processes underlie symptoms in both responsive and resistant patients, but that these processes occur to a greater degree in resistant patients and so treatment is less effective. The other, that can be considered the categorical hypothesis, is that resistant schizophrenia has a fundamentally different pathophysiology to responsive schizophrenia, and thus current treatments are ineffective as they target the wrong processes.70
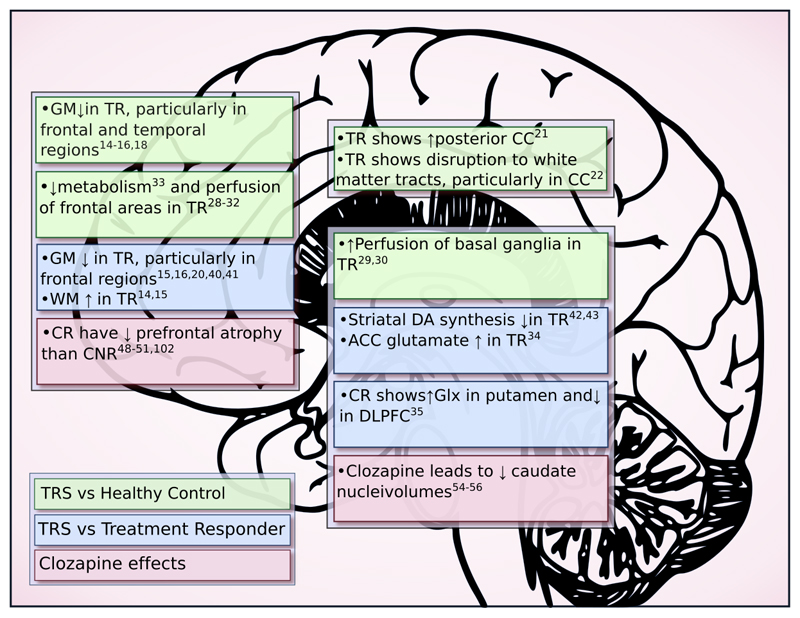
Figure 2 summarises the findings that have support from more than a single study. When resistant patients are compared to healthy controls, structural studies uniformly show gray matter reductions relative to controls, which is consistent with findings seen in schizophrenia in general.71 It is important to note, however, that volume reductions may not be universally detrimental, with one study showing an association between symptom severity and larger orbitofrontal cortex voumes.72 Functional changes were also similar to those reported in schizophrenia in general.26,27
Figure 2. Summary of neuroimaging findings in treatment resistant schizophrenia.
ACC – anterior cingulate cortex; CC – corpus callosum; CR – clozapine responder; CNR – clozapine non-responder; DLPFC – dorsolateral prefrontal cortex; Glx – glutamate+glutamine; GM – grey matter; TR – treatment resistant; WM – white matter
In comparing resistant and responsive patients the most replicated finding was a greater reduction in gray matter in resistant patients, predominantly in frontal areas. One fMRI27 and one EEG study14 also suggested a continuum of pathology – with differences observable in the treatment responders compared to controls, but more marked in the resistant group.
In terms of neurochemistry, two PET studies are consistent in suggesting that resistant patients might have different dopaminergic functioning relative to responsive patients.42,43 One F-DOPA PET study showed raised dopamine synthesis capacity in schizophrenia patients in general but no evidence of increased capacity in resistant patients.43 A FDG PET study of the effect of a haloperidol challenge showed marked metabolic decreases in resistant but not responsive patients.42 This can be interpreted as being secondary to responsive patients having elevated presynaptic dopamine reserves, and thus being able to accommodate the antidopaminergic effects of haloperidol; while treatment-resistant patients do not, resulting in decreased metabolism. If resistant patients do indeed have a normally functionally dopaminergic system, this raises the question of what neurochemical abnormalities underlie treatment resistance. Glutamatergic dysfunction has been implicated in the development of schizophrenia, in relation to both positive and negative symptoms.73–81 The two 1H-MRS studies were consistent in showing glutamatergic elevations in resistant compared to responsive patients.34,35 As elevations in glutamate have been associated with excitotoxicity and structural brain changes82, glutamate elevations in resistant patients could account for the relative gray matter reductions found in some studies of resistant patients. Whilst this supports the idea that glutamatergic dysfunction underlies resistance, it needs to be tested further.
Support for both continuum and categorical hypotheses can be found outside of neuroimaging. Recent research has suggested a potential genetic framework on which categorically different schizophrenia subtypes could sit,83 and neurochemically it is possible that categorical differences in dopaminergic and glutamatergic function could account for differences in treatment response.70 Conversely other studies provide support for a continuum - demonstrating that patients with greater exposure to both environmenta,84 and genetic85 risk factors are more likely to be treatment resistant.
Recent studies have employed multimodal imaging techniques to more precisely delineate the neurobiological processes underlying psychotic disorders.86–88 An expansion of this approach to include both thorough phenotypic characterization, and measurement of environmental and genetic factors may be needed to gain a fuller understanding of the causative factors leading to treatment resistance.
Treatment resistant patients will, by definition, have greater symptom severity but may also have longer illness duration, and greater cumulative antipsychotic exposure than responsive patients. Long term exposure to antipsychotics has been shown to cause both increases in basal ganglia volume89 and atrophy of cortical gray matter.90,91 As such the brain differences observed could reflect these confounds, as opposed to identifying pathophysiologically different illness types.
The effects of clozapine on brain structure and function and predictors of clozapine response
Two studies showed an association between clozapine treatment and reductions in caudate volume, while other antipsychotics were associated with enlargement.54–56 In addition, reductions in caudate volume were associated with a good clinical response. Furthermore, two SPECT studies demonstrated response to clozapine is predicted by increased pre-treatment perfusion of the basal ganglia, that decreases with successful treatment.30,59
This suggests that clozapine’s superior efficacy may be related to its normalising effect on striatal structure and function, consistent with its reduced affinity for the D2 receptor.92 Some patients seem to show an initial good response to antipsychotic treatment and then develop treatment resistance after a number of years of treatment.8,93 It has been suggested that this secondary treatment resistance is due to D2/3 receptor supersentivity due to receptor up-regulation or other changes. Antipsychotic exposure is associated with dopamine D2/3 receptor up-regulation in rodents94 and, whilst the degree to which this happens in humans is unclear, antipsychotic treatment is associated with changes in striatal volume and functional indices in patients.89 Clozapine has a relatively low affinity for and fast dissociation from the D2/3 receptor.92 Thus, putatively, these actions at the D2/3 receptor could allow D2/3 supersensitivity to resolve, and underlie clozapine’s efficacy for individuals who have developed secondary treatment resistance following sustained antipsychotic treatment. Whilst this is consistent with the normalisation of the striatal functional and structural changes seen with clozapine, this needs testing in patients. Moreover this is unlikely to explain all of clozapine’s clinical efficacy, not least because enhanced efficacy in treatment resistance is not seen with quetiapine, which also shows relatively low affinity for D2/3 receptors.95
Clozapine has effects on a large number of other neurotransmitter systems, including glutamate.96 Given the glutamatergic abnormalities that have been associated with resistant schizophrenia34,35 this is a element of its pharmacology which could may contribute to its superior efficacy.
The apparent inconsistency between the two studies that showed increased perfusion following clozapine treatment57,58 and the others can potentially be explained by differences in participants’ medication status at the time of scan. The findings of Lahti et al. neatly illustrate the fact that striatal perfusion increases with greater D2 antagonism.97 Therefore if a baseline scan is performed while participants are receiving non-clozapine antipsychotics and are then scanned again when receiving clozapine a reduction in perfusion might be expected (due to a relative reduction in D2 antagonism). If, however, the baseline scan occurs when participants are receiving no antipsychotic treatment, the scan following clozapine treatment might be expected to show increased perfusion, due to the relative increase in D2 antagonism.
In terms of predicting response, early studies suggested that individuals with the most marked frontal atrophy were less likely to benefit from clozapine treatment;14,48–51 but later studies have produced conflicting results.15,53 The findings regarding clozapine’s longitudinal effects on global gray matter studies are too inconsistent to draw conclusions from. Electrophysiological studies showed that clozapine has widespread effects on spectral power66–68 and connectivity65,66. It appears that a good clinical response to clozapine is accompanied by normalization of various EEG measures towards the values seen in healthy controls.36,38,98 No consistent findings, however, show markers predicting treatment response at baseline.
Current research limitations and future directions
Our review highlights the heterogeneity that permeates neuroimaging research into treatment resistance. Some of this may be inherent to the problem under examination. There may be many ways for an illness to be treatment resistant, but only one route to treatment response. In particular individuals with similar clinical presentations may show treatment resistance due to different pharmacokinetic and pharmacodynamic factors, and/or vary markedly in the underlying pathoaetiology. Some of the heterogeneity is, however, as a result of the methods used. The cohorts studied vary widely in illness duration, in previous drug treatment and treatment at time of scan, in sample sizes, in imaging techniques, and in analysis methods used. A further issue contributing to heterogeneity is that many studies were underpowered to detect even moderate effect sizes (e.g. Cohen’s d=0.5). Another limitation is the variable definition of treatment-resistance among studies. It is important that, for research purposes, treatment-resistance is defined using standardised, quantifiable criteria. This would allow for more direct comparisons across studies, which is particularly important in the identification of biomarkers.
Cross-sectional comparisons of treatment resistant and responsive patients can potentially indicate differences that may underlie treatment resistance. They cannot, however, determine causality. Furthermore, it is difficult to exclude certain confounders in cross-sectional studies, for example, the finding that higher clozapine doses correlate with greater gray matter loss is confounded by the association of higher doses with disease severity.17 In view of this, where there are differences, we cannot exclude that they may be secondary to other factors. We did not identify any studies that prospectively investigated brain structure or function from illness onset to the development of treatment resistance. A logical strategy is to start with cross-sectional studies to identify brain differences between responders and resistant patients but, ultimately, prospective studies from illness onset are needed to identify what biologically underlies treatment resistance. Furthermore, prospective studies will be required to evaluate whether any neurobiological markers have the potential for clinically relevant prediction of treatment resistance.
The available imaging evidence provides some limited support for both continuum and categorical hypotheses. This suggests a hybrid of both hypotheses may best describe the neurobiology of resistant schizophrenia, with some aspects such as structural changes on a continuum, whilst other aspects, such as presynaptic dopamine function, may be categorically different. It is also apparent, that whilst there have been fifty-nine imaging studies of resistant schizophrenia, few have attempted to replicate prior findings. Well controlled, ideally prospective, studies from illness onset are required to definitively determine the key aspects of the neurobiology underlying treatment resistance and identify reliable biomarkers for treatment resistance.
Supplementary Material
Footnotes
Conflicts of interest: Dr Howes has received investigator-initiated research funding from and/or participated in advisory/ speaker meetings organised by Astra-Zeneca, Autifony, BMS, Eli Lilly, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, and Roche. Neither Dr Howes or his family have been employed by or have holdings/ a financial stake in any biomedical company. Drs McCutcheon and Mouchlianitis do not report any conflicts of interest.
References
- 1.Howes O, Murray R. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;6736:1–11. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howes O, Egerton A, Allan V. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15:2550–9. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kane JM. Addressing nonresponse in schizophrenia. J Clin Psychiatry. 2012;73:e07. doi: 10.4088/JCP.11076tx2c. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy J, Altar C, Taylor D. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin. 2014;29:63–76. doi: 10.1097/YIC.0b013e32836508e6. [DOI] [PubMed] [Google Scholar]
- 5.Black K, Peters L, Rui Q, Milliken H, Whitehorn D, Kopala LC. Duration of untreated psychosis predicts treatment outcome in an early psychosis program. Schizophr Res. 2001;47:215–22. doi: 10.1016/s0920-9964(00)00144-4. [DOI] [PubMed] [Google Scholar]
- 6.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 7.Agid O, Foussias G, Singh S, Remington G. Where to position clozapine: re-examining the evidence. Can J Psychiatry. 2010;55:677–84. doi: 10.1177/070674371005501007. [DOI] [PubMed] [Google Scholar]
- 8.Beck K, McCutcheon R, Bloomfield MA, et al. The practical management of refractory schizophrenia–the Maudsley Treatment REview and Assessment Team service approach. Acta Psychiatr Scand. 2014;130:427–38. doi: 10.1111/acps.12327. [DOI] [PubMed] [Google Scholar]
- 9.Howes ODO, Kambeitz J, Stahl D, et al. The Nature of Dopamine Dysfunction in Schizophrenia and What This Means for Treatment. Arch Gen Psychiatry. 2012;69:776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howes OD, Vergunst F, Gee S, McGuire P, Kapur S, Taylor D. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201:481–5. doi: 10.1192/bjp.bp.111.105833. [DOI] [PubMed] [Google Scholar]
- 11.National Institute For Clinical Excellence. Schizophrenia: The NICE guideline on core interventions in the treatment and management of schizophrenia in primary and secondary care; National Clinical Practice Guidelines Number CG82. 2014 [Google Scholar]
- 12.Howes OD, Vergunst F, Gee S, McGuire P, Kapur S, Taylor D. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J psychiatry J Ment Sci. 2012;201:481–5. doi: 10.1192/bjp.bp.111.105833. [DOI] [PubMed] [Google Scholar]
- 13.Kane JM, Honigfeld G, Singer J, Meltzer H. Clozapine in treatment-resistant schizophrenics. Psychopharmacol Bull. 1988;24:62–7. [PubMed] [Google Scholar]
- 14.Molina V, Reig S, Sanz J, et al. Differential clinical, structural and P300 parameters in schizophrenia patients resistant to conventional neuroleptics. Prog Neuro-Psychopharmacology Biol Psychiatry. 2008;32:257–66. doi: 10.1016/j.pnpbp.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Anderson VM, Goldstein ME, Kydd RR, Russell BR. Extensive Gray Matter Volume Reduction in Treatment-Resistant Schizophrenia. Int J Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyv016. published online Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quarantelli M, Palladino O, Prinster A, et al. Patients with poor response to antipsychotics have a more severe pattern of frontal atrophy: a voxel-based morphometry study of treatment resistance in schizophrenia. Biomed Res Int. 2014;2014:325052. doi: 10.1155/2014/325052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed M, Cannon DM, Scanlon C, et al. Progressive Brain Atrophy and Cortical Thinning in Schizophrenia after Commencing Clozapine Treatment. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.90. published online April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maller JJ, Daskalakis ZJ, Thomson RHS, Daigle M, Barr MS, Fitzgerald PB. Hippocampal volumetrics in treatment-resistant depression and schizophrenia: The devil’s in De-Tail. Hippocampus. 2012;22:9–16. doi: 10.1002/hipo.20873. [DOI] [PubMed] [Google Scholar]
- 19.Kubera KM, Sambataro F, Vasic N, et al. Source-based morphometry of gray matter volume in patients with schizophrenia who have persistent auditory verbal hallucinations. Prog Neuro-Psychopharmacology Biol Psychiatry. 2014;50:102–9. doi: 10.1016/j.pnpbp.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Zugman A, Gadelha A, Assunção I, et al. Reduced dorso-lateral prefrontal cortex in treatment resistant schizophrenia. Schizophr Res. 2013;148:81–6. doi: 10.1016/j.schres.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Maller JJ, Daskalakis ZJ, Furtado CC, Fitzgerald PB. Morphology of the corpus callosum in treatment-resistant schizophrenia and major depression. Acta Psychiatr Scand. 2009;120:265–73. doi: 10.1111/j.1600-0447.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- 22.Holleran L, Ahmed M, Schmidt H, et al. Altered Interhemispheric and Temporal Lobe White Matter Microstructural Organisation in Severe Chronic Schizophrenia. Neuropsychopharmacology. 2014;39:944–54. doi: 10.1038/npp.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alonso-Solis A, Vives-Gilabert Y, Grasa E, et al. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr Res. 2015;161:261–8. doi: 10.1016/j.schres.2014.10.047. [DOI] [PubMed] [Google Scholar]
- 24.Vercammen A, Knegtering H, den Boer Ja, Liemburg EJ, Aleman A. Auditory Hallucinations in Schizophrenia Are Associated with Reduced Functional Connectivity of the Temporo-Parietal Area. Biol Psychiatry. 2010;67:912–8. doi: 10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Wolf ND, Sambataro F, Vasic N, et al. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J Psychiatry Neurosci. 2011;36:366–74. doi: 10.1503/jpn.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald PB, Sritharan A, Benitez J, et al. A preliminary fMRI study of the effects on cortical activation of the treatment of refractory auditory hallucinations with rTMS. Psychiatry Res - Neuroimaging. 2007;155:83–8. doi: 10.1016/j.pscychresns.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Wolf ND, Grön G, Sambataro F, et al. Magnetic resonance perfusion imaging of auditory verbal hallucinations in patients with schizophrenia. Schizophr Res. 2012;134:285–7. doi: 10.1016/j.schres.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Klirova M, Horacek J, Novak T, et al. Individualized rTMS neuronavigated according to regional brain metabolism (18FGD PET) has better treatment effects on auditory hallucinations than standard positioning of rTMS: A double-blind, sham-controlled study. Eur Arch Psychiatry Clin Neurosci. 2013;263:475–84. doi: 10.1007/s00406-012-0368-x. [DOI] [PubMed] [Google Scholar]
- 29.Molina Rodríguez V, Montz Andrée R, Pérez Castejón MJ, et al. Cerebral perfusion correlates of negative symptomatology and parkinsonism in a sample of treatment-refractory schizophrenics: An exploratory 99mTc-HMPAO SPET study. Schizophr Res. 1997;25:11–20. doi: 10.1016/s0920-9964(96)00115-6. [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez VM, Andrée RM, Pérez Castejón MJ, et al. Fronto-striato-thalamic perfusion and clozapine response in treatment- refractory schizophrenic patients. A 99mTc-HMPAO study. Psychiatry Res - Neuroimaging. 1997;76:51–61. doi: 10.1016/s0925-4927(97)00057-7. [DOI] [PubMed] [Google Scholar]
- 31.Molina V, Tamayo P, Montes C, et al. Clozapine may partially compensate for task-related brain perfusion abnormalities in risperidone-resistant schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:948–54. doi: 10.1016/j.pnpbp.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 32.Zhao J, He X, Liu Z, Yang D. The effects of clozapine on cognitive function and regional cerebral blood flow in the negative symptom profile schizophrenia. Int J Psychiatry Med. 2006;36:171–81. doi: 10.2190/1AA0-UW9Q-1CNK-3E2N. [DOI] [PubMed] [Google Scholar]
- 33.Molina V, Sanz J, Sarramea F, Palomo T. Marked hypofrontality in clozapine-responsive patients. Pharmacopsychiatry. 2007;40:157–62. doi: 10.1055/s-2007-984399. [DOI] [PubMed] [Google Scholar]
- 34.Demjaha A, Egerton A, Murray RM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75:e11–3. doi: 10.1016/j.biopsych.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein ME, Anderson VM, Pillai A, Kydd RR, Russell BR. Glutamatergic neurometabolites in clozapine-responsive and -resistant schizophrenia. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galletly Ca, Clark CR, McFarlane AC. Clozapine improves working memory updating in schizophrenia. Eur Neuropsychopharmacol. 2005;15:601–8. doi: 10.1016/j.euroneuro.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Horton J, Millar A, Labelle A, Knott VJ. MMN responsivity to manipulations of frequency and duration deviants in chronic, clozapine-treated schizophrenia patients. Schizophr Res. 2011;126:202–11. doi: 10.1016/j.schres.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Umbricht D, Javitt D, Novak G, et al. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1998;44:716–25. doi: 10.1016/s0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 39.Milovan DL, Baribeau J, Roth RM, Stip E. ERP study of pre-attentive auditory processing in treatment-refractory schizophrenia. Brain Cogn. 2004;55:355–7. doi: 10.1016/j.bandc.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 40.Lawrie SM, Ingle GT, Santosh CG, et al. Magnetic resonance imaging and single photon emission tomography in treatment-responsive and treatment-resistant schizophrenia. Br J Psychiatry. 1995;167:202–10. doi: 10.1192/bjp.167.2.202. [DOI] [PubMed] [Google Scholar]
- 41.Mitelman Sa, Shihabuddin L, Brickman AM, Hazlett Ea, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005;72:91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Bartlett EJ, Brodie JD, Simkowitz P, et al. Effect of a Haloperidol Challenge on Regional Brain Metabolism in Neuroleptic-Responsive and Nonresponsive Schizophrenic Patients. Am J Psychiatry. 1998;155:337–43. doi: 10.1176/ajp.155.3.337. [DOI] [PubMed] [Google Scholar]
- 43.Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–10. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- 44.Ramos J, Cerdán LF, Guevara MA, Amezcua C, Sanz A. Abnormal EEG patterns in treatment-resistant schizophrenic patients. Int J Neurosci. 2001;109:47–59. doi: 10.3109/00207450108986524. [DOI] [PubMed] [Google Scholar]
- 45.Hong LE, Summerfelt A, McMahon RP, Thaker GK, Buchanan RW. Gamma/beta oscillation and sensory gating deficit in schizophrenia. Neuroreport. 2004;15:155–9. doi: 10.1097/00001756-200401190-00030. [DOI] [PubMed] [Google Scholar]
- 46.Lee S-H, Wynn JK, Green MF, et al. Quantitative EEG and low resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophr Res. 2006;83:111–9. doi: 10.1016/j.schres.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 47.Lee S-H, Choo J-S, Im W-Y, Chae J-H. Nonlinear analysis of electroencephalogram in schizophrenia patients with persistent auditory hallucination. Psychiatry Investig. 2008;5:115–20. doi: 10.4306/pi.2008.5.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Honer WG, Smith GN, Lapointe JS, Macewan GW, Kopala L, Altman S. Regional cortical anatomy and clozapine response in refractory schizophrenia. Neuropsychopharmacology. 1995;13:85–7. doi: 10.1016/0893-133X(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 49.Konicki PE, Kwon KY, Steele V, et al. Prefrontal cortical sulcal widening associated with poor treatment response to clozapine. Schizophr Res. 2001;48:173–6. doi: 10.1016/s0920-9964(00)00130-4. [DOI] [PubMed] [Google Scholar]
- 50.Friedman L, Knutson L, Shurell M, Meltzer HY. Prefrontal sulcal prominence is inversely related to response to clozapine in schizophrenia. Biol Psychiatry. 1991;29:865–77. doi: 10.1016/0006-3223(91)90053-o. [DOI] [PubMed] [Google Scholar]
- 51.Arango C, Breier A, McMahon R, Carpenter WT, Buchanan RW. The relationship of clozapine and haloperidol treatment response to prefrontal, hippocampal, and caudate brain volumes. Am J Psychiatry. 2003;160:1421–7. doi: 10.1176/appi.ajp.160.8.1421. [DOI] [PubMed] [Google Scholar]
- 52.Molina V. Anatomical and functional brain variables associated with clozapine response in treatment-resistant schizophrenia. Psychiatry Res Neuroimaging. 2003;124:153–61. doi: 10.1016/s0925-4927(03)00108-2. [DOI] [PubMed] [Google Scholar]
- 53.Lauriello J, Mathalon DH, Rosenbloom M, et al. Association between regional brain volumes and clozapine response in schizophrenia. Biol Psychiatry. 1998;43:879–86. doi: 10.1016/s0006-3223(97)00491-5. [DOI] [PubMed] [Google Scholar]
- 54.Chakos MH, Lieberman Ja, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345:456–7. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- 55.Scheepers FE, De Wied CCG, Pol HEH, Van De Flier W, Van Der Linden Ja, Kahn RS. The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology. 2001;24:47–54. doi: 10.1016/S0893-133X(00)00172-X. [DOI] [PubMed] [Google Scholar]
- 56.Scheepers FE, Gispen de Wied CC, Hulshoff Pol HE, Kahn RS. Effect of clozapine on caudate nucleus volume in relation to symptoms of schizophrenia. Am J Psychiatry. 2001;158:644–6. doi: 10.1176/appi.ajp.158.4.644. [DOI] [PubMed] [Google Scholar]
- 57.Buchsbaum MS, Potkin SG, Marshall JF, et al. Effects of clozapine and thiothixene on glucose metabolic rate in schizophrenia. Neuropsychopharmacology. 1992;6:155–63. [PubMed] [Google Scholar]
- 58.Potkin SG, Buchsbaum MS, Jin Y, et al. Clozapine effects of glucose metabolic rate in striatum and frontal cortex. J Clin Psychiatry. 1994;55:63–6. [PubMed] [Google Scholar]
- 59.Molina Rodríguez V, Andreé RM, Jesús Pérez Castejón M, Capdevila García E, Carreras Delgado JL, Rubia Vila J. SPECT study of regional cerebral perfusion in neuroleptic-resistant schizophrenic patients who responded or did not respond to clozapine. Am J Psychiatry. 1996;153:1343–6. doi: 10.1176/ajp.153.10.1343. [DOI] [PubMed] [Google Scholar]
- 60.Kim E, Howes OD, Turkheimer FE, et al. The relationship between antipsychotic D2 occupancy and change in frontal metabolism and working memory : A dual [(11)C]raclopride and [(18) F]FDG imaging study with aripiprazole. Psychopharmacology (Berl) 2013;227:221–9. doi: 10.1007/s00213-012-2953-0. [DOI] [PubMed] [Google Scholar]
- 61.Ertugrul A, Volkan-Salanci B, Basar K, et al. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: relationship with treatment response. Psychiatry Res. 2009;174:121–9. doi: 10.1016/j.pscychresns.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Ergün EL, Volkan-Salanci B, Ertugrul A, Demir B, Erbas B. Evaluation of SISCOM in routine regional cerebral blood flow alterations after clozapine, in schizophrenia. Hell J Nucl Med. 2010;13:35–9. [PubMed] [Google Scholar]
- 63.Lahti AC, Holcomb HH, Weiler Ma, Medoff DR, Tamminga Ca. Functional effects of antipsychotic drugs: Comparing clozapine with haloperidol. Biol Psychiatry. 2003;53:601–8. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- 64.Potkin SG, Basile VS, Jin Y, et al. D1 receptor alleles predict PET metabolic correlates of clinical response to clozapine. Mol Psychiatry. 2003;8:109–13. doi: 10.1038/sj.mp.4001191. [DOI] [PubMed] [Google Scholar]
- 65.Lacroix D, Chaput Y, Rodriguez JP, et al. Quantified EEG changes associated with a positive clinical response to clozapine in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:861–76. doi: 10.1016/0278-5846(95)00116-d. [DOI] [PubMed] [Google Scholar]
- 66.Knott VJ, LaBelle A, Jones B, Mahoney C. EEG coherence following acute and chronic clozapine in treatment-resistant schizophrenics. Exp Clin Psychopharmacol. 2002;10:435–44. doi: 10.1037//1064-1297.10.4.435. [DOI] [PubMed] [Google Scholar]
- 67.Gross A, Joutsiniemi SL, Rimon R, Appelberg B. Clozapine-induced QEEG changes correlate with clinical response in schizophrenic patients: A prospective, longitudinal study. Pharmacopsychiatry. 2004;37:119–22. doi: 10.1055/s-2004-818989. [DOI] [PubMed] [Google Scholar]
- 68.Knott V, Labelle a, Jones B, Mahoney C. Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Schizophr Res. 2001;50:41–53. doi: 10.1016/s0920-9964(00)00165-1. [DOI] [PubMed] [Google Scholar]
- 69.MacCrimmon D, Brunet D, Criollo M, Galin H, Lawson JS. Clozapine Augments Delta, Theta, and Right Frontal EEG Alpha Power in Schizophrenic Patients. ISRN Psychiatry. 2012;2012:1–8. doi: 10.5402/2012/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howes OD, Kapur S. A neurobiological hypothesis for the classification of schizophrenia: Type a (hyperdopaminergic) and type b (normodopaminergic) Br J Psychiatry. 2014;205:1–3. doi: 10.1192/bjp.bp.113.138578. [DOI] [PubMed] [Google Scholar]
- 71.Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK. Mapping of grey matter changes in schizophrenia. Schizophr Res. 1999;35:1–14. doi: 10.1016/s0920-9964(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 72.Hoptman MJ, Volavka J, Weiss EM, et al. Quantitative MRI measures of orbitofrontal cortex in patients with chronic schizophrenia or schizoaffective disorder. Psychiatry Res - Neuroimaging. 2005;140:133–45. doi: 10.1016/j.pscychresns.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Egerton A, Stone JM. The glutamate hypothesis of schizophrenia: neuroimaging and drug development. Curr Pharm Biotechnol. 2012;13:1500–12. doi: 10.2174/138920112800784961. [DOI] [PubMed] [Google Scholar]
- 74.Egerton A, Fusar-Poli P, Stone JM. Glutamate and psychosis risk. Curr Pharm Des. 2012;18:466–78. doi: 10.2174/138161212799316244. [DOI] [PubMed] [Google Scholar]
- 75.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. 1994 doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 76.Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–42. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Newcomer JW, Farber NB, Jevtovic-Todorovic V, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 1999;20:106–18. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 80.Stone JM, Dietrich C, Edden R, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–5. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stone JM, Raffin M, Morrison P, McGuire PK. Review: The biological basis of antipsychotic response in schizophrenia. J Psychopharmacol. 2010;24:953–64. doi: 10.1177/0269881109106959. [DOI] [PubMed] [Google Scholar]
- 82.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 83.Arnedo J, Svrakic DM, del Val C, et al. Uncovering the Hidden Risk Architecture of the Schizophrenias: Confirmation in Three Independent Genome-Wide Association Studies. Am J Psychiatry. 2015;172:139–53. doi: 10.1176/appi.ajp.2014.14040435. [DOI] [PubMed] [Google Scholar]
- 84.Hassan AN, De Luca V. The effect of lifetime adversities on resistance to antipsychotic treatment in schizophrenia patients. Schizophr Res. 2015;161:496–500. doi: 10.1016/j.schres.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 85.Frank J, Lang M, Witt SH, et al. Identification of increased genetic risk scores for schizophrenia in treatment-resistant patients. Mol Psychiatry. 2015;20:150–1. doi: 10.1038/mp.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lubeiro A, Rueda C, Hernández JA, Sanz J, Sarramea F, Molina V. Identification of two clusters within schizophrenia with different structural, functional and clinical characteristics. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;64:79–86. doi: 10.1016/j.pnpbp.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Allen P, Chaddock Ca, Howes OD, et al. Abnormal relationship between medial temporal lobe and subcortical dopamine function in people with an ultra high risk for psychosis. Schizophr Bull. 2012;38:1040–9. doi: 10.1093/schbul/sbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fusar-Poli P, Howes OD, Allen P, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 89.Ebdrup BH, Nørbak H, Borgwardt S, Glenthøj B. Volumetric changes in the basal ganglia after antipsychotic monotherapy: a systematic review. Curr Med Chem. 2013;20:438–47. doi: 10.2174/0929867311320030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beng-Choon H, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term Antipsychotic Treatment and Brain Volumes. Arch Gen Psychiatry. 2011;68:128–37. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dorph-Petersen K-A, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis Da. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–61. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 92.Kapur S, McClelland RA, VanderSpek SC, et al. Increasing D2 affinity results in the loss of clozapine’s atypical antipsychotic action. Neuroreport. 2002;13:831–5. doi: 10.1097/00001756-200205070-00019. [DOI] [PubMed] [Google Scholar]
- 93.Sheitman BB, Lieberman Ja. The natural history and pathophysiology of treatment resistant schizophrenia. J Psychiatr Res. 1998;32:143–50. doi: 10.1016/s0022-3956(97)00052-6. [DOI] [PubMed] [Google Scholar]
- 94.Samaha a-N, Seeman P, Stewart J, Rajabi H, Kapur S. ‘Breakthrough’ Dopamine Supersensitivity during Ongoing Antipsychotic Treatment Leads to Treatment Failure over Time. J Neurosci. 2007;27:2979–86. doi: 10.1523/JNEUROSCI.5416-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163:600–10. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- 96.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–77. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 97.Lahti AC, Holcomb HH, Weiler Ma, et al. Clozapine but not haloperidol Re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29:171–8. doi: 10.1038/sj.npp.1300312. [DOI] [PubMed] [Google Scholar]
- 98.Ravan M, Hasey G, Reilly JP, MacCrimmon D, Khodayari-Rostamabad A. A machine learning approach using auditory odd-ball responses to investigate the effect of Clozapine therapy. Clin Neurophysiol. 2015;126:721–30. doi: 10.1016/j.clinph.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 99.Cachia A, Paillère-Martinot ML, Galinowski A, et al. Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage. 2008;39:927–35. doi: 10.1016/j.neuroimage.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 100.Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973–8. doi: 10.2147/NDT.S69784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lacroix D, Chaput Y, Rodriguez JP, et al. Quantified EEG changes associated with a positive clinical response to clozapine in schizophrenia. Prog Neuro-Psychopharmacology Biol Psychiatry. 1995;19:861–76. doi: 10.1016/0278-5846(95)00116-d. [DOI] [PubMed] [Google Scholar]
- 102.Molina V, Reig S, Sarramea F, et al. Anatomical and functional brain variables associated with clozapine response in treatment-resistant schizophrenia. Psychiatry Res. 2003;124:153–61. doi: 10.1016/s0925-4927(03)00108-2. [DOI] [PubMed] [Google Scholar]
- 103.Molina V, Gispert JD, Reig S, et al. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl) 2005;178:17–26. doi: 10.1007/s00213-004-1981-9. [DOI] [PubMed] [Google Scholar]
- 104.Tsekou H, Angelopoulos E, Paparrigopoulos T, et al. Sleep EEG and spindle characteristics after combination treatment with clozapine in drug-resistant schizophrenia: a pilot study. J Clin Neurophysiol. 2015;32:159–63. doi: 10.1097/WNP.0000000000000145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.