Abstract
Escherichia albertii is a recently discovered species with a limited number of well characterized strains. The aim of this study was to characterize four of the E. albertii strains, which were among 41 identified Escherichia strains isolated from the feces of living animals on James Ross Island, Antarctica, and Isla Magdalena, Patagonia. Sequencing of 16S rDNA, automated ribotyping, and rep-PCR were used to identify the four E. albertii isolates. Phylogenetic analyses based on multi-locus sequence typing showed these isolates to be genetically most similar to the members of E. albertii phylogroup G3. These isolates encoded several virulence factors including those, which are characteristic of E. albertii (cytolethal distending toxin and intimin) as well as bacteriocin determinants that typically have a very low prevalence in E. coli strains (D, E7). Moreover, E. albertii protein extracts caused cell cycle arrest in human cell line A375, probably because of cytolethal distending toxin activity.
Keywords: Antarctica, bacteriocins, cytolethal distending toxin, Escherichia albertii
Strains of Escherichia albertii were originally classified as Hafnia alvei-like stains, which were isolated from human stool specimens in the early 1990s and were suspected of being causative agents of diarrhea [1]. Based on DNA-DNA hybridization analyses, the Hafnia alvei-like strains were reclassified as a new species−Escherichia albertii [15]. Even though E. albertii is a relative of E. coli, it is phylogenetically distinct from other Escherichia species [16, 23, 37, 56].
In general, Escherichia albertii is a potential human pathogen with a limited number of characterized isolates. Since 2003, when the new E. albertii species was proposed, only 282 isolates have been described and more than half of them (n=144) were associated with diarrhea and/or gastroenteritis in humans [2, 3, 6, 9, 10, 13,14,15, 17, 20, 21, 24, 25, 31,32,33,34,35, 37, 42, 52, 55,56,57]. E. albertii has also been shown to be responsible for epidemic mortality among birds [33]. It has been isolated from pig, cat, environmental samples, and found as a contaminant of various raw meats [14, 23, 33, 36, 42, 57].
However, the real frequency of E. albertii in clinical samples remains unclear. E. albertii ferments D-mannitol but not D-xylose and does not produce indole. Because strains of E. albertii are not included in the databases of majority of commercial diagnostic tests, E. albertii strains are often misidentified as Hafnia, Salmonella, Escherichia coli or Yersinia ruckeri [reviewed in 59]. E. albertii possesses a specific set of virulence genes including intimin and the eae-encoded outer membrane protein, and thus many strains of E. albertii might be misidentified as enterohemorrhagic (EHEC) or enteropathogenic E. coli (EPEC) [36, 58]. However, compared to the EPEC (and not EHEC), E. albertii often encodes cytolethal distending toxin that causes cell cycle arrest in eukaryotic cells, which leads to cell distention and cell death [4, 8, 16, 46, 60].
Escherichia species, as well as many other bacterial species, are known to produce antimicrobial agents called bacteriocins. In the genus Escherichia, bacteriocins include colicins and microcins. Although the exact role of bacteriocin production in bacterial populations remains unclear, there is increasing evidence of the role of bacteriocins in bacterial virulence [28, 49], in probiotic phenotype of E. coli strains [50], and in colonization of the gastrointestinal tract [11].
Recently, we isolated E. albertii from feces of Antarctic animals and published preliminary data regarding characterization [44]. In this work, we have analyzed a larger sample set of isolates and characterized, in greater detail, four isolates of E. albertii from animals living in Antarctica and Patagonia.
MATERIALS AND METHODS
Samples collection
Fecal specimens and rectal or cloacal swabs from seals (mostly Leptonychotes weddelli), penguins (Pygoscelis adeliae, P. papua, Spheniscus magellanicus), skuas (Stercorarius maccormicki), and gulls (Larus dominicanus) were collected on James Ross Island and Seymour Island, Antarctica, and Isla Magdalena, Patagonia, during austral summers in 2013 and 2014. This sampling was a part of Cultivable Fecal Bacteria Communities study, which was part of the CzechPolar project. The samples were collected using swab/transport tube system E-Swabs (Dispolab, Brno, Czech Republic), kept at 4°C and transported to the Czech Republic for further analyses.
Reference and type strains used in this study were obtained from the Czech Collection of Microorganisms (Masaryk University, Brno, Czech Republic).
Isolation and biochemical identification of strains
Samples were cultivated on Columbia blood agar and sub-cultivated on Columbia blood agar supplemented with 7% sheep blood (Bio-Rad, Praha, Czech Republic) at 30°C for 24 hr. Different colonies revealing the macroscopic morphology typical for enteric bacteria were randomly picked up from smears of faecal samples grown on Endo agar and MacConkey agar. Two commercial identification kits, ENTEROtest 24 (Erba Lachema, Brno, Czech Republic) and Biolog Identification System, GN2 MicroPlate (Biolog, Hayward, CA, U.S.A.), were used (according to the manufacturers’ instructions). The list of 41 identified Escherichia isolates is shown in Table S1.
16S rDNA and multi-locus sequence typing (MLST) analyses
The 16S rRNA gene, from all 41 Escherichia isolates, was amplified as described previously [41]. Additionally, a MLST analysis of E. albertii isolates was performed by amplifying and sequencing six housekeeping genes, aspC, clpX, fadD, icdA, lysP and mdh, using a previously described protocol [16, 33]. All PCR products were sequenced using the Sanger method (Elisabeth® Pharmacon, Brno, Czech Republic and GATC Biotech AG, Konstanz, Germany). Sequences were analyzed using Lasergene software (DNASTAR v.7.1.0., Madison, WI, U.S.A.).
Automated ribotyping, repetitive element PCR (rep-PCR) and pulse-field gel electrophoresis (PFGE)
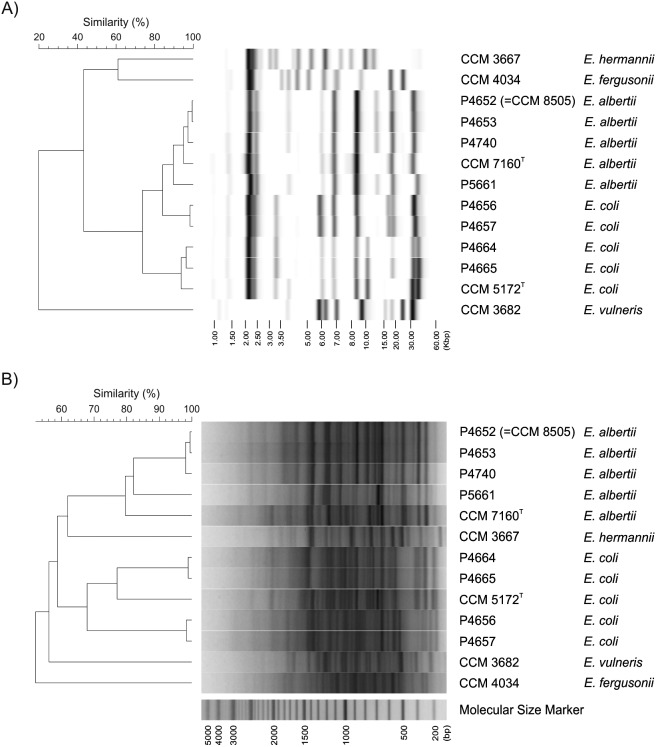
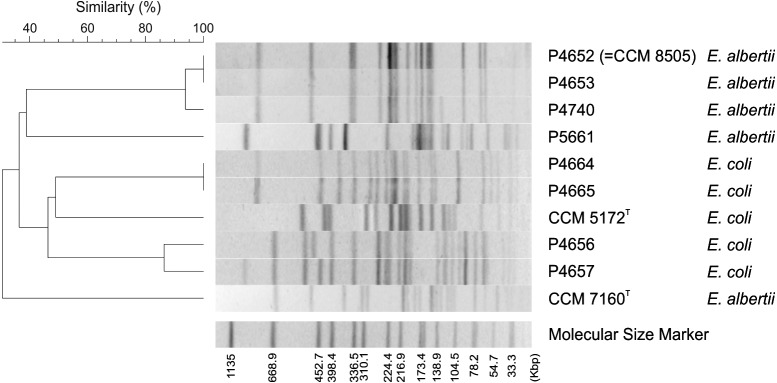
All 41 isolates, which had been previously classified to the Escherichia genus by commercial identification kits, were used for automated ribotyping with the EcoRI restriction enzyme and rep-PCR fingerprinting as described previously [53, 54]. PFGE was performed using the XbaI enzyme and the standardized PulseNet protocol for E. coli O157:H7 (http://www.pulsenetinternational.org/protocols). The dendrograms of automated ribotyping and rep-PCR were constructed with Pearson’s correlation coefficient and analysis of PFGE macrorestriction patterns was done with Jaccard similarity coefficient. All dendrograms were constructed with UPGMA clustering method (BioNumerics v. 7.5, Applied Maths, Sint-Martens-Latem, Belgium).
PCR detection of virulence markers
Our set of E. albertii isolates and the E. albertii type strain, CCM 7160T, were tested for the presence of 21 virulence markers, which are typical for the Enterobacteriaceae. This included genes encoding virulence factors found in enteroaggregative E. coli (pCVD432), enterotoxigenic E. coli (heat-labile enterotoxin (lt) and heat-stable enterotoxin (st)), enteroinvasive E. coli (invasivity antigen (ial) and invasion plasmid antigen H (ipaH)), enteropathogenic E. coli (colibactin, bundle-forming pillus A (bfpA) and intimin (eaeA)), enterohemorrhagic E. coli (enterohemolysin (ehly) and Shiga toxins (stx1, stx2)), diffusely adherent E. coli (afimbrial adhesin (afa)) and other genes coding virulence factors (α-hemolysin (α-hly), aerobactin (aer), B unit of the cytolethal distending toxin (cdtB), cytotoxic necrosis factor 1 (cnf1), type I fimbriae (fimA), aerobactin iron transport system (iucC), P-fimbriae (pap), S-fimbriae (sfa) and uropathogenic specific protein (usp)).The primer pair sequences, PCR product lengths and PCR protocols were previously described [5, 19, 22, 24, 26, 38, 39, 43, 59].
Bacteriocin production and identification of bacteriocin types
Detection of bacteriocin production was performed phenotypically as described previously [49] using bacteriocin indicator strains E. coli K12 - Row, E. coli C6 (φ), Shigella sonnei 17, E. coli P400, E. coli S40 and E. coli 5K. For characterization of individual bacteriocin types, PCR amplifications of bacteriocin determinants were performed as described previously [28]. This screening detected most of the known colicins and microcin determinants (n=32). The bacteriocin ‘control’ producers, used for PCR detection of bacteriocin genes, were previously described in a detail [27, 49].
Whole protein extraction
E. albertii strains examined in this study, E. albertii type strain CCM 7160T, and E. coli CCM 4825 (K12) were grown overnight at 37°C in 3 ml of TY broth (Himedia, Mumbai, India) and centrifugated at 5,000 g for 10 min. Total bacterial proteins from 1 g of wet bacterial biomass were extracted using B-PER Complete bacterial protein extraction reagent (Thermo Fisher Scientific, Waltham, MA, U.S.A.) according to the manufacturer’s recommendations. Final suspension was filtered using 0.45 µm bacterial filters (Nalgene filters, Thermo Fisher Scientific, Waltham, MA, U.S.A.).
Cultivation of cell line A375
The human malignant melanoma cancer cell line, A375 (European Collection of Cell Cultures, Salisbury, U.K.), was used. Cells were grown in RPMI 1640 medium (HyClone Laboratories, Inc., South Logan, UT, U.S.A.) supplemented with 2 mmol l−1 L-glutamine (PAA Laboratories, Pasching, Austria), 10% fetal calf serum, penicillin (final concentration of 100 IU ml−1), and streptomycin (final concentration of 100 µg ml−1). Cells were incubated at 37°C under 5% CO2 in a high-humidity-atmosphere and subcultured three times per week.
Cell cycle analysis
A375 cells were plated in concentration of 7 × 104 cells per ml and cultivated for 24 and 48 hr. Subsequently, A375 cells were treated with protein extracts (final dilution 1:1,000) for 24 and 48 hr. Both detached and attached cells were harvested into ice-cold PBS, fixed and processed as described previously [47]. A Cytomics FC 500 flow cytometry system (Beckman Coulter, Inc., Prague, Czech Republic) was used for cell cycle analysis. The cell cycle phases were determined using a Multicycle AV for Windows software (Phoenix Flow system, San Diego, CA, U.S.A.).
RESULTS
Identification of Escherichia isolates
As a part of Cultivable Fecal Bacteria Communities study project, we collected, during 2013–2014, 83 swabs of fecal specimens of randomly sampled animals from James Ross (23 seals, 14 penguins and 20 undetermined feces) and Seymour Islands (10 seals and 14 penguins), Antarctica, and Isla Magdalena, Patagonia (two penguins). From eight phenotypically identified genera of enteric bacteria (Aeromonas, Citrobacter, Edwardsiella, Enterobactre, Escherichia, Leclercia, Raoultella and Serratia), 41 isolates were classified as members of the Escherichia genus using two commercial identification kits, ENTEROtest 24 and Biolog GN2 MicroPlate system. This set of Escherichia strains was isolated from perianal or cloacal smears of seals (n=14), penguins (n=13), skuas (n=8), and gulls (n=2), and from the environment (n=4). Samples were collected on James Ross Island (n=25), Seymour Island (n=13), Antarctica, and Isla Magdalena, Patagonia (n=3) (Table S1). In addition to the 37 isolates, which were classified as E. coli species using both commercial biochemical kits, four isolates were classified as “atypical/inactive” E. coli (9.7%). Compared to the other E. coli species, the four isolates were beta-glucuronidase negative and unable to ferment sorbitol and melibiose (data not shown).
The 16S rDNA region (1,352 out of 1,494 bp) in all 41 Escherichia isolates was analyzed. While 37 isolates showed 99.5–99.8% nucleotide sequence similarity to the 16S rDNA sequence of E. coli (E. coli type strain; GenBank Accession No. X80725), the 16S rDNA sequences of the four above mentioned isolates, previously characterized using commercial tests as atypical/inactive E. coli (P4652, P4653, P4740 and P5661), showed the greatest similarity (99.6–99.7%) to the 16S rDNA sequence of E. albertii (E. albertii type strain; GenBank Accession No. AJ508775). Three of the E. albertii isolates were obtained from seal feces (James Ross Island, Antarctica) and one E. albertii isolate came from the feces of a penguin (Isla Magdalena, Patagonia) (Table 1).
Table 1. Characteristics of Escherichia albertii isolates analyzed in this study.
| Isolate | Source | Similarity level −16S rDNA (%)a) | MLSTb) | Detected virulence factor determinants | Detected bacteriocin determinants |
|---|---|---|---|---|---|
| P4652 | Seal, Antarctica | 99.6 | 99.99% similarity to E. albertii in Corvus sp. | eae, cdtB, iucC | D, E7 |
| P4653 | Seal, Antarctica | 99.6 | 99.99% similarity to E. albertii in Corvus sp. | eae, cdtB,iucC | D, E7 |
| P4740 | Seal, Antarctica | 99.6 | 99.99% similarity to E. albertii in Corvus sp. | eae, cdtB, fimA | D, E7 |
| P5661 | Penguin, Patagonia | 99.7 | Identical to E. albertii in Egretta garzetta | eae, cdtB, aer, fimA, ipaH | B, M |
a) Similarity was calculated according to the number of single nucleotide variants in 16S rDNA sequences of E. albertii isolates compared to the 16S rDNA sequence of E. albertii type strain CCM 7160T (according to GenBank Accession No. AJ508775 in coordinates 58-1409). b) MLST − Multi Locus Sequence Typing based on six concatenated housekeeping genes (aspC, clpX, fadD, icdA, lysP and mdh). MLST data were compared to the data published in [37].
To confirm the classification of E. albertii based on 16S rDNA sequencing, two DNA fingerprinting techniques, automated ribotyping and rep-PCR were used to show the differences between the isolates and their similarity to related Escherichia spp. The results of both methods clearly grouped isolates P4652, P4653, P4740, and P5661 with the E. albertii CCM 7160T type strain (Fig. 1). Moreover, PFGE of XbaI restriction fragments revealed identical fingerprints for isolates P4652 and P4653, suggesting that these two isolates are representatives of the same clone (Fig. 2). The P4652 isolate was deposited in the Czech Collection of Microorganisms (Brno, Czech Republic), under accessional number CCM 8505, as a representative of E. albertii strains isolated from seals in Antarctica.
Fig. 1.
Dendrograms based on cluster analysis of A) ribotype profiles and B) rep-PCR fingerprints. Four strains previously identified as E. albertii by 16S rDNA analysis (P4661, P4652, P4653 and P4740) were used as well as strain CCM 7160T (type strain of E. albertii), strain CCM 5172T (type strain of E. coli), four randomly selected strains examined in this study previously identified as E. coli by 16S rDNA analysis (P4656, P4657, P4664 and P4665; Table 1) and E. vulneris, E. hermanni and E. fergusonii reference strains as outgroups.
Fig. 2.
PFGE dendrogram based on cluster analysis of macro-restriction patterns obtained from investigated E. albertii and E. coli strains. Four E. albertii isolates (P4661, P4652, P4653 and P4740) were used as well as strain CCM 7160T (type strain of E. albertii), strain CCM 5172T (type strain of E. coli), and four randomly selected strains examined in this study previously identified as E. coli by 16S rDNA analysis (P4656, P4657, P4664 and P4665; Table 1).
Comparison of Antarctic isolates to previously characterized E. albertii isolates
Based on a genome-wide analysis of 34 E. albertii strains isolated from 3 different geographic areas (Japan, Germany, and Brazil) and from 3 different hosts (humans, birds, and cats), E. albertii can be further divided into five different phylogroups (G1–G5), as recently described [37]. We compared six concatenated housekeeping gene sequences from E. albertii isolates examined in this study (with a total length of 2,040 bp) to corresponding sequences present in the 34 draft genomes examined in [37]. While penguin isolate P5661 was completely identical to the silver heron isolate (Egretta garzetta) sampled in Japan (NIAH_Bird_8, GeneBank Accession No. BBVQ01000001-BBVQ01000167), seal isolates P4652, P4653 and P4740 were most similar (99.99% of sequence identity) to a raven isolate (Corvus sp.) found in Japan (E2675, GeneBank Accession No. BBVT01000001-BBVT01000119). All Antarctic isolates were genetically most similar to the members of phylogroup G3 (99.99% of sequence identity and more) and shared less than 99% of sequence identity with samples from other phylogroups.
Other characteristics of E. albertii identified in this study
Detection of virulence determinants: Out of 21 tested virulence factor determinants (see Material and Methods section), all isolates were PCR positive for subunit B of cytolethal distending toxin (CDT, cdtB) and intimin (eae). In addition, the aerobactin determinant (aer) was found in P5661, type I fimbriae (fimA) in P4740 and P5661, invasion plasmid antigen H (ipaH) in P5661, and the aerobactin iron transport system (iucC) in P4652, P4653 and P4740 (Table 1).
Production of antimicrobial agents: While Escherichia albertii type strain CCM 7160T did not produced bacteriocins or phages, Antarctic and Patagonian E. albertii isolates inhibited the growth of indicator strains E. coli and S. sonnei. Using PCR screening, the presence of 32 determinants of known bacteriocin types (i.e. 25 colicin and 7 microcin determinants) was screened and four different bacteriocin types were identified. While colicin D and E7 determinants were found in E. albertii isolated from seals in Antarctica, the penguin isolate from Patagonia contained determinants encoding colicins B and M (Table 1).
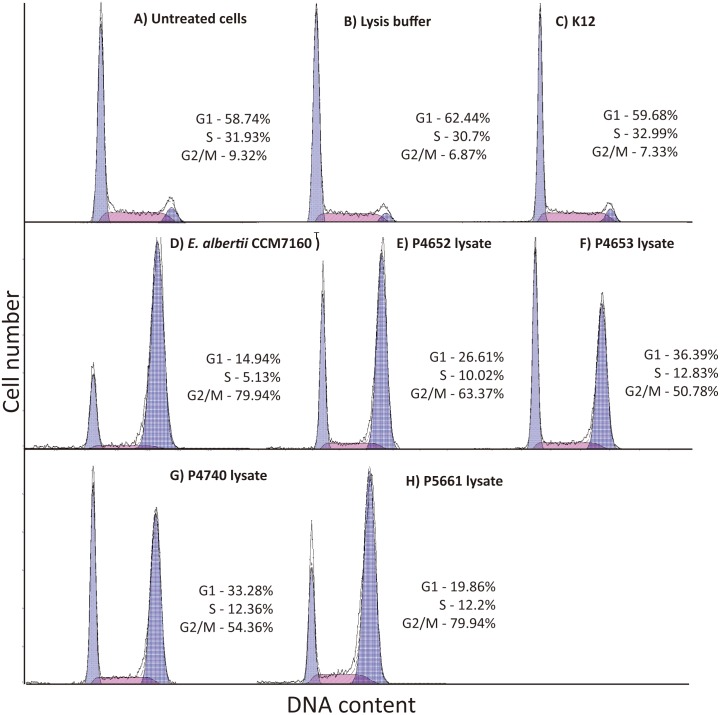
Analyses of bacterial extracts activity on human cells: Since we detected DNA sequences encoding CDT in all E. albertii isolates, which has been found to affect the cell cycle [4, 8, 16, 40, 60], we analyzed cell cycles of human malignant melanoma A375 cells treated with protein extracts from E. albertii isolates (P4652, P4653, P4740 and P5661). Unlike the ‘control’ E. coli K12 protein extract, extracts from E. albertii isolates as well as lysates from positive controls (E. albertii type strain CCM 7160T) caused accumulation of A375 cells in G2/M transition after 24 and 48 hr of treatment (Fig. 3).
Fig. 3.
Cell cycle analysis. All negative controls: non-treated cells (A), cells treated with bacterial lysis buffer (B) and lysate from E. coli K12 (non-producing strain; C), did not affect cell cycle of cell line A375, and showed normal distribution of phases. Whole protein extract from E. albertii type strain CCM 7160T (CDT producing strain; D) [16] and extracts from PCR cdtB−positive E. albertii isolates (P4652, P4653, P4740 and P5661; E-H) caused cell cycle arrest and accumulation of A375 cells in G2/M transition. Only results after 48 hr of treatment are shown.
DISCUSSION
Escherichia albertii is considered to be a food-borne pathogen of the human gastrointestinal tract that causes gastroenteritis around the word (Table 2). In this study, we identified three strains of E. albertii in feces of seals on James Ross Island, Antarctica, and one strain in feces of a penguin on Isla Magdalena, Patagonia, as E. albertii species, using three independent diagnostic tools−16S rDNA sequencing, ribotyping, and rep-PCR.
Table 2. Strains of E. albertii published since 2003.
| No. of isolates | Host | Geographic area | Clinical impact | Year of isolation | Reference |
|---|---|---|---|---|---|
| 5 | Human | Bangladesh | Diarrhea | 1990–1991 | [15] |
| 21 | Human | Bangladesh | Diarrhea | 1990–1993 | [52] |
| 1 | Drinking water in hospital | Hungary | NA | 2005 | [9] |
| 7 | Birds | U.S.A. | Death | 2005–2007 | [33] |
| 2 | Birds | Canada | Healthy | 2005 | [33] |
| 5 | Birds | Scotland | Death | 1998–2000 | [20] |
| 9 | Birds | Australia | Healthy | 2001–2002 | [33] |
| 3 | Human | Guinea-Bissau | Healthy | 1997 | [56] |
| 2 | Human | U.S.A. | Diarrhea | NA | [33] |
| 7 | Birds | Australia | NA | NA | [57] |
| 9 | Birds | Korea | Healthy | 2009–2010 | [34] |
| 6 | Human | Japan | Gastroenteritis | 2011 | [35] |
| 1 | Environmental fresh water | Bangladesh | NA | 2006 | [42] |
| 1 | Human | Poland | Diarrhea | NA | [10] |
| 6 | Human | Japan | Diarrhea | 2003 | [2] |
| 1 | Swine | Japan | Healthy | 2004 | [14] |
| 18 | Environmental water | Canada | NA | 2009 | [25] |
| 27 | Chicken carcass | U.S.A. | NA | 2009–2010 | [21] |
| 39 | Human | Norway | Diarrhea | 2008–2014 | [6] |
| 2 | Chicken food | Japan | NA | 2014 | [24] |
| 1 | Raw chicken liver | Japan | NA | 2013 | [3] |
| 14 | Human | Japan, Germany, Brazil | Gastroenteritis | 1993–2009 | [35] |
| 11 | Bird | Japan | NA | 1993–2009 | [35] |
| 1 | Cat | Brazil | Healthy | 2004 | [31] |
| 48 | Human | NA | Diarrhea | 1997–2007 | [32] |
| 30 | Raw meat (duck, chicken, mutton) | China | NA | NA | [58] |
| 3 | Human | Japan | NA | 2008–2009 | [37] |
| 1 | Human | Australia | Febrile infection | NA | [17] |
| 1 | Human | Japan | Diarrhea | 2008 | [13] |
NA; not available.
According to the analyses of six housekeeping genes (aspC, clpX, fadD, icdA, lysP and mdh), all examined E. albertii isolates were most similar to the members of phylogroup G3, described in [37]. However, until now, no associations of group G3 and bacterial hosts, with infectious symptoms and geographic areas, have been described [37].
E. albertii isolates are often classified as atypical enteropathogenic E. coli (aEPEC). This classification is based on the fact that both groups share a specific set of virulence genes including intimin encoding genes located within the LEE pathogenicity island [45, 58]. Unlike EPEC, E. albertii often encodes cytolethal distending toxin. The gene encoding subunit B of CDT (cdtB) was detected in all E. albertii isolates examined in this study. There are few other bacterial genotoxins; to date, in addition to CDTs, we have the uropathogenic-specific protein (usp) and colibactin [4]. Above mentioned results are in concordance with our results showing that Antarctic and Patagonia E. albertii whole protein extracts blocked cell cycles in the G2/M phase. Since we detected the cdtB subunit in the bacterial DNA and did not find any other DNA determinants coding known genotoxins (usp or colibactin), we propose that the cell cycle arrest was caused by CDTs. Except of eae and cdtB determinants, genes encoding other virulence factors including aerobactin synthesis (aer) and aerobactin iron transport system (iucC), and fimbriae type I (fimA) were detected. In addition, the large invasive plasmid (detected by presence of ipaH), typical for all virulent Shigella and enteroinvasive E. coli (EIEC) isolates, was found.
The frequency of bacteriocin determinants in pathogenic E. coli isolates is higher compared to commensal E. coli [12, 18, 27, 30, 48, 51]. In our study, all four identified E. albertii were found to be bacteriocin double-producers (Table 1), encoding colicins B, D, E7, and M. Interestingly, E. albertii type strain CCM 7160T, which was also included in our bacteriocin detection assay, did not produce any tested antimicrobial substances. Production of colicin D is extremely rare in human E. coli strains. Occurrence of the colicin D determinant among extraintestinal pathogenic E. coli (ExPEC) isolates have revealed two colicin D producers among 407 examined strains (0.5%) [29] and similar analysis among fecal strains of E. coli only identified one colicin D determinant among 1,283 examined strains (0.08%) [29]. Similarly, frequency of E7 production in human E. coli was shown to be quite low (0.6–2.3%) [12, 27, 29]. On the other hand, colicins B and M are known to be encoded on large plasmids, which are present in many E. coli, including commensal (1.1–8.6%) [12, 28], uropathogenic (4.1–12.8%) [7, 49], and other ExPEC (5.0–11.7%) [27, 29]. However, the presence of rare colicin determinants among tested E. albertii isolates may correspond to the geographical differences between the tested E. albertii and E. coli strains [48].
Isolates P4652 and P4653, which came from the different seal feces collected on the same day at small beach nearby Lachman Cape, James Ross Island, Antarctica, shared the following four characteristics: (1) identical 16S rDNA sequences, (2) same sequences of six housekeeping genes (aspC, clpX, fadD, icdA, lysP and mdh), (3) identical ribotype, rep-PCR, and PFGE profiles, and (4) harbored same virulence factors and bacteriocin determinants (Table 1, Figs. 1 and 2). These facts imply that P4652 and P4653 are isolates of the same strain obtained from different faeces of one seal. Antarctic seal isolate P4740 had different ribotype, rep-PCR, and PFGE profiles compared to the P4652 and P4653 isolates. However, P4740 was shown to be phylogenetically related to the P4652 and P4653 isolates, since it possessed identical 16S rDNA sequences, identical sequences of six housekeeping genes, and the presence of identical bacteriocin determinants as the P4652 and P4653 isolates. On the other hand, isolate P5661 was found to be distantly related based on its 16S rDNA sequence, MLST data, ribotyping result, rep-PCR and PFGE profile as well as the presence of different virulence factors and bacteriocin determinants (Table 1 and Figs. 1 and 2). However, since P5661 was isolated from a penguin in Patagonia, the observed differences could simply reflect host and geographical differences.
Supplementary
Acknowledgments
The authors want to thank the staff of the J.G. Mendel Czech Antarctic Station for their assistance (part of the Czech Polar Research Infrastructure (CzechPolar2), supported by the Ministry of Education, Youth and Sports of the Czech Republic (LM2015078)). Additional support was provided by the Grant Agency of the Czech Republic to DS (16-21649S), by the National Sustainability Program of the Czech Ministry of Education, Youth and Sports (MEYS, CETOCOEN, LO1214) and by founds from the Faculty of Medicine, Masaryk University (ROZV/25/LF/2017) to junior researcher Juraj Bosák. We also thank to Thomas Secrest (Secrest Editing, Ltd.) for English editing of the manuscript.
REFERENCES
- 1.Albert M. J., Alam K., Islam M., Montanaro J., Rahaman A. S., Haider K., Hossain M. A., Kibriya A. K., Tzipori S.1991. Hafnia alvei, a probable cause of diarrhea in humans. Infect. Immun. 59: 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asoshima N., Matsuda M., Shigemura K., Honda M., Yoshida H., Oda T., Hiwaki H.2015. Isolation of Escherichia albertii from Raw Chicken Liver in Fukuoka City, Japan. Jpn. J. Infect. Dis. 68: 248–250. doi: 10.7883/yoken.JJID.2014.530 [DOI] [PubMed] [Google Scholar]
- 3.Asoshima N., Matsuda M., Shigemura K., Honda M., Yoshida H., Hiwaki H., Ogata K., Oda T.2014. Identification of Escherichia albertii as a causative agent of a food-borne outbreak occurred in 2003. Jpn. J. Infect. Dis. 67: 139–140. doi: 10.7883/yoken.67.139 [DOI] [PubMed] [Google Scholar]
- 4.Bezine E., Vignard J., Mirey G.2014. The cytolethal distending toxin effects on Mammalian cells: a DNA damage perspective. Cells 3: 592–615. doi: 10.3390/cells3020592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birosová E., Siegfried L., Kmet’ová M., Makara A., Ostró A., Gresová A., Urdzík P., Liptáková A., Molokácová M., Bártl R., Valanský L.2004. Detection of virulence factors in alpha-haemolytic Escherichia coli strains isolated from various clinical materials. Clin. Microbiol. Infect. 10: 569–573. doi: 10.1111/j.1469-0691.2004.00922.x [DOI] [PubMed] [Google Scholar]
- 6.Brandal L. T., Tunsjø H. S., Ranheim T. E., Løbersli I., Lange H., Wester A. L.2015. Shiga toxin 2a in Escherichia albertii. J. Clin. Microbiol. 53: 1454–1455. doi: 10.1128/JCM.03378-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budič M., Rijavec M., Petkovšek Z., Zgur-Bertok D.2011. Escherichia coli bacteriocins: antimicrobial efficacy and prevalence among isolates from patients with bacteraemia. PLoS One 6: e28769. doi: 10.1371/journal.pone.0028769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comayras C., Tasca C., Pérès S. Y., Ducommun B., Oswald E., De Rycke J.1997. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect. Immun. 65: 5088–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felföldi T., Heéger Z., Vargha M., Márialigeti K.2010. Detection of potentially pathogenic bacteria in the drinking water distribution system of a hospital in Hungary. Clin. Microbiol. Infect. 16: 89–92. doi: 10.1111/j.1469-0691.2009.02795.x [DOI] [PubMed] [Google Scholar]
- 10.Fiedoruk K., Daniluk T., Swiecicka I., Murawska E., Sciepuk M., Leszczynska K.2014. First Complete Genome Sequence of Escherichia albertii Strain KF1, a New Potential Human Enteric Pathogen. Genome Announc. 2: 2. doi: 10.1128/genomeA.00004-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillor O., Giladi I., Riley M. A.2009. Persistence of colicinogenic Escherichia coli in the mouse gastrointestinal tract. BMC Microbiol. 9: 165. doi: 10.1186/1471-2180-9-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon D. M., O’Brien C. L.2006. Bacteriocin diversity and the frequency of multiple bacteriocin production in Escherichia coli. Microbiology 152: 3239–3244. doi: 10.1099/mic.0.28690-0 [DOI] [PubMed] [Google Scholar]
- 13.Hinenoya A., Yasuda N., Hibino T., Shima A., Nagita A., Tsukamoto T., Yamasaki S.2017. Isolation and Characterization of an Escherichia albertii Strain Producing Three Different Toxins from a Child with Diarrhea. Jpn. J. Infect. Dis. 70: 252–257. doi: 10.7883/yoken.JJID.2016.186 [DOI] [PubMed] [Google Scholar]
- 14.Hinenoya A., Shima K., Asakura M., Nishimura K., Tsukamoto T., Ooka T., Hayashi T., Ramamurthy T., Faruque S. M., Yamasaki S.2014. Molecular characterization of cytolethal distending toxin gene-positive Escherichia coli from healthy cattle and swine in Nara, Japan. BMC Microbiol. 14: 97. doi: 10.1186/1471-2180-14-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huys G., Cnockaert M., Janda J. M., Swings J.2003. Escherichia albertii sp. nov., a diarrhoeagenic species isolated from stool specimens of Bangladeshi children. Int. J. Syst. Evol. Microbiol. 53: 807–810. doi: 10.1099/ijs.0.02475-0 [DOI] [PubMed] [Google Scholar]
- 16.Hyma K. E., Lacher D. W., Nelson A. M., Bumbaugh A. C., Janda J. M., Strockbine N. A., Young V. B., Whittam T. S.2005. Evolutionary genetics of a new pathogenic Escherichia species: Escherichia albertii and related Shigella boydii strains. J. Bacteriol. 187: 619–628. doi: 10.1128/JB.187.2.619-628.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inglis T. J. J., Merritt A. J., Bzdyl N., Lansley S., Urosevic M. N.2015. First bacteraemic human infection with Escherichia albertii. New Microbes New Infect. 8: 171–173. doi: 10.1016/j.nmni.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohoutová D., Šmajs D., Morávková P., Cyrany J., Morávková M., Forstlová M., Cihák M., Rejchrt S., Bureš J.2014. Escherichia coli strains of phylogenetic group B2 and D and bacteriocin production are associated with advanced colorectal neoplasia. BMC Infect. Dis. 14: 733. doi: 10.1186/s12879-014-0733-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhnert P., Hacker J., Mühldorfer I., Burnens A. P., Nicolet J., Frey J.1997. Detection system for Escherichia coli-specific virulence genes: absence of virulence determinants in B and C strains. Appl. Environ. Microbiol. 63: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Ragione R. M., McLaren I. M., Foster G., Cooley W. A., Woodward M. J.2002. Phenotypic and genotypic characterization of avian Escherichia coli O86:K61 isolates possessing a gamma-like intimin. Appl. Environ. Microbiol. 68: 4932–4942. doi: 10.1128/AEM.68.10.4932-4942.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsey R. L., Fedorka-Cray P. J., Abley M., Turpin J. B., Meinersmann R. J.2015. Evaluating the occurrence of Escherichia albertii in chicken carcass rinses by PCR, Vitek analysis, and sequencing of the rpoB gene. Appl. Environ. Microbiol. 81: 1727–1734. doi: 10.1128/AEM.03681-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Saucedo C., Cerna J. F., Villegas-Sepulveda N., Thompson R., Velazquez F. R., Torres J., Tarr P. I., Estrada-García T.2003. Single multiplex polymerase chain reaction to detect diverse loci associated with diarrheagenic Escherichia coli. Emerg. Infect. Dis. 9: 127–131. doi: 10.3201/eid0901.010507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo C., Walk S. T., Gordon D. M., Feldgarden M., Tiedje J. M., Konstantinidis K. T.2011. Genome sequencing of environmental Escherichia coli expands understanding of the ecology and speciation of the model bacterial species. Proc. Natl. Acad. Sci. U.S.A. 108: 7200–7205. doi: 10.1073/pnas.1015622108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda E., Murakami K., Sera N., Ito K., Fujimoto S.2015. Detection of Escherichia albertii from chicken meat and giblets. J. Vet. Med. Sci. 77: 871–873. doi: 10.1292/jvms.14-0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maheux A. F., Boudreau D. K., Bergeron M. G., Rodriguez M. J.2014. Characterization of Escherichia fergusonii and Escherichia albertii isolated from water. J. Appl. Microbiol. 117: 597–609. doi: 10.1111/jam.12551 [DOI] [PubMed] [Google Scholar]
- 26.Martínez J. L., Herrero M., de Lorenzo V.1994. The organization of intercistronic regions of the aerobactin operon of pColV-K30 may account for the differential expression of the iucABCD iutA genes. J. Mol. Biol. 238: 288–293. doi: 10.1006/jmbi.1994.1290 [DOI] [PubMed] [Google Scholar]
- 27.Micenková L., Bosák J., Vrba M., Ševčíková A., Šmajs D.2016. Human extraintestinal pathogenic Escherichia coli strains differ in prevalence of virulence factors, phylogroups, and bacteriocin determinants. BMC Microbiol. 16: 218. doi: 10.1186/s12866-016-0835-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micenková L., Beňová A., Frankovičová L., Bosák J., Vrba M., Ševčíková A., Kmeťová M., Šmajs D.2017. Human Escherichia coli isolates from hemocultures: Septicemia linked to urogenital tract infections is caused by isolates harboring more virulence genes than bacteraemia linked to other conditions. Int. J. Med. Microbiol. 307: 182–189. doi: 10.1016/j.ijmm.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 29.Micenková L., Štaudová B., Bosák J., Mikalová L., Littnerová S., Vrba M., Ševčíková A., Woznicová V., Šmajs D.2014. Bacteriocin-encoding genes and ExPEC virulence determinants are associated in human fecal Escherichia coli strains. BMC Microbiol. 14: 109. doi: 10.1186/1471-2180-14-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micenková L., Bosák J., Štaudová B., Kohoutová D., Čejková D., Woznicová V., Vrba M., Ševčíková A., Bureš J., Šmajs D.2016. Microcin determinants are associated with B2 phylogroup of human fecal Escherichia coli isolates. MicrobiologyOpen 5: 490–498. doi: 10.1002/mbo3.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morato E. P., Leomil L., Beutin L., Krause G., Moura R. A., Pestana de Castro A. F.2009. Domestic cats constitute a natural reservoir of human enteropathogenic Escherichia coli types. Zoonoses Public Health 56: 229–237. doi: 10.1111/j.1863-2378.2008.01190.x [DOI] [PubMed] [Google Scholar]
- 32.Nimri L. F.2013. Escherichia albertii, a newly emerging enteric pathogen with poorly defined properties. Diagn. Microbiol. Infect. Dis. 77: 91–95. doi: 10.1016/j.diagmicrobio.2013.06.028 [DOI] [PubMed] [Google Scholar]
- 33.Oaks J. L., Besser T. E., Walk S. T., Gordon D. M., Beckmen K. B., Burek K. A., Haldorson G. J., Bradway D. S., Ouellette L., Rurangirwa F. R., Davis M. A., Dobbin G., Whittam T. S.2010. Escherichia albertii in wild and domestic birds. Emerg. Infect. Dis. 16: 638–646. doi: 10.3201/eid1604.090695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh J. Y., Kang M. S., Hwang H. T., An B. K., Kwon J. H., Kwon Y. K.2011. Epidemiological investigation of eaeA-positive Escherichia coli and Escherichia albertii strains isolated from healthy wild birds. J. Microbiol. 49: 747–752. doi: 10.1007/s12275-011-1133-y [DOI] [PubMed] [Google Scholar]
- 35.Ooka T., Tokuoka E., Furukawa M., Nagamura T., Ogura Y., Arisawa K., Harada S., Hayashi T.2013. Human gastroenteritis outbreak associated with Escherichia albertii, Japan. Emerg. Infect. Dis. 19: 144–146. doi: 10.3201/eid1901.120646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ooka T., Seto K., Kawano K., Kobayashi H., Etoh Y., Ichihara S., Kaneko A., Isobe J., Yamaguchi K., Horikawa K., Gomes T. A. T., Linden A., Bardiau M., Mainil J. G., Beutin L., Ogura Y., Hayashi T.2012. Clinical significance of Escherichia albertii. Emerg. Infect. Dis. 18: 488–492. doi: 10.3201/eid1803.111401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ooka T., Ogura Y., Katsura K., Seto K., Kobayashi H., Kawano K., Tokuoka E., Furukawa M., Harada S., Yoshino S., Seto J., Ikeda T., Yamaguchi K., Murase K., Gotoh Y., Imuta N., Nishi J., Gomes T. A., Beutin L., Hayashi T.2015. Defining the Genome Features of Escherichia albertii, an Emerging Enteropathogen Closely Related to Escherichia coli. Genome Biol. Evol. 7: 3170–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paciorek J.2002. Virulence properties of Escherichia coli faecal strains isolated in Poland from healthy children and strains belonging to serogroups O18, O26, O44, O86, O126 and O127 isolated from children with diarrhoea. J. Med. Microbiol. 51: 548–556. doi: 10.1099/0022-1317-51-7-548 [DOI] [PubMed] [Google Scholar]
- 39.Paton A. W., Paton J. C.1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36: 598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickett C. L., Cottle D. L., Pesci E. C., Bikah G.1994. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect. Immun. 62: 1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pontes D. S., Pinheiro F. A., Lima-Bittencourt C. I., Guedes R. L. M., Cursino L., Barbosa F., Santos F. R., Chartone-Souza E., Nascimento A. M. A.2009. Multiple antimicrobial resistance of gram-negative bacteria from natural oligotrophic lakes under distinct anthropogenic influence in a tropical region. Microb. Ecol. 58: 762–772. doi: 10.1007/s00248-009-9539-3 [DOI] [PubMed] [Google Scholar]
- 42.Rahman M. Z., Akter S., Azmuda N., Sultana M., Weill F. X., Khan S. I., Grimont P. A. D., Birkeland N. K.2013. Serological cross-reaction between O-antigens of Shigella dysenteriae type 4 and an environmental Escherichia albertii isolate. Curr. Microbiol. 67: 590–595. doi: 10.1007/s00284-013-0405-7 [DOI] [PubMed] [Google Scholar]
- 43.Schmidt H., Knop C., Franke S., Aleksic S., Heesemann J., Karch H.1995. Development of PCR for screening of enteroaggregative Escherichia coli. J. Clin. Microbiol. 33: 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sedláček I., Grillová L., Kroupová E., Černohlávková J., Šmajs D.2013. Escherichia albertii from feces of seals (Leptonychotes weddelli) in James Ross Island, Antarctica. Czech Polar Reports 3: 173–183. doi: 10.5817/CPR2013-2-18 [DOI] [Google Scholar]
- 45.Sharma M., Kniel K. E., Derevianko A., Ling J., Bhagwat A. A.2007. Sensitivity of Escherichia albertii, a potential food-borne pathogen, to food preservation treatments. Appl. Environ. Microbiol. 73: 4351–4353. doi: 10.1128/AEM.03001-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shima A., Hinenoya A., Asakura M., Sugimoto N., Tsukamoto T., Ito H., Nagita A., Faruque S. M., Yamasaki S.2012. Molecular characterizations of cytolethal distending toxin produced by Providencia alcalifaciens strains isolated from patients with diarrhea. Infect. Immun. 80: 1323–1332. doi: 10.1128/IAI.05831-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slaninová I., Brezinová L., Koubíková L., Slanina J.2009. Dibenzocyclooctadiene lignans overcome drug resistance in lung cancer cells--study of structure-activity relationship. Toxicol. In Vitro 23: 1047–1054. doi: 10.1016/j.tiv.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 48.Šmajs D., Cejková D., Micenková L., Lima-Bittencourt C. I., Chartone-Souza E., Šmarda J., Nascimento A. M. A.2012. Human Escherichia coli strains of different geographical and time source: bacteriocin types and their gene sequences are population-specific. Environ. Microbiol. Rep. 4: 459–466. doi: 10.1111/j.1758-2229.2012.00365.x [DOI] [PubMed] [Google Scholar]
- 49.Šmajs D., Micenková L., Šmarda J., Vrba M., Sevčíková A., Vališová Z., Woznicová V.2010. Bacteriocin synthesis in uropathogenic and commensal Escherichia coli: colicin E1 is a potential virulence factor. BMC Microbiol. 10: 288. doi: 10.1186/1471-2180-10-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Šmajs D., Strouhal M., Matejková P., Cejková D., Cursino L., Chartone-Souza E., Šmarda J., Nascimento A. M. A.2008. Complete sequence of low-copy-number plasmid MccC7-H22 of probiotic Escherichia coli H22 and the prevalence of mcc genes among human E. coli. Plasmid 59: 1–10. doi: 10.1016/j.plasmid.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 51.Štaudová B., Micenková L., Bosák J., Hrazdilová K., Slaninková E., Vrba M., Ševčíková A., Kohoutová D., Woznicová V., Bureš J., Šmajs D.2015. Determinants encoding fimbriae type 1 in fecal Escherichia coli are associated with increased frequency of bacteriocinogeny. BMC Microbiol. 15: 201. doi: 10.1186/s12866-015-0530-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stock I., Rahman M., Sherwood K. J., Wiedemann B.2005. Natural antimicrobial susceptibility patterns and biochemical identification of Escherichia albertii and Hafnia alvei strains. Diagn. Microbiol. Infect. Dis. 51: 151–163. doi: 10.1016/j.diagmicrobio.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 53.Švec P., Nováková D., Zácková L., Kukletová M., Sedlácek I.2008. Evaluation of (GTG)5-PCR for rapid identification of Streptococcus mutans. Antonie van Leeuwenhoek 94: 573–579. doi: 10.1007/s10482-008-9275-6 [DOI] [PubMed] [Google Scholar]
- 54.Švec P., Černohlávková J., Busse H. J., Vojtková H., Pantůček R., Cnockaert M., Mašlaňová I., Králová S., Vandamme P., Sedláček I.2015. Classification of strain CCM 4446T as Rhodococcus degradans sp. nov. Int. J. Syst. Evol. Microbiol. 65: 4381–4387. doi: 10.1099/ijsem.0.000584 [DOI] [PubMed] [Google Scholar]
- 55.Valentiner-Branth P., Steinsland H., Fischer T. K., Perch M., Scheutz F., Dias F., Aaby P., Mølbak K., Sommerfelt H.2003. Cohort study of Guinean children: incidence, pathogenicity, conferred protection, and attributable risk for enteropathogens during the first 2 years of life. J. Clin. Microbiol. 41: 4238–4245. doi: 10.1128/JCM.41.9.4238-4245.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walk S. T., Alm E. W., Gordon D. M., Ram J. L., Toranzos G. A., Tiedje J. M., Whittam T. S.2009. Cryptic lineages of the genus Escherichia. Appl. Environ. Microbiol. 75: 6534–6544. doi: 10.1128/AEM.01262-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H., Li Q., Bai X., Xu Y., Zhao A., Sun H., Deng J., Xiao B., Liu X., Sun S., Zhou Y., Wang B., Fan Z., Chen X., Zhang Z., Xu J., Xiong Y.2016. Prevalence of eae-positive, lactose non-fermenting Escherichia albertii from retail raw meat in China. Epidemiol. Infect. 144: 45–52. doi: 10.1017/S0950268815001120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto D., Hernandes R. T., Liberatore A. M. A., Abe C. M., Souza R. B., Romão F. T., Sperandio V., Koh I. H., Gomes T. A. T.2017. Escherichia albertii, a novel human enteropathogen, colonizes rat enterocytes and translocates to extra-intestinal sites. PLOS ONE 12: e0171385. doi: 10.1371/journal.pone.0171385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto S., Terai A., Yuri K., Kurazono H., Takeda Y., Yoshida O.1995. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 12: 85–90. doi: 10.1111/j.1574-695X.1995.tb00179.x [DOI] [PubMed] [Google Scholar]
- 60.Young V. B., Knox K. A., Schauer D. B.2000. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect. Immun. 68: 184–191. doi: 10.1128/IAI.68.1.184-191.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.