Abstract
Obesity is a highly prevalent non-communicable disease worldwide and is commonly associated with male infertility. Several etiopathological theories have been mentioned in the literature by which obesity affects spermatogenesis, thus affecting the male fertility potential. Mechanisms for explaining the effect of obesity on male infertility include endocrinopathy, increased aromatization activity, associated erectile dysfunction, psychological and thermal effects, obstructive sleep apnea, increased leptin and oxygen free radicals, and associated inflammatory and obstructive elements of epididymitis. Treatment of such a complex problem includes weight reduction (by lifestyle modification and increased physical activity), optimization of altered testosterone-to-estradiol ratio using aromatase inhibitors and/or gonadotropins, treatment of associated comorbidities by phosphodiesterase inhibitors for erectile dysfunction, and insulin-sensitizing agents for the management of diabetes. The aim of this mini-review is to highlight the pathological basis of this problem and to focus on obesity as an etiology of male infertility.
Keywords: Body Mass Index, Male Infertility, Review, Obesity, Spermatogenesis
Introduction
Obesity is a prevalent medical condition characterized by abnormal or excessive fat accumulation leading to negative impact on health, reduction of life expectancy, and/or increased risk for health problems.1 Nowadays, obesity and overweight are considered rapidly growing epidemics due to their significant increase between 1980 and 2013 from 28.8% to 36.9% in men, and from 29.8% to 38.0% in women.2
Body mass index (BMI) is an anthropometric measurement used to assess obesity and is calculated by dividing body weight in kilograms by the squared height in meters. The general population is classified into five categories based on their BMI: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), obese (BMI 30.0–39.9 kg/m2), and morbid obesity (BMI > 40 kg/m2).3
The main cause of obesity is the ‘energy imbalance’ when energy input exceeds expenditure. The global increase in the prevalence of obesity is due to multiple factors including a shift in diet toward high-calorie junk food containing excess fat and sugars with low fiber content and a trend toward decreased physical activity and a sedentary lifestyle. However, several factors may attribute to obesity such as smoking, sleep, drugs, and environmental factors.4 Additionally, obesity has been suggested to have a genetic predisposition.5 In the era of metagenomics, some studies in both rodents and humans have suggested that interaction between dietary habits, environmental factors, genetic predisposition, and gut microbiota (intestinal commensalism that regulates the rate of digestion, absorption, and metabolism of nutrients) may also contribute to obesity. This topic is still a promising area of research that may help in understanding pathogenesis of obesity and find novel therapeutic modalities for obesity-related metabolic syndrome.6
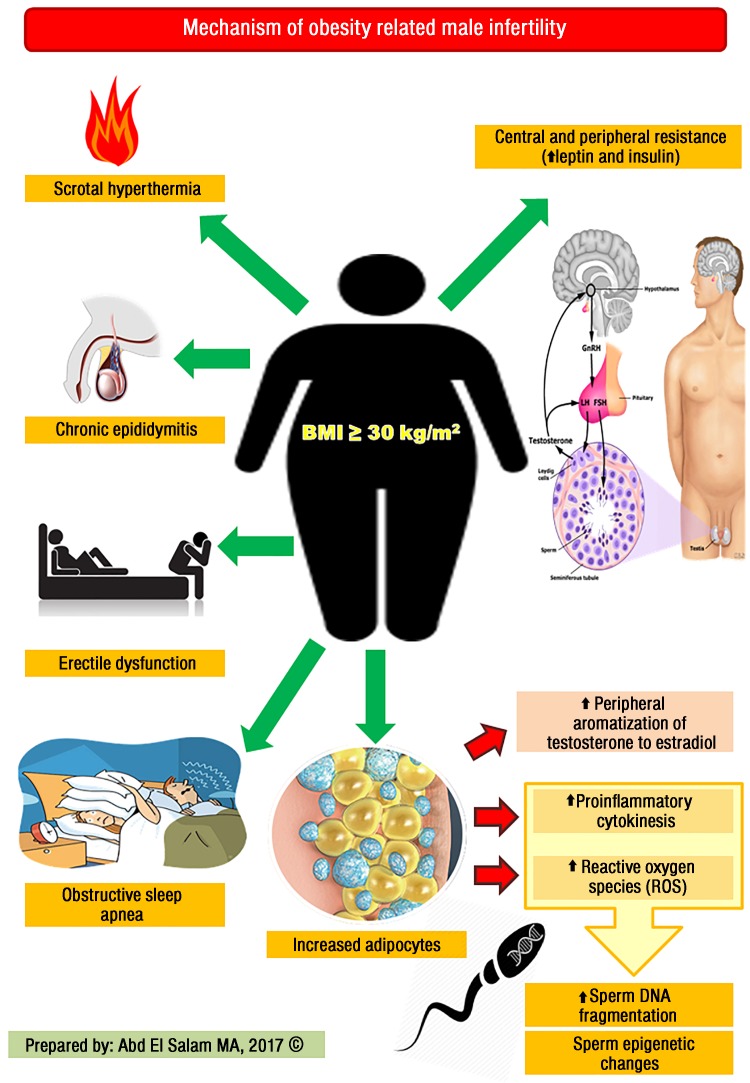
Obesity leads to an increased risk for a wide spectrum of diseases, particularly ischemic heart disease, dyslipidemia, hypertension, type 2 diabetes, stroke, obstructive sleep apnea, certain types of cancer, and osteoarthritis. In addition, obesity may have a negative impact on psychological health due to negative body image, decreased self-esteem, social withdrawal, and depression.7 Several mechanisms have been suggested to have a role in the pathogenesis of obesity-related male infertility and are discussed below [Figure 1].
Figure 1.
Mechanisms by which obesity causes male infertility.
Endocrinopathies
Excess body fat is associated with many endocrinal disturbances that lead to altered spermatogenesis. The most common mechanism is increased peripheral aromatization of testosterone into estrogen by aromatase enzyme with subsequent inhibition of the hypothalamo-hypophyseal-gonadal (HPG) axis.8 Another key hormone that affects male fertility is leptin which is normally secreted by white adipose tissue for regulation of appetite and energy homeostasis. Physiologically, leptin stimulates the secretion of gonadotropin hormones by acting centrally on the hypothalamus to regulate gonadotropin-releasing hormone, neuronal activity, and secretion. However, in obese patients there are elevated levels of serum leptin which leads to the disruption of HPG axis and decreased production of testosterone from Leydig cells.9
Additionally, increased adipose tissue is associated with excess production of cytokines known as adipokines such as resistin, adiponectin, ghrelin, chemerin, visfatin, tumor necrosis factor-a (TNFa), and interleukin-6(IL-6), which have a negative influence on the function of HPG axis with a subsequent effect on spermatogenesis. However, the exact mechanism by which they affect the axis is still unknown.10
Obese men with type 2 diabetes may have secondary hypogonadism due to central and peripheral insulin resistance and the negative effect of proinflammatory cytokines (TNFa and IL-6) on the HPG axis.11 Some studies showed that obesity is associated with reduced levels of sex hormone binding globulin and testosterone with a concomitant rise in the level of estrogen that leads to alteration of spermatogenesis.12
Sperm DNA fragmentation
Obesity is associated with excess production of reactive oxygen species (ROS), which increases sperm DNA damage. The exact mechanisms by which ROS cause DNA damage are not well established. One of these mechanisms is through the production of lipid degradation by-products especially malonaldehyde which either causes oxidation of DNA bases (mainly guanosine) into 8′-hydroxyguanosine (which is promutagenic) or through direct interaction with the DNA strand leading to non-specific single- and double-strand breaks.13
Erectile dysfunction
Erectile dysfunction (ED) has been commonly associated with obesity leading to coital infertility in men. Obesity may cause organic ED due to associated metabolic syndrome, which is a combination of at least three of five of the following: abdominal (central) obesity, hypertension, diabetes, high serum triglycerides, and low high-density lipoprotein levels.14 On the other hand, obesity may cause psychogenic ED as obese people have a negative body image and lack self-esteem. Thus, they consider themselves sexually unattractive and undesirable, causing them to avoid potential or actual sexual relationships.15
Obstructive sleep apnea
Obstructive sleep apnea is a sleep disorder commonly affecting obese patients. It is characterized by sudden pauses of breathing during sleep with interrupted sleep rhythm.16 The exact pathological mechanism is not well established; however, it has been suggested that chronic hypoxia leads to dysfunction of the HPG axis and disruption of nocturnal testosterone rhythm due to sleep fragmentation.17
Hyperthermia
Normally, both testes are located inside the scrotum at a lower temperature ranging between 34–35 °C, which is suitable for the spermatogenesis. In the case of marked obesity, there is excess lower abdominal, suprapubic and medial thigh fat, which increases the intrascrotal temperature altering the process of spermatogenesis. Additionally, increased intrascrotal temperature leads to increased DNA fragmentation and increased oxidative stress with subsequent alteration of semen parameters and sperm functions.18
Chronic epididymitis
Obesity is associated with excess fat in the inner medial aspects of the thighs and suprapubic area, which causes repeated trauma to the scrotal contents (i.e., testis and epididymis). This mechanical trauma may induce inflammation of the epididymis with subsequent alteration of epididymal functions, thus affecting sperm maturation and fertilization. In addition, this chronic inflammation may cause scarring and fibrosis of the epididymal tubules. However, to date, there is no solid evidence for this assumption.19
Epigenetic changes
Epigenetics refers to changes or modifications in a chromosome that affect gene activity and expression that can also be inheritable to offspring. These changes may include methylation, carboxylation and hydroxylation of DNA nucleotides, and histone modifications. Recent research in the field of epigenetics have suggested that paternal obesity can affect offspring metabolic and reproductive phenotypes by the epigenetic reprogramming of spermatogonial stem cells.20
Several approaches have been suggested for the treatment of obesity and its related male infertility including:
Lifestyle modification: The main strategy of losing weight in obese patients is decreasing caloric intake and increasing energy expenditure.21 Few controlled studies have been conducted on obese patients to evaluate the effect of weight reduction strategies on male reproductive capacity. Despite this lack of definitive evidence, there is a consensus among these studies that men who lost weight through diet control and exercise experienced a high increase in androgen and inhibin B levels as well as an improvement in semen parameters.22
Pharmacotherapy: Several therapeutic agents (e.g., orlistat, topiramate/phentermine, lorcaserin, bupropion/naltrexone, and liraglutide) have been suggested for weight reduction and work either by suppression of appetite, decreasing fat absorption from the gut, or increasing caloric expenditure.23 No studies have been conducted concerning their effect on semen parameters or male fertility. However, they may have an indirect effect on male fertility by aiding weight reduction, thus normalizing the testosterone-to-estradiol ratio and decreasing the harmful effects of adipose tissue.
Bariatric surgery: Weight reduction surgeries such as gastric ballooning/banding, sleeve gastrectomy, or gastric bypass are effective lines of treatment in severely or morbidly obese patients after failure of diet and/or exercise and pharmacotherapy to aid weight loss, or if they have associated comorbidities preventing them from achieving their goal. Few studies have been conducted to verify the effect of bariatric surgery and its effects on semen parameters is still debatable.24,25
Aromatase inhibitors: Aromatase inhibitors are a group of drugs used in the treatment of male infertility by inhibiting aromatase P450 enzymes, thus normalizing the testosterone-to-estradiol ratio. They are classified chemically into steroidal and nonsteroidal groups, but currently available aromatase inhibitors include testolactone, anastrozole, and letrozole.26,27 Testolactone as an aromatase inhibitor has been shown to be effective in alleviating infertility as a result of hypogonadotropic hypogonadism of obese male subjects.28 On the other hand, Raman et al29 conducted a study to evaluate the effect of anastrozole on the hormone profile and semen parameters in nonobstructive azoospermic patients who presented with normal or decreased levels of testosterone and elevated estradiol. Anastrozole treatment was found to be effective in normalizing the testosterone-to-estradiol (E2) ratio and total testosterone levels, thus improving semen parameters.
Additionally, letrozole was suggested to normalize serum testosterone levels in severely obese men with hypogonadotropic hypogonadism, and short-term letrozole treatment normalized serum testosterone levels in all obese men in a study carried out by Loves et al.30 However, the clinical significance of this intervention remains to be established in controlled, long-term studies.31
New insights in the pharmacological treatment of male infertility due to obesity might include gonadotropin and/or testosterone replacement therapy, which has been shown to decrease the level of circulating leptin.32 However, not enough information regarding the effect of the treatment on semen parameters is reported.
Assisted reproductive techniques: Finally, assisted reproductive techniques (ART) may be reserved for morbidly obese patients who can not achieve conception and show no improvement in either semen parameters or sperm function after failure of the treatment modalities discussed previously.10
Conclusion
Obesity is a widely spreading pandemic worldwide that has a negative impact on multiple organs, total quality of life, and the fertility potential of males via variable mechanisms. Preventing obesity is key to abolish its potential complications. Variable curative modalities for the negative effects of obesity on semen parameters have been suggested ranging from diet, exercise, and the use of anti-estrogens and/or aromatase inhibitors. Additionally, bariatric surgery is still an option after failure of above measures in morbidly obese men. ART should be spared for cases resistant to treatment.
Disclosure
The author declared no conflicts of interest. No funding was received for this study.
References
- 1.Haslam DW, James WP. Obesity. Lancet 2005. Oct;366(9492):1197-1209. 10.1016/S0140-6736(05)67483-1 [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014. Aug;384(9945):766-781. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008. Dec;32(Suppl 7):S120-S126. 10.1038/ijo.2008.247 [DOI] [PubMed] [Google Scholar]
- 4.Ali A, Crowther N. Factors predisposing to obesity: a review of the literature. S Afr Fam Pract 2010;52(3):193-197 . 10.1080/20786204.2010.10873970 [DOI] [Google Scholar]
- 5.Goni L, Cuervo M, Milagro FI, Martínez JA. A genetic risk tool for obesity predisposition assessment and personalized nutrition implementation based on macronutrient intake. Genes Nutr 2015. Jan;10(1):445. 10.1007/s12263-014-0445-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covasa M. Gut microbiota modulates intestinal signalling in obesity. Appetite 2016;96:643 . 10.1016/j.appet.2015.08.019 [DOI] [Google Scholar]
- 7.Kopelman P. Health risks associated with overweight and obesity. Obes Rev 2007. Mar;8(s1)(Suppl 1):13-17. 10.1111/j.1467-789X.2007.00311.x [DOI] [PubMed] [Google Scholar]
- 8.Phillips K, Tanphaichitr N. Mechanisms of obesity-induced male infertility. Expert Rev Endocrinol Metab 2010;5(2):229-251 . 10.1586/eem.09.65 [DOI] [PubMed] [Google Scholar]
- 9.Hausman GJ, Barb CR, Lents CA. Leptin and reproductive function. Biochimie 2012. Oct;94(10):2075-2081. 10.1016/j.biochi.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 10.Cabler S, Agarwal A, Flint M, du Plessis SS. Obesity: modern man’s fertility nemesis. Asian J Androl 2010. Jul;12(4):480-489. 10.1038/aja.2010.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison CD, Brannigan RE. Metabolic syndrome and infertility in men. Best Pract Res Clin Obstet Gynaecol 2015. May;29(4):507-515. 10.1016/j.bpobgyn.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab 1993. May;76(5):1140-1146. [DOI] [PubMed] [Google Scholar]
- 13.Niederberger C. Re: Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. J Urol 2012. Oct;188(4):1272-1273. [DOI] [PubMed] [Google Scholar]
- 14.Katsiki N, Athyros VG, Karagiannis A. Metabolic syndrome in clinical practice. Cardiol J 2014;21(2):209-209. 10.5603/CJ.2014.0032 [DOI] [PubMed] [Google Scholar]
- 15.Kolotkin RL, Binks M, Crosby RD, Østbye T, Gress RE, Adams TD. Obesity and sexual quality of life. Obesity (Silver Spring) 2006. Mar;14(3):472-479. 10.1038/oby.2006.62 [DOI] [PubMed] [Google Scholar]
- 16.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med 2010. May;11(5):441-446. 10.1016/j.sleep.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 17.Luboshitzky R, Aviv A, Hefetz A, Herer P, Shen-Orr Z, Lavie L, et al. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J Clin Endocrinol Metab 2002. Jul;87(7):3394-3398. 10.1210/jcem.87.7.8663 [DOI] [PubMed] [Google Scholar]
- 18.Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia 2007. Dec;39(6):203-215. 10.1111/j.1439-0272.2007.00794.x [DOI] [PubMed] [Google Scholar]
- 19.Katib A. Mechanisms linking obesity to male infertility. Cent European J Urol 2015;68(1):79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig JR, Jenkins TG, Carrell DT, Hotaling JM. Obesity, male infertility, and the sperm epigenome. Fertil Steril 2017. Apr;107(4):848-859. 10.1016/j.fertnstert.2017.02.115 [DOI] [PubMed] [Google Scholar]
- 21.Lee G, Choi HY, Yang SJ. [Effects of Dietary and Physical Activity Interventions on Metabolic Syndrome: A Meta-analysis]. J Korean Acad Nurs 2015. Aug;45(4):483-494. 10.4040/jkan.2015.45.4.483 [DOI] [PubMed] [Google Scholar]
- 22.Hofny ER, Ali ME, Abdel-Hafez HZ, Kamal Eel-D, Mohamed EE, Abd El-Azeem HG, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010. Jul;94(2):581-584. 10.1016/j.fertnstert.2009.03.085 [DOI] [PubMed] [Google Scholar]
- 23.Patel D. Pharmacotherapy for the management of obesity. Metabolism 2015. Nov;64(11):1376-1385. 10.1016/j.metabol.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 24.Bastounis EA, Karayiannakis AJ, Syrigos K, Zbar A, Makri GG, Alexiou D. Sex hormone changes in morbidly obese patients after vertical banded gastroplasty. Eur Surg Res 1998;30(1):43-47. 10.1159/000008556 [DOI] [PubMed] [Google Scholar]
- 25.di Frega AS, Dale B, Di Matteo L, Wilding M. Secondary male factor infertility after Roux-en-Y gastric bypass for morbid obesity: case report. Hum Reprod 2005. Apr;20(4):997-998. 10.1093/humrep/deh707 [DOI] [PubMed] [Google Scholar]
- 26.Elkhiat Y, Fahmy I. Aromatase inhibitors in the treatment of male infertility. Human Andrology. 2011;1(2):35-38 . 10.1097/01.XHA.0000397087.63955.45 [DOI] [Google Scholar]
- 27.Schlegel PN. Aromatase inhibitors for male infertility. Fertil Steril 2012. Dec;98(6):1359-1362. 10.1016/j.fertnstert.2012.10.023 [DOI] [PubMed] [Google Scholar]
- 28.Zumoff B, Miller LK, Strain GW. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism 2003. Sep;52(9):1126-1128. 10.1016/S0026-0495(03)00186-0 [DOI] [PubMed] [Google Scholar]
- 29.Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol 2002. Feb;167(2 Pt 1):624-629. 10.1097/00005392-200202000-00038 [DOI] [PubMed] [Google Scholar]
- 30.Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol 2008. May;158(5):741-747. 10.1530/EJE-07-0663 [DOI] [PubMed] [Google Scholar]
- 31.Du Plessis SS, Cabler S, McAlister DA, Sabanegh E, Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol 2010. Mar;7(3):153-161. 10.1038/nrurol.2010.6 [DOI] [PubMed] [Google Scholar]
- 32.Isidori AM, Caprio M, Strollo F, Moretti C, Frajese G, Isidori A, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999. Oct;84(10):3673-3680. [DOI] [PubMed] [Google Scholar]