Abstract
Posttraumatic stress disorder (PTSD) is a chronic, debilitating condition for which Prolonged Exposure (PE) therapy is highly efficacious. However, for some individuals, premature dropout and residual PTSD symptoms remain obstacles. The neuropeptide oxytocin is a promising candidate to enhance PE due to its ability to enhance 1) prosocial cognition and behavior, which are theorized to promote positive working alliance, and 2) extinction learning, which is the central mechanism of action underlying successful PE treatment. Despite a robust theoretical rationale, no studies to date have combined evidence-based psychotherapy for PTSD with oxytocin. This randomized, placebo-controlled, double-blind pilot trial examined the feasibility, safety, and preliminary efficacy of augmenting PE with oxytocin. Participants were 17 individuals with diverse index traumas. Participants self-administered intranasal oxytocin (40 IU) or matching placebo 45 minutes prior to each weekly PE therapy session. One adverse event occurred in the placebo group and three individuals dropped out (17.6%; 2 oxytocin group and 1 placebo group). The oxytocin group demonstrated lower PTSD and depression symptoms during PE, and had higher working alliance scores, although these differences did not reach statistical significance. Although preliminary, the findings support the feasibility of oxytocin combined with PE. Adequately powered studies are necessary to determine whether oxytocin enhances PE treatment outcomes and to examine potential mechanisms, such as accelerating extinction learning, enhancing early response, and preventing premature dropout. NCT03238924.
Keywords: PTSD, Prolonged Exposure, oxytocin, translational research, treatment
1. Introduction
Posttraumatic stress disorder (PTSD) is a chronic, prevalent, and debilitating disorder (Kessler et al., 2012). If left untreated, individuals with PTSD incur risk for other psychiatric problems (e.g., depression, substance use disorder), neuropsychological impairment, suicidality, physical health problems and increased mortality, reduced resiliency, unemployment, and family/couples impairment (Marx et al., 2009; Monson et al., 2009; Pietrzak et al., 2009; Pietrzak et al., 2011).
Prolonged Exposure (PE; Foa et al., 2007) is a manualized, cognitive-behavioral therapy that is considered a “gold standard” treatment for PTSD (Institute of Medicine, 2008; The Management of Posttraumatic Stress Disorder Work Group, 2017). PE consistently outperforms control and waitlist conditions in randomized controlled trials (Powers et al., 2010; Resick et al., 2002) and typically demonstrates robust improvement of 1–2 standard deviations in symptom severity (Eftekhari et al., 2013; Foa et al., 2005; Powers et al., 2010). However, there is a critical need to improve retention and substantial room to improve outcomes in PE. Dropout rates are approximately 30% across populations and treatment settings (Bradley et al., 2005; Eftekhari et al., 2013; Hembree et al., 2003) and a substantial proportion of patients maintain clinically significant residual symptoms and/or continue to meet diagnostic criteria for PTSD following PE (Bradley et al., 2005; Goodson et al., 2013).
As proposed by Olff and colleagues (2010), the neuropeptide oxytocin is a promising candidate for improving retention and outcomes in PE for two reasons. First, oxytocin may enhance retention by reducing excessive distress and facilitating therapeutic alliance. Many patients struggle with distress and avoidance during treatment, and fail to receive an adequate dose of PE (Foa et al., 2005; Tuerk, 2014). The capacity to establish and navigate an effective working alliance is essential to maximize the benefits of behavioral intervention (Horvath and Luborsky, 1993), and particularly integral to the success of PE (Cloitre et al., 2004; McLaughlin et al., 2014). Extensive data indicate that oxytocin exerts prosocial properties such as enhancing affiliative behavior, trust, and warmth (Bartz and Hollander, 2006; MacDonald and MacDonald, 2010). Interpersonal challenges and impaired relational functioning are deficits among individuals with PTSD (Beck et al., 2009). One small laboratory study among participants with PTSD found that a single dose of oxytocin had positive effects on anxiety, irritability, mood, intensity of intrusive thoughts, and desire for social interaction (Yatzkar and Klein, 2010).
The development of PTSD is often conceptualized as a function of Pavlovian fear conditioning (Rauch et al., 2006), while effective fear extinction is the foundation of PE treatment (Foa and Kozak, 1986; Rothbaum and Davis, 2003; Smith et al., 2017). Oxytocin has demonstrated the ability to enhance fear extinction in preclinical models (Eskandarian et al., 2013) and among healthy individuals (Acheson et al., 2013; Eckstein et al., 2014). These findings suggest that oxytocin has potential as an adjunctive therapy for extinction-based PTSD treatments such as PE (Koch et al., 2014; Olff et al., 2010).
Some medications have been examined for the specific purpose of enhancing exposure-based treatments (de Kleine et al., 2013; Tuerk, 2014; Zoellner et al., in press). For example, preliminary studies investigating medications such as yohimbine and d-cycloserine to enhance treatment efficiency have shown some promise (Litz et al., 2012; Wangelin et al., 2013). While these medications are similar to oxytocin in that they also have a short half-life and are administered prior to therapy sessions, both yohimbine and d-cycloserine are stimulating medications designed to increase within-session arousal. No medications to date have demonstrated the ability to improve patient tolerability or retention in psychotherapy for PTSD (Hetrick et al., 2010; Litz et al., 2012; Wangelin et al., 2013; Zoellner et al., in press). Prevailing hypotheses suggest that oxytocin may uniquely help mitigate barriers to engaging in behavioral treatments that specifically target avoidance (Preckel et al., 2014), which would thereby allow patients who might otherwise be unable to tolerate PE to engage effectively, complete the treatment, obtain an adequate therapeutic dose, and experience long-term remission of PTSD (Frijling et al., 2014; Koch et al., 2014; Olff et al., 2010).
While both preclinical and clinical oxytocin studies have expanded tremendously in recent years, several critical gaps in the literature remain with regard to translating oxytocin into a meaningful therapeutic application for PTSD treatment. First, no previous studies pairing oxytocin with a behavioral intervention have taken place among PTSD populations, and studies in related clinical populations have not utilized more than a single intervention session. For example, two small preliminary studies augmented a single-session exposure therapy for social anxiety (N=25) and arachnophobia (N=44) with only one low dose (24 IU) of oxytocin with null findings (Acheson et al., 2015; Guastella et al., 2009). One previous study paired a dose of oxytocin with a single 20-minute therapy session among men with depression with mixed findings (MacDonald et al., 2013). No studies to date have paired oxytocin with a manualized, evidence-supported behavioral therapy to treat PTSD.
Second, only two previous studies have examined oxytocin in the context of PTSD. One study found that a single dose of oxytocin was not effective in reducing physiological reactivity during one-session exposure therapy for PTSD (Pitman et al., 1993). A more recent study by van Zuiden and colleagues (van Zuiden et al., 2016) targeting the prevention of PTSD found that twice daily doses of 40 IU oxytocin for 8 days did not significantly attenuate the onset on PTSD compared to placebo among emergency department patients. However, oxytocin was more effective among individuals who had more severe baseline PTSD severity in this sample.
Finally, while some studies among patients with other psychiatric disorders (e.g., autism, alcohol use disorder) have utilized a repeated dosing strategy in the form of 1–3 daily doses, most of these studies are only 8 days or less (Dadds et al., 2014; Guastella et al., 2015; Pedersen et al., 2011). While the overall safety of single doses of intranasal oxytocin are well established (MacDonald et al., 2011), the feasibility, safety and efficacy of repeated dosing at weekly intervals is less clear.
This pilot study addressed these gaps in the literature by examining the synergistic effects of combining oxytocin with PE using a randomized, placebo-controlled, double-blind design. We hypothesized that individuals randomized to the oxytocin condition would demonstrate significantly greater improvement in PTSD symptom severity during treatment. Because depression commonly co-occurs with PTSD, and is typically identified as an important outcome in clinical trials treating these disorders (see Stander et al., 2014, for review), we also examined depression symptom severity as a treatment outcome. In order to assess feasibility, we compared client satisfaction and working alliance among individuals in the oxytocin versus placebo conditions.
2. Material and methods
2.1 Participants
Of 36 respondents evaluated, 9 were ineligible and 10 declined participation. Reasons for declining participation included being unable to commit to weekly therapy sessions (n=3), already engaged in another therapy that they did not want to discontinue (n=4), not wanting to discuss their trauma (n=1), and being uncomfortable taking a medication (n=2). Seventeen individuals enrolled in the study and were randomized in a 1:1 manner to receive oxytocin (OT; n=8) or placebo (n=9) conditions as well as 10 individual, manualized PE therapy sessions. Three participants dropped out during treatment (at sessions 3, 4, and 6), and one participant was withdrawn after the first PE session and referred to more intensive clinical services. The remaining 13 participants (OT=6) completed all 10 PE sessions. Participants were 82.4% male (n=14) and 58.8% (n=10) were military veterans, among whom 38.5% (n=5) had served in the Iraq or Afghanistan conflicts. An equal number of participants were White or African American (n=6; 35.3%, respectively), and Native American or Pacific Islander (5.9%; n=1, respectively), while 17.6% (n=3) identified as more than one race. Most participants were single/never married (n=10; 58.8%) and were either unemployed (n=6; 35.3%) or part/full-time employed (n=8; 47.1%). Eight participants reported an index trauma related to combat exposure (47.1%), 23.5% (n=4) reported sexual assault, and the remaining five participants endorsed transportation accidents, assault, witnessing sudden violent death, and service as a first responder in the World Trade Center attacks on September 11, 2001. All participants had achieved a minimum of 12 years of education. There were no significant differences in demographic or baseline clinical characteristics between groups (see Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics (N=17)
| Characteristics | Overall N=17 |
Treatment Group | |
|---|---|---|---|
|
| |||
| Placebo n=9 |
Oxytocin n=8 |
||
| Demographics | M (SD) or n (%) | ||
| Age (years) | 43.82 ± 14.54 | 45.78 ± 15.09 | 41.63 ± 14.58 |
| Gender (Male) | 14 (82.3%) | 7 (77.8%) | 7 (87.5%) |
| Veteran | 10 (58.8%) | 5 (55.6%) | 5 (62.5%) |
| Employment Status | |||
| Unemployed | 6 (35.3%) | 4 (44.4%) | 2 (25.0%) |
| Part-time | 2 (11.8%) | 1 (11.1%) | 1 (12.5%) |
| Full-time | 6 (35.3%) | 2 (22.2%) | 4 (50.0%) |
| Retired/Disabled | 3 (17.6%) | 2 (22.2%) | 1 (12.5%) |
| Race, n (%) | |||
| Black/African American | 6 (35.3%) | 3 (33.3%) | 3 (37.5%) |
| White | 6 (35.3%) | 3 (33.3%) | 3 (37.5%) |
| Multiracial/Other | 4 (23.5%) | 2 (22.2%) | 2 (25.0%) |
| Relationship Status, n (%) | |||
| Single/Never married | 10 (58.8%) | 4 (44.4%) | 6 (75.0%) |
| Married | 4 (23.5%) | 2 (22.2%) | 2 (25.0%) |
| Divorced | 2 (11.8%) | 2 (22.2%) | 0 (0%) |
| Clinical Characteristics | |||
| CAPS-5 | 39.29 (10.65) | 39.56 (13.85) | 39.00 (6.32) |
| PCL | 52.82 (13.33) | 53.11 (15.12) | 52.50 (12.04) |
| BDI-II | 29.71 (10.87) | 30.22 (12.51) | 29.13 (9.51) |
| CSQ | 28.64 (2.50) | 27.88 (2.80) | 29.67 (1.75) |
| HAQ | 98.43 (8.71) | 96.38 (7.25) | 101.17 (10.38) |
Note. CAPS= Clinician Administered PTSD Scale. PCL =PTSD Checklist. BDI-II= Beck Depression Inventory II. CSQ= Client Satisfaction Questionnaire. HAQ=Helping Alliance Questionnaire, Client Version. CSQ and HAQ values were obtained at Session 5.
2.2 Measures
Psychiatric diagnoses were assessed for inclusion/exclusion using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). DSM-5 PTSD diagnosis was assessed using the Clinician Administered PTSD Scale for DSM-5 (Weathers et al., 2013). The CAPS-5 was administered at baseline, session 5 and session 10 by trained evaluators blind to treatment condition. Weekly self-reported PTSD and depression symptoms were assessed with the PTSD Checklist for DSM-5 (PCL-5; Weathers, 2013) and Beck Depression Inventory, 2nd edition (BDI-II; Beck et al., 1996). The Helping Alliance Questionnaire (HAQ-II; Luborsky et al., 1996) and Client Satisfaction Questionnaire (CSQ; Nguyen et al., 1983) were administered at sessions 5 and 10 to assess feasibility and acceptability. Safety was assessed by monitoring adverse events (AEs) each week.
2.3 Procedures
All study procedures were IRB-approved, and all participants provided written informed consent. Participants were recruited from treatment clinics in the community and in the Department of Veterans Affairs (VA), and with newspaper and internet advertisements. Participation was open to individuals 1) male or female; any race or ethnicity; age 18 years or older, 2) able to comprehend English and function at an intellectual level sufficient to provide informed consent and accurately complete assessment instruments, and 3) met DSM-5 diagnostic criteria for current PTSD. Participants taking psychotropic medications were required to be maintained on a stable dose for at least eight weeks before study initiation. Exclusion criteria included 1) meeting diagnostic criteria for a history of or current psychotic or bipolar affective disorders, 2) serious risk of harm to self or others, 3) meeting diagnostic criteria for a current substance use disorder, except caffeine or nicotine, 4) pregnancy or breastfeeding for women, or 5) enrollment in another PTSD treatment.
2.3.1. Medication
Oxytocin is a hypothalamic neuropeptide that initiates physiologic events necessary for parturition and lactation (Carter, 1992; Kendrick et al., 1992). Intranasal oxytocin spray (40 IU) and matching placebo were compounded and dispensed by the research pharmacy, which also oversaw randomization. Participants self-administered medication in the research office, guided by trained research staff, 45 minutes prior to weekly PE sessions (sessions 2–9). Medication administration was observed by research staff to ensure proper procedures were followed. The dose and timing of medication administration is based on past research in our group and others (Cardoso et al., 2013; Flanagan et al., 2015; MacDonald et al., 2011).
2.3.2 Study Intervention
Participants received 10 individual, 90-minute weekly sessions of PE therapy (Foa et al., 2007). Study therapists were three female Masters or Doctoral-level clinicians who received weekly supervision during the trial.
2.3.3 Statistical Analyses
Descriptive statistics were calculated for demographic variables and baseline clinical characteristics for the two conditions. Wilcoxon ranked sum tests were used to examine changes over time in the feasibility outcomes (i.e., the CSQ and HAQ) which were collected at mid- and end- of treatment (Sessions 5 and 10, respectively). Adverse events and patient retention rates were monitored weekly throughout the study.
To assess preliminary efficacy, general linear mixed models (GLMMs) controlling for baseline values and repeated measurement effects were used to examine treatment group, time, and treatment group*time interactions. After controlling for baseline values, we also examined group differences at specific time points using appropriate linear combinations of the GLMM estimated parameters to explore group differences in the trajectory of symptom severity during treatment. Given the small sample size and pilot nature of this project, alpha was set at .05.
3. Results
3.1 Feasibility
One adverse event (i.e., chest pains) was reported during the study by a participant randomized to the placebo condition. At end of treatment, participants in both treatment conditions reported high satisfaction (oxytocin group M=29.67, SD=1.75; placebo group M=28.86 SD=2.91) and therapeutic alliance (oxytocin group M=101.17, SD=10.38; placebo group M=105.57, SD=9.62), and there were no significant between-group differences.
3.2 Preliminary Efficacy
When examining group differences in treatment outcomes, the treatment group*time interactions were not significant; therefore, the interactions were removed from the models. While no statistically significant differences between groups emerged on any of the three symptom variables, the oxytocin group had a marginally lower estimated mean PCL-5 score at end of treatment as compared to the placebo group (31.0 v. 40.9, p=0.09). For all three models, both baseline and time were significantly related to the outcomes, indicating that while there were no treatment group differences, scores on these instruments changed significantly over time among participants in both treatment conditions, and higher baseline values were related to higher scores at end of treatment.
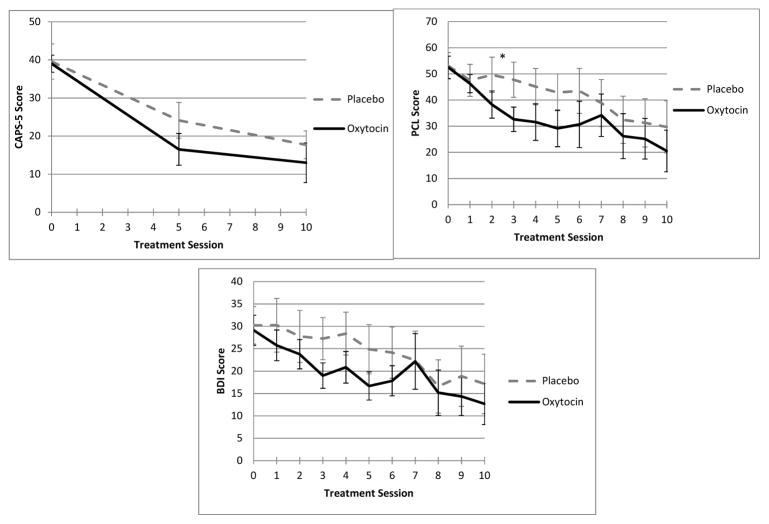
When estimating between-group differences in the trajectory of symptom improvement, session 3 PCL scores showed a statistically significant group difference (OT=30.3 vs. PL=45.4, p=0.03). As illustrated in Figure 1, mean scores between groups varied over time and were largely similar for all outcomes of interest.
Figure 1.
Mean total PTSD and depression symptom scores over time by treatment group. CAPS=Clinician-Administered PTSD Scale. PCL=PTSD Checklist. BDI-II=Beck Depression Inventory-II. Session 0= Baseline assessment. *p<.05.
4. Discussion
This exploratory pilot study was the first to pair an evidence-based psychotherapy for PTSD with weekly doses of intranasal oxytocin, which could serve to improve treatment outcomes via hypothesized mechanisms including enhanced extinction, treatment retention, and therapeutic alliance (Koch et al., 2014; Olff et al., 2010). Results support the safety, feasibility, and preliminary efficacy of augmenting PE with weekly doses of oxytocin in a sample of individuals who were diverse with respect to trauma exposure, race and ethnicity, and veteran vs. non-veteran status. Only one adverse event, which occurred in the placebo group, was reported. Participants in both the oxytocin and placebo conditions showed good retention in treatment compared to previous PE trials, improved symptom severity during treatment, and reported high treatment satisfaction. While statistically significant group differences in treatment outcomes were limited, results demonstrate that participants randomized to the oxytocin condition reported consistently lower symptom severity despite similar baseline PTSD symptom severity. These preliminary findings support the rationale for a larger randomized controlled trial to assess the efficacy of oxytocin for enhancing PE outcomes.
In addition to consistently lower PTSD and depression symptom severity, oxytocin was associated with significantly lower PTSD symptom severity at session 3. Previous trials of therapy-enhancing medications for PTSD have similarly shown medication-related differential trajectories of improvement (Difede et al., 2014; Zoellner et al., in press). One recent trial found that exposure therapy for PTSD combined with methylene blue was associated with accelerated recovery in later sessions and overall more reliable change. The authors attributed the non-linear effects to methylene blue-facilitated fear expression in early sessions and extinction in later sessions, thus proposing that methylene blue may be more effectively paired with later therapy sessions. A similar pattern was shown in healthy individuals administered oxytocin after fear conditioning: participants showed increased fear expression during early-stage extinction and then facilitated decline of physiological distress at the late stage of extinction (Eckstein et al., 2014). Participants in the current study self-administered oxytocin starting in session 2, a design selected so that oxytocin would coincide with exposure sessions of PE based on extinction being the hypothesized target of oxytocin; the differential symptom severity at session 3 supports this hypothesis. Future research is necessary to assess oxytocin’s ability to facilitate fear extinction specifically compared to placebo or other therapy enhancers, and the efficacy of utilizing oxytocin at specific timepoints in conjunction with behavioral interventions. This information will be useful to maximize oxytocin’s utility among patients with PTSD including identifying patients (such as those with higher baseline PTSD symptom severity) who might benefit more from oxytocin treatment.
It is possible that limiting the medication only to sessions 2–9 led to a missed opportunity for potential beneficial effects on therapeutic alliance—another potential mechanism of action in oxytocin-enhanced psychotherapy (Olff et al., 2010). This is a particularly important consideration because existing literature suggests that the rapport in PE is critical (Keller et al., 2010; McLaughlin et al., 2014). However, oxytocin effects have also been shown to be moderated by context (Shamay-Tsoory and Abu-Akel, 2016). Individuals with PTSD often perceive their environments as threatening and experience distrust of others (Ehlers and Clark, 2000). It is possible that administering oxytocin after providers and patients have had opportunities to develop rapport is more effective than earlier administration, when the salience-enhancing effects of oxytocin could potentially be harmful.
Although the present study did not employ neuroimaging to examine indicators of treatment response, an additional potential treatment mechanism that could be enhanced by oxytocin is connectivity within the emotion regulation network (Koch et al., 2014). Dysregulation of prefrontal cortex-amygdala connectivity is a salient correlate of PTSD (Pitman et al., 2012; Sripada et al., 2012; Woodward et al., 2006), and evidence suggests that oxytocin mitigates prefrontal cortex-amygdala dysregulation in healthy samples (Sripada et al., 2013) and in individuals with PTSD (Koch et al., 2016). Oxytocin may thus further promote the changes in prefrontal cortex function that has been observed in individuals who have undergone PE (Fonzo et al., in press). Additional studies testing the effects of oxytocin on mechanisms of PTSD treatment outcomes in PTSD patients are critical, as are investigations of biomarkers of oxytocin response.
The primary limitation of this study is the small sample size. This study was not designed or sufficiently powered to adequately examine between-group differences in treatment outcomes. Our limited ability to detect medication effects was also coupled with a high level of effectiveness of the intervention. Larger randomized controlled trials of PE similarly show large effects, but have also revealed a subset of patients who show limited or no benefit (Jonas et al., 2013). Future studies of oxytocin-enhanced PE should be powered to examine not only group differences in outcome, but also moderators of oxytocin effects. Such findings could inform precision medicine approaches to treating PTSD. For example, previous research has shown that oxytocin effects can vary as a function of emotion regulation skills (Quirin et al., 2011), social context history of childhood maltreatment (Ebert et al., 2013; Flanagan et al., 2015), and sex (Ditzen et al., 2012; Rilling et al., 2014). It is therefore possible that there are subpopulations of individuals with PTSD who might derive more benefit from oxytocin-enhanced treatment, as has been the case for other therapy enhancers like d-cycloserine and methylene blue. For example, previous studies suggest that d-cycloserine enhances responses to PE among patients who have more severe symptoms at baseline (de Kleine et al., 2012) and who exhibit within-session extinction learning (Rothbaum et al., 2014), while methylene blue effects have been shown to be moderated by baseline working memory (Zoellner et al., in press). Taken together, these findings suggest that various therapy enhancers may be better suited to some patients than others. Future research can assess whether oxytocin may be best suited, for example, for patients who are most at-risk for PE dropout. Although some studies have found no relationship between patient-therapist gender match and psychotherapy retention or outcome (Shiner et al., 2017; Sterling et al., 1998), adequately powered studies should consider examining this factor (Fujino et al., 1994; Greenfield et al., 2007; Wintersteen et al., 2005), particularly in light of the role that sex plays in oxytocin response in preclinical and laboratory studies.
Finally, future studies could consider daily dosing of oxytocin rather than weekly dosing to maximize potential therapeutic effects, particularly if oxytocin administration could be linked to specific treatment mechanisms. For example, investigators may wish to study whether patients’ self-administration of oxytocin before daily in vivo exposure exercises maximizes outcomes, guided again by the premise that oxytocin enhances extinction learning. Safety and feasibility of daily dosing has been demonstrated in other human OT studies including those with alcohol-dependent patients (Pedersen et al., 2013), patients with schizophrenia (Pedersen et al., 2011) and individuals with trauma exposure (van Zuiden et al., 2016).
In conclusion, this pilot study illustrated the feasibility, safety and preliminary efficacy of oxytocin-enhanced PE for PTSD. The findings inform future methodological approaches for adequately powered randomized controlled trials. Oxytocin could enhance early treatment response via promoting extinction learning and/or enhance retention in treatment via its anxiolytic and prosocial properties. Adequately powered studies that can effectively detect medication effects, assess mechanisms of action, and test moderating variables to identify subgroups for whom oxytocin may be most effective as an adjunctive treatment for PTSD are needed.
Highlights.
This study is the first to combine an evidence-based therapy for PTSD with oxytocin
We augmented Prolonged Exposure therapy with weekly oxytocin doses
We employed a randomized, placebo-controlled, double-blind design
Findings support the feasibility and safety of oxytocin combined with PE
Adequately powered studies are necessary to determine efficacy and mechanisms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson D, Feifel D, de Wilde S, Mckinney R, Lohr J, Risbrough V. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology. 2013;229(1):199–208. doi: 10.1007/s00213-013-3099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson DT, Feifel D, Kamenski M, Mckinney R, Risbrough VB. Intranasal oxytocin administration prior to exposure therapy for arachnophobia impedes treatment response. Depression and Anxiety. 2015;32(6):400–407. doi: 10.1002/da.22362. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Hormones and Behavior. 2006;50(4):518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. BDI-II, Beck depression inventory: manual. Psychological Corp; San Antonio, TX: 1996. [Google Scholar]
- Beck JG, Grant DM, Clapp JD, Palyo SA. Understanding the interpersonal impact of trauma: Contributions of PTSD and depression. Journal of Anxiety Disorders. 2009;23(4):443–450. doi: 10.1016/j.janxdis.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry. 2005;(162):214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: A dose–response study. Psychoneuroendocrinology. 2013;38(3):399–407. doi: 10.1016/j.psyneuen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Carter CS. Oxytocin and sexual behavior. Neuroscience and biobehavioral reviews. 1992;16(2):131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- Cloitre M, Stovall-McClough KC, Miranda R, Chemtob CM. Therapeutic alliance, negative mood regulation, and treatment outcome in child abuse-related posttraumatic stress disorder. Journal of consulting and clinical psychology. 2004;72(3):411. doi: 10.1037/0022-006X.72.3.411. [DOI] [PubMed] [Google Scholar]
- Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J. Nasal Oxytocin for Social Deficits in Childhood Autism: A Randomized Controlled Trial. J Autism Dev Disord. 2014;44:521–531. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- de Kleine RA, Hendriks G, Kusters WC, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biological Psychiatry. 2012;71(11):962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- de Kleine RA, Rothbaum BO, van Minnen A. Pharmacological enhancement of exposure-based treatment in PTSD: a qualitative review. European journal of psychotraumatology. 2013:4. doi: 10.3402/ejpt.v4i0.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difede J, Cukor J, Wyka K, Olden M, Hoffman H, Lee FS, Altemus M. D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: A pilot randomized clinical trial. Neuropsychopharmacology. 2014;39(5):1052–1058. doi: 10.1038/npp.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Nater UM, Schaer M, La Marca R, Bodenmann G, Ehlert U, Heinrichs M. Sex-specific effects of intranasal oxytocin on autonomic nervous system and emotional responses to couple conflict. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert A, Kolb M, Heller J, Edel M, Roser P, Brüne M. Modulation of interpersonal trust in borderline personality disorder by intranasal oxytocin and childhood trauma. Social neuroscience. 2013;8(4):305–313. doi: 10.1080/17470919.2013.807301. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE, Grinevich V, Kendrick KM, Maier W, Hurlemann R. Oxytocin Facilitates the Extinction of Conditioned Fear in Humans. Biological Psychiatry. 2014;78(3):194–202. doi: 10.1016/j.biopsych.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Eftekhari A, Ruzek JI, Crowley JJ, Rosen CS, Greenbaum MA, Karlin BE. Effectiveness of national implementation of prolonged exposure therapy in Veterans Affairs care. JAMA Psychiatry. 2013;70(9):949–955. doi: 10.1001/jamapsychiatry.2013.36. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behaviour research and therapy. 2000;38(4):319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Eskandarian S, Vafaei AA, Vaezi GH, Taherian F, Kashefi A, Rashidy-Pour A. Effects of systemic administration of oxytocin on contextual fear extinction in a rat model of post-traumatic stress disorder. Basic and Clinical Neuroscience. 2013;4(4):315. [PMC free article] [PubMed] [Google Scholar]
- Flanagan JC, Baker NL, McRae AL, Brady KT, Moran-Santa Maria M. Effects of Adverse Childhood Experiences on the Association between Intranasal Oxytocin and Social Stress Reactivity among Individuals with Cocaine Dependence. Psychiatry Research. 2015;229(1–2):94–100. doi: 10.1016/j.psychres.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Hembree EA, Cahill SP, Rauch SA, Riggs DS, Feeny NC, Yadin E. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: outcome at academic and community clinics. Journal of Consulting and Clinincal Psychology. 2005;73(5):953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- Foa EB, Hembree EA, Rothbaum BO. Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide. Oxford University Press; 2007. [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99(1):20. [PubMed] [Google Scholar]
- Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, Weiss ME, Thompson AL, Zack SE, Mills-Finnerty CE, Rosenberg BM, Edelstein R, Wright RN, Kole CA, Lindley SE, Arnow BA, Jo B, Gross JJ, Rothbaum BO, Etkin A. Selective Effects of Psychotherapy on Frontopolar Cortical Function in PTSD. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2017.16091073. in press. appi.ajp.2017.16091073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling JL, van Zuiden M, Koch S, Nawijn L, Goslings JC, Luitse JS, Biesheuvel TH, Honig A, Bakker FC, Denys D. Efficacy of oxytocin administration early after psychotrauma in preventing the development of PTSD: study protocol of a randomized controlled trial. BMC psychiatry. 2014;14(1):92. doi: 10.1186/1471-244X-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino DC, Okazaki S, Young K. Asian–American women in the mental health system: An examination of ethnic and gender match between therapist and client. Journal of Community Psychology. 1994;22(2):164–176. [Google Scholar]
- Goodson JT, Lefkowitz CM, Helstrom AW, Gawrysiak MJ. Outcomes of prolonged exposure therapy for veterans with posttraumatic stress disorder. Journal of Traumatic Stress. 2013;26(4):419–425. doi: 10.1002/jts.21830. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Trucco EM, McHugh RK, Lincoln MF, Gallop R. The Women’s Recovery Group Study: A Stage I trial of women-focused group therapy for substance use disorders versusmixed-gender group drug counseling. Drug and Alcohol Dependence. 2007;90:39–47. doi: 10.1016/j.drugalcdep.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Gray KM, Rinehart NJ, Alvares GA, Tonge BJ, Hickie IB, Keating CM, Cacciotti-Saija C, Einfeld SL. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. Journal of Child Psychology and Psychiatry. 2015;56(4):444–452. doi: 10.1111/jcpp.12305. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34(6):917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, Tu X. Do patients drop out prematurely from exposure therapy for PTSD? Journal of Traumatic Stress. 2003;16(6):555–562. doi: 10.1023/B:JOTS.0000004078.93012.7d. [DOI] [PubMed] [Google Scholar]
- Hetrick SE, Purcell R, Garner B, Parslow R. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2010;7(7) doi: 10.1002/14651858.CD007316.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath AO, Luborsky L. The Role of Alliance in Psychotherapy. Journal of Consulting and Clinical Psychology. 1993;4(61):561–573. doi: 10.1037//0022-006x.61.4.561. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. Washington, DC: 2008. [Google Scholar]
- Jonas DE, Cusack K, Forneris CA, Wilkins TM, Sonis J, Middleton JC, Feltner C, Meredith D, Cavanaugh J, Brownley K. Psychological and pharmacological treatments for adults with posttraumatic stress disorder (PTSD) Agency for Healthcare Research and Quality; Rockville, MD: 2013. [PubMed] [Google Scholar]
- Keller SM, Zoellner LA, Feeny NC. Understanding factors associated with early therapeutic alliance in PTSD treatment: Adherence, childhood sexual abuse history, and social support. Journal of Consulting and Clinical Psychology. 2010;78(6):974–979. doi: 10.1037/a0020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Hinton MR, Goode JA. Oxytocin, amino acid and monoamine release in the region of the medial preoptic area and bed nucleus of the stria terminalis of the sheep during parturition and suckling. Brain research. 1992;569(2):199–209. doi: 10.1016/0006-8993(92)90631-i. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelvemonth and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: salience processing and fear inhibition processes. Psychoneuroendocrinology. 2014;40:242–256. doi: 10.1016/j.psyneuen.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Koch S, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal oxytocin normalizes amygdala functional connectivity in posttraumatic stress disorder. Neuropsychopharmacology. 2016;41(8):2041–2051. doi: 10.1038/npp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, Hofmann SG. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. Journal of Psychiatric Research. 2012;46(9):1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Luborsky L, Barber JP, Siqueland L, Johnson SM, Najavits LM, Frank A, Daley D. The revised Helping Alliance questionnaire (HAq-II): psychometric properties. The Journal of Psychotherapy Practice and Research. 1996;5(3):260. [PMC free article] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36(8):1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry. 2010;18(1):1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM, Brüne M, Lamb K, Wilson MP, Golshan S, Feifel D. Oxytocin and psychotherapy: a pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology. 2013;38(12):2831–2843. doi: 10.1016/j.psyneuen.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Marx BP, Brailey K, Proctor SP, MacDonald HZ, Graefe AC, Amoroso P, Heeren T, Vasterline JJ. Association of time since deployment, combat intensity, and posttraumatic stress symptoms with neuropsychological outcomes following Iraq War deployment. Arch Gen Psychiatry. 2009;66:996–1004. doi: 10.1001/archgenpsychiatry.2009.109. [DOI] [PubMed] [Google Scholar]
- McLaughlin AA, Keller SM, Feeny NC, Youngstrom EA, Zoellner LA. Patterns of therapeutic alliance: Rupture–repair episodes in prolonged exposure for posttraumatic stress disorder. Journal of consulting and clinical psychology. 2014;82(1):112. doi: 10.1037/a0034696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson CM, Taft CT, Fredman SJ. Military-related PTSD and intimate relationships: From description to theory-driven research and intervention development. Clinical Psychology Review. 2009;29(8):707–714. doi: 10.1016/j.cpr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TD, Attkisson CC, Stegner BL. Assessment of patient satisfaction: development and refinement of a service evaluation questionnaire. Evaluation and Program Planning. 1983;6(3):299–313. doi: 10.1016/0149-7189(83)90010-1. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Witteveen A, Denys D. A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectrums. 2010;15(8):522–530. doi: 10.1017/s109285290000047x. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophrenia research. 2011;132(1):50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcoholism: Clinical and Experimental Research. 2013;37(3):484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein MB, Malley JC, Johnson DC, Southwick SM. Subsyndromal posttraumatic stress disorder is associated with health and psychosocial difficulties in veterans of Operations Enduring Freedom and Iraqi Freedom. Depression and Anxiety. 2009;26(8):739–744. doi: 10.1002/da.20574. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: Results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25(3):456–465. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Lasko NB. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Research. 1993;48:107–117. doi: 10.1016/0165-1781(93)90035-f. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review. 2010;30(6):635–641. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Preckel K, Scheele D, Kendrick KM, Maier W, Hurlemann R. Oxytocin facilitates social approach behavior in women. Frontiers in Behavioral Neuroscience. 2014;8:191. doi: 10.3389/fnbeh.2014.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Düsing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36(6):898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. Journal of Consulting and Clinical Psychology. 2002;70:867–879. doi: 10.1037//0022-006x.70.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, Kessler KJ. A Randomized, Double-blind Evaluation of D-cycloserine or Alprazolam Combined with Virtual Reality Exposure Therapy for Posttraumatic Stress Disorder (PTSD) in Iraq and Afghanistan War Veterans. The American Journal of Psychiatry. 2014;171(6):640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biological Psychiatry. 2016;79(3):194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shiner B, Westgate CL, Harik JM, Watts BV, Schnurr PP. Effect of patient-therapist gender match on psychotherapy retention among United States veterans with posttraumatic stress disorder. Administration and Policy in Mental Health and Mental Health Services Research. 2017;44(5):642–650. doi: 10.1007/s10488-016-0761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NB, Doran JM, Sippel LM, Harpaz-Rotem I. Fear extinction and memory reconsolidation as critical components in behavioral treatment for posttraumatic stress disorder and potential augmentation of these processes. Neuroscience letters. 2017;649:170–175. doi: 10.1016/j.neulet.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Sripada CS, Phan KL, Labuschagne I, Welsh RC, Nathan PJ, Wood AG. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. The International Journal of Neuropsychopharmacology. 2013;16(2):255–260. doi: 10.1017/S1461145712000533. [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of Psychiatry and Neuroscience. 2012;37(4):241. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stander VA, Thomsen CJ, Highfill-McRoy RM. Etiology of depression comorbidity in combat-related PTSD: a review of the literature. Clinical psychology review. 2014;34(2):87–98. doi: 10.1016/j.cpr.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Sterling RC, Gottheil E, Weinstein SP, Serota R. Therapist/patient race and sex matching: Treatment retention and 9-month follow-up outcome. Addiction. 1998;97(7):1043–1050. doi: 10.1046/j.1360-0443.1998.93710439.x. [DOI] [PubMed] [Google Scholar]
- The Management of Posttraumatic Stress Disorder Work Group. VA/DoD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. Washington, D.C: 2017. [Google Scholar]
- Tuerk PW. Starting From Something: Augmenting Exposure Therapy and Methods of Inquiry. American Journal of Psychiatry. 2014;171(10):1034–1037. doi: 10.1176/appi.ajp.2014.14070880. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Frijling JL, Nawijn L, Koch S, Goslings JC, Luitse JS, Biesheuvel TH, Honig A, Veltman D, Olff M. Intranasal oxytocin to prevent PTSD symptoms: a randomized controlled trial in emergency department patients. Biological Psychiatry. 2016;81(12):1030–1040. doi: 10.1016/j.biopsych.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Wangelin BC, Powers MB, Smits JJ, Tuerk PW. Enhancing exposure therapy for PTSD with yohimbine HCL: Protocol for a double-blind, randomized controlled study implementing subjective and objective measures of treatment outcome. Contemporary Clinical Trials. 2013;36(2):319–326. doi: 10.1016/j.cct.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Weathers FW. The PTSD checklist for DSM-5 (PCL-5): development and initial psychometric analysis. The 29th Annual meeting of the International Society for Traumatic Stress Studies; Philadelphia, PA. 2013. [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP. PTSD: National Center for PTSD. US Department of Veterans Affairs; 2013. Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) [Google Scholar]
- Wintersteen MB, Mensinger JL, Diamond GS. Do gender and racial differences between patient and therapist affect therapeutic alliance and treatment retention in adolescents? Professional Psychology: Research and Practice. 2005;36(4):400. [Google Scholar]
- Woodward SH, Neylan TC, Mellman TA, Ross RJ. Distinguishing current from remitted posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63(8):940–941. doi: 10.1001/archpsyc.63.8.940. [DOI] [PubMed] [Google Scholar]
- Yatzkar U, Klein E. P.3.026 Intranasal oxytocin in patients with post traumatic stress disorder: a single dose, pilot double blind crossover study. European Neuropsychopharmacology. 2010;20:S84. [Google Scholar]
- Zoellner LA, Telch M, Foa EB, Farach FJ, McLean CP, Gallop R, Bluett EJ, Cobb A, Gonzalez-Lima F. Enhancing extinction learning in posttraumatic stress disorder with brief daily imaginal exposure and methylene blue: a randomized controlled trial. Journal of Clinical Psychiatry. doi: 10.4088/JCP.16m10936. in press. [DOI] [PubMed] [Google Scholar]