Abstract
Multiple genetic variations have been identified in FTO (fat mass and obesity-associated) gene. Among them, FTO rs9939609 polymorphism is shown to be associated with the risk of primary venous thromboembolism (VTE). However, its role in recurrent VTE is not known. The aim of our study was to investigate the association between FTO rs9939609 polymorphism and the risk of VTE recurrence in a prospective follow-up study in both male and female patients. FTO rs9939609 polymorphism (T/A) was analyzed in the Malmö thrombophilia study (MATS, followed for ~10 years) by using TaqMan PCR. MATS patients (n=1,050) were followed from the discontinuation of anticoagulant treatment until diagnosis of VTE recurrence or the end of follow-up. A total of 126 patients (12%) had VTE recurrence during follow-up. Cox regression analyses showed that sex modified the potential effect of FTO rs9939609 polymorphism on VTE recurrence. Male patients with the AA genotype for the FTO rs9939609 polymorphism had significantly higher risk of VTE recurrence as compared to the TT or AT genotypes (univariate hazard ratio [HR]=2.05, 95% confidence interval [CI]=1.2–3.5, p=0.009 and adjusted HR=2.03, 95% CI 1.2–3.6, p=0.013). There was no association between FTO rs9939609 polymorphism and VTE recurrence in female patients. In conclusion, our results show that FTO rs9939609 polymorphism in recurrent VTE may differ according to gender and FTO polymorphism may predict VTE recurrence in male patients.
Keywords: Obesity, venous thromboembolism, multivariate analysis
Introduction
Venous thromboembolism (VTE) that includes deep vein thrombosis (DVT) and pulmonary embolism (PE) is the third most common vascular disease after coronary artery disease and stroke (1). Its consequences include recurrence, post-thrombotic syndrome, fatal PE, and severe bleeding owing to anticoagulant treatment (2). Patients who have experienced one episode of VTE are at risk of recurrent, and the risk is highest during the first 6–12 months after the first diagnosis. Around 30% of patients with primary VTE experience recurrence within 10 years (3). VTE recurrence is fatal in approximately 5–9% of cases (4). Unprovoked VTE patients (without known acquired risk factors for VTE, e.g. immobilization, trauma, major surgery, female hormone therapy, pregnancy etc.) are at higher risk of VTE recurrence than provoked VTE (5).
Standard treatment protocol for VTE patients is the use of anticoagulant therapy for 3–6 months. Prolongation of treatment time, for instance in the case of unprovoked VTE, protects patients from VTE recurrence at the cost of increased bleeding risk (6, 7). Despite several identified risk factors and prediction models such as male sex, increased D-dimers level, residual thrombosis, HERDOO2 score, Vienna prediction model and DASH score, it is still difficult to precisely predict the risk of VTE recurrence after anticoagulation therapy stops (8, 9).
Familial and twin studies have shown a substantial role of genetic factors in the development of VTE (10–12). In a recent Swedish population study, heritability of VTE was reported as 47% for male and 40% for female VTE patients (13). Despite a number of genes being identified as risk factors for VTE, a major portion of the heritability remains unknown. Moreover, results from previous studies demonstrated that many genetic risk factors for primary VTE are less important for risk prediction of recurrent VTE (14, 15). Therefore, it is important to identify new biomarkers for a better stratification of the risk of VTE recurrence in order to tailor the anti-coagulant therapy accordingly.
Obesity is the measure of body mass index (BMI) >30 kg m−2. Being overweight or obese is a major and increasingly prevalent risk factor for multiple disorders, including VTE (16). Obesity is connected to the raised intra-abdominal pressure, decreased blood velocity in legs, inactivity, as well as prothrombotic and proinflammatory states. These factors are also known to contribute to the risk of VTE (17). Obesity is associated with almost doubling the risk of primary VTE (18). There are a lack of consistent data available on the role of BMI as a risk factor for VTE recurrence though most studies reported a slightly increased risk of VTE recurrence (19–22). Recent reports argue that BMI is not the most accurate measure of obesity and is not always feasible to measure in clinical settings. Furthermore, visceral adiposity is now suggested as more accurate measure of obesity but it needs CT/MRI that is not possible in all settings (23, 24). Therefore, the genetic factors associated with lifelong obesity are important to investigate for their role in risk prediction of VTE recurrence.
Heritability studies have provided evidence for a substantial genetic contribution (60–70%) to the obesity-related phenotypes (25, 26). Obesity is associated with a large number of common genetic variants, each with a small effect size. FTO (fat mass and obesity associated gene), positioned on chromosome 16, was the first common obesity susceptibility gene identified through genome-wide association studies, discovered by Frayling et al. (27). FTO protein is expressed in several tissues, mainly in specific parts of the muscles and brain, involved in fatty acid metabolism, energy homoeostasis, and hypothalamic regulation of food intake and appetite (27, 28). Frayling et al. showed that genetic variants in FTO gene were associated with risk of type 2 diabetes mellitus (DM). However, the primary effect was due to BMI rather than DM (27). The major contribution to this association with BMI was a cluster of 10 SNPs present in the first intron of FTO gene that were tightly linked to each other. This association was replicated by analyzing a single SNP (rs9939609 polymorphism) present in the first intron of FTO gene in 3757 type 2 diabetes patients and 5346 controls; the diabetes risk allele was significantly associated with BMI as well (27). The FTO gene continues to be the locus with the largest effect on obesity risk and BMI. However, the pathway whereby the rs9939609 and other FTO variants influence the risk of obesity remains unknown.
In a recent study, conducted by Klovaite J et al. in a group of 87,574 individuals of Danish descent, they found that the FTO rs9939609 polymorphism is significantly associated with higher risk of primary VTE (HR=1.86; 95% CI= 1.14–3.02) (29). However, the FTO rs9939609 variant has not been studied in recurrent VTE. The aim of the present study was to analyze the FTO rs9939609 variant in VTE patients and determine its possible association with VTE recurrence in both male and female patients.
Materials and Methods
Study subjects
The Malmö thrombophilia study (MATS) is a well-characterized cohort including 1465 VTE patients that were followed after inclusion in this study (March 1998) until VTE recurrence or death or the end of the study (December 2008) (30, 31). This study was performed at Skåne University Hospital Malmö, Sweden. At the time of inclusion, VTE events prior to the inclusion in the study, immobilization and cast therapy, location of DVT, surgical intervention, hospitalization, malignancies (past or prevalent), hormonal therapy, use of contraceptive pills, pregnancy and postpartum period (first six weeks after delivery), family history of VTE (history of VTE in first-degree relatives), and VTE recurrence during the follow-up period were recorded for all VTE patients.
The inclusion criteria in MATS were objective diagnosis of DVT and/or PE by one or more of the following methods: phlebography, computed tomography (CT), lung scintigraphy, magnetic resonance imaging (MRI) or duplex ultrasonography. MATS patients were required to answer a questionnaire and leave blood samples. The rate of consensual participation in this study was 70%. The remaining patients (30%) were excluded from the study because of the following: language problems, <18 years of age, did not participate in the blood sampling, questionnaire and complete risk factor analysis due to dementia and the presence of other severe diseases, and unwillingness to participate in the study.
Treatment of patients was performed according to the standard treatment protocol of Malmö University Hospital, i.e. initial treatment with low molecular weight heparin or unfractionated heparin and then with warfarin as an oral anticoagulant. According to hospital treatment protocol, therapy was recommended for 3–6 months for first-time VTE, with consideration of extended treatment in case of recurrent VTE. Thrombophilia was defined as presence of the factor V Leiden (FVL, rs6025) or factor II G20210A (rs1799963), or a level below the laboratory reference range of protein C (<0.7 kilo international unit (kIU)/L) or antithrombin (<0.82 kIU/L) or free protein S (female <0.5 kIU/L, male <0.65 kIU/L) in VTE patients without warfarin treatment.
Follow-up period was counted in years (Mean ± SD, 3.9 ± 2.5) after stopping the anticoagulant treatment until the diagnosis of VTE, death of the patient or the end of the study (December, 2008). The ethical committee of Lund University approved this study and all the participants provided written permission before their inclusion in the study according to the declaration of Helsinki.
Laboratory methods
DNA was extracted from the whole blood using QiAmp 96 DNA Blood Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. TaqMan® SNP Genotyping Assay was used to perform genotyping of FTO rs9939609 polymorphism according to the manufacturer’s protocol (Applied Biosystems, Life Technologies Corporation, Carlsbad, CA, USA). To summarize, a polymerase chain reaction (PCR) master mix (3µL) was prepared as 2.5µL Taqman master mix, 0.25µL Taqman gene-specific assay (including VIC and FAM probes for FTO rs9939609 polymorphism) and 0.25µL deionized water. Master Mix was then added to each well in 384 PCR plate followed by addition of 10ng genomic DNA. PCR plate (384 wells) was vortexed followed by centrifugation at 1000 rpm (revolutions per minute) for 30 seconds. For polymorphism analysis, BioRad CFX384 real-time PCR (1000 Alfred Nobel Drive Hercules, California 94547 USA) was used according to the manufacturer’s instructions with the following temperature conditions, 95°C for 10 minutes followed by 40× (92°C for 15 sec, 60°C for 1min). Different alleles of FTO rs9939609 polymorphism were determined by using BioRad CFX manager software.
Analysis of known thrombophilic variants
Factor II G20210A and FVL in MATS patients were analyzed by TaqMan allele discrimination assays (Applied Biosystems) as described previously (32). Protein C activities were analyzed by the chromogenic method using the Berichrom® Protein C reagent (Siemens Healthcare Diagnostics, Upplands Väsby, Sweden) (33). Analysis of free Protein S antigen concentration was performed by latex immunoassay with Coamatic® Protein S-Free (Chromogenix, Haemochrom Diagnostica AB, Gothenburg, Sweden) (34). A thrombin-based method using Berichrom Antithrombin reagent (Siemens Healthcare Diagnostics) was used for antithrombin III antigen concentration analysis (35).
Statistical analysis
Statistical analyses were performed by using SPSS version 21 (IBM, Armonk, NY, USA). Dichotomous variables were compared by Chi-square test or Fisher's exact test, where appropriate and continuous variables were compared by Student T-test (if data was normally distributed) or Mann-Whitney U test (if data was not normally distributed). For normally distributed variable (BMI), Mean±SD is presented whereas for skewed variable (age), median and IQR (interquartile range) is presented. Testing for effect modification between included variables was done with inclusion of interaction term. Survival curves for time to recurrent VTE by FTO rs9939609 genotypes are presented and log-rank test was used to compare recurrence-free survival between genotypes. Univariate and multivariate Cox regression analyses (after adjusting for BMI, family history of VTE, mild [heterozygous prothrombin G20210A or FVL] and severe thrombophilia [homozygous carriers of FVL or those patients who had natural anticoagulant deficiencies, e.g., protein C, antithrombin, protein S deficiency, and/or carriers of multiple abnormalities] and acquired risk factors for VTE) were performed using Cox proportional hazards models. For each group of patients, Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. Sensitivity analyses were performed by Multivariate Cox regression analyses including all VTE patients with an exception for those who had thrombotic events before inclusion. The follow-up period for sensitivity analyses was calculated from the time of inclusion and was adjusted for the duration of anticoagulation treatment. Hardy–Weinberg equilibrium analysis was performed to see the genotypic distribution by using web based calculator (36). To calculate the power of our results, we used online software (www.openepi.com). Linear regression analysis was performed to find the association between FTO rs9939609 polymorphism and BMI. FTO rs9939609 polymorphism has three different genotypic forms, TT (homozygous wild type), AT (heterozygous) and AA (homozygous mutated form). During data analysis, all three genotypic forms were analyzed separately as well as a recessive model for genotype analysis was also used (by combining TT+AT genotypes and compared with AA genotype).
Results
Clinical data of the patients
Out of 1465 VTE patients, those who had one or more thrombotic events before inclusion to this study (n=154) were excluded. Among the remaining patients (n=1311), 148 (11.3%) developed recurrent VTE during the follow-up period. In recurrent VTE patients, frequency of thrombophilia and family history of VTE was significantly higher as compared to non-recurrent VTE patients (P<0.05). No significant difference, however, was observed among recurrent and non-recurrent VTE patients in age, BMI, sex, DVT and PE. Table 1.
Table 1.
Characteristics of studied population including the distribution of FTO rs9939609 polymorphism genotypes stratified by recurrent and non-recurrent status
| Parameters | Non recurrent VTE n (%) |
Recurrent VTE n (%) |
Total n (%) |
¶P- value |
|---|---|---|---|---|
| FTO genotype rs9939609 | ||||
| TT | 375 (32.5) | 44 (29.9) | 419 (32.2) | |
| AT | 575 (49.9) | 70 (47.6) | 645 (49.6) | 0.352 |
| AA | 203 (17.6) | 33 (22.4) | 236 (18.2) | |
| TT and AT | 950 (82.4) | 114 (77.6) | 1064 (81.8) | 0.172 |
| AA | 203 (17.6) | 33 (22.4) | 236 (18.2) | |
| Age a inclusion | ||||
| Years, Median (IQR) | 66.4 (24) | 63.4 (24) | 65.8 (24) | 0.088† |
| BMI | ||||
| Mean±SD | 26.6±4.7 | 27.4±5.1 | 26.6±4.8 | 0.066* |
| Sex | ||||
| Male | 565 (48.6) | 78 (52.7) | 643 (49.0) | 0.383 |
| Female | 598 (51.4) | 70 (47.3) | 668 (51.0) | |
| DVT+PE | ||||
| DVT | 736(68.2) | 98 (68.5) | 834 (68.2) | 0.323 |
| PE | 277 (25.7) | 32 (22.4) | 309 (25.3) | |
| DVT+PE | 66 (6.1) | 13 (9.1) | 79 (6.5) | |
| Thrombophilia | ||||
| Yes | 390 (36.9) | 68 (49.6) | 458 (38.3) | 0.005 |
| No | 668 (63.1) | 69 (50.4) | 737 (61.7) | |
| Family history | ||||
| Yes | 269 (23.5) | 47 (32.4) | 316 (24.5) | 0.024 |
| No | 875 (76.5) | 98 (67.6) | 973 (75.5) |
DNA was not enough for genotyping in 11 samples for FTO rs9939609 polymorphism, DVT, deep vein thrombosis; PE, pulmonary embolism; BMI, body mass index. P-value, Chi square test until unless indicated,
Student T-test,
Mann-Whitney U test.
comparing non-recurrent with recurrent VTE. IQR, Interquartile range. Thrombophilia, presence of factor V Leiden, factor II G20210A, or antithrombin, protein C or free protein S levels below the laboratory reference ranges.
Genotypic distributions in FTO rs9939609 polymorphism did not deviate significantly in Hardy-Weinberg equilibrium analyses (P= 0.656).
The distribution of FTO rs9939609 genotypes according to sex and their association with the basic characteristics of VTE patients (age, BMI, DVT, PE and family history) are presented in Table 2. Male patients with AA genotype were at significantly higher risk of pulmonary embolism (P=0.040) as compared to females. No statistically significant association was found between the distribution of FTO genotypes and other variables including BMI in the whole study population or when associations were analyzed separately by sex (Table 2). Furthermore, no association was found between FTO rs9939609 polymorphism and BMI (P>0.05, result not shown) in linear-regression analysis.
Table 2.
Distribution of different genotypes of FTO rs rs9939609 polymorphism in studied population
| FTO genotypes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Males (n=643) |
¶P value |
Females (n=668) |
¶P value |
All patients (n=1311) |
¶P value |
||||
|
|
|
|
|||||||
| TT & AT | AA | TT & AT | AA | TT & AT | AA | ||||
| Age a inclusion | |||||||||
| Years, Median (IQR) | 64.9 (20) | 63.7 (24) | 0.650† | 67.5 (30) | 67.5 (28) | 0.872† | 65.7 (24) | 65.8 (24) | 0.895† |
| BMI | |||||||||
| (Mean±SD) | 26.6±4.0 | 26.9±4.2 | 0.482* | 26.6±5.2 | 26.9±5.9 | 0.581* | 26.6±4.7 | 26.9±5.2 | 0.402* |
| DVT | |||||||||
| No | 100 (76.9) | 30 (23.1) | 0.163 | 150 (84.7) | 27 (15.3) | 0.305 | 250 (81.4) | 57 (18.6) | 0.865 |
| Yes | 420 (82.7) | 88 (17.3) | 394 (81.2) | 91 (18.8) | 814 (82) | 179 (18) | |||
| PE | |||||||||
| No PE | 388 (83.4) | 77 (16.6) | 0.040 | 365 (80.9) | 86 (19.1) | 0.233 | 753 (82.2) | 163 (17.8) | 0.636 |
| PE | 132 (76.3) | 41 (23.7) | 179 (84.8) | 32 (15.2) | 311 (81) | 73 (19) | |||
| Thrombophilia | |||||||||
| No | 277 (81.2) | 64 (18.8) | 0.915 | 329 (83.9) | 63 (16.1) | 0.062 | 606 (82.7) | 127 (17.3) | 0.167 |
| Yes | 194 (80.8) | 46 (19.2) | 167 (77.7) | 48 (22.3) | 361 (79.3) | 94 (20.7) | |||
| Family history | |||||||||
| No | 395 (80.3) | 97 (19.7) | 0.267 | 289 (81.9) | 86 (18.1) | 0.818 | 784 (81.1) | 183 (18.9) | 0.314 |
| Yes | 116 (84.7) | 21 (15.3) | 145 (82.9) | 30 (17.1) | 261 (83.7) | 51 (16.3) | |||
P-value, Chi square test until unless indicated, DVT, deep vein thrombosis; PE, pulmonary embolism; BMI, body mass index.
Mann-Whitney U test,
comparing different genotypes of FTO rs9939609 rs polymorphism.
Student T-test,
Mann-Whitney U test.
comparing non-recurrent with recurrent VTE. IQR, Interquartile range. Thrombophilia, presence of factor V Leiden, factor II G20210A, or antithrombin, protein C or free protein S levels below the laboratory reference ranges.
FTO rs9939609 polymorphism and risk of VTE recurrence
For the risk assessment analyses from 1311 patients, we excluded those patients who died, had VTE recurrence during anticoagulant treatment, or for whom complete information was missing (n=261). Remaining 1050 patients were followed after stopping the anticoagulant treatment and 12% (126) had VTE recurrence during the follow-up.
In the whole population, no significant association between FTO rs9939609 polymorphism and risk of VTE recurrence was observed (Table 3). However, in the Cox regression model, inclusion of an interaction term between FTO rs9939609 polymorphism and sex of the patients showed a modifying effect of sex on FTO rs9939609 polymorphism (FTO rs9939609 polymorphism *sex: HR= 0.42, 95 % CI=0.18–0.99, P=0.049). Consequently, data was stratified according to the sex and FTO rs9939609 polymorphism was significantly associated with higher risk of VTE recurrence in male patients (HR =1.90 and 95% CI = 1.0–3.58, P= 0.048). In multivariate analysis also (after adjusting for BMI, family history of VTE, mild and severe thrombophilia and acquired risk factors for VTE), FTO rs9939609 polymorphism was associated with high risk of VTE recurrence in male patients however, it didn’t reach significant level when 3 genotypes were analyzed individually (HR=1.87, CI=0.95–3.54, P= 0.072). However, as T allele containing genotypes (TT and AT) has similar risk and on the Kaplan-Meier survival curve, both genotypes had similar recurrence free survival, we combined TT and AT genotypes and compared with the AA homozygous mutated genotype. Male patients that had the FTO rs9939609 polymorphism were at significantly higher risk of VTE recurrence in both uni- and multivariate Cox regression analyses (HR= 2.05, CI= 1.20–3.49, P= 0.009 and HR=2.03, CI= 1.16–3.55, P= 0.013 respectively) (Table 3).
Table 3.
Uni- and multivariate Cox regression analyses of FTO rs9939609 polymorphism in recurrent VTE patients
| All patients | Male | Female | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Univariate | P | Multivariate | P* | Univariate | P | Multivariate | P* | Univariate | P | Multivariate | P* | |
|
|
|
|
|
|
|
|||||||
| Genotypes | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| rs9939609 | ||||||||||||
| TT | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| AT | 0.94 (0.63–1.41) | 0.775 | 0.86 (0.56–1.30) | 0.469 | 0.88 (0.49–1.58) | 0.674 | 0.84 (0.46–1.55) | 0.575 | 1.01 (0.58–1.77) | 0.978 | 0.89 (0.50–1.59) | 0.692 |
| AA | 1.35 (0.84–2.19) | 0.219 | 1.30 (0.80–2.13) | 0.289 | 1.90 (1.0–3.58) | 0.048 | 1.83 (0.95–3.54) | 0.072 | 0.88 (0.41–1.88) | 0.741 | 0.84 (0.39–1.79) | 0.645 |
| TT & AT | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| AA | 1.40 (0.93–2.12) | 0.111 | 1.43 (0.93–2.19) | 0.100 | 2.05 (1.20–3.49) | 0.009 | 2.03 (1.16–3.55) | 0.013 | 0.88 (0.44–1.73) | 0.701 | 0.90 (0.45–1.78) | 0.752 |
P* Adjusted for BMI, acquired risk factors, mild and severe thrombophilia, and family history of VTE.
To detect the power of our results, we made power calculation analyses in whole populations and male patients separately. We found that with our sample size, we could detect a risk difference as low as 1.4 and 1.9 with 95% confidence interval and 80% power in whole population and male patients respectively. Our study results showed a risk difference of 2.05 in male patients, therefore we have had enough power to detect the differences between non-recurrent VTE and recurrent VTE patients.
FTO rs9939609 polymorphism and risk of VTE recurrence in unprovoked VTE patients
We also performed a sub-analysis on unprovoked first VTE patients (n=618). In this analysis, we excluded VTE patients who had a provoked first VTE (see above). A non-significant association between FTO polymorphism and high risk of VTE recurrence in male patients (P= 0.059, HR= 1.94, 95% CI= 0.98–3.86) was found on univariate Cox regression analysis. However, on multivariate Cox regression analysis (after adjusting our data with BMI, family history, and risk of thrombophilia), this association became statistically significant (P= 0.046, HR= 2.09, 95% CI= 1.04–4.21). (Table 4)
Table 4.
Uni- and multivariate Cox regression analyses of FTO rs9939609 polymorphism in Unprovoked VTE patients
| All patients | Male | Female | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Univariate |
P |
Multivariate |
P* |
Univariate |
P |
Multivariate |
P* |
Univariate |
P |
Multivariate |
P* |
|
|
|
|
|
|
|
|
|||||||
| Genotypes | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| rs9939609 | ||||||||||||
| TT | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| AT | 1.03 (0.63–1.70) | 0.900 | 0.85 (0.51–1.43) | 0.542 | 1.03 (0.50–2.12) | 0.939 | 0.92 (0.44–1.94) | 0.832 | 1.12 (0.56–2.32) | 0.740 | 0.90 (0.43–1.88) | 0.784 |
| AA | 1.24 (0.67–2.32) | 0.495 | 1.22 (0.65–2.27) | 0.541 | 1.97 (0.87–4.48) | 0.103 | 1.99 (0.88–4.53) | 0.101 | 0.67 (0.24–1.87) | 0.447 | 0.65 (0.23–1.82) | 0.412 |
| TT & AT | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| AA | 1.22 (0.70–2.11) | 0.478 | 1.33 (0.76–2.32) | 0.311 | 1.94 (0.98–3.86) | 0.059 | 2.09 (1.04–4.21) | 0.046 | 0.63 (0.25–1.61) | 0.333 | 0.69 (0.27–1.78) | 0.440 |
P* Adjusted for BMI, mild and severe thrombophilia, and family history of VTE.
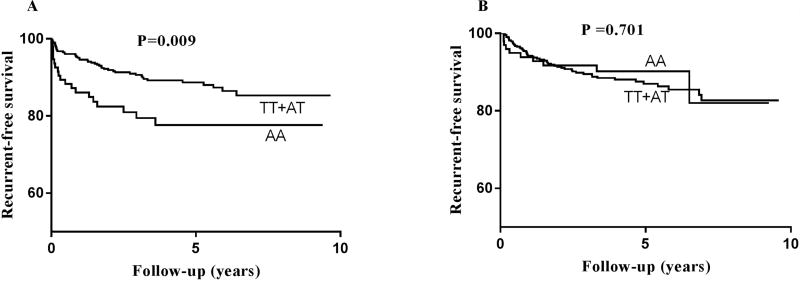
Survival analysis by Kaplan-Meier curve was performed to analyze whether FTO rs9939609 polymorphism effects recurrence-free survival. Patients having AA and TT+AT genotypes were compared and results showed a significant difference in recurrence-free survival (Figure 1A, Log-rank test, P =0.009) in male patients. Male patients having AA genotype had significantly shorter recurrence-free survival as compared to TT and AT genotypes whereas no significant difference was observed between FTO genotypes and risk of VTE recurrence in female patients (Figure 1B, Log-rank test, P =0.701).
Figure 1.
Survival curves representing the different genotypes in FTO rs9939609 polymorphism and their association with risk of VTE recurrence in male (Figure 1A) and in female patients (Figure 1B). P=log-rank test.
Furthermore, sensitivity analyses were performed including all MATS patients (n=1311) except those who had one or more episodes of VTE before inclusion (n=154). For sensitivity analyses, follow-up time was calculated from the time of inclusion and was adjusted for duration of anticoagulant treatment. Cox regression analyses showed that FTO rs9939609 polymorphism was associated with risk of VTE recurrence in male patients (P= 0.007, HR= 2.05, 95% CI= 1.21–3.46) and this association was independent of duration of anticoagulant treatment, BMI, family history of VTE, mild and severe thrombophilia and acquired risk factors for VTE (Table 1 in the Supplementary Appendix).
Discussion
In the present follow-up study, we have investigated the role of FTO rs9939609 polymorphism in risk of recurrent VTE. We found that AA genotype of the FTO rs9939609 polymorphism was significantly associated with higher risk of VTE recurrence in male patients and this association was not attenuated after adjustment with BMI, acquired risk factors for VTE, family history, and risk of thrombophilia. To our knowledge, this is the first study in which the FTO rs9939609 polymorphism has been analyzed in a prospective follow-up study of VTE patients whilst taking into account the confounders that are involved in VTE recurrence.
We could not find any study investigating the role of FTO rs9939609 polymorphism in recurrent VTE. However, we found one Danish study in which this polymorphism was reported to be associated with high risk of primary VTE but they have not stratified the data according to the sex; therefore, it is unclear whether this polymorphism is also associated with risk of primary VTE in a sex dependent manner (29). In a prospective cohort study, Fisher et al. reported an association between FTO rs9939609 polymorphism and increased levels of C-reactive protein (CRP); a well-known inflammatory marker involved in the pathogenesis of VTE (37, 38). The mechanisms underlying the association of the FTO variant in the pathophysiology of VTE remain unclear. Considering the intronic location of FTO rs9939609 polymorphism, it can be involved in transcription of the gene as previous reports have shown (39), however, it needs to be confirmed. Another possibility could be that this polymorphism is in linkage disequilibrium with other variants in FTO or in neighboring obesity related genes (40). For example, this polymorphism is present close to another gene with undefined function i.e. KIAA1005 (also known as RPGRIP1L, lies 200 base pairs away from 5’ untranslated region of FTO gene) that opens up the possibility that genetic variation in FTO may affect the regulatory part of KIAA1005 (27). However, at present, there is no obvious mechanism that explains how this intronic variant alters the function of KIAA1005, FTO, or any other distant genes.
Gene knockout and over expression studies in mice models have shown that FTO protein is highly expressed in the central nervous system, involved in the regulation of energy intake and metabolism (41–44). One possible explanation for the association between FTO polymorphism and obesity could be that carriers of this polymorphism not only consume more food but also select the energy-dense palatable food, which suggests that FTO variants affect the sensing of micronutrients of the diet (45). Another suggested explanation could be the interaction of FTO polymorphisms with the unhealthy lifestyle factors, i.e. lack of physical activity, dietary habits and smoking that may affect the epigenetic status (DNA methylation), ultimately contributing to the development of disease (46). However, to what extent FTO polymorphisms interact with these factors remains to be answered in future studies.
Genetic variation in FTO has been the subject of several studies for a plethora of phenotypes including obesity and obesity-related phenotypes, e.g. high BMI and a significant association between FTO polymorphism and BMI has been previously reported (27, 29). In our study, we also analyzed FTO rs9939609 polymorphism for its association with BMI. However, we did not find a significant association between FTO rs9939609 polymorphism and BMI. As shown in previous studies, genetic factors can vary according to gender, environment and population (47–49); e.g. Factor V Leiden frequency was found to be higher in a Swedish population as compared to the populations of other geographical regions (47, 50). In agreement with our findings, Jacobsson et al. reported no association between FTO rs9939609 polymorphism and BMI in a Swedish population (51). Therefore, there is a possibility that an association between FTO polymorphism and BMI may be population dependent. Further studies on larger population groups are warranted to confirm the association between FTO rs9939609 polymorphism and BMI.
It has been reported that male patients have 2–4 fold higher risk of VTE recurrence as compared to females (52, 53). In our study, we found a marker for the risk prediction of VTE recurrence among male patients. It remains unclear why FTO polymorphism is associated with higher risk of disease in males only and why male patients have higher risk of VTE recurrence. Sex-specific effects of the FTO variants have been reported, but the importance of these effects remains unclear (54, 55). It has been reported that in female VTE patients if the cause of primary VTE is a reversible risk factor (e.g. hormone-mediated primary VTE), the risk of VTE recurrence is reduced once the hormonal exposure is removed (56). Moreover, a previous study showed that men had a higher risk of VTE recurrence if the cause of primary VTE is unprovoked (57) suggesting that male patients have persistent but still undetermined risk factors for the development of VTE and its recurrence. Our findings, i.e. male patients with FTO rs9939609 polymorphism have about 2 times higher risk of VTE recurrence, could at least partially contribute to the understanding of higher risk of VTE recurrence in males.
Although our study contains several strengths, including its relatively large sample size, objective diagnosis of VTE and long follow-up period, we acknowledge its limitations as well. One possible limitation of our study was the lack of data on the functional role of FTO rs9939609 polymorphism in VTE patients. The present study showed that FTO rs9939609 polymorphism was associated with the risk of VTE recurrence in male but not in female VTE patients but we do not know the potentially differential functional role of FTO rs9939609 polymorphism in male and female patients.
In conclusion, this is the first study in which FTO rs9939609 polymorphism has been studied in recurrent VTE and our results indicate that FTO rs9939609 polymorphism was associated with higher risk of VTE recurrence in males but not in females independent of other well-known risk factors for VTE. Further studies in other populations are needed to confirm this association as well as to examine the functional role of FTO rs9939609 polymorphism for VTE recurrence in male and female patients.
Supplementary Material
Acknowledgments
We would like to thank biobank services at Biobank, Lab medicine Skåne, Sweden. We also thank science editor Patrick Reilly for language editing of this manuscript.
Funding
This work was supported by grants from NIH awarded to Dr. Kristina Sundquist, grants awarded to Dr. Bengt Zöller by the Swedish Heart-Lung Foundation, ALF funding from Region Skåne awarded to Dr. Bengt Zöller and Dr. Kristina Sundquist, grants awarded to Dr. Bengt Zöller and Dr. Kristina Sundquist by the Swedish Research Council, and grants awarded to Dr. Jan Sundquist by King Gustaf V and Queen Victoria’s Foundation of Freemasons. The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
List of abbreviations
- A
Adenine
- BMI
Body mass index
- CT
Computed tomography
- CI
Confidence interval
- CRP
C-reactive protein
- DVT
Deep vein thrombosis
- DNA
Deoxyribonucleic acid
- FTO
fat mass and obesity-associated gene
- FVL
factor V Leiden
- HR
Hazard ratio
- kIU
kilo international unit
- MRI
Magnetic resonance imaging
- MATS
Malmö thrombophilia study
- ng
Nano gram
- PE
pulmonary embolism
- rpm
revolutions per minute
- SD
Standard deviation
- T
Thymine
- VTE
venous thromboembolism
- µL
Micro liter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Authors declare no conflict of interest.
References
- 1.van Schouwenburg IM, Gansevoort RT, Mahmoodi BK, et al. Increased risk of arterial thromboembolism after a prior episode of venous thromboembolism: results from the Prevention of REnal and Vascular ENd stage Disease (PREVEND) Study. British journal of haematology. 2012;159(2):216–22. doi: 10.1111/bjh.12005. [DOI] [PubMed] [Google Scholar]
- 2.Martinelli I, De Stefano V, Mannucci PM. Inherited risk factors for venous thromboembolism. Nature reviews Cardiology. 2014;11(3):140–56. doi: 10.1038/nrcardio.2013.211. [DOI] [PubMed] [Google Scholar]
- 3.Heit JA. Epidemiology of venous thromboembolism. Nature reviews Cardiology. 2015;12(8):464–74. doi: 10.1038/nrcardio.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douketis JD, Gu CS, Schulman S, et al. The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Annals of internal medicine. 2007;147(11):766–74. doi: 10.7326/0003-4819-147-11-200712040-00007. [DOI] [PubMed] [Google Scholar]
- 5.Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Archives of internal medicine. 2010;170(19):1710–6. doi: 10.1001/archinternmed.2010.367. [DOI] [PubMed] [Google Scholar]
- 6.Carrier M, Le Gal G, Wells PS, et al. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Annals of internal medicine. 2010;152(9):578–89. doi: 10.7326/0003-4819-152-9-201005040-00008. [DOI] [PubMed] [Google Scholar]
- 7.Kearon C, Gent M, Hirsh J, et al. A Comparison of Three Months of Anticoagulation with Extended Anticoagulation for a First Episode of Idiopathic Venous Thromboembolism. New England Journal of Medicine. 1999;340(12):901–7. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- 8.Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet. 2010;376(9757):2032–9. doi: 10.1016/S0140-6736(10)60962-2. [DOI] [PubMed] [Google Scholar]
- 9.Ensor J, Riley RD, Moore D, et al. Systematic review of prognostic models for recurrent venous thromboembolism (VTE) post-treatment of first unprovoked VTE. BMJ Open. 2016;6(5) doi: 10.1136/bmjopen-2016-011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heit JA, Phelps MA, Ward SA, et al. Familial segregation of venous thromboembolism. Journal of thrombosis and haemostasis : JTH. 2004;2(5):731–6. doi: 10.1111/j.1538-7933.2004.00660.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsen TB, Sorensen HT, Skytthe A, et al. Major genetic susceptibility for venous thromboembolism in men: a study of Danish twins. Epidemiology. 2003;14(3):328–32. [PubMed] [Google Scholar]
- 12.Zoller B, Li X, Ohlsson H, et al. Epidemiology of Familial Aggregation of Venous Thromboembolism. Seminars in thrombosis and hemostasis. 2016;42(8):821–32. doi: 10.1055/s-0036-1593543. [DOI] [PubMed] [Google Scholar]
- 13.Zoller B, Ohlsson H, Sundquist J, et al. A sibling based design to quantify genetic and shared environmental effects of venous thromboembolism in Sweden. Thrombosis research. 2017;149:82–7. doi: 10.1016/j.thromres.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Eichinger S, Minar E, Hirschl M, et al. The risk of early recurrent venous thromboembolism after oral anticoagulant therapy in patients with the G20210A transition in the prothrombin gene. Thrombosis and haemostasis. 1999;81(1):14–7. [PubMed] [Google Scholar]
- 15.Baglin T. Unraveling the thrombophilia paradox: from hypercoagulability to the prothrombotic state. Journal of Thrombosis and Haemostasis. 2010;8(2):228–33. doi: 10.1111/j.1538-7836.2009.03702.x. [DOI] [PubMed] [Google Scholar]
- 16.Lewis CE, McTigue KM, Burke LE, et al. Mortality, health outcomes, and body mass index in the overweight range: a science advisory from the American Heart Association. Circulation. 2009;119(25):3263–71. doi: 10.1161/CIRCULATIONAHA.109.192574. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzet R, Napoleone E, Cutrone A, et al. Thrombosis and obesity: cellular bases. Thrombosis research. 2012;129(3):285–9. doi: 10.1016/j.thromres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 19.Cannegieter SC, van Hylckama Vlieg A. Venous thrombosis: understanding the paradoxes of recurrence. Journal of thrombosis and haemostasis : JTH. 2013;11(Suppl 1):161–9. doi: 10.1111/jth.12263. [DOI] [PubMed] [Google Scholar]
- 20.Heit JA, Silverstein MD, Mohr DN, et al. The epidemiology of venous thromboembolism in the community. Thrombosis and haemostasis. 2001;86(1):452–63. [PubMed] [Google Scholar]
- 21.Fahrni J, Husmann M, Gretener SB, et al. Assessing the risk of recurrent venous thromboembolism – a practical approach. Vascular Health and Risk Management. 2015;11:451–9. doi: 10.2147/VHRM.S83718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romualdi E, Squizzato A, Ageno W. Abdominal obesity and the risk of recurrent deep vein thrombosis. Thrombosis research. 2007;119(6):687–90. doi: 10.1016/j.thromres.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Shah NR, Braverman ER. Measuring Adiposity in Patients: The Utility of Body Mass Index (BMI), Percent Body Fat, and Leptin. PLOS ONE. 2012;7(4):e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuster A, Patlas M, Pinthus JH, et al. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. The British journal of radiology. 2012;85(1009):1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behavior genetics. 1997;27(4):325–51. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 26.Segal NL, Allison DB. Twins and virtual twins: bases of relative body weight revisited. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(4):437–41. doi: 10.1038/sj.ijo.0801941. [DOI] [PubMed] [Google Scholar]
- 27.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science (New York, NY) 2007;316(5826):889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loos RJF, Yeo GSH. The bigger picture of FTO – the first GWAS-identified obesity gene. Nature reviews Endocrinology. 2014;10(1):51–61. doi: 10.1038/nrendo.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klovaite J, Benn M, Nordestgaard BG. Obesity as a causal risk factor for deep venous thrombosis: a Mendelian randomization study. Journal of internal medicine. 2015;277(5):573–84. doi: 10.1111/joim.12299. [DOI] [PubMed] [Google Scholar]
- 30.Isma N, Svensson PJ, Gottsater A, et al. Prospective analysis of risk factors and distribution of venous thromboembolism in the population-based Malmo Thrombophilia Study (MATS) Thrombosis research. 2009;124(6):663–6. doi: 10.1016/j.thromres.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad A, Sundquist K, Zoller B, et al. Identification of Genetic Aberrations in Thrombomodulin Gene in Patients With Recurrent Venous Thromboembolism. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2017;23(4):319–28. doi: 10.1177/1076029616686716. [DOI] [PubMed] [Google Scholar]
- 32.Sveinsdottir SV, Saemundsson Y, Isma N, et al. Evaluation of recurrent venous thromboembolism in patients with Factor V Leiden mutation in heterozygous form. Thrombosis research. 2012;130(3):467–71. doi: 10.1016/j.thromres.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Francis RB, Jr, Seyfert U. Rapid amidolytic assay of protein C in whole plasma using an activator from the venom of Agkistrodon contortrix. Am J Clin Pathol. 1987;87(5):619–25. doi: 10.1093/ajcp/87.5.619. [DOI] [PubMed] [Google Scholar]
- 34.Giri TK, Hillarp A, Hardig Y, et al. A new direct, fast and quantitative enzyme-linked ligandsorbent assay for measurement of free protein S antigen. Thrombosis and haemostasis. 1998;79(4):767–72. [PubMed] [Google Scholar]
- 35.Odegard OR, Lie M, Abildgaard U. Heparin cofactor activity measured with an amidolytic method. Thrombosis research. 1975;6(4):287–94. doi: 10.1016/0049-3848(75)90078-x. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. American journal of epidemiology. 2009;169(4):505–14. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher E, Schulze MB, Stefan N, et al. Association of the FTO rs9939609 single nucleotide polymorphism with C-reactive protein levels. Obesity (Silver Spring, Md) 2009;17(2):330–4. doi: 10.1038/oby.2008.465. [DOI] [PubMed] [Google Scholar]
- 38.Folsom AR, Lutsey PL, Astor BC, et al. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thrombosis and haemostasis. 2009;102(4):615–9. doi: 10.1160/TH09-04-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park SG, Hannenhalli S, Choi SS. Conservation in first introns is positively associated with the number of exons within genes and the presence of regulatory epigenetic signals. BMC Genomics. 2014;15(1):526. doi: 10.1186/1471-2164-15-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephens JC, Schneider JA, Tanguay DA, et al. Haplotype Variation and Linkage Disequilibrium in 313 Human Genes. Science (New York, NY) 2001;293(5529):489–93. doi: 10.1126/science.1059431. [DOI] [PubMed] [Google Scholar]
- 41.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41(1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Church C, Moir L, McMurray F, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42(12):1086–92. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer J, Koch L, Emmerling C, et al. Inactivation of the Fto gene protects from obesity. Nature. 2009;458(7240):894–8. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 44.Ronkainen J, Mondini E, Cinti F, et al. Fto-Deficiency Affects the Gene and MicroRNA Expression Involved in Brown Adipogenesis and Browning of White Adipose Tissue in Mice. International Journal of Molecular Sciences. 2016;17(11):1851. doi: 10.3390/ijms17111851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanofsky-Kraff M, Han JC, Anandalingam K, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. The American journal of clinical nutrition. 2009;90(6):1483–8. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hubacek JA, Stanek V, Gebauerova M, et al. A FTO variant and risk of acute coronary syndrome. Clinica chimica acta; international journal of clinical chemistry. 2010;411(15–16):1069–72. doi: 10.1016/j.cca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 47.Holm J, Zoller B, Berntorp E, et al. Prevalence of factor V gene mutation amongst myocardial infarction patients and healthy controls is higher in Sweden than in other countries. Journal of internal medicine. 1996;239(3):221–6. doi: 10.1046/j.1365-2796.1996.470808000.x. [DOI] [PubMed] [Google Scholar]
- 48.Zoller B, Li X, Sundquist J, et al. Age- and gender-specific familial risks for venous thromboembolism: a nationwide epidemiological study based on hospitalizations in Sweden. Circulation. 2011;124(9):1012–20. doi: 10.1161/CIRCULATIONAHA.110.965020. [DOI] [PubMed] [Google Scholar]
- 49.Hunter DJ. Gene-environment interactions in human diseases. Nat Rev Genet. 2005;6(4):287–98. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- 50.Hallam PJ, Millar DS, Krawczak M, et al. Population differences in the frequency of the factor V Leiden variant among people with clinically symptomatic protein C deficiency. Journal of medical genetics. 1995;32(7):543–5. doi: 10.1136/jmg.32.7.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobsson JA, Riserus U, Axelsson T, et al. The common FTO variant rs9939609 is not associated with BMI in a longitudinal study on a cohort of Swedish men born 1920–1924. BMC Med Genet. 2009;10:131. doi: 10.1186/1471-2350-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyrle PA, Minar E, Bialonczyk C, et al. The risk of recurrent venous thromboembolism in men and women. The New England journal of medicine. 2004;350(25):2558–63. doi: 10.1056/NEJMoa032959. [DOI] [PubMed] [Google Scholar]
- 53.Baglin T, Luddington R, Brown K, et al. High risk of recurrent venous thromboembolism in men. Journal of thrombosis and haemostasis : JTH. 2004;2(12):2152–5. doi: 10.1111/j.1538-7836.2004.01050.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang M, Zhao X, Cheng H, et al. Age- and Sex-Dependent Association between FTO rs9939609 and Obesity-Related Traits in Chinese Children and Adolescents. PLoS ONE. 2014;9(5):e97545. doi: 10.1371/journal.pone.0097545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saldana-Alvarez Y, Salas-Martinez MG, Garcia-Ortiz H, et al. Gender-Dependent Association of FTO Polymorphisms with Body Mass Index in Mexicans. PLoS One. 2016;11(1):e0145984. doi: 10.1371/journal.pone.0145984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lijfering WM, Veeger NJ, Middeldorp S, et al. A lower risk of recurrent venous thrombosis in women compared with men is explained by sex-specific risk factors at time of first venous thrombosis in thrombophilic families. Blood. 2009;114(10):2031–6. doi: 10.1182/blood-2009-04-215418. [DOI] [PubMed] [Google Scholar]
- 57.Tagalakis V, Kondal D, Ji Y, et al. Men had a higher risk of recurrent venous thromboembolism than women: a large population study. Gender medicine. 2012;9(1):33–43. doi: 10.1016/j.genm.2011.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.