Abstract
Background
Insulin-like growth factor I (IGF-I) is a key regulator of chondrogenesis, but its therapeutic application to articular cartilage damage is limited by rapid elimination from the repair site. The human IGF-I gene gives rise to three IGF-I propeptides (proIGF-IA, proIGF-IB and proIGF-IC) that are cleaved to create mature IGF-I. In this study, we elucidate the processing of IGF-I precursors by articular chondrocytes, and test the hypotheses that proIGF-I isoforms bind to heparin and regulate articular chondrocyte biosynthesis.
Methods
Human IGF-I propeptides and mutants were overexpressed in bovine articular chondrocytes. IGF-I products were characterized by ELISA, western blot and FPLC using a heparin column. The biosynthetic activity of IGF-I products on articular chondrocytes was assayed for DNA and glycosaminoglycan that the cells produced.
Results
Secreted IGF-I propeptides stimulated articular chondrocyte biosynthetic activity to the same degree as mature IGF-I. Of the three IGF-I propeptides, only one, proIGF-IA, strongly bound to heparin. Interestingly, heparin binding of proIGF-IA depended on N-glycosylation at Asn92 in the EA peptide. To our knowledge, this is the first demonstration that N-glycosylation determines the binding of a heparin-binding protein to heparin.
Conclusion
The biosynthetic and heparin binding abilities of proIGF-IA, coupled with its generation of IGF-I, suggest that proIGF-IA may have therapeutic value for articular cartilage repair.
General significance
These data identify human pro-insulin-like growth factor IA as a bifunctional protein. Its combined ability to bind heparin and augment chondrocyte biosynthesis make it a promising therapeutic agent for cartilage damage due to trauma and osteoarthritis.
Keywords: Insulin-like growth factor (IGF), IGF-I propeptide, E peptide, chondrocyte, biosynthesis, heparin-binding protein, protein processing, glycosylation
1. Introduction
Insulin-like growth factor I (IGF-I) plays a central role in skeletal growth and development. Global IGF-I knockout in mice produces marked embryonic and postnatal growth failure [1]. The liver is the main source of circulating IGF-I and liver–specific IGF-I gene knockout mice have a greater than 75% reduction of circulating IGF-I levels. Despite the low levels of circulating IGF-I, these mice develop and grow normally through the autocrine/paracrine action of locally produced IGF-I [2, 3]. IGF-I is produced by chondrocytes [4] and is an important growth factor for cartilage development and homeostasis [5]. IGF-I also has the potential to serve as a therapeutic agent for articular cartilage repair. Delivery of exogenous IGF-I, or production of endogenous IGF-I by gene transfer, stimulates chondrocyte proliferation and the synthesis and production of glycosaminoglycan and collagen, the two major components of cartilage matrix [6, 7]. In addition, IGF-I inhibits the endogenous catabolic breakdown of articular cartilage matrix and the catabolic actions of inflamatory cytokines that are thought to mediate cartilage damage in arthritis [8, 9]. IGF-I promotes articular cartilage repair in both in vitro and in vivo cartilage repair models [10–12]. These features make IGF-I an attractive candidate for treating damaged articular cartilage in joint disease.
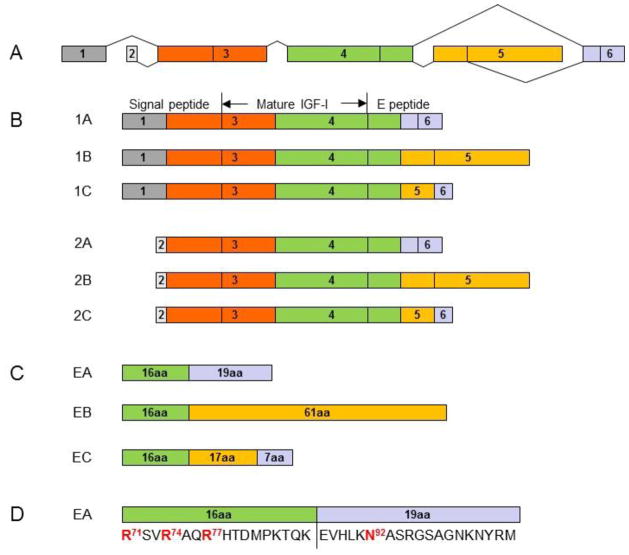
The human IGF1 gene has six exons. Alternative splicing at the 5′ and 3′ ends of the gene gives rise to two classes and three groups of IGF-I mRNA transcript variants (1A, 1B and 1C, and 2A, 2B and 2C). Classes 1 and 2 variants arise from exon 1 and exon 2, respectively. Group A variants exclude exon 5 while group B variants exclude exon 6. Group C variants are produced by an internal splice site within exon 5, which causes a frame shift and premature termination in exon 6 (Figure 1). After translation, the six IGF-I mRNA variants give rise to six corresponding IGF-I prepropeptides, each consisting of an N-terminal signal peptide, mature peptide and a C-terminal E peptide. The removal of signal peptides from the six IGF-I prepropeptides results in three IGF-I propeptides (proIGF-IA, proIGF-IB and proIGF-IC), each containing a different E peptide (EA peptide, EB peptide or EC peptide). The EA peptide (35 amino acids), EB peptide (77 amino acids) and EC peptide (40 amino acids) share the first 16 amino acid sequence from Exon 4. Cleavage of the E peptides from the three IGF-I propeptides results in the same mature IGF-I peptide (Figure 1). Secreted proIGF-I has been detected in vitro in medium from cultured cell lines [13, 14], and in vivo in serum [15]. IGF-I precursor processing by articular chondrocytes and the potential role of proIGF-I peptides in articular chondrocyte function remain unknown.
Figure 1.
Alternative splicing of the human IGF-I gene. The human IGF-I gene has six exons (A). Alternative splicing of the gene gives rise to six IGF-I mRNA variants for six corresponding IGF-I prepropeptides (1A, 1B and 1C, and 2A, 2B and 2C) in two classes and three groups (B). Classes 1 and 2 variants arise from exon 1 and exon 2, respectively. Group A variants exclude exon 5 while group B variants exclude exon 6. Group C variants produced by an internal splice site within exon 5, which causes a frame shift and premature termination in exon 6. Each of IGF-I prepropeptides consists of an N-terminal signal peptide, mature peptide and a C-terminal E peptide. Proteolytic processing at both N- and C-terminal ends of the IGF-I prepropeptides produces the same 70 amino acid mature IGF-I peptide. The removal of signal peptides from the 6 IGF-I prepropeptides results in 3 IGF-I propeptides (proIGF-IA, proIGF-IB and proGF-IC), each containing a different E peptide (EA peptide, EB peptide or EC peptide) in addition to the mature IGF-I peptide. The EA peptide (35 amino acids), EB peptide (77 amino acids) and EC peptide (40 amino acids) share the first 16 amino acid sequence from Exon 4 (C). The three Arg (Arg71, Arg74 and Arg77) in the 16 amino acid sequence are involved in the processing of proIGF-IA to produce the mature IGF-I. Only the EA peptide contains a putative N-glycosylation site, Asn92 in the 19 amino acid region from Exon 6 (D).
In articular cartilage, the bioavailability of growth factors is determined, in part, by their interaction with matrix molecules that retain them in the vicinity of articular chondrocytes. Prominent among these matrix molecules is the family of polyanionic sulfated glycosaminoglycans. Heparin and the closely related molecule, heparan sulfate (HS), are the most negatively charged members of this family [16]. Heparin and HS bind to multiple protein ligands, termed heparin-binding proteins (HBPs). HBPs are a diverse group of molecules that include growth factors as well as extracellular matrix components, enzymes, and the cell surface proteins of pathogens [17, 18]. Mutational analysis of heparin-binding segments in these HBPs by site–directed mutagenesis has revealed specific surface motifs enriched in clusters of basic amino acids, especially arginines and lysines. Some of the common putative heparin-binding segments are XBBXBX, XBBBXXBX, and XBBXXBBBXXBBX, where B is one of the three basic amino acids (mainly arginine and lysine) and X is any of the other 17 natural amino acids. These heparin binding segments function by electrostatic interaction with the negatively charged sulfate and carboxyl groups in heparin or heparan sulfate [19].
Several growth factors, including some bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs), and transforming growth factor betas (TGF-βs), have been identified as heparin-binding proteins [20]. Cell surface heparan sulfate proteoglycans often modulate the binding of these growth factors to their receptors and regulate their biologic activities [17]. Mature IGF-I lacks a heparin binding motif and does not bind to heparin or heparin sulfate [21].
For many glycoproteins, glycosylation is a critical step in producing a fully functional protein [22]. Human proIGF-IA contains one putative N-glycosylation site at Asn92, located in the EA peptide in the 19 amino acid region from Exon 6 (Figure 1D). Glycosylated human proIGF-IA and its glycosylated cleaved EA peptide have been detected in culture medium from IGF-I-overexpressing HEK293 cells and IM9 B-lymphocytes [14, 23]. There is no putative N-glycosylation site in human proIGF-IB or proIGF-IC. It is not known if glycosylation plays a role in the processing or function of proIGF-I isoforms.
The goals of this study were to 1) elucidate the processing of IGF-I precursors by articular chondrocytes, 2) determine whether proIGF-I isoforms bind to heparin and 3) test the hypothesis that proIGF-I isoforms regulate articular chondrocyte biosynthesis.
2. Materials and methods
2.1. Expression vector construction
The cDNAs encoding each of the 6 human IGF-I prepropeptides: 1A (Genebank accession number NM_000618), 1B (Genebank accession number NM_001111285), 1C (Genebank accession number NM_001111283), 2A (Genebank accession number NM_001111284), 2B and 2C, were generated by reverse transcription and PCR using a total RNA preparation of human articular chondrocytes. The human articular chondrocytes were isolated from de-identified articular cartilage discarded at the time of total knee arthroplasty. Because the tissue was de-identified medical waste, this protocol was determined to be exempt by the Indiana University Institutional Review Board. Forward primer IGF-I-F1 with reverse primer IGF-I-R(A), IGF-I-R(B) or IGF-I-R(C) (Table 1) were used to amplify IGF-I1A, 1B and 1C cDNAs, respectively. Similarly, forward primer IGF-I-F2 (Table 1) with the reverse primer IGF-I-R(A), IGF-I-R(B) or IGF-I-R(C) were used to amplify IGF-I2A, 2B and 2C cDNAs, respectively. The PCR products were cloned into pCR2.1-TOPO TA vector (Life Technologies) respectively, and the sequences were confirmed. Based on prior work with IGF-I and transfection of articular chondrocytes [24], the adeno-associated virus-based plasmid vector, pAAV-MCS, was selected for cDNA delivery and the cytomegalovirus (CMV) was used as the promoter. Each of the IGF-I cDNAs was subcloned into pAAV-MCS (Stratagene) to obtain pAAV-IGF-I1A, 1B, 1C, 2A, 2B and 2C.
Table 1.
Primers used for PCR
| Primer | Sequence (5′ to 3′) |
|---|---|
| IGF-I-F1 | CAGAATTCACCATGGGAAAAATCAGCAGTCTTCCAAC |
| IGF-I-F2 | CAGAATTCACCATGATTACACCTACAGTGAAGATG |
| IGF-I-R(A) | CTAGATCTACATCCTGTAGTTCTTGTTTCCTGCAC |
| IGF-I-R(B) | CTAGATCTCATTTTCCTTTTTTGCCTCTGCATTCAG |
| IGF-I-R(C) | CTAGATCTACTTGCGTTCTTCAAATGTACTTCCTTTCCTTC |
| IGF-I-R(noEA) | CAAGATCTAAGCTGACTTGGCAGGCTTGAGG |
| IGF-I-R(noEx6) | CTAGATCTACTTCTGGGTCTTGGGCATGTCGGTG |
| IGF-I-R(flag) | TAGATCTCTACTTATCGTCGTCATCCTTGTAATCCATCCTGTAGTTCTTG-TTTCCTGCAC |
| IGF-I-F-N92A | GAAGTACATTTGAAGGCCGCAAGTAGAGGGAGTGCAGGAAACAAG |
| IGF-I-R-N92A | CTCCCTCTACTTGCGGCCTTCAAATGTACTTCCTTCTGGGTCTTG |
| IGF-I-F-R/A | AGCAGTCGGAGGGCACCTCAGACAGGCATCGTGGATGAGTGC |
| IGF-I-R-R/A | ATGCCTGTCTGAGGTGCCCTCCGACTGCTGGAGCCATACCCTG |
| IGF-I-F-R/K | AAATCTGTCAAGGCCCAGAAACACACCGACATGCCCAAGACCCAGAAG |
| IGF-I-R-R/K | TTTCTGGGCCTTGACAGATTTAGCTGACTTGGCAGGCTTGAGGGGTG |
The cDNA encoding human IGF-I prepropeptide 1A without E peptide (IGF-I1A-noEA) was generated by PCR using pAAV-IGF-I1A as a template and forward primer IGF-I-F1 with reverse primer IGF-I-R(noEA) (Table 1). The cDNA encoding human IGF-I prepropeptide 1A without the C-terminal 19 amino acid sequence of the Exon 6 in human IGF-I gene (IGF-I1A-noEx6) was generated by PCR using pAAV-IGF-I1A as a template and forward primer IGF-I-F1 with reverse primer IGF-I-R(noEx6) (Table 1). The cDNA encoding human IGF-I prepropeptide 1A, including a flag tag at C-terminus (IGF-I1A-flag), was generated by PCR using pAAV-IGF-I1A as a template and forward primer IGF-I-F1 with reverse primer IGF-I-R(flag) (Table 1). The PCR products were cloned into pCR2.1-TOPO TA vector (Life Technologies), respectively. After confirming the sequences, the cDNAs were subcloned into pAAV-MCS (Stratagene) to obtain pAAV-IGF-I1A-noEA, pAAV-IGF-I1A-noEx6 and pAAV-IGF-I1A-flag, respectively.
The cDNA encoding a mutant of human IGF-I prepropeptide 1A, in which the putative N-glycosylation site Asn92 in EA peptide was replaced by Ala (IGF-I1A-N92A), was generated by PCR-based mutagenesis using forward primer IGF-I-F-N92A with reverse primer IGF-I-R-N92A (Table 1). In order to perform PCR-based mutagenesis, IGF-I1A cDNA in pAAV-IGF-I1A was sub-cloned into pCMV-MCS (Stratagene) and the resulting plasmid pCMV-IGF-I1A was used as as a template. The cDNA encoding a mutant of human IGF-I prepropeptide 1A, in which the three Arg (Arg71, Arg74 and Arg77) in the EA peptide were replaced by Ala (IGF-I1A-R/A), was generated by PCR-based mutagenesis using pCMV-IGF-I1A as a template and forward primer IGF-I-F-R/A with reverse primer IGF-I-R-R/A (Table 1). The cDNA encoding a mutant of human IGF-I prepropeptide 1A, in which the three Arg (Arg71, Arg74 and Arg77) in the EA peptide were replaced by Lys (IGF-I1A-R/K), was generated by PCR-based mutagenesis using pCMV-IGF-I1A as a template and forward primer IGF-I-F-R/K with reverse primer IGF-I-R-R/K (Table 1). The mutation in each of the resulting expression vectors pCMV-IGF-I1A-N92A, pCMV-IGF-I1A-R/A and pCMV-IGF-I1A-R/K, was confirmed by sequencing.
2.2. Chondrocyte cell culture and transfection
Dulbecco’s minimum essential medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin, glutamine and proteinase k were from Life Technologies. Ascorbic acid was from Sigma. Basal medium was prepared with DMEM, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine and 50 μg/ml ascorbic acid. Complete medium was prepared with basal medium supplemented with 10% FBS. Bovine articular chondrocytes were isolated as previously described [24]. Briefly, chondrocytes were isolated from the carpal articular cartilage of skeletally mature (physes closed) bovids and placed in monolayer culture in 6 - well plates at a density of 3 × 105 cells/well in 4 ml of complete medium. After 3 days, cells were transfected using FuGENE 6 (Roche Applied Science) and 2 μg of each plasmid DNA per well. The ratio of plasmid DNA (μg) to FuGENE 6 reagent (μl) was 1:3. Transfection with empty vector pAAV-MCS was used as a control. After 16 hours, transfection was stopped by replacing the medium with 4 ml of fresh complete medium. On day 2 and day 4 after transfection, conditioned medium (CM) was collected and replaced by basal medium. On day 6, CM was collected and the cell layer was digested in 2 ml proteinase k solution (0.5 mg/ml proteinase k in 10 mM Tris, pH 8.2, and 5 mM EDTA) at 65°C for 2 h. CM was stored at −20°C for analyses of IGF-I and proteoglycan in medium. The cell digest was stored at −20°C for analyses of proteoglycan and DNA in the cell layer.
2.3. IGF-I analysis
IGF-I in conditioned medium was measured by human IGF-I ELISA (R&D Systems, Cat.#: D291) according to the manufacturer’s procedures. Specifically, the capture antibody of the sandwich ELISA was mouse anti-human IGF-I monoclonal antibody raised using human mature IGF-I as antigen. The detection antibody was goat anti-human IGF-I polyclonal antibody raised using human mature IGF-I as antigen. Phosphate buffered saline (PBS, 11.9 mM phosphate, 137 mM sodium chloride, 2.7 mM potassium chloride, pH 7.4) was used to dilute capture antibody for plate coating. 0.05% Tween 20 in PBS was used as wash buffer. 5% Tween 20 in PBS was used as blocking buffer, and also as a diluent for samples, IGF-I standard, detection antibody and streptavidin-HRP. The sensitivity of this assay is 31.25 pg/ml.
IGF-I-to-heparin binding activity was measured by a functional ELISA using heparin in place of the anti-IGF-I capture antibody in the human IGF-I ELISA. Specifically, heparin (Sigma) at 0.5 mg/ml in PBS was used instead of anti-IGF-I capture antibody to coat plates. Heparin binding activity was calculated based on the IGF-I standard curve of the human IGF-I ELISA conducted on the same ELISA plate. The resulting value, expressed in arbitrary units (AU), reflects the IGF-I concentration in the medium, the heparin amount relative to the amount of anti-IGF-I capture antibody coated on the ELISA plates, and the affinity of IGF-I with heparin relative to that of IGF-I with anti-IGF-I capture antibody.
IGF-I in conditioned medium (CM) was analyzed by western blotting using anti-IGF-I antibody. Recombinant IGF-I (rIGF-I) was used as control. One part of 6X SDS-Sample Buffer (375 mM Tris-HCl pH 6.8, 6% SDS, 48% glycerol, 9% 2-Mercaptoethanol, and 0.03% bromophenol blue) was mixed with 5 parts of sample, and heated at 95°C for 10 min. The prepared samples were electrophoresed on a 10–20% precast SDS-polyacrylamide gel (Bio-Rad) or 15% SDS-polyacrylamide gel, and the proteins were transferred to nitrocellulose membranes. The membranes were blocked with 5% fat-free milk (Bio-Rad) in Tris-buffered saline/Tween (TBST) for 1 hour at room temperature and were then separately probed with primary antibodies against to IGF-I (Epitomics, Cat.#: 5217-1) or flag tag (Sigma, Cat.#: F1804 ) overnight at 4°C. Horseradish peroxidase-conjugated donkey anti-rabbit IgG (Amersham Bioscience, Cat.#: NA934V) or sheep anti-mouse IgG (Amersham Bioscience, Cat.#: NA931V), was used as a second antibody. Chemiluminescent substrate (Thermo Scientific, Cat.#: 34080) was used for detection of horseradish peroxidase on immunoblots. CL-X posure films (Thermo Scientific) were exposed to immunoblots and developed using Kodak Medical X-ray processor 104.
2.4. Heparin binding activity analysis by FPLC
Heparin binding activity of the IGF-I in pAAV-IGF-I1A transfected chondrocyte conditioned medium, was analyzed by fast performance liquid chromatography (FPLC) using a HiTrap Heparin High Performance (HP) column (GE Heatthcare Life Science) and a NGC 10 Quest Chromatography System (Bio-Rad). The HiTrap Heparin HP column (1 ml) was equilibrated with PBS. IGF-I in conditioned medium from pAAV-IGF-I1A transfected bovine chondrocytes, recombinant human BMP-2 (gift from Drs. Eric Vanderploeg and Howard Seeherman) and recombinant human TGF-β1 (R&D Systems, Minneapolis, MN) dissolved in PBS, were separately applied to the HiTrap Heparin HP column for comparison. After sample application, the column was washed with 10 ml PBS. Then the column was eluted with a 10-ml linear gradient of sodium chloride from 0 to 2 M in PBS. Protein eluted from the column was monitored by absorbance at 280 nm (A280 nm) and ionic strength of eluate was monitored by conductivity. The column eluate was collected in 0.5 ml fractions. IGF-I, BMP-2 and TGF-β1 in eluate fractions was measured by human IGF-I ELISA, human BMP-2 ELISA (R&D Systems, Cat.#: DY355) and human TGF-β1 ELISA (R&D Systems, Cat.#: DY240 ), respectively. IGF-I in the pass-through was also measured by human IGF-I ELISA.
2.5. Proteoglycan and DNA analysis
Glycosaminoglycan (GAG) released into the medium (released GAG) and retained with the chondrocytes (cell-associated GAG) were separately measured by dimethylmethylene blue (DMB) assay of conditioned medium or proteinase k cell digest, as previously described [7]. Chondroitin sulfate A (Sigma) was used as the standard. DMB solution was prepared as follows: 1,9-dimethylmethylene blue (32 mg) was dissolved in 5 ml ethanol; 4 g sodium formate and 4 ml formic acid were added, and the volume was made up to 1 liter with water. DMB solution was stored in a brown glass bottle at room temperature. Each sample or sample diluted with water, was mixed with DMB solution (1:1 volume) and the absorbance at 535 nm was read using DU640 Spectrophotometer (Beckman Coulter) or ELX808 Ultra Microplate Reader (BioTek Instruments). Cell proliferation was assessed by DNA analysis of the cell digest as previously described [7] by Picogreen dsDNA assay (Molecular Probes) according to the manufacturer’s instructions using pure phage λ DNA as the standard.
2.6. Statistical Analysis
Three independent experiments were performed using three different batches of bovine articular chondrocytes isolated from three different animals at different times. Data are presented as means ± SD of three independent experiments. The overall group effect was evaluated using ANOVA, followed by pair-wise comparisons between groups using Fisher’s Protected Least Significant Differences. A logarithmic transformation of the data was used for the analyses. A 5% significance level was used for all tests.
3. Results
3.1. Transfected articular chondrocytes synthesize and process IGF-I precursors from their respective transgenes
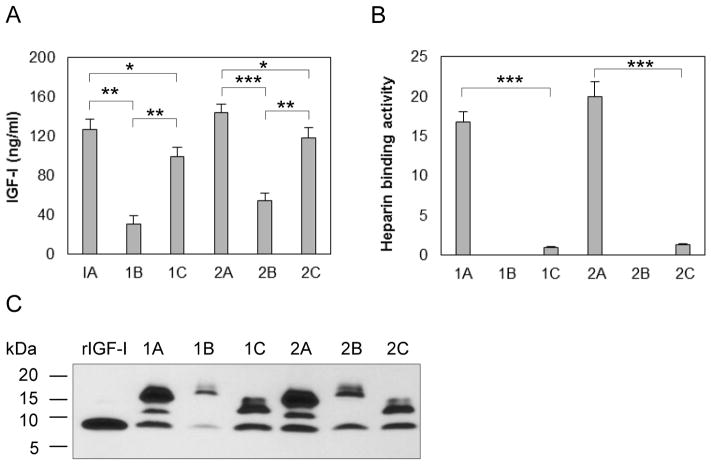
To determine how chondrocytes process IGF-I precursors, we transfected primary bovine chondrocytes with each of pAAV-IGF-I1A, 1B, 1C, 2A, 2B and 2C, and measured IGF-I in the CM by ELISA. IGF-I1A and IGF-I2A CM contained the highest IGF-I levels (127.0 ng/ml and 143.7 ng/ml respectively), followed by IGF-I1C and IGF-I2C CM (99.6 ng/ml and 118.6 ng/ml respectively) and IGF-I1B and IGF-I2B CM (31.0 ng/ml and 54.9 ng/ml, respectively) (Figure 2A).
Figure 2.
Production and characterization of six human IGF-I prepropeptides in transfected bovine chondrocytes. Bovine chondrocytes were transfected by each of pAAV-IGF-I1A, 1B, 1C, 2A, 2B and 2C. The respective human IGF-I products were assessed in day 2 conditioned medium (CM) by (A) human IGF-I ELISA, (B) functional ELISA using heparin in place of the anti-IGF-I capture antibody in the human IGF-I ELISA and (C) western blotting of CM samples probed with ant-IGF-I antibody, using recombinant IGF-I (rIGF-I) for comparison. ELISA data are presented as mean ± SD of three independent experiments. Western blot data are from a representative experiment and equal amounts (20 ul) of CM were loaded for each sample. Human recombinant IGF-I (rIGF-I, 2 ng) was loaded for comparison. The western blot shows that the cells transfected with pAAV-IGF-I1A or 2A, produced mature IGF-I and two forms of IGF-I propeptide A (proIGF-IA). The cells transfected with pAAV-IGF-I1B or 2B, produced mature IGF-I and one IGF-I propeptide B (proIGF-IB). The cells transfected with pAAV-IGF-I1C or 2C, produced mature IGF-I and IGF-I propeptide C (proIGF-IC). *: p < 0.05; **: p < 0.01; ***: p < 0.001. Detailed p values are provided in STable 1.
To characterize IGF-I isoforms produced from the different cDNAs, we analyzed the chondrocyte CM by western blot using anti-IGF-I antibody. The western blots showed three bands in IGF-I1A and IGF-I2A CM, two bands in IGF-I1B and IGF-I 2B CM, and two bands in IGF-I1C and IGF-I2C CM (Figure 2C). The relative molecular mass (Mr) of the bands was the same for classes 1 and 2 of the 3 IGF-I isoforms (1A vs 2A, 1B vs 2B and 1C vs 2C). The lowest Mr band in all western blots was similar to the Mr of recombinant human IGF-I, and was interpreted as mature human IGF-I. Thus, the western blot data suggest that, in addition to mature human IGF-I, the transfected chondrocytes secreted two proIGF-IA forms in IGF-I1A and IGF-I2A CM, one proIGF-IB form in IGF-I1B and IGF-I2B CM and one proIGF-IC form in IGF-I1C and IGF-I2C CM, respectively.
3.2. Human IGF-I propeptide A binds to heparin
To determine whether the IGF-I produced by transfected chondrocytes binds to heparin, we analyzed the CM by functional ELISA. Both IGF-I1A and IGF-I2A produced IGF-I with high heparin binding activity (16.8 AU/ml and 20.0 AU/ml respectively). In contrast, IGF-I in IGF-I1C and IGF-I2C CM possessed minimal heparin binding activity (1.0 and 1.33 AU/ml respectively). No heparin binding activity was detectable in the IGF-I1B or IGF-I2B CM (Figure 2B).
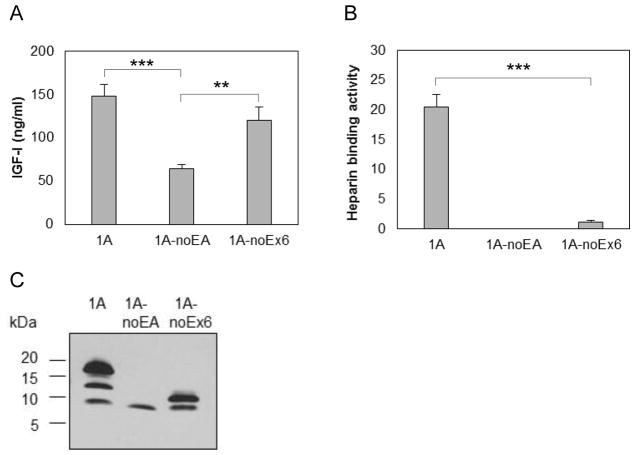
3.3. The 19 amino acid sequence from Exon 6 in EA peptide mediates proIGF-IA binding to heparin
To examine the effect of the two parts of the EA peptide (Figure 1D) on heparin binding activity by proIGF-IA, we measured IGF-I (Figure 3A) and heparin binding activity (Figure 3B) in CM from chondrocytes producing IGF-I1A without the EA peptide, and from chondrocytes producing IGF-I1A without the 19 amino acids from exon 6. The deletion of the 35 amino acid EA peptide eliminated the heparin binding activity of proIGF-IA. The deletion of the 19 amino acid sequence from Exon 6 in the EA peptide decreased pro-IGF-I heparin binding activity from 20.5 AU/ml to 1.1 AU/ml.
Figure 3.
Production and characterization of human IGF-I prepropeptide 1A truncated mutants. Bovine chondrocytes were transfected by each of pAAV-IGF-I1A-noEA and pAAV-IGF-I1A-noEx6 to express human IGF-I prepropeptide 1A without EA peptide (1A-NoEA) and human IGF-I prepropeptide 1A without the C-terminal 19 amino acid sequence of the Exon 6 (1A-noEx6). pAAV-IGF-IA transfection was included for comparison. The respective human IGF-I products were assessed in day 2 conditioned medium (CM) by (A) human IGF-I ELISA, (B) functional ELISA using heparin in place of the anti-IGF-I capture antibody in the human IGF-I ELISA and (C) western blotting of CM samples probed with ant-IGF-I antibody. ELISA data are presented as mean ± SD of three independent experiments. Western blot data are from a representative experiment and equal amounts (20 ul) of CM were loaded for each sample. The western blot shows that the cells transfected with pAAV-IGF-I1A-noEA (1A-NoEA) produced only mature IGF-I. The cells transfected with pAAV-IGF-I1A-noEx6 (1A-noEx6) produced mature IGF-I and the IGF-I containing the 16 AA sequence of Exon 4. *: p < 0.05; **: p < 0.01; ***: p < 0.001. Detailed p values are provided in STable 2.
Examination of the IGF-I products in the CM by western blot showed that cells transfected with pAAV-IGF-I1A-noEA produced only mature IGF-I while cells transfected with pAAV-IGF-I1A-noEx6 produced mature IGF-I and the IGF-I containing the 16 AA sequence of Exon 4 (Figure 3C). Taken together, the above data suggest that the EA peptide confers the heparin binding activity on proIGF-I1A, and that the 19AA sequence from Exon 6 plays an important role in the heparin binding activity.
3.4. Chondrocytes expressing IGF-I1A produce mature IGF-I and two proIGF-IA with intact EA peptide
Western blot of IGF-I1A CM revealed three bands. The lowest Mr band corresponded to the 70 amino acid mature IGF-I. To characterize the two higher Mr bands, we transfected chondrocytes with the vector pAAV-IGF-I1A-flag. The original pAAV-IGF-I1A was used as control. The CM was analyzed by western blot using anti-IGF-I antibody (Figure 4A) and flag antibody (Figure 4B). IGF-I antibody detected three bands in both the IGF-I1A-flag and the IGF-I1A CM. The lowest Mr bands in each transfection matched recombinant mature IGF-I. The Mr of the two higher bands in the IGF-I1A-flag CM was slightly greater than that of the two higher Mr bands in the IGF-I1A CM. Flag tag antibody recognized the two higher Mr bands in the IGF-I1A-flag CM. These data suggest that the two higher Mr bands in the IGF-I1A-Flag CM are proIGF-IA containing the EA peptide with the flag tag. Taken together, the western blot data indicate that the two higher Mr bands in IGF-I1A CM are proIGF-IA, both of which contain the intact EA peptide.
Figure 4.
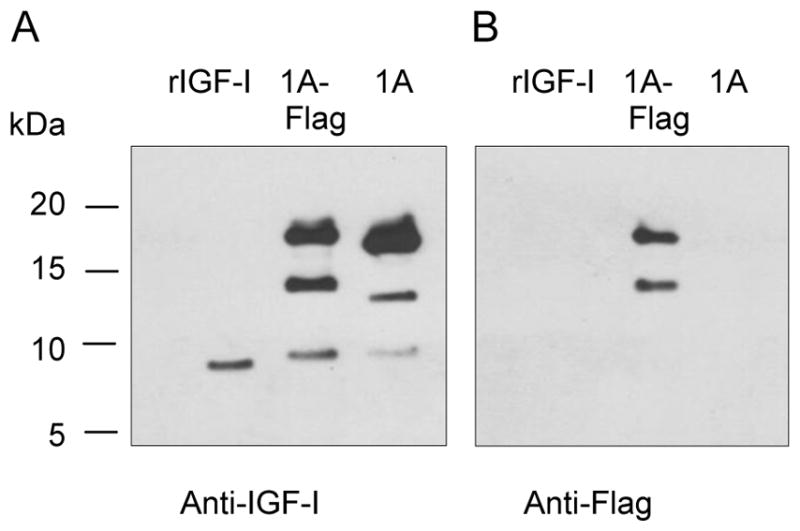
Production and characterization of human IGF-I prepropeptide 1A with a C-terminal flag tag. Bovine chondrocytes were transfected by pAAV-IGF-I1A-flag (1A-flag). The human IGF-I product was assessed in day 2 conditioned medium (CM) by western blotting of CM samples probed with anti-IGF-I antibody (A) or anti-flag antibody (B). Recombinant IGF-I (rIGF-I) and pAAV-IGF-I1A transfection were included for comparison. The data are from a representative experiment and equal amounts (40 ul) of CM were loaded for each sample. Human recombinant IGF-I (rIGF-I, 2 ng) was loaded for comparison. The data shows that the cells transfected with pAAV-IGF-I1A-flag produced mature IGF-I and two forms of IGF-I propeptide A (proIGF-IA) with flag tag. The cells transfected with pAAV-IGF-I1A produced mature IGF-I and two forms of proIGF-IA.
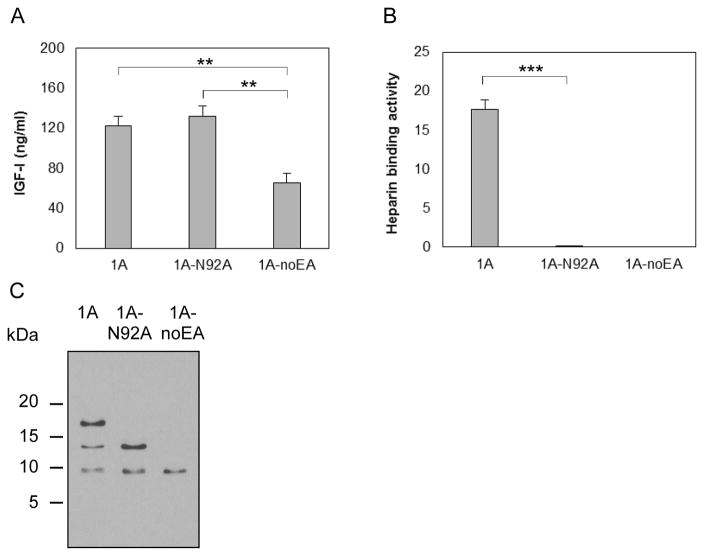
3.5. N-glycosylation in EA peptide plays an important role in mediating proIGF-IA binding to heparin
To determine whether the EA peptide is glycosylated and, if so, whether glycosylation is involved in proIGF-IA binding to heparin, we transfected chondrocytes with a vector carrying IGF-I1A mutated to Ala at Asn92 (pCMV-IGF-I1A-N92A), the putative N-glycosylation site in EA peptide (Figure 1D). Vectors pAAV-IGF-I1A and pAAV-IGF-I1A-noEA were used for comparison. The IGF-I ELISA results showed that the change of Asn92 to Ala abrogated heparin binding activity without affecting IGF-I expression (Figure 5A and Figure 5B). Western blot showed only two bands in IGF-I1A-N92A CM, corresponding to the two lower Mr bands of the three bands present in IGF-I1A CM. The highest Mr band in IGF-I1A CM disappeared in IGF-I1A-N92A CM (Figure 5C). These results further suggest that the highest Mr band in IGF-I1A CM is glycosylated proIGF-IA, and that the heparin binding activity of proIGF-IA is dependent on this glycosylation.
Figure 5.
Production and characterization of a human IGF-I prepropeptide 1A mutant (1A-N92A), in which the putative N-glycosylation site Asn92 in EA peptide was replaced by Ala. Bovine chondrocytes were transfected by pCMV-IGF-I1A-N92A. pAAV-IGF-I1A transfection and pAAV-IGF-I1A-noEA transfection were included for comparison. The respective human IGF-I products were assessed in day 2 conditioned medium (CM) by (A) human IGF-I ELISA, (B) functional ELISA using heparin in place of the anti-IGF-I capture antibody in the human IGF-I ELISA and (C) western blotting of CM samples probed with ant-IGF-I antibody. ELISA data are presented as mean ± SD of three independent experiments. Western blot data are from a representative experiment and equal amounts (20 ul) of CM were loaded for each sample. The western blot showed that the cells transfected with pCMV-IGF-I1A-N92A produced mature IGF-I and one IGF-I propeptide A (proIGF-IA) without N-glycosylation at Asn92 in the EA peptide. The cells transfected with pAAV-IGF-I1A produced mature IGF-I and two IGF-I propeptide A forms (proIGF-IA), one without N-glycosylation at Asn92 in the EA peptide and one with N-glycosylation at Asn92 in the EA peptide. *: p < 0.05; **: p < 0.01; ***: p < 0.001. Detailed p values are provided in STable 3.
3.6. Comparison of proIGF-IA, BMP-2 and TGF-β1 binding to heparin
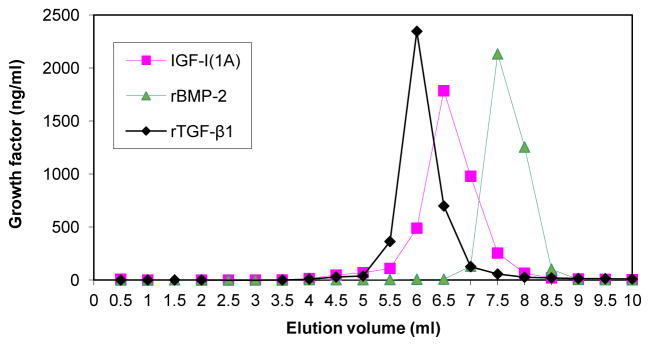
We evaluated the interactions of proIGF-IA, BMP-2 and TGF-β1 with heparin by FPLC analysis. The results indicate that BMP-2, TGF-β1 and proIGF-IA, each specifically bind to heparin and that the heparin binding affinity of proIGF-IA is higher than that of TGF-β1, but lower than that of BMP-2 (Figure 6).
Figure 6.
Comparison of proIGF-IA, BMP-2 and TGF-β1 heparin binding activity. CM from chondrocytes transfected with pAAV-IGF-I1A or PBS containing recombinant BMP-2 (10 ug) or TGF-β1 (8 ug), was analyzed by FPLC using a heparin-sepharose column. CM was applied to the column and the column was washed with 10 ml PBS. The column was eluted with a 10-ml linear gradient of sodium chloride from 0 to 2 M in PBS. The eluate was collected in 0.5 ml fractions. IGF-I, BMP-2 and TGF-β1 in eluate fractions were measured by human IGF-I ELISA, human BMP-2 ELISA and human TGF-β1 ELISA, respectively. Data are expressed as ng/ml of the designated growth factors in each fraction of the eluate.
FPLC also separated the IGF-I forms produced by pAAV-IGF-I1A transfected chondrocytes. Of the total IGF-I detected in the conditioned medium by IGF-I ELISA and applied to the FPLC column, 30.3% did not bind to the heparin column and was present in the pass-through and a single IGF-I peak was observed in the FPLC sodium chloride eluate. As noted (Figure 5), western blot analysis and ELISA demonstrated that the pAAV-IGF-I1A transfected chondrocytes produced three forms of IGF-I: glycosylated proIGF-IA, non-glycosylated proIGF-IA and mature IGF-I, and that only glycosylated proIGF-IA bound to heparin. Taken together, the data most likely reflect the appearance of the glycosylated proIGF-IA, heparin-binding form as the single peak in the FPLC eluate (Figure 6), and the “loss” of the other two forms in the FPLC column pass-through.
3.7. ProIGF-IA mutated at Arg71, Arg74 and Arg77 is resistant to EA peptide cleavage
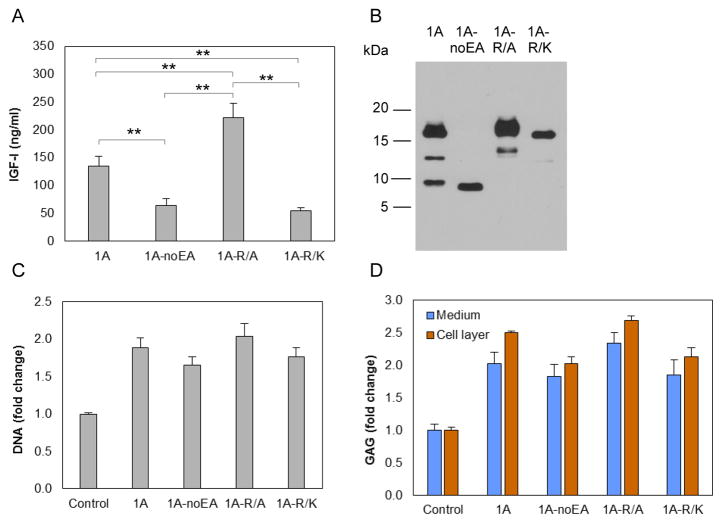
Chondrocytes were transfected with vectors carrying IGF-I1A mutated at Arg71, Arg74 and Arg77 (Figure 1D), three amino acids that are involved in EA peptide cleavage [23], to either Ala (pCMV-IGF-I1A-R/A) or Lys (pCMV-IGF-I1A-R/K). The latter was selected to retain basic amino acids at these sites. pAAV-IGF-I1A and pAAV-IGF-I1A-noEA were used for comparison. IGF-I production was measured by IGF-I ELISA of CM (Figure 7A) and examined by western blot using IGF-I antibody (Figure 7B). The western blots showed one major, high Mr band, consistent with N-glycosylated proIGF-IA, and one minor, medium Mr band consistent with unglycosylated proIGF-IA in CM from both IGF-I1A-R/A and IGF-I1A-R/K transfected chondrocytes. No band representing mature peptide was detected (Figure 7B). These data are consistent with the absence of proIGF-I cleavage by the chondrocytes. Interestingly, the data also showed that the change of Arg71, Arg74 and Arg77 to Ala increased IGF-I production by the cells, whereas the change of Arg71, Arg74 and Arg77 to Lys decreased IGF-I production, compared to production from IGF-I1A.
Figure 7.
Production and characterization of two human IGF-I prepropeptide 1A mutants, in which the three Arg (Arg71, Arg74 and Arg77) in the EA peptide were replaced by Ala (1A-R/A) or Lys (1A-R/K). Bovine chondrocytes were transfected by pCMV-IGF-I1A-R/A or pCMV-IGF-I1A-R/K. pAAV-IGF-I1A transfection and pAAV-IGF-I1A-noEA transfection were included for comparison. The respective human IGF-I products were assessed in day 2 conditioned medium (CM) by (A) human IGF-I ELISA and (B) western blotting of CM samples probed with ant-IGF-I antibody. ELISA data are presented as mean ± SD of three independent experiments. Western blot data are from a representative experiment and equal amounts (20 ul) of conditioned medium were loaded for each sample. The western blot showed that the cells transfected with each of pCMV-IGF-I1A-R/A and pCMV-IGF-I1A-R/K, produced one major proIGF-IA with N-glycosylation at Asn92 in the EA peptide and one minor proIGF-IA without N-glycosylation at Asn92 in the EA peptide, but did not produce mature IGF-I. Additional transfected cells were cultured for 6 days and assayed for DNA content (C) and glycosaminoglycan (GAG) production (D). GAG released into the medium (Medium) and GAG retained with the chondrocytes in the cell layer (Cell layer) were measured separately. Chondrocytes transfected by empty vector were used as control. Data are normalized to control and presented as mean ± SD of three independent experiments. *: p < 0.05; **: p < 0.01; ***: p < 0.001. Detailed p values are provided in STable 4.
3.8. ProIGF-IA stimulates biosynthetic activity by articular chondrocytes
To determine whether the IGF-I propeptides possess biological activity, chondrocytes were transfected with the above vectors and the cell layer was assayed for DNA content as an index of cell proliferation. The cell layer and conditioned medium were assayed for glycosaminoglycan (GAG) content as an index of cartilage matrix production. Chondrocytes transfected with pCMV-IGF-I1A-R/A, pCMV-IGF-I1A-R/K, pAAV-IGF-I1A, or pAAV-IGF-I1A-noEA increased DNA content over a range of 1.7–2.0 times controls (p < 0.001) (Figure 7C). The transfected cells produced 1.8–2.3 times more released GAG (p < 0.005) and 2.0–2.7 times more cell-associated GAG (p < 0.001) than controls (Figure 7D). Empty vector pAAV-MCS was used as control.
Western blot data demonstrate that chondrocytes transfected by pAAV-IGF-I1A produced both proIGF-IA and mature IGF-I. In contrast, chondrocytes transfected by pAAV-IGF-I1A-noEA produced only mature IGF-I, and those transfected by pCMV-IGF-I1A-R/A or pCMV-IGF-I1A-R/K produced only proIGF-IA. Taken together, these results indicate that IGF-I propeptide A (proIGF-IA) has a biological activity that is similar in type and magnitude to that of mature IGF-I in stimulating chondrocytes.
4. Discussion
These data demonstrate that, when human IGF-I is overexpressed in bovine articular chondrocytes, the transfected cells secrete human IGF-I propeptides and mature IGF-I peptide. The data further show that both possess biological activity in stimulating chondrocytes. We found that, of the three human IGF-I propeptides (proIGF-IA, B and C), only proIGF-IA, strongly bound to heparin, and the heparin binding affinity was in the same range as that of two other heparin-binding growth factors, BMP-2 and TGF-β1.
In general consensus, heparin-binding proteins bind to heparin through the interaction of positively charged amino acids in the heparin-binding proteins and the negatively charged sulfate and carboxyl groups in heparin. Although there is no putative heparin-binding segment in the IGF-I EA peptide, we found that human proIGF-IA binds strongly to heparin. Mutating its single putative N-glycosylation site at Asn92 in the EA peptide abrogated this heparin binding activity. These results indicate that N-glycosylation is involved in the heparin binding activity of human proIGF-IA. Prior studies showed that mouse glycosylated proIGF-IA is the predominant form in mouse skeletal muscle and suggested that this glycosylation on the EA peptide has a physiological role in retaining mouse proIGF-IA in muscle by interacting with the extracellular matrix[25]. The finding that human proIGF-IA exhibits glycosylation-dependent heparin binding activity suggests that human proIGF-IA that is glycosylated on the EA peptide may also interact with extracellular matrix. To our knowledge, this is the first demonstration of the interaction of a heparin binding protein with heparin that is mediated through an N-linked oligosaccharide. The molecular mechanism by which the N-linked oligosaccharide in human proIGF-A mediates the interaction of proIGF-IA with heparin remains to be determined. There are 20 positively charged amino acids in human proIGF-IA, 9 of them in mature IGF-I and 11 of them in the EA peptide including two histidines (Figure 1D). The N-linked glycosylation in EA peptide may alter protein folding or conformation so as to coordinate these positively charged amino acids to form a high affinity heparin binding site.
We dissected the roles of the different signal peptides and E peptides of preproIGF-I on IGF-I production by overexpressing each of the six proIGF-I isoforms and their truncated mutants. The similar levels of production observed for class 1 and class 2 IGF-I isoforms suggest that the different signal peptides do not differentially regulate IGF-I expression and secretion in these cells. The finding that these cells overexpressed and secreted mutant IGF-I1A lacking the EA peptide suggests that the E peptide is not required for the secretion of IGF-I.
ELISA and western blot data for IGF-I1A, IGF-I1B, IGF-I1C (Figure 2A and 2C) and for the IGF-I1A-noEA and IGF-I1A-noEx6 mutants (Figure 3A and 3C) indicate that both the EA and EC peptides promote IGF-I production, whereas the EB peptide inhibits IGF-I production. The finding that IGF-I1A-noEx6 was produced as efficiently as IGF-I1A, suggests that the N-terminal 16 amino acid sequence from Exon 4 is sufficient for efficient IGF-I production. Taken together, these results suggest that the different E peptides and parts of the E peptides exert distinct effects on IGF-I production.
Many polypeptide hormones and growth factors are initially synthesized as larger, inactive protein precursors, usually in the form of pre-pro-proteins, from which the biologically active mature molecules have to be liberated by proteolysis. IGF-I is also initially synthesized in the form of pre-pro-proteins. However, the finding that proIGF-IA mutants that are resistant to EA peptide cleavage possessed biological activity indicates that the biological effects of the mutants are due to the propeptide rather than to mature IGF-I. These data suggest that the E peptide in proIGF-I does not prevent IGF-I binding to its receptor and subsequent internalization. A prior report similarly suggests that pro-IGF-IC (also termed full length mechano-growth factor), which includes an EC peptide, stimulated HEK 293 cells by binding to the IGF-I receptor [26].
Mouse IGF-I has served as a model for the investigation of IGF-I processing. Human proIGF-IA and proIGF-IC are the corresponding pro forms to mouse proIGF-IA and proIGF-IB respectively [27]. We found that human proIGF-IA bound strongly to heparin, but proIGF-IC possessed minimal heparin binding activity. In a previous study [16] mouse proIGF-IA also bound to heparin, but unlike human IGF-IC, mouse proIGF-IB bound to heparin with greater affinity than mouse proIGF-IA. This species difference is consistent with the presence in mouse proIGF-IB of a putative heparin-binding segment (NKKTKL) in its EB peptide, and of a corresponding sequence in the EC peptide of human proIGF-IC of NKNTKS. The difference in sequence between NKKTKL and NKNTKS may explain the difference in heparin binding activity.
Although proIGF-IA is the corresponding isoform in both humans and rats, rat proIGF-IA has an N-glycosylation site at Asn100 in addition to the Asn92 that is present in human proIGF-IA. This second N-glycosylation site at Asn100 also exists in mouse proIGF-IA [28]. Both rat Asn92 and Asn100 have been reported to be glycosylated [29]. The present data show that the N-glycosylation at the Asn92 is sufficient for human proIGF-IA binding to heparin. It is not known what function, if any, the N-glycosylation at Asn100 in rat EA peptide contributes to rat proIGF-A, or whether the glycosylation at the conserved Asn92 mediates binding of rat proIGF-IA to heparin. Since neither human proIGF-IC nor rat proIGF-IB have putative N-glycosylation sites, this glycosylation-based mechanism of interaction with heparin would not apply to these isoforms.
Human proIGF-IB has no analogue in rats or mice. In the present studies, CM from pAAV-IGF-I1B or pAAV-IGF-I2B transfected chondrocytes contained no heparin binding activity. In addition, these chondrocytes produced less IGF-I than chondrocytes transfected with pAAV-IGF-I1A, pAAV-IGF-I2A, pAAV-IGF-I1C or pAAV-IGF-I2C. To rule out the possibility that the absence of heparin binding activity was due to the low IGF-I levels in the CM of pAAV-IGF-I1B or pAAV-IGF-I2B transfected chondrocytes, we delivered pAAV-IGF-I1B to HEK 293 cells that produce high levels of IGF-I. The cells produced 654 ng/ml of IGF-I, yet no heparin binding activity was detected.
The E-peptides that are cleaved from proIGF-I isoforms to produce mature IGF-I may themselves have biological activities that are independent of IGF-I. Several studies have shown that E-peptides themselves have biological activities that are independent of IGF-I. Human EB-peptide has been reported to increase human bronchial epithelial cell proliferation [30], localize to nucleoli [31], induce neuroblastoma cell differentiation, stimulate neurite growth, increase ERK1/2 phosphorylation [32], and augment proliferation and migration in multiple human cell lines [33]. Human EC-peptide, (mechano-growth factor), has been reported to increase stem cell migration, proliferation and differentiation and promote tissue repair and regeneration [34–37].
Heparin is closely related to heparan sulfate, a component of heparan sulfate proteoglycans (HSPGs) that are found within cartilage matrix (eg perlecan) and also on the surface of the chondrocytes (eg syndecans) [38, 39]. Future studies will be required to determine whether such HSPGs bind N-glycosylated proIGF-IA and, if so, whether they help control IGF-I bioactivity in the tissue.
Growth factors have been widely investigated for use in tissue repair and regeneration. In vitro and in vivo studies have identified IGF-I as an attractive chondrogenic agent [6, 7, 10, 11]. However, the application of IGF-I to articular cartilage repair is currently limited by rapid elimination from the repair site [40]. Some growth factors, including BMP-2, FGF-2 and TGF-β1, bind to heparin. Others, including IGF-I, have been genetically modified through the addition of a heparin binding domain, to bind to heparin [41]. This feature has been employed to create new therepeutic agents by providing a sustained delivery of such growth factors from heparin-bearing vehicles to improve their reparative actions [42]. The present data indicate that, in addition to serving as the source of mature IGF-I, IGF-I propeptide A possesses the ability to both stimulate articular chondrocyte reparative activity and to bind to heparin. These properties suggest that proIGF-IA may hold potential as a therapeutic agent for articular cartilage repair.
Supplementary Material
Highlights.
Human proIGF-IA stimulates articular chondrocyte biosynthesis.
Human proIGF-IA strongly binds to heparin.
This heparin binding depends on N-glycosylation at Asn92 in the EA peptide.
The bifunctional properties of proIGF-IA may offer therapeutic benefit.
Acknowledgments
This work was supported in part by the National Institutes of Health, National Institute of Arthritis Musculoskeletal and Skin Diseases Grant AR047702, US Department of Veterans Affairs, BRL&D Merit Review Award I01 BX000447, and the Indiana University School of Medicine Research Infrastructure Fund.
Abbrreviations
- IGF-I
Insulin-like growth factor I
- GAG
glycosaminoglycan
- ELISA
enzyme-linked immunosorbent assay
- HBP
heparin-binding protein
- FPLC
fast performance liquid chromatography
- HSPG
heparan sulfate proteoglycan
Footnotes
Conflict of interest
Dr. Trippel is a consultant for ELK Orthobiologics and Fidia Pharma USA. The other authors declare that they have no conflicts of interest with the contents of this article.
Author contributions
SS and SBT designed the study, analyzed the data and wrote the paper. SS performed the experiments and supervised the work of BJK, CW, KK, and AC who provided technical assistance. GJE performed the statistical analyses. All authors reviewed the results and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 2.Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinecke M, Schmid AC, Heyberger-Meyer B, Hunziker EB, Zapf JR. Effect of Growth Hormone and Insulin-Like Growth Factor I (IGF-I) on the Expression of IGF-I Messenger Ribonucleic Acid and Peptide in Rat Tibial Growth Plate and Articular Chondrocytes in Vivo. Endocrinology. 2000;141:2847–2853. doi: 10.1210/endo.141.8.7624. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Bikle DD, Chang W. Autocrine and Paracrine Actions of IGF-I Signaling in Skeletal Development. Bone Res. 2013;1:249–259. doi: 10.4248/BR201303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 7.Shi S, Mercer S, Eckert GJ, Trippel SB. Growth factor transgenes interactively regulate articular chondrocytes. J Cell Biochem. 2013;114:908–919. doi: 10.1002/jcb.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308:137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- 9.Tyler JA. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989;260:543–548. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 11.Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8:1443–1449. doi: 10.1038/sj.gt.3301535. [DOI] [PubMed] [Google Scholar]
- 12.Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 13.Conover CA, Baker BK, Bale LK, Clarkson JT, Liu F, Hintz RL. Human hepatoma cells synthesize and secrete insulin-like growth factor Ia prohormone under growth hormone control. Regul Pept. 1993;48:1–8. doi: 10.1016/0167-0115(93)90330-b. [DOI] [PubMed] [Google Scholar]
- 14.Wilson HE, Westwood M, White A, Clayton PE. Monoclonal antibodies to the carboxy-terminal Ea sequence of pro-insulin-like growth factor-IA (proIGF-IA) recognize proIGF-IA secreted by IM9 B-lymphocytes. Growth Horm IGF Res. 2001;11:10–17. doi: 10.1054/ghir.2000.0182. [DOI] [PubMed] [Google Scholar]
- 15.Powell DR, Lee PD, Chang D, Liu F, Hintz RL. Antiserum developed for the E peptide region of insulin-like growth factor IA prohormone recognizes a serum protein by both immunoblot and radioimmunoassay. J Clin Endocrinol Metab. 1987;65:868–875. doi: 10.1210/jcem-65-5-868. [DOI] [PubMed] [Google Scholar]
- 16.Hede MS, Salimova E, Piszczek A, Perlas E, Winn N, Nastasi T, Rosenthal N. E-peptides control bioavailability of IGF-1. PLoS One. 2012;7:e51152. doi: 10.1371/journal.pone.0051152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 18.Rostand KS, Esko JD. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris J, Jayanthi S, Langston R, Daily A, Kight A, McNabb DS, Henry R, Kumar TK. Heparin-binding peptide as a novel affinity tag for purification of recombinant proteins. Protein Expr Purif. 2016;126:93–103. doi: 10.1016/j.pep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Billings PC, Pacifici M. Interactions of signaling proteins, growth factors and other proteins with heparan sulfate: mechanisms and mysteries. Connect Tissue Res. 2015;56:272–280. doi: 10.3109/03008207.2015.1045066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arai T, Busby W, Jr, Clemmons DR. Binding of insulin-like growth factor (IGF) I or II to IGF-binding protein-2 enables it to bind to heparin and extracellular matrix. Endocrinology. 1996;137:4571–4575. doi: 10.1210/endo.137.11.8895319. [DOI] [PubMed] [Google Scholar]
- 22.Imperiali B, O’Connor SE, Hendrickson T, Kellenberger C. Chemistry and biology of asparagine-linked glycosylation. Pure Appl Chem. 1999;71:777–787. [Google Scholar]
- 23.Duguay SJ, Lai-Zhang J, Steiner DF. Mutational analysis of the insulin-like growth factor I prohormone processing site. J Biol Chem. 1995;270:17566–17574. doi: 10.1074/jbc.270.29.17566. [DOI] [PubMed] [Google Scholar]
- 24.Shi S, Mercer S, Trippel SB. Effect of transfection strategy on growth factor overexpression by articular chondrocytes. J Orthop Res. 2010;28:103–109. doi: 10.1002/jor.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brisson BK, Barton ER. New modulators for IGF-I activity within IGF-I processing products. Front Endocrinol (Lausanne) 2013;4 doi: 10.3389/fendo.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen JA, Hofland LJ, Strasburger CJ, van den Dungen ES, Thevis M. Potency of Full-Length MGF to Induce Maximal Activation of the IGF-I R Is Similar to Recombinant Human IGF-I at High Equimolar Concentrations. PLoS One. 2016;11:e0150453. doi: 10.1371/journal.pone.0150453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. 2006;31:791–797. doi: 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- 28.Bell GI, Stempien MM, Fong NM, Rall LB. Sequences of liver cDNAs encoding two different mouse insulin-like growth factor I precursors. Nucleic Acids Res. 1986;14:7873–7882. doi: 10.1093/nar/14.20.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bach MA, Roberts CT, Jr, Smith EP, LeRoith D. Alternative splicing produces messenger RNAs encoding insulin-like growth factor-I prohormones that are differentially glycosylated in vitro. Mol Endocrinol. 1990;4:899–904. doi: 10.1210/mend-4-6-899. [DOI] [PubMed] [Google Scholar]
- 30.Siegfried JM, Kasprzyk PG, Treston AM, Mulshine JL, Quinn KA, Cuttitta F. A mitogenic peptide amide encoded within the E peptide domain of the insulin-like growth factor IB prohormone. Proc Natl Acad Sci U S A. 1992;89:8107–8111. doi: 10.1073/pnas.89.17.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan DS, Cook A, Chew SL. Nucleolar localization of an isoform of the IGF-I precursor. BMC Cell Biol. 2002;3:17. doi: 10.1186/1471-2121-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo YH, Chen TT. Novel activities of pro-IGF-I E peptides: regulation of morphological differentiation and anchorage-independent growth in human neuroblastoma cells. Exp Cell Res. 2002;280:75–89. doi: 10.1006/excr.2002.5628. [DOI] [PubMed] [Google Scholar]
- 33.Durzynska J, Wardzinski A, Koczorowska M, Gozdzicka-Jozefiak A, Barton ER. Human Eb peptide: not just a by-product of pre-pro-IGF1b processing? Horm Metab Res. 2013;45:415–422. doi: 10.1055/s-0032-1331699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 2002;522:156–160. doi: 10.1016/s0014-5793(02)02918-6. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Wu K, Lin F, Luo Q, Yang L, Shi Y, Song G, Sung KL. Mechano-growth factor induces migration of rat mesenchymal stem cells by altering its mechanical properties and activating ERK pathway. Biochem Biophys Res Commun. 2013;441:202–207. doi: 10.1016/j.bbrc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Lei M, Luo Z, Wu S, Zhong L, Xu Z, Lv Y, Yang L. Mechano-growth factor enhances differentiation of bone marrow-derived mesenchymal stem cells. Biotechnol Lett. 2015;37:2341–2348. doi: 10.1007/s10529-015-1915-0. [DOI] [PubMed] [Google Scholar]
- 37.Luo Z, Jiang L, Xu Y, Li H, Xu W, Wu S, Wang Y, Tang Z, Lv Y, Yang L. Mechano growth factor (MGF) and transforming growth factor (TGF)-β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model. Biomaterials. 2015;52:463–475. doi: 10.1016/j.biomaterials.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Farach-Carson MC, Carson DD. Perlecan--a multifunctional extracellular proteoglycan scaffold. Glycobiology. 2007;17:897–905. doi: 10.1093/glycob/cwm043. [DOI] [PubMed] [Google Scholar]
- 39.Pap T, Bertrand J. Syndecans in cartilage breakdown and synovial inflammation. Nat Rev Rheumatol. 2013;9:43–55. doi: 10.1038/nrrheum.2012.178. [DOI] [PubMed] [Google Scholar]
- 40.Caldwell J, Offenberg H, Ramanathan-Girish S, Yoshizawa C, Seely L. Arthritis Rheum. LIPPINCOTT WILLIAMS & WILKINS; 530 WALNUT ST, PHILADELPHIA, PA 19106-3621 USA: 2000. A safety, tolerability and pharmacokinetic study of intra-articular recombinant human insulin-like growth factor I (rhIGF-I) in patients with severe osteoarthritis (OA) of the knee; pp. S223–S223. [Google Scholar]
- 41.Tokunou T, Miller R, Patwari P, Davis ME, Segers VF, Grodzinsky AJ, Lee RT. Engineering insulin-like growth factor-1 for local delivery. Faseb j. 2008;22:1886–1893. doi: 10.1096/fj.07-100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jha AK, Tharp KM, Ye J, Santiago-Ortiz JL, Jackson WM, Stahl A, Schaffer DV, Yeghiazarians Y, Healy KE. Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels. Biomaterials. 2015;47:1–12. doi: 10.1016/j.biomaterials.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.