Abstract
Surfaces covered by epithelial cells, termed mucosal surfaces, serve special functions as selectively permeable barriers that partition the host and the outside world. Given its close association to microbial antigens, the intestinal mucosa has evolved creative mechanisms to maintain homeostasis, to prevent excessive inflammatory responses, and to promote rapid and full inflammatory resolution. In recent years, an active role for the epithelium has been attributed to the local generation of specialized pro-resolving mediators (SPMs) in the maintenance of immunological homeostasis. In this brief review, we highlight evidence that the epithelium actively contributes to coordination and resolution of inflammation, principally through the generation of SPMs. These autacoids are derived from omega-6 and omega-3 polyunsaturated fatty acids. Acting through widely expressed G-protein coupled receptors, SPMs are implicated in the resolution of acute inflammation that manifests specific, epithelial-directed actions focused on mucosal-homeostasis, including regulation of leukocyte trafficking, the generation of antimicrobial peptides, the dampening of endotoxin signaling, and the attenuation of mucosal cytokine responses.
1. Introduction
Acute inflammation is the body’s immediate and well-coordinated response to injury. The active process involves proinflammatory lipid mediators (prostaglandins and leukotrienes) that increase vascular permeability and orchestrate neutrophil infiltration to eliminate the source of inflammation. While vital, if unchecked and without resolution, acute inflammation can be prolonged and result in chronic inflammation that has been implicated in the pathogenesis of a wide range of diseases including cardiovascular disease, metabolic disorders, and cancer. Thus, resolution of inflammation involving the removal of cellular debris and restoration of tissue integrity is equally crucial (Kumar et al., 2014; Medzhitov, 2008; Serhan et al., 2007). It is now appreciated that inflammatory resolution is also a biosynthetically active process regulated by lipid mediators known as specialized pro-resolving mediators (SPMs), instead of the passive decrease in proinflammatory signals as previously believed (Serhan et al., 2000a, 2006). Appropriately termed a lipid mediator class switch, resolution begins with a transition from the proinflammatory prostaglandins and leukotrienes to the pro-resolving SPMs consisting of the resolvins, lipoxins, protectins, and maresins (Levy et al., 2001). SPMs exert many specialized actions including blocking neutrophil recruitment and activating efferocytosis that all ultimately brings the body back to homeostasis (Schwab et al., 2007).
The human gut embodies the concept of homeostasis, balancing mucosal immune responses whilst maintaining an equilibrium between host and microbiota (Garrett et al., 2010). Mucosal surfaces exist in the unique environment of encountering numerous foreign antigens via the luminal surface while concurrently being intimately associated with the immune system via the subepithelial lymphoid tissue. While coexisting with more than 1000 different bacterial phylotypes, the intestinal mucosa optimizes eliminating harmful pathogens while at the same time avoids unnecessary immune activity in a setting of regular contact with commensal microbes (Hakansson and Molin, 2011; Lozupone et al., 2012). Crucial to this homeostasis are the intestinal epithelial cells. Forming a physical barrier at the mucosal surface, the intestinal epithelial cells prevents the frivolous mixing of luminal antigens with the mucosal immune system housed within the lamina propia (McCole and Barrett, 2007). Inflammation disrupts this barrier, and a key component of inflammatory resolution is the restoration of barrier integrity and function.
In this review, we highlight the role of SPMs in modulating timely and controlled inflammatory resolution not only by restoring barrier but also by regulating the immune system and microbial environment to ultimately preserve the tenuous but vital balance of the human gut. Unrestricted inflammation contributes to numerous disease states, and a complete understanding of these endogenous mediators of inflammatory resolution holds immense potential to direct new therapeutic opportunities.
2. SPMs in intestinal mucosal inflammation
2.1. Overview of the intestinal mucosa
The gastrointestinal (GI) mucosal surface provides an optimal setting to define the features of inflammatory resolution. With a surface area of approximately 300m2, the GI tract is the largest mucosal surface in the adult human, while also representing the largest mass of lymphoid tissue with over 106 lymphocytes per gram of tissue. In the process of digestion and waste removal, the GI tract faces a constant flux of new non-pathogenic antigenic material atop an environment filled with diverse microorganisms and necessarily hones the mucosal immune system to dampen immunological reactions to these innocuous ingested antigens. Commensal microbes also present a barrage of antigenic material. As a result of this constant antigenic exposure, the GI tract is considered to exist normally in a state of low-grade inflammation (Salminen et al., 1998).
The intestinal epithelium is central to coordinating both inflammation and resolution. As a monolayer, the polarized intestinal epithelial cells through intercellular tight junctions form a dynamic barrier coated with a thick mucus layer that regulates what can reach the lamina propia from the lumen (Ivanov et al., 2010; Koch, 2012). Although the mucosa-associated lymphoid tissue (MALT) develops tolerance to harmless signals from the external environment, the ability to rapidly and effectively to respond to harm that penetrates this barrier is also primed.
Like other mucosal organs lined by an epithelium, the intestines witness the transepithelial migration of polymorphonuclear leukocytes (PMNs) to the apical surface for regular immune surveillance and elimination of invading pathogens. The transmigration occurs physiologically in the basolateral-to-apical direction, which facilitates the retention of neutrophils in the crypt for mucosal defense. Due to the nature of the paracellular migration of these cells, the epithelial barrier is altered by loosening the apical junction complex consisting of tight junctions and adherens junctions between adjacent epithelial cells. Excessive transepithelial migration of PMNs disrupts the barrier by causing epithelial damage, which perpetuates inflammation, as seen in many active inflammatory intestinal diseases (Sumagin and Parkos, 2015). However, a unique feature of the GI tract from other mucosal organs such as the lungs is how crucial activated PMNs and their transepithelial migration is to resolution (reviewed in Colgan et al., 2013). PMNs generate important anti-inflammatory and pro-resolving molecules including SPMs via local interactions with epithelial and endothelial cells. Depletion of circulating PMNs exacerbated symptoms in murine inflammatory bowel disease (IBD) models, whereas the opposite was seen in acute lung injury models, where PMN depletion seemed to attenuate damage (Kuhl et al., 2007; Zemans et al., 2009). With this context, the gut is the ideal setting for the ultimate exploration of balance.
2.2. SPM production in the gut
SPMs fall into several distinct families of locally and temporally regulated lipid mediators known as the lipoxins, resolvins, protectins, and maresins. Although all actively involved in resolving inflammation, SPMs arise from different precursors. Lipoxins are known as the classic mediators and are formed from the omega-6 fatty acid arachidonic acid (AA), which also gives rise to well-established proinflammatory lipid mediators such as leukotrienes (LTs) and prostaglandins (PGs). LTB4 is a potent chemoattractant for neutrophils, and PGE2, PGI2 are involved in early phases of inflammation by regulating vascular responses in terms of blood flow and permeability. Resolvins, protectins, and maresins are known as the novel local mediators and are made from omega-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). E-series resolvins are derived from EPA, and D-series resolvins are derived from DHA. Resolvin E1 (RvE1) was the first resolvin to be identified in murine resolving exudate as a potent inhibitor of PMN migration (Serhan et al., 2000b).
Because both proinflammatory and proresolution signals can arise from the same precursor such as AA, temporal control is crucial for the inflammatory response. SPMs thus are produced through transcellular biosynthesis during specific time frames and in transient coordination between different cells. SPM production often requires the actions of fatty acid lipoxygenases (LOXs) and cyclooxygenase-2 (COX-2), and the joint effort of these enzymes occurs through transcellular interactions between migrating cells from the vasculature and the cells of the inflamed tissue site (Bannenberg and Serhan, 2010). Key lipoxygenases include the 5-LOXs found in leukocytes, the 12-LOXs found in platelets, and the 15-LOXs found in monocytes, eosinophils, and epithelial cells (Claria and Serhan, 1995; Serhan et al., 1984; Serhan and Sheppard, 1990). COX-2 expression plays a crucial role in proinflammatory responses and has been shown to be rapidly induced in apical epithelial cells of inflamed foci in IBD by TNF-α and IL-1β, while normally undetectable in the normal ileum or colon (Gronert et al., 1998a; Singer et al., 1998). 15-lipoxygenase isoform 2 (15-LOX-2) is found in the colonic mucosa (Mangino et al., 2006). LOXs also prove to be a key enzyme in SPM production, as exogenous plant LOX treatment induces lipoxin generation, overexpression of LOX reduces inflammation and tissue damage, and the parasitic Toxoplasma gondii hijacks lipoxin production to quench the human host’s inflammatory response (Serhan et al., 2003). Proteomic analysis of T. gondii extract revealed the presence of peptides resembling LOXs, and these extracts exhibited 15-LOX activity by functionally producing lipoxins (Bannenberg et al., 2004a,b). T. gondii infection begins as the parasites initially cross the intestinal epithelium to then become widely disseminated after evading immune detection in part by generating pro-resolving mediators (Egan et al., 2012). Further investigation could potentially reveal if lipoxin production is a balancing point between host and gut inhabitants.
The nature of SPM production was best revealed through aspirin (acetyl-salicylic acid) treatment. Aspirin is known for its anti-inflammatory effects via its actions on the cyclooxygenases (COX-1 and COX-2) that produce proinflammatory mediators (PGs and LTs). While irreversibly inhibiting COX-1, aspirin acetylates serine residue 516 in the active site of COX-2, modifying the enzymatic activity of COX-2, which results in the aspirin induced production of SPMs, furthering the anti-inflammatory effects of aspirin by now also promoting resolution. Acetylation of COX-2 inhibits its oxygenase activity but leaves its peroxidase activity intact (Serhan and Petasis, 2011). COX-2 normally generates PGs from AA released from cell membranes, but in the presence of aspirin, acetylated COX-2 forms 15R-hydroxyeicosatetraenoate (15R-HETE), which then is catalyzed by 5-LOX into pro-resolving 15-epi-lipoxin, belonging to the class of aspirin-triggered lipoxins (ATLs), which are more resistant to local degradation and often more potent compared to endogenous lipoxins (Maddox et al., 1998; Schottelius et al., 2002; Serhan et al., 1995). Endogenous lipoxins are degraded within minutes by myeloid cells as part of the temporally fine-tuned system of inflammation and resolution (Louis et al., 2005). Aspirin-triggered production of resolvins from EPA and DHA also occurs (Serhan et al., 2000a, 2002). This biosynthetic route functions due to COX-2 expressing cells (epithelial cells) producing precursors that are then acted upon by 5-LOX from cells such as neutrophils to produce SPMs.
Although exogenous aspirin plays a major role in SPM production, endogenous transcellular biosynthesis occurs as well. Cytochrome P450 and S-nitrosylation of COX-2 have also been shown to contribute to 15-epi-lipoxin formation (Birnbaum et al., 2007; Titos et al., 1999). LOXs alone can initiate the synthesis of resolvins, as well as protectins and maresins from omega-3 PUFAs. This is the major pathway for SPM production in the absence of aspirin treatment (Campbell et al., 2011; Serhan et al., 2008).
SPM production in the gut is crucial for maintaining homeostasis. In colonic mucosa from patients with ulcerative colitis characterized by persistent colonic inflammation, lipoxin production was reduced 12-fold with a concomitant decreased protein expression of 15-LOX-2, suggesting defective lipoxin biosynthesis contributes to disease pathogenesis (Mangino et al., 2006). Transcellular biosynthesis of SPMs provides the temporal control necessary to allow for the gut to respond to potential threats but not persist in excessive inflammation. The specific signal can only result from the physical proximity of inflammatory cells with cells of the tissue undergoing inflammation and acts to restrict further inflammatory insult locally. When PMNs transmigrate to the intestinal mucosa, SPMs can be generated to resolve the inflammatory response and preserve the balance of the immune system and the microbial environment in the gut.
2.3. Receptors for SPMs
SPMs exert their action as molecular signals by being endogenous receptor agonists for G-protein coupled membrane-spanning receptors at low concentrations in the pM to low nM range (Bannenberg and Serhan, 2010). Endogenous lipoxin A4 (LXA4) and aspirin-triggered 15-epi-LXA4 binds to the lipoxin A4 receptor/formyl peptide receptor 2 (ALX/FPR2) with high affinity. ALX/FPR2 is a G-protein coupled receptor that when activated by these lipid mediators reduces neutrophil chemotaxis (Chiang et al., 2006). ALX/FPR2 was first isolated from human PMNs but since then has also been found in monocytes, T cells, macrophages, and synovial fibroblasts (Serhan et al., 2011). In the intestines, ALX/FPR2 receptors are expressed on the basolateral surface of epithelial cells and are induced by interleukin-13 (IL-13) and interferon gamma (IFN-γ) (Gronert et al., 1998b; Kucharzik et al., 2003). The receptor shows positive feedback regulation, as low doses of LXA4 in vivo enhances its expression (Bannenberg et al., 2004a,b). The ALX/FPR2 receptor behaves as a promiscuous receptor and can be activated by other endogenous anti-inflammatory factors such as glucocorticoid-derived annexin A1 (AnxA1) and several non-lipid ligands such as synthetic peptides and serum amyloid A (Bena et al., 2012; Chiang et al., 2000).
A high affinity receptors also exists for resolvin E1 (RvE1), known as the chemokine-like receptor 1 (ChemR23) (Arita et al., 2005). RvE1 functions as a selective agonist of ChemR23, which shares 36.4% amino acid sequence homology with ALX/FPR2. ChemR23 is expressed in the cardiovascular system, brain, kidney, liver, lung, testis, prostate, and gastrointestinal tissues (Serhan et al., 2011; Serhan and Petasis, 2011). ChemR23 is abundantly expressed in intestinal epithelial cells and primarily localizes to the apical surface (Campbell et al., 2010). ChemR23 is also highly expressed in antigen-presenting cells (APCs) such as macrophages, dendritic cells, as well as monocytes, with lower amounts in neutrophils and T lymphocytes. RvE1 can also bind to leukotriene B4 receptor 1 (BLT1) on PMNs (Chiang et al., 1999).
Resolvin D1 (RvD1) is a selective agonist for orphan G protein-coupled receptor GPR32, a member of the chemoattractant receptor family (Chiang et al., 2015). RvD1 also binds ALX/FPR2 with high affinity, but does not bind to ChemR23 (Bento et al., 2011). Resolvin D2 (RvD2) is selective for N-arachidonyl glycine receptor GPR18, which is expressed on PMNs, monocytes, and macrophages (Chiang et al., 2015).
The actions of SPMs interacting with their specific receptors are all dose regulated and dependent on cell type and tissue environment. The different mechanisms of SPM mediated resolution specifically in the gut revolve around a decrease in infiltrating inflammatory cells and the restoration of barrier that separates the microbes from the immune system.
2.4. Actions of SPMs on leukocyte trafficking
A key step of acute inflammation is the migration of PMNs into the tissue and the activation of these cells to neutralize the offending agents. As a class of mediators, SPMs share common functional themes by limiting neutrophil infiltration, promoting macrophage phagocytosis of cellular debris and microorganisms, and stimulating production of anti-inflammatory cytokines.
Each class of SPMs regulates leukocyte trafficking in the resolution process. The intestinal mucosa is the site of constant interactions between microbes and the human host. Immune responses are primed to be quickly activated, beginning with PMNs infiltration. However, prolonged PMN presence is implicated in chronic inflammatory conditions.
LXA4, lipoxin analogs, and ATLs block human PMN transmigration across intestinal epithelial cells in a polarized manner (Colgan et al., 1993; Fierro et al., 2003; Schottelius et al., 2002). TNF-α is a potent proinflammatory cytokine implicated in many chronic inflammatory conditions. LXA4 downregulates TNF-α mRNA expression level in intestinal epithelial cells and reduces the degradation of IκB, and thus prevents the activation of NF-κB (Kure et al., 2010). Lipoxins inhibit TNF-α-stimulated neutrophil adherence to epithelial monolayers and potently attenuate the release of chemotactic IL-8 from activated intestinal epithelial cells (Goh et al., 2001). The ALX/FPR2 receptor is expressed basolaterally in intestinal epithelial cells allowing for the LXA4 generated at the initiation of neutrophil paracellular transmigration to immediately act on epithelial cells to dampen inflammation (Kucharzik et al., 2003). LXA4 inhibits PMN β2 integrin (CD11b/18) expression, an integral adhesion protein necessary for cell-cell interactions that initiate neutrophil transmigration (Fiore and Serhan, 1995).
The omega-3 PUFA-derived SPMs also modulate leukocyte trafficking. RvE1 signaling also inhibits TNF-α stimulated NF-κB activation in intestinal epithelial cells that stimulates PMN adherence and mobilization through release of chemokines and chemoattractants (Tessier et al., 1997). Because RvE1 acts as a partial agonist of the BLT1 receptor, RvE1 competitively inhibits LTB4-induced proinflammatory signals (Chiang et al., 1999). RvE1 promotes the mucosal epithelium to upregulate the expression of decay accelerating factor (CD55), an anti-adhesive molecule, which accelerates the clearance of apically adherent activated neutrophils from the epithelium into the intestinal lumen (Lawrence et al., 2003). Interestingly, unlike ALX/FPR2 and most other G protein-coupled receptors, which exhibit basolateral localization in polarized cells, ChemR23 is expressed apically on intestinal epithelial cells. Thus, the transit of PMNs through the mucosa to the lumen seems to naturally promote a pro-resolving signature that temporally produces RvE1 apically that initiates the termination and clearance of PMNs after transmigration (Campbell et al., 2007).
RvD1 and its aspirin-triggered form (AT-RvD1) dramatically change PMN shape and cease PMN migration by decreasing actin polymerization (Kasuga et al., 2008; Sun et al., 2007). RvD1 also significantly blocks LTB4-induced expression of b2 integrin CD11b/18 adhesion molecules on PMNs (Krishnamoorthy et al., 2010). Although RvD2 itself does not alter PMN adhesion molecule expression, RvD2 potently reduces leukocyte adherence and emigration in cells activated by platelet activating factor (PAF). Protection D1 (PD1), another DHA-derived SPM, also attenuates human neutrophil transmigration after initiation of inflammation and the effect is additive with RvE1 (Serhan et al., 2006).
In addition to the cessation of neutrophil infiltration, resolution of intestinal inflammation depends on the apoptosis of the accumulated neutrophils and the clearance of the cellular debris through efferocytosis. Although SPMs have the most significant effects in halting the migration of PMNs, SPMs also limit the inflammatory actions of the PMNs and promote the clearance of these activated PMNs from the inflamed tissue. RvD1 in vivo gave a significant reduction in the levels of a number of proinflammatory mediators including prostaglandins and LTB4 (Norling et al., 2012). Lipoxins as well as RvE1 and RvD1 enhance macrophage phagocytosis of apoptotic PMNs (Godson et al., 2000). RvD1 interacting with GPR32 on macrophages steers macrophages toward a pro-resolving phenotype to reduce secretion of proinflammatory cytokines IL-1β and IL-8. RvD2 potently reduces C5a-stimulated extracellular superoxide generation, which has been implicated in tissue damage seen in unresolved inflammation (Serhan and Petasis, 2011). A recent study found that 15-epi-LXA4 and RvD1 even promote autophagy in both murine and human macrophages by favoring the fusion of autophagosomes with lysosomes, the crucial and final step of autophagic vesicle processing (Prieto et al., 2015). SPMs not only regulate the resolution of acute inflammation by clearing PMNs but also monitor the lifespan of the macrophages cleaning up in the tissue to ultimately reestablish homeostasis.
2.5. Actions of SPMs on epithelial restoration
The intestinal epithelial cell regulates what can enter the body by creating a selective barrier that prevents the passage of potentially harmful agents while allowing for the passage of nutrients. These cells are polarized, with the apical surface primed to interact with the lumen while barring enteric bacteria via intercellular junctions, directional membrane transport systems, and mucus secretion, and with the basolateral surface optimized to interface with the mucosa and immune cell repertoire below (Colgan et al., 2015). Due to the extravasation of leukocytes in an inflammatory response, the structure and function of the epithelial barrier is compromised in that the intercellular apical junction complex must open to allow for the passage of the PMNs. Excessive inflammation often results in increased PMN transmigration and tissue damage, both of which compromise the barrier (Chin and Parkos, 2007). SPMs promote the restoration of the epithelial monolayer and barrier function after subduing the inflammatory response.
Crucial to intestinal epithelial wound healing is the precise balance of the initial migration of cells surrounding the wound (epithelial restitution), followed by the proliferation of cells to repair the defect, all culminating in the maturation and differentiation of the newly generated epithelial cells to regain barrier function (Iizuka and Konno, 2011). Keratinocytes, or skin epithelial cells, show increased migration that accelerates wound healing after exposure to AT-RvD1 delivered in nanoparticles (Norling et al., 2011). Similarly, treatment with LXA4 and PD1 increase re-epithelialization of denuded cornea after 24 h in mice (Gronert et al., 2005). A recent study found that intestinal mucosal wound closure is delayed in mice lacking the RvE1 receptor ChemR23. RvE1 treatment of intestinal epithelial cells promotes wound repair by increasing cell proliferation through activation of the mTOR signaling pathway. Local administration of RvE1-encapsulated synthetic nanoparticle into in vivo healing intestinal wounds promotes mucosal wound repair (Quiros et al., 2016). Similarly, AnxA1 is released as a component of extracellular vesicles derived from intestinal epithelial cells. Delivery of encapsulated AnxA1 analogs within targeted nanoparticles accelerates healing of murine colonic wounds, and cells treated with AnxA1 show increased migration to restore the wound (Leoni et al., 2015). AnxA1, which also binds to the formyl peptide receptor (FPR) in intestinal epithelium, through activation of reactive oxygen species (ROS) by the intestinal NADPH oxidase (NOX1) results in epithelial migration and facilitates wound restoration (Leoni et al., 2013). While promoting migration of epithelial cells to restore the barrier, SPMs also support the survival of existing epithelium. Exposure of colonic tissue to lipoxins attenuates TNF-α mediated epithelial cell apoptosis and protects the mucosa against TNF-α induced mucosal damage (Goh et al., 2001).
While supporting epithelial barrier restoration amidst limiting PMN infiltration, SPMs do not alter barrier function or agonist-stimulated chloride secretion and preserve the function of the epithelium (Gronert et al., 1998b).
2.6. Actions of SPMs on antimicrobial peptide generation
On top of balancing the immune system, the intestinal mucosa keeps the luminal microbes in check with the host, promoting mutualism with the nonpathogenic microbes while armed to eliminate pathogenic ones. Although the immune system is well equipped to face the challenge, the epithelial barrier acts as the first line of defense against invading pathogens and infection and actually contributes significantly to the orchestration of inflammatory processes. Epithelial cells express bactericidal permeability-increasing protein (BPI), an antibacterial and endotoxin-neutralizing molecule. The BPI protein was originally characterized on the neutrophil surface as well as in neutrophil azurophilic granules. BPI expression is transcriptionally upregulated by lipoxins and ATLs. BPI blocks endotoxin-mediated signaling in epithelium and kills Salmonella typhimurium (Levy et al., 2003). RvE1 also induces expression of BPI (Campbell et al., 2010). BPI thus acts as a “molecular shield” for protection of mucosal surfaces against Gram-negative bacteria and their lipopolysaccharide (LPS) endotoxin.
BPI exerts antimicrobial actions against Gram-negative bacteria through damaging the bacterial membranes, neutralizing endotoxin, and opsonizing bacteria for phagocytosis by neutrophils (Elsbach and Weiss, 1998). BPI has a high affinity to the conserved lipid A region of LPS, targeting its actions to a wide array of Gram-negative bacteria (Gazzano-Santoro et al., 1992).
In addition to BPI, microarray analysis of RvE1 treated cells reveals that RvE1 also potently upregulates the expression of intestinal alkaline phosphatase (ALPI). ALPI is a well-known epithelial differentiation marker and is a GPI-anchored protein expressed apically in epithelial cells (Goldberg et al., 2008). Because of its luminal location, ALPI has been shown to provide a key role in microbial homeostasis, as it halts Gram-negative growth such as Escherichia coli and potently neutralizes LPS through dephosphorylation of a moiety in lipid A (Bates et al., 2007). RvE1 promotes significant improvement of disease activity indices in the dextran sulfate colitis (DSS) murine model of colitis (body weight, colon length) concomitant with ALPI expression induction in the intestinal epithelium. Inhibition of ALPI activity worsens colitic disease and negates the protective influences of RvE1 (Campbell et al., 2010).
By facilitating the gatekeeping aspect of the intestinal mucosa that is the generation of antimicrobial peptides, SPMs guarantee that the immune system will only be activated when absolutely necessary. The intestinal epithelial cells play an active and integral role in maintaining homeostasis via SPMs, which is summarized in Fig. 1.
Fig. 1.
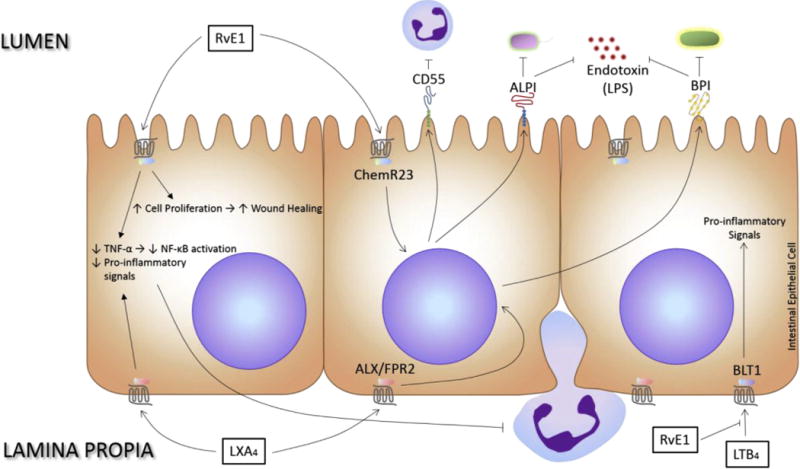
Active role of the intestinal epithelium in promoting immunological and host-microbial homeostasis via special pro-resolving mediators. SPMs, including LXA4 and RvE1, modulate the transmigration of leukocytes, the generation of antimicrobial peptides, the dampening of endotoxin signaling, the attenuation of mucosal cytokine responses, and the restoration of barrier integrity and function.
3. SPMs in colitis
Inflammatory bowel disease is divided into two main subtypes, ulcerative colitis (UC) and Crohn’s disease (CD). Both UC and CD are characterized by chronic, non-resolving inflammation and differs in the location of activity within the intestines (Abraham and Cho, 2009). Although the exact pathogenesis is unknown, it is accepted that IBD arises from an imbalance between the immune system and gut flora that is disrupted due to greater intestinal permeability and breakdown of epithelial tight junctions that leads to an inappropriate immune response of increased infiltration of immune cells and elevated levels of proinflammatory signals (Cho, 2008). As SPMs target all aspects of maintaining the homeostasis in the gut, colitis provides the ideal disease system in which to apply such coordination.
In many murine colitis studies, SPMs have shown significant protection. Treatment with lipoxin analog significantly reduces weight loss, hematochezia, and mortality in the DSS model of inflammatory colitis, most likely due to the down-regulation of NF-κB mediated proinflammatory gene expression (Gewirtz et al., 2002). Lipoxins also limit the proinflammatory signals by inducing macrophages towards the resolution phenotype instead of the proinflammatory phenotype, characterized by secretion of proinflammatory cytokines (IL-6, IL-8, IL-13, IL-18, and TNF-α) (Allison and Poulter, 1991; Godson et al., 2000; Mahida, 2000). A recent study demonstrated that the action of SPMs on eosinophils also attenuates inflammatory responses in experimental colitis. Eosinophils generally reside in the mucosa and increase significantly with disease. In this study, eosinophil-deficient mice develop significantly more severe colitis. Lipid profiling reveals virtually absent colonic levels of PD1 in colitic eosinophil-deficient mice. Exogenous PD1 administration in these mice reduces the severity of colitis by attenuating neutrophil infiltration and reducing levels of proinflammatory cytokines (TNF-α, IL-1β, IL-6) (Masterson et al., 2015). The actions of SPMs extend beyond limiting proinflammatory signals however, as LXA4 and AnxA1 have been shown to enhance IL-10 production, a key anti-inflammatory cytokine. IL-10 regulates the lack of inflammatory response in germfree mice, and these mice show enhanced expression of both LXA4 and AnxA1. When LXA4 and AnxA1 are blocked, germ free mice show a partial reversal of lacking immune responses. This suggests that SPMs coordinate both proinflammatory and anti-inflammatory responses, and that the immune system is tightly honed by the presence of microbiota, which ultimately depends on the endogenous production of SPMs (LXA4 and AnxA1 in this case) to stimulate IL-10 (Souza et al., 2007). In UC patients in remission, LXA4 and AnxA1 expression are increased (Vong et al., 2012).
Thus, SPMs appear to be a promising therapeutic option for IBD remission. However, the clinical data has not been as clear as the experimental data. Experimental data shows promise as diets supplemented with omega-6 fatty acids increase LXA4 production and attenuation of inflammatory injury (Gobbetti et al., 2015). Systemic treatment with D-series resolvins improves disease activity score with reduced colonic damage and decreased PMN infiltration in murine colitis models (Bento et al., 2011). Early studies in which rats with trinitrobenzene sulphonic acid (TNBS) induced colitis were fed diets supplemented with cod liver oil show reduced colonic damage and inflammation compared to control sunflower oil (Vilaseca et al., 1990). However, clinical supplementation studies with omega-3 PUFAs in patients with IBD have been inconclusive with not enough data to claim benefit (Yates et al., 2014). Additionally, there is conflicting evidence in regards to using NSAIDs in patients with IBD. While generally not prescribed, there is debate as to if nonsteroidal anti-inflammatory drugs (NSAIDs) truly exacerbate chronic IBD. Studies have found that NSAIDs, in particular COX-2 specific inhibitors, may promote relapses of IBD, but other studies are less clear (Kefalakes et al., 2009). A recent study in mice found that NSAID therapy increases colonic mucosal LXA4 synthesis and decreases disease indices. Elevated LXA4 levels negatively correlate with disease progression, suggesting NSAID-induced LXA4 synthesis to be favorable for healing in UC (Aʇiş et al., 2015). Because aspirin, a widely prescribed NSAID, triggers the production of more potent and stable SPMs that have shown improvement in disease activity, the use of NSAIDs and the relationship between such use, aspirin-triggered SPMs, and mucosal inflammation and resolution should be explored more in lieu of the conflicting results.
4. SPMs in mucosal infections
When harmful pathogens manage to invade the epithelial barrier and infect the underlying lamina propia, the appropriate immune response must be mounted. SPMs help fight off infections through the generation of antimicrobial peptides, but also additionally limits the inflammatory response as to not incure tissue damage and to avoid chronic inflammation. Stable LXA4 analogs downregulate active inflammation at mucosal surfaces after S. typhimurium infection by inhibiting the epithelial secretion of chemoattractants either apically (pathogen-elicited epithelial chemoattractant) or basolaterally (IL-8). This inhibits PMN infiltration and mutes the immune response, but bacterial adherence to and subsequent internalization by the epithelium to then be eliminated through interactions with the innate immune system is not altered (Gewirtz et al., 1998). SPMs also modulate the immune cells involved. When infected with Gram-negative bacteria, LPS from the bacteria interacts with toll-like receptor 4 (TLR4) on macrophages, which then produces proinflammatory signals. Lipoxins and RvD1 inhibit LPS-induced TNF-α production from macrophages. However, interestingly, not all SPMs inhibit the LPS-TLR4 interaction, as macrophages exposed to E. coli and pretreated with AT-RvD1 show increased TNF-α resulting in enhanced internalization and killing of the bacteria (Palmer et al., 2011). In E. coli and Staphylococcus aureus infections, RvD2 limits PMN infiltration while enhancing phagocytosis of bacteria, and accelerates resolution by stimulating efferocytosis (Chiang et al., 2015). All the families of SPMs block IL-12 production and suppress dendritic cell migration (Aliberti et al., 2002; Gu et al., 2016). Overall, a prominent anti-inflammatory response is elicited by SPMs in infection, as RvD1, RvD2, and maresin 1 all increase production of the anti-inflammatory cytokine IL-10 in monocytes while increasing the phosphorylation (anti-inflammatory state) of glycogen synthase kinase 3β (GSK3 β) (Gu et al., 2016).
While SPMs can promote the killing of invading and harmful pathogens, most of their actions are focused on keeping the immune response in check. Because of this tenuous balance, the temporal control of SPM production is once again highlighted, as too early of a response could impede microbial clearance.
5. SPMs in sepsis
The gut has been implicated as a central player to the development of sepsis due to dysregulation between the mucosal epithelium, the immune system, and endogenous microbes. The imbalance between these highly regulated systems not only leads to the chronic inflammation thus far discussed but can also progress to fatal systemic manifestations, reaching far beyond the intestine (Mittal and Coopersmith, 2014). Sepsis is an extremely serious and quite often fatal complication of bacterial infection resulting in systemic immune dysregulation. SPMs have been found to be protective in regulating this systemic detour from homeostasis.
Both RvD1 and RvD2 improve the survival of mice in the cecal ligation and puncture initiated sepsis model. The resolvins enhance phagocyte killing of microbes while limiting an increase in neutrophils and reducing the release of inflammatory cytokines such as IL-6 and IFN-γ (Chen et al., 2014; Spite et al., 2009). The immune response is facilitated by the resolvins but still held in check as sepsis is already an uncontrolled inflammatory response. The SPMs coordinate balance to destroy the microbes while keeping the body from excessive damage. Recently, it was elucidated that RvD2 enhances CREB, ERK1/2, and STAT3 phosphorylation in macrophages after interaction with the GPR18 receptor to enhance phagocytosis of E. coli, which is dependent on protein kinase A and STAT3 signal transduction (Chiang et al., 2017).
A recent clinical study analyzed peripheral blood of sepsis patients within 48 h of admission to the ICU for lipid mediator profiling and revealed that in septic non-survivors, the peripheral blood contains significantly higher proinflammatory mediators such as LTB4 and PGF2α than survivors. Interestingly, pro-resolving mediators are also elevated in non-survivors, including RvE1, RvD5, PD1, than survivors (Dalli et al., 2017). Such a profile hints at the development of biomarkers for septic disease and presents novel biologic targets for this systemic disease.
SPMs regulate pro-resolving responses to dampen the overwhelming inflammatory response seen in sepsis while still maintaining a possibly improved clearance of systemic bacterial load. The ability to clear an infection while keeping system integrity is an ideal therapeutic model. Synthetic peptides that are selective agonists of the lipoxin receptor ALX/FPR2 have shown therapeutic effects in both the murine sepsis model and ulcerative colitis model via modulating the cytokine profile as well as decreasing epithelial permeability (Kim et al., 2013). Therapeutics designed to promote resolution and mimic the way inflammation naturally subsides in the body may thus prove to be better tolerated.
6. Conclusion
Given the close association of microbial products to intestinal surfaces, the maintenance of tissue homeostasis presents a significant challenge. Studies in recent years have identified a central role for the local generation and action of SPMs as a catalyst for resolution of inflammation. This review highlights the multi-functional role of SPMs in inflammation and unveils the critical role of lipid mediator signaling as an active participant in mucosal homeostasis. Through activation of G-protein coupled receptors, SPMs provide balanced, integrated and enduring responses. These same features also provide promising therapeutic potential – whether as novel biomarkers or as disease-targeted drugs, SPMs hold great promise in this arena. One of the more promising roles for SPMs drugs may be the lowering of antibiotic thresholds during infection to combat the evolution of resistant bacterial strains (Blaser, 2016). Indeed, Chiang et al. (2012) demonstrated that RvD1, RvD5 and PD1 enhance antimicrobial activity in peritoneal infection models to the extent that antibiotics can be used at suboptimal doses and for shortened periods of time. These findings provide but one example of how SPMs may function to promote multiple aspects of mucosal homeostasis. Studies in the future addressing the challenges of SPM stability, distribution, and selectivity should be revealing.
Acknowledgments
This work was supported by National Institutes of Health Grants DK50189, DK104713, DK095491, and VA Merit Award I01BX002182.
Footnotes
Disclosure statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as influencing the objectivity of this review.
References
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. http://dx.doi.org/10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol. 2002;3:76–82. doi: 10.1038/ni745. http://dx.doi.org/10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- Allison MC, Poulter LW. Changes in phenotypically distinct mucosal macrophage populations may be a prerequisite for the development of inflammatory bowel disease. Clin Exp Immunol. 1991;85:504–509. doi: 10.1111/j.1365-2249.1991.tb05757.x. http://dx.doi.org/10.1111/j.1365-2249.1991.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. http://dx.doi.org/10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aʇiş ER, Savaş B, Melli M. Impact of colonic mucosal lipoxin A4 synthesis capacity on healing in rats with dextran sodium sulfate-induced colitis. Prostagl Other Lipid Mediat. 2015;121:63–69. doi: 10.1016/j.prostaglandins.2015.04.001. http://dx.doi.org/10.1016/j.prostaglandins.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, Bauman JG, Subramanyam B, Perez HD, Parkinson JF, Serhan CN. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004a;143:43–52. doi: 10.1038/sj.bjp.0705912. http://dx.doi.org/10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta Mol Cell Biol Lipids. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. http://dx.doi.org/10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Aliberti J, Hong S, Sher A, Serhan C. Exogenous pathogen and plant 15-lipoxygenase initiate endogenous lipoxin A4 biosynthesis. J Exp Med. 2004b;199:515–523. doi: 10.1084/jem.20031325. http://dx.doi.org/10.1084/jem.20031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–382. doi: 10.1016/j.chom.2007.10.010. http://dx.doi.org/10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bena S, Brancaleone V, Wang JM, Perretti M, Flower RJ. Annexin A1 interaction with the FPR2/ALX receptor: identification of distinct domains and downstream associated signaling. J Biol Chem. 2012;287:24690–24697. doi: 10.1074/jbc.M112.377101. http://dx.doi.org/10.1074/jbc.M112.377101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol. 2011;187:1957–1969. doi: 10.4049/jimmunol.1101305. http://dx.doi.org/10.4049/jimmunol.1101305. [DOI] [PubMed] [Google Scholar]
- Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Huang MH, Perez-Polo JR, Uretsky BF. Aspirin augments 15-epi-lipoxin A4 production by lipopolysaccharide, but blocks the pioglitazone and atorvastatin induction of 15-epi-lipoxin A4 in the rat heart. Prostagl Other Lipid Mediat. 2007;83:89–98. doi: 10.1016/j.prostaglandins.2006.10.003. http://dx.doi.org/10.1016/j.prostaglandins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544–545. doi: 10.1126/science.aad9358. http://dx.doi.org/10.1007/s13398-014-0173-7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. http://dx.doi.org/10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- Campbell EL, Macmanus CF, Kominsky DJ, Keely S, Glover LE, Bowers BE, Scully M, Bruyninckx WJ, Colgan SP. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci USA. 2010;107:14303. doi: 10.1073/pnas.0914730107. http://dx.doi.org/10.1073/pnas.0914730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EL, Serhan CN, Colgan SP. Antimicrobial aspects of inflammatory resolution in the mucosa: a role for proresolving mediators. J Immunol. 2011;187:3475–3481. doi: 10.4049/jimmunol.1100150. http://dx.doi.org/10.4049/jimmunol.1100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Fan XH, Wu YP, Zhu JL, Wang F, Bo LL, Li JB, Bao R, Deng XM. Resolvin D1 improves survival in experimental sepsis through reducing bacterial load and preventing excessive activation of inflammatory response. Eur J Clin Microbiol Infect Dis. 2014;33:457–464. doi: 10.1007/s10096-013-1978-6. http://dx.doi.org/10.1007/s10096-013-1978-6. [DOI] [PubMed] [Google Scholar]
- Chiang N, de la Rosa X, Libreros S, Serhan C. Novel resolvin D2 receptor axis in infectious inflammation. J Immunol. 2017;198:842–851. doi: 10.4049/jimmunol.1601650. http://dx.doi.org/10.4049/jimmunol.1601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212:1203–1217. doi: 10.1084/jem.20150225. http://dx.doi.org/10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. http://dx.doi.org/10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. http://dx.doi.org/10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Gronert K, Clish CB, O’Brien JA, Freeman MW, Serhan CN. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104:309–316. doi: 10.1172/JCI7016. http://dx.doi.org/10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlén SE, Drazén JM, Hay DWP, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. http://dx.doi.org/10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol. 2007;2:111–143. doi: 10.1146/annurev.pathol.2.010506.091944. http://dx.doi.org/10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. http://dx.doi.org/10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. http://dx.doi.org/10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Curtis VF, Lanis JM, Glover LE. Metabolic regulation of intestinal epithelial barrier during inflammation. Tissue Barriers. 2015;3:e970936. doi: 10.4161/21688362.2014.970936. http://dx.doi.org/10.4161/21688362.2014.970936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Ehrentraut SF, Glover LE, Kominsky DJ, Campbell EL. Contributions of neutrophils to resolution of mucosal inflammation. Immunol Res. 2013;55:75–82. doi: 10.1007/s12026-012-8350-2. http://dx.doi.org/10.1007/s12026-012-8350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. http://dx.doi.org/10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Quintana C, Barragan-Bradford D, Hurwitz S, Levy BD, Choi AM, Serhan CN, Baron RM. Human sepsis eicosanoid and pro-resolving lipid mediator temporal profiles: correlations with survival and clinical outcomes. Crit Care Med. 2017;45:58–68. doi: 10.1097/CCM.0000000000002014. http://dx.doi.org/10.1097/CCM.0000000000002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CE, Cohen SB, Denkers EY. Insights into inflammatory bowel disease using Toxoplasma gondii as an infectious trigger. Immunol Cell Biol. 2012;90:668–675. doi: 10.1038/icb.2011.93. http://dx.doi.org/10.1038/icb.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P, Weiss J. Role of the bactericidal/permeability-increasing protein in host defence. Curr Opin Immunol. 1998;10:45–49. doi: 10.1016/s0952-7915(98)80030-7. http://dx.doi.org/10.1016/S0952-7915(98)80030-7. [DOI] [PubMed] [Google Scholar]
- Fierro IM, Colgan SP, Bernasconi G, Petasis NA, Clish CB, Arita M, Serhan CN. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J Immunol. 2003;170:2688–2694. doi: 10.4049/jimmunol.170.5.2688. http://dx.doi.org/10.4049/jimmunol.170.5.2688. [DOI] [PubMed] [Google Scholar]
- Fiore S, Serhan CN. Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4-lipoxin A4 receptor interaction. Biochemistry. 1995;34:16678–16686. doi: 10.1021/bi00051a016. http://dx.doi.org/10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. http://dx.doi.org/10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzano-Santoro H, Parent JB, Grinna L, Horwitz A, Parsons T, Theofan G, Elsbach P, Weiss J, Conlon PJ. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60:4754–4761. doi: 10.1128/iai.60.11.4754-4761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, Williams IR, Neish AS, Madara JL. Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. http://dx.doi.org/10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL. Pathogen-induced chemokine secretion for model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101:1860–1869. doi: 10.1172/JCI1339. http://dx.doi.org/10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbetti T, Ducheix S, le Faouder P, Perez T, Riols F, Boue J, Bertrand-Michel J, Dubourdeau M, Guillou H, Perretti M, Vergnolle N, Cenac N. Protective effects of n-6 fatty acids-enriched diet on intestinal ischaemia/reperfusion injury involve lipoxin A4 and its receptor. Br J Pharmacol. 2015;172:910–923. doi: 10.1111/bph.12957. http://dx.doi.org/10.1111/bph.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. http://dx.doi.org/10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- Goh J, Baird AW, O’Keane C, Watson RWG, Cottell D, Bernasconi G, Petasis NA, Godson C, Brady HR, MacMathuna P. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 antagonize TNF-alpha-stimulated neutrophil-enterocyte interactions in vitro and attenuate TNF-alpha-induced chemokine release and colonocyte apoptosis in human intestinal mucosa ex vivo. J Immunol. 2001;167:2772–2780. doi: 10.4049/jimmunol.167.5.2772. http://dx.doi.org/10.4049/jimmunol.167.5.2772. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Austen WG, Jr, Zhang X, Munene G, Mostafa G, Biswas S, McCormack M, Eberlin KR, Nguyen JT, Tatlidede HS, Warren HS, Narisawa S, Millan JL, Hodin RA. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA. 2008;105:3551–3556. doi: 10.1073/pnas.0712140105. http://dx.doi.org/10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronert Karsten, Maheshwari Neha, Khan Nabeela, Hassan Iram R, Dunn Michael, Schwartzman ML. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. http://dx.doi.org/10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- Gronert K, Colgan SP, Serhan CN. Characterization of human neutrophil and endothelial cell ligand-operated extracellular acidification rate by microphysiometry: impact of reoxygenation. J Pharmacol Exp Ther. 1998a;285:252–261. [PubMed] [Google Scholar]
- Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alpha-induced IL-8 release. J Exp Med. 1998b;187:1285–1294. doi: 10.1084/jem.187.8.1285. http://dx.doi.org/10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Lamont GJ, Lamont RJ, Uriarte SM, Wang H, Scott DA. Resolvin D1, resolvin D2 and maresin 1 activate the GSK3 anti-inflammatory axis in TLR4-engaged human monocytes. Innate Immun. 2016;22:186–195. doi: 10.1177/1753425916628618. http://dx.doi.org/10.1177/1753425916628618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients. 2011;3:637–682. doi: 10.3390/nu3060637. http://dx.doi.org/10.3390/nu3060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol. 2011;17:2161–2171. doi: 10.3748/wjg.v17.i17.2161. http://dx.doi.org/10.3748/wjg.v17.i17.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. http://dx.doi.org/10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalakes H, Stylianides TJ, Amanakis G, Kolios G. Exacerbation of inflammatory bowel diseases associated with the use of nonsteroidal anti-inflammatory drugs: myth or reality? Eur J Clin Pharmacol. 2009;65:963–970. doi: 10.1007/s00228-009-0719-3. http://dx.doi.org/10.1007/s00228-009-0719-3. [DOI] [PubMed] [Google Scholar]
- Kim SD, Kwon S, Lee SK, Kook M, Lee HY, Song KD, Lee HK, Baek SH, Park CB, Bae YS. The immune-stimulating peptide WKYMVm has therapeutic effects against ulcerative colitis. Exp Mol Med. 2013;45:e40. doi: 10.1038/emm.2013.77. http://dx.doi.org/10.1038/emm.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SNA. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol. 2012;7:35–60. doi: 10.1146/annurev-pathol-011811-120905. http://dx.doi.org/10.1146/annurev-pathol-011811-120905. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. http://dx.doi.org/10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T, Gewirtz AT, Merlin D, Madara JL, Williams IR. Lateral membrane LXA4 receptors mediate LXA4’s anti-inflammatory actions on intestinal epithelium. Am J Physiol Cell Physiol. 2003;284:C888–C896. doi: 10.1152/ajpcell.00507.2001. http://dx.doi.org/10.1152/ajpcell.00507.2001. [DOI] [PubMed] [Google Scholar]
- Kuhl AA, Pawlowski NN, Grollich K, Loddenkemper C, Zeitz M, Hoffmann JC. Aggravation of intestinal inflammation by depletion/deficiency of gammadelta T cells in different types of IBD animal models. J Leukoc Biol. 2007;81:168–175. doi: 10.1189/jlb.1105696. http://dx.doi.org/10.1189/jlb.1105696. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Aster JC. Robbins And Cotran Pathologic Basis of Disease. ninth. Philadelphia, PA: Saunders; 2014. pp. 1–1408. Ipswich, MA. [Google Scholar]
- Kure I, Nishiumi S, Nishitani Y, Tanoue T, Ishida T, Mizuno M, Fujita T, Kutsumi H, Arita M, Azuma T. Lipoxin A 4 reduces lipopolysaccharide-induced inflammation in macrophages and intestinal epithelial cells through inhibition of nuclear factor-κB activation. J Pharmacol Exp Ther. 2010;332:541–548. doi: 10.1124/jpet.109.159046. http://dx.doi.org/10.1124/jpet.109.159046.tion. [DOI] [PubMed] [Google Scholar]
- Lawrence DW, Bruyninckx WJ, Louis NA, Lublin DM, Stahl GL, Parkos CA, Colgan SP. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J Exp Med. 2003;198:999–1010. doi: 10.1084/jem.20030380. http://dx.doi.org/10.1084/jem.20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, Reutelingsperger C, Perretti M, Parkos CA, Neish AS, Nusrat A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. http://dx.doi.org/10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G, Argyris I, Rijcken E, Kusters D, Reutelingsperger C, Perretti M, Parkos CA, Farokhzad OC, Neish AS, Nusrat A. Annexin A1’containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015;125:1215–1227. doi: 10.1172/JCI76693. http://dx.doi.org/10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. http://dx.doi.org/10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Levy O, Canny G, Serhan CN, Colgan SP. Expression of BPI (bactericidal/permeability-increasing protein) in human mucosal epithelia. Biochem Soc Trans. 2003;31:795–800. doi: 10.1042/bst0310795. http://dx.doi.org/10.1042/. [DOI] [PubMed] [Google Scholar]
- Louis NA, Hamilton KE, Colgan SP. Lipid mediator networks and leukocyte transmigration. Prostagl Leukot Essent Fat Acids. 2005;73:97–202. doi: 10.1016/j.plefa.2005.05.006. http://dx.doi.org/10.1016/j.plefa.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. http://dx.doi.org/10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox JF, Colgan SP, Clish CB, Petasis NA, Fokin VV, Serhan CN. Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: design of stable lipoxin B4 analogs with increased biologic activity. FASEB J. 1998;12:487–494. doi: 10.1096/fasebj.12.6.487. [DOI] [PubMed] [Google Scholar]
- Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Mangino MJ, Brounts L, Harms B, Heise C. Lipoxin biosynthesis in inflammatory bowel disease. Prostagl Other Lipid Mediat. 2006;79:84–92. doi: 10.1016/j.prostaglandins.2005.10.004. http://dx.doi.org/10.1016/j.prostaglandins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Masterson JC, McNamee EN, Fillon SA, Hosford L, Harris R, Fernando SD, Jedlicka P, Iwamoto R, Jacobsen E, Protheroe C, Eltzschig HK, Colgan SP, Arita M, Lee JJ, Furuta GT. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut. 2015;64:1236–1247. doi: 10.1136/gutjnl-2014-306998. http://dx.doi.org/10.1136/gutjnl-2014-306998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCole DF, Barrett KE. Varied role of the gut epithelium in mucosal homeostasis. Curr Opin Gastroenterol. 2007;23:647–654. doi: 10.1097/MOG.0b013e3282f0153b. http://dx.doi.org/10.1097/MOG.0b013e3282f0153b. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. http://dx.doi.org/10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20:214–223. doi: 10.1016/j.molmed.2013.08.004. http://dx.doi.org/10.1016/j.molmed.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits PMN recruitment to inflammatory loci: receptor dependent actions. Arterioscler Thromb Vasc Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. http://dx.doi.org/10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. http://dx.doi.org/10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CD, Mancuso CJ, Weiss JP, Serhan CN, Guinan EC, Levy O. 17(R)-Resolvin D1 differentially regulates TLR4-mediated responses of primary human macrophages to purified LPS and live E. coli. J Leukoc Biol. 2011;90:459–470. doi: 10.1189/jlb.0311145. http://dx.doi.org/10.1189/jlb.0311145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto P, Rosales-Mendoza CE, Terrón V, Toledano V, Cuadrado A, López-Collazo E, Bannenberg G, Martín-Sanz P, Fernández-Velasco M, Boscá L. Activation of autophagy in macrophages by pro-resolving lipid mediators. Autophagy. 2015;11:1729–1744. doi: 10.1080/15548627.2015.1078958. http://dx.doi.org/10.1080/15548627.2015.1078958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros M, Nishio H, Leoni G, Agarwal R, Bernal G, Gerner-Smith C, Colas RA, Graham K, Serhan CN, Garcia A, Parkos CA, Nusrat A. The specialized pro-resolving lipid mediator resolvin e1 promotes intestinal mucosal wound repair. FASEB J. 2016;30:305–307. [Google Scholar]
- Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, Isolauri E, Moreau MC, Roberfroid M, Rowland I. Functional food science and gastrointestinal physiology and function. Br J Nutr. 1998;80(Suppl 1):S147–S171. doi: 10.1079/bjn19980108. http://dx.doi.org/10.1079/BJN19980108. [DOI] [PubMed] [Google Scholar]
- Schottelius AJ, Giesen C, Asadullah K, Fierro IM, Colgan SP, Bauman J, Guilford W, Perez HD, Parkinson JF. An aspirin-triggered lipoxin a4 stable analog displays a unique topical anti-inflammatory profile. J Immunol. 2002;169:7063–7070. doi: 10.4049/jimmunol.169.12.7063. http://dx.doi.org/10.4049/jimmunol.169.12.7063. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. http://dx.doi.org/10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LaJ, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. http://dx.doi.org/10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. http://dx.doi.org/10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000a;192:1197–1204. doi: 10.1084/jem.192.8.1197. http://dx.doi.org/10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Gronert K, Chiang N. Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: a novel mechanism for unsaid and N-3 pufa therapeutic actions. J Physiol Pharmacol. 2000b;51:643–654. [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A. 1984;81:5335–5339. doi: 10.1073/pnas.81.17.5335. http://dx.doi.org/10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. http://dx.doi.org/10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. http://dx.doi.org/10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory–pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. http://dx.doi.org/10.1016/j.bbi.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. http://dx.doi.org/10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. http://dx.doi.org/10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Sheppard KA. Lipoxin formation during human neutrophil-platelet interactions: evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J Clin Invest. 1990;85:772–780. doi: 10.1172/JCI114503. http://dx.doi.org/10.1172/JCI114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. http://dx.doi.org/10.1016/S0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- Souza DG, Fagundes CT, Amaral FA, Cisalpino D, Sousa LP, Vieira AT, Pinho V, Nicoli JR, Vieira LQ, Fierro IM, Teixeira MM. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol. 2007;179:8533–8543. doi: 10.4049/jimmunol.179.12.8533. [DOI] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. http://dx.doi.org/10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumagin R, Parkos CA. Epithelial adhesion molecules and the regulation of intestinal homeostasis during neutrophil transepithelial migration. Tissue Barriers. 2015;3:e969100. doi: 10.4161/21688362.2014.969100. http://dx.doi.org/10.4161/21688362.2014.969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. http://dx.doi.org/10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- Titos E, Chiang N, Serhan CN, Romano M, Gaya J, Pueyo G, Clària J. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A4. Am J Physiol Cell Physiol. 1999;277:C870–C877. doi: 10.1152/ajpcell.1999.277.5.C870. http://dx.doi.org/10.1016/S0090-6980(99)90306-4. [DOI] [PubMed] [Google Scholar]
- Vilaseca J, Salas A, Guarner F, Rodríguez R, Martínez M, Malagelada JR. Dietary fish oil reduces progression of chronic inflammatory lesions in a rat model of granulomatous colitis. Gut. 1990;31:539–544. doi: 10.1136/gut.31.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L, Ferraz JGP, Dufton N, Panaccione R, Beck PL, Sherman PM, Perretti M, Wallace JL. Up-regulation of Annexin-A1 and lipoxin A4 in individuals with ulcerative colitis may promote mucosal homeostasis. PLoS One. 2012;7:e39244. doi: 10.1371/journal.pone.0039244. http://dx.doi.org/10.1371/journal.pone.0039244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–282. doi: 10.1016/j.pharmthera.2013.10.010. http://dx.doi.org/10.1016/j.pharmthera.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol. 2009;40:519–535. doi: 10.1165/rcmb.2008-0348TR. http://dx.doi.org/10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]


