Significance
Current conflict in the heavily HIV-affected regions of eastern Ukraine, which caused the relocation of 1.7 million people, may have increased the exportation of the virus from war-affected regions to other parts of the country. We show that the large-scale war-initiated movement of people (including those HIV-infected) was associated with patterns of HIV movement reconstructed from gene-sequence data. Our results further suggest that virus dissemination was directed to the locations with the highest prevalence of risky sexual practices in people who inject drugs (PWID). Recently, the Ukrainian HIV epidemic has been transitioning from being PWID-concentrated to sexually driven. Our study helps to understand the factors that might facilitate this shift.
Keywords: HIV, Ukraine, war, people who inject drugs, phylogeography
Abstract
Ukraine has one of the largest HIV epidemics in Europe, historically driven by people who inject drugs (PWID). The epidemic showed signs of stabilization in 2012, but the recent war in eastern Ukraine may be reigniting virus spread. We investigated the movement of HIV-infected people within Ukraine before and during the conflict. We analyzed HIV-1 subtype-A pol nucleotide sequences sampled during 2012–2015 from 427 patients of 24 regional AIDS centers and used phylogeographic analysis to reconstruct virus movement among different locations in Ukraine. We then tested for correlations between reported PWID behaviors and reconstructed patterns of virus spread. Our analyses suggest that Donetsk and Lugansk, two cities not controlled by the Ukrainian government in eastern Ukraine, were significant exporters of the virus to the rest of the country. Additional analyses showed that viral dissemination within the country changed after 2013. Spearman correlation analysis showed that incoming virus flow was correlated with the number of HIV-infected internally displaced people. Additionally, there was a correlation between more intensive virus movement and locations with a higher proportion of PWID practicing risky sexual behaviors. Our findings suggest that effective prevention responses should involve internally displaced people and people who frequently travel to war-affected regions. Scale-up of harm reduction services for PWID will be an important factor in preventing new local HIV outbreaks in Ukraine.
The HIV epidemic in Ukraine is one of the largest in Europe: In 2015, the country had an estimated 220,000 people living with HIV and the highest rate of new infections in Europe (∼30 cases per 100,000 people) (1). After independence in 1991, the population of people who inject drugs (PWID) grew quickly; in 1994, the number of new HIV cases in this group rocketed to 12,000, and by 1997, HIV in PWID was reported in all administrative regions of Ukraine (2). This was the beginning of one of the largest HIV in PWID epidemics recorded, characterized by multiple transmissions within the first month after infection and driven by risky injecting practices (3–5). From 2002, Ukraine scaled up a harm-reduction approach (6) that has been shown to reduce risky injecting practices and HIV transmission among PWID (7). Consequently, the fraction of newly diagnosed cases attributed to illicit drug use declined; since 2010, more newly diagnosed infections have been attributed to sexual than parenteral transmission (8). In 2012, the number of newly registered HIV cases dropped for the first time in the history of the epidemic in Ukraine, indicating that the epidemiological situation had stabilized (8).
However, this epidemiological equilibrium was disrupted by the Maidan protests in late 2013 (9), the Crimean Peninsula annexation in early 2014, and the ongoing military conflict in eastern Ukraine, in the Donetsk and Lugansk regions. During the conflict, health care provision and harm-reduction services were continuously interrupted (9–12). HIV incidence has grown 15–54% in some areas affected by war (13). Notably, the conflict caused massive internal human migration: Since 2014, >1.7 million people have been internally displaced in Ukraine (14), out of a total population of 45 million (15). HIV prevalence in the general population stands at >0.5% (16), suggesting that >8,000 of these displaced individuals might be HIV-infected. However, only 1,153 HIV-infected internally displaced people (IDP) have registered with AIDS centers at their new places of residence as of early 2017 (16). Large-scale human migration is likely to have had an impact on epidemic dynamics within and among the regions of Ukraine. Within cities and well-established transmission networks, chronically infected people protect individuals susceptible to infection from highly infectious recently infected cases, creating the so-called “firewall” effect (17, 18). Changes to established networks could disrupt this effect and trigger further viral transmission or even provoke local outbreaks. Furthermore, as infected people move to new places, they might disseminate new viral strains. In addition, new groups of susceptible individuals may be generated—e.g., disadvantaged IDP, affected by war, with potentially poor access to care. In general, we might expect an increased export of HIV from eastern Ukraine (historically the most heavily HIV-affected region) to other locations.
There have been some efforts to study the effect of the political turmoil on HIV epidemics elsewhere (18) and some reports of the response of Ukrainian nongovernmental organizations to the conflict (12). Here, we analyze 427 HIV-1 subtype-A pol sequences collected from almost all administrative regions of Ukraine to investigate how viral infections have been redistributed among geographical locations in recent years. We then combine these findings with epidemiological and behavioral data to explore the current epidemiological situation in those regions where increased viral gene flow was detected.
Results
HIV-1 Dissemination Among Locations in Ukraine.
A total of 448 HIV-1 pol gene sequences were available from the Ukrainian HIV drug-resistance database: Of these, 427; 19; and 2 were identified as subtypes A, B, and circulating recombinant form 03_AB, respectively. Only subtype A sequences were used in our phylogenetic/phylogeographic analyses. These data were collected between 2012 and 2015 from 22 administrative regions of Ukraine, from the capital city of Kyiv, and from the Autonomous Republic of Crimea, representing 24 of the 27 Ukrainian administrative units. For the phylogeographic analysis, we grouped sequences into seven geographic locations: Center, Crimea, East (including Donetsk and Lugansk—two regions in eastern Ukraine involved in military conflict), Kyiv, Odessa, South, and West. Hereafter, we refer to these groups as “locations” and to the Ukrainian administrative units as “regions.” Full details of the sequences used are provided in Dataset S1, Table S1 A and B and Supporting Information.
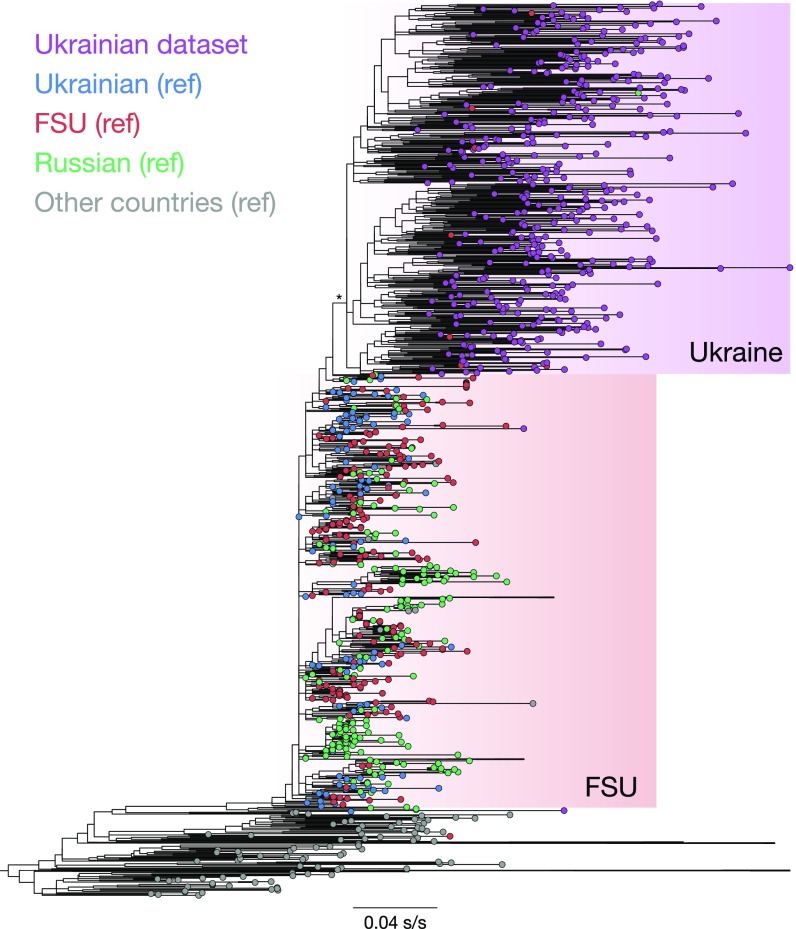
An initial maximum-likelihood (ML) phylogenetic tree included 2,199 reference and 427 Ukrainian subtype-A sequences (Fig. 1). The tree revealed that the Ukrainian samples formed a distinct monophyletic clade within sequences from countries of the former Soviet Union (FSU). The FSU sequences in turn formed a clade within the global HIV-1 subtype-A dataset, as expected from previous reports (19, 20).
Fig. 1.
ML phylogenetic reconstruction of global HIV-1 subtype A genetic diversity based on genetic sequences (n = 2,626) from the Ukrainian dataset (n = 427) and publicly available sequence (n = 2,199; reference dataset). The bar represents the genetic distance. The asterisk marks the Ukrainian dataset further used for phylogeographical analysis. Some clades are merged to improve the graphic representation of the figure.
Furthermore, we selected 36 HIV subtype-A sequences sampled in 1986–2014 and added them to the Ukrainian sequences to improve molecular clock calibration (n = 463; combined dataset). The dataset showed sufficient temporal signal (root-to-tip correlation coefficient = 0.5), supporting the use of molecular clock models. Phylodynamic analysis showed that the most recent common ancestor of the sequences in the Ukrainian dataset was 2001 [95% Bayesian credible interval (BCI): 1999–2002], for the FSU countries was 1998 (95% BCI: 1997–2000), and for the combined dataset was 1977 (95% BCI: 1972–1981). The resulting empirical posterior distribution of dated phylogenies was used in subsequent phylogeographic analyses, after the removal of the non-Ukrainian reference sequences (n = 427; Ukrainian dataset). Given the k = 7 sampling locations, we investigated virus dissemination among the k(k − 1) = 42 possible pathways of virus spread using discrete phylogeographic models (21, 22). More specifically, we investigated the main exporters of viral lineages—i.e., the locations from which the majority of migration events originated.
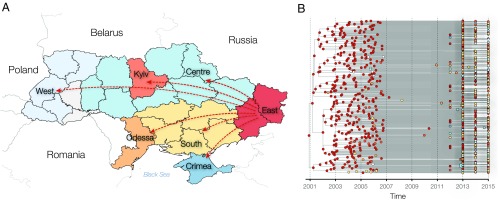
In our phylogeographic analysis, the East (Donetsk and Lugansk regions) was the main exporter, accounting on average for 92% (95% BCI: 87–96%) of the migration events in the Ukrainian dataset (Fig. 2, Table 1, and Dataset S1, Table S2). In particular, viral lineage movements were most frequently observed from the East to the Center, South, Kyiv, and Odessa locations, accounting for 26% (95% BCI: 24–28%), 24% (95% BCI: 21–26%), 22% (95% BCI: 20–24%), and 17% (95% BCI: 16–18%) of migration events, respectively. Very few migration events were observed as originating from the Crimea and West locations (<1% each), but the West received a significant proportion of translocated viral lineages (7% of viral lineage movement events; 95% BCI: 6–8%).
Fig. 2.
(A) A geographical map of Ukraine. Colors indicate the grouping of the sequences in locations used in phylogeographic analysis. The arrows indicate the directionality of virus gene flow movement from the East. (B) Results of the discrete trait phylogeographic analysis based on the Ukrainian dataset (n = 427). Colors indicate the ancestral state locations of the internal nodes reconstructed by robust count phylogeographic method and the sampling locations of the tips.
Table 1.
Lineage migration events in the Ukrainian HIV-1 dataset, 2012–2015
| From | Export. events, n [95% BCI] | Export. events, % | Import. events, n [95% BCI] | Import. events, % |
| Center | 4.83 [0, 11] | 1.59 | 78.76 [74, 84] | 25.96 |
| Crimea | 0.52 [0, 2] | 0.17 | 10.39 [10, 12] | 3.43 |
| East | 279.73 [264, 292] | 92.27 | 1.83 [0, 5] | 0.60 |
| Kyiv | 7.00 [1, 13] | 2.31 | 67.65 [61, 74] | 22.31 |
| Odessa | 4.13 [0, 9] | 1.36 | 50.88 [46, 55] | 16.78 |
| South | 5.36 [1, 12] | 1.76 | 72.24 [65, 78] | 23.83 |
| West | 1.74 [0, 5] | 0.57 | 21.60 [19, 24] | 7.12 |
| All, n | 303.18 [289, 315] | |||
Exportation (Export.) and importation (Import.) events are the total number of lineage migration events that originated from and received by a given location, respectively.
Given that locations were represented by different numbers of sequences, we explored estimates of among-location viral lineage movement in more balanced datasets. To this end, we conducted additional analyses in which the number of sequences per location were randomly down-sampled to match the number of sequences in the third smallest location (Odessa; n = 57). We conducted 10 replicate analyses with different random subsamples (all n = 317) of the dataset and found that the East remained as the main viral lineage export location (accounting on average for 91% of migration events) in 7 of 10 subsampled replicates. For the number of migration events estimated for each subsampled dataset, see Dataset S1, Table S3.
Temporal Change in Virus Lineage Movement After the Beginning of the Conflict.
We aimed to investigate whether viral gene flow among regions has changed after the initiation of war, a phenomenon we call hereafter “a phylogeographic transition.” We estimated rates of viral lineage movement using models with time-dependent lineage transition rates. Specifically, we compared the “single-epoch” model (in which lineage migration rates remained constant between 2012–2015) with the “two-epoch” model (in which lineage migration rates were allowed to be different before and after the start of the conflict in 2013). The results of the epoch analysis showed that the two-epoch model fitted the data better than the single-epoch model, as demonstrated by a Bayes factor (BF) support of >30 in favor of the more complex model. Standard terminology defines the strength of support for a particular model as “very strong” for BF >30 (23). This result was consistent across all 10 replicate datasets generated by random subsampling (Dataset S1, Table S4).
Correlation Between Observed Virus Flow and PWID Behaviors.
We further investigated whether among-location viral lineage movement was correlated with important epidemiological characteristics of the HIV-1 epidemic in Ukraine. Here, we calculated Spearman correlation coefficients between exporting and importing viral gene flow and all behavioral covariates of interest. In particular, we were interested in the total number of lineage migration events that originated in a given location (exportation events) and the total number of lineage migration events received by a given location (importation events). Behavioral data relating to PWID and regional epidemiological characteristics were aggregated to the same geographic units (locations) as used in the phylogeographic analysis.
The behavioral variables came from (i) the national survey of PWID (Integrated Bio-Behavioral Surveillance; IBBS), (ii) the regional HIV surveillance systems, and (iii) data on the war-associated movement of IDP. We chose the IBBS data that were collected closer to the phylogeographic transition (i.e., IBBS-2013) as an indicator of behaviors at the time when viral movement was hypothesized to have changed.
We found that injecting behaviors of PWID were not associated with the observed viral migration flow. Risky sexual behaviors of PWID, on the contrary, were associated with the number of importation events. Specifically, the higher proportion of PWID who always use condoms with casual and commercial partners was associated with a lower number of importation events [Spearman correlation P = 0.03 and 0.05, respectively, after adjustment for multiple comparisons using the false discovery rate method (24)]. The number of importation events per location was surprisingly negatively correlated with the proportion of PWID who reported participating in group sex (P = 0.04). Importantly, we found that the number of exportation events to a location was positively correlated with the HIV prevalence in the general population (P = 0.05) and the number of IDP relocated to that location (P < 0.01). At the same time, the number of HIV-infected IDP registered at that location was positively correlated with a higher number of importation events (P = 0.03). Table 2 shows statistically significant correlations at P ≤ 0.05; see Dataset S1, Table S5 for all correlation coefficients.
Table 2.
Spearman correlation coefficient for correlation between behavioral variables and the number of lineage migration events
| Variables | No. of export. events | No. of import. events | ||
| r | P | r | P | |
| Risky sexual practices | ||||
| A proportion of PWID who reported participating in group sex | 0.21 | 0.64 | −0.82 | 0.04 |
| Safe sexual practices | ||||
| A proportion of PWID who always used condoms with casual partners | 0.04 | 0.94 | −0.86 | 0.03 |
| A proportion of PWID who always used condoms with commercial partners | 0.07 | 0.88 | −0.75 | 0.05 |
| Regional level epidemiological characteristics | ||||
| No. of IDP relocated to and registered in a location | 0.96 | <0.01 | 0.14 | 0.76 |
| No. of HIV-positive IDP relocated to and registered in a location | 0.43 | 0.34 | 0.86 | 0.03 |
| HIV prevalence in general population | 0.75 | 0.05 | 0.18 | 0.7 |
r, correlation coefficient. Significant P values are shown in bold.
Discussion
Here, we explored HIV dissemination in Ukraine by analyzing geographical patterns of viral genetic flow in recent years. Our results suggest that viral lineages have moved from the regions engaged in the war to the rest of Ukraine: Many strains sampled in Ukraine during 2012–2015 appear to have originated, directly or indirectly, from Donetsk and Lugansk (i.e., the ancestral sequence location of the Ukrainian dataset was found to be in the East location). The East was the main exporter of viral lineages, and this remained the case when the number of sequences per location was made more balanced. The most intensive virus-lineage movement was observed from the eastern to the central and southern regions and to Odessa and Kyiv. The East location is heavily affected by HIV: 24% of all nationally registered HIV cases, and the largest number of HIV-positive PWID (45,000), live or have lived in the Donetsk and Lugansk regions. Donetsk is the largest city in the area and has some of the highest HIV prevalences in Ukraine among PWID (33.5% in 2015), sex workers (17% in 2015), and the general population [0.9% of pregnant women were HIV-positive in Donetsk in 2015 (13, 16, 25)].
Our data support a transition in the pattern of viral lineage movement around the end of 2013, the time of the beginning of several major events in Ukraine: the Maidan protests in Kyiv, the annexation of Crimea, and an initiation of the war in the east. The movement of people who relocated from the areas not controlled by the Ukrainian government to locations elsewhere corresponds to the observed virus movement patterns. Furthermore, we believe that the observed virus-lineage movement is not a result of recent transmissions, but reflects the redistribution of preexisting infections within the country. Viral gene sequences in the Ukrainian HIV database come from patients who experienced antiretroviral therapy (ART) failure and whose blood samples were sent for HIV drug-resistance testing. In Ukraine before 2017, ART followed an older treatment guideline (i.e., patients received ART when CD4 count <350), hence, it is likely that the patients included in our study were infected some years before their virus was sequenced. Overall, patients in Ukraine are diagnosed with HIV late in infection: 41% of the newly diagnosed patients are in the third or fourth stage of HIV (26). Thus, patients in our analysis are unlikely to reflect recent transmission events, and the viral lineage movement from Donetsk and Lugansk suggests that many people (e.g., residents) who were initially infected in the eastern regions have moved to the central and southern regions due to the war, resulting in a redistribution of HIV viral lineages within Ukraine.
The number of virus-lineage exportations correlated well with the overall number of IDP registered in a region. Thus, we saw that more people moved to the locations from which the virus is originating. This observation is explained by the fact that a large proportion of IDP moved away from the front line that divided government- and nongovernment-controlled regions, but stayed within the Donetsk and Lugansk regions, just in different counties. Surprisingly, this observation was not repeated for the HIV-infected IDP population: Higher numbers of HIV-infected IDP were registered in the locations that imported more viral lineages. Thus, even though more IDP moved to the main exporting locations (East), more HIV-infected people moved to the main importing locations in our analysis (Center, Kyiv, South, and Odessa). It is crucial to understand the nature of this difference: Either we see more HIV-infected IDP move fully outside of the eastern regions compared with noninfected people, or we are simply not linking the HIV-infected IDP who stayed within the Donetsk and Lugansk regions to care.
The stress of the displacement might result in treatment failures for some HIV-infected patients and cause overrepresentation of IDP in our sample. Patients who had to relocate because of the conflict may be more likely to reduce treatment adherence or drop out of treatment for some time. With the nonnucleoside reverse-transcriptase inhibitors that are currently in use in Ukraine for most first-line regimens, mutations can occur within weeks of nonadherence. On the other hand, in Ukraine, 9–10% of ART patients experience virological failure, which, given 79,000 patients in treatment, would lead to ∼7,000 resistance tests a year. However, only ∼500 tests are available annually. Therefore, every region receives a quota of tests, in proportion to the number of infected people in the region. Physicians then decide who is tested, based on the current state and medical history of a patient. Patients with multiple changes of drug schemes are prioritized. Thus, a patient failing ART due to apparent adherence issues is unlikely to be prioritized for resistance testing over patients with failures under treatment. Indeed, the percentage of sequences with no resistance mutations at the time of failure did not change significantly over time (17% and 20% before and after the beginning of war, respectively), suggesting that severe adherence issues are not overrepresented in our data (Dataset S1, Table 6B). More details on the drug-resistance-associated mutations in our sample can be found in Supporting Information.
Regrettably, the viral genetic data from patients of the AIDS centers in Ukraine do not have associated risk group information. Thus, we cannot be confident as to what risk population our sample mostly represents. We know that HIV-infected PWID have poor access to treatment compared with other patients; only 9.4% of people in treatment report the use of illicit drugs at any point, while it is estimated that at least half of the people living with HIV in Ukraine have a history of drug use (27). At the same time, PWID might be more likely to experience treatment failure (due to social, psychological, and economical challenges), and thus they may be more likely to be part of our sample than other patients on ART.
In 2003, the Joint United Nations Programme on HIV/AIDS highlighted the potential contribution of conflict to the spread of HIV and other sexually transmitted diseases (28), and the topic has been intensively discussed since then (29–32). Our finding, that viral lineage imports are correlated with some risky sexual practices, suggests that regions that have received new HIV lineages also have appropriate conditions for those lineages to flourish. While there might be further variability in behaviors within a location, the small amount of data available from some administrative regions limited statistical power to detect significant correlations between virus movement and behaviors. Furthermore, there were no available regional data on sexual behaviors in the general population, but the IBBS PWID behavior data provided a useful insight. It is important to mention that IBBS data represent only urban PWID behaviors, since the survey is conducted only in regional capitals. Nonetheless, the HIV epidemic in Ukraine is largely urbanized: In 2013, a total of 77% of all HIV cases were registered among the urban population (33). Regional capitals are also human mobility hubs that accommodate the majority of IDP (34). It could be argued that the dominance of sexual HIV transmission in Ukraine [the proportion of sexually attributed HIV incidence is growing every year (8)] can be attributed to the behaviors prevalent in the PWID group (35). This is a widespread scenario in Eastern European countries: HIV prevailed first in PWID and then shifted toward the general population through unsafe sex practices (27, 36–38). The war and internal movement of people to regions with more reported high-risk sexual behaviors among PWID might create a favorable environment for new HIV transmission and facilitate the generalization of the Ukrainian epidemic.
Conclusions
Recent movement of HIV-infected people might change existing network structures, disturbing the firewall effect and accelerating HIV flows within Ukraine. Enabling sustainable prevention services and treatment provision in locations where services have been physically disrupted because of the armed conflict is a priority (39). However, we highlight the necessity of urgent preventive measures in other parts of the country as well. Proactive integration into routine HIV testing of people who have relocated due to the war, or who frequently travel to the war zone, should become a component of HIV prevention. Since our analysis likely reflects the redistribution of existing infections, not the location of new transmissions, we suggest that there is a need to continuously monitor the effect of the war on HIV incidence and prevalence in Ukraine at both national and regional levels.
Methods
Data.
Sequences sampling and phylogenetic analysis.
We used 448 HIV-1 genetic sequences sampled in 2012–2015 from patients of 24 Ukrainian Regional Centers to Prevent and Fight AIDS (AIDS Centers) (52% males) and available from the Ukrainian HIV drug-resistance database. All of the available high-quality (<5% of ambiguous nucleotides) subtype A (n = 427) sequences were used in the analysis. All sequence IDs and accession numbers are in Dataset S1, Table S1 A and B.
We aligned the sequences using MEGA software (Version 7.0) (40) and then manually edited the alignment, deleting 43 codon positions associated with drug resistance (41) (the positions are listed in Supporting Information). The final alignment consisted of 866 codons that corresponded to the 2,253–3,216 positions of the HXB2 HIV reference sequence (pol gene). We used the REGAv3 (42) and COMET (43) subtyping tools to identify subtypes and recombinant sequences.
To explore the position of the 427 sequences from the Ukrainian HIV database within the global subtype-A epidemic, we merged our sequences with a globally representative subtype-A reference dataset, which consisted of 2,199 sequences, using Clustal W (44). We then reconstructed an ML phylogenetic tree with 2,626 sequences (427 Ukrainian and 2,199 reference) using RAxML (45) to check the position of our sequences within the global subtype-A diversity (Fig. 1). For further analyses, we chose 36 subtype-A HIV-1 reference sequences sampled in 1987–2013 that were publicly available from the Los Alamos database to improve the molecular clock calibration. Sequences were selected to ensure a balanced geographical and temporal representation. We used RAxML to build the ML tree of the 427 Ukrainian and 36 reference sequences (Fig. S1) (the combined dataset; n = 463).
Behavioral data.
Data on PWID behaviors came from the IBBS of PWID (n = 9,002) conducted biyearly in 25 administrative regions of Ukraine in 2013. In IBBS-2013, participants were asked about their injecting and sexual behaviors and tested for HIV and hepatitis C virus antibodies by using rapid tests CITO TEST HIV 1/2/07.
More information on sampling, phylogenetic analysis, and reference dataset, as well as additional information on the IBBS survey, can be found in Supporting Information and elsewhere (25).
Phylogeographic Analysis.
Dating phylogenetics.
We conducted molecular clock and discrete trait analyses in BEAST (Version 1.8.4) (21). We used the general time-reversible nucleotide substitution model with a gamma-distributed rate variation among sites. We used a log-normal uncorrelated relaxed clock model to account for rate heterogeneity among lineages (46). Analyses were run for 200,000,000 generations (burn-in of 30,000,000) until appropriate mixing and convergence of the Markov chain Monte Carlo sampler in the posterior target distribution was obtained (effective sample size > 150). We used LogCombiner (22) to subsample the posterior tree distribution, and after removing 15% of burn-in, the resulting empirical tree distribution (n = 1,700 trees) was used in subsequent phylogeographic analysis, after reference sequences were pruned by using PAUP (47). The maximum clade credibility tree for the Ukrainian dataset was reconstructed in TreeAnnotator and is presented in Fig. 2.
Discrete trait analysis.
For the discrete trait phylogeographic analysis, we grouped the 427 Ukrainian sequences from 24 different administrative regions into seven geographic locations, as shown in Fig. 2 and Dataset S1, Table S1B. We grouped the data into seven locations to avoid overparameterization of the model and to ensure that every geographic location had at least 10 sequences. We also made sure that this grouping was appropriate in the cultural and social context of Ukraine.
We computed a discrete phylogeographic analysis with the robust counting method as implemented in BEAST, using an empirical tree distribution as described (48–51). This approach inferred the expected number of viral migrations for every pathway on a branch-by-branch basis. The location-exchange process throughout the phylogeny was estimated by using asymmetric (nonreversible) discrete-time Markov chains, which quantified the number of exporting and importing virus-lineage movements for each pair of locations. We estimated viral gene flow as the number of migration events, which is the number of viral-lineage movements from one location to another.
In the first step of the analysis, we included the full Ukrainian dataset, n = 427. In an attempt to account for bias that might arise from unequal allocation of sequences per location, we ran additional analyses with reduced numbers of sequences per location. Specifically, we conducted 10 replicate analyses, in which we reduced the number of sequences in each location to the third smallest number in the dataset (n = 57 for Center, East, Kyiv, Odessa, and South; n = 10 for Crimea; and n = 22 for West). For each of these analyses, we randomly selected different subsets of the sequences for each location, with the same n = 314 for each subset. Phylogeographic analyses using the empirical tree distributions were then performed for 5,000,000 generations (burn-in 5 × 106).
Phylogeographic transition.
To check for changes in viral gene flow that might be associated with the military conflict in the East, or with any Maidan-related effects, we applied models with time-dependent lineage transition rates, i.e., an “epoch analysis” as implemented in BEAST (52). Specifically, we compared the single-epoch model (in which pairwise rates of lineage movement remained constant between 2012 and 2015) with the two-epoch model (in which pairwise rates of lineage movement could be different before and after the start of the conflict in 2013). This approach allowed us to test for a change in viral gene flow after a specified time point. For the two-epoch model, we chose the year 2013 as the transition time between the two epochs. For this analysis, we merged the sequences from two locations: East (Donetsk and Lugansk) and Other (all other sequences). We created 10 subsamples, down-sampling the number of sequences in the “Other” location to meet the number of sequences in the East. We used path-sampling (53) and stepping-stone-sampling (54) marginal likelihood estimators to determine which epoch model fit the data best. The results of the epoch analysis showed that the two-epoch model fit the data better than the single-epoch model. This result was consistent across all 10 replicate datasets generated by random subsampling (Dataset S1, Table S4). Additional data on epoch analysis and model selection are available in Supporting Information.
Spearman Correlation Analysis.
We performed a Spearman correlation test to check whether the numbers of viral lineage migration exportation and importation events were correlated with some of the behavioral and epidemiological characteristics of locations. We ran two separate analyses of correlation to individually explore factors that are associated with the number of exportation and importation events. All of the P values for the correlation analyses were adjusted for multiple comparisons by using the false discovery rate method (24).
For every location, we looked at the following:
-
(i)
The sum of exportation viral lineage migration events from a location to all of the other locations.
-
(ii)
The sum of importation viral lineage migration events from a location to all of the other locations.
Details of behavioral variable construction can be found in Supporting Information.
Ethical Approval.
HIV drug-resistance testing was performed at the L. V. Gromashevskij Institute of Epidemiology and Infectious Diseases, National Academy of Sciences of Ukraine, following all Ukrainian legal and ethical requirements. Fully anonymized viral gene sequences were transferred to Oxford through a Material Transfer Agreement for molecular evolution analyses. Fully anonymized aggregated behavioral data (IBBS) were obtained from the Alliance for Public Health. The IBBS protocol and questionnaires were examined by the Committee on Sociologist’s Professional Ethics at the Sociological Association of Ukraine. The epidemiological component was examined by the Committee on Medical Ethics of the L. V. Gromashevskij Institute of Epidemiology and Infectious Diseases, National Academy of Sciences of Ukraine.
Supplementary Material
Acknowledgments
We thank Louis du Plessis (Department of Zoology, University of Oxford) for assistance. T.I.V. is supported by the Clarendon Fund and Hertford College of the University of Oxford; S.R.F. is supported by NIH National Institute on Drug Abuse Grant DP1DA034989; G.M. is supported by Medical Research Council Clinician Scientist Fellowship MR/K010565/1; and N.R.F. is supported by the Medical Research Council/Wellcome Trust/Newton Fund (Grant MC_PC_15100/ZK/16-078). IBBS data collection was administered by the Ukrainian Institute for Social Research (UISR) after O. Yaremenko (UISR) under International Charitable Foundation Contract “Alliance for Public Health.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.J.C. is a guest editor invited by the Editorial Board.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KY370106–KY370532).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701447115/-/DCSupplemental.
References
- 1.European Centre for Disease Prevention and Control/WHO Regional Office for Europe 2016. HIV/AIDS surveillance in Europe 2015 (European Centre for Disease Prevention and Control, Stockholm)
- 2.Barnett T, Whiteside A, Khodakevich L, Kruglov Y, Steshenko V. The HIV/AIDS epidemic in Ukraine: Its potential social and economic impact. Soc Sci Med. 2000;51:1387–1403. doi: 10.1016/s0277-9536(00)00104-0. [DOI] [PubMed] [Google Scholar]
- 3.Booth RE, et al. Predictors of risky needle use following interventions with injection drug users in Ukraine. Drug Alcohol Depend. 2006;82:S49–S55. doi: 10.1016/s0376-8716(06)80009-8. [DOI] [PubMed] [Google Scholar]
- 4.Vasylyeva TI, et al. Reducing HIV infection in people who inject drugs is impossible without targeting recently-infected subjects. AIDS. 2016;30:2885–2890. doi: 10.1097/QAD.0000000000001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehne KL, Grund JPC, Khodakevich L, Kobyshcha Y. The HIV/AIDS epidemic among drug injectors in Eastern Europe: Patterns, trends and determinants. J Drug Issues. 1999;29:729–776. [Google Scholar]
- 6.World Health Organization . Good Practices in Europe: HIV Prevention for People Who Inject Drugs Implemented by the International HIV/AIDS Alliance in Ukraine. World Health Organization; Copenhagen: 2014. [Google Scholar]
- 7.MacArthur GJ, et al. Interventions to prevent HIV and hepatitis C in people who inject drugs: A review of reviews to assess evidence of effectiveness. Int J Drug Policy. 2014;25:34–52. doi: 10.1016/j.drugpo.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Vitek CR, et al. Slowing of the HIV epidemic in Ukraine: Evidence from case reporting and key population surveys, 2005-2012. PLoS One. 2014;9:e103657. doi: 10.1371/journal.pone.0103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzi M. 2015 War in Ukraine threatens to worsen HIV crisis. Aljazeera America. Available at america.aljazeera.com/articles/2015/1/26/war-in-ukraine-threatens-to-worsen-hiv-crisis.html. Accessed May 11, 2016.
- 10.Owczarzak J, Karelin M, Phillips SD. A view from the frontlines in Slavyansk, Ukraine: HIV prevention, drug treatment, and help for people who use drugs in a conflict zone. Int J Drug Policy. 2015;26:6–7. doi: 10.1016/j.drugpo.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippovych S. Impact of armed conflicts and warfare on opioid substitution treatment in Ukraine: Responding to emergency needs. Int J Drug Policy. 2015;26:3–5. doi: 10.1016/j.drugpo.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Duchenko A, Deshko T, Braga M. Crisis management by HIV/AIDS non-governmental organisations in the post-Euromaidan Ukraine led to opening new horizons. Drugs Alcohol Today. 2017;17:149–156. [Google Scholar]
- 13.Alliance for Public Health 2015. Annual report (Alliance Public Health, Kyiv, Ukraine)
- 14.Internal Displacement Monitoring Centre 2017 Ukraine: Country information 2015. Available at www.internal-displacement.org/countries/ukraine. Accessed May 22, 2016.
- 15.State Service for Statistics of Ukraine 2016 All-Ukrainian population service. Available at www.ukrcensus.gov.ua/eng/. Accessed January 17, 2017.
- 16.Ministry of Health of Ukraine 2016. HIV-infection in Ukraine (Ukrainian Center for Control for Socially Dangerous Diseases of Ministry of Health of Ukraine, Institute of Epidemiology and Infectious Diseases named after L. V. Gromashevskiy, Kyiv, Ukraine), Informational Bulletin N46.
- 17.Friedman SR, et al. Network-related mechanisms may help explain long-term HIV-1 seroprevalence levels that remain high but do not approach population-group saturation. Am J Epidemiol. 2000;152:913–922. doi: 10.1093/aje/152.10.913. [DOI] [PubMed] [Google Scholar]
- 18.Khan B, Dombrowski K, Saad M, McLean K, Friedman S. Network firewall dynamics and the subsaturation stabilization of HIV. Discrete Dyn Nat Soc. 2013;2013:720818. doi: 10.1155/2013/720818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomson MM, et al. New insights into the origin of the HIV type 1 subtype A epidemic in former Soviet Union’s countries derived from sequence analyses of preepidemically transmitted viruses. AIDS Res Hum Retroviruses. 2007;23:1599–1604. doi: 10.1089/aid.2007.0166. [DOI] [PubMed] [Google Scholar]
- 20.Bobkov A, et al. An HIV type 1 epidemic among injecting drug users in the former Soviet Union caused by a homogeneous subtype A strain. AIDS Res Hum Retroviruses. 1997;13:1195–1201. doi: 10.1089/aid.1997.13.1195. [DOI] [PubMed] [Google Scholar]
- 21.Lemey P, Rambaut A, Drummond AJ, Suchard MA. Bayesian phylogeography finds its roots. PLOS Comput Biol. 2009;5:e1000520. doi: 10.1371/journal.pcbi.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffreys H. Theory of Probability. 3rd Ed. Clarendon; Oxford: 1961. p. 447. [Google Scholar]
- 24.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 25.Balakirieva O, Bondar T, Loktieva I, Sazonova Y, Sereda Y. 2014 Summary of the analytical report “Monitoring the behaviour and HIV-infection prevalence among people who inject drugs as a component of HIV second generation surveillance”. Available at aph.org.ua/wp-content/uploads/2016/07/zvit-IDU_obl_eng.pdf. Accessed May 12, 2016.
- 26.Ministry of Health of Ukraine 2016. Report on monitoring and evaluation of effectiveness of national program against HIV/AIDS for 2014-2018, as of 2015 (Ministry of Health of Ukraine, Kyiv, Ukraine)
- 27.Graw F, Leitner T, Ribeiro RM. Agent-based and phylogenetic analyses reveal how HIV-1 moves between risk groups: Injecting drug users sustain the heterosexual epidemic in Latvia. Epidemics. 2012;4:104–116. doi: 10.1016/j.epidem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UNAIDS Office on AIDS and Humanitarian Response 2003. HIV/AIDS and conflict (UNAIDS Office on AIDS and Humanitarian Response, Copenhagen)
- 29.Mock NB, et al. Conflict and HIV: A framework for risk assessment to prevent HIV in conflict-affected settings in Africa. Emerg Themes Epidemiol. 2004;1:6. doi: 10.1186/1742-7622-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripodi P, Patel P. HIV/AIDS, peacekeeping and conflict crises in Africa. Med Confl Surviv. 2004;20:195–208. doi: 10.1080/1362369042000248802. [DOI] [PubMed] [Google Scholar]
- 31.Mills EJ, Singh S, Nelson BD, Nachega JB. The impact of conflict on HIV/AIDS in sub-Saharan Africa. Int J STD AIDS. 2006;17:713–717. doi: 10.1258/095646206778691077. [DOI] [PubMed] [Google Scholar]
- 32.Betsi NA, et al. Effect of an armed conflict on human resources and health systems in Côte d’Ivoire: Prevention of and care for people with HIV/AIDS. AIDS Care. 2006;18:356–365. doi: 10.1080/09540120500200856. [DOI] [PubMed] [Google Scholar]
- 33.Ministry of Health 2017. Overall number of people receiving ART in Ukraine as of 01.04.2017 (Ministry of Health, Kyiv, Ukraine)
- 34.Smal V, Poznyak O. 2016. Internally displaced people: social and economical integration in host communities based on data from Vinnytsya, Zaporizzhya, and Ivano-Frankivsk regions (Project Promis, Kyiv, Ukraine)
- 35.Simmons R, et al. CASCADE Collaboration in EuroCoord HIV testing and diagnosis rates in Kiev, Ukraine: April 2013-March 2014. PLoS One. 2015;10:e0137062. doi: 10.1371/journal.pone.0137062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niccolai LM, Shcherbakova IS, Toussova OV, Kozlov AP, Heimer R. The potential for bridging of HIV transmission in the Russian Federation: Sex risk behaviors and HIV prevalence among drug users (DUs) and their non-DU sex partners. J Urban Health. 2009;86:131–143. doi: 10.1007/s11524-009-9369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Des Jarlais DC, et al. Transitions from injection-drug-use-concentrated to self-sustaining heterosexual HIV epidemics: Patterns in the international data. PLoS One. 2012;7:e31227. doi: 10.1371/journal.pone.0031227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toussova O, et al. Potential bridges of heterosexual HIV transmission from drug users to the general population in St. Petersburg, Russia: Is it easy to be a young female? J Urban Health. 2009;86:121–130. doi: 10.1007/s11524-009-9364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.UNAIDS 2016. Operational research: Systems of service to prevent and treat HIV/AIDS under the situation of the armed conflict in the east of Ukraine (UNAIDS, Kyiv, Ukraine)
- 40.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett DE, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One. 2009;4:e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pineda-Peña AC, et al. Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: Performance evaluation of the new REGA version 3 and seven other tools. Infect Genet Evol. 2013;19:337–348. doi: 10.1016/j.meegid.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 43.Struck D, Lawyer G, Ternes AM, Schmit JC, Bercoff DP. COMET: Adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nucleic Acids Res. 2014;42:e144. doi: 10.1093/nar/gku739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 45.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drummond AJ, Ho SY, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swofford DL. 2002. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods) (Sinauer Associates, Sunderland, MA), Version 4.
- 48.Minin VN, Suchard MA. Counting labeled transitions in continuous-time Markov models of evolution. J Math Biol. 2008;56:391–412. doi: 10.1007/s00285-007-0120-8. [DOI] [PubMed] [Google Scholar]
- 49.O’Brien JD, Minin VN, Suchard MA. Learning to count: Robust estimates for labeled distances between molecular sequences. Mol Biol Evol. 2009;26:801–814. doi: 10.1093/molbev/msp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faria NR, et al. HIV epidemiology. The early spread and epidemic ignition of HIV-1 in human populations. Science. 2014;346:56–61. doi: 10.1126/science.1256739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raghwani J, et al. Endemic dengue associated with the co-circulation of multiple viral lineages and localized density-dependent transmission. PLoS Pathog. 2011;7:e1002064. doi: 10.1371/journal.ppat.1002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bielejec F, Lemey P, Baele G, Rambaut A, Suchard MA. Inferring heterogeneous evolutionary processes through time: From sequence substitution to phylogeography. Syst Biol. 2014;63:493–504. doi: 10.1093/sysbio/syu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gelman A, Meng XL. Simulating normalizing constants: From importance sampling to bridge sampling to path sampling. Stat Sci. 1998;13:163–185. [Google Scholar]
- 54.Xie W, Lewis PO, Fan Y, Kuo L, Chen MH. Improving marginal likelihood estimation for Bayesian phylogenetic model selection. Syst Biol. 2011;60:150–160. doi: 10.1093/sysbio/syq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rambaut A, Lam TT, Max Carvalho L, Pybus OG. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen) Virus Evol. 2016;2:vew007. doi: 10.1093/ve/vew007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu G, Smith DK, Zhu H, Guan Y, Tsan-Yuk Lam T. ggtree: An r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol. 2016;8:28–36. [Google Scholar]
- 57.Ministry of Health of Ukraine 2014. HIV-infection in Ukraine (Ukrainian Center for Control for Socially Dangerous Diseases of Ministry of Health of Ukraine; Institute of Epidemiology and Infectious Diseases named after L. V. Gromashevskiy, Kyiv, Ukraine), Informational Bulletin N41.
- 58.UNAIDS 2015 Ukraine Global AIDS Response Progress Reporting (GARPR). Available at www.unaids.org/sites/default/files/country/documents/UKR_narrative_report_2015.pdf. Accessed April 19, 2017.
- 59.Berleva G, et al. Analytical report: “Estimation of the Size of Populations Most-at-Risk for HIV Infection in Ukraine” as of 2012 based on the results of 2011 survey. Alliance for Public Health; Kyiv, Ukraine: 2012. [Google Scholar]
- 60.Balakiryeva OM, Bondar TV, Sereda YuV, Sazonova YaO. Analytical report “Behaviour monitoring and HIV-prevalence among injecting drug users as a component of secondgeneration surveillance” (based on results of the 2011 biobehavioral survey) International HIV/AIDS Alliance; Kyiv, Ukraine: 2012. [Google Scholar]
- 61.UN High Commissioner for Refugees 2014. Preliminary analysis and needs assessment of Internally displaced persons.
- 62.Ministry of Health of Ukraine 2014. HIV-infection in Ukraine (Ukrainian Center for Control for Socially Dangerous Diseases of Ministry of Health of Ukraine; Institute of Epidemiology and Infectious Diseases named after L. V. Gromashevskiy, Kyiv, Ukraine), Informational Bulletin N44.
- 63.State Statistics Service of Ukraine 2016 Statistics 2016. Available at https://ukrstat.org/en/operativ/operativ2016/ds/kn/kn_e/kn1216_e.html. Accessed June 12, 2017.
- 64.Ruane PJ, Luber AD. K65R-associated virologic failure in HIV-infected patients receiving tenofovir-containing triple nucleoside/nucleotide reverse transcriptase inhibitor regimens. MedGenMed. 2004;6:31. [PMC free article] [PubMed] [Google Scholar]
- 65.del Rio C. Current concepts in antiretroviral therapy failure. Top HIV Med. 2006;14:102–106. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.