Abstract
Tissues have a natural capacity to replace dying cells and to heal wounds. This ability resides in resident stem cells, which self-renew, preserve, and repair their tissue during homeostasis and following injury. The skin epidermis and its appendages are subjected to daily assaults from the external environment. A high demand is placed on renewal and regeneration of the skin’s barrier in order to protect the body from infection and dehydration and to heal wounds. This review focuses on the epithelial stem cells of skin, where they come from, where they reside, and how they function in normal homeostasis and wound repair.
Introduction
Wound management is a major world health problem, sparing no country or individual. While acute wounds from accidental cuts and burns are commonplace and heal spontaneously, millions suffer from serious and large wounds from major accidents, surgeries, and warfare that take time to heal and are prone to infection. Even worse are chronic wounds, such as ulcers in elderly and diabetic patients that require repetitive medical intervention to prevent complications. Wound management thus imposes a huge economic burden worldwide.
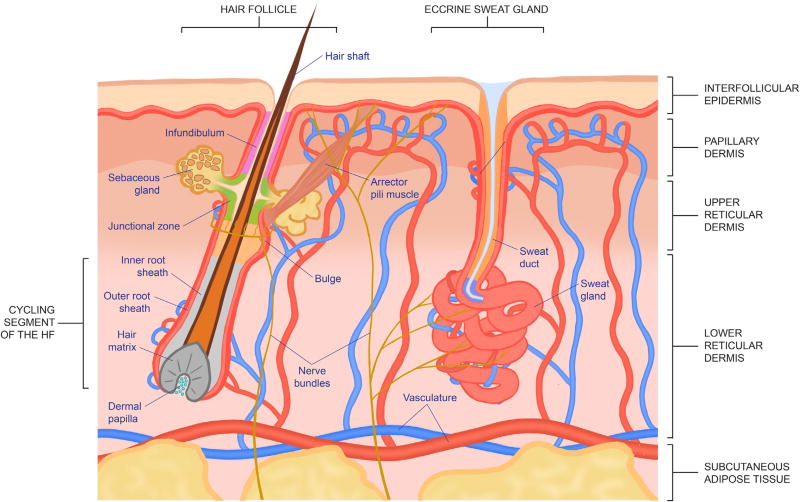
The skin is a complex organ comprised of various tissues that act in harmony to provide protection from daily wear and tear, harmful microbes, and other assaults from the external environment (Figure 1). Thus, when this barrier is breached during wounding, great coordination between various cell types, signaling factors, and matrix interactions is required to re-establish tissue integrity and function. Key players in this process are tissue-resident stem cells, which have the ability to self-renew and maintain their population during homeostasis, and to give rise to one or more specialized cell types to maintain and repair tissue function.
Figure 1. Structure and Components of the Skin.
Under homeostatic conditions, each stem cell population generally contributes only to the differentiation program that exists within its turf. However, following injury, when the systemic and local environments change dramatically, these stem cell populations display remarkable plasticity (Adam et al., 2015; Ge et al., 2017). Indeed, even if their own niche is not perturbed, nearby stem cells respond to wound-induced stimuli by exiting their niche and participating in re-epithelializing damaged tissue (Horsley et al., 2006; Ito et al., 2005; Jensen et al., 2009; Levy et al., 2005; Lu et al., 2012; Nowak et al., 2008; Page et al., 2013). In some cases, they must switch their tissue regeneration program to do so, a feature that can either be transient or permanent, depending upon the particular type of wound.
In this regard, harnessing the plasticity of resident stem cells in the skin promises an attractive alternative avenue for wound therapeutics, but doing so relies on a deep understanding of how stem cells behave in physiological and wound settings. In this review, we piece together current knowledge on the distinct characteristics of the various skin epithelial stem cells and their dynamics under homeostasis and injury. We then discuss the implications of these features on skin biology and therapy.
Stem Cells of the Epidermis and Its Appendages
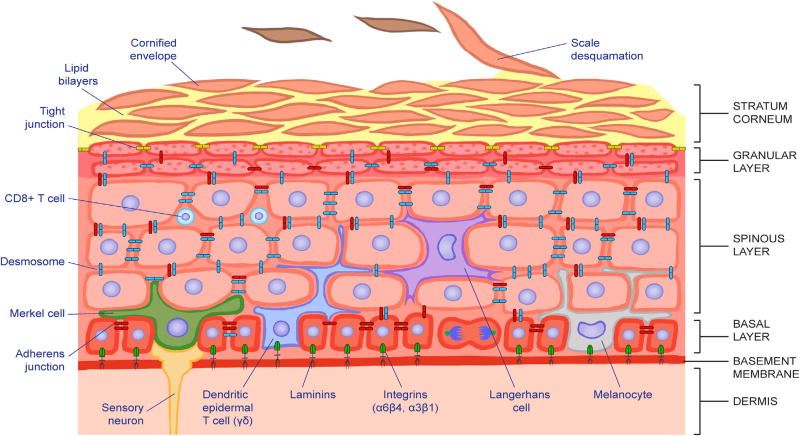
The adult mammalian epidermis is a stratified squamous epithelium, which provides the skin with its barrier (Figure 2). It consists of an inner layer of proliferative basal cells (keratinocytes) that are separated from the underlying dermis by a basement membrane. Only the innermost (basal) layer is proliferative. When basal cells detach (delaminate), they cease to proliferate and embark upon an upward path of differentiation, giving rise to the spinous, granular, and stratum corneum layers. In transit, they undergo a programmed series of morphological and biochemical changes that culminate in the production of dead squames. Each squame is a cellular ghost that has lost all its organelles including the nucleus, and is composed of a durable γ-glutamyl-ε-lysine-cross-linked proteinaceous sac (cornified envelope) that is packed full of insoluble bundles of keratin filaments. Squames are sealed to one another through lipid bilayers that are extruded during the last stages of terminal differentiation. The result is an impenetrable molecular fortress that excludes harmful microbes from entering and protect fluids from leaving. To constantly rejuvenate the barrier, squames are sloughed from the skin surface and replenished by inner differentiating cells moving outward (reviewed by Blanpain and Fuchs, 2009).
Figure 2. The Epidermal Progenitor Niche.
The epidermis is a stratified squamous epithelium. It is divided into four main layers that are distinguished morphologically according to the differentiation status of the keratinocytes as they cease to proliferate and move upward to produce the skin’s barrier. Keratinocytes within the basal layer experience a unique niche distinguished by their contact with the basement membrane, composed of extracellular matrix components and growth factors, contributed by both the epidermis and underlying dermis. This feature maintains their proliferative status. By contrast, keratinocytes that have exited the basal layer embark upon a terminal differentiation program, culminating in the production of dead squames that are sloughed from the skin surface and replaced by inner cells moving upward. Immune cells, mechanosensory cells, and melanocytes also populate discrete layers of the epidermis, reflective of their as yet poorly understood, but vital cross-talk with the keratinocytes and with the microbiota at the skin surface.
The epidermis produces an array of appendages, which differ for different vertebrate species. Epidermal appendages include hair follicles (HFs), sebaceous glands (SGs), sweat glands (SwGs), scales, feathers, fins, beaks, and nails (Chuong and Noveen, 1999). Most mammals produce and maintain an elaborate hair coat, which provides thermal protection from the cold. With the exception of a few mammals such as lemurs, each HF attaches to an arrector pili muscle, which, in addition to enabling the animal to provide a social warning in response to aggression, also positions the hairs off the skin surface to allow thermal cooling (Chaplin et al., 2014; Fujiwara et al., 2011). SGs are HF appendages that reside just above the arrector pili muscle (Figure 1). As sebocyte progenitors at the juncture between the HF and gland differentiate and move inward, they terminally differentiate and then degenerate, releasing sebum into the lumen. The sebum is then extruded through the same orifice as the hair to provide an oily lubricant to the skin surface and hairs. Together, HFs, SGs, and the arrector pili muscle comprise the pilosebaceous unit.
Separate from the pilosebaceous unit, each eccrine sweat gland has its own duct from which salty sweat protrudes to cool the body surface (Figure 1). In contrast to the mammary gland, which undergoes tremendous branching and expansion of glandular tissue to meet the demands of milk production during lactation, the SwG hardly fluctuates in tissue size in response to climate and exercise status. Instead, secretory sweat production by each gland is adjusted through sensory nerve stimuli to fulfill the particular cooling need. For most mammals, SwGs are largely confined to the paws, limiting their cooling capacity. For humans, however, SwGs are prevalent over most of the body’s skin, endowing us with optimal thermoregulation over a broader climate range as well as a remarkable endurance for exercise. As beneficial as it has been, this recent evolutionary change came at the concomitant expense of human body hairs, which are less developed and fewer, thus baring the skin to increased risk of environmental and physical assaults (Lu et al., 2016). To compensate, human epidermis also became considerably thicker and more resilient than the interfollicular epidermis (IFE) of other mammals.
The epidermis and its appendages undergo frequent turnover to rejuvenate their tissue and replace dying cells (Figure 2). Given the diversity of epidermal appendages, it is intriguing that despite their distinct niches and different roles in tissue homeostasis, most if not all adult skin epithelial progenitors express keratins KRT5 and KRT14, among the first skin epithelial progenitor markers ever characterized and cloned (Fuchs and Green, 1980; Fuchs et al., 1981). These progenitors reside along a basement membrane rich in extracellular matrix (ECM) and growth factors. The basement membrane separates epithelium and dermal mesenchyme and likely derives from both compartments. That said, many of the basement membrane components are transcribed by the skin epithelial genome, and progenitors use integrins α6β4 and α3β1 to adhere to and assemble the basement membrane (Watt and Jones, 1993). This attachment polarizes the progenitors and signals transmitted to them. Additional polarity is imparted through the apical-lateral network of adherens junctions and desmosomes that interconnects the epithelial cells within the tissue (Green et al., 2010).
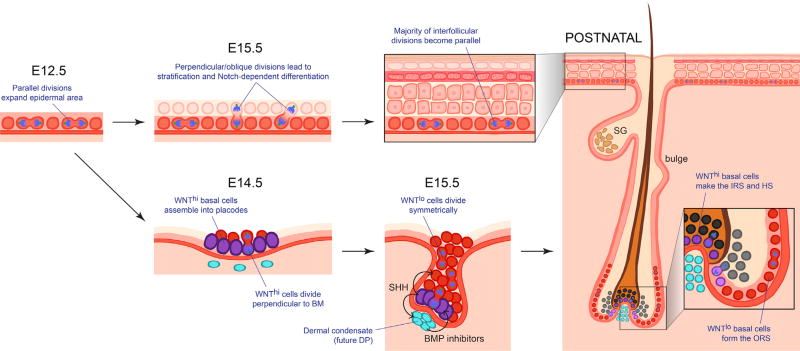
The similarities in progenitors are rooted in embryogenesis (Figure 3). Shortly after gastrulation, the skin exists as a single layer of unspecified epithelial progenitors, which soon begin to express KRT5/KRT14 and other progenitor-specific markers. As the epithelium develops, scattered cells within the epidermal plane produce a higher WNT signal than their neighbors, and these cells cluster into placodes (Ahtiainen et al., 2014; Jamora et al., 2003). If the underlying mesenchyme produces elevated levels of bone morphogenetic protein (BMP) inhibitors, the WNThi cells will form an HF (Jamora et al., 2003; Noramly and Morgan, 1998). If the underlying mesenchyme produces a strong BMP signal, the WNThi cells will make an SwG (Lu et al., 2016). The epidermal cells in the plane that do not receive as potent a morphogen gradient stratify to form the epidermis.
Figure 3. Skin Morphogenesis.
Shortly after gastrulation, the skin begins as a single layer of epidermal progenitors that divide exclusively parallel to the basement membrane underneath. Within several days, divisions become first oblique and then more perpendicular, leading to asymmetric fates, stratification, and differentiation of the epidermis. During this time, hair placodes also form from gathering WNThigh cells within the basal layer. When these cells begin to divide, they do so perpendicular to the basement membrane, leading to asymmetrically fated daughters. WNThigh cells produce SHH, but only neighboring cells appear to respond. SHH prompts the mesenchyme underneath to organize into a dermal condensate and produce BMP inhibitors. SHH also prompts the overlying daughter cells that lose contact with the basement membrane to dampen WNT signaling and divide symmetrically. These WNTlow daughters will generate the outer root sheath (ORS) which develops a niche (bulge) of stem cells, while the WNThigh daughters generate the inner root sheath (IRS) and hair shaft (HS). DP, dermal papilla; SG, sebaceous gland.
By the time morphogenesis is complete, the progenitors of the epidermis and appendages maintain their contact with the basement membrane and the fundamental markers that identify them as keratinocyte progenitors. However, within the adult, the progenitors have now diversified considerably in their additional molecular features. Along the basement membrane, they reside in discrete niches (homes) whose cellular components and other sources of signaling factors heavily influence their behavior with respect to the genes they express and the tissues that they make and maintain (Hsu et al., 2014; Yang et al., 2017).
Many of these progenitors can both self-renew and sustain long-term tissue maintenance, and hence qualify as bona fide stem cell compartments (Blanpain et al., 2004; Horsley et al., 2006; Jensen et al., 2009; Lu et al., 2012; Morris et al., 2004; Snippert et al., 2010; Tumbar et al., 2004). The progenitors that reside in the lower half of the outer root sheath (ORS) and the mature hair bulb appear to be transient and thus exceptions, as demonstrated by the degeneration of these tissues during the destructive (catagen) phase of the hair cycle (described below) (Greco et al., 2009; Rompolas et al., 2016; Yang et al., 2017). In the following sections, we discuss how each stem cell compartment instructs its residents to perform a distinct tissue maintenance task during homeostasis, and how injury changes their behavior to favor plasticity during wound healing.
Contribution and Dynamics of Skin Stem Cells during Homeostasis and Wounding
Epidermal Stem Cells in Development and Adult Tissue Homeostasis
During mouse embryonic development, the epidermis stratifies when progenitors from the single layered epithelium begin to divide obliquely relative to the underlying basement membrane (Lechler and Fuchs, 2005; Williams et al., 2011, 2014). Shortly thereafter, >50% of the basal epidermal mitoses become perpendicular to the basement membrane, generating one basal and one suprabasal daughter (Figure 3). These perpendicularly dividing mammalian epidermal cells utilize a mechanism similar to Drosophila neuroblast asymmetric cell divisions, whose differentially fated daughter cells arise when NOTCH signaling becomes differentially partitioned (Knoblich, 2008). Interestingly, concomitant with perpendicular divisions in the embryonic epidermis, NOTCH signaling becomes elevated in and essential for the emerging spinous layer daughters, while basal daughters retain their progenitor status (Williams et al., 2011). When the epidermis is mature, a shift to largely parallel divisions occurs, although basal cells still appear to partition NOTCH signaling differentially to their daughters (Clayton et al., 2007) (Figure 3). KRT14/KRT5 basal cells that dually express differentiation markers, e.g., KRT10, INVOLUCRIN, and NOTCH, appear to be fated to move upward and terminally differentiate (Asare et al., 2017; Clayton et al., 2007; Mascre et al., 2012; Watt and Green, 1982).
In adult primate skin, it takes ~4 weeks for a committed epidermal cell to exit the basal layer and be sloughed from the skin surface. For mouse back skin, the homeostatic flux of epidermal cells in upward vertical columns has been estimated to be considerably faster, typically a week (Potten et al., 1987; Sada et al., 2016). However, turnover rates have been hard to measure accurately at densely hairy body sites, where the epidermis only consists of a few layers. In addition, the mouse’s propensity for frequent scratching creates an unpredictable wound-induced tissue landscape for the surface epithelium, which could shorten estimates.
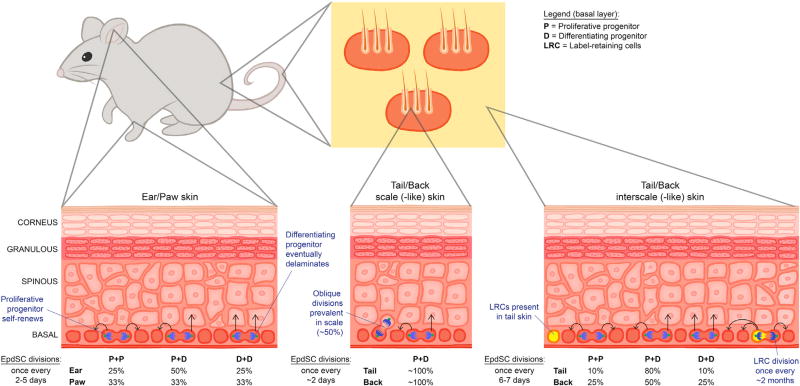
To avoid some of these caveats, most mouse studies on the dynamics of epidermal homeostasis have focused on analyzing the epidermis from tail, ear, or paw skin, where HFs are sparser and the epidermis is thicker. In these studies, lineage tracing has been a powerful means of measuring the potential of a basal keratinocyte progenitor by genetically tagging it and then following its marked progeny. The method involves using a promoter to drive an inducible Cre recombinase transgene in the progenitor of interest and then inducing Cre. When on the background of a genetically manipulated Rosa26 locus, a stop codon is recombined out, resulting in constitutive Rosa26-driven expression of YFP, Tomato, or a randomly chosen fluor from Confetti or Rainbow (Hsu, 2015).
Lineage tracing and mathematical modeling of randomly marked basal cells within the interscale region of adult mouse tail skin epidermis suggest that every 6 to 7 days, basal keratinocytes divide stochastically along the basement membrane to generate one basal daughter that stays attached to the basement membrane and one committed daughter that eventually delaminates (Clayton et al., 2007) (Figure 4). Similar asymmetric fates were observed by mathematical modeling of clones derived from basal cells marked by Involucrin-CreER (Mascre et al., 2012). Although some basal expansion was seen among 10% of the clones, the majority of these divisions gave rise to one proliferative progenitor and one fated daughter with a high propensity to be suprabasally located. This was consistent with the promoter used, as INVOLUCRIN is a cornified envelope protein expressed very early in the terminal differentiation program.
Figure 4. Unified Model for the Dynamics of Epidermal Homeostasis.
The basal layer is composed of progenitors that divide parallel to the basement membrane. Division rates (number of divisions in a given amount of time) vary significantly across different body locations and are not coupled with fate (whether daughter cells retain a proliferative [P] status or differentiate [D] and delaminate). Instead, fate determination appears to be mostly stochastic, although outcome probabilities also vary in different skin locales. In contrast to the hair follicle or to human epidermis, label-retaining cells (LRCs) with properties of long-lived stem cells have only been found in the interscale regions of mouse tail. That said, some rapidly proliferating progenitors persist for up to a year in other body sites, including back skin, and thus merit assignment as bona fide EpdSCs.
Additional support for a stochastic model of epidermal progenitors comes from serial optical sectioning of the ear and paw epidermis from live H2BGFP pulse-chased mice assayed at successive time points to monitor cells by position and cellular morphology (Rompolas et al., 2016). The data support a simple model where both basal daughters have an equal chance to proliferate or differentiate. That said, in this study, morphology and location were used to distinguish proliferative versus differentiating, and these criteria overlook basal daughters expressing dual differentiation markers (Asare et al., 2017), which may still have some proliferative potential but have been referred to as “committed progenitors” (Mascre et al., 2012).
In contrast to these observations which favor homogeneity and stochastic behavior of basal progenitors, studies on interscale tail skin epidermis have yielded signs of a hierarchy in the progenitor lineage. Thus, when basal cells of interscale tail skin epidermis were pulse chased with either a nucleotide analog or tetracycline-regulatable fluorescent histone (Tumbar et al., 2004), and then lineage traced with a Krt14-CreER driver, a longer-lived, label-retaining population of progenitors was discovered within the basal layer (Mascre et al., 2012). These rarer cells divided 10–20 times less frequently than Involucrin-CreER marked progenitors and displayed considerably greater expansion of marked basal cells. Mathematically, modeling was consistent with ~10% of these divisions generating two basal progenitors, and ~80% of the divisions giving rise to a basal and suprabasal daughter. In comparing the clonal expansion kinetics of Krt14-CreER and Involucrin-CreER populations, a hierarchy was suggested in which a small number of label-retaining epidermal progenitors can both self-renew and also generate intermediate progenitors with a greater propensity to delaminate and differentiate (Mascre et al., 2012).
For the mouse, this existence of infrequently dividing progenitors that retain label for >4 weeks seems to be an aberration of the interscale tail skin epidermis. When pulse-chase analyses were used to examine radiolabeled nucleotide or H2BGFP fluorescence dilution within the proliferative basal populations of paw, ear, or scale regions of tail skin epidermis, progenitors divided more frequently, with estimates of divisions every 2–5 days (Doupe et al., 2010; Lim et al., 2013; Sada et al., 2016). Interestingly, however, when Roy et al. (2016) crossed Krt14-CreER and Rainbow mice to conduct multicolor lineage tracing and track individual back skin epidermal clones, they discovered that the clones nearest to actively cycling HFs were considerably more proliferative than the ones more distant (Roy et al., 2016). Given the variation in HF densities at different body sites, this may explain some of the heterogeneity seen among basal cell populations from different body sites. Moreover, since a clone that begins near an HF can expand to produce more distal progeny, faster and slower growing cells may be dynamically shifting in a spatial and temporal fashion.
The notion that cycling rates are not a reliable measure of evaluating stemness within the epidermis has also been suggested by Sada et al. (2016), who noted that basal layer progeny of even very fast cycling (on average once per 2 days) progenitors can self-renew and persist in back skin for at least a year (Sada et al., 2016). Another key point is that basal location is not synonymous with progenitor status. Indeed, when fluorescence-activated cell sorting was recently used to purify dually positive KRT14/KRT10 cells from Krt14-eGFP and Krt10-RFP mice, this cohort comprised some cells that showed polarized integrins to substantiate their basal layer identity but also displayed a transcriptional profile reflective of a differentiation-poised state (Asare et al., 2017).
In reviewing the collective studies on epidermal progenitors, it seems clear that most of the differences in interpretation have arisen from whether progenitor fates were assigned according to molecular differentiation markers (Clayton et al., 2007; Mascre et al., 2012), basal residence status (Roy et al., 2016; Sada et al., 2016), or the ability of two daughters to divide at least once prior to exiting the basal layer (Rompolas et al., 2016). Additional confusion has arisen from whether researchers have assigned stemness according to cell-cycling rates (Lim et al., 2013) or according to clonal longevity (Sada et al., 2016). When considering that (1) epidermal progenitors can give rise to long-lived clones irrespective of cycling rates, (2) cycling rates and clonal expansion can vary markedly depending upon proximity to HFs, and possibly other appendages such as SwGs and scales, and (3) progenitors can acquire molecular signs of commitment prior to exiting the basal layer, the results from these various studies are remarkably in unison (Figure 4).
Overall, the data point to a model whereby mouse skin epidermis is replenished long term from a pool of relatively fast cycling undifferentiated progenitors, effectively epidermal stem cells (EpdSCs), which in normal homeostasis, show no signs of contributing to HF cells (Mascre et al., 2012; Sada et al., 2016). These EpdSCs divide to generate an EpdSC daughter and a poised progenitor that has an elevated propensity to exit the basal layer and embark upon a terminal differentiation pathway (Figure 4). While expansion of some basal clones and shrinkage of others occurs over time, the process seems to be largely stochastic for most body sites, with EpdSCs dividing symmetrically whenever a vacancy arises from delamination and differentiation of a basal progenitor. Single-cell analyses, fluorescence in situ hybridizations, and additional lineage tracings will be needed to provide further insights into the heterogeneity that exists within the basal layer. Examining the relevance of these ideas to human skin will also be pertinent. In a recent study, keratinocytes from the skin of a patient with the blistering disease junctional epidermolysis bullosa were genetically corrected in vitro and then used in vivo to reconstitute the entire epidermis. Intriguingly, long-term epidermal homeostasis post-graft was achieved by only a subset of the cultured progenitors, specifically those able to form large clones (holoclones), a feature of self-renewing stem cells (Hirsch et al., 2017). Together, these findings support the existence of EpdSC heterogeneity in human.
A major question still unaddressed is what controls the balance of epidermal growth and differentiation. The EpdSC “niche” (Figure 2) consists not only of the underlying basement membrane and neighboring basal keratinocytes but also the overlying, terminally differentiating spinous cells. In addition, the murine basal layer is also home to γδ T cells and mechanoreceptors (Merkel cells), while the suprabasal layers contain specialized dendritic (Langerhans) cells as well as sensory nerve endings that sense pain and temperature. Besides these close-range inputs, longer range signals and/or signal relays could emanate from the microbiota on the skin surface (Belkaid and Segre, 2014; Kong and Segre, 2017) and/or dermal components, such as upper reticular fibroblasts (Driskell et al., 2013), blood vessels, and adipose tissue. Any one or more of these inputs could affect how basal daughter cell fates are coupled to epidermal homeostasis in ways that in most cases have yet to be explored.
Epidermal Stem Cells in Wound Healing
Following injury, a wound-healing response must be triggered to rapidly repair the epidermis and restore the skin barrier. While repair is ongoing, the damaged skin must also guard against infection, and even so, delays in the re-epithelialization process can lead to higher incidence of infection and chronic wound formation (Heath and Carbone, 2013).
The complex biological process is generally subdivided into four independent yet interconnected phases: (1) underlying dermal contraction and coagulation of platelets to form an eschar (scab) shortly after wounding, processes that restore hemostasis and provide a temporary barrier; (2) activation of resident T cells and infiltration of macrophages, monocytes, and neutrophils in immune surveillance; (3) local migration and proliferation of epidermal keratinocytes to re-epithelialize the damaged barrier; and finally (4) resolution of the wound with repair of underlying damaged dermis and remodeling of its ECM to restore homeostatic function and architecture (reviewed in Gurtner et al., 2008).
Basal epidermal progenitors at the wound edge are primary respondents in the re-epithelialization process. Immunofluorescence analyses of back skin and intravital videomicroscopy of ear skin shows that the process begins at ~12 hr post wounding with migration rates being highest (~1.6 µm/day) at the wound edge by day 3 (Aragona et al., 2017; Keyes et al., 2016; Park et al., 2017). By contrast, in a fashion similar to that seen in closure of the embryonic epidermis over the eyelid (Heller et al., 2014), the proliferative response occurs in the region behind and only partially overlapping with the migrating epidermal front (Park et al., 2017). Similar migration and proliferation kinetics occur in wounded tail skin, where activated EpdSCs undergo rapid asymmetric fate outcome, visible by lineage-traced streams of cells that span from the proliferative zone to the wound center (Aragona et al., 2017).
Besides enhanced migration and proliferation rates, the healing process is facilitated by the direction of both the division plane and cellular shape toward the wound site. Many details remain unclear, but WNT signaling is thought to play an important role (Ito et al., 2007; Miyoshi et al., 2012; Petersen and Reddien, 2009; Stoick-Cooper et al., 2007). Canonical WNTs function by inhibiting GSK3β kinase, resulting in the accumulation of stabilized, unphosphorylated β-catenin, which then acts as a nuclear cofactor for the LEF1/TCF family of DNA-binding proteins (Nusse and Clevers, 2017). However, by inhibiting GSK3β, WNTs can also promote accumulation of the active (unphosphorylated) state of key microtubule-binding proteins which stabilize and polarize microtubules at the wound front to facilitate wound repair (Wu et al., 2011). Further solidifying the importance of the cytoskeleton in migration and wound repair is the participation of the small GTPase and actin regulator RAC (Park et al., 2017). Integrins α5β1 cluster at the leading edge of the keratinocyte, where they participate in polarizing cell shape and cytoskeletal movements needed for effective epithelial migration and fibronectin assembly (Aragona et al., 2017; Coles et al., 2006; Heller et al., 2014; Qiao et al., 2014). Finally, RNA-seq analyses of microdissected tissue in the migrating zone suggests that a number of metalloproteinases are expressed, where they likely function in the ECM remodeling that takes place in the wound repair process (Aragona et al., 2017; Ge et al., 2017; Hattori et al., 2009; Okada et al., 1997; Stevens and Page-McCaw, 2012).
Although all cells in the basal epidermis appear to respond to wounding, their contributions differ. Thus, for example, both scale and interscale tail skin progenitors participate in re-epithelialization following a punch wound, but only the EpdSCs in the compartment closest to the wound site appear to contribute long term (Mascre et al., 2012; Sada et al., 2016) (Figure 5). Examples of such wound-induced plasticity in epithelial progenitors now abound, being described not only for the skin epidermis but also the sweat gland (Lu et al., 2012) and intestinal epithelium (reviewed by Beumer and Clevers, 2016). By essentially making an emergency “911” call to all nearby progenitors in the vicinity of the wound, optimal restoration of epithelial barriers is ensured. In the future, it will be interesting to dissect the molecular nature of this tissue emergency signal. The ultimate resolution of this plasticity following wound repair suggests that epithelial remodeling may continue long after the wound has healed, another topic for future study.
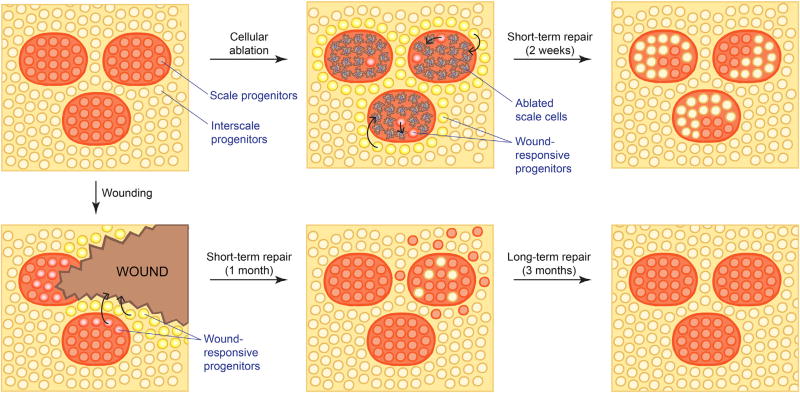
Figure 5. Wound Response from Scale and Interscale EpdSCs.
Cellular (laser) ablation and wounding induce a re-epithelialization response from all nearby EpdSCs, regardless of origin. However, only local cells contribute to long-term homeostasis of the injured site. Thus, interscale contribution to the injured scale region is only transient and vice versa. Diagrams represent a top-down view of EpdSCs of the tail/back interfollicular epidermis.
Hair Follicle Morphogenesis and the Adult Hair Cycle
In the mouse, HF morphogenesis occurs in waves, which begin at ~E14.5 and continue until birth. The first divisions seen in embryonic WNT-activated hair placode cells are exclusively perpendicular and give rise to asymmetrically fated daughters (Ouspenskaia et al., 2016) (Figure 3). Correlations between WNT signaling and asymmetric cell divisions have also been described in the early Caenorhabditis elegans embryo (Lam and Phillips, 2017; Sugioka et al., 2011), as well as in ectodermal fate determination in Xenopus (Huang and Niehrs, 2014).
Intriguingly, these early WNT-driven divisions appear to lead to differential levels of WNT signaling in their progeny and differential fates of the two daughter cells (Ouspenskaia et al., 2016). In this regard, these placode divisions seem to resemble what happens when a cultured human embryonic stem cell encounters a WNT bead placed on its surface, which preferentially localizes WNT signaling to the daughter cell maintaining contact with the bead, leaving a paucity of WNT signaling in the liberated daughter (Habib et al., 2013). It is tempting to speculate that in the hair placode, WNTs and/or WNT receptors may be naturally polarized by the basement membrane, since the basal daughter is the one that retains the WNThigh state of its parent.
In the hair placode, the basal WNThigh daughters produces Sonic hedgehog (SHH), which prompts the WNTlow daughters to proliferate and expand this newly fated cell population, which will become the ORS and the stem cells of the adult HF niche, called the bulge. At these early times, SHH also signals to the underlying mesenchyme, which responds by coalescing to form the dermal papilla (DP) (St-Jacques et al., 1998). The DP also responds to SHH by producing NOGGIN, a BMP inhibitor that fuels the proliferation of overlying WNThigh hair germ (HG) cells (Noramly and Morgan, 1998; Woo et al., 2012). As the HG expands and grows, it begins to generate the progenitors that form first the inner root sheath (IRS) and subsequently the hair shaft. As the follicle matures shortly after birth, the growing hair shaft begins to protrude through the skin surface, fueled by short-lived progeny within the hair bulb that have enveloped and maintained contact with the DP (Figure 3). Following several weeks of hair growth, ~2/3 of the follicle degenerates, a process poorly understood but thought to be linked to the exhaustion of proliferative capacity of the short-lived progeny that generate the hair and its channel.
Many of these steps are recapitulated in the adult hair follicle, which cycles through sequential phases of active HF regeneration and hair growth (anagen), destruction (catagen), and then rest (telogen) (Muller-Rover et al., 2001) (Figure 6). Deceptively simple as an epidermal appendage, the regenerated portion of the HF consists of eight morphologically distinct lineages, which are spatially organized and coordinately regulated in an orchestrated continuum of tissue production (Chuong and Widelitz, 2009). Each hair is composed of three of these lineages, forming concentric layers of terminally differentiated, dead hair cells. At the peak of anagen, hair growth from the core of the regenerated HF can reach an astounding 20 µm/hr, a process elegantly captured by live imaging (Rompolas et al., 2013).
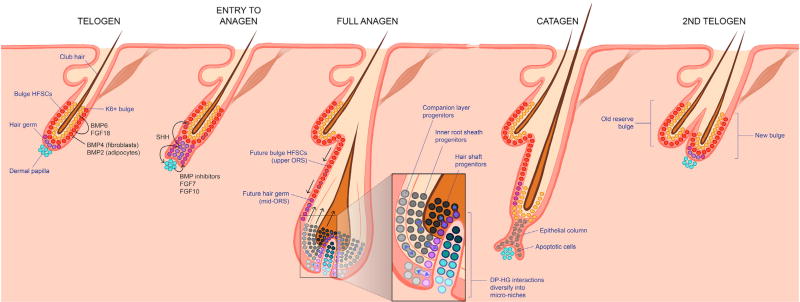
Figure 6. The Hair Cycle.
During the resting phase (telogen), bulge HFSCs are kept inactive by BMP and FGF18 signals from the neighboring K6+ bulge and from nearby fibroblasts and adipocytes. BMP inhibitors and pro-activating FGFs from the dermal papilla (DP) overcome the inhibitory cues, leading to entry into anagen. At the base of the bulge, some hair germ (HG) cells become WNThigh multipotent progenitors, which express SHH. SHH triggers bulge HFSCs to divide symmetrically and grow the outer root sheath (ORS), which then pushes the signaling center away, returning the bulge to quiescence, and then progressively returning the ORS cells to quiescence in a cascade as it grows downward. Progenitors that maintain contact with the DP continue to produce SHH, which fuels the DP to elevate signaling and expand the multipotent progenitor pool. As the hair bulb grows, it envelops the DP. Interactions at the elaborated interface between the DP and hair bulb establish micro-niches, each of which harbors unipotent progenitors that produce the seven concentric differentiating layers of the hair shaft and its channel, or inner root sheath (IRS). By mechanisms still unclear, these progenitors exhaust their proliferative capacity and an apoptotic phase (catagen) ensues, during which the follicle is degenerated and restored back to its resting size as it enters the next telogen. Note that some cells within the upper/mid ORS are spared, and these become the new bulge and new HG for the next hair cycle.
Hair cycles are fueled by HF stem cells (HFSCs) that reside in the “bulge” niche located at the base of the telogen phase HF (Cotsarelis et al., 1990) (Figure 6). Cells along the basement membrane in the outer bulge layer display traits often associated with long-term stem cell populations, including (1) their ability to give rise to all lineages of the regenerating lower HF when traced either by dilution of H2BGFP fluorescence, or by K15-CrePGR or Lgr5-CreER, both of which mark the lower bulge and HG (Morris et al., 2004; Nowak et al., 2008; Tumbar et al., 2004); (2) their clonogenic nature when cultured in vitro (Blanpain et al., 2004; Claudinot et al., 2005); and (3) their versatility upon transplantation; they are able to regenerate epidermis, SGs, and cycling HFs that are replete with their own HFSC niche (Blanpain et al., 2004; Oshima et al., 2001). By single-cell RNA-sequencing, bulge HFSCs were found to be homogeneous and high in their levels of integrins α3β1 and α6β4, KRT5/14, LGR5, SOX9, TCF3/4, NFATc1, and LHX2 (Joost et al., 2016; Yang et al., 2017).
In mice, the first few hair cycles are synchronized, a process that is thought to emanate from signals in the skin dermis (Greco et al., 2009; Hsu et al., 2011; Plikus and Chuong, 2014; Shook et al., 2016). This synchrony makes HFs the perfect model system to probe how stem cells transition through bouts of quiescence and tissue regeneration in a physiologically unperturbed state. In addition to macro-environmental signals, there are local ones within the niche. During telogen, HFSCs are maintained in quiescence by BMP6 and FGF18, which are expressed by the “inner bulge” layer of terminally differentiated cells associated with the hair remnant (Hsu et al., 2011) (Figure 6).
Throughout this resting phase, however, HFSCs at the bulge base (HG) undergo cross-talk with the DP, involving WNT and inhibitory BMP signals, as well as receiving signals from other cell types surrounding the niche (Chi et al., 2013; Festa et al., 2011; Greco et al., 2009; Sennett et al., 2015; Woo et al., 2012). Once the level of these activating cues overpowers the inhibitory signals, stem cells within the HG are activated to divide and launch a new hair cycle (Greco et al., 2009).
Recent single-cell RNA-sequencing and lineage tracing suggest that the spatially polarized cross-talk at the base of the quiescent stem cell niche compartmentalizes the HG into micro-niches that together possess the blueprint for their downstream specification into discrete lineages (Yang et al., 2017) (Figure 6). The organization of the bulge and HG into microniches ensures that once activated, each HFSC will receive slightly different levels of morphogens to help choreograph the specification of their fates in the complex journey of HF regeneration. The bulge HFSCs and the contiguous HFSCs in the upper HG will fuel production of the ORS, reflective of their lower WNT signaling. By contrast, the HG cells closest to the DP with the highest WNT signaling will divide asymmetrically to the basement membrane and give rise to multipotent progenitors, which initially show dual signs of both the hair shaft (3 lineages) and its channel, the IRS (three lineages) (Yang et al., 2017).
In early anagen (Anagen I), these multipotent progenitors are a transient component of the stem cell niche (Hsu et al., 2014). Similar at this stage to the embryonic HG, the multipotent progenitors of the activated HG are not only WNThigh but also express SHH, which fuels the proliferation of the bulge stem cells and emerging ORS, now in Anagen II–III (Hsu et al., 2014). As in embryogenesis, proliferation in these SHH responding cells is symmetric relative to the basement membrane. Soon thereafter, the downward growing ORS distances the DP signaling center away from the bulge and upper ORS, which then return to quiescence, preserving their stem cell potential for the next hair cycle (Hsu et al., 2011). By contrast, the HG maintains contact with the DP stimulus to sustain its proliferation (Woo et al., 2012; Hsu et al., 2014).
As the HG expands, it envelops the DP, thereby diversifying the WNT-mediated epithelial-mesenchymal interactions to elaborate upon the distinct micro-niches that line the elongated basement membrane interface. In addition, from the start of the regenerative phase through full anagen, the WNT-stimulated cell divisions that are linked to IRS and hair shaft fates are perpendicular relative to the basement membrane (Yang et al., 2017). Lineage tracing reveals that these micro-niche progenitor divisions fuel the coordinated growth of the individual onionskin- like layers that constitute the hair and its channel (Legue et al., 2010; Yang et al., 2017). Studies to date suggest that the asymmetric cell division prompted by WNT signaling in hair progenitors differs in mechanism from that seen in the embryonic epidermis (Byrd et al., 2016).
Much still remains to be clarified as to the complex signals, cellular interactions, and mechanisms that progressively restrict and compartmentalize the options afforded to the multipotent HG progenitors during the regenerative phase of the HF. However, it is notable that during this time, the epithelium undergoes continual remodeling. Moreover, in addition to the changes in DP, there are many other dermal changes that downward growing hair bulbs experience, including a shift from the surrounding reticular dermis to the adipose layer. This macro-environmental change has been implicated not only in synchronizing the HFs across the hair coat but also in fueling anagen (Festa et al., 2011; Plikus et al., 2009; Shook et al., 2016).
Another feature poorly understood is the destructive phase that follows the period of active hair growth by the mature HF (Figure 6). During this time, most of the cycling portion of the hair follicle is lost, with the exception of the upper ORS, which houses cells with HFSC potential and which form a new bulge and HG for the next cycle (Hsu et al., 2011; Mesa et al., 2015). The destructive phase of the hair cycle has served as a convenient means of distinguishing long- and short-term progenitors in the HF.
HFSCs during Wound Repair
In striking contrast to homeostasis, injury provokes a remarkable plasticity in HFSCs. Thus, whereas the bulge-derived upper ORS HFSCs form the HG at the end of each hair cycle (Hsu et al., 2011), if bulge HFSCs are ablated either by depilation or by laser, HG cells move up and become bulge residents (Ito et al., 2004; Rompolas et al., 2013) (Figure 7A). Similarly, when LGR5+ lower bulge/HG SCs are targeted for ablation, they can be replaced by CD34+ upper bulge cells (Hoeck et al., 2017). These findings exemplify how the HF harnesses cellular malleability to preserve stem cell function and further illuminate the power of the niche in dictating cellular behavior, whether its occupants are native residents or immigrants.
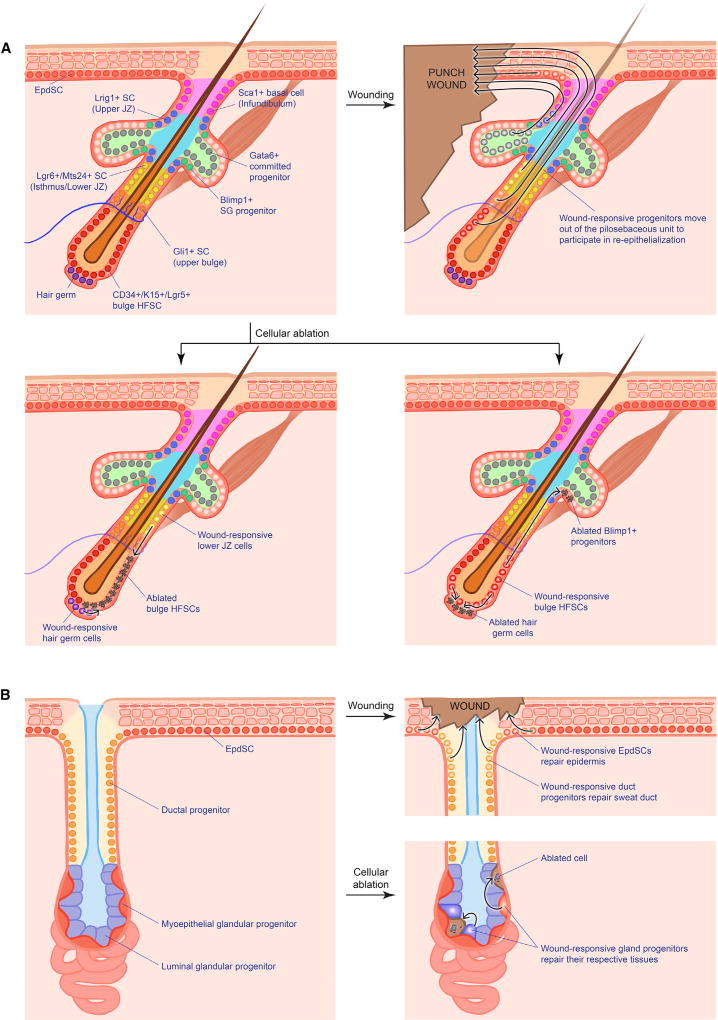
Figure 7. Wound Response from Hair Follicle and Sweat Gland Progenitors.
(A) Different niches within the pilosebaceous unit house various progenitors that all display plasticity and contribute to wound-induced re-epithelialization, albeit at varying degrees. Ablation of these populations likewise trigger neighbor progenitors to refill the vacated niche.
(B) In contrast, the sweat gland displays stricter compartmentalization in terms of injury response. Wounding of the sweat gland orifice only triggers duct (but not gland) progenitors to reconstruct the sweat duct. Similarly, ablation of specific gland progenitors only activates the same type of progenitor for repair.
SC, stem cell; JZ, junctional zone; SG, sebaceous gland.
The importance of the niche is further illustrated from studies in which HFSCs were removed from their native niche, cultured, and then engrafted, resulting in the generation of de novo IFE, SGs, and HFs (Blanpain et al., 2004). In addition, when the epidermis is wounded in vivo, HFSCs can exit the bulge and migrate upward to participate in the re-epithelialization process (Claudinot et al., 2005; Ito et al., 2005; Taylor et al., 2000; Tumbar et al., 2004).
Although these findings build a compelling case in favor of bulge HFSC participation, it has been argued that bulge contributions are largely short-term, and hence not reflective of a genuine stem cell fate switch (Ito et al., 2005; Page et al., 2013). It has even been put forward that bulge HFSCs are dispensable for the early phase (first 3 days) of the re-epithelialization response (Garcin et al., 2016). Moreover, even the HFs that form de novo during a WNT-induced re-epithelialization of more substantive wounds appear to emanate from EpdSCs and not Krt15-labeled bulge HFSCs (Ito et al., 2007).
What accounts for the paradoxical long-term plasticity displayed by bulge HFSCs after culturing but not in the disruption and/or elimination of the bulge niche caused by wounding in vivo? While the jury is still out on this question, several explanations are possible. A priori, the answer could be rooted in the striking proliferative differences between proliferating bulge HFSCs in vitro versus HFSCs in their native niche, where they spend much of their time in quiescence. In this regard, it could be relevant that anagen skin closes full-thickness wounds faster than telogen or catagen skin (Ansell et al., 2011). Adding to these effects, WNT signaling is also known to be higher during anagen, where it not only promotes proliferation of HG HFSCs but also enhances the re-epithelialization process of tissue regeneration (Ito et al., 2007; Kawakami et al., 2006; Stoick-Cooper et al., 2007; Whyte et al., 2013; Wu et al., 2011).
Another possibility is that the epigenetic and/or translational state of native bulge HFSCs leaves them less equipped to withstand the injury stress. Recent studies have profiled the histone modifications that occur as bulge HFSCs are taken from their niche and placed in culture. A dramatic shift in the chromatin landscape occurs as transcription factors normally expressed in quiescent bulge HFSCs are dampened, and those known to be induced during injury stress are activated (Adam et al., 2015). In addition, the colony-forming efficiency of quiescent bulge HFSCs is poor, suggesting that they experience stress prior to coping with it (Blanpain et al., 2004). Finally, it is notable that stress-experiencing epidermal stem cells in vivo or in vitro activate an alternative translation route that their normal stem cell counterparts do not (Sendoel et al., 2017). While this ultimately enables them to produce higher levels of stress-coping factors, the stressed cells repress global protein synthesis, a feature that could make them less adept at wound repair (Sendoel et al., 2017). In the future, it will be interesting to interrogate the extent to which these features eventually confer long-term cultured HFSCs an advantage over in vivo bulge HFSCs in rapid tissue regeneration in response to injury.
A final factor worth considering is that most of the lineage-tracing studies thus far have been performed on mice subjected to full-thickness wounds, in which an entire skin segment was excised. If there is a hierarchy, and stem cells closest to the wound are the ones to respond first, the bulge HFSCs would be the last to participate in a full-thickness wound. If this latter explanation has merit, bulge HFSCs might be expected to be among the first to participate in a shallower (partial-thickness) wound that removes the epidermis but leaves HFs intact.
Regardless of these currently open questions about bulge HFSCs in wound repair, it is indubitable that the participation of the HF as a whole in wound healing is physiologically significant. In either split thickness grafts of skin lacking Sox9 (and therefore also HFSCs), or alternatively, in tail wounds of mutant skin lacking HFs altogether, the re-epithelialization process is delayed relative to wild-type grafts (Langton et al., 2008; Nowak et al., 2008). In addition, long-term contribution of HF cells to epidermal wound repair has been observed in adult mice whose HFs were uniformly and exclusively marked by Rosa-lacZ+ activated in embryogenesis with a Shh-Cre reporter (Levy et al., 2005). Whatever the identity of the contributing lacZ-expressing HF cells, they are predicted to also express SOX9, since conditional ablation of Sox9 abrogates the ability of HF cells to contribute to epidermal repair (Nowak et al., 2008).
How Many Other Stem Cell Compartments in the Skin Are There, and What Do They Do during Normal Homeostasis and Wound Repair?
Lineage-tracing experiments with Krt14-Cre have long underscored the multipotency of early embryonic skin progenitors, which give rise to the adult skin epidermis and its complete array of appendages (Snippert et al., 2010; Vasioukhin et al., 1999). That said, progenitors appear to become increasingly more restricted during the course of embryonic development. Thus, when skin was lineage traced with Shh- or Sox9-driven reporters, which are activated in HF cells as they first emerge, exclusive labeling of progeny was seen in the HF and SG but not IFE (Levy et al., 2005; Nowak et al., 2008). When lineage tracing was performed with Blimp1-Cre, which is activated even later in embryonic development, it first marked the base of emerging SGs, and then labeled the differentiating progeny of the SG, with no labeling seen in the IFE or HF (Horsley et al., 2006) (Figure 7A).
Postnatally, distinct stem cell compartments also exist within the SG and non-cycling portion of the HF (Figure 7A). Lgr6-Cre is expressed largely in the adult skin cells within the HF junctional zone, located between the SG and the bulge. Lineage tracing has revealed that during homeostasis, these junctional zone SCs sustain not only the junctional zone but also the differentiating progeny of the SG (Snippert et al., 2010). MTS24 marks a similar group of cells within the junctional zone (Nijhof et al., 2006), while Lrig1-CreER marks SCs within the upper junctional zone and extending just above the SGs. During normal homeostasis, LRIG1-marked cells govern the replenishment of this upper junctional zone and the SG (Page et al., 2013). At present, markers have not been developed that distinguish the progenitors within the infundibulum, residing between the upper junctional zone and the IFE, although most markers including SCA-1, show a strong resemblance to IFE EpdSCs. Thus, for the IFE and the pilosebaceous unit, there appear to be multiple SC populations that each display specialized roles in governing their own tissue turf during homeostasis.
None of these other SC compartments are as infrequently cycling as the SCs of the bulge and HG, reflective of the fact that the upper HF does not undergo the cyclical regenerative behavior of the HF below the bulge. However, all of these SC compartments share the requisite keratinocyte SC features of residing along the basement membrane and expressing KRT5/14 and basal progenitor integrins.
Like the IFE and bulge/HG SCs, these other SCs of the HF show signs of considerable plasticity in an injury situation. When Blimp1 progenitors are targeted for diphtheria-toxin-mediated ablation, they are replenished by bulge HFSCs (Horsley et al., 2006) (Figure 7A). Intriguingly, the reverse also appears to be the case: lineage tracing coupled with live imaging and temporal monitoring has shown that when bulge HFSCs are laser ablated, Krt14-CreER-marked cells within the lower junctional zone can migrate into and restock the bulge (Rompolas et al., 2013). Moreover, these cells are not simply alien idle residents but rather appear to assume the active duties of bulge HFSCs; they participate in hair cycling, just like the previous bona fide bulge residents (Rompolas et al., 2013).
Several additional experiments have yielded further insights into the behavior of different SC compartments in response to injury. One intriguing finding is that the IFE is unaffected when HFSCs are targeted to express a suicide gene (Ito et al., 2005). Another is that in response to a full-thickness wound, bulge progenitors marked by K15-CreER do not appear to contribute effectively to long-term repair of the IFE (Ito et al., 2007; Page et al., 2013). At first glance, these results might seem to indicate intrinsic differences among stem cell populations. An equally plausible explanation, however, is that when SC compartments are too far from the site of injury, they do not contribute to wound repair unless there are no other SC compartments nearby to do so.
In this regard, it is notable that HF progenitors uniformly labeled in embryogenesis do contribute long term to wound repair (Levy et al., 2005; Nowak et al., 2008), underscoring a role for some SCs of the HF in the re-epithelialization of a full-thickness wound. That said, the wound-provoked contribution of upper junctional zone SCs to epidermal re-epithelialization is largely short term in full-thickness wounds (Page et al., 2013). Intriguingly, however, when LRIG1 is absent, mice develop spontaneous epidermal hyperplasia, and in hyperplastic states, the upper junctional SCs do contribute to the IFE long term (Jensen et al., 2009). Signs of versatility have also been reported for lower junctional zone and/or upper bulge SCs (Brownell et al., 2011; Snippert et al., 2010).
In perhaps the most remarkable illustration of plasticity, a recent study suggests long-term contribution by fate-committed suprabasal (Gata6+) cells within the SG duct (Donati et al., 2017) (Figure 7A). This result is surprising, given that suprabasal IFE cells have not been observed to dedifferentiate in response to wounding (Aragona et al., 2017). However, only a few studies to date have tackled the question of when along a program of differentiation does a cell reach the point of no return where it cannot backtrack and become a stem cell (Hsu et al., 2011). The issue is a fascinating one, and resolving it will require temporal live imaging and/or other means of marking and monitoring individual stem cells and their clonal progeny over time.
Apart from the pilosebaceous unit, the skin also contains SwGs, which contain their own stem cell populations. Within the murine paw skin, the epidermis and sweat duct each contain KRT14/5+ SCs, which in normal homeostasis, replenish only their local tissue segments (Lu et al., 2012) (Figure 7B). Within the secretory coil of the gland itself, both KRT14/5+ myoepithelial and KRT8/18+ luminal cells also have unipotent progenitors, but during homeostasis, little turnover occurs in these glandular cells, marking them as among the highest label-retaining cells of the skin.
When subjected to wounding, different SwG stem cell populations respond. In shallow wounds, stem cells in the paw skin and upper SwG duct are mobilized to repair the local tissue damage, with no activity elicited from the myoepithelial or luminal progenitors of the coiled gland. However, when either of the two glandular progenitors were selectively ablated, neighboring glandular cells of the corresponding progenitor type became mobilized to repair the damage. Moreover, when myoepithelial SCs were purified from SwGs and then engrafted onto different body sites, multipotency was unleashed (Lu et al., 2012).
Engraftment of myoepithelial SwG SCs onto back skin resulted in reconstitution of the epidermis, while transplantation into a cleared mammary fat pad resulted in regeneration of an entire gland replete with a duct-like structure (Lu et al., 2012). Initially, these de novo SwGs produced sweat proteins, but over time, they began to turn on milk protein synthesis, reflective of the power of the host microenvironment in dictating what epithelial stem cells will do (Lu et al., 2012). It will be interesting in the future to assess whether myoepithelial stem cells also have the potential to reconstitute HFs under permissive conditions.
Finally, the skin is also host to non-epithelial cells such as dermal cells, immune cells, and other stem cells such as those of the melanocyte and adipocyte lineages. Albeit outside the scope of this review, they have all been found to impact skin homeostasis (Ali et al., 2017; Chang et al., 2013; Driskell et al., 2013; Festa et al., 2011; Plikus et al., 2008; Rabbani et al., 2011). How they contribute to wound healing, whether through direct contribution to re-epithelialization or through interactions with epithelial stem cells, is an exciting topic for future study.
Conclusions
Our review of epithelial stem cell dynamics in the skin culminates toward a single moral: during homeostasis, epithelial stem cell populations govern only their specific tissue territory, but they possess a plasticity that is unleashed during wound repair. If this versatility is intrinsic to stem cell identity, what prevents stem cell potency from going rogue? As discussed in this review, the answers all point to the niche microenvironment in which the stem cell finds itself. Thus, when skin epithelial stem cells are ablated, their neighbors happily adopt each other’s responsibility and move into vacated niches.
How neighbors sense these vacancies is still not clear. However, the signals appear to be local, as the closest neighbors nearly if not always seem to win in these resettlements. Moreover, the new squatters appear to gain all rights of their bona fide prior residents, underscoring the domineering role of the niche in instructing stem cell behavior. Indeed, if niche cells are ablated or otherwise irrevocably damaged, the stem cells cannot exert their tissue regenerating potential. In the many engraftment studies described in this review, epithelial stem cell plasticity was only displayed through the addition or existing presence of the appropriate mesenchymal niche cells. These two facets—the more rapid response of neighboring SCs to injury and the need for the requisite niche components—explain why de novo HFs are such a rare occurrence in regenerated skin from full-thickness wounds, and why SwG myoepithelial SCs are refractory to shallow wounds and will only make glands when placed in a glandular permissive environment.
Indeed, the research over the past decade has illuminated the resilient ability of the body to rapidly restore the skin’s barrier following injury and to return to normal tissue homeostasis following re-epithelialization. In these processes, the interactions between epithelial stem cells and their microenvironment are critical. In fact, simply viewed, the field is converging on the view that the progenitors of the epidermis and its appendages may be intrinsically equivalent, sharing certain core structural features, including expression of KRT5 and KRT14, and integrins α6β4 and α3β1, and residing on a basement membrane that demarcates the dermo-epidermal border. According to this model, their differences in behavior emanate from their cross-talk with their local niche environment.
Although there are still some findings that are inconsistent with this notion, the evidence is increasingly beginning to point in this direction. In this view, the versatility of a stem cell in a wound response may then simply be a reflection of changes in this environment. When stem cells are removed from their native context, either in a wound response or in culture, they then must adjust their chromatin dynamics and change their program of gene expression in order to survive in their new environment (Adam et al., 2015; Ge et al., 2017). Given that not all epigenetic adjustments are rapid, this could account for why aging keratinocytes in vitro are initially readily distinguishable from their younger counterparts, but with time and passage in culture, these differences wane (Keyes et al., 2013).
The famed British philosopher John Locke (1632–1704) suggested that the individual is strongly influenced by social and cultural factors rather than by innate mechanisms. Similarly, the research shows that tissue stem cells are malleable, responding to their niche microenvironment rather than exhibiting a predetermined, innate behavior. If this model is correct, as seems increasingly likely, it prompts us to reconsider the steps necessary to make strides in regenerative medicine and wound repair. For under this view, it will not be sufficient merely to identify and culture stem cells. Rather, in addition, it will be essential either to recreate the stem cell niches or to recreate the environment that is needed to generate the desired stem cell behavior.
This concept provides a clear explanation for why a spray harboring millions of tiny skin fragments—each replete with stem cells in their native niches—has replaced passaged cultured epidermal keratinocytes as the favored method of burn therapy (Zielins et al., 2014). However, the importance of the niche microenvironment need not necessarily require that all niche components are present, as long as the necessary factors they produce are provided. In organotypic cultures, ranging from human epidermal equivalents to mammary and intestinal epithelia, this is in fact what is being done.
The challenge now is to exploit this newfound knowledge to harness the natural potential of stem cells for the desired task at hand. For homeostasis, the future challenge is squarely placed in the arena of unearthing a better understanding of SC niches. Will this knowledge be sufficient to regenerate skin appendages from scratch, or are there features of development that impose irreversible restrictions on fate options? The challenges are as great in the field of wound repair, where further characterization of wound-stimulated alterations to the microenvironment is needed, as is a deeper understanding of the relative contributions of different stem cell populations to the re-epithelialization process. The solutions to these unresolved biological problems will occupy researchers for the coming decade, but will provide the molecular soil that will nurture new and improved clinical applications for wound healing and regenerative medicine.
Acknowledgments
We are grateful to the many friends and colleagues in the skin biology field whose work inspired us to write this review. Given the reference constraints, we regret that we could not cite all the worthy papers on this topic. However, we have tried to present a balanced view of the field, attempting to clarify areas of seeming discrepancies and present a collective view of where the field stands and the exciting areas of research that await us all. K.A.U.G. is a recipient of the Human Frontier Science Program Postdoctoral Fellowship. E.F. is an Investigator of the Howard Hughes Medical Institute. This work was supported by grants from the NIH (R37-AR27883, R01-AR050452, and R01-AR31737).
References
- Adam RC, Yang H, Rockowitz S, Larsen SB, Nikolova M, Oristian DS, Polak L, Kadaja M, Asare A, Zheng D, et al. Pioneer factors govern super-enhancer dynamics in stem cell plasticity and lineage choice. Nature. 2015;521:366–370. doi: 10.1038/nature14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahtiainen L, Lefebvre S, Lindfors PH, Renvoise E, Shirokova V, Vartiainen MK, Thesleff I, Mikkola ML. Directional cell migration, but not proliferation, drives hair placode morphogenesis. Dev. Cell. 2014;28:588–602. doi: 10.1016/j.devcel.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell. 2017;169:1119–1129. doi: 10.1016/j.cell.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the “hair growth-wound healing connection”: anagen phase promotes wound re-epithelialization. J. Invest. Dermatol. 2011;131:518–528. doi: 10.1038/jid.2010.291. [DOI] [PubMed] [Google Scholar]
- Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascre G, Simons BD, Blanpain C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017;8:14684. doi: 10.1038/ncomms14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asare A, Levorse J, Fuchs E. Coupling organelle inheritance with mitosis to balance growth and differentiation. Science. 2017;355 doi: 10.1126/science.aah4701. https://doi.org/10.1126/science.aah4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346:954–959. doi: 10.1126/science.1260144. [DOI] [PubMed] [Google Scholar]
- Beumer J, Clevers H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development. 2016;143:3639–3649. doi: 10.1242/dev.133132. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd KM, Lough KJ, Patel JH, Descovich CP, Curtis TA, Williams SE. LGN plays distinct roles in oral epithelial stratification, filiform papilla morphogenesis and hair follicle development. Development. 2016;143:2803–2817. doi: 10.1242/dev.136010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Pasolli HA, Giannopoulou EG, Guasch G, Gronostajski RM, Elemento O, Fuchs E. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. 2013;495:98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin G, Jablonski NG, Sussman RW, Kelley EA. The role of piloerection in primate thermoregulation. Folia Primatol (Basel) 2014;85:1–17. doi: 10.1159/000355007. [DOI] [PubMed] [Google Scholar]
- Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–1683. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Noveen A. Phenotypic determination of epithelial appendages: genes, developmental pathways, and evolution. J. Investig. Dermatol. Symp. Proc. 1999;4:307–311. doi: 10.1038/sj.jidsp.5640235. [DOI] [PubMed] [Google Scholar]
- Chuong CM, Widelitz RB. The river of stem cells. Cell Stem Cell. 2009;4:100–102. doi: 10.1016/j.stem.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl. Acad. Sci. USA. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Coles EG, Gammill LS, Miner JH, Bronner-Fraser M. Abnormalities in neural crest cell migration in laminin alpha5 mutant mice. Dev. Biol. 2006;289:218–228. doi: 10.1016/j.ydbio.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Donati G, Rognoni E, Hiratsuka T, Liakath-Ali K, Hoste E, Kar G, Kayikci M, Russell R, Kretzschmar K, Mulder KW, et al. Wounding induces dedifferentiation of epidermal Gata6+ cells and acquisition of stem cell properties. Nat. Cell Biol. 2017;19:603–613. doi: 10.1038/ncb3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe DP, Klein AM, Simons BD, Jones PH. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell. 2010;18:317–323. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fuchs EV, Coppock SM, Green H, Cleveland DW. Two distinct classes of keratin genes and their evolutionary significance. Cell. 1981;27:75–84. doi: 10.1016/0092-8674(81)90362-7. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin CL, Ansell DM, Headon DJ, Paus R, Hardman MJ. Hair follicle bulge stem cells appear dispensable for the acute phase of wound re-epithelialization. Stem Cells. 2016;34:1377–1385. doi: 10.1002/stem.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Gomez NC, Adam RC, Nikolova M, Yang H, Verma A, Lu CP, Polak L, Yuan S, Elemento O, et al. Stem cell lineage infidelity drives wound repair and cancer. Cell. 2017;169:636–650. doi: 10.1016/j.cell.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb. Perspect. Biol. 2010;2:a000125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E, Nusse R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D’Armiento J, Okada Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am. J. Pathol. 2009;175:533–546. doi: 10.2353/ajpath.2009.081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013;14:978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- Heller E, Kumar KV, Grill SW, Fuchs E. Forces generated by cell intercalation tow epidermal sheets in mammalian tissue morphogenesis. Dev. Cell. 2014;28:617–632. doi: 10.1016/j.devcel.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, Scaglione D, Reichelt J, Klausseger A, Kneisz D, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017 doi: 10.1038/nature24487. Published online November 8, 2017. https://doi.org/10.1038/nature24487. [DOI] [PMC free article] [PubMed]
- Hoeck JD, Biehs B, Kurtova AV, Kljavin NM, de Sousa EMF, Alicke B, Koeppen H, Modrusan Z, Piskol R, de Sauvage FJ. Stem cell plasticity enables hair regeneration following Lgr5+ cell loss. Nat. Cell Biol. 2017;19:666–676. doi: 10.1038/ncb3535. [DOI] [PubMed] [Google Scholar]
- Horsley V, O’Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC. Theory and practice of lineage tracing. Stem Cells. 2015;33:3197–3204. doi: 10.1002/stem.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014;157:935–949. doi: 10.1016/j.cell.2014.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Niehrs C. Polarized Wnt signaling regulates ectodermal cell fate in Xenopus. Dev. Cell. 2014;29:250–257. doi: 10.1016/j.devcel.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost S, Zeisel A, Jacob T, Sun X, La Manno G, Lonnerberg P, Linnarsson S, Kasper M. Single-cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst. 2016;3:221–237.e9. doi: 10.1016/j.cels.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Rodriguez Esteban C, Raya M, Kawakami H, Marti M, Dubova I, Izpisua Belmonte JC. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 2006;20:3232–3237. doi: 10.1101/gad.1475106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes BE, Liu S, Asare A, Naik S, Levorse J, Polak L, Lu CP, Nikolova M, Pasolli HA, Fuchs E. Impaired epidermal to dendritic T cell signaling slows wound repair in aged skin. Cell. 2016;167:1323–1338.e14. doi: 10.1016/j.cell.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes BE, Segal JP, Heller E, Lien WH, Chang CY, Guo X, Oristian DS, Zheng D, Fuchs E. Nfatc1 orchestrates aging in hair follicle stem cells. Proc. Natl. Acad. Sci. USA. 2013;110:E4950–E4959. doi: 10.1073/pnas.1320301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kong HH, Segre JA. The molecular revolution in cutaneous biology: investigating the skin microbiome. J. Invest. Dermatol. 2017;137:e119–e122. doi: 10.1016/j.jid.2016.07.045. [DOI] [PubMed] [Google Scholar]
- Lam AK, Phillips BT. Wnt signaling polarizes C. elegans asymmetric cell divisions during development. Results Probl. Cell Differ. 2017;61:83–114. doi: 10.1007/978-3-319-53150-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J. Invest. Dermatol. 2008;128:1311–1318. doi: 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legue E, Sequeira I, Nicolas JF. Hair follicle renewal: authentic morphogenesis that depends on a complex progression of stem cell lineages. Development. 2010;137:569–577. doi: 10.1242/dev.044123. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342:1226–1230. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CP, Polak L, Keyes BE, Fuchs E. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science. 2016;354 doi: 10.1126/science.aah6102. https://doi.org/10.1126/science.aah6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N, Blanpain C, Fuchs E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- Mesa KR, Rompolas P, Zito G, Myung P, Sun TY, Brown S, Gonzalez DG, Blagoev KB, Haberman AM, Greco V. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature. 2015;522:94–97. doi: 10.1038/nature14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Nijhof JG, Braun KM, Giangreco A, van Pelt C, Kawamoto H, Boyd RL, Willemze R, Mullenders LH, Watt FM, de Gruijl FR, et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development. 2006;133:3027–3037. doi: 10.1242/dev.02443. [DOI] [PubMed] [Google Scholar]
- Noramly S, Morgan BA. BMPs mediate lateral inhibition at successive stages in feather tract development. Development. 1998;125:3775–3787. doi: 10.1242/dev.125.19.3775. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Okada A, Tomasetto C, Lutz Y, Bellocq JP, Rio MC, Basset P. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J. Cell Biol. 1997;137:67–77. doi: 10.1083/jcb.137.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Ouspenskaia T, Matos I, Mertz AF, Fiore VF, Fuchs E. WNT-SHH antagonism specifies and expands stem cells prior to niche formation. Cell. 2016;164:156–169. doi: 10.1016/j.cell.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13:471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Gonzalez DG, Guirao B, Boucher JD, Cockburn K, Marsh ED, Mesa KR, Brown S, Rompolas P, Haberman AM, et al. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat. Cell Biol. 2017;19:155–163. doi: 10.1038/ncb3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. A wound-induced Wnt expression program controls planarian regeneration polarity. Proc. Natl. Acad. Sci. USA. 2009;106:17061–17066. doi: 10.1073/pnas.0906823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Widelitz RB, Maxson R, Chuong CM. Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity. Int. J. Dev. Biol. 2009;53:857–868. doi: 10.1387/ijdb.072564mp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Chuong CM. Macroenvironmental regulation of hair cycling and collective regenerative behavior. Cold Spring Harb. Perspect. Med. 2014;4:a015198. doi: 10.1101/cshperspect.a015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Saffhill R, Maibach HI. Measurement of the transit time for cells through the epidermis and stratum corneum of the mouse and Guinea-pig. Cell Tissue Kinet. 1987;20:461–472. doi: 10.1111/j.1365-2184.1987.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Qiao L, Gao H, Zhang T, Jing L, Xiao C, Xiao Y, Luo N, Zhu H, Meng W, Xu H, et al. Snail modulates the assembly of fibronectin via alpha5 integrin for myocardial migration in zebrafish embryos. Sci. Rep. 2014;4:4470. doi: 10.1038/srep04470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, Taketo MM, Ito M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Kawaguchi K, Park S, Gonzalez D, Brown S, Boucher J, Klein AM, Greco V. Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science. 2016;352:1471–1474. doi: 10.1126/science.aaf7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy E, Neufeld Z, Cerone L, Wong HY, Hodgson S, Livet J, Khosrotehrani K. Bimodal behaviour of interfollicular epidermal progenitors regulated by hair follicle position and cycling. EMBO J. 2016;5:2658–2670. doi: 10.15252/embj.201693806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada A, Jacob F, Leung E, Wang S, White BS, Shalloway D, Tumbar T. Defining the cellular lineage hierarchy in the interfollicular epidermis of adult skin. Nat. Cell Biol. 2016;18:619–631. doi: 10.1038/ncb3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendoel A, Dunn JG, Rodriguez EH, Naik S, Gomez NC, Hurwitz B, Levorse J, Dill BD, Schramek D, Molina H, et al. Translation from unconventional 5’ start sites drives tumour initiation. Nature. 2017;541:494–499. doi: 10.1038/nature21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennett R, Wang Z, Rezza A, Grisanti L, Roitershtein N, Sicchio C, Mok KW, Heitman NJ, Clavel C, Ma’ayan A, et al. An integrated transcriptome atlas of embryonic hair follicle progenitors, their niche, and the developing skin. Dev. Cell. 2015;34:577–591. doi: 10.1016/j.devcel.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook B, Rivera Gonzalez G, Ebmeier S, Grisotti G, Zwick R, Horsley V. The role of adipocytes in tissue regeneration and stem cell niches. Annu. Rev. Cell Dev. Biol. 2016;32:609–631. doi: 10.1146/annurev-cellbio-111315-125426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Curr. Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Stevens LJ, Page-McCaw A. A secreted MMP is required for re-epithelialization during wound healing. Mol. Biol. Cell. 2012;23:1068–1079. doi: 10.1091/mbc.E11-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Sugioka K, Mizumoto K, Sawa H. Wnt regulates spindle asymmetry to generate asymmetric nuclear beta-catenin in C. elegans. Cell. 2011;146:942–954. doi: 10.1016/j.cell.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. USA. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982;295:434–436. doi: 10.1038/295434a0. [DOI] [PubMed] [Google Scholar]
- Watt FM, Jones PH. Expression and function of the keratinocyte integrins. Dev. Suppl. 1993:185–192. [PubMed] [Google Scholar]
- Whyte JL, Smith AA, Liu B, Manzano WR, Evans ND, Dhamdhere GR, Fang MY, Chang HY, Oro AE, Helms JA. Augmenting endogenous Wnt signaling improves skin wound healing. PLoS One. 2013;8:e76883. doi: 10.1371/journal.pone.0076883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Ratliff LA, Postiglione MP, Knoblich JA, Fuchs E. Par3-mInsc and Galphai3 cooperate to promote oriented epidermal cell divisions through LGN. Nat. Cell Biol. 2014;16:758–769. doi: 10.1038/ncb3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo WM, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012;26:1235–1246. doi: 10.1101/gad.187401.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Shen QT, Oristian DS, Lu CP, Zheng Q, Wang HW, Fuchs E. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell. 2011;144:341–352. doi: 10.1016/j.cell.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Adam RC, Ge Y, Hua ZL, Fuchs E. Epithelial-mesenchymal micro-niches govern stem cell lineage choices. Cell. 2017;169:483–496.e413. doi: 10.1016/j.cell.2017.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielins ER, Atashroo DA, Maan ZN, Duscher D, Walmsley GG, Hu M, Senarath-Yapa K, McArdle A, Tevlin R, Wearda T, et al. Wound healing: an update. Regen. Med. 2014;9:817–830. doi: 10.2217/rme.14.54. [DOI] [PubMed] [Google Scholar]