Abstract
Pulmonary complications are a significant cause of morbidity, mortality, and resource utilization after hematopoietic stem cell transplantation (HSCT). The objective of this study was to compare antemortem clinical suspicion of pulmonary complications and postmortem findings in a modern HSCT cohort. All patients who underwent allogeneic HSCT at our institution (n = 1854) between January 1, 2000 and June 30, 2010 were reviewed and patients who died of any cause greater than 1 year after HSCT and had an unrestricted autopsy available for analysis were included. Presence of pulmonary graft-versus-host disease (GVHD) was assessed by a pathologist blinded to the autopsy report, as previously described by Yousem (1995). A total of 35 (1.9%) patients had autopsies available for review. Airway disease, vascular disease, and interstitial disease were all clinically under-recognized compared with the pathological findings on autopsy. Varying degrees of pathological changes were detected, including 10 (28.6%) patients having bronchiolitis obliterans (BO) and 12 (34.3%) patients having pulmonary veno-occlusive disease (PVOD). Pulmonary manifestations of chronic GVHD, particularly BO and PVOD, were clinically under-recognized in our cohort. Our results suggest that PVOD, which has traditionally been considered a rare complication, may be clinically and histologically under-recognized.
Keywords: Autopsy, Hematopoietic stem cell, transplantation, Graft-versus-host disease
INTRODUCTION
Pulmonary complications after hematopoietic stem cell transplantation (HSCT) are a major cause of morbidity, mortality, and resource utilization [1]. They are estimated to occur in nearly one-half of patients and account for approximately 50% of transplantation-related deaths and diminished quality of life in those who survive [2].
There is a wide spectrum of pulmonary complications associated with HSCT and the etiologies are believed to be multifactorial, including pretransplantation chemo radiation, infection, and graft-versus-host disease (GVHD) [3]. Late-onset clinical syndromes affecting lungs in patients after HSCT also include bronchiolitis obliterans syndrome (BOS), cryptogenic organizing pneumonia (COP), and pulmonary veno-occlusive disease (PVOD) [4,5]. The histological spectrum of pulmonary GVHD was described by Yousem [6] and includes manifestations in the airways, vasculature, and interstitium. Airway features include lymphocytic bronchitis/bronchiolitis (LB), lymphocytic bronchitis/bronchiolitis with intraluminal granulation tissue, and cicatricial bronchiolitis obliterans (BO) [6]. Vasculature manifestations include veno-occlusive disease [6]. Pathological changes within the interstitium include cellular interstitial pneumonitis (CIP), airspace granulation tissue/organizing pneumonia (OP), and patchy interstitial fibrosis (IF) [6].
Among these, only BOS/BO is currently considered diagnostic of chronic GVHD, according to the 2005/2014 National Institutes of Health (NIH) consensus criteria [5,7].
The objective of this study was to compare antemortem clinical suspicion of pulmonary complications with postmortem findings in a retrospective autopsy cohort of patients status post HSCT who survived more than 1 year after transplantation.
METHODS
All patients who underwent allogeneic HSCT at our institution (n = 1854) between January 1, 2000 and June 30, 2010 and survived more than 1 year were reviewed for autopsies available for analysis. Histological findings were assessed by a pathologist blinded to the autopsy report, according to the categories of injury previously described by Yousem [6] as present or absent. Acute and chronic GVHD were graded by consensus grading criteria [8,9]. Lymphocytic infiltration and structural remodeling were scored separately for the airways, vasculature, and interstitium. International Society of Heart Lung Transplant criteria were used for grading venopathy [10,11]. Patients with extensive lung involvement by infection, malignancy, or other defined process (ie, aspiration injury) were excluded from analysis.
Clinical variables were collected prospectively as part of the usual care and stored in a centralized data repository. Premortem diagnosis of BOS, PVOD, and interstitial disease was based on treating physician’s clinical interpretation and diagnosis based on medical record review. Additional clinical variables not stored in the centralized data repository were collected retrospectively by review of the electronic medical record.
This study (protocol 09-316) was approved by the institutional review board at Dana Farber/Harvard Cancer Center, Boston, MA.
Statistical Analysis
Data are presented as median and interquartile range for continuous variable and as percent for categorical variables. Categorical and continuous variables were compared between groups using 2-sided Fisher’s exact test and 2-sided Wilcoxon rank-sum test, respectively. The main analysis was performed using logistic regression where the outcome was airway disease, vascular disease, or interstitial disease. We first performed univariate logistic analysis with the following variables: patient age, gender, race, pretransplantation forced expiratory volume in 1 second as percent, pretransplantation carbon monoxide diffusion capacity as percent, patient cytomegalovirus (CMV) status at transplantation, high-risk disease, peripheral blood stem cell source, myeloablative conditioning regimen, busulfan-based conditioning regimen, presence of acute GVHD, presence of chronic GVHD, donor age, gender, donor-recipient gender mismatch, donor CMV status, related donor, and matched donor. A multivariable model was built using forward selection based upon their statistical significance (P < .05) on univariate analysis. All statistical analyses were performed using SAS9.3.
RESULTS
A total of 37 (2%) patients were noted to have an autopsy with lung specimens available for analysis. Two of these patients were noted to have diffuse parenchymal involvement by infection and were excluded, leaving a sample size of 35 for analysis. The median age of the cohort was 51 (42; 57) and the subjects survived for a median of 610 days (452; 892) after transplantation. Pulmonary complications were felt to contribute to death in 17 (49%) cases, and 32 (91%) of the patients had a diagnosis of chronic GVHD affecting organ systems other than the lungs before death (Table 1 and Table 2).
Table 1.
Underlying Disease at Time of Transplantation, Cause of Death, and Days after Transplantation of the Autopsy Cohort
| Subject | Disease | Cause of Death as Determined by Autopsy | Days after Transplantation at Time of Death |
|---|---|---|---|
| 1 | AML | Hepatic failure secondary to probable VOD and a minor component of GVHD | 438 |
| 2 | MDS | Myocardial infarction and a cerebrovascular accident most likely as a result of thromboembolic events in the setting of myelodysplasia | 497 |
| 3 | CML | Renal and hepatic insufficiency in the setting of high-output congestive heart failure and diffuse serosanguinous effusions | 836 |
| 4 | CML | Pneumococcus sepsis | 929 |
| 5 | AML | Respiratory failure associated with marked DAD and superimposed acute bronchopneumonia | 140 |
| 6 | NHL | Sepsis in the setting of infective endocarditis, complicated by a large ring abscess, with myocardial seeding from septic emboli, ultimately ending in compromise of cardiac and respiratory function | 2801 |
| 7 | AML | Multiorgan system failure, including early DAD, renal failure, and centrilobular vascular congestion of the liver and hepatocyte dropout, that developed in the setting of relapse/persistence of AML, mild-to- moderate GVHD, and VOD with centrilobular venous congestion of the liver | 391 |
| 8 | CML | Candida albicans fungal pneumonia with superimposed Pseudomonas aeruginosa infection, complicated by acute renal failure and liver dysfunction | 2667 |
| 9 | ALL | Bronchopneumonia and sepsis in the setting of documented anaplasmosis | 2666 |
| 10 | CML | Progressive respiratory failure secondary to bilateral, mild acute and chronic GVHD with focal areas of airway obliteration consistent with BO with organizing pneumonia and organizing DAD | 1433 |
| 11 | CLL | Acute, bilateral uncal, and cerebellar tonsil herniation in the setting of diffuse cerebral edema after complications arising from hemorrhagic pancreatitis in the setting CLL status post bone marrow transplantation | 603 |
| 12 | NHL | Acute myocardial infarction in the setting of coronary artery disease secondary to chronic GVHD | 598 |
| 13 | CML | Respiratory failure with DAD and acute liver failure in the setting of chronic GVHD | 638 |
| 14 | NHL | Bilateral bronchopneumonia complicated by pleural fluid accumulation, multifocal healing myocardial microinfarcts, renal failure, and hemorrhagic colitis leading to multiorgan failure | 3454 |
| 15 | AML | DAD compounded by bacteremia and renal failure | 136 |
| 16 | HD | Acute and organizing DAD arising in the setting of extensive relapse of HD | 374 |
| 17 | NHL | Organizing DAD with focal acute pneumonitis in the setting of lung and liver GVHD | 686 |
| 18 | NHL | Hypoxemic respiratory failure due to bilateral organizing pneumonia with early organizing DAD | 610 |
| 19 | CLL | VOD in the setting of myeloablative stem cell transplantation for CLL | 666 |
| 20 | CLL | Bilateral acute bronchopneumonia | 700 |
| 21 | MDS | GVHD and organizing DAD, died in cardiorespiratory failure | 452 |
| 22 | HD | Extensive bilateral pulmonary emboli from a left femoral vein thrombosis, associated with severe acute bilateral bronchopneumonia superimposed on infarcted lung parenchyma | 1379 |
| 23 | CML | Progressive respiratory failure (clinical) secondary to severe bilateral upper lobe emphysema (COPD), pulmonary infarction associated with invasive zygomycosis, and pulmonary hypertension | 602 |
| 24 | ALL | Respiratory failure in the setting of acute and organizing DAD, subacute pulmonary embolism, and superimposed fungal infection | 760 |
| 25 | CLL | Liver failure due to GVHD, with polymicrobial sepsis as a contributing factor | 509 |
| 26 | CML | Acute respiratory distress syndrome with DAD and progressive respiratory failure | 396 |
| 27 | MDS | E. coli sepsis of gastrointestinal origin secondary to involvement of the bowel by PTLD | 705 |
| 28 | AML | Klebsiella pneumonia sepsis and pneumonia | 432 |
| 29 | MDS | Sepsis, suspect E. coli as etiology, with ischemic end-organ damage, including bowel | 586 |
| 30 | AML | Acute respiratory distress syndrome due to DAD and organizing pneumonia | 485 |
| 31 | NHL | Hypoxemic respiratory failure due to adenoviral pneumonia and DAD/diffuse alveolar hemorrhage | 892 |
| 32 | AML | Staphylococcus aureus bronchopneumonia with DAD in the setting of PVOD | 486 |
| 33 | MPD | Gram-negative bacteremia and sepsis, likely arising from a cecal ulcer, consistent with typhlitis, and secondary bronchopneumonia | 794 |
| 34 | MDS | Bilateral DAD and focal hemorrhage, left main pulmonary artery thromboembolism, left upper lobe hemorrhagic infarct with smaller thromboemboli and bilateral pleural effusions in the setting of MDS status post bone marrow transplantation complicated by chronic mucocutaneous GVHD | 377 |
| 35 | AML | Multiorgan involvement by AML (which included extensive pulmonary leukemic infiltrates), focal bronchopneumonia in the setting of emphysematous changes in the lung, and adrenal cortical hemorrhage consistent with Waterhouse-Friderichsen syndrome | 449 |
AML indicates acute myeloid leukemia; VOD, veno-occlusive disease; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; DAD, diffuse alveolar damage; NHL, non-Hodgkin lymphoma; ALL, acute lymphocytic leukemia; CLL, chronic lymphocytic leukemia; HD, Hodgkin disease; COPD, chronic obstructive pulmonary disease; PTLD, post-transplantation lymphoproliferative disease; MPD, myeloproliferative disease.
Table 2.
Clinical Characteristics of Autopsy Cohort
| Characteristics | All Patients N = 35 |
Airway Disease n = 23 (66%) |
Venopathy n = 14 (40%) |
Interstitial Disease n = 13 (37%) |
|---|---|---|---|---|
| Age, yr | 51 (42–57) | 49 (57–38) | 47 (38–56) | 56 (51–57)* |
| Male gender | 21 (60%) | 15 (65%) | 10 (71%) | 8 (62%) |
| White race | 32 (91%) | 2/0 (87%) | 14 (100%) | 12 (92%) |
| Days after Transplantation | 610 (452–892) | 686 (596–929) | 606 (438–929) | 610 (485–929) |
| Pretransplantation FEV1% | 97 (84–109) | 96 (83–105) | 102 (95–109) | 99 (94–109) |
| Pretransplantation DLCO% | 82 (70–101) | 81 (71–100) | 89 (71–106) | 74 (70–106) |
| CMV positive | 16 (46%) | 15 (65%)* | 6 (43%) | 8 (62%) |
| High-risk disease† | 21 (60%) | 16 (70%) | 11 (79%) | 8 (62%) |
| Cell source | ||||
| PBSC | 26 (74%) | 16 (70%) | 9 (64%) | 11 (85%) |
| Conditioning | ||||
| Myeloablative‡ | 20 (57%) | 15 (66%) | 12 (86%)* | 7 (54%) |
| Busulfan based | 14 (40%) | 7 (30%) | 2 (14%)* | 6 (46%) |
| GVHD | ||||
| Acute GVHD§ | 19 (54%) | 14 (61%) | 11 (79%)* | 7 (54%) |
| Chronic GVHD§ | 32 (91%) | 23 (100%)* | 14 (100%) | 12 (92%) |
| Donor | ||||
| Age, yr | 37 (29–43) | 39 (29–45) | 41 (33–45) | 39 (33–43) |
| Donor male gender | 21 (60%) | 13 (57%) | 6 (43%) | 4 (31%)* |
| Gender mismatch | 10 (29%) | 8 (35)%) | 7 (50)% | 6 (46%) |
| Related | 7 (20%) | 4 (17%) | 4 (29%) | 4 (31%) |
| Matched | 31 (89%) | 19 (83%) | 14 (100%) | 11 (85%) |
| Donor CMV positive | 10 (29%) | 7 (30%) | 4 (29%) | 4 (31%) |
FEV1 indicates forced expiratory volume in 1 second; DLCO, carbon monoxide diffusion capacity; PBSC, peripheral blood stem cell.
P < .05.
All diseases other than CML first chronic phase, AML/ALL in first complete remission MDS refractory anemia with ringed sideroblasts, or aplastic anemia are considered high risk in this analysis.
Myeloablative conditioning regimens: cyclophosphamide + total body irradiation 1400 cGY; high-dose busulfan cyclophosphamide.
A total of 23 (66%) patients had evidence of airway disease, 14 (40%) subjects had venopathy, and 13 (37%) patients had disease of the interstitium. Of these, only 8 (34.8%), 1 (7.1%), and 5 (38.5%), respectively, were suspected before death.
Airway Disease
Of the 35 patients, 23 (66%) had evidence of airway disease (Figure 1), LB was present in 17 (48.6%), LB/bronchiolitis with intraluminal granulation tissue was found in 5 (14.3%), and BO was identified in 10 (28.6%) (Table 3). A total of 20.4% (7 of 23) of the subjects with airway disease had more than 1 manifestation of airway disease present at the time of autopsy. A total of 8.7% (2 of 23) patients had evidence of all 3 manifestations of airway disease present at the time of autopsy. Multivariate analysis revealed that CMV-positive status (P < .02) and presence of chronic GVHD (P < .01) were both independent factors associated with the development of airway disease.
Figure 1.
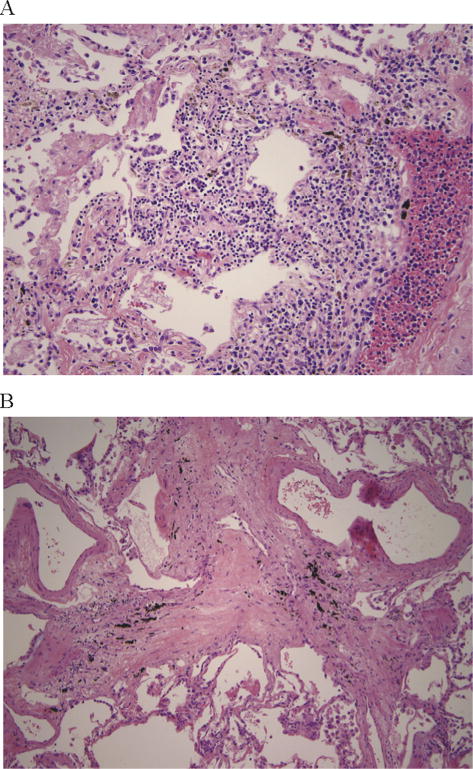
Airways disease. (A) Shows lymphocytic bronchitis/bronchiolitis and (B) shows bronchiolitis obliterans.
Table 3.
Autopsy Findings
| Autopsy Findings: Pathological Findings | |
|---|---|
| Airway disease, n = 23 (66%) | |
| LB | 17 of 35 (48.6%) |
| Lymphocytic bronchitis/bronchiolitis with intraluminal granulation tissue | 5 of 35 (14.3%) |
| BO | 10 of 35 (28.6%) |
| Pulmonary venous disease, n = 14 (40%) | |
| Perivascular lymphoplasmacytic infiltrates | 3 of 35 (8.6%) |
| Septal obliteration (PVOD) | 12 of 35 (34.3%) |
| Interstitial disease, n = 13 (37%) | |
| CIP | 6 of 35 (17.1%) |
| OP | 6 of 35 (17.1%) |
| IF | 8 of 35 (22.9%) |
Venopathy
Fourteen (40%) patients had venopathy (Figure 2), perivascular lymphoplasmacytic infiltrates were identified in 8.6% (3 of 35), and PVOD was identified in 34.3% (12 of 35) of the subjects (Table 3). Multivariate analysis revealed that myeloablative conditioning (P < .01) and presence of acute GHVD (P < .04) were independent factors associated with the development of venopathy. There was also a trend towards an association with airway disease and pulmonary venous disease (P = .07).
Figure 2.
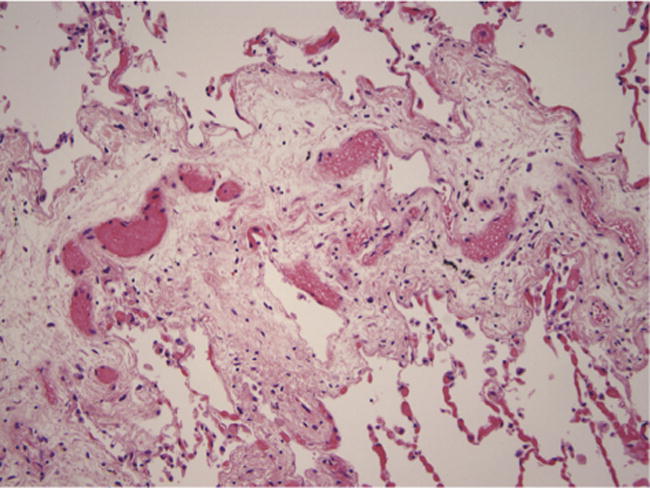
Pulmonary venous disease. Septal edema and narrowing of septal veins by myxoid connective tissue.
Disease of the Interstitium
Thirteen (37%) patients had disease of the interstitium (Figure 3). CIP was found in 17.1% (6 of 35), OP in 17.1% (6 of 35), and IF in 22.9% (8 of 35). Multivariate analysis revealed only female donors remained as an independent factor associated with the development of disease of the interstitium (P < .01).
Figure 3.
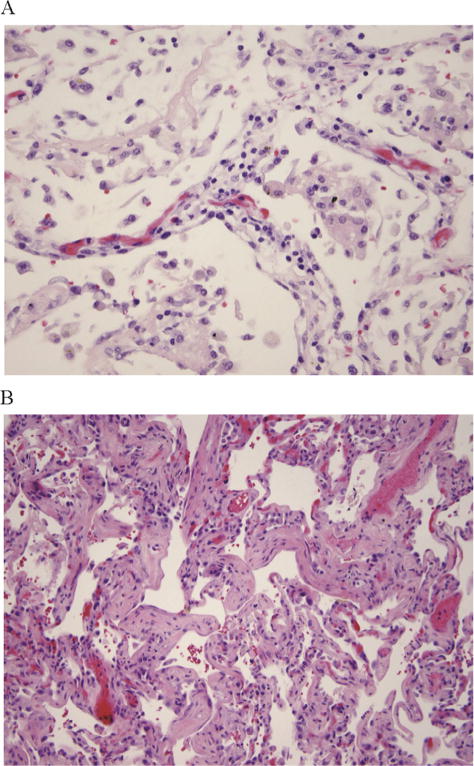
Disease of the interstitium. (A) Shows cellular interstitial pneumonitis and (B) shows interstitial fibrosi.
Upon univariate analysis the presence of OP was associated with BO (P < .05). Upon univariate analysis the presence of CIP was associated with IF (P < .02).
DISCUSSION
Pulmonary complications and respiratory failure after HSCT are a major cause of post-transplantation morbidity, mortality, and resource utilization [1,12]. We sought to characterize the pathologic pulmonary findings in patients who survived more than 1 year after allogeneic HSCT. We found that airway disease, vascular disease, and interstitial disease were all clinically under-recognized in our cohort.
BOS is a form of irreversible airflow obstruction and is a late noninfectious pulmonary complication after HSCT that is associated with a significant increase in morbidity and mortality [12–15].
Yousem described GVHD of the airways to follow a spectrum from LB to BO. He speculated that LB represents an early manifestation of airway disease and may be amenable to therapy before the onset of fibrosis and BO [6]. This theory is supported by the findings of Holbro et al. [16], who demonstrated that patients with LB were more likely to respond to therapy and had a significant improvement in overall survival compared with patients with constrictive BO. Our results also suggest that airway disease is a spectrum, all 3 forms of airway disease can coexist in the same subject and, airway disease is clinically under-recognized in our HSCT cohort. Our study was performed before the initiation of a standard screening protocol at our institution. We previously published the prevalence of BOS in this cohort to be 4.8% [17]. Previous estimates of the prevalence of BOS range from 2% to 30% and up to 30% of patients are asymptomatic at the time of meeting NIH criteria [13–15,18–22]. In the absence of routine spirometric screening, reports of disease prevalence likely underestimate the true burden of BOS, since symptomatic patients are typically already suffering from moderate-to-severe airflow obstruction [19]. Kuzmina et al. found the cumulative incidence of BOS to be 27% at 3 years using a strict 3-month screening protocol, suggesting we may be vastly underestimating the true prevalence of BOS [21]. Within our autopsy series, only 34.3% (12 of 35) subjects had any post-transplantation pulmonary function test performed. Thus, our results suggest that airway disease and BO may have been clinically under-recognized in our previously published retrospective cohort [17].
The development of BOS has been found to be highly associated with the presence of chronic GVHD at other sites and is currently considered to be a form of chronic GVHD of the lungs, according to NIH consensus criteria [5,7,12–15,19,23–25]. Other risk factors reported to be associated with BOS include acute GVHD, busulfan-based conditioning regimens, use of methotrexate for GVHD prophylaxis, CMV, respiratory viral infections within the first 100 days after transplantation, peripheral blood stem cell source, history of pneumonitis, low immunoglobulin levels, and reduced values on pretransplantation pulmonary function tests [13,14,17–20,23–27]. Our results confirm that chronic GVHD and CMV status are associated with the development of airway disease.
PVOD is considered a rare complication of HSCT. PVOD and is pathologically identified by intimal proliferation and fibrosis of the pulmonary venules and small veins leading to progressive vascular obstructions. PVOD can lead to severe pulmonary arterial hypertension; radiographic evidence of pulmonary edema and a normal pulmonary artery wedge pressure are the clinical criteria for diagnosis of PVOD [28,29]. The etiology has been speculated to be the result of regimen-related toxicity or possibly related to the inflammatory nature of GVHD [29–32]. Our data suggests that both regimen-related toxicity and GVHD may play a role in the development of vasculopathy and PVOD. We demonstrated that myeloablative conditioning regimen was associated with PVOD. Cyclophosphamide/total body irradiation was the standard myeloablative regimen used in all patients in our cohort. This is consistent with prior reports suggesting an association between cyclophosphamide and PVOD [28,32]. A striking finding was that the chart review revealed that clinically only 7% (1 of 14) patients with PVOD were identified before death. This may suggest that PVOD is either clinically under-recognized or the pathological findings may vary in clinical significance.
COP is a disorder that involves the alveoli, alveolar ducts, and bronchioles, which become filled with buds of granulation tissue consisting of fibroblasts and an associated matrix of loose connective tissue [33]. Currently consensus diagnostic criteria are lacking for the diagnosis of COP after HSCT. The incidence of COP is estimated at 1% to 2% based upon single-center reports [34,35]. Our data suggests that OP is also under-recognized in our HSCT cohort. In addition we found an association between OP and BO, which is considered part of the NIH diagnostic criteria for BOS and may represent a manifestation of chronic GVHD [7]. However, OP often seen with BO may not reflect COP, which is often amenable to steroid treatment. Further study between the association of OP, BO, and COP is warranted.
Within our cohort, we also found an association between CIP and IF, suggesting a possible correlation. Pulmonary fibrosis after HSCT has been described and it has been postulated to be a manifestation of late toxicity of total body irradiation or chemotherapy or the result of interstitial pneumonitis secondary to infection or GVHD [36]. Similar to LB progressing to airway fibrosis and BOS, a similar process with CIP and the subsequent development of pulmonary fibrosis is suggested by our results.
Our data consist of a retrospective assessment of clinically acquired data. The premortem clinical diagnoses were at the discretion of the treating physicians and obtained from chart review. As such, it likely biased in nature to those patients who underwent clinical evaluation or an autopsy. In addition, diffuse alveolar damage was present to some degree in the majority of autopsy cases (15 of 35; 43%), often as a secondary result of the immediate cause of death and, thus, may confound our results. We made every effort to score these samples away from areas of diffuse alveolar damage, but the possibility remains that this process may lead to a bias towards overcalling pathologic findings. Finally, although the pathologic findings were present on autopsy, their clinical significance is not clear and the pathological findings may have been truly under-recognized or of an unclear clinical significance.
Given the limitations, our study suggests that pulmonary pathology of the airways, vasculature, and interstitium after HSCT may be clinically under-recognized and warrants further study.
Acknowledgments
The authors thank Qiheng Yang for her contributions to this study.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Financial disclosure statement: There is nothing to disclose.
References
- 1.Allareddy V, Roy A, Rampa S, Lee MK, Nalliah RP, Rotta AT. Outcomes of stem cell transplant patients with acute respiratory failure requiring mechanical ventilation in the United States. Bone Marrow Transplant. 2014;49:1278–1286. doi: 10.1038/bmt.2014.130. [DOI] [PubMed] [Google Scholar]
- 2.Yanik G, Kitko C. Management of noninfectious lung injury following hematopoietic cell transplantation. Curr Opin Oncol. 2013;25:187–194. doi: 10.1097/CCO.0b013e32835dc8a5. [DOI] [PubMed] [Google Scholar]
- 3.Soubani AO, Pandya CM. The spectrum of noninfectious pulmonary complications following hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther. 2010;3:143–157. doi: 10.1016/s1658-3876(10)50025-6. [DOI] [PubMed] [Google Scholar]
- 4.Uhlving HH, Andersen CB, Christensen IJ, et al. Biopsy-verified bronchiolitis obliterans and other noninfectious lung pathologies after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:531–538. doi: 10.1016/j.bbmt.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Shulman HM, Cardona DM, Greenson JK, et al. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transplant. 2015;21:589–603. doi: 10.1016/j.bbmt.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousem SA. The histological spectrum of pulmonary graft-versus-host disease in bone marrow transplant recipients. Hum Pathol. 1995;26:668–675. doi: 10.1016/0046-8177(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 7.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 9.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 10.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 11.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2015;21:1167–1187. doi: 10.1016/j.bbmt.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark JG, Schwartz DA, Flournoy N, Sullivan KM, Crawford SW, Thomas ED. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med. 1987;107:648–656. doi: 10.7326/0003-4819-107-5-648. [DOI] [PubMed] [Google Scholar]
- 15.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 16.Holbro A, Lehmann T, Girsberger S, et al. Lung histology predicts outcome of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:973–980. doi: 10.1016/j.bbmt.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazourian L, Rogers AJ, Ibanga R, et al. Factors associated with bronchiolitis obliterans syndrome and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Am J Hematol. 2014;89:404–409. doi: 10.1002/ajh.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudek A. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant. 2003;9:657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 19.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstructive lung disease after allogeneic marrow transplantation. Clinical presentation and course. Ann Intern Med. 1989;111:368–376. doi: 10.7326/0003-4819-111-5-368. [DOI] [PubMed] [Google Scholar]
- 20.Santo Tomas LH, Loberiza FR, Jr, Klein JP, et al. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest. 2005;128:153–161. doi: 10.1378/chest.128.1.153. [DOI] [PubMed] [Google Scholar]
- 21.Kuzmina Z, Krenn K, Petkov V, et al. CD19+CD21low B cells and patients at risk for NIH-defined chronic graft-versus-host disease with bronchiolitis obliterans syndrome. Blood. 2013;121:1886–1895. doi: 10.1182/blood-2012-06-435008. [DOI] [PubMed] [Google Scholar]
- 22.Diab KJ, Yu Z, Wood KL, et al. Comparison of pulmonary complications after nonmyeloablative and conventional allogeneic hematopoietic cell transplant. Biol Blood Marrow Transplant. 2012;18:1827–1834. doi: 10.1016/j.bbmt.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood. 1988;72:621–627. [PubMed] [Google Scholar]
- 24.Nakaseko C, Ozawa S, Sakaida E, et al. Incidence, risk factors and outcomes of bronchiolitis obliterans after allogeneic stem cell transplantation. Int J Hematol. 2011;93:375–382. doi: 10.1007/s12185-011-0809-8. [DOI] [PubMed] [Google Scholar]
- 25.Nishio N, Yagasaki H, Takahashi Y, et al. Late-onset non-infectious pulmonary complications following allogeneic hematopoietic stem cell transplantation in children. Bone Marrow Transplant. 2009;44:303–308. doi: 10.1038/bmt.2009.33. [DOI] [PubMed] [Google Scholar]
- 26.Ringden O, Remberger M, Ruutu T, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Nordic Bone Marrow Transplantation Group. Blood. 1999;93:2196–2201. [PubMed] [Google Scholar]
- 27.Soubani AO, Uberti JP. Bronchiolitis obliterans following haematopoietic stem cell transplantation. Eur Respir J. 2007;29:1007–1019. doi: 10.1183/09031936.00052806. [DOI] [PubMed] [Google Scholar]
- 28.Bunte MC, Patnaik MM, Pritzker MR, Burns LJ. Pulmonary veno-occlusive disease following hematopoietic stem cell transplantation: a rare model of endothelial dysfunction. Bone Marrow Transplant. 2008;41:677–686. doi: 10.1038/sj.bmt.1705990. [DOI] [PubMed] [Google Scholar]
- 29.Hosokawa K, Yamazaki H, Nishitsuji M, et al. Pulmonary veno-occlusive disease following reduced-intensity allogeneic bone marrow transplantation for acute myeloid leukemia. Intern Med. 2012;51:195–198. doi: 10.2169/internalmedicine.51.6302. [DOI] [PubMed] [Google Scholar]
- 30.Gutman JA, Allen CT, Madtes DK, Schramm J, Delaney C. Pulmonary veno-occlusive disease following reduced-intensity cord blood transplantation. Bone Marrow Transplant. 2008;42:559–561. doi: 10.1038/bmt.2008.210. [DOI] [PubMed] [Google Scholar]
- 31.Troussard X, Bernaudin JF, Cordonnier C, et al. Pulmonary veno-occlusive disease after bone marrow transplantation. Thorax. 1984;39:956–957. doi: 10.1136/thx.39.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahab N, Haider S, Doll DC. Vascular toxicity of antineoplastic agents. Semin Oncol. 2006;33:121–138. doi: 10.1053/j.seminoncol.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Freudenberger TD, Madtes DK, Curtis JR, Cummings P, Storer BE, Hackman RC. Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood. 2003;102:3822–3828. doi: 10.1182/blood-2002-06-1813. [DOI] [PubMed] [Google Scholar]
- 35.Jinta M, Ohashi K, Ohta T, et al. Clinical features of allogeneic hematopoietic stem cell transplantation-associated organizing pneumonia. Bone Marrow Transplant. 2007;40:465–472. doi: 10.1038/sj.bmt.1705768. [DOI] [PubMed] [Google Scholar]
- 36.Wolff SN. Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transplant. 2002;29:545–552. doi: 10.1038/sj.bmt.1703389. [DOI] [PubMed] [Google Scholar]


