Abstract
Circadian clocks are ubiquitous in eukaryotic organisms where they are used to anticipate regularly occurring diurnal and seasonal environmental changes. Nevertheless, little is known regarding pathways connecting the core clock to its output pathways. Here, we report that the HAD family phosphatase CSP-6 is required for overt circadian clock output but not for the core oscillation. The loss of function Δcsp-6 deletion mutant is overtly arrhythmic on race tubes under free running conditions; however, reporter assays confirm that the FREQUENCY-WHITE COLLAR COMPLEX core circadian oscillator is functional, indicating a discrete block between oscillator and output. CSP-6 physically interacts with WHI-2, Δwhi-2 mutant phenotypes resemble Δcsp-6, and the CSP-6/WHI-2 complex physically interacts with WC-1, all suggesting that WC-1 is a direct target for CSP-6/WHI-2-mediated dephosphorylation and consistent with observed WC-1 hyperphosphorylation in Δcsp-6. To identify the source of the block to output, known clock-controlled transcription factors were screened for rhythmicity in Δcsp-6, identifying loss of circadian control of ADV-1, a direct target of WC-1, as responsible for the loss of overt rhythmicity. The CSP-6/WHI-2 complex thus participates in the clock output pathway by regulating WC-1 phosphorylation to promote proper transcriptional/translational activation of adv-1/ADV-1; these data establish an unexpected essential role for post-translational modification parallel to circadian transcriptional regulation in the early steps of circadian output.
Author summary
Though molecules and components in the core circadian oscillator are well studied in Neurospora, the mechanisms through which output pathways are coupled with core components are less well understood. In this study we investigated a HAD phosphatase, CSP-6; loss-of-function Δcsp-6 strains are overtly arrhythmic but have a functional core circadian oscillation. CSP-6 in association with WHI-2 dephosphorylates the core clock component WC-1 to regulate light-responses and development. To dissect the functions of CSP-6 in core clock and output, we screened known WC-1 targets and found that loss of CSP-6 causes misregulation of transcriptional/translational activation of ADV-1, a key regulator of output. Thus, loss of CSP-6-mediated dephosphorylation of WC-1 leads to loss of ADV-1 activation and is responsible for the complete loss of overt developmental rhythmicity in Δcsp-6.
Introduction
Circadian clocks are endogenous timekeepers that control a wide variety of daily biochemical, physiological, molecular and behavioral rhythms in mammals, plants, insects, fungi and cyanobacteria. The circadian system consists of three essential parts, input, a central oscillator and output [1–4]. In fungi and animals, the backbone of the oscillator mechanism is a transcriptional and translational autoregulatory feedback loop driven by positive and negative elements. The positive element, a heterodimeric transcription factor in which the proteins interact via PAS domains, drives expression of the negative element, a complex of proteins that physically interacts with the positive element to reduce its activity. In the case of Neurospora crassa, the positive elements are the PAS domain containing transcription factors White Collar-1 (WC-1) and White Collar-2 (WC-2) that form the White Collar Complex (WCC). WCC in turn activates transcription of the negative element gene frequency (frq); FRQ nucleates formation of a complex including FRQ Interacting RNA Helicase (FRH) and Casein Kinase 1 (the FRQ/FRH Complex or FFC) that feeds back to physically interact with WCC and suppress frq transcription [5–7]. FRQ is progressively phosphorylated over time, modifications that provide the long time constant for the cycle and that ultimately reduce the affinity of the FFC for the WCC, releasing it to initiate the next cycle of transcription. Eventually hyperphosphorylated FRQ is turned over via a ubiquitin-mediated pathway, but in a normal circadian cycle the kinetics of this turnover is not believed to influence the period length of the clock [8,9].
Both WC-1 and WC-2 are phosphorylated in vivo under circadian conditions and become hyperphosphorylated after a short light exposures [10,11]. In the current model of the circadian feedback loop, the FRQ-FRH complex (FCC) closes the loop by inhibiting WCC activity via the promotion of phosphorylation of WCC, primarily through kinases CK-1a and CKII [12–14]. The importance of WCC phosphorylation for circadian oscillation has been argued based on short period, low amplitude, phase shifted and arrhythmic phenotypes resulting from mutations of phosphorylation sites on WCC [15–17]. In the current model, hyperphosphorylated WCC is believed to be inactive but stable whereas hypophosphorylation WCC is active and supports transcriptional activation of frq and other genes [12–14]. PP2A (protein phosphatase 2A) is believed to dephosphorylate WC-1 in vivo and this is correlated with an increase frq RNA levels [2,18].
In addition to its clock functions, WC-1 and WC-2 (WCC) comprise the blue light photoreceptor that initiates the organism’s principal photoresponse. Upon illumination the WCC undergoes a rapid conformation change, binding to light-responsive elements (LREs) via WC-2 and functioning as a TF to bind to and regulate the expression of hundreds of light-responsive genes [10,19–22]. Similar to WCC functioning in the dark, hyperphosphorylated WC-1 is believed to be transcriptionally inactive and hypophosphorylated WC-1 transcriptionally active. Consistent with this are reports that hyperphosphorylated WC-1 binds less strongly to target promoters while dephosphorylation of WC-1 increases promoter binding [23,24]. VVD (VIVID), a small PAS/LOV protein and another blue light receptor, acts as a repressor of the light response through its physical interaction with the WCC [11], and recent studies have shown the photocycle length of VVD plays a dominant role in determining the utility of the photoreceptor [25]. Though VVD is not required for clock rhythmicity, it modulates various WCC-mediated circadian clock properties such as gating of light input of clock and phasing light resetting responses. Loss of function vvd mutants exhibit a 4-hour delay of clock-controlled conidiation [26,27].
Time of day information generated by the circadian clock is transduced to clock control genes (ccgs) whose time-of-day specific expression yields products, the output pathway, that generate overt rhythmicity in the cell [28,29]. The best-characterized and most easily monitored output of the FFC/WCC Oscillator is the conidiation rhythm [30,31]. Though enormous advances have been seen in understanding core oscillators of Neurospora in the past two decades, how circadian oscillators signal through output pathways to control rhythmic activity of those ccgs remains only partially understood at molecular level [9,30]. Most recently, clock-controlled genes showing consistent rhythms and comprising as much as 40% of the genome have been identified in N. crassa by RNA-seq [32]. While these will provide a great resource for studying rhythmic behavior in the future they have afforded limited insight into the connection between the core clock and individual outputs.
Here we characterize functions of a HAD-domain family phosphatase protein, CSP-6, revealing its essential role in regulating circadian output pathways including conidiation rhythms and phase resetting. CSP-6 physically interacts with a partner, WHI-2, and this phosphatase complex, which interacts with WC-1, is important for maintaining WC-1 protein and phosphorylation levels. Loss of function of csp-6 results in constitutively hyperphosphorylated WC-1 but does not ablate core oscillator function, although the clock shows a 3.5-h phase delay due to reduced amounts and hyperphosphorylation of WC-1. This indicates that the regulation of genes and proteins acting downstream of WC-1 to control circadian developmental processes has been disrupted in Δcsp-6, and our results are consistent with a model in which ADV-1, a direct target of WC-1, plays this direct role in regulating overt clock output [33]. Prior to this finding the early steps of circadian output have been viewed primarily as a transcriptional network of activators and repressors. CSP-6 demonstrates an essential role for post-translational modifications in the early steps of output.
Results
CSP-6 is required for overt rhythmicity but retains a functional clock
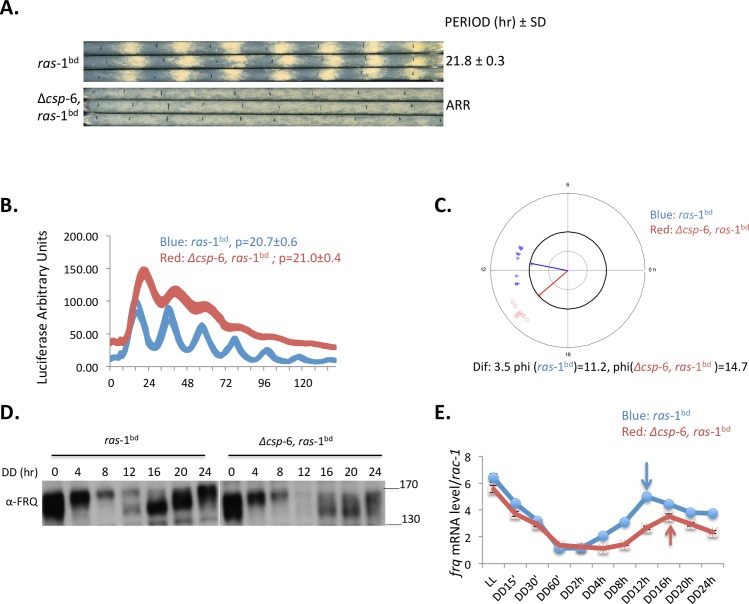
The csp-6 gene (NCU08380) encodes a member of the haloacid dehalogenase (HAD) superfamily containing a conserved dullard-like phosphatase domain, (S1A Fig, HAD domain 222–388 aa). It was first reported in a screen of putative phosphatases as having a conidial separation phenotype [34] and was later reported (under the name psr-1) to be more broadly involved in female sexual development, cell-cell-fusion and autophagy [35]. Because the conidial separation defect was similar to that seen in mutants of csp-1 and csp-2, two transcription factors important for conidiation and the circadian clock, we asked whether Δcsp-6 has similar phenotypes. We crossed the Δcsp-6 deletion mutant with the ras-1bd mutant and performed race tube assays (Fig 1A) revealing that, unlike the ras-1bd strain, Δcsp-6, ras-1bd show reduced hyphal growth and arrhythmic conidiation. Use of frq-luc to report clock core oscillator function [36] in this background (Δcsp-6, ras-1bd, csr::c-box-luc), however, revealed rhythmic frq expression with a wild type period length around 22 h, albeit having a somewhat reduced amplitude and a 3.5-hour phase delay (Fig 1B and 1C). While luciferase reporter data as seen in Fig 1B is an excellent indicator of rhythmicity because of the density of time points, the amount of bioluminescence produced is a function of growth and development as well as the clock; Δcsp-6-induced changes in growth characteristics could thus influence bioluminescent output, so we also examined frq and FRQ biochemically to more directly view the effect of Δcsp-6 on the oscillator components. Analysis of mRNA by RT-qPCR and protein by Western blotting confirmed core clock rhythmicity, showing that FRQ oscillated with a small loss of amplitude in Δcsp-6 mutants and displayed a delay of around 4 hours (Fig 1D), and frq mRNA levels rhythmic but again phase delayed about 4 hours (Fig 1E).
Fig 1. Loss of csp-6 abolishes conidiation rhythms and results in a phase delay in frq transcriptional and FRQ translational rhythms.
A: Race tube assays of WT ras-1bd and Δcsp-6, ras-1bd strains. Period is reported in hours ± one standard deviation, ARR is arrhythmic. B: Analyses of luciferase activity rhythms of frq C-box-luc in WT (blue) and Δcsp-6 (red). Strains were grown on race tube medium in a 96 plate under constant light for 48 hrs and then transferred to constant dark. The luciferase signals were captured every hour for 6 days. Three replicates for each strain are shown and ‘p’ stands for period. C: Determination of phase delay in Δcsp-6. Primary time series data from the experiments shown in panel B were analyzed using a Matlab-based program [60]; Dif. corresponds to the phase difference between ras-1bd and Δcsp-6, ras-1bd, and phi reports the average phase of the rhythm in each genetic background. D: FRQ protein remains rhythmic in the Δcsp-6 mutant. Shown is a Western blot of FRQ protein in ras-1bd and Δcsp-6, ras-1bd, the hours in constant darkness are shown above the blots. Protein maker(s) were used as with the molecular weight (KDa) as indicated. E: frq mRNA accumulates with a circadian rhythm in the Δcsp-6 mutant as it does in wild type. frq mRNA expression was assayed by qRT-PCR and normalized to rac-1 in ras-1bd and ras-1bd,Δcsp-6; frq mRNA peaks at DD16 in Δcsp-6 instead of DD12 in WT.
We also followed FRQ and frq levels from DD24h to DD48h during the second day after moving from LL to constant DD. The results showed FRQ protein levels were reduced but we still can observe rhythmicity in FRQ amounts along with its phosphorylation (S1B Fig), consistent with c-box-luc luciferase traces in Δcsp-6. The frq mRNA level was reduced in Δcsp-6 but the amount still oscillated with a peak time of DD36h, 4 hours delay compared to wild type (S1C Fig). These data indicated that CSP-6 plays a role in maintaining robust frq/FRQ expression but is not required for the clock core oscillation. So we concluded that the arrhythmic conidiation on race tubes in the Δcsp-6 mutant was caused by disruption of a circadian output pathway.
The csp-6 promoter shows very weak circadian regulation when assayed by luciferase fusion (S1D Fig), but csp-6 is not a typical clock-controlled gene involved in circadian output. The weak transcriptional regulation detected by luciferase is also seen in a translational fusion reporter of CSP-6 but not confirmed by Western analysis (S1E and S1F Fig), so its action appears more likely to facilitate output rather than to drive it. Saccharomyces contains both an ortholog and a paralog of csp-6, psr1 and psr2 respectively, both of which arose together in screens and share similar mutant phenotypes, interactors, and functions (http://www.yeastgenome.org/locus/S000004009/overview); in yeast these phosphatases function to regulate the stress response [37]. Likewise, Neurospora has a csp-6 paralog (NCU08948 provisionally named pph-11 [34]) that contains the same conserved HAD phosphatase domain as csp-6 (S2A Fig, underlined sequences). To investigate whether csp-6 and its paralog are both involved in circadian clock output, conidiation rhythms were followed on race tubes in ΔNCU08948 (psr-2, ras-1bd) (S2B Fig). The ΔNCU08948, ras-1bd mutant showed normal overt rhythmicity although it grew a bit slower compared to the wild type. Assay of the core circadian oscillator via a frq-luc reporter confirmed a robust circadian rhythm (S2C Fig) indicating that only csp-6 but not its paralog is involved in circadian function in N. crassa, and unlike Saccharomyces, the two paralogs have some distinct functions.
CSP-6 associates with the WCC and acts as a phosphatase on WC-1
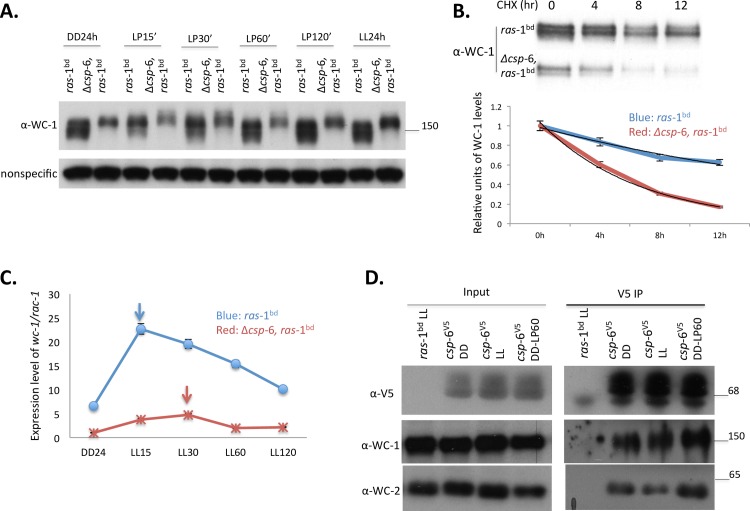
Because the WCC drives frq expression and we observed delayed transcriptional and translational rhythms of frq/FRQ (Fig 1C–1E), we examined the expression of WC-1. As shown in Fig 2A, the protein levels of WC-1 in DD (constant dark), LL (constant light) or following a light pulse (LP) were significantly reduced in Δcsp-6, and WC-1 was hyperphosphorylated in Δcsp-6 in all conditions we examined (Fig 2A). To confirm that the mobility shift of WC-1 in Δcsp-6 is caused by phosphorylation, total protein of wild type and Δcsp-6 were treated with λ-phosphatase. The results showed that WC-1 in both wild type and Δcsp-6 collapsed to the same level in both tested time points, constant dark 24h and light pulse 15min, indicating the low mobility form of WC-1 in Δcsp-6 is a hyperphosphorylation form of WC-1 (S3A Fig).
Fig 2. CSP-6 interacts with WC-1 to regulate its phosphorylation status, stability and expression.
A: Western blot analysis showing the amount and degree of modification of WC-1 in ras-1bd and Δcsp-6, ras-1bd strains. Nonspecific bands were shown for equal loading. The cultures were harvested at the indicated time points. (DD24: constant darkness for 24h; LP15-120’: Light pulse for 15-120min after DD24; LL: constant light. B: WC-1 is less stable absent CSP-6. Representative Western blots of WC-1 following addition of CHX show the rate of WC-1 degradation (Upper). Results of densitometric analysis (Lower) for ras-1bd (blue) and Δcsp-6, ras-1bd (red) +/- SD. C: Light-induction of wc-1 is reduced in Δcsp-6. RNA was isolated from ras-1bd and Δcsp-6, ras-1bd, and amounts of wc-1 mRNA quantified by qRT-PCR. D: Co-IP demonstrating physical interaction between CSP-6 and WC-1 in vivo. CSP-6 was tagged with a V5 epitope at its C-terminal and immunoprecipitation was performed using V5 agarose beads. Western blot of V5-purified CSP-6 shows WC-1 was pulled down in all indicated conditions together with WC-2. Protein maker(s) were shown as with the molecular weight (KDa) as indicated.
In addition, WC-1 is less stable in Δcsp-6 than in wild type indicating that CSP-6 stabilized the WC-1 protein (Fig 2B). Furthermore and consistent with Western analysis, wc-1 mRNA levels were significantly reduced in Δcsp-6 under all conditions as well, and, although wc-1 is still light-induced, it took longer (LP30 min) to reach peak in the Δcsp-6, indicating that the light response was affected in Δcsp-6 (Fig 2C). WC-1 is required for light-induced carotenogenesis and cultures of Δcsp-6 were pale pink instead of orange, so we tested expression level of three albino genes in the Δcsp-6 mutant (S3B Fig). All three albino genes (al-1, al-2, al-3) showed reduced mRNA levels in Δcsp-6 consistent with reduced carotenoid accumulation and the pale color in Δcsp-6 (S3C Fig). Additionally, we examined the mRNA expression level of a few light induced genes al-1, al-3, sub-1 and frq, following light pulses in order to understand whether hyperphosphorylated WC-1 in Δcsp-6 would affect their light response. Interestingly, sub-1 and frq showed normal kinetics in Δcsp-6 while al-1 and al-3 showed a delayed light response compared to the wild type, and al-1, al-3, sub-1 all had reduced expression in Δcsp-6 (S3D Fig). These data suggested that the inability to dephosphorylate WC-1 in Δcsp-6 impacted expression its downstream targets.
The hyperphosphorylation of WC-1 observed in the Δcsp-6 background suggested that WC-1 could be a direct target of CSP-6. To determine whether CSP-6 interacts with WC-1, co-immunoprecipitation (co-IP) assays were performed using CSP-6 epitope tagged with V5 on its C-terminus, under conditions of DD, LL and LP60min. The data show that CSP-6 can interact with WC-1 to regulate WC-1 phosphorylation under all tested conditions (Fig 2D).
Additionally, although we noticed a reduced level of WC-2 in Δcsp-6, the phosphorylation level of WC-2 was not significantly changed in the Δcsp-6 mutant indicating that CSP-6 more specifically targets WC-1 (S3E Fig). Interaction was also detected between CSP-6 and WC-2 (Fig 2D), although we expect this is most likely indirect, reflecting the interaction between CSP-6 and WC-1 which heterodimerizes with WC-2.
Structure function analysis of CSP-6 shows the HAD domain is sufficient partially to rescue output defects in a manner dependent on glucose concentration
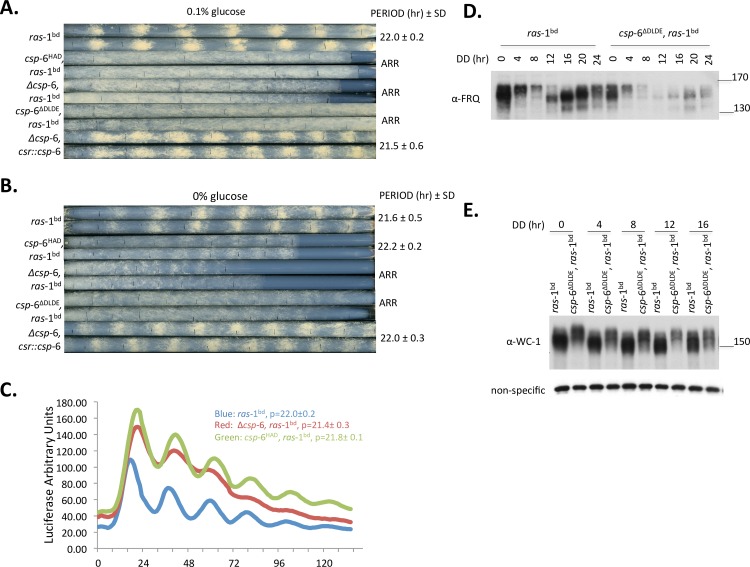
In CSP-6, only the HAD phosphatase domain is predicted to dephosphorylate its substrates (S1A Fig). To determine whether the HAD domain was sufficient to rescue defects caused by deletion of csp-6, we transferred a construct bearing only the HAD domain (csp-6HAD, amino acids 222–388, under the control of 1.5 kb of the native csp-6 promoter) to the csr locus. The resulting transformants failed to produce overt rhythmicity on race tubes while the full length csp-6 construct successfully complemented the banding defect though with slightly reduced growth rate (Fig 3A). To exclude the possibility that the N-terminal region of csp-6 might be sufficient for circadian function, we also generated construct csp-6ΔHAD (amino acids 1–221), lacking the HAD domain, and transformed it into Δcsp-6. The resulting transformants were as arrhythmic as Δcsp-6 on race tubes.
Fig 3. The catalytic HAD phosphatase domain can partially rescue Δcsp-6 phenotypes dependent on glucose concentration.
A: Race tube assays of wild type ras-1bd; Δcsp-6, ras-1bd; Δcsp-6, csr::csp-6HAD,ras-1bd; Δcsp-6,csr::csp-6ΔDLDE, ras-1bd; and Δcsp-6, csr::csp-6, ras-1bd(a full-length csp-6 complementary strain) on race tube medium containing 0.1% glucose. All complementation constructs were transformed into Δcsp-6 by targeting to the csr locus. Duplicate race tubes for each strain are shown. Period is reported in hours ± one standard deviation; ARR is arrhythmic. B: Race tube assay for the same sets strains on race tube medium without glucose, showing csp-6HAD expresses conidiation rhythms after 2-day under free running in constant darkness. C: Analyses of frq transcriptional rhythmicity in, Δcsp-6, ras-1bd and csp-6HAD, ras-1bd by luciferase activity. ‘p’ stands for period. D: Western blot analysis showed that FRQ protein remains rhythmic in csp-6ΔDLDE, ras-1bd. Cultures were harvested in constant darkness at the indicated time. E: Western blot analysis showing hyperphosphorylated WC-1 in csp-6ΔDLDE, ras-1bd strains at the indicated times in darkness. Protein maker(s) were used with the molecular weight (KDa) as indicated.
However, one interesting phenomenon emerged when transformants were examined under conditions of glucose depletion. After a few days, transformants bearing csp-6HAD showed weak conidiation rhythms whereas no banding was detected in Δcsp-6 under the same conditions (Fig 3B). To confirm core clock function in csp-6HAD we used the frq c-box-luc transcriptional reporter, showing that csp-6HAD had the delayed phase seen with Δcsp-6 but with more robust rhythmicity than that of Δcsp-6. (Fig 3C). These results indicated that the HAD domain itself could partially rescue clock defects caused by loss function of CSP-6 under tested conditions.
HAD phosphatases, which have essential Asp residues in their catalytic domains, are emerging as a large family existing in plants, prokaryotes and mammals. Their conserved active sites have a consensus sequence hhhDxDx (T/V)(L/V) h, where h represents a hydrophobic residue, and x indicates any amino acid [38], the two aspartates coordinating the essential Mg2+ in the active site. In the yeast, mutation of DXDX(T/V) motif cannot functionally complement the psr1/psr2 mutant. It is essential for its sodium stress response suggesting that mutation of DXDX disrupted its phosphatase activity [37]. To investigate if the DxDx motif (DLDE) in CSP-6 that is essential for phosphatase activity is also essential for its circadian function (S2A Fig), we generated a csp-6ΔDLDE construct and transferred it into the csr locus of a Δcsp-6 mutant. Race tubes of csp-6ΔDLDE failed to show overt rhythmicity with or without glucose (Fig 3A and 3B). Western blot analyses showed that compared to the wild type, FRQ protein along with phosphorylation level of csp-6ΔDLDE was rhythmic as that shown in Δcsp-6 (Fig 3D). However, similar to Δcsp-6, the FRQ level csp-6ΔDLDE was reduced significantly after moving to dark, which is consistent to the lower level WC-1 in csp-6ΔDLDE (Fig 1D, Fig 3D and 3E). Additionally, we detected hyperphosphorylated WC-1 in csp-6ΔDLDE under constant dark and light (Fig 3E, S3A Fig). These data confirmed that the conserved DLDE motif essential for phosphatase activity was critical for CSP-6 circadian activity.
CSP-6 interacts genetically with VVD and reduces the physical interaction between VVD and WC-1
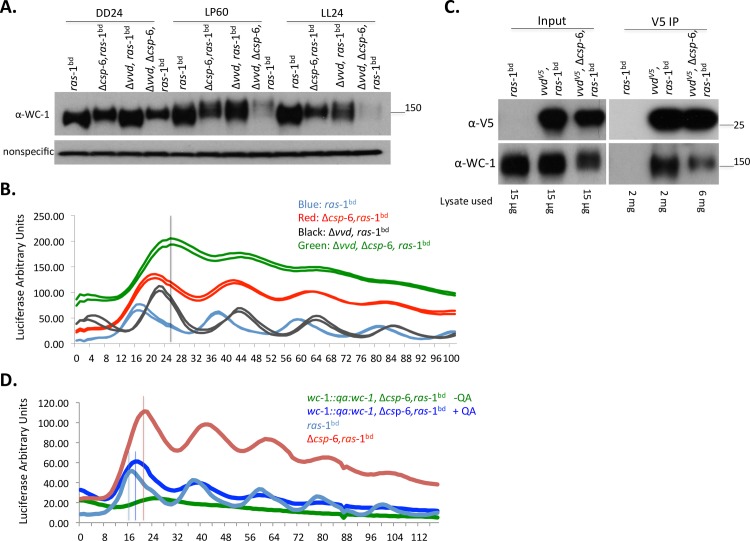
The Δcsp-6 strain displayed a four-hour phase delay, as well as a reduced level of WC-1 that is hyperphosphorylated (Figs 1 and 2), all similar to phenotypes observed in the Δvvd mutant [26]. Evidence for genetic interaction between csp-6 and vvd was seen when Δcsp-6, Δvvd, ras-1bd, csr::c-box-luc cultures growing on minimal slants showed enhanced carotenoid accumulation (S3B Fig), and this was confirmed at the protein level when Western blot analyses showed much less WC-1 in the double mutant especially in the light, indicating that deletion of csp-6 and vvd together had synergistic effects compared to the individual mutants (Fig 4A). Surprisingly, even with dramatically reduced WC-1 levels, the luciferase activity driven by frq c-box in the Δcsp-6, Δvvd double mutant appeared robust. Furthermore, a nearly 8 hr phase delay was detected in the double mutant and rhythmicity was dampened after three cycles, a plainly additive or synergistic effect as neither was observed in the single mutants Δcsp-6 or Δvvd (Fig 4B). These data suggest that CSP-6 and VVD contribute to the separate but parallel pathways, based on the synergistic phenotype of double mutants.
Fig 4. Reduced interaction between WC-1 and VVD in the Δcsp-6 mutant affects WC-1 phosphorylation and the phase of frq expression.
A: Western blot analyses showing WC-1 levels in ras-1bd; Δcsp-6 ras-1bd, Δvvd, ras-1bd and Δcsp-6, Δvvd, ras-1bd under indicated conditions. B: Representative luciferase traces of frq c-box-luc showing a further phase delay in the Δcsp-6, Δvvd double mutant as compared to either single mutant. C: VVD-WC-1 interaction at LP60min in both vvdV5 and vvdV5,Δcsp-6 in the ras-1bd background; ras-1bd was used as negative control. VVD-V5 was immunoprecipitated using anti-V5 agarose beads. Immunoprecipitates were analyzed by Western blot using WC-1 antibody. Because reduced WC-1 was detected in the Δcsp-6 mutant, we used three times more extract for the IP of vvdV5, Δcsp-6 (6mg) than those of wild type (2mg) background, based on the quantification by Western blot (S4A Fig) to make sure similar amounts of WC-1 were available for IP. D: Representative luciferase activity assays of frq-luc in strain wc-1::qa:wc-1, Δcsp-6 in the presence or absence of 0.01M QA, showing that increased WC-1 levels can partially rescue the phase delay phenotype of Δcsp-6.
VVD governs photoadaptation and influences light responses [26] and does so by interacting physically with WC-1 [11]. Because weaker light-induction and delayed photoadaptation was detected in the Δcsp-6 mutant (Fig 2C), we hypothesized that a disruption of the interaction between VVD and WC-1 could be a contributing factor. To test this, we used a vvdV5, Δcsp-6 strain and performed a Co-IP assay between VVD and WC-1, adjusting the input amounts to 3 times (6mg) more than that used in the wild type (2mg) so that equivalent WC-1 was present in all samples (Fig 4C, S4A Fig). These data indicate a substantial reduction in the amount of WC-1 interacting with VVD in the Δcsp-6 mutant compared to wild type, even with comparable amounts of WC-1 input (Fig 4C). This reduced interaction between VVD and WC-1 might result in the weaker light response seen in the Δcsp-6 mutant, and combined with the reduced level of WC-1 seen in Δcsp-6 could also underlie the additive phase delay.
To test this hypothesis we used a strain in which wc-1 expression was driven by the inducible qa-2 promoter at the native wc-1 locus [14] in the Δcsp-6, ras-1bd, csr::C-box-luc background. Under quinic acid (QA) induction (10-2M QA), high levels of WC-1 comparable to wild type can be expressed constitutively in the Δcsp-6 mutant (S4B Fig) and hyperphosphorylated WC-1 can be detected as well in the qawc-1, Δcsp-6 strains. The wc-1::qa:wc-1, Δcsp-6 strain was arrhythmic absent inducer and rhythmic with inducer for the first 3 cycles and the enhanced WC-1 expression in wc-1::qa:wc-1, Δcsp-6 substantially rescued the phase delay phenotype, reducing the 4 hr phase delay to around two hours (Fig 4D). To exclude the possibility that quinic acid caused the dampening in wc-1::qa:wc-1, Δcsp-6, we also tested the rhythmicity of wc-1::qa:wc-1 grown with 10-2M QA; the results showed that wc-1::qa:wc-1 displayed robust rhythmicity through tested 5 days (S4C Fig). Although the QA-induced increase in WC-1 protein level in the Δcsp-6 mutant largely rescued the phase delay phenotype, race tube analysis showed that QA-induced WC-1 failed to rescue the overt conidiation rhythm in Δcsp-6 (S4D Fig), indicating that downstream WCC targets or certain clock-control genes regulating circadian output were misregulated in the Δcsp-6 mutant and this most likely was caused by the hyperphosphorylated WC-1 but not the reduced WC-1 amount.
CSP-6 forms a complex with WHI-2 and plays a major role in the complex
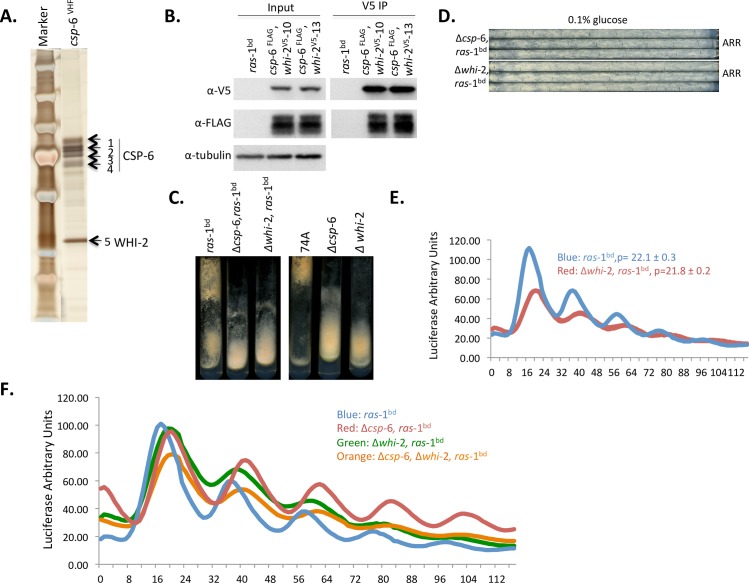
To identify CSP-6-associated proteins, CSP-6 was epitope tagged with VHF (V5, His and FLAG tandem tag) and used to purify CSP-6 by tandem affinity purification using FLAG agarose followed by V5 magnetic beads. Mass spectrometric analysis showed that the four bands (labeled as 1–4 from silver staining) clustered together around 70kDa are all CSP-6, and the one band below was identified as a weak ortholog (BLASTP e-11) of Saccharomyces WHI-2 encoded by NCU10518 (Fig 5A). Two translational start sites were found in the csp-6 5’UTR suggesting that CSP-6 has two protein isoforms with a size difference of 5.5 kDa (S5A Fig). We also performed phosphatase treatment on V5-purified CSP-6 and the results showed that the proteins can apparently be dephosphorylated indicating that, like its Saccharomyces ortholog Psr1p, post-translational modification occurs to CSP-6 after it is synthesized (S5B Fig).
Fig 5. CSP-6 associates with WHI-2 in vivo, and loss of whi-2 results in phenotypes similar to Δcsp-6.
A: Silver stained SDS-PAGE gel showing CSP-6 associated with WHI-2 (band 5) as confirmed by MS-based protein sequencing. Bands 1–4 correspond to CSP-6 itself based on Mass Spec data (S2 Table). B: Co-immunoprecipitation assay showing CSP-6 physically interacts with WHI-2 in vivo. A wild type strain (ras-1bd) that lacks the epitope tag was used as a negative control. Two individual transformants (-10 and -13) of csp-6FLAG, whi-2V5 were used in Co-IPs and Western analyses. C: Cultures of WT (ras-1bd or 74A), Δcsp-6 and Δwhi-2 (with or without ras-1bd respectively) were grown on solid minimal slants showing similar growth morphology between Δcsp-6 and Δwhi-2. D: Race tube data showing loss of overt conidiation rhythms in Δwhi-2; triplicates of each strain are shown. E: Luciferase traces showing frq-luc was circadianly rhythmic but expressed with a delayed phase in the Δwhi-2 mutant. ‘p’ stands for period. F: Luciferase activity assay of frq C-box-luc in strains of ras-1bd, the Δcsp-6, ras-1bd strain, the Δwhi-2, ras-1bd strain, and double mutant Δcsp-6, Δwhi-2, ras-1bd strain, showing the phase delay seen in Δcsp-6 and in the double mutant Δcsp-6, Δwhi-2 was similar.
Saccharomyces Whi2p is a general stress regulator protein known to interact physically and genetically with Psr1p and Psr2p, the ortholog and paralog of CSP-6 respectively, and is believed to activate them; knockouts of any members of the Psr(s)/Whi-2 complex in yeast share similar phenotypes [39,40]. We confirmed the interaction between CSP-6 and WHI-2 in Neurospora by performing Co-IP (Fig 5B), and then used a Δwhi-2 strain obtained from the Neurospora knockout collection [41] to ask whether WHI-2 played a role in the circadian system. Δwhi-2 showed growth, morphology, and circadian phenotypes similar to Δcsp-6 including slow growth, reduced conidiation, circadian output defects on race tubes, a phase delay in the core clock (assayed by frq c-box-luc reporter), and increased WC-1 phosphorylation level (Fig 5C–5E, S6A Fig). However, in most cases the defects in Δwhi-2 were not as severe and were in all cases hypostatic to those seen in Δcsp-6: e.g., the phase delay in Δwhi-2 was not as severe as Δcsp-6 (Fig 5F), and WC-1 protein amounts were not reduced significantly in the Δwhi-2 mutant (S6A Fig). The double mutant Δcsp-6, Δwhi-2 had WC-1 levels similar to Δcsp-6, and no further hyperphosphorylation of WC-1 was detected in the Δcsp-6, Δwhi-2 double mutant (S6B Fig) compared to Δcsp-6. These results suggest that in the CSP-6/WHI-2 complex, CSP-6 plays a major role in regulating circadian related phenotypes including phase and conidiation rhythmicity, while WHI-2 is more like an assistant to fully activate CSP-6.
WCC-mediated circadian and light regulation of adv-1 is abolished in the Δcsp-6 mutant
Although driving WC-1 protein amounts to wild type levels can partially rescue the phase delay phenotype of Δcsp-6 mutant (Fig 4D), it failed to rescue circadian rhythmicity on the race tubes (S4D Fig). These data indicated a break in circadian output control downstream from the WCC in the absence of CSP-6, so we sought the source of the break in control. Because in Saccharomyces Psr1p functions together with Whi2p to activate stress responses and mediate gene expression through the stress-responsive transcription factor MSN2p [39], we asked whether the ortholog of yeast MSN2 in N. crassa, that is MSN-1, a cutinase G-box binding protein encoded by NCU02671, was involved in clock output. The Δmsn-1 mutant, however, displayed normal circadian banding and period length (though with a reduced growth rate; S7A Fig) and frq c-box-luc reporter assays showed that Δmsn-1 had a robust circadian rhythm (S7B Fig), all data indicating that in Neurospora CSP-6 does not act through msn-1 to regulate circadian output. Because Ghosh et al (2014) had suggested that csp-6 might act in the same pathway as csp-1 [42] to regulate growth and conidiation, we also confirmed that transcriptional rhythmicity of csp-1 was not affected in the Δcsp-6 mutant (S7C Fig). After this and following the genetic principle of epistasis, we screened transcription factors known to be targets of the WCC [21,22] for circadian output defects, showing regulation of fluffy (a major regulator of conidiation) to be still weakly rhythmic in Δcsp-6 (S7D Fig) before rediscovering that deletion of adv-1 (NCU07392) results in defects similar to Δcsp-6 (Fig 6A) [22]. Though deletion of csp-6 did not affect the oscillation of csp-1 and fluffy promoter activity, we found that the transcriptional expression level of csp-1 and fluffy was reduced in Δcsp-6 compared to that in the wide type (S7E Fig).
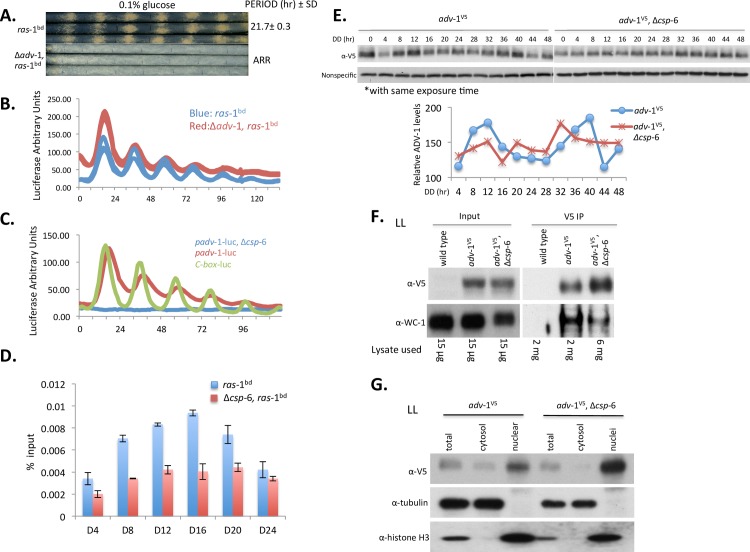
Fig 6. ADV-1 is a direct downstream target of CSP-6-WC-1 that regulates circadian output.
A: Race tube data showing loss of overt conidiation rhythmicity in Δadv-1; triplicate race tubes are shown. Period is reported in hours ± one standard deviation, ARR is arrhythmic. B: Luciferase traces of frq c-box-luc in the ras-1bd and Δadv-1, ras-1bd genetic backgrounds confirming that the clock functions normally in Δadv-1. C: Representative luciferase activity assays showing circadian rhythmicity of adv-1 promoter activity was significantly impaired in the Δcsp-6 mutant. D: ChIP assays were performed in a time course across a circadian cycle from DD4 to DD24 to show the recruitment of WC-2 to the adv-1 promoter in the ras-1bd and Δcsp-6, ras-1bd. Error bars reflect +/- 1SD, n = 3. E: Western blot analysis of protein expression of V5-tagged ADV-1 in WT and Δcsp-6 (Upper). The data shows protein ADV-1 is expressed rhythmically in the wild type strains but the rhythmicity was lost in Δcsp-6. Densitometric analysis of these data is shown in the lower panel. F: Co-IP assay demonstrating ADV-1 and WC-1 interaction using DSP crosslinking in constant LL. V5-tagged ADV-1 was co-immunoprecipitated with anti-V5 agarose beads and WC-1 was detected with anti-WC-1 antibody. To compensate for the reduced WC-1 present in Δcsp-6, 3 fold more lysate was used for the V5-IP in adv-1V5, Δcsp-6 as compared to adv-1V5; even so, reduced interaction between ADV-1 and WC-1 was detected in Δcsp-6 compared to WT. G: ADV-1 protein is enriched in nuclei. Subcellular fractions from adv-1V5 in WT and Δcsp-6 were analyzed with the indicated antibodies. Tubulin was used as a control to show nuclear fraction was not contaminated with cytosolic protein and histone H3 was used to show enrichment of the nuclear fraction.
ADV-1 is a transcription factor previously shown broadly to affect development [41], to be robustly regulated by light [21] and the clock, and to be required for the overt rhythm in conidiation on race tubes [22,33]. Consistent with prior data [22] the frq-luc reporter remained robustly rhythmic in Δadv-1 indicating that ADV-1-regulated circadian output does not impact the core oscillation (Fig 6B) but importantly, by comparing adv-1 transcriptional activity in wild type and Δcsp-6 backgrounds, it is clear that the normally rhythmic transcription of adv-1 is lost in Δcsp-6 (Fig 6C).
Because adv-1 is a direct downstream target of WC-1 [22], we hypothesized that the arrhythmicity of adv-1/ADV-1 in Δcsp-6 might be caused by the misregulation of WCC binding efficiency at the adv-1 promoter. ChIP assays using WC-2 antibody were performed across a time course from 4h to 24h in darkness. The results showed that rhythmic WCC-binding at adv-1 promoter sites was disrupted in the Δcsp-6 mutant as compared to wild type (Fig 6D). Binding was also attenuated following light pulses (LP15min) (S8A Fig). The loss of circadian regulation of ADV-1 in Δcsp-6 was also confirmed at the protein level (Fig 6E) demonstrating that CSP-6 is essential for rhythmicity of adv-1/ADV1-1 at both transcriptional and translational levels.
Because of the disruption of WCC binding to the adv-1 promoter elicited by loss of csp-6 we asked whether there was a corresponding disruption of oscillator-relevant binding to the C-box within the frq promoter in Δcsp-6 (S8B and S8C Fig); overall binding was reduced roughly half in first 24h in darkness and even more significantly reduced WCC binding was detected in day two from 28-48h in darkness. Consistent with the frq-luc luciferase data in Fig 1B, deletion of csp-6 did not affect the rhythmicity of FRQ, but its robust expression. These data again suggest that the phosphorylation status of WC-1 as impacted by loss of CSP-6 has a discrete effect on circadian output, and moreover the effect may not be only on the ability of WC-1 to bind to the adv-1 promoter but also on its ability to activate expression from it.
We asked separately whether CSP-6 can physically interact with ADV-1 to regulate its transcriptional and translational activity and confirmed via pull down assays using an epitope tagged adv-1V5, csp-6FLAG strain that no direct interaction between CSP-6 and ADV-1 was detected under the conditions used (S9A Fig). In addition, CSP-6 does not regulate the light response of ADV-1 though slightly reduced ADV-1 protein level was detected in Δcsp-6 (S9B Fig). A dephosphorylation assay was also performed to further confirm that CSP-6 did not function on ADV-1 directly as a phosphatase (S9C Fig). Taken altogether these data are most easily interpreted as showing that hyperphosphorylation of WC-1 caused by loss of CSP-6 reduced the binding efficacy of the WCC at the adv-1 promoter and that CSP-6 regulates circadian output and light-regulation via impacting transcription of adv-1 through the WCC. However, in addition to disruption of rhythmic WCC binding to the adv-1 promoter, reduced interaction between ADV-1 and WC-1 was also detected by Co-IP (Fig 6F); these experiments used three times more IP protein from the adv-1V5, Δcsp-6 (6mg) strain as compared to adv-1V5 (2mg) to make up for the reduced WC-1 expression seen in adv-1V5, Δcsp-6. This result confirmed that loss of csp-6 substantially interrupted the interaction between WC-1 and ADV-1.
We then examined the subcellular distribution of ADV-1 in cultures grown in LL. Aliquots of total, cytosol and nuclear fractions were analyzed by Western blotting (Fig 6G). Two proteins, tubulin and histone-H3 were used as cytoplasmic and nuclear protein markers, respectively, to confirm the quality of the nuclei and to control for cytoplasmic contamination in the nuclear preparation. ADV-1 was enriched in nuclei, which was consistent with its function as a transcription factor (Fig 6G). Interestingly however, although slightly less total ADV-1 was detected in Δcsp-6, more of it was enriched in nuclei, suggesting that loss of csp-6 resulted in mildly misregulated localization such that ADV-1 was more strongly partitioned to nuclei rather than to the cytoplasm. Furthermore, the Δcsp-6 mutant displayed only slightly less ADV-1 protein and no difference in ADV-1 phosphorylation as compared to WT, suggesting that CSP-6 did not directly work on ADV-1 as a phosphatase (S9B Fig). Therefore, the reduced interaction between ADV-1 and WC-1, and increased amount of nuclear ADV-1 in Δcsp-6 (Fig 6F and 6G) may reflect the action of CSP-6 on WC-1 rather than directly on ADV-1.
CSP-6 is found in the nucleus
As reflected in the gene name, Saccharomyces PSR1 (Plasma membrane Sodium Response 1) localizes to the plasma membrane [37,39] and we were interested to know whether CSP-6 has similar localization in Neurospora. Nuclei were isolated from csp-6V5, ras-1bd and the cytoplasmic and nuclear fractions analyzed by SDS-PAGE [43] (Fig 7A). CSP-6 was enriched in the nuclear fraction but was still present in the cytoplasm. Therefore, unlike yeast, Neurospora CSP-6 localized to both the cytoplasm and nucleus.
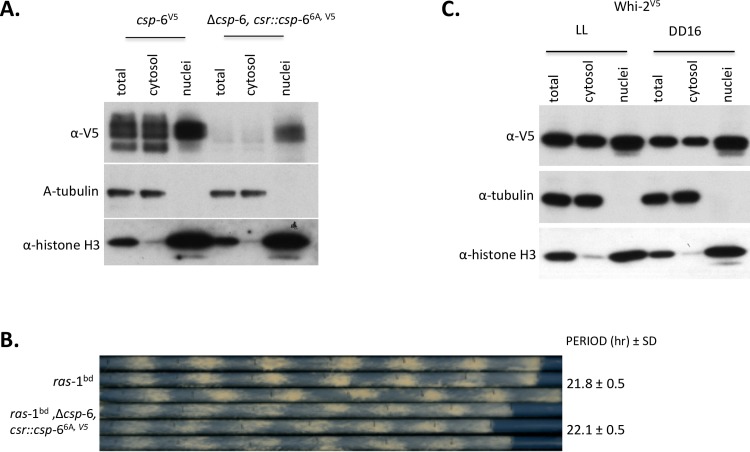
Fig 7. CSP-6 localizes inside nuclei in Neurospora.
A: Western blots show CSP-6 and two control proteins (tubulin and histone-H3) in total cell lysates (Total), cytoplasm (Cyto) and nuclear fractions of csp-6V5 and Δcsp-6, csr::csp-66A, V5. CSP-6 was detected in the nuclei; there was no tubulin signal in the nuclear fraction suggesting that isolated nuclei have little cytosolic contamination. B: Race tube assay showing Δcsp-6 ras-1bd; csr::csp-66A, V5 has normal overt circadian rhythmicity with the same period as wild type (ras-1bd); triplicate race tubes are shown. Period is reported in hours ± one standard deviation, ARR is arrhythmic. C: Subcellular distribution of WHI-2 was analyzed by Western blot with indicated antibodies. Cellular fractions were prepared by differential centrifugation by standard techniques (57), but the final nuclear pellet was resuspended in just 300 μl of buffer versus 40 ml for total and cytoplasmic fractions. In all cases the same volume of extract (10μl) was loaded on the gels, so estimates of the total amount of protein in each compartment must reflect both the amount seen on the gel and the total amount of extract prepared.
Sequence analysis of CSP-6 revealed a putative nuclear localization signal ‘PKKKKG’ (9-14aa) near the N-terminus that is absent from yeast Psr1p (S10 Fig). To determine whether ‘PKKKKG’ is a nuclear localization signal (NLS) in CSP-6, ‘PKKKKG’ was replaced by ‘AAAAAA’ (6A) and the construct (including a C-terminal V5 tag) transformed into the csr locus of Δcsp-6 driven by its own promoter. The resulting transformant (Δcsp-6, csr:: csp-66A, V5) rescued the banding defect and growth rate of the Δcsp-6 mutant (Fig 7B); however, ‘PKKKKG’ with 6A does not abolish the nuclear localization of CSP-6 indicating that PKKKKG is not a NLS (Fig 7A). In addition, we noticed that CSP-6 was reduced significantly in amount in Δcsp-6, csr:: csp-66A, V5 suggesting PKKKKG may be essential for CSP-6 stability though not as a NLS. Cellular fractionation showed that WHI-2 displayed localization similar to that of CSP-6 (Fig 7C).
Discussion
csp-6 encodes a phosphatase required for Neurospora’s major overt circadian output, the daily cycle of asexual development. However, CSP-6 is constitutively expressed over the day without obvious rhythmicity and is thus not a typical clock-controlled gene [30]. Previous studies associated two protein phosphatases PP1 and PP2A, with the Neurospora core circadian oscillator, PP1 implicated in regulating stability of FRQ and PP2A impacting negative feedback and acting on WC-1 in vitro [18]. CSP-6 plainly has a major effect on WC-1 phosphorylation; however, deletion of csp-6 has relatively less effect on core clock oscillations (a reduced amplitude and delayed phase but normal period length), instead only having a significant discrete effect on circadian output including phase setting and conidiation rhythms. Because of the high likelihood that WC-1 appears to be a direct target of CSP-6 in vivo, interpretation of its actions is revealing both in the context of the oscillator and of output and these are addressed sequentially.
The Neurospora blue light photoreceptor and clock protein WC-1, in association with WC-2, regulates expression and oscillation of FRQ. FRQ then undergoes a cycle of phosphorylation that eventually impacts its ability to interact with WCC [44]. Biochemical analyses of cell extracts have suggested that dephosphorylation of WCC may enhance its DNA binding activity to the frq promoter [13,2,24]; however, these effects could be mediated by other proteins in the extracts such as FRQ whose phosphorylation status affects WCC’s activity. Similarly, hyperphosphorylated WCC observed in cell extracts of phosphatase mutants including PP1, PP2A was reported to have a reduced binding activity to the frq promoter, although again is not possible to say whether the effect on WCC is direct or via interacting proteins. In another correlation, activation of WCC was reported to be dependent on RGB-1, a regulatory subunit of PP2A, and was correlated with dephosphorylation of WC-1 and WC-2, data supporting a model in which the rhythmic activity of WCC is controlled by a dynamic equilibrium of phosphorylation and dephosphorylation mediated by several kinases and phosphatases [45]. In all these studies, then, a correlation was developed between phosphorylation of WCC and reduced activity of WCC. However, direct in vivo interaction between these phosphatases and WC-1 has not been demonstrated, correlation does not establish cause and effect, and data presented here are not entirely consistent with this model for WC-1 regulation. We saw significantly hyperphosphorylated WC-1 in the strain lacking CSP-6, a phosphatase protein that interacts with WC-1 (Fig 2). This suggests that CSP-6 dephosphorylates WC-1 to a significant degree, and yet the clock still functions with no period lengthening (Fig 1). WC-2 ChIP analysis in the Δcsp-6 deletion mutant revealed moderately (1.5–2 fold) reduced WC-2 binding occupancy at the frq promoter C-box site as compared to wild type but no loss of rhythm (S9 Fig). The reduced binding is roughly consistent with previous work correlating hyperphosphorylated WCC with reduced DNA binding activity at frq C-box promoter [10,13]; however, the WC-1 hyperphosphorylation plainly has little effect on the core clock feedback loop. Previous reports had suggested that transcriptionally active hypophosphorylated WCCs are unstable and that active WCC leads to very low WCC levels [13,16,24]; however, here we saw the reverse, where hyperphosphorylated WCC present in Δcsp-6 is much less stable (Fig 2B) but still binds to DNA and supports a robust clock at least for the first few days though not perfectly as that in the wild type. Additionally, we noticed that the FRQ protein level and frq mRNA expression level were reduced after moving to constant darkness suggesting that CSP-6 affected the expression of FRQ though not enough to disrupt the clock oscillation (S1B and S1C Fig). Different from other phosphatase proteins so far examined such as PP1, PP2A, PPP-1 or PP4, CSP-6 did not regulate the phosphorylation level of FRQ. Furthermore, we also examined WCC binding to the c-box region over two days. The results showed reduced binding at frq C-box in Δcsp-6 compared to the wild type (S8B and S8C Fig), so most likely CSP-6 plays a role in maintaining robust FRQ expression through aiding WC-1 binding activity [18,46]. It should be noted, however, that the hyperphosphorylated WC-1 seen in Δcsp-6 still binds to the frq promoter and still drives a circadian clock.
Loss of CSP-6 resulted in an around 4 hr phase delay in the rhythm, a phenotype similar to that seen strains lacking VVD, the small blue light photoreceptor protein consisting of LOV domain and an N-terminal cap that physically interacts with WC-1 to reduce its ability to activate transcription to regulate photoadaptation [26,27]. Indeed, an additive phase delay was observed in the double mutant Δvvd, Δcsp-6 (Fig 4B), and there was plainly much less WC-1 in Δvvd, Δcsp-6 compared to either single mutant, suggesting that the low level of WC-1 seen in Δcsp-6 might be the reason for the phase delay. However, elevated expression of WC-1 in wc-1::qa-wc-1, Δcsp-6 only partially rescued the phase defect (Fig 4D), and this suggested that the reduced VVD-WC-1 interaction also observed in the Δcsp-6 mutant, independent of the low level of WC-1, might also underlie the phase delay. This seems to be the case, because even the addition of three times more WC-1 in immunoprecipitations from Δcsp-6 (to make up for the reduced WC-1 level in this strain) failed to recover a full level of interaction (Fig 4C, S4A Fig). Therefore we hypothesize that hyperphosphorylation of WC-1 might independently impact the interaction between VVD and WC-1 resulting in the phase delay and weaker light response phenotypes.
WC-1 and WC-2 play multiple roles in the circadian system; their protein levels contribute to the robustness and stability of the clock and they are at the top of the hierarchy of transcription factors that governs circadian output. The importance of the WCC to output was demonstrated by Cheng et al. (2001) who examined the strains qa-WC-1 or qa-WC-2 in which ORFs of WC-1 or WC-2 were under the control of quinic acid-inducible promoter (qa-2); these strains were arrhythmic on race tubes when the QA concentration was less than 1X10-7M (corresponding to << 10% of wt WC-1 levels), but the conidiation rhythm became overt and robust as inducer was increased to yield even 30% of normal levels. Our initial observation of less WC-1 in the Δcsp-6 mutant suggested that this was the cause of the loss of the overt rhythm; however, we were unable to find evidence for limiting WC-1. Westerns (Fig 6E) showed ADV-1 levels in Δcsp-6 comparable to wt and light-induction of WCC target genes was only slightly reduced, not severely reduced as is seen when WC-1 becomes truly limiting as in the wc-1[MK1] allele (56). When even full induction of WC-1 in qa-wc-1 failed to rescue the conidiation rhythmicity (S4B and S4C Fig) a role for CSP-6 specifically in output was suggested. This was supported by finding that the core oscillator runs with a normal period length in the Δcsp-6 mutant, although the amplitude of the daily cycle in WCC binding to frq, the positive arm in the cycle was reduced compared to wide type (S8B and S8C Fig). Precedents exist for target gene-specific effects of post-translational modifications of transcription factors, for instance in mutants defective in phosphorylation of Ser(276) of NF-kB subunit p65 [47,48].
Circadian output is a consequence of the negative feedback of the central clock that results in the daily cycle of WCC activity. Output happens when the WCC drives expression of clock-controlled genes (ccgs) whose products do not impact the core oscillator itself [30,49]. A number of ccgs have been identified as involved in circadian output regulation [50–53] including genes encoding transcription factors (TFs) directly controlled by WCC involved in clock and light regulation. From these data emerged the model where WCC sits atop an interconnected hierarchy of TFs that governs light and clock regulation [22]. The results of our study indicate that the transcriptional activity of some TFs within this hierarchy that are required for conidiation rhythms and are downstream of CSP-6-WC-1 were disrupted by deletion of csp-6. Examination of candidate TF genes including csp-1, fluffy, msn-1, and adv-1 revealed that both transcriptional and translational rhythmicity of adv-1/ADV-1 was significantly disrupted in the Δcsp-6 mutant (Fig 6C and 6E, S7 Fig). We confirmed previous results showing arrhythmicity of Δadv-1 by race tube assay though with normal core clock function [22] as well as rhythmic WCC binding at the promoter of adv-1 [32] that we here show is weakened in Δcsp-6 (Fig 6D). These data lead to the surprising conclusion that the phosphorylation status of WC-1 differentially affects its two functions: Loss of CSP-6-mediated dephosphorylation has little impact on WC-1 action in the core circadian oscillator but it abrogates the ability of WCC to regulate a salient circadian output, the daily cycle of conidiation. We also noticed that though the rhythmic expression of csp-1 and fluffy promoter was not affected by deletion of csp-6, the mRNA expression level was reduced for both genes in Δcsp-6 (S7E Fig), further suggesting that CSP-6 mediated dephosphorylation of WC-1 has distinct role in output through ADV-1, but also generally affects the expression of other downstream targets.
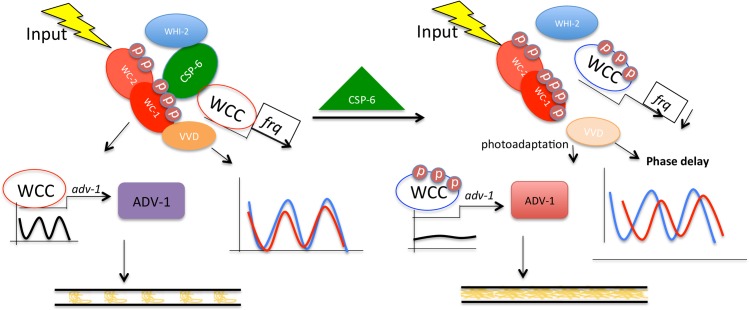
Based on these data, a working model is summarized in Fig 8. CSP-6 physically interacts with and dephosphorylates WC-1 in vivo so that it can interact with VVD to regulate photoadaptation and phase resetting. WHI-2, as an assisting protein, associates with CSP-6 to adjust the WC-1 protein amount and phosphorylation level. adv-1, as a ccg, is one of the direct targets of the WCC that regulates circadian output. The promoter of adv-1 is rhythmically recruited by WCC, and ADV-1 directly functions downstream of CSP-6/WHI-2/WC-1 to control overt rhythmic conidiation on race tubes. In contrast, hyperphosphorylated WC-1 seen in the Δcsp-6 deletion mutant shows disrupted rhythmic DNA binding activity at the adv-1 promoter and arrhythmic transcription/translation of adv-1/ADV-1, the essential cause of the circadian output defect in the Δcsp-6 mutant. However, hyperphosphorylated WCC in Δcsp-6 still sufficiently drives rhythmic frq expression for the clock to run, though with reduced FRQ levels indicating that CSP-6 plays a role in maintaining robust frq/FRQ expression.
Fig 8. A working model: CSP-6 functions upstream of WC-1-ADV-1 in regulation of the circadian output pathway leading to control of development.
(Left) CSP-6 physically interacts with and dephosphorylates WC-1 to an optimal level facilitating its interaction with VVD, regulation of photoadaptation, and circadian phase. Meanwhile, the promoter of adv-1 is rhythmically recruited by WC-1 to active the rhythmic transcription and translation of adv-1/ADV-1 that is essential for overt rhythms. WHI-2 directly interacts with and assists CSP-6 fully to dephosphorylate WC-1 but does not directly interact with WC-1. (Right) Deletion of CSP-6 results in hyperphosphorylated WC-1 and reduced amounts of WC -1 protein, weakening the interaction between WC-1 and VVD and resulting in a 3.5-hour delayed phase. Furthermore, the hyperphosphorylated WC-1 in Δcsp-6 results in disrupted rhythmic binding of WCC to the adv-1 promoter and to arrhythmic expression of adv-1/ADV-1 and resulting in loss of overt circadian output. CSP-6 functions upstream of WC-1/ADV-1 and regulates through WC-1 the expression of adv-1/ADV-1.
These data present some unexpected paradoxes and compel a more nuanced view of the role of phosphorylation of WC-1 in the core clock and in output. The existing model of the circadian feedback loop posits that WC-1 is active and unstable early in the circadian cycle prior to its undergoing changes in phosphorylation that cause it to bind less tightly to DNA. The feedback loop closes when, because of these alterations in the phosphorylation state, WC-1 becomes stable and inactive. It is plausible that the differing combinations of phosphorylation states seen at times throughout the circadian cycle could differentially affect sequence-specific binding affinities of WC-1 to target genes, thereby modulating amplitudes in a hierarchical manner such that some genes are only modestly affected whereas others are more severely affected. Hence, we cannot rule out that the effects on output–specifically the loss of the overt developmental rhythm–arising from loss of CSP-6 are the result of the reduced amount of WC-1 seen in this strain. However, this seems less likely because the reduction in the absolute levels of both WC-1 (including all of it isoforms, Figs 2A and 4A) and ADV-1 (Fig 6E, 6F and 6G), each on the order of three-fold, are not so severe. Instead it seems that it is the quality of WC-1 and not its quantity that is important. Implicit in this statement is the prediction of different classes of phosphosites in WC-1, some involved more directly in oscillator function and some more in output so that output, more than the oscillator itself, is modulated based on the ability of CSP-6 to dephosphorylate WC-1.
CSP-6 is plainly required to dephosphorylate WC-1 because WC-1 is hyperphosphorylated in Δcsp-6. The existing model of the circadian feedback loop posits that WC-1 is active and unstable early in the circadian cycle when it is hypophosphorylated, and the feedback loop closes when, because of hyperphosphorylation, WC-1 becomes stable and inactive. Here, paradoxically, WC-1 is hyperphosphorylated, active, and unstable, and the clock runs, indicating that the class of phosphosites normally dephosphorylated by CSP-6 is distinct from the phosphosites mediating closure of the feedback loop. Different combinatorial states of WC-1 phosphorylation affecting DNA affinity at different promoters, along with turnover, replenishment, and inactivation, are all likely to contribute to distinguishing between oscillator and output functions of WC-1.
Materials and methods
Strains and culture conditions
The ras-1bd and 74A strains were used as clock WT strains in this study. The Δcsp-6, Δpsr-2, Δwhi-2, Δadv-1, Δvvd strains were obtained from the Fungal Genetics Stock Center [41]. These KO strains were backcrossed to ras-1bd to obtain band phenotype for race tube assays. The newly created double knock-out strains were Δcsp-6, Δpsr-2 and Δcsp-6, Δwhi-2 in background #1497 (mus52::natamycin). Neurospora transformation was done as previously described [41].
Race tube medium contained 1xVogel’s salts, 0.1% glucose, 0.17% arginine, 50ng/mL biotin and 1.5% (w/v) agar. Race tube assays were carried out as previously described [54]. Liquid cultures were grown in medium containing 1xVogel’s, 0.5% arginine, and 50ng/mL biotin with 2% glucose.
Protein isolation and detection
Protein extraction, quantification, Western blot analysis and Co-IP were performed as described previously [55,56]. For Western blot analysis, equal amounts of total proteins (30 μg) were loaded to protein gels that were transferred to PVDF membrane after electrophoresis. The V5 antibody (Invitrogen, NY) was used at dilution of 1:5000. Other antibodies including antisera directed at WC-1, WC-2, and FRQ were generated by our own lab [57]. For Co-IP, 2mg total protein was incubated with 30 μl V5 agarose beads (Sigma, MO) for 2h to overnight at 4°C in PEB buffer (50mM HEPES, pH7.4, 150mM NaCl, 10% glycerol, 0.4% NP-40). The V5 agarose beads were washed with wash buffer (50mM HEPES, pH7.4, 150mM NaCl, 0.4% NP-40) four times and eluted with 4xLDS buffer (Thermo Fisher, MA) at 95°C for 5min. In testing the interaction between WC-1 and VVD in Δcsp-6, because of the reduced WC-1 level detected in the Δcsp-6 mutant and to make sure that similar amounts of WC-1 were available based on the quantification by western blot (S4A Fig) we used three fold more input of vvdV5, Δcsp-6 than of vvdV5, and of the wild type (ras-1bd). The same treatment was performed for the interaction between WC-1 and ADV-1 with DSP crosslink as described previously [11].
For protein purified by tandem affinity tag, total protein was isolated from 10–15 g of fresh tissue and incubated with FLAG agarose beads first, followed by V5 magnetic beads. A small amount of the final V5 precipitates were separated by SDS-PAGE and the gel was silver-stained followed manufacturer’s instruction for purification quality examination (SilverQuest, Invitrogen). For Mass Spectrometry, the remainder of the V5 precipitate preparation was separated by SDS-PAGE and the specific bands were excised from a Coomassie blue stained gel and sent for Mass Spectrometry as previously reported [44]. Nuclear preparation was performed as reported [58].
RNA isolation and quantitative RT-PCR
Total RNA was isolated by Trizol according to the manufacturer’s protocol (15596–026; Invitrogen). For quantitative RT-PCR, 2–3 μg RNA were treated with DNase at 37°C for 60min and then incubated with inactivation buffer for 5 min following instructions (AM2239; Life Technology). cDNA was generated by reverse transcription reaction according to the manufacturer’s protocol (18080–051; Invitrogen). Expression levels of genes of interest were analyzed by quantitative real-time PCR with primers listed in S1 Table in the Supplemental Material.
Luciferase reporter assay
The luciferase reporter assay was performed as described previously [36]. The C-box-luc, csr::C-box-luc, ras-1bd, A or his-3::pfrq-luc, ras-1bd, A/a were used as control strains. Knock out strains were crossed with these as appropriate to place frq-luc at the csr or his-3 locus. Camera runs showed there was no difference in rhythmicity of the frq-luc reporter at csr versus the his-3 locus. Race tube medium was used for luciferase assays and 0.01M quinic acid (QA) was added for qa-2 promoter-driven strains as appropriate. All cultures were grown in LL for 2 days and then transferred to constant darkness and luminescence was recorded every hour for six days.
ChIP and region-specific ChIP PCR
ChIP assays were performed as described previously [22,59]. Briefly, the Neurospora tissues were fixed with 1% formaldehyde for 15 min and quenched by glycine at final concentration of 125mM for 5 min. Around fifty-mg of cross-linked tissue was used for each sample and were suspended in 500 μl ChIP lysis buffer. Chromatin was sheared by sonication to 100–500 bp fragments. The immunoprecipitation was performed using 5μl WC-2 antibody [57]. Immunoprecipitated DNA was quantified using real time PCR with primer sets listed in S1 Table. ChIP quantitative PCR data were normalized to a sample of input DNA as described in instructions from the Life Technology website (https://www.lifetechnologies.com/us/en/home/life-science/epigenetics-noncoding-rna-research/chromatin-remodeling/chromatin-immunoprecipitation-chip/chip-analysis.html). Each experiment was independently performed at least three times.
Supporting information
A: Schematic depiction of the domain architecture of CSP-6 protein based on NCBI BLAST. B. Western blot analysis showing FRQ remains rhythmicity but with low protein levels in Δcsp-6. Cultures were harvested in constant darkness at the indicated times during the second day in darkness.
C: frq mRNA accumulates with a circadian rhythm in the Δcsp-6 mutant but with reduced amplitude compared to wild type in the darkness from 24-48hrs. frq mRNA expression was assayed by qRT-PCR and normalized to rac-1 in ras-1bd and ras-1bd,Δcsp-6. The rhythm in Δcsp-6 mutant is weaker than that in the wild type and the phase is around 4-hour delay. D: Luciferase activity of frq C-box-luc and the csp-6 promoter driving luciferase under free running conditions shows that csp-6 is only very weakly circadianly regulated. E: Luciferase activity of frq C-box-luc and the CSP-6 translational luciferase showing CSP-6 is not a rhythmically expressed protein. F: Western blot analysis showing a time course (LL-DD48h) of CSP-6 protein expression at 4 h resolution; hours after light to dark transfer are shown above the blots and the densitometric analysis of these Western blot data are shown in the left panel.
(TIF)
A: Amino acid sequence alignment of CSP-6 and its paralog PSR-2 (NCU08948) showing they are conserved within the C-terminal phosphatase domain. Protein sequence alignment was performed by EBI-cluster. Four amino acids DLDE in red frame depicting the conservation of the active site motif in both HAD phosphatase proteins, CSP-6 and PSR-2. B: Race tube assays of WT (ras-1bd) and Δpsr-2, ras-1bd. Normal conidiation rhythms were observed in strains lacking psr-2, though with slight growth defect; duplicate race tubes are shown for each strain. Period is reported in hours ± one standard deviation C: Luciferase traces of frq-luc in ras-1bd and Δpsr-2, ras-1bd.
(TIF)
A. Western blot analysis showing WC-1 in WT, Δcsp-6 and csp-6ΔDLDE with or without the λ-phosphatase treatment under indicated conditions. DD24: constant darkness for 24hr; LP30: light pulse for 30min. B: Strains of ras-1bd; Δcsp-6, ras-1bd; Δvvd, ras-1bd, and the double mutant Δcsp-6, Δvvd, ras-1bd were grown on minimal slants showing their carotenoid accumulation and growth defect. C: Strains (ras-1bd and Δcsp-6, ras-1bd) exposed to a 30 min light pulse (LP30) were subjected to RT-PCR to determine mRNA expression levels of three albino genes (al-1, al-2, al-3) as a measure of impaired light responses and carotenoid biosynthesis. D: Real time PCR analysis of light inducible genes (al-1, al-3, sub-1, frq) in strains ras-1bd and Δcsp-6, ras-1bd with light pulse samples. E: Western blot analysis showing reduced amounts of WC-2 protein but no significant effect on WC-2 phosphorylation in Δcsp-6.
(TIF)
A: Western blot analysis showing approximately three times more WC-1 protein in ras-1bd as in Δcsp-6, ras-1bd. B: Western blot showing WC-1 protein expression in WT and in a qa-2-driven wc-1 strain at the native locus in Δcsp-6. In the presence of 10−2 M QA, WC-1 levels in the qa-2 driven wc-1 strain in Δcsp-6 were similar to those in wild type. The WC-1 was still hyperphosphorylated in Δcsp-6, and a dephosphorylation assay with λPPase showed the lower mobility of WC-1 in Δcsp-6 was caused by phosphorylation. The protein level of WC-1 was not affected by exogenous QA in the clock wild type strain. The unspecific band was used to validate the quantity of protein loading. C: Luciferase traces for three technical replicates of frq-luc in wc-1::qa:wc-1 with 10-2M QA; the only source of WC-1 in this strain is the QA-induced construct. D: Race tube assay showing that even in the presence of QA to elevate WC-1 expression, no conidiation banding was observed in the wc-1::qa:wc-1, Δcsp-6 strain while ras-1bd showed rhythmic banding on race tube with 10-2M QA.
(TIF)
A: Two translational start sites, labeled as S1 and S2, were found based on CSP-6 sequence as reported by FungiDB [http://fungidb.org/fungidb/]; the difference in size between the two translational isoforms was 5.5kDa. B: Western blots showing the two isoforms of CSP-6 and their modification. Isoform S1 is obviously phosphorylated, and isoform S2 likely phosphorylated, based on results from phosphatase treatment. Buffer: protein extraction buffer; PP: phosphatase; PPI: phosphatase inhibitor.
(TIF)
A: WC-1 is hyperphosphorylated in the Δwhi-2. Shown is a Western blot of WC-1 in ras-1bd and Δwhi-2, ras-1bd, the conditions are label as indicated on the top of the western. B: Western blot analysis showing no difference in WC-1 protein amount, or in degree of hyperphosphorylation, between Δcsp-6 and the double mutant Δcsp-6, Δwhi-2.
(TIF)
A: Race tube assay showing Δmsn-1 displays normal overt rhythmic banding but a significantly reduced growth rate; triplicate race tubes are shown. B: Luciferase traces elucidating that a functional clock was running in the Δmsn-1 mutant; duplicate assays are shown. C-D: Representative luciferase activity assays showing circadian rhythmicity of csp-1 (C) and fluffy (D) promoter activity was not abolished in the Δcsp-6 mutant. E: Strains ras-1bd and Δcsp-6, ras-1bd were subjected to RT-PCR to determine mRNA expression levels of csp-1 and fluffy normalize to WT (ras-1bd), error bars represent +/- S.D.
(TIF)
A: ChIP analysis showing the recruitment of WC-2 to the adv-1 promoter in ras-1bd and ras-1bd, Δcsp-6 under indicated conditions. LL: constant light for 24h, LP15’: light pulse for 15min after moving from DD24. B. C: Chip analysis showing the recruitment of WC-2 to the frq promoter C-box in Δcsp-6 is reduced compared to WT but is still rhythmic with the same peak phase. The examined time points are in darkness from 8h-24h (B) and 28-48h (C). Error bars represent +/- S.D.
(TIF)
A: Co-IP assay demonstrating that ADV-1 failed to interact with CSP-6 even using DSP crosslink. ADV-1 was purified by V5 agarose beads from strain adv-1V5, csp-6FLAGand no CSP-6 was detected in IP sample. B: Western blot analysis showing there was no change in the phosphorylation status of ADV-1 between WT and Δcsp-6 under the indicated conditions. C: A phosphorylation assay showed there was no difference of ADV-1 before and after treatment in wild type and Δcsp-6 under the indicated conditions.
(TIF)
Amino acid PKKKKG in red frame indicates a putative nuclear localization site in CSP-6.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank the Fungal Genetics Stock Center for provision of strains and service to the fungal community.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Institutes of Health to JJL (R35GM118022) and to JCD (R35GM118021). http://www.nigms.nih.gov/Pages/default.aspx. We acknowledge use of materials generated by P01 GM068087 from National Institutes of Health to JCD. http://www.nigms.nih.gov/Pages/default.aspx. We thank the Fungal Genetics Stock Center for providing Neurospora knockout strains. http://www.fgsc.net/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dunlap JC. Molecular Bases for Circadian Clocks. Cell. 1999;96: 271–290. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Bell-Pedersen D. Circadian Rhythms in Neurospora crassa and Other Filamentous Fungi. Eukaryot Cell. 2006;5: 1184–1193. doi: 10.1128/EC.00133-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardin PE, Panda S. Circadian timekeeping and output mechanisms in animals. Curr Opin Neurobiol. 2013;23: 724–731. doi: 10.1016/j.conb.2013.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaix A, Zarrinpar A, Panda S. The circadian coordination of cell biology. J Cell Biol. 2016;215: 15–25. doi: 10.1083/jcb.201603076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha J, Zhou M, Liu Y. Mechanism of the Neurospora Circadian Clock, a FREQUENCY-centric View. Biochemistry (Mosc). 2015;54: 150–156. doi: 10.1021/bi5005624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montenegro-Montero A, Canessa P, Larrondo LF. Around the Fungal Clock. Advances in Genetics. Elsevier; 2015. pp. 107–184. doi: 10.1016/bs.adgen.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 7.Dunlap JC, Loros JJ. Making Time: Conservation of Biological Clocks from Fungi to Animals. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.FUNK-0039-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larrondo LF, Olivares-Yanez C, Baker CL, Loros JJ, Dunlap JC. Decoupling circadian clock protein turnover from circadian period determination. Science. 2015;347: 1257277–1257277. doi: 10.1126/science.1257277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurley JM, Loros JJ, Dunlap JC. Circadian Oscillators: Around the Transcription–Translation Feedback Loop and on to Output. Trends Biochem Sci. 2016;41: 834–846. doi: 10.1016/j.tibs.2016.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Q, Liu Y. Molecular mechanism of light responses in Neurospora: from light-induced transcription to photoadaptation. Genes Dev. 2005;19: 2888–2899. doi: 10.1101/gad.1369605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C-H, DeMay BS, Gladfelter AS, Dunlap JC, Loros JJ. Physical interaction between VIVID and white collar complex regulates photoadaptation in Neurospora. Proc Natl Acad Sci. 2010;107: 16715–16720. doi: 10.1073/pnas.1011190107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng P, He Q, He QY, Wang LX, Liu Y. Regulation of the Neurospora circadian clock by an RNA helicase. Genes Dev. 2005;19: 234–241. doi: 10.1101/gad.1266805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Q, Cha J, He QY, Lee HC, Yang YH, Liu Y. CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev. 2006;20: 2552–2565. doi: 10.1101/gad.1463506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi M, Collett M, Loros JJ, Dunlap JC. FRQ-Interacting RNA Helicase Mediates Negative and Positive Feedback in the Neurospora Circadian Clock. Genetics. 2010;184: 351–361. doi: 10.1534/genetics.109.111393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Q, Shu H, Cheng P, Chen S, Wang L, Liu Y. Light-independent Phosphorylation of WHITE COLLAR-1 Regulates Its Function in the Neurospora Circadian Negative Feedback Loop. J Biol Chem. 2005;280: 17526–17532. doi: 10.1074/jbc.M414010200 [DOI] [PubMed] [Google Scholar]
- 16.Huang G, Chen S, Li S, Cha J, Long C, Li L, et al. Protein kinase A and casein kinases mediate sequential phosphorylation events in the circadian negative feedback loop. Genes Dev. 2007;21: 3283–3295. doi: 10.1101/gad.1610207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sancar G, Sancar C, Brunner M, Schafmeier T. Activity of the circadian transcription factor White Collar Complex is modulated by phosphorylation of SP-motifs. FEBS Lett. 2009;583: 1833–1840. doi: 10.1016/j.febslet.2009.04.042 [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, He Q, Cheng P, Wrage P, Yarden O, Liu Y. Distinct roles for PP1 and PP2A in the Neurospora circadian clock. Genes Dev. 2004;18: 255–260. doi: 10.1101/gad.1152604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froehlich AC. White Collar-1, a Circadian Blue Light Photoreceptor, Binding to the frequency Promoter. Science. 2002;297: 815–819. doi: 10.1126/science.1073681 [DOI] [PubMed] [Google Scholar]
- 20.He Q. White Collar-1, a DNA Binding Transcription Factor and a Light Sensor. Science. 2002;297: 840–843. doi: 10.1126/science.1072795 [DOI] [PubMed] [Google Scholar]
- 21.Chen C-H, Ringelberg CS, Gross RH, Dunlap JC, Loros JJ. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J. 2009;28: 1029–1042. doi: 10.1038/emboj.2009.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith KM, Sancar G, Dekhang R, Sullivan CM, Li S, Tag AG, et al. Transcription Factors in Light and Circadian Clock Signaling Networks Revealed by Genomewide Mapping of Direct Targets for Neurospora White Collar Complex. Eukaryot Cell. 2010;9: 1549–1556. doi: 10.1128/EC.00154-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong CI, Ruoff P, Loros JJ, Dunlap JC. Closing the circadian negative feedback loop: FRQ-dependent clearance of WC-1 from the nucleus. Genes Dev. 2008;22: 3196–3204. doi: 10.1101/gad.1706908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafmeier T, Diernfellner A, Schafer A, Dintsis O, Neiss A, Brunner M. Circadian activity and abundance rhythms of the Neurospora clock transcription factor WCC associated with rapid nucleo-cytoplasmic shuttling. Genes Dev. 2008;22: 3397–3402. doi: 10.1101/gad.507408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dasgupta A, Chen C-H, Lee C, Gladfelter AS, Dunlap JC, Loros JJ. Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity. Crane B, editor. PLOS Genet. 2015;11: e1005215 doi: 10.1371/journal.pgen.1005215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heintzen C, Loros JJ, Dunlap JC. The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating, and regulates clock resetting. Cell. 2001;104: 453–464. [DOI] [PubMed] [Google Scholar]
- 27.Elvin M, Loros JJ, Dunlap JC, Heintzen C. The PAS/LOV protein VIVID supports a rapidly dampened daytime oscillator that facilitates entrainment of the Neurospora circadian clock. Genes Dev. 2005;19: 2593–2605. doi: 10.1101/gad.349305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell-Pedersen D, Shinohara ML, Loros JJ, Dunlap JC. Circadian clock-controlled genes isolated from Neurospora crassa are late night-to early morning-specific. Proc Natl Acad Sci. 1996;93: 13096–13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Correa A, Bell-Pedersen D. Distinct Signaling Pathways from the Circadian Clock Participate in Regulation of Rhythmic Conidiospore Development in Neurospora crassa. Eukaryot Cell. 2002;1: 273–280. doi: 10.1128/EC.1.2.273-280.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitalini MW, de Paula RM, Park WD, Bell-Pedersen D. The Rhythms of Life: Circadian Output Pathways in Neurospora. J Biol Rhythms. 2006;21: 432–444. doi: 10.1177/0748730406294396 [DOI] [PubMed] [Google Scholar]
- 31.Baker CL, Loros JJ, Dunlap JC. The circadian clock of Neurospora crassa. FEMS Microbiol Rev. 2012;36: 95–110. doi: 10.1111/j.1574-6976.2011.00288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurley JM, Dasgupta A, Emerson JM, Zhou X, Ringelberg CS, Knabe N, et al. Analysis of clock-regulated genes in Neurospora reveals widespread posttranscriptional control of metabolic potential. Proc Natl Acad Sci. 2014;111: 16995–17002. doi: 10.1073/pnas.1418963111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dekhang R, Wu C, Smith KM, Lamb TM, Peterson M, Bredeweg EL, et al. The Neurospora Transcription Factor ADV-1 Transduces Light Signals and Temporal Information to Control Rhythmic Expression of Genes Involved in Cell Fusion. G3:Genes|Genomes|Genetics. 2017;7: 129–142. doi: 10.1534/g3.116.034298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh A, Servin JA, Park G, Borkovich KA. Global Analysis of Serine/Threonine and Tyrosine Protein Phosphatase Catalytic Subunit Genes in Neurospora crassa Reveals Interplay Between Phosphatases and the p38 Mitogen-Activated Protein Kinase. G3:Genes|Genomes|Genetics. 2014;4: 349–365. doi: 10.1534/g3.113.008813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinnici JL, Fu C, Caccamise LM, Arnold JW, Free SJ. Neurospora crassa Female Development Requires the PACC and Other Signal Transduction Pathways, Transcription Factors, Chromatin Remodeling, Cell-To-Cell Fusion, and Autophagy. Pöggeler S, editor. PLoS ONE. 2014;9: e110603 doi: 10.1371/journal.pone.0110603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gooch VD, Mehra A, Larrondo LF, Fox J, Touroutoutoudis M, Loros JJ, et al. Fully Codon-Optimized luciferase Uncovers Novel Temperature Characteristics of the Neurospora Clock. Eukaryot Cell. 2008;7: 28–37. doi: 10.1128/EC.00257-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siniossoglou S, Hurt EC, Pelham HRB. Psr1p/Psr2p, Two Plasma Membrane Phosphatases with an Essential DXDX(T/V) Motif Required for Sodium Stress Response in Yeast. J Biol Chem. 2000;275: 19352–19360. doi: 10.1074/jbc.M001314200 [DOI] [PubMed] [Google Scholar]
- 38.Seifried A, Schultz J, Gohla A. Human HAD phosphatases: structure, mechanism, and roles in health and disease: Human HAD phosphatases. FEBS J. 2013;280: 549–571. doi: 10.1111/j.1742-4658.2012.08633.x [DOI] [PubMed] [Google Scholar]
- 39.Kaida D, Yashiroda H, Toh-e A, Kikuchi Y. Yeast Whi2 and Psr1-phosphatase form a complex and regulate STRE-mediated gene expression. Genes Cells. 2002;7: 543–552. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Stabryla L, Wei N. Improved Acetic Acid Resistance in Saccharomyces cerevisiae by Overexpression of the WHI2 Gene Identified through Inverse Metabolic Engineering. Cullen D, editor. Appl Environ Microbiol. 2016;82: 2156–2166. doi: 10.1128/AEM.03718-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, et al. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci. 2006;103: 10352–10357. doi: 10.1073/pnas.0601456103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madi L, Ebbole DJ, White BT, Yanofsky C. Mutants of Neurospora crassa that alter gene expression and conidia development. Proc Natl Acad Sci. 1994;91: 6226–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo C, Loros JJ, Dunlap JC. Nuclear localization is required for function of the essential clock protein FRQ. EMBO J. 1998;17: 1228–1235. doi: 10.1093/emboj/17.5.1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker CL, Kettenbach AN, Loros JJ, Gerber SA, Dunlap JC. Quantitative Proteomics Reveals a Dynamic Interactome and Phase-Specific Phosphorylation in the Neurospora Circadian Clock. Mol Cell. 2009;34: 354–363. doi: 10.1016/j.molcel.2009.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schafmeier T, Haase A, Káldi K, Scholz J, Fuchs M, Brunner M. Transcriptional Feedback of Neurospora Circadian Clock Gene by Phosphorylation-Dependent Inactivation of Its Transcription Factor. Cell. 2005;122: 235–246. doi: 10.1016/j.cell.2005.05.032 [DOI] [PubMed] [Google Scholar]
- 46.Cha J, Chang S-S, Huang G, Cheng P, Liu Y. Control of WHITE COLLAR localization by phosphorylation is a critical step in the circadian negative feedback process. EMBO J. 2008;27: 3246–3255. doi: 10.1038/emboj.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong J, Jimi E, Zhong H, Hayden MS, Ghosh S. Repression of gene expression by unphosphorylated NF- B p65 through epigenetic mechanisms. Genes Dev. 2008;22: 1159–1173. doi: 10.1101/gad.1657408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smale ST. Hierarchies of NF-κB target-gene regulation. Nat Immunol. 2011;12: 689–694. doi: 10.1038/ni.2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loros J, Denome S, Dunlap J. Molecular cloning of genes under control of the circadian clock in Neurospora. Science. 1989;243: 385–388. doi: 10.1126/science.2563175 [DOI] [PubMed] [Google Scholar]
- 50.Zhu H, Nowrousian M, Kupfer D, Colot HV, Berrocal-Tito G, Lai H, et al. Analysis of expressed sequence tags from two starvation, time-of-day-specific libraries of Neurospora crassa reveals novel clock-controlled genes. Genetics. 2001;157: 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Correa A, Lewis ZA, Greene AV, March IJ, Gomer RH, Bell-Pedersen D. Multiple oscillators regulate circadian gene expression in Neurospora. Proc Natl Acad Sci. 2003;100: 13597–13602. doi: 10.1073/pnas.2233734100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nowrousian M, Duffield GE, Loros JJ, Dunlap JC. The frequency gene is required for temperature-dependent regulation of many clock-controlled genes in Neurospora crassa. Genetics. 2003;164: 923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loros JJ, Dunlap JC, Larrondo LF, Shi M, Belden WJ, Gooch VD, et al. Circadian Output, Input, and Intracellular Oscillators: Insights into the Circadian Systems of Single Cells. Cold Spring Harb Symp Quant Biol. 2007;72: 201–214. doi: 10.1101/sqb.2007.72.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belden WJ, Larrondo LF, Froehlich AC, Shi M, Chen C-H, Loros JJ, et al. The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes Dev. 2007;21: 1494–1505. doi: 10.1101/gad.1551707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garceau NY, Liu Y, Loros JJ, Dunlap JC. Alternative Initiation of Translation and Time-Specific Phosphorylation Yield Multiple Forms of the Essential Clock Protein FREQUENCY. Cell. 1997;89: 469–476. [DOI] [PubMed] [Google Scholar]
- 56.Hurley JM, Larrondo LF, Loros JJ, Dunlap JC. Conserved RNA Helicase FRH Acts Nonenzymatically to Support the Intrinsically Disordered Neurospora Clock Protein FRQ. Mol Cell. 2013;52: 832–843. doi: 10.1016/j.molcel.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee K, Dunlap JC, Loros JJ. Roles for WHITE COLLAR-1 in Circadian and General Photoperception in Neurospora crassa. Genetics. 2003;163: 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong CI, Ruoff P, Loros JJ, Dunlap JC. Closing the circadian negative feedback loop: FRQ-dependent clearance of WC-1 from the nucleus. Genes Dev. 2008;22: 3196–3204. doi: 10.1101/gad.1706908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Z, Liu X, Hu Q, Zhang N, Sun G, Cha J, et al. Suppression of WC-independent frequency transcription by RCO-1 is essential for Neurospora circadian clock. Proc Natl Acad Sci. 2013;110: E4867–E4874. doi: 10.1073/pnas.1315133110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levine JD, Funes P, Dowse HB, Hall JC. Signal analysis of behavioral and molecular cycles. BMC Neurosci. 2002;3: 1–25. doi: 10.1186/1471-2202-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Schematic depiction of the domain architecture of CSP-6 protein based on NCBI BLAST. B. Western blot analysis showing FRQ remains rhythmicity but with low protein levels in Δcsp-6. Cultures were harvested in constant darkness at the indicated times during the second day in darkness.
C: frq mRNA accumulates with a circadian rhythm in the Δcsp-6 mutant but with reduced amplitude compared to wild type in the darkness from 24-48hrs. frq mRNA expression was assayed by qRT-PCR and normalized to rac-1 in ras-1bd and ras-1bd,Δcsp-6. The rhythm in Δcsp-6 mutant is weaker than that in the wild type and the phase is around 4-hour delay. D: Luciferase activity of frq C-box-luc and the csp-6 promoter driving luciferase under free running conditions shows that csp-6 is only very weakly circadianly regulated. E: Luciferase activity of frq C-box-luc and the CSP-6 translational luciferase showing CSP-6 is not a rhythmically expressed protein. F: Western blot analysis showing a time course (LL-DD48h) of CSP-6 protein expression at 4 h resolution; hours after light to dark transfer are shown above the blots and the densitometric analysis of these Western blot data are shown in the left panel.
(TIF)
A: Amino acid sequence alignment of CSP-6 and its paralog PSR-2 (NCU08948) showing they are conserved within the C-terminal phosphatase domain. Protein sequence alignment was performed by EBI-cluster. Four amino acids DLDE in red frame depicting the conservation of the active site motif in both HAD phosphatase proteins, CSP-6 and PSR-2. B: Race tube assays of WT (ras-1bd) and Δpsr-2, ras-1bd. Normal conidiation rhythms were observed in strains lacking psr-2, though with slight growth defect; duplicate race tubes are shown for each strain. Period is reported in hours ± one standard deviation C: Luciferase traces of frq-luc in ras-1bd and Δpsr-2, ras-1bd.
(TIF)
A. Western blot analysis showing WC-1 in WT, Δcsp-6 and csp-6ΔDLDE with or without the λ-phosphatase treatment under indicated conditions. DD24: constant darkness for 24hr; LP30: light pulse for 30min. B: Strains of ras-1bd; Δcsp-6, ras-1bd; Δvvd, ras-1bd, and the double mutant Δcsp-6, Δvvd, ras-1bd were grown on minimal slants showing their carotenoid accumulation and growth defect. C: Strains (ras-1bd and Δcsp-6, ras-1bd) exposed to a 30 min light pulse (LP30) were subjected to RT-PCR to determine mRNA expression levels of three albino genes (al-1, al-2, al-3) as a measure of impaired light responses and carotenoid biosynthesis. D: Real time PCR analysis of light inducible genes (al-1, al-3, sub-1, frq) in strains ras-1bd and Δcsp-6, ras-1bd with light pulse samples. E: Western blot analysis showing reduced amounts of WC-2 protein but no significant effect on WC-2 phosphorylation in Δcsp-6.
(TIF)
A: Western blot analysis showing approximately three times more WC-1 protein in ras-1bd as in Δcsp-6, ras-1bd. B: Western blot showing WC-1 protein expression in WT and in a qa-2-driven wc-1 strain at the native locus in Δcsp-6. In the presence of 10−2 M QA, WC-1 levels in the qa-2 driven wc-1 strain in Δcsp-6 were similar to those in wild type. The WC-1 was still hyperphosphorylated in Δcsp-6, and a dephosphorylation assay with λPPase showed the lower mobility of WC-1 in Δcsp-6 was caused by phosphorylation. The protein level of WC-1 was not affected by exogenous QA in the clock wild type strain. The unspecific band was used to validate the quantity of protein loading. C: Luciferase traces for three technical replicates of frq-luc in wc-1::qa:wc-1 with 10-2M QA; the only source of WC-1 in this strain is the QA-induced construct. D: Race tube assay showing that even in the presence of QA to elevate WC-1 expression, no conidiation banding was observed in the wc-1::qa:wc-1, Δcsp-6 strain while ras-1bd showed rhythmic banding on race tube with 10-2M QA.
(TIF)
A: Two translational start sites, labeled as S1 and S2, were found based on CSP-6 sequence as reported by FungiDB [http://fungidb.org/fungidb/]; the difference in size between the two translational isoforms was 5.5kDa. B: Western blots showing the two isoforms of CSP-6 and their modification. Isoform S1 is obviously phosphorylated, and isoform S2 likely phosphorylated, based on results from phosphatase treatment. Buffer: protein extraction buffer; PP: phosphatase; PPI: phosphatase inhibitor.
(TIF)
A: WC-1 is hyperphosphorylated in the Δwhi-2. Shown is a Western blot of WC-1 in ras-1bd and Δwhi-2, ras-1bd, the conditions are label as indicated on the top of the western. B: Western blot analysis showing no difference in WC-1 protein amount, or in degree of hyperphosphorylation, between Δcsp-6 and the double mutant Δcsp-6, Δwhi-2.
(TIF)
A: Race tube assay showing Δmsn-1 displays normal overt rhythmic banding but a significantly reduced growth rate; triplicate race tubes are shown. B: Luciferase traces elucidating that a functional clock was running in the Δmsn-1 mutant; duplicate assays are shown. C-D: Representative luciferase activity assays showing circadian rhythmicity of csp-1 (C) and fluffy (D) promoter activity was not abolished in the Δcsp-6 mutant. E: Strains ras-1bd and Δcsp-6, ras-1bd were subjected to RT-PCR to determine mRNA expression levels of csp-1 and fluffy normalize to WT (ras-1bd), error bars represent +/- S.D.
(TIF)
A: ChIP analysis showing the recruitment of WC-2 to the adv-1 promoter in ras-1bd and ras-1bd, Δcsp-6 under indicated conditions. LL: constant light for 24h, LP15’: light pulse for 15min after moving from DD24. B. C: Chip analysis showing the recruitment of WC-2 to the frq promoter C-box in Δcsp-6 is reduced compared to WT but is still rhythmic with the same peak phase. The examined time points are in darkness from 8h-24h (B) and 28-48h (C). Error bars represent +/- S.D.
(TIF)
A: Co-IP assay demonstrating that ADV-1 failed to interact with CSP-6 even using DSP crosslink. ADV-1 was purified by V5 agarose beads from strain adv-1V5, csp-6FLAGand no CSP-6 was detected in IP sample. B: Western blot analysis showing there was no change in the phosphorylation status of ADV-1 between WT and Δcsp-6 under the indicated conditions. C: A phosphorylation assay showed there was no difference of ADV-1 before and after treatment in wild type and Δcsp-6 under the indicated conditions.
(TIF)
Amino acid PKKKKG in red frame indicates a putative nuclear localization site in CSP-6.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.