Abstract
Background
Autism spectrum disorder (ASD) is a mental disorder that has long been considered to result from brain underconnectivity. However, volumetric analysis of structural MRI data has failed to find consistent white matter alterations in patients with ASD. The present study aims to examine whether there are consistent focal white matter alterations as measured by diffusion tensor imaging (DTI) in individuals with ASD compared with typically developing (TD) individuals.
Method
Coordinate-based meta-analysis was performed on 14 studies that reported fractional anisotropy (FA) alterations between individuals with ASD and TD individuals. These studies have in total 297 subjects with ASD and 302 TD subjects.
Results
Activation likelihood estimation (ALE) analysis identified two clusters of white matter regions that showed consistent reduction of FA in individuals with ASD compared with TD individuals: the left splenium of corpus callosum and the right cerebral peduncle.
Conclusions
Consistent focal white matter reductions in ASD could be identified by using FA, highlighting the cerebral peduncle which is usually overlooked in studies focusing on major white matter tracts. These focal reductions in the splenium and the cerebral peduncle may be associated with sensorimotor impairments seen in individuals with ASD.
Keywords: Autism spectrum disorder, corpus callosum, DTI, fractional anisotropy, meta-analysis, white matter
1. Introduction
Autism spectrum disorder (ASD) is a mental disorder thought to be the result of underconnectivity between brain regions (1). Under this premise, different lines of studies have been performed to understand brain white matter anatomical connectivity and functional connectivity under resting-state and task conditions. Earlier functional MRI (fMRI) studies have reported lower functional connectivity between the frontal lobe and posterior cortical regions in ASD patients compared with typically developing (TD) controls during performance of executive function related tasks (2; 3). In contrast, studies of resting-state functional connectivity typically found largely similar connectivity patterns with healthy controls (4), and most of the detectable connectivity were reduced functional connectivity in individuals with ASD (5) (but see (6)).
In addition to functional connectivity, another line of studies has focused on the infrastructure of brain connectivity, i.e. white matter anatomy. One commonly used approach is to study morphological differences of the white matter structures using voxel-based morphometry (VBM), which examines morphometrical differences in every voxel of the structural MRI (7). However, inconsistent results have been reported in VBM-based studies in ASD. Several early structural MRI studies showed widespread white matter volume reductions in ASD compared with healthy controls (8; 9), whereas later meta-analyses identified both increased and decreased white matter volumes in patients with ASD compared with controls (10–13). The inconsistence of these early studies may be caused by the heterogeneity of different study samples. Recent large-scale multi-site MRI data sharing makes it possible to accumulate large samples of subjects (5). However, studies using the Autism Brain Imaging Data Exchange (ABIDE) did not find reliable white matter volumetric alterations (14–16).
Apart from the morphormetric examinations of white matter, diffusion tensor imaging (DTI) has made it possible to study white matter microstructures and properties of large-scale white matter tracts (17–19). Instead of looking at macro level white matter morphology, DTI offers information on the microstructural properties of white matter tracts. Reduced fractional anisotropy (FA) of white matter in individuals with ASD has been consistently reported in many white matter tracts: the corpus callosum, cingulum, uncinate fasciculus, and superior longitudinal fasciculus (20; 21). However, these two meta-analytical studies used an ROI-based (regions of interest) approach, which relies on authors’ defined ROIs and precludes whole brain evaluations of white matter alterations (22). It is possible that white matter alterations may occur outside the major white matter tracts. Therefore, voxel-wise meta-analysis is needed to provide more comprehensive mapping of white matter microstructure alterations in ASD.
Activation likelihood estimation (ALE) analysis (23) is a voxel-based meta-analysis method for examining the consistency of reported spatial coordinates across neuroimaging studies. Although originally proposed for fMRI studies, it can be applied to other neuroimaging modalities as well, such as VBM studies (24–27) and DTI studies (28; 29). Since a large portion of DTI studies performed voxel-based analysis (VBA) or tract-based spatial statistics (TBSS) on spatial properties such as FA, we can examine consistency of FA alterations in patients with ASD compared with TD controls. In the current study, we performed a voxel-based meta-analysis on DTI studies that used VBA or TBSS to compare FA differences between individuals with ASD and TD individuals. Based on the results of former studies (18–20) and the underconnectivity theory (1), we hypothesize that there would be focal white matter reductions in ASD. We are looking for such focal white matter alterations in either previously reported white matter tracts, such as the corpus callosum, or other white matter regions that might be overlooked with ROI-based meta-analyses.
2. Materials and methods
2.1 Literature search
We performed two PubMed searches: using key words “autism” combined with “fractional anisotropy” and the other using “autism” with “diffusion tensor imaging” to identify relevant papers. The original search was performed in December 2015. Papers published after the initial search with the same key words were identified by PubMed generated RSS (rich site summary) feeds. We chose to study FA differences because most of the VBA or TBSS studies compared FA, whereas only a small portion of studies reported other measures, e.g. mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD).
Criteria for inclusion and exclusion are as follows: first, a study was included if it examines FA differences between a group of subjects with ASD and a group of TD subjects. Second, the study should perform VBA or TBSS, instead of ROI-based analysis. Third, results were reported as stereoscopic coordinates in either MNI (Montreal Neurological Institute) or Talairach space. Fourth, the ASD groups were high-functioning ASD, defined as mean full-scale intelligence quotient (IQ) greater than 70 (30). One study was discarded because the mean nonverbal IQ of the autistic group is 49 (31). Another study did not report IQ, possibly because the mean age of the sample is too young to perform IQ test (28 months) (32). The age range of this study (2 – 3 years old) is also quite different from all other studies included in the current analysis. Therefore, this paper was also excluded from the analysis.
As a result, 14 papers were included in the current meta-analysis (Table 1). Among these studies, all reported clusters of smaller FA in the ASD group than in the TD group. Within these, four studies also reported clusters of greater FA in ASD group than TD group. Due to the limited number of studies, we only performed meta-analysis on FA reductions in the ASD group compared with the TD group. In total there are 132 foci reported from the 14 studies. Together, the 14 studies included a total of 297 subjects with ASD and 302 TD subjects. Seven studies included only male subjects, while the other 7 studies included a small portion of female subjects. Eight studies used VBA analysis, and 6 studies adopted TBSS method. The details of DTI imaging and statistical analysis in these papers are outlined in Table 2.
Table 1.
Study and subject information of the studies included in the current meta-analysis.
| Study | ASD | TD | ↓↑ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | F | age (y) | IQ | n | F | age (y) | IQ | |||
| 1 | Bakhtiari 2012a | 16 | 1 | 15.5 | 108.1 | 18 | 1 | 15.5 | 111.8 | ↑ |
| 2 | Bamea-Goraly 2004 | 7 | 0 | 14.6 | 101 | 9 | 0 | 13.4 | 107 | ↑ |
| 3 | Bloemen 2010 | 13 | 0 | 39 | 110 | 13 | 0 | 37 | 115 | ↑ |
| 4 | Cheng 2010 | 25 | 0 | 13.71 | 101.6 | 25 | 0 | 13.51 | 109.04 | ↓↑ |
| 5 | Cheung 2009 | 13 | 1 | 9.3 | 99.5 | 14 | 1 | 9.9 | 111.9 | ↓↑ |
| 6 | Fitzgerald 2016 | 45 | 0 | 15.91 | 109.5 | 45 | 0 | 16.55 | 115 | ↑ |
| 7 | Itahashi 2015 | 46 | 0 | 30.21 | 106 | 46 | 0 | 30.54 | 109.22 | ↑ |
| 8 | Ke 2009 | 12 | 0 | 8.75 | 100.6 | 10 | 0 | 9.4 | 99.83 | ↓↑ |
| 9 | Keller 2007 | 34 | 0 | 18.9 | 102 | 31 | 0 | 18.9 | 109.5 | ↑ |
| 10 | Kleinhans 2013 | 25 | 9 | 21.29 | 109.88 | 28 | 6 | 21.31 | 113.25 | ↑ |
| 11 | Mueller 2013 | 12 | 3 | 35.5 | 111.3 | 12 | 4 | 33.3 | 110.8 | ↑ |
| 12 | Nickel 2017 | 30 | 11 | 35.4 | 124.5 | 30 | 11 | 35.53 | 123.63 | ↑ |
| 13 | Noriuchi 2010 | 7 | 1 | 13.96 | 92.71 | 7 | 1 | 13.36 | 116.43 | ↑ |
| 14 | Thakkar 2008 | 12 | 2 | 30 | 116 | 14 | 6 | 27 | 114 | ↓↑ |
↓ and ↑ indicate a study reported clusters with increased or increased FA in ASD compared with TD, respectively.
Bakhtiariet al. (2012) recruited two age groups, but only adolescent group showed statistically significant results. Therefore, only adolescent groups of patients and controls were included in the current analysis.
Table 2.
Diffusion tensor imaging (DTI) parameters and analysis methods in the studies of the current meta-analysis.
| Study | Tesla | Resolution | No. direction | B0 | Analysis | Software | |
|---|---|---|---|---|---|---|---|
| 1 | Bakhtiari 2012 | 3 | 2 × 2 × 2 | 70 | 700 | TBSS | FSL |
| 2 | Barnea-Goraly 2004 | 3 | 1.875 × 1.875 × 6.5 | 6 | 900 | VBA | SPM |
| 3 | Bloemen 2010 | 1.5 | 1.875 × 1.875 × 2.5 | 64 | 1300 | VBA | SPM |
| 4 | Cheng 2010 | 1.5 | 2 × 2 × 2.2 | 70 | 900 | TBSS | FSL |
| 5 | Cheung 2009 | 1.5 | 2.1875 × 2.1875 × 6.5 | 25 | 1200 | VBA | SPM |
| 6 | Fitzgerald 2016 | 3 | 1.94 × 1.94 × 2 | 61 | 1500 | TBSS | FSL |
| 7 | Itahashi 2015 | 1.5 | 1.875 × 1.875 × 3 | 30 | 1000 | TBSS | FSL |
| 8 | Ke 2009 | 1.5 | 1.875 × 1.875 × 3 | 15 | 1000 | VBA | SPM |
| 9 | Keller 2007 | 3 | 1.5625 × 1.5625 × 3 | 6 | 850 | VBA | SPM |
| 10 | Kleinhans 2013 | 3 | 1.875 × 1.875 × 2 | 32 | 1000 | TBSS | FSL |
| 11 | Mueller 2013 | 3 | 1.8 × 1.8 × 4 | 20 | 1000 | TBSS | FSL |
| 12 | Nickel 2017 | 3 | 2 × 2 × 2 | 61 | 1000 | VBA | SPM |
| 13 | Noriuchi 2010 | 3 | 1.8 × 1.8 × 2 | 32 | 800 | VBA | SPM |
| 14 | Thakkar 2008 | 3 | 2 × 2 × 2 | 72 | 700 | VBA | FreeSurfer |
2.2 Activation likelihood estimation analysis
We performed a simple ALE analysis to estimate consistent FA alterations in ASD compared with TD across the 14 studies. GingerALE 2.3.6 was used for ALE analysis (33–35). Turkeltaub et al.’s non-addtive method was used (34), and the spatial smoothness for each study was estimated based on the number of subjects (33). The number of subjects was approximated as the minimum number of subjects in either the ASD or TD group. The analysis was performed in MNI space. One study used Brett’s transformation to convert the results from MNI space into Talairach space. The results were transformed back into MNI space. The ALE analysis results were first thresholded using uncorrected p < 0.001, and a cluster-level threshold of p < 0.05 was used for multiple comparison correction. The resulting clusters were labeled based on the JHU (Johns Hopkins University) DTI-based white-matter atlases (36; 37).
2.3 Sample age and IQ
For each study, we extracted the mean and standard deviation (SD) of the age of each group. The age ranges were not available for all the studies. Based on the available age ranges and information from the text, we assigned the sample into three groups, i.e. preadolescent children (age less than 10 years old), adolescents (age between 10 to 20 years old), and adults (age greater than 20). The assignment of age groups was not clear-cut. Three studies included both preadolescents and adolescents. Three studies included only adolescents. Three studies included adolescents and adults. While five studies included only adults. Because there are only a few studies in each group, it is not possible to perform moderator analysis on the effects of age. We then performed a post-hoc analysis on the studies that contributed to the consistent results in the meta-analysis to see whether these studies were from particular age groups.
Most of the studies reported full scale IQ from some versions of the Wechsler Intelligence Scales. However, four studies used different methods. One study reported only performance IQ (38). One study used Raven’s progressive matrices (39). One study used Multiple choice vocabulary test (MWT) (40). And one study used American National Adult Reading Test to estimate verbal IQ (41). For each study and each group, mean and SD of IQ scores were extracted. Cohen’s d was calculated for each study. We performed post-hoc analysis on whether the studies that showed consistent meta-analysis results were due to IQ differences between case and control groups.
3. Results
Two clusters were identified that showed consistent FA reductions in individuals with ASD compared with TD individuals (Figure 1 and Table 3). The first cluster was located in the left splenium of the corpus callosum, as three out of the total of 14 studies contributed to it. The second cluster was located in the right cerebral peduncle, with four out of 14 studies having contributed to it. The studies that contributed to each of the clusters are also listed in Table 3.
Figure 1.
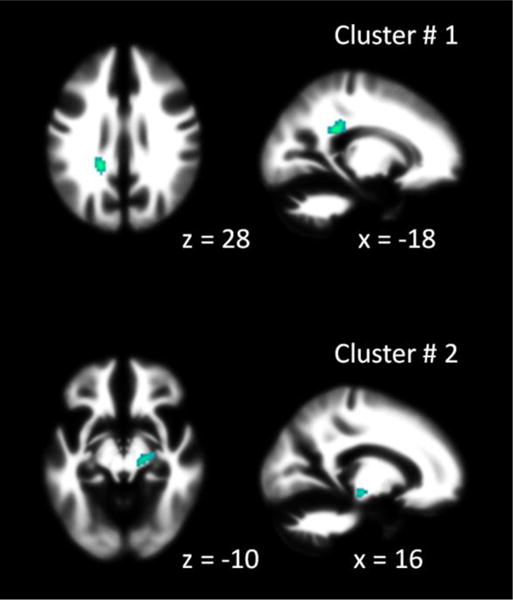
Regions that showed consistent fractional anisotropy (FA) reduction in individuals with autism spectrum disorder (ASD) compared with typically developing (TD) individuals. The x or z values under each slide indicates x or z coordinates in MNI space (Montreal Neurology Institute). Cluster numbers correspond to what reported in Table 3.
Table 3.
Clusters that showed consistent smaller fractional anisotropy (FA) in individuals with autism spectrum disorder (ASD) and typically developing (TD) individuals. Contributed studies correspond to the study numbers in Table 1 & 2.
| Cluster # | Volume (mm3) | Extrema | x | y | z | Label | Contributed studies |
|---|---|---|---|---|---|---|---|
| 1 | 760 | 0.016214 | −18 | −38 | 28 | Left splenium of corpus callosum | 1, 6, 10 |
| 2 | 640 | 0.013563 | 16 | −20 | −10 | Right cerebral peduncle | 1, 7, 10, 11 |
We sorted the 14 studies according to the mean age of the ASD group, and highlighted the studies that have contributed to the consistent clusters in the ALE analysis (Figure 2A). There is no clear specific age range for these studies, and the age ranges of these studies covered both adolescence and adulthood. We also sorted the 14 studies according to the IQ differences (Cohen’s d) between the two groups (Figure 2B). The studies that contributed to the consistent clusters had only small effects sizes of IQ differences. There is one study that has extremely different IQ scores between the ASD and TD groups (Cohen’s d = 2.89) (42). We also discarded this study, and re-run the meta-analysis with the remaining 13 studies. The results remained the same.
Figure 2.
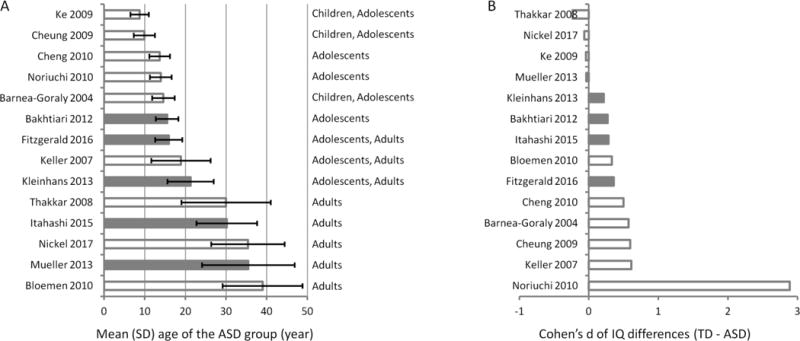
Mean ages of the ASD group (A) and IQ differences (Cohen’s d) between the TD group and ASD group (B) in different studies. The studies were ranked according to the mean age and IQ differences, respectively. The error bars in A represent standard deviations of the samples’ age. The age range of each study can be roughly categorized into preadolescent children, adolescents, or adults based on the descriptions in the paper. Gray bars indicate that these studies contribute to the resultant consistent clusters in the meta-analysis.
We also examined several methodological factors, including the number of subjects in the ASD group, publication year, the number of DTI directions, and the analysis methods (TBSS vs. VBA) (Figure 3). Among these factors, we found an interesting trend that all the studies that contributed to the consistent clusters were published after 2011 and used the TBSS method.
Figure 3.
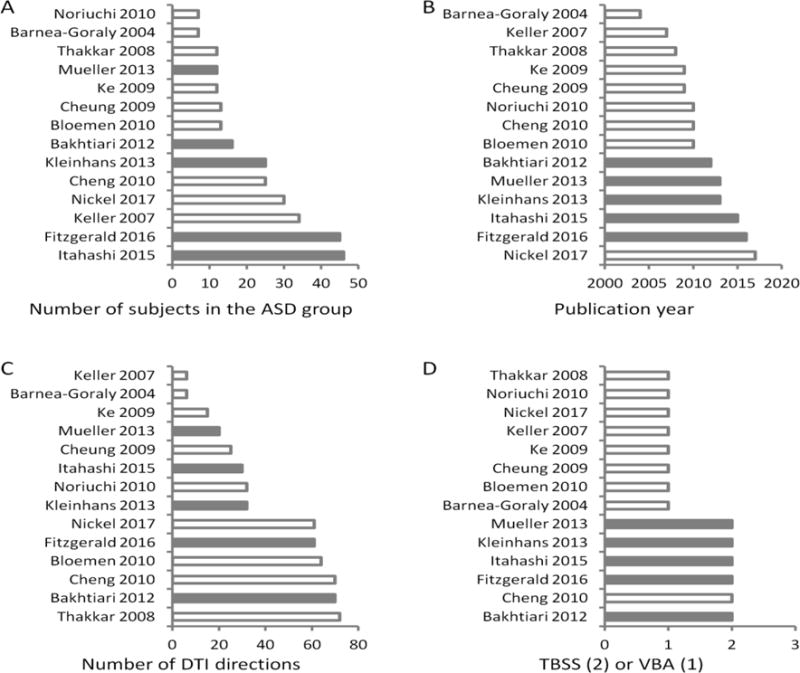
Study and methodological characteristics for each study. A) Number of subjects in the ASD group. B) Publication year. C) Number of directions in DTI imaging. D) Analysis methods with 1 representing VBA (voxel-based analysis) and 2 representing TBSS (tract-based spatial statistics). The studies were ranked according to respective variables in each plot. Gray bars indicate that these studies contribute to the resultant consistent clusters in the meta-analysis.
4. Discussion
Using voxel-based meta-analysis, the current study searched for consistent brain white matter FA alterations in ASD across 14 studies, with a total of 297 subjects with ASD and 302 TD subjects. All studies reported reduced FA in the ASD group compared with TD group, while only four studies reported increased FA in different brain regions. Consistent with our prediction, we identified focal white matter regions that showed consistent FA reductions in the ASD group, the left splenium of the corpus callosum and the right cerebral peduncle.
Existing meta-analyses on VBM studies have failed to report consistent white matter reductions in the two regions observed in the current analysis (10–13). A large-scale analysis of corpus callosum volumes on the ABIDE data did not demonstrate clear evidence of smaller corpus callosum volume in ASD subjects either (14). In contrast, reduced FA, as well as increased MD, in the corpus callosum have been consistently reported in DTI studies (20; 21). Adopting a voxel-wise analysis approach, the current analysis identified altered FA consistently in the posterior portion of the corpus callosum, i.e. the splenium. In addition to the corpus callosum, voxel-wise analysis also enabled us to identify FA reductions in the cerebral peduncle, an area overlooked in previous reviews and meta-analyses (20; 21). A closer look at previous DTI studies not included in the current studies also suggests FA reductions in the cerebral peduncle (43–45).
The splenium is the most posterior portion of the corpus callosum, which connects homotopic regions of the occipital, parietal, and temporal areas of the brain (46; 47). The main role of the corpus callosum is to enable inter-hemispheric communications, either in an excitatory or inhibitory manor (48). The splenium is mainly served as a relay between homotopic regions of the occipital, parietal, and temporal lobe regions, which mainly involve visual processing. The reduction in the splenium in subjects with ASD may be associated with abnormalities of visual processing in ASD, specifically the deficit of higher visual processing such as face processing (49–51). The second cluster of the right cerebral peduncle is in the anterior part of the midbrain, which acts as a conductor between the midbrain, thalamic nuclei, and cerebrum. Largely responsible for refining motor movements, white matter integrity of the cerebral peduncle was found to be related to motor function recovery after stroke (52). The reduction of white matter in the cerebral peduncle in ASD may be associated with impaired motor functions in individuals with ASD (53). However, such a link needs to be directly tested in future studies.
Several factors may moderate brain structural or functional alterations in ASD, e.g. age range, IQ differences between groups, and biological sex. Due to the limited number of studies, it is not possible to perform moderation analysis to directly examine these effects. To get some insights, we identified the studies that contributed to the resulting clusters, and explored whether these studies were biased in these sample characteristics. As can be seen in Figure 2, the two consistent clusters were contributed by studies with participants at both adolescent and adult age range. Given the limited number of studies that cover the preadolescent or even younger age range, it is not possible to directly compare FA alterations at different age ranges. Several studies of brain structural and functional alterations in ASD have shown developmental effects, not only in DTI studies (54; 55), but also in gray matter volume studies (15) and fMRI studies (56; 57). The effects of age need to be further explored when more studies are available.
ASD includes a wide range of IQ. The mean IQs of the samples in the current study are all above 90. In addition, only two studies reported statistical significant differences in IQ between the ASD and TD groups (18; 42). Among them, one study reported an extreme IQ difference in term of effect size (Figure 2B) (42). The studies that contributed to the consistent clusters, on the other hand, only showed small effects of IQ differences. This suggests that the differences observed in the current analysis are not due to IQ differences between the case and control samples. Inclusion of low IQ samples may increase the variations so that the sensitivity of detecting consistent FA alterations reduced.
The effects of sex on brain structures and functions have been a hot topic due to epidemiological differences of autism in males and females (58). However, 7 out of 14 studies in the current analysis only included male subjects, and the remaining 7 studies only recruited a small portion of female subjects in their sample. A recent study did suggest gender differences of corpus callosum connectivity to the frontal cortex in ASD (55). Further research is necessary to understand gender differences in individuals with ASD.
In addition to the study sample characteristics, methodological factors regarding DTI data acquisition and analysis may also affect the results. We did not see a clear pattern in the number of DTI directions on the consistent results. However, we found a clear pattern in which analysis methods may affect the consistency of results. Specifically, all the studies that showed consistent results in the current analysis used the TBSS approach, and were published after 2011. One difference between VBA and TBSS methods is that inter-subject registration and normalization is based on whole brain tissue maps for VBA and based on white matter “skeleton” for TBSS. In addition, VBA performs statistical analysis on every voxel in brain, while TBSS only performs analysis on the “skeleton” of white matter tracts (59). Therefore, the TBSS method may have better inter-subject registration and less severe problem of multiple comparison. This may explain why the TBSS method produces more consistent results compared with VBA. However, other factors may also contribute to this observation, e.g. TBSS analyses in the current analysis were all performed using FSL (FMRIB Software Library) (60), but VBA analyses were typically performed using SPM (http://www.fil.ion.ucl.ac.uk/spm/). Statistical models and inference methods may also be different in the two packages. The analysis of DTI data actually has great flexibility regarding the choose of comparing parameters, analysis method, and so on. New method such as Automating Fiber-Tract Quantification (AFQ) (61; 62) have also been proposed and are currently being used. These different methodologies may also make it difficult to compare results between DTI studies, thus making them less reproducible.
ASD is a complex and highly heterogeneous mental condition, both in terms of underlying genetics (63) and brain structures (64). Overall, two regions of clinical interest across the 14 studies were found: the left splenium of the corpus callosum and the right cerebral peduncle. Further inquiries on the methods of analysis as well as the software used may reduce variance and clarify inconsistencies across studies.
Highlights.
Meta-analysis on voxel-wise FA differences in ASD
Consistent FA reductions in the left splenium and right cerebral peduncle
DTI data analysis pipeline may affect the consistency of results
Acknowledgments
The research is funded by a New Jersey Autism Center of Excellence (NJ ACE) grant CAUT16APL019 and National Institute of Health (NIH) grants R01 AG032088 and R01 DA038895.
Abbreviations
- ABIDE
Autism Brain Imaging Data Exchange
- AD
axial diffusivity
- AFQ
Fiber-Tract Quantification
- ALE
Activation likelihood estimation
- ASD
Autism spectrum disorder
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- fMRI
function magnetic resonance imaging
- IQ
intelligence quotient
- JHU
Johns Hopkins University
- MD
mean diffusivity
- MNI
Montreal Neurological Institute
- RD
radial diffusivity
- ROI
regions of interest
- RSS
rich site summary
- TBSS
tract-based spatial statistics
- TD
typically developing
- VBA
voxel-based analysis
- VBM
voxel-based morphometry
Appendix Studies included in the current meta-analysis
- Bakhtiari R, Zürcher NR, Rogier O, Russo B, Hippolyte L, Granziera C, Araabi BN, Nili Ahmadabadi M, Hadjikhani N. Differences in white matter reflect atypical developmental trajectory in autism: A Tract-based Spatial Statistics study. NeuroImage Clin. 2012;1:48–56. doi: 10.1016/j.nicl.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Bloemen OJN, Deeley Q, Sundram F, Daly E, Barker GJ, Jones DK, van Amelsvoort TAMJ, Schmitz N, Robertson D, Murphy KC, Murphy DGM. White matter integrity in Asperger syndrome: a preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res. 2010;3:203–213. doi: 10.1002/aur.146. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010;50:873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TKW, Ho TP, McAlonan GM. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry. 2009;50:1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Gallagher L, McGrath J. Widespread Disrupted White Matter Microstructure in Autism Spectrum Disorders. J Autism Dev Disord. 2016 May 20; doi: 10.1007/s10803-016-2803-8. [DOI] [PubMed] [Google Scholar]
- Itahashi T, Yamada T, Nakamura M, Watanabe H, Yamagata B, Jimbo D, Shioda S, Kuroda M, Toriizuka K, Kato N, Hashimoto R. Linked alterations in gray and white matter morphology in adults with high-functioning autism spectrum disorder: A multimodal brain imaging study. NeuroImage Clin. 2015;7:155–169. doi: 10.1016/j.nicl.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, Zhou Z, Ruan Z, Lu Z, Tao G, Liu Y. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Ph D, Pauley G, Richards T, Martin N, Corrigan NM, Shaw DW. Age-related abnormalities in white matter microstructure in autism spectrum disorders. Brain Res. 2013;1479:206–221. doi: 10.1016/j.brainres.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Keeser D, Samson AC, Kirsch V, Blautzik J, Grothe M, Erat O, Hegenloh M, Coates U, Reiser MF, Hennig-Fast K, Meindl T. Convergent Findings of Altered Functional and Structural Brain Connectivity in Individuals with High Functioning Autism: A Multimodal MRI Study. PLoS One. 2013;8:e67329. doi: 10.1371/journal.pone.0067329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel K, Tebartz van Elst L, Perlov E, Endres D, Müller GT, Riedel A, Fangmeier T, Maier S. Altered white matter integrity in adults with autism spectrum disorder and an IQ >100: a diffusion tensor imaging study. Acta Psychiatr Scand. 2017;135:573–583. doi: 10.1111/acps.12731. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, Tobimatsu S, Kamio Y. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res. 2010;1362:141–149. doi: 10.1016/j.brainres.2010.09.051. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJS, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36:1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 3.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an fmri study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyszka JM, Kennedy DP, Paul LK, Adolphs R. Largely Typical Patterns of Resting-State Functional Connectivity in High-Functioning Adults with Autism. Cereb Cortex. 2014;24:1894–1905. doi: 10.1093/cercor/bht040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Martino A, Yan C-G, Li Q, Denio E, Castellanos FX, Alaerts K, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2014;19:659–67. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, et al. Brain Hyperconnectivity in Children with Autism and its Links to Social Deficits. Cell Rep. 2013;5:738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 8.Chung MK, Dalton KM, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. Neuroimage. 2004;23:242–251. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: A voxel-based morphometry MRI study. Neuroimage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Radua J, Via E, Catani M, Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2011;41:1539–1550. doi: 10.1017/S0033291710002187. [DOI] [PubMed] [Google Scholar]
- 11.DeRamus TP, Kana RK. Anatomical likelihood estimation meta-analysis of grey and white matter anomalies in autism spectrum disorders. NeuroImage Clin. 2014 doi: 10.1016/j.nicl.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nickl-Jockschat T, Habel U, Maria Michel T, Manning J, Laird AR, Fox PT, et al. Brain structure anomalies in autism spectrum disorder-a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp. 2012;33:1470–1489. doi: 10.1002/hbm.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duerden EG, Mak-Fan KM, Taylor MJ, Roberts SW. Regional differences in grey and white matter in children and adults with autism spectrum disorders: An activation likelihood estimate (ALE) meta-analysis. Autism Res. 2012;5:49–66. doi: 10.1002/aur.235. [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre A, Beggiato A, Bourgeron T, Toro R. Neuroanatomical Diversity of Corpus Callosum and Brain Volume in Autism: Meta-analysis, Analysis of the Autism Brain Imaging Data Exchange Project, and Simulation. Biol Psychiatry. 2015;78:126–34. doi: 10.1016/j.biopsych.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Di X, Biswal BB. Similarly Expanded Bilateral Temporal Lobe Volumes in Female and Male Children With Autism Spectrum Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:178–185. doi: 10.1016/j.bpsc.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haar S, Berman S, Behrmann M, Dinstein I. Anatomical Abnormalities in Autism? Cereb Cortex. 2014:1–13. doi: 10.1093/cercor/bhu242. [DOI] [PubMed] [Google Scholar]
- 17.Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- 19.Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Aoki Y, Abe O, Nippashi Y, Yamasue H. Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Mol Autism. 2013;4:25. doi: 10.1186/2040-2392-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travers BG, Adluru N, Ennis C, Tromp DPM, Destiche D, Doran S, et al. Diffusion Tensor Imaging in Autism Spectrum Disorder: A Review. Autism Res. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A comparison of label-based review and ALE meta-analysis in the stroop task. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-Analysis of the Functional Neuroanatomy of Single-Word Reading: Method and Validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 24.Chan RCK, Di X, McAlonan GM, Gong Q. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37:177–88. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di X, Chan RCK, Gong Q. White matter reduction in patients with schizophrenia as revealed by voxel-based morphometry: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1390–4. doi: 10.1016/j.pnpbp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. Meta-Analysis of Gray Matter Anomalies in Schizophrenia: Application of Anatomic Likelihood Estimation and Network Analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di X, Rypma B, Biswal BB. Correspondence of executive function related functional and anatomical alterations in aging brain. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:41–50. doi: 10.1016/j.pnpbp.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, et al. Is depression a disconnection syndrome? Meta- analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai MC, Lombardo MV, Suckling J, Ruigrok ANV, Chakrabarti B, Ecker C, et al. Biological sex affects the neurobiology of autism. Brain. 2013;136:2799–2815. doi: 10.1093/brain/awt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardini M, Garaci FG, Bonzano L, Roccatagliata L, Palmieri MG, Pompili E, et al. White matter reduced streamline coherence in young men with autism and mental retardation. Eur J Neurol. 2009;16:1185–1190. doi: 10.1111/j.1468-1331.2009.02699.x. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Z, Qiu T, Ke X, Xiao X, Xiao T, Liang F, et al. Autism spectrum disorder as early neurodevelopmental disorder: Evidence from the brain imaging abnormalities in 2–3 years old toddlers. J Autism Dev Disord. 2014;44:1633–1640. doi: 10.1007/s10803-014-2033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–47. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakhtiari R, Zürcher NR, Rogier O, Russo B, Hippolyte L, Granziera C, et al. Differences in white matter reflect atypical developmental trajectory in autism: A Tract-based Spatial Statistics study. NeuroImage Clin. 2012;1:48–56. doi: 10.1016/j.nicl.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TKW, et al. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry. 2009;50:1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- 40.Nickel K, Tebartz van Elst L, Perlov E, Endres D, Müller GT, Riedel A, et al. Altered white matter integrity in adults with autism spectrum disorder and an IQ >100: a diffusion tensor imaging study. Acta Psychiatr Scand. 2017;135:573–583. doi: 10.1111/acps.12731. [DOI] [PubMed] [Google Scholar]
- 41.Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJS, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, et al. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res. 2010;1362:141–149. doi: 10.1016/j.brainres.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 43.Shukla DK, Keehn B, Lincoln AJ, Müller R-A. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:1269–78. 1278–2. doi: 10.1016/j.jaac.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, Pugliese L, et al. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage. 2008;41:1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 45.Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Jr, de Rodrigues L S, Gasparetto EL, Calçada CABP. Diffusion Tensor Imaging Findings in School-Aged Autistic Children. J Neuroimaging. 2009;19:337–343. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- 46.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–32. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Knyazeva MG. Splenium of Corpus Callosum: Patterns of Interhemispheric Interaction in Children and Adults. Neural Plast. 2013;2013:1–12. doi: 10.1155/2013/639430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- 49.Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual Scanning of Faces in Autism. J Autism Dev Disord. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- 50.Hubl D, Bölte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, et al. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- 51.Bölte S, Hubl D, Dierks T, Holtmann M, Poustka F. An fMRI-study of locally oriented perception in autism: altered early visual processing of the block design test. J Neural Transm. 2008;115:545–552. doi: 10.1007/s00702-007-0850-1. [DOI] [PubMed] [Google Scholar]
- 52.Kusano Y, Seguchi T, Horiuchi T, Kakizawa Y, Kobayashi T, Tanaka Y, et al. Prediction of Functional Outcome in Acute Cerebral Hemorrhage Using Diffusion Tensor Imaging at 3T: A Prospective Study. Am J Neuroradiol. 2009;30:1561–1565. doi: 10.3174/ajnr.A1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor Coordination in Autism Spectrum Disorders: A Synthesis and Meta-Analysis. J Autism Dev Disord. 2010;40:1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- 54.Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010;50:873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Nordahl CW, Iosif A-M, Young GS, Perry LM, Dougherty R, Lee A, et al. Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Mol Autism. 2015;6:26. doi: 10.1186/s13229-015-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nomi JS, Uddin LQ. Developmental changes in large-scale network connectivity in autism. NeuroImage Clin. 2015;7:732–741. doi: 10.1016/j.nicl.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai M-C, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. Sex/Gender Differences and Autism: Setting the Scene for Future Research. J Am Acad Child Adolesc Psychiatry. 2015;54:11–24. doi: 10.1016/j.jaac.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 60.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62 doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract Profiles of White Matter Properties: Automating Fiber-Tract Quantification. In: Beaulieu C, editor. PLoS One. Vol. 7. 2012. p. e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Libero LE, Burge WK, Deshpande HD, Pestilli F, Kana RK. White Matter Diffusion of Major Fiber Tracts Implicated in Autism Spectrum Disorder. Brain Connect. 2016;6:691–699. doi: 10.1089/brain.2016.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol. 2014;10:74–81. doi: 10.1038/nrneurol.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez-Murcia FJ, Lai M-C, Górriz JM, Ramírez J, Young AMH, Deoni SCL, et al. On the brain structure heterogeneity of autism: Parsing out acquisition site effects with significance-weighted principal component analysis. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23449. [DOI] [PMC free article] [PubMed] [Google Scholar]


