Abstract
Background
Matrix vesicles (MVs) are released from hypertrophic chondrocytes and from mature osteoblasts, the cells responsible for endochondral and membranous ossification. Under pathological conditions, they can also be released from cells of non-skeletal tissues such as vascular smooth muscle cells. MVs are extracellular vesicles of approximately 100–300 nm diameter harboring the biochemical machinery needed to induce mineralization.
Scope of the review
The review comprehensively delineates our current knowledge of MV biology and highlights open questions aiming to stimulate further research. The review is constructed as a series of questions addressing issues of MVs ranging from their biogenesis and functions, to biomimetic models. It critically evaluates experimental data including their isolation and characterization methods, like lipidomics, proteomics, transmission electron microscopy, atomic force microscopy and proteoliposome models mimicking MVs.
Major conclusions
MVs have a relatively well-defined function as initiators of mineralization. They bind to collagen and their composition reflects the composition of lipid rafts. We call attention to the as yet unclear mechanisms leading to the biogenesis of MVs, and how minerals form and when they are formed. We discuss the prospects of employing upcoming experimental models to deepen our understanding of MV-mediated mineralization and mineralization disorders such as the use of reconstituted lipid vesicles, proteoliposomes and, native sample preparations and high-resolution technologies.
Graphical abstract legend
Matrix vesicles are extracellular vesicles that bind to collagen and can induce formation of apatitic mineral during physiological and ectopic mineralization. Lipid and protein compositions in matrix vesicles resemble those of lipid rafts. Mechanisms of the biogenesis of matrix vesicles and processes leading to mineral/apatite formation are still unclear. Proteoliposomes can serve as biomimetic models to understand matrix vesicle-mediated mineralization.
Keywords: Mineralization, Matrix vesicles, Lipid raft, Proteoliposomes, Atomic force microscopy, Electron microscopy
Graphical abstract
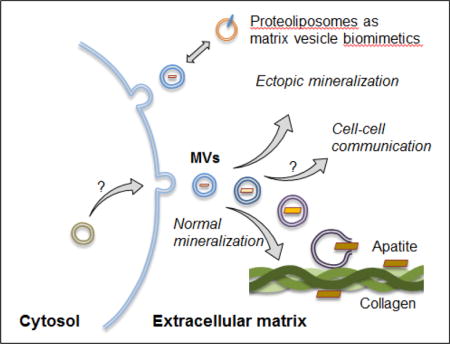
1. Introduction
Matrix vesicles (MVs) are released by hypertrophic chondrocytes and from mature osteoblasts, i.e., the cells responsible for endochondral and membranous ossification [1, 2]. In addition, MVs from odontoblasts contribute to initiate the mineralization of mantle dentin during dentinogenesis [3, 4]. Under pathological conditions, MVs can also be released from non-skeletal tissues such as vascular smooth muscle cells (VSMCs) [5–7]. The similarities and differences between MVs released from skeletal and non-skeletal tissues have been well covered previously [2, 8]. Since there are already excellent reviews on MVs released by skeletal and cartilaginous tissues [8–12], we will focus our discussion on issues that are still controversial in MV biology. This review is addressing a series of questions, all of which seek to stimulate further research on MV function and their clinical utility.
2. Description of MVs
2.1 What do we mean by MVs?
Spherical microstructures can be defined as structures of 40–1,000 nm diameter. Extracellular vesicles (EVs) are membrane-enclosed microstructures which can be broadly classified into at least into four main classes [13], based primarily on their size and presumed biogenetic pathways: (a) apoptotic bodies (ABs) of 500–1,000 nm diameter and released by cells undergoing programmed cell death, (b) microvesicles as membranous vesicles of 50–1,000 nm diameter that are produced by budding from the plasma membrane, (c) exosomes (EXs), 40–100 nm diameter vesicles considered to be of endocytosis origin and, lastly, (d) other types of vesicles including plasma membrane vesicles (PMVs) and organelle membrane fractions (OMFs). MVs are of 100–300 nm diameter [1, 2, 9–11] (Fig 1 and 2), able to bind to collagen in vitro [14–17] and to form apatite within one to three hours in a medium containing 2 mM calcium and 1–2 mM phosphate [18–20]. Three phosphatases have to-date been implicated in the concerted regulation of inorganic pyrophosphate (PPi) as evidenced by studies in genetically modified mice [21–24] and in their isolated MVs [23, 25]. One is orphan phosphatase 1 (PHOSPHO1) present in the lumen of MVs [26], which releases inorganic phosphate (Pi) from phosphocholine, derived from sphingomyelin (SM) by the action of sphingomyelin phosphodiesterase 3 (SMPD3) located at the inner surface of the MV membrane (Fig. 3). Two ectophosphatases, namely, ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1, or NPP1) and tissue-nonspecific alkaline phosphatase (TNAP), act in concert on the outer surface of MVs to regulate the extracellular PPi/Pi ratio. ENPP1 produces PPi as well as Pi from adenosine 5′- triphosphate (ATP), while TNAP hydrolyzes both ATP and PPi to form Pi [27]. Phosphate transporter 1 (PiT-1) helps incorporate Pi into MVs [28, 29] (Fig. 3A), while annexins may be involved in the binding and transport of Ca2+ and in the biophysical process that initiates mineralization in the MV lumen [30] (Fig. 3B). Several members of the vertebrate annexin (AnxA) family (AnxA1, AnxA2, AnxA5, Anx A6 and AnxA7) are present in MVs [31, 32]. They may be found in the lumen, on the inner leaflet of the bilayer in contact with phosphatidylserine (PS), or outside on the surface of MVs (Fig. 3B). AnxA5 [33, 34], AnxA6 or an unidentified calcium channel (UCC) may function as a calcium carrier. Annexins [35] and TNAP [36] also have collagen-binding capacity, a property that may help align MVs along collagen fibers (Fig. 3C) to promote propagation of mineralization onto the extracellular matrix (ECM) scaffold. Fetuin A [37, 38] and osteopontin (OPN) [39–41] are inhibitors of apatite formation and can restrict further propagation of mineralization within the ECM (Fig. 3C). For instance fetuin A has been proposed to be responsible for the restriction of mineralization to the collagen scaffold in bone [42].
Figure 1. Electron transmission micrographs of MVs.
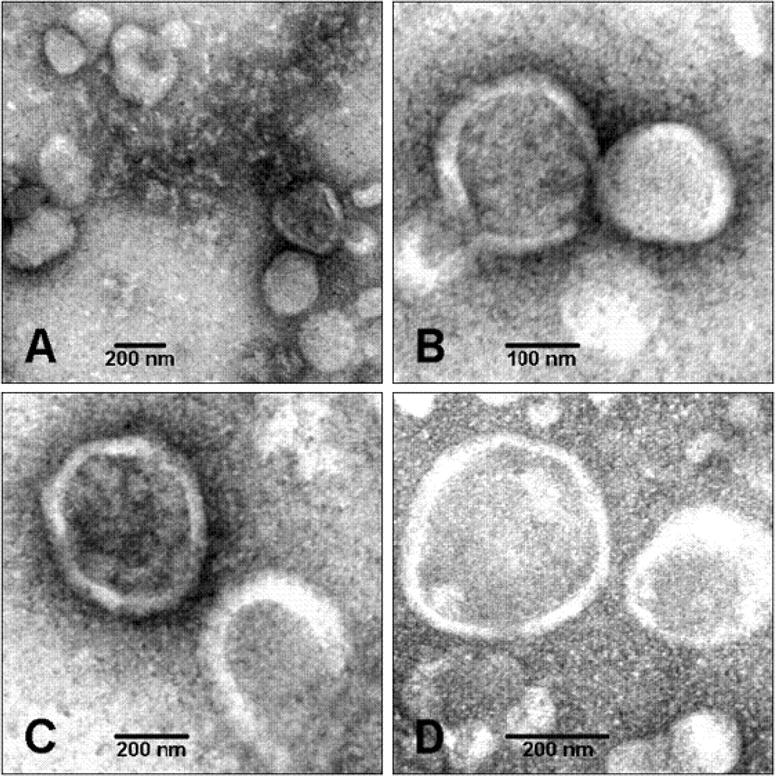
MVs extracted from chicken embryo growth plate cartilages were round structures with a diameter ranging from 100 to 250 nm. (Magnifications: (A), × 53,000; (B), × 100,000; (C), × 75,000; (D), × 100,000). Adapted from [20].
Figure 2. Atomic force microscope image of an MV released by primary chondrocytes.
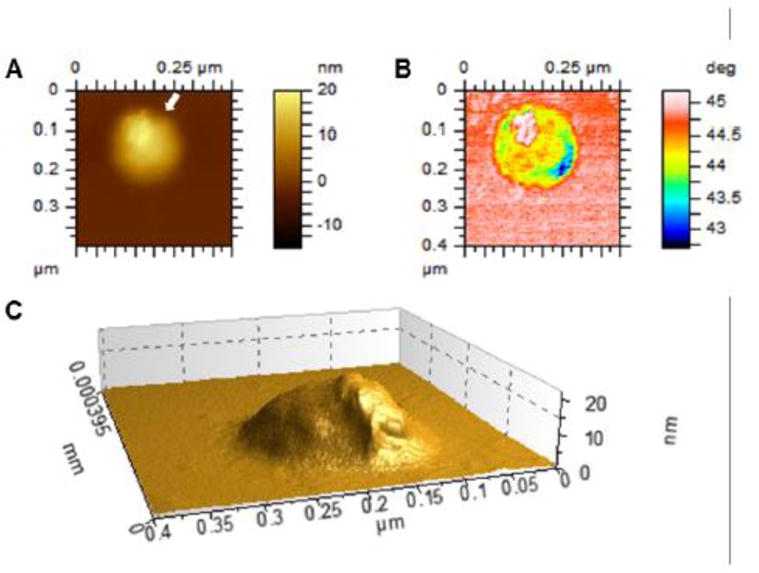
Cells were isolated from WT mouse and grown in differentiation medium (α-MEM with 10% FBS and 50 mg/mL ascorbic acid) for 18 days. MVs were isolated through digestion of the cell monolayer with collagenase followed by two-step differential ultracentrifugation, and dropped onto a freshly cleaved mica substrate. The substrate was dried under vacuum and scanned by means of an AFM (model 5500 AFM, Keysight Technologies, Santa Rosa, CA). (A), topography image; (B), phase image; (C), three-dimensional reconstruction of the topography image along the direction indicated by the white arrow in A.
Figure 3. Schematic depiction of our current understanding of the biochemical pathways involved in MV-mediated initiation of skeletal, dental and vascular mineralization.
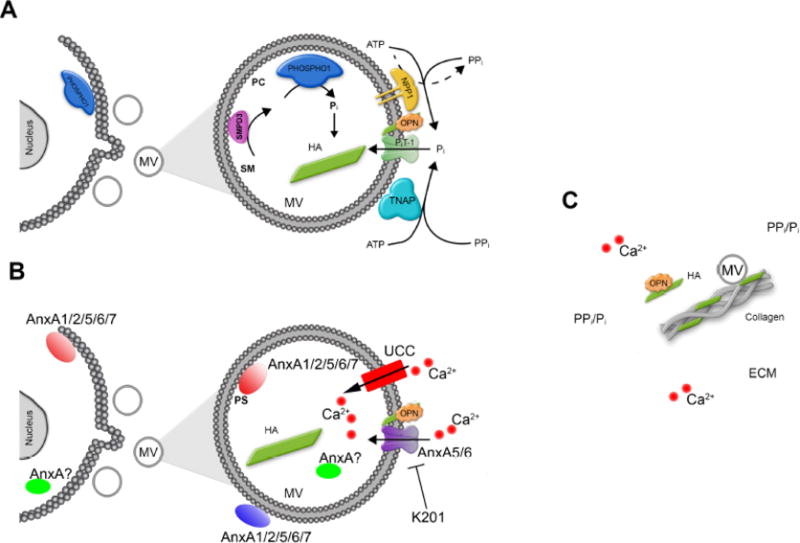
For the sake of simplicity, the main functional components in MVs are divided in two parts: (A), Pi turnover. Currently available data are compatible with the following sequence of events: MVs initiate mineral deposition by accumulation of Pi generated intravesicularly by the action of PHOSPHO1 on phosphocholine (PC) derived from sphingomyelin (SM) by the action of SMPD3, and also via PiT-1-mediated incorporation of Pi generated extravesicularly by TNAP and/or NPP1. The extravesicular propagation of mineral onto the collagenous matrix is mainly controlled by the pyrophosphatase activity of TNAP that restricts the concentration of PPi, a potent mineralization inhibitor, to establish a PPi/Pi ratio conducive to controlled mineralization. How MVs are formed is still unclear but emerging evidence indicates that PHOSPHO1 is involved in MV biogenesis. (B), Ca2+ turnover. Mineral deposition in MVs is initiated by accumulation of Ca2+ intravesicularly by the action of calcium carriers like annexins (AnxA), or unidentified calcium carriers (UCC). Annexins may be located in the lumen of MV or may bind to phosphoserine (PS)-rich membrane domains on the inner surface, or the outer surface of MVs. Some annexins at slightly intracellular acidic pH may protrude MV membrane and form transmembrane like ion channels. Benzodiazepine derivative K201 was known to be a potential inhibitor of annexin calcium channel activity and reduced the ability of the MVs to subsequently mineralize collagen fibers. (C), Propagation of apatite crystals in the ECM. It is unclear how apatite crystals formed within MVs propagate onto the collagenous matrix. The attachment of MV to the collagenous extracellular matrix (ECM) via a number of collagen-binding proteins has been proposed. Osteopontin (OPN), or fetuin A (not shown), which are potent mineralization inhibitors that bind to apatite as soon as it is exposed to the extracellular fluid, further restricts the degree of ECM mineralization.
2.2 Are there several types of MVs?
There is only one type of MVs. During extraction and purification of chondrocyte-released MVs without collagenase, [43–45], usually two bands of membranous layers are visible on Percoll gradients [44], while four bands of membranous layers (Fig. 4) are found in sucrose gradients [45]. The less dense membrane fraction with the highest lipid-to-protein ratio (around 3 mg/mg), the highest specific TNAP activity and the highest ability to form apatite, corresponds to MVs [45]. Addition of collagenase increases the yield of released MVs. There are other types of EVs released into the culture media, which are different from those trapped in the ECM [46–49]. These EVs, called media vesicles by Wuthier [10], include ABs, EXs, PMVs and OMFs, and have different lipid compositions [47] than mineralization-competent MVs. MVs are enriched in cholesterol (CHOL), SM and phosphatidylserine (PS) [10]. Consistent with this, other types of vesicles may have detectable TNAP activity but they usually do not form apatite [45].
Figure 4. Membrane fraction profiles of collagenase-released MVs or of MV enriched microsomes without collagenase treatment.
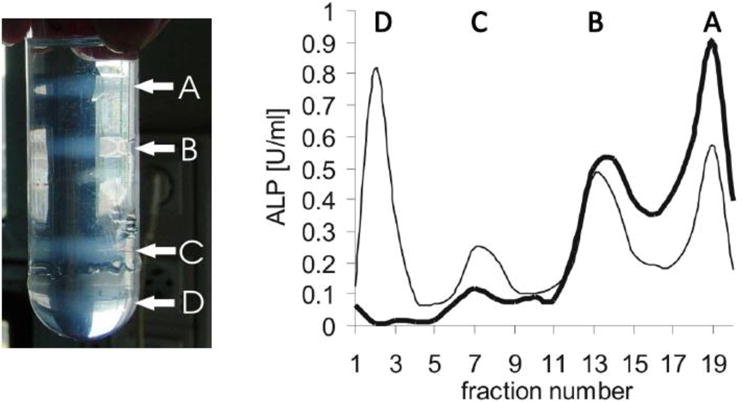
Femurs from 17-old day chicken embryo were washed in synthetic cartilage lymph medium. They were homogenized and subjected with or without collagenase treatments. Then, membrane fractions were isolated during several differential ultracentrifugations. The pellet was subjected by a sucrose gradient composed (from the bottom to the top) of 1.6 M (3 ml), 1.2 M (8 ml), 0.9 M (9 ml), and 0.6 M (9 ml) sucrose dissolved in synthetic cartilage lymph. Then it was centrifuged at 100,000×g for 60 min at 4°C. Left panel: MV enriched microsomes obtained without collagenase treatment indicated four membranous fractions (A-D) after ultracentrifugation. Right panel: Collagenase-treatment released two membrane fractions (A, B, bold line). One fraction (A) corresponds to MVs as indicated by high alkaline phosphatase activity (ALP). Omission of collagenase treatment leaded to four membrane fractions (A, B, C, D, normal line) having less MVs as indicated by lower ALP activity in fraction A. Fraction A was unambiguously assigned to MVs due to its mineralization property. Adapted from [45].
3. Biogenesis of MVs
3.1 At what stages of cellular differentiation are MVs produced?
The biogenesis of MVs coincides with the sequence of events leading to apoptosis or programmed cell death in the case of chondrocytes [50], hence the formation of MVs from hypertrophic chondrocytes [51–55]. Hypertrophic chondrocyte differentiation is associated with high TNAP activity, and the synthesis and secretion of type X collagen following that of type II collagen by proliferating and pre-hypertrophic chondrocytes [17, 51–53]. Expression of type I collagen by hypertrophic chondrocytes might be associated with differentiation into osteoblast-like cells [55–57]. The release of MVs by osteoblasts is stimulated in osteogenic medium containing ascorbic acid and beta-glycerophosphate [58]. Type I collagen and fibronectin matrix deposition in the ECM are required for osteoblast differentiation [59, 60]. Since MVs are produced during the late stages of cell differentiation, this prompted the question of their origin as compared to that of ABs [61]. Available data point to a different provenance of these two vesicular entities [62, 63]. MVs and ABs can probably be formed concomitantly since only mature osteoblasts mineralize their matrix and only terminally differentiated growth plate chondrocytes release MVs [64]. Moreover, ABs might also be involved in mineralization in vitro since inhibition of apoptosis reduced mineralization in cultured hypertrophic chondrocytes [65] and in trans-differentiated VSMCs [66], which are involved in medial vascular mineralization. Immature chondrocytes and osteoblasts, which exhibit low TNAP activity, do not mineralize and therefore EVs produced by these not fully differentiated cells are less likely to have MV characteristics and should not be called MVs.
3.2. How homogenous are MVs?
Most of the controversy about the biogenesis of MVs arises from the fact that EVs remain poorly characterized and from difficulties in obtaining a homogeneous vesicle population. The majority of the purification methods do not yield a single type of EVs [67]. This is also true for the purification of MVs. As pointed out by Wuthier, despite intensive efforts during several decades to optimize methods of extraction [18, 43, 45, 68–74], there is still no perfect method to isolate MVs [10]. The comparison between different extraction methods of MVs including collagenase-treatment with those used to isolate other types of EVs, including EXs, have been already reported [45, 71]. From a historical point of view, the earlier findings on MVs were done using collagenase treatment on material isolated from chicken embryo or rachitic rat epiphyseal growth plates by collagenase treatment, which contained a larger proportion of MVs compared to extraction from primary cells [10]. Furthermore, one of the most challenging aspects of sample preparation is establishing approaches that faithfully preserve biological material in situ in a fully hydrated state. This is of particular importance when handling biological samples like MVs that include membranes, to prevent the collapse of the three-dimensional volume and extraction of the contents upon dehydration. It is tempting to speculate that sample preparations underlie much of controversial issues in MV biology.
3.3 Are MVs released by budding from the plasma membrane?
Based on data from freeze-fracture electron microscopy samples, it was proposed that MVs are released by budding from the plasma membrane of hypertrophic chondrocytes [47, 74–78]. The budding takes place at the polarized apical side of these cells [74, 75]. Microvilli-like membranes were found to be the precursor of MVs [47]. MV release is triggered by depolymerization of the actin cytoskeleton [47]. A proteomic analysis of MVs isolated from growth plates of chicken embryos revealed several markers for microvilli including TNAP, 5′-nucleotidase, annexins [AnxA2, AnxA5, AnxA6)], transporters (e.g., Na+/K+ ATPase, monocarboxylate transporter 1, transient receptor potential channel V member 4), receptors (e.g., scavenger receptor class B, integrin alpha-V, glycoprotein HT7, CD9), cytoskeleton proteins (e.g., actin, actinin, tubulins, radixin), regulatory proteins (e.g., G proteins, protein kinase C), heat shock proteins, alpha-2 macroglobulin and translation elongation factor 2 [31]. Lipidomic analysis of MVs from chondrocytes revealed similar composition of fatty acids in microvilli-like membranes and MVs [79]. In contrast, MVs, as well as microvilli-like membranes, have different lipid compositions compared to those of basolateral membranes [79] or plasma membranes [80], confirming that MVs originate from apical microvilli-like membranes of hypertrophic chondrocytes [80]. Concerning the biogenesis of MVs released from osteoblasts, most of the findings were obtained from human osteoblast-like cells, Saos-2 cells [32, 81] and from MC3T3 cells [82–85] or bone tissues [86, 87]. TEM revealed budding of clustered MVs from MC3T3 osteoblasts that were either disassembled spontaneously or became attached to collagen fibers [85]. In bone tissues, vesicular protrusions arising from areas of the plasma membrane of osteoblasts were detected by immunostaining using a monoclonal antibody against TNAP [86] or by the freeze-fracture method [87] suggesting MV budding. MV formation from Saos-2 cells was stimulated by addition of cytochalasin D, an inhibitor of actin microfilament polymerization, indicating that destabilization in cytoskeletal structures induces MV release from Saos-2 cells [81]. The MV budding occurs at the apical side of microvilli-like membranes of Saos-2 cells since the lipid composition in MVs is very similar to that of apical membrane with relatively high content of CHOL (Table 1), PS and SM (Table 2) [81]. This is corroborated by the enzymatic activities of markers of plasma membrane (TNAP), inner mitochondrial membrane (succinate dehydrogenase), outer mitochondrial membrane and endoplasmic reticulum (NADH oxidase), lysosome (acid phosphatase) and apical membrane (leucine aminopeptidase) (Table 3). MVs have no significant enzymatic activities characteristic of mitochondrial, lysosomal or endoplasmic reticulum membranes (Table 3). However, MVs possess significant enzymatic activity of leucine aminopeptidase as in the case of microvilli, pointing out that MVs from osteoblasts originate from apical microvilli-like membranes, and not from lysosomes nor from the endoplasmic reticulum (Table 3). Employing emerging imaging technologies such as super resolution light or high resolution cryo electron microscopies [88–90] will provide the means to faithfully preserve the sample content as well as provide accurate three-dimensional spatial perspective for these fascinating vesicles in their native environment.
Table 1. Lipid composition of basolateral membranes, microvilli and matrix vesicles.
BLM, basolateral membranes; Microvilli (from apical membranes); MVs, matrix vesicles; TAG, triacylglycerols; FFA, free fatty acids; CHOL, cholesterol; DAG, diacylglycerols; MAG, monoacylglycerols. Chromatogram scans were analyzed by densitometry using ImageJ software. Values are expressed as apparent percentages (intensity of the lipid to intensity of total lipids). (Results are mean ± SD, n=3). Adapted from [81].
|
|
|||
|---|---|---|---|
| BLM | Microvilli | MVs | |
|
| |||
| % of total lipids | |||
| TAG | 24.4 ± 1.6 | 14.8 ± 2.4 | 14.3 ± 1.2 |
|
| |||
| FFA | 23.7 ± 1.9 | 23.6 ± 2.2 | 22.1 ± 1 |
|
| |||
| CHOL | 27.5 ± 1.5 | 45.9 ± 3.6 | 46.7 ± 3.2 |
|
| |||
| DAG | 10.2 ± 1.2 | 10.2 ± 0.8 | 10 ± 0.9 |
|
| |||
| MAG | 14.2 ± 1 | 5.5 ± 0.6 | 6.8 ± 0.9 |
Table 2. Phospholipid composition of basolateral membrane, microvilli-like membranes and matrix vesicles extracted from Saos-2 cells.
BLM, basolateral membranes; Microvilli (from apical membranes); MVs, matrix vesicles; PE, phosphatidylethanolamine; PA, phosphatidic acid; PI, phosphatidylinositol; PS, phosphatidylserine; PC, phosphatidylcholine; SM, sphingomyelin. Chromatogram scans were analyzed by densitometry using ImageJ software. Values are expressed as apparent percentages (intensity of the lipid to intensity of total phospholipids). (Results are mean ± SD, n=3). Adapted from [81].
|
|
|||
|---|---|---|---|
| BLM | Microvilli | MVs | |
|
| |||
| % of total phospholipids | |||
| PE | 38.1 ± 2.6 | 35.9 ± 2.5 | 35.8 ± 1.9 |
|
| |||
| PA | 5.1 ± 1.1 | 3.4 ± 0.3 | 3.5 ± 0.6 |
|
| |||
| PI | 9.9 ± 0.9 | 7.4 ± 1.5 | 6.7 ± 0.3 |
|
| |||
| PS | 9.9 ± 0.4 | 16.4 ± 1.2 | 16.3 ± 1.3 |
|
| |||
| PC | 32.6 ± 1.8 | 26.9 ± 2.2 | 26.2 ± 1 |
|
| |||
| SM | 4.4 ± 0.6 | 9.9 ± 0.9 | 11.5 ± 1 |
Table 3. Enzyme activities in basolateral membrane (pellet B), microvilli and matrix vesicles extracted from Saos-2 cells.
Succinate dehydrogenase (marker of inner mitochondrial membrane), NADH oxidase (marker of both outer mitochondrial membrane and endoplasmic reticulum), acid phosphatase (marker of lysosomes), leucine aminopeptidase (marker of apical membranes) and alkaline phosphatase (marker of plasma membranes and MVs). Pellet A stands for cell debris, pellet B corresponds to basolateral membranes, supernatant C corresponds to microsomal and cytosolic fractions, pellet 2 corresponds to cell debris and supernatant 3 stands for extracellular matrix (see Fig. 4). ND: not detectable; E: enrichment. (Results are mean ± SD, n=3). Adapted from [32].
| Digest | Pellet A | Pellet B | Microvilli | Supernatant C | Pellet 2 | MVs | Supernatant 3 | ||
|---|---|---|---|---|---|---|---|---|---|
| Proteins | mg | 30.4 ± 0.1 | 12.2. ± 0.8 | 2.4 ± 0.6 | 0.5 ± 0.1 | 5.8 ± 1.1 | 2.2 ± 0.2 | 0.7 ± 0.2 | 7.7 ± 1.7 |
| % | 100 | 40 | 8 | 2 | 19 | 7 | 2 | 25 | |
|
| |||||||||
| Succinate dehydrogenase | Umg−1 | 2.1 ± 0.1 | 4.3. ± 0.8 | 2.3 ± 0.4 | ND | ND | 2.1 ± 0.2 | ND | ND |
| % | 100 | 80.7 | 8.7 | ND | ND | 7 | ND | ND | |
| E | 1 | 2 | 1.1 | ND | ND | 1 | ND | ND | |
|
| |||||||||
| NADH oxidase | Umg−1 | 13.5 ± 1.2 | 24.4. ± 4.1 | 20.1 ± 5.8 | 5.6 ± 1.0 | 8.1 ± 2.2 | 7.2 ± 1.1 | 1.9 ± 0.6 | ND |
| % | 100 | 72.6 | 11.8 | 0.6 | 11.5 | 3.8 | 0.3 | ND | |
| E | 1 | 1.8 | 1.5 | 0.4 | 0.6 | 0.5 | 0.1 | ND | |
|
| |||||||||
| Acide phosphatase | Umg−1 | 2.3 ± 0.2 | 4.4. ± 0.3 | 2.6 ± 0.6 | 1.1 ± 0.2 | ND | 4.42 ± 0.8 | 0.7 ± 0.02 | ND |
| % | 100 | 79 | 8.7 | 0.7 | ND | 12.7 | 0.7 | ND | |
| E | 1 | 2 | 1.2 | 0.5 | ND | 1.8 | 0.3 | ND | |
|
| |||||||||
| Leucine aminopeptidase | Umg−1 | 1.0 ± 0.1 | 0.7. ± 0.1 | 1.2 ± 0.2 | 6.8 ± 0.8 | 0.8 ± 0.8 | 2.1 ± 0.3 | 5.3 ± 0.9 | 0.5 ± 0.1 |
| % | 100 | 28.5 | 9.4 | 9.7 | 15 | 14.6 | 11.8 | 11.3 | |
| E | 1 | 0.7 | 1.2 | 6.6 | 0.8 | 2 | 5.1 | 0.5 | |
|
| |||||||||
| Alkaline phosphatase | U Umg−1 | 8.4 ± 0.4 | 3.2 ± 0.2 | 7.1 ± 0.2 | 110 ± 6 | 2.1 ± 0.4 | 14.1 ± 2.4 | 121 ± 13 | 1.22 ± 1 |
| % | 100 | 15 | 6.7 | 19.3 | 4.9 | 11.9 | 33.4 | 3.7 | |
| E | 1 | 0.4 | 0.9 | 13.1 | 0.3 | 1.7 | 14.4 | 0.1 | |
3.4 What are the differences between EXs and MVs?
The biogenesis of EXs differs from that of MVs [84, 85]. EXs are formed during endocytosis from plasma membrane, are broken into smaller vesicles and repackaged into larger multivesicular structures in the cytoplasm of the cells and finally they are secreted to the extracellular medium [85]. We cannot exclude the possibility that mineral-competent cells can produce MVs and EXs concomitantly, together with ABs and other types of vesicles. EXs (or other types of EVs purified by a two-three ultracentrifugation steps) and MVs (collagenase treatment followed by a two-three ultracentrifugation steps) have distinct properties [45, 71]. MVs bind to surrounding ECM [14–17], while EXs do not bind to the ECM [12]. Comparisons based on the same material (growth plates of femurs and epiphyseal cartilage from chicken embryo) between the procedure of extraction of EXs (without the use of collagenase) and the procedure of extraction of MVs (with the use of collagenase) yielded different types of vesicles, having distinct mineralization properties [45]. After sucrose gradient fractionation, four distinct membranous fractions were obtained following the usual extraction method of EXs (Fig. 4), while two membrane fractions were obtained after collagenase treatment [45]. The less-dense membrane fraction had the highest TNAP activity, highest CHOL content and the highest mineralization rate as compared to the other membrane fractions [45]. In contrast, the four membrane fractions obtained by sucrose gradient from the usual extraction method of EXs, yielded membrane fractions having less TNAP activity and poor mineralizing properties compared to collagenase-released MVs [45]. Thus, the method of extraction of EXs and the method of extraction of collagenase-released MVs produced distinct types of vesicles from the same bone tissues [45], confirming that several types of EVs can be produced by mineralization-competent cells. This does not rule out the possibility of a selective apical targeting of lipids and proteins from the trans-Golgi network or from the endosomal compartments to MV membranes, prior to MV budding. However, MVs which are obtained after collagenase treatment are distinct from EXs (obtained without collagenase treatment). Skeletal mineralization takes place through a carefully orchestrated process thought to begin within the confines of the chondrocyte-containing growth plate. MVs then bind to the ECM via collagen fibers that serve as a scaffold. Available biochemical and genetic data are compatible with this sequence of events. However, it is unclear how MVs are formed and little is known about how the apatite crystals are formed within MVs, how they are propagated from the MVs onto the collagenous matrix and how the apatite or other types of minerals are propagated. While in vitro apatite formation can proceed via a chemical transition from amorphous calcium phosphate (ACP) during the induction phase to octacalcium phosphate (OCP) during the rapid acquisition stage and finally to apatite during the plateau phase [10], these transitions have never been observed in the ECM in vivo.
4. Properties and functions of MVs
4.1 Do MVs correspond to lipid raft structures?
MVs isolated from growth plate cartilage have a higher content of CHOL, SM, and PS [45, 80, 91] and longer fatty acids [79] than EVs isolated from homogeneous membranes. Among the usual protein markers of lipid rafts [92], AnxA6, carboxypeptidase M, H+ ATPase, G-proteins, and TNAP are found in MVs isolated from epiphyseal growth plate cartilage [31]. Proteomic analysis of MVs isolated from osteoblast-like Saos-2 cells indicated the presence of glycosylphosphatidylinositol (GPI) anchored, myristoylated and palmitoylated proteins targeting sphingolipid- and CHOL-enriched membranes [32]. MVs from osteoblasts are also enriched in CHOL, SM and PS (Tables 1 and 2). The fact that MVs are enriched in SM is significant as SM, via the enzymatic action of SMPD3, is converted to phosphocholine that is then used by PHOSPHO1 to produce Pi intravesicularly. Importantly, insufficient PHOSPHO1 activity leads not only to suppressed MV-mediated mineralization but also to severely reduced MV biogenesis [29, 93, 94]. SMPD3 has also been implicated in the production of vesicles by VSMCs [95], so it seems that SM metabolism may be a crucial component of MV biogenesis. The lipid-raft characteristics [96, 97] of MVs indicate that MVs from both chondrocytes and osteoblasts originate from lipid raft domains. MVs can be considered as a model of lipid raft domain due to their selective lipid and protein recruitments, with the additional advantage of being extracted without detergent.
4.2 Are MVs necessary to induce mineralization?
It is generally agreed that formation of apatite as well as in vitro mineralization necessitates a nucleation process [1, 98–100]. Calcium (~1–2 mM) and phosphate (~1–2 mM) concentrations are metastable under physiological conditions [101] and facilitate formation of calcium phosphate complexes. Several inhibitors of mineralization including PPi [102], phosphorylated osteopontin (OPN) [103], matrix Gla protein [104, 105], and fetuin A [106] can prevent apatite formation. The formation of apatite is preceded by a nucleation step [1, 98–101, 107] and/or by an intermediate formation of ACP [100, 101, 108, 109]. Apatite in a crystalline phase is induced by a nucleation step, even if it is generated from ACP. The nucleation can be induced without MVs in vitro by chemical agents such as citrate [110, 111], dimethyl sulfoxide [112] and other natural pro-nucleation agents such as positively charged amino acids [113], aspartic acid [113, 114], acidic carboxyproteins and phosphoproteins [115–117], bone sialioprotein [118], proteins of the enamel matrix (ameloblastin, amelogenin, dentin sialophosphoprotein, enamelin, tuftelin) [119] and hydrophobic fusion protein [120]. However, the contribution of these proteins in mineralization in vivo is highly hypothetical. Lipids, especially PS [121, 122] and CHOL [123, 124] may also participate in the nucleation of apatite, which indicates that vesicles other than MVs may have the ability to induce the nucleation of apatites. On the other hand, MVs concentrate a very high TNAP activity [10, 125, 126]. Also, an adequate supply of intravesicular Pi to initiate mineralization is ensured by a dual mechanism, i.e., accumulation of Pi generated intravesicularly by the action of PHOSPHO1 on PC derived from SM and by PiT-1-mediated incorporation of Pi from extracellular medium and generated extravesicularly by TNAP and/or NPP1 (Fig 3A). The extravesicular propagation of mineral onto the collagenous ECM is mainly controlled by the pyrophosphatase activity of TNAP that restricts the concentration of the potent mineralization inhibitor PPi by maintaining a Pi/PPi ratio conducive to controlled mineralization. Indeed, the Pi/PPi ratio during biomineralization is a turning point between physiological and pathological mineralization [127, 128]. With a Pi/PPi ratio above 142, MVs induce the formation of apatite only. When the ratio is between 24 and 124, MVs induce poorly crystalline apatite as well as other types of minerals but not calcium pyrophosphate dihydrate (CPPD, Ca2P2O7 × 2 H2O). When the ratio is below 24, MVs induce CPPD [20]. Excess of PPi can lead to CPPD deposits in cartilage as seen in chondrocalcinosis [129] or in acute calcium pyrophosphate crystal arthritis, often referred to pseudo-gout [130, 131]. Additionally, phosphorylated OPN, another potent mineralization inhibitor that binds to apatite mineral as soon as it is exposed to the extracellular fluid [132], further restricts the degree of ECM mineralization (Fig. 3C). Furthermore, MVs bind strongly to collagen [14–17] contributing to the propagation of mineralization (Fig. 3C). Taken together, these facts indicate that MVs are involved with the initiation of the mineralization process [10] in an efficient manner. At least in vitro, other types of vesicles [98], including unidentified types of vesicles [133], intracellular vesicles [98] or natural pro-nucleating agents [113–120] as well as necrotic cells and cellular remnants [98] may also contribute to the mineralization process. All these patterns of mineralization are the result of different activities of one cell type [98].
4.3 Why and how can MVs bind to collagen?
Chondrocytes are able to synthetize an ECM rich in type II collagen [51–53], while osteoblasts produce a type I collagen rich ECM. Type I collagen and fibronectin matrix deposition are required for MC3T3-E1 osteoblast differentiation [59, 60]. Co-expression of TNAP and a fibrillar collagen are required to induce mineralization [134]. TNAP can bind to collagen contributing to maintain MVs linked to the ECM [135]. MVs released by chondrocytes [31] and by osteoblast-like cells [32] contain calcium-binding proteins of the annexin family: AnxA2, AnxA5 and AnxA6. MVs from chondrocytes can bind to type II collagen and to type X collagen [16]. AnxA5 [136] and proteoliposomes in the presence of AnxA5, but not in the presence of AnxA2 or AnxA6 [17], can bind to type II and type X collagens. However, AnxA5 and AnxA6 may not represent the essential annexins that promote mineralization in vivo [137, 138]. Neither the development of skeletal elements nor the in vitro mineralization properties of isolated chondrocytes is significantly impaired by the absence of AnxA5 [139, 140]. Therefore, AnxA5 is dispensable for the formation and maintenance of skeletal elements in the mouse, which points to a possible compensatory effect of other members from the annexin family due to their high functional and structural similarity. Consistent with this hypothesis, primary chondrocyte cultures isolated from rib cartilage of newborn AnxA6−/− mice showed delayed terminal differentiation as indicated by reduced terminal differentiation markers, including TNAP, matrix metalloproteinase-13, osteocalcin and Runx2, and reduced mineralization [141]. Besides AnxA2, AnxA5 and AnxA6, also AnxA1 and AnxA7, but not AnxA4, are selectively enriched in MVs of Saos-2 cells upon stimulation for mineralization [142]. So far, AnxA2, AnxA5, AnxA6 and TNAP are among the very few known proteins of MVs able to bind collagen (Fig. 3C). This does not exclude the possibility that other MV proteins may bind to collagen since there is a strong association of MVs with collagen [14–17]. Collagenase-treatment of growth plate tissues releases a large amount of functional MVs, while omission of collagenase-treatment leads to vesicles displaying poor mineralization ability [45].
4.4 What are the functions of MVs?
A well-established function of MVs is the initiation of apatite formation due to their high TNAP activity that hydrolyzes extracellular PPi, a physiological inhibitor of apatite formation [1, 2, 8–10]. It was recently speculated that MVs might mediate cell signaling [143]. The presence of bone morphogenetic proteins (BMP-2 and BMP-4) ascertained by Western Blot analyses of MVs indicated that MVs may serve as a carriers of morphogenetic information to nearby chondrocytes and osteoblasts [143]. A similar line of reasoning might apply to many other MV proteins, among them TNAP, which can stimulate not only mineralization but may also affect cell differentiation. The presence of microRNA (miRNAs) in MVs [144] suggests that MVs can function as signalosomes in cell-cell communication during cartilage and bone development via transfer of specific miRNAs. However, it remains to be determined whether such cell-cell communication occurs in vivo. The average life span of MVs as determined in vitro is one-to-three hours [18–20]. Once apatite forms, MV membranes break, releasing their lumen content, and possibly also signaling molecules. Secondly, MVs bind to collagen [14–17] produced by the cells from which they originate, preventing long-distance displacement. Thirdly, MVs are probably surrounded by many dying cells from which they were released during late stages of differentiation [50–54, 58]. Taken together, these findings suggest that cell-cell communication mediated by MVs, if it exists, would be of a limited occurrence and of a short distance, i.e., rather autocrine or paracrine in nature. This does not exclude the possibility that other types of EVs participate in cell-cell communications during differentiation and maturation of cells [145]. Chondrocytes can produce EVs including EXs and microvesicles, which may act as mediators in cell-to-cell communication by transporting various proteins, miRNAs and mRNAs [146]. It was proposed that EXs participate in communication networks integrating bone cells (osteoclasts, osteoblasts, osteocytes) as well as to other tissues [147]. The identification of proteins, miRNAs or other molecules in MVs and/or in EVs, isolated from healthy and diseased tissues, and comparison of their contents will reveal markers of human diseases and possible components of cell-to-cell signaling.
4.5 When is apatite formed?
Accumulation of Ca2+ and Pi was observed by X-ray micro-analytical mapping of chondrocyte surface blebs suggesting that initial Ca2+/Pi complexes and apatite are formed by chondrocytes [148]. Similar findings were observed in osteoblast-like Saos-2 cells (Fig. 5). Saos-2 cells [149] and osteoblasts [150] produced vesicles containing electron-dense material as probed by TEM. Ca2+ and Pi were mostly co-localized inside vesicles as monitored by X-ray analysis (Fig. 5) indicating possible formation of apatite or Ca2+/Pi complexes (Fig. 5). One possible mechanism of transporting Ca2+ despite the very low Ca2+ concentration in the cytoplasm, assumes that AnxA5 may act as a carrier protein and transport Ca2+ from mitochondria and/or other organelle to the inner leaflet of the plasma membrane where it binds to PS. In vitro modeling confirmed the possibility of inducing the formation of Ca2+/Pi complexes in proteoliposomes containing PS, AnxA5, Ca2+ and Pi [151]. It is tempting to suggest that Ca2+, Pi, AnxA5 and PS complexes, accumulated in lipid rafts within microvilli-like membranes, constitute the MV nucleation core. Of note, analysis of neonatal mouse calvaria osteoblasts by high angle-annular dark-field scanning TEM and electron-loss spectroscopy revealed the presence of dense materials containing Ca2+ and Pi in intracellular vesicles (IVs) and in mitochondria [152]. It was speculated that a direct transport of Ca2+ and possibly Pi between mitochondria and IVs toward the ECM might initiate mineralization. However, this speculation is not substantiated by experimental facts. Indeed, there are many unanswered questions: How and where are IVs formed? Are they guided to the apical side of the cells? Do they contain TNAP and, eventually, is TNAP anchored inside? What are the lipid and protein compositions of the IVs? Do they target plasma membranes or organelle membranes or are directly exocytosed? On the other hand, MVs extracted from chondrocytes in medium without Ca2+ revealed a population of MVs devoid of mineral deposits [45], suggesting that MVs may bud off from chondrocytes without formation of apatite. One likely mechanism is that either empty MVs or MVs with pre-formed NC are released from the apical microvilli-like cell membrane. Once in the extracellular medium, MVs accumulate Ca2+ and Pi ions to initiate the nucleation process giving rise to apatite [1]. Either mechanical stress or actions of phospholipases within MVs may contribute to the breaking of MV membranes [153] enabling the release of apatite from MVs to extracellular medium and spreading mineralization in the vicinity of collagen [1].
Figure 5. Transmission electron microscopy coupled with X-ray microanalysis of osteoblast like Saos-2 cells.
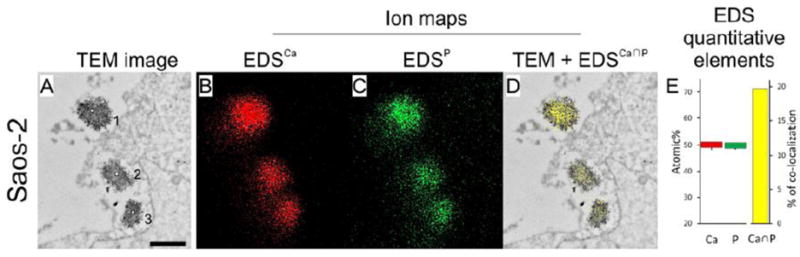
Saos-2 osteosarcoma cells were stimulated for mineralization by treatment with 50 μg mL−1 ascorbic acid and 7.5 mM beta-glycerophosphate for 7 days. Cells were washed in physiological desensitization medium, fixed with paraformaldehyde/glutaraldehyde mixture for 1h and postfixed with osmium tetroxide for 20 min. After dehydration in ethanol series probes were embedded in LR White resin at 56°C for 48 hrs. Finally the samples were cut with ultramicrotome for 700Å sections and counterstained with uranyl acetate for 1h followed by lead citrate for 2 min. The cells with vesicles were observed under high performance TEM JEM-1400 (JEOL Co., Japan) with a magnification of x50,000 (A, bar 500 nm) and elemental maps (B, Calcium, red and C, Phosphorus, green) were performed using energy-dispersive full range X-ray microanalysis system EDS INCA ENERGY TEM (Oxford Instruments, UK). Presence of calcium and phosphoreus were evidenced in vesicles containing dense materials. Co-localization of both elements (D, yellow) and quantitative determinations (E, Calcium, red, Phosphorus, green, Calcium to Phosphorus, yellow), provided evidence of calcium and phosphorus deposition inside vesicles, suggesting apatite deposition in MVs. Adapted from [149].
4.6 How is the luminal content of MVs established?
Proteomic analysis of chondrocytes [31] and of osteoblast-like cells [32] revealed the presence of some cytoplasmic proteins in MVs. Just to mention a few, there are those implicated in glucose and energy metabolism such as aldolase A, enolase 1, creatine kinase, glucose phosphate isomerase, lactate dehydrogenase A, phosphoglycerate kinase, pyruvate kinase, and those involved in cytoskeleton elements such as beta-actin, filamin B, actinin alpha-1, actinin alpha-4, tubulin, radixin, plastin, gelsolin and myristoylated alanine-rich C-kinase substrate. Considering the proximity of genesis of MVs near cytoskeletal structures and microvilli-like membranes, we hypothesize that the proteins localized close to the actin cytoskeleton in the vicinity of microvilli-like membranes may be captured by MVs during exocytosis. Indeed most of the annexins found in MVs are colocalized with actin [154–158]. Several enzymes related to energy metabolism and to the actin cytoskeleton found in MVs are markers of microvilli [31]. There is, probably, a specific recruitment of proteins toward the actin cytoskeleton and microvilli-like membranes due to their respective affinities. Such specific recruitment of proteins may perform particular functions prior to MV release. The question remains as to whether or not these cytosolic proteins perform their function also in the lumen of MVs and if cells finely control their recruitment.
5 Functions of MVs during pathological mineralization
5.1 Is TNAP activity in MVs essential for mineralization?
TNAP activity is a straightforward indication of the ability of cells to mineralize the ECM. Therefore, TNAP activity in MVs often correlates with mineralization ability of mineralization competent cells. In humans, the severe inherited absence of functional TNAP leads to perinatal death of fetuses devoid of minerals in their skeleton [159]. On the other hand, the ablation of the gene encoding for TNAP in mice does not prevent completely the ability of chondrocyte-derived and osteoblast-derived MVs to mineralize [29, 160, 161].
5.2 What are the characteristics of pathological mineralization?
Pathological mineralization concerns not only skeletal but also non-skeletal tissues [1, 2, 5–8, 162]. They are manifested by apatite deposition, often mediated by MVs [163], in soft tissues of tendons and/or ligaments (calcific tendinitis and ankylosing spondylitis) [164], in arterial media, in vascular mineralization induced by chronic kidney disease [165, 166] or by type 2 non-insulin-dependent diabetes mellitus [167, 168], in atherosclerosis [5, 7, 169, 170], and in articular cartilage [171]. Ectopic deposition of calcium-containing crystals, mostly comprising calcium pyrophosphate dehydrate (CPPD), basic calcium phosphate (BCP) and apatite, is observed in soft tissues during the development of articular and cardiovascular diseases.
5.3 What are the characteristics of ectopic calcium-containing mineral deposits by MVs in osteoarthritis?
Ectopic deposition of calcium-containing crystals occurs in the midzone of the articular cartilage ECM and periarticular soft tissues in several articular diseases. CPPD crystals are almost exclusively located in cartilage ECM and observed in up to 20% of patients with chronic pyrophosphate arthritis at the time of joint replacement [172, 173], in 25% of patients with acute gout-like episodes of inflammation (pseudo-gout), in approximately 5% of patients with rheumatoid arthritis (RA) and in less than 5% of patients with neuropathic osteoarthropathy [174]. BCP and apatite crystals are more widely distributed and found in more types of calcific deposits than CPPD. The presence of BCP and apatite crystals closely relates to OA severity and they are very common in joint fluid and cartilage of patients with end-stage disease [172]. Indeed, calcium-containing crystals are present in the synovial fluid extracted from the knee joints of up to 70 % of OA patients [175]. Deposits of BCP are also present in periarticular soft tissues and can elicit tendonitis, bursitis and acute calcific periarthritis [174]. Despite the acknowledged role of calcium-containing crystals in OA, their precise role in pathogenesis is still not known. Ali observed MVs in OA cartilage (articular cartilage vesicles or ACVs) associated with “cuboid” crystals and mineral nodules, but not with needle-shaped apatite crystals, approximately thirty years ago for the first time [176]. Since then several reports demonstrated the ability of ACVs to release crystals in OA, but not in normal cartilage [177–179]. Under physiological conditions, ACVs originate from autophagosomes and are externalized through membrane blebbing in a caspase-3 and Rho/ROCK-dependent way [180]. Nevertheless, OA chondrocytes have been demonstrated to have defective autophagic machinery, thus other mechanisms other than autophagy may regulate ACV and/or MV biogenesis and release in OA joints [181]. Although clear evidence is still lacking, crystal deposition by ACVs has been postulated to be controlled by the PPi/Pi ratio through the same network of enzymes (e.g., TNAP, NPP1, PHOSPHO1 and SMPD3) and membrane transporters (e.g., PiT1) regulating mineral generation by MVs in growth plate cartilage. Indeed, based on in vitro observations, CPPD and BCP or apatite crystals cannot be formed at the same time and same place due to the Pi/PPi ratio [127, 128, 182] regulated by the activities of antagonistic enzymes such as TNAP and ANK or NPP1 [183]. It was suggested that CPPD crystals may form in places distinct from those typical for BCP and apatite crystal formation or may appear sequentially during ageing [163, 182]. Therefore a distinction between CPPD deposits and BCP or apatite deposits is warranted due to their distinct conditions of formation. Recent works showed that the interaction of calcium-containing crystals with chondrocyte toll-like receptors (TLRs) regulates the composition of cartilage ECM [184]. TLR activation leads to increased expression of the pro-inflammatory cytokine interleukin-1 beta (IL-1beta) and matrix metalloproteinases (MMPs) such as a MMP1 and MMP13. These enzymes directly or indirectly alter cartilage ECM composition by decreasing proteoglycans and type-II collagen content and increasing type-I collagen content, as in the case of OA. Calcium-containing crystals may also contribute to the composition of cartilage ECM by affecting the secretion of other types of EVs, including exosomes and non-mineralizing microvesicles, which act as immunomodulators [185]. Conversely, the composition of cartilage ECM regulates the release of calcium-containing crystals by ACV. Rosenthal et al. showed that the presence of type I collagen in the matrix surrounding ACVs stimulates the release of CPPD and BCP crystals from vesicles treated with ATP and beta-glycerophosphate, respectively, whereas the presence of type II collagen and proteoglycans inhibited ACV-induced mineralization [186]. Additionally, studies of mineralization on non-articular cells showed that IL-1beta induces mesenchymal stem cells to undergo a massive mineralization driven by a decrease in the activity of NPP1 [187] and/or a stimulation of TNAP [188]. IL-1beta might regulate ACV mineralization in OA joints through a similar mechanism. Taken together, these studies suggest an important role of ACVs in OA development due to ability of ACV-released calcium-containing crystals to induce a positive feedback loop of events that ultimately leads to joint inflammation and cartilage degradation and altered biomechanics.
5.4 What are the characteristics of MVs in ectopic vascular mineralization?
Ectopic vascular mineralization involves accumulation of apatites in the medial and intimal layers of the wall of blood vessels and is common in the elderly and patients with chronic kidney disease (CKD), atherosclerosis and diabetes mellitus [5]. The earliest steps of this process are thought to be driven by a network of tightly regulated events resembling that regulating normal endochondral mineralization of bone. Indeed vascular cells can transdifferentiate to an osteogenic phenotype and form nodules for the deposition of BCP crystals in the form of apatites. Microvascular pericytes, human aortic valve interstitial cells (ICs), vascular smooth muscle cells (VSMCs) and multipotent vascular mesenchymal progenitors are among the cells capable of osteoblastic differentiation [66, 189–191]. Similar to endochondral mineralization of bone, vascular mineralization may be mediated by MV-like bodies [95]. Several in vitro and in vivo experiments showed that both healthy (proliferative) and mineralizing VSMCs release MV-like bodies [166, 192, 193]. The mechanism of release of MV-like bodies from VSMCs is not fully understood. The presence of vesicles in the vessels of children with CKD on dialysis was ascertained and were in the range size of 0.1 to 1 μm, filled with HA crystals and budding from VSMC plasma membrane, consistent with their classification as MVs [194]. Although they share similarities with osteoblast-released MVs with enrichment of proteins implicated in mineralization and bone development (e.g., annexins A1, A5 and A6, collagens type-I, V, VI and XII), MV-like bodies released from VSMCs were 100-nm in diameter and expressed the exosomal tetraspanins CD9 and CD63 on the surface [95]. These data suggested that VSMCs can release more than one class of mineralizing vesicle during vessel mineralization. MVs released by healthy VSMCs do not mineralize because of the presence in their lumen of mineralization inhibitors, such as vitamin K-dependent and apatite-binding protein, matrix gamma-carboxyglutamic acid (GLA) protein (MGP) and fetuin A [195–197]. However an increase in Ca2+ and Pi leads to VSMC apoptosis, formation of apatite nodules and increased local Ca2+ concentration. This, in turn, stimulates SMPD3 expression, which further promotes mineralization by increasing the release of MVs from VSMCs, by accumulation of uncarboxylated MGP and by blocking fetuin A loading [198]. Accumulation of uncarboxylated MGP and the block of fetuin A loading contribute both to vascular mineralization by VSMCs transdifferentiated to an osteogenic phenotype. Remarkably, previous genetic reports have revealed a decrease in expression and in nucleoside triphosphate pyrophosphohydrolase activity of NPP1 is associated with apatite deposition in a 25-month-old boy with idiopathic infantile arterial calcification (IIAC) [199, 200]. These studies also note the absence of an effect of NPP1 transfection in VSMCs on cell proliferation in vitro. Although the effect of NPP1 deficiency on VSMC phenotype was not validated in vivo, these studies shed the light on an alternative pathway of vascular mineralization that is driven by the deficiency of mineralization inhibitors and is independent of and/or preceding VSMC osteogenic trans-differentiation.
Key osteogenic markers, including TNAP, PHOSPHO1 and PiT1 also play a critical role in regulating the process of VSMC mineralization [201–203]. VSMC-driven arterial mineralization is aided by an additional mechanism in patients with CKD. During initial stages of atherosclerotic plaque mineralization, inflammation recruits macrophages, which, upon Ca2+/Pi stimulation, release mineralizing membrane-bound vesicles expressing CD9, tumor susceptibility gene 101 (TSG101) and PS-S100A9-AnxA5 membrane complexes [204].
6. Can MVs be mimicked by proteoliposomes?
Proteoliposomes provide a means of reconstituting lipid vesicles [205, 206] that function like MVs, making these structures an advantageous and convenient experimental model to understand MV-mediated mineralization. They may be constituted of a single type of lipid or a mixture of lipids [207], with proteins and/or electrolytes [208–216]. Liposomes mimicking biomembranes [25, 29, 210, 211, 213–220] and multilamellar liposomes [221–224] stimulating MV mineralization have been described. Since no proteins responsible for Ca2+ uptake into MVs have been unequivocally identified, it was proposed that one member of the annexin family could be responsible. Liposomes containing anionic and neutral phospholipids, CHOL and AnxA5 served as a model of Ca2+ uptake and confirmed the hypothesis that AnxA5 may participate in Ca2+ influx in MVs [29, 30]. Insertion of TNAP into dipalmitoylphosphatidylcholine (DPPC), CHOL, SM and ganglioside liposomes [214, 225] resulted in lateral phase segregation with the formation of CHOL-rich microdomains, typical of lipid rafts. The same phenomenon was observed by fluorescence microscopy in giant liposomes (Fig. 6A) and giant TNAP-harboring proteoliposomes (Fig. 6B) consisting of dioleoylphosphatidylcholine (DOPC), CHOL and SM. The presence of the enzyme fluidified the vesicles, while the gradual increase in the complexity of the vesicles decreased the activity of incorporated TNAP [214]. Electron spin resonance measurements with spin labeled phospholipids showed that GPI-anchored-TNAP probably localizes close to the membrane surface [220], which can be related to the modulation of TNAP activity by the lipid composition of the vesicles as previously reported [29, 205, 210, 211, 213, 214]. Comparisons of proteoliposomes and osteoblast-derived MVs or MVs deficient in TNAP, NPP1 or PHOSPHO1, using natural substrates such as ATP, adenosine 5′-diphosphate (ADP) and PPi confirmed the validity of proteoliposome models [23]. TNAP- and PHOSPHO1-deficient MVs showed reduced mineralization ability, while NPP1-deficient MVs hypercalcified. This demonstrates that the cooperativity as well as the competition of TNAP, NPP1 and PHOSPHO1 for the biomineralization substrates provides an additional level of regulation of metabolite flow for the control of the mineralization process. The size of DPPC proteoliposomes harboring TNAP is around 300 nm, as determined by dynamic light scattering [213, 219], and this is comparable to the median size of natural MVs [210, 219]. Therefore such DPCC proteoliposomes can adequately serve as a model to examine TNAP function in the context of a lipid membrane environment that mimics the MV environment. TEM of empty DPPC liposomes (Fig. 6C) and of TNAP-proteoliposomes (Fig. 6D) showed that enzyme reconstitution did not affect the morphology of the liposomes [211]. The NC consisted mostly of AnxA5, amorphous calcium phosphate complexes such as Ca3(PO4)2 and PS [18, 72]. These findings contributed to the design of proteoliposome models containing PS, Ca2+ and Pi complexes. Addition of other lipids such as PE or SM with PS strongly inhibited the nucleation activity while addition of AnxA5 promoted it [151, 226, 227]. Proteoliposomes formed by PS, Ca2+ and Pi containing either TNAP, NPP1, or both together induced mineral formation when incubated in synthetic cartilage lymph containing 1 mM ATP as substrate; but the induction of mineralization rate was equivalent at pH 7.5 and 8, whereas it was considerably less at pH 9 [212]. Yet, proteoliposomes harboring both TNAP and NPP1 triggered significantly more extensive Pi-dependent mineralization than TNAP-proteoliposomes, suggestive of additive hydrolytic activities for NPP1 and TNAP. In DPPC and DPPC:diphosphatidylserine (DPPS) -proteoliposomes harboring AnxA5 and TNAP, the presence of AnxA5 affected the hydrolysis of TNAP substrates at physiological pH [29]. AnxA5 was able to mediate Ca2+-influx into the DPPC and DPPC:DPPS 10%-vesicles at physiological Ca2+ concentrations (~2 mM). This process was not affected by the presence of TNAP in the proteoliposomes. These findings indicated that proteins such as AnxA5 and lipids could modulate TNAP activity.
Figure 6. Characterization of liposomes and proteoliposomes by different techniques.
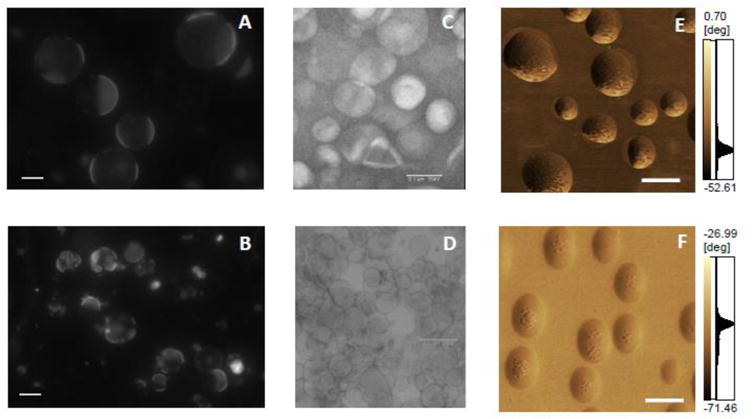
Fluorescence microscopy (60× magnification, scales bar of 20 μm, labeled with 2% rhodamine) of (A) giant liposomes consisting of DOPC, CHOL and SM (8:1:1, molar ratio) and (B) 8:1:1 DOPC:CHOL:SM-giant liposomes harboring TNAP. Electron microscopy using negative staining of: (C) Liposomes consisting of DPPC (D) DPPC-proteoliposomes harboring TNAP, both with 50× magnification. 3D topographic AFM images of (E) liposomes consisting of DPPC and DPPS (9:1, molar ratio) (xy area is 1 μm × 11 μm and z axis from 0 to 84.50 nm) and scale bar of 250 nm and (F) 9:1 DPPC:DPPS-proteoliposomes harboring TNAP (xy area is 2.50 μm × 2.50 μm and z axis from 0 to 40.81 nm) and scale bar of 250 nm.
To understand how microdomains would affect the interaction of proteoliposomes with type II collagen fibers, which corresponds to the early MV activity during biomineralization, AFM images of proteoliposomes were recorded [228]. AFM images (Fig. 6E and 6F) revealed the presence of microdomains with distinct viscoelasticity and suggested that the presence of TNAP and AnxA5 induced local changes in membrane fluidity, compatible with the generation of microdomains prominent in TNAP-proteoliposomes (Fig. 6F) but were barely detectable in AnxA5-proteoliposomes or in liposomes (Fig. 6E). A more complex microdomain structure was observed for the proteoliposomes harboring TNAP and AnxA5 concomitantly, resulting in a lower affinity for type II collagen fibers compared to exclusive proteoliposomes harboring AnxA5 alone [228].
The ultimate goal of the in vitro MVs biomimetic models is to replicate in vitro the key events leading to the domain-induced MV budding, and the initiation of apatite crystal formation in chondrocyte- and osteoblast-derived MVs. Once these proteoliposomes are obtained and characterized, they can be added to fixed amounts of MVs, either WT or deficient in specific enzymes, to modulate their in vitro mineralization properties. Such MVs biomimetic proteoliposomes would be useful also for many important translational applications. The enzymatic defects associated with disease causing mutations in the TNAP molecule, such as those found in hypophosphatasia [229] could be further elucidated in a membrane vesicle that mimics the in vivo MVs to test their biological consequences. Since these artificial vesicles adequately mimic the kinetic behavior of the enzymes in the natural vesicular MV environment [211, 219], these proteoliposomes could also be used for the screening of small molecule compounds able to modulate (inhibit or activate) the activity of MV enzymes for potential therapeutic usage [225, 230].
7. Concluding remarks
Here we highlight some of the remaining open questions of MVs biogenesis and (mal) function. One of the difficulties encountered, when working with MVs is that most cells can produce various types of microparticles ranging from ABs, EVs, EXs and MVs, with distinct properties. These difficulties are exacerbated by the fact that the MVs’ properties depend on the level of maturation and differentiation of cells: MVs are released at the end stage of differentiation, e.g., hypertrophic chondrocytes and fully differentiated osteoblasts, thus overlapping with possible release of ABs as well as other types of EVs. Additionally, reliable purification of MVs remains a pressing issue, thus making a clear understanding of the differences between the properties of MVs and other types of EVs still a difficult task.
This review focused on the MVs released from osteoblasts and chondrocytes owing to the similarities of their properties. These MVs have a very distinct function with respect to other types of nanoparticles or microparticles which are a means to initiate mineralization. In addition, unlike other types of microparticles, MVs reflect very well the composition of lipid rafts with a lipid to protein ratio of around 2 as compared to that of the plasma membrane around 0.5 [45]. MVs contain several protein markers of lipid rafts (AnxA6, carboxypeptidase M, H+ ATPase, G-proteins, TNAP as a GPI anchored protein, myristoylated and palmitoylated proteins), as well has high CHOL and SM content to be considered as markers of lipid rafts.
In this review, we also call attention to the yet unclear mechanisms leading to the biogenesis of MVs. Although data have generally shown MVs budding off the cell membrane [47, 74–78], thus pointing to a biogenesis pathway of MVs distinct from the endo/lysosomal pathway of EXs [13], a recent report about the role of MVs in the pathogenesis of OA suggests the possibility of an alternative pathway for the release of MVs based on autophagosomes [180]. This has also shed light on potential alternative functions of MVs in arthritic joints. Based on the notion that chondrocytes acquire a hypertrophic phenotype during OA, it is clear that the main role of MVs in OA is the pathological mineralization of the cartilage ECM. MV function has not been directly linked to cell-cell communication. However, MVs may indirectly behave as conveyers of information among joint cells during OA development. Experimental findings have started shedding light on the complex exchange of molecular signals among joint cells mediated by EXs, whose biogenesis, content and release is controlled by the presence of cytokines, chemokines and other soluble factors in joint tissues. In this respect, MV-released calcium-containing crystals can interact with chondrocyte TLRs and stimulate the release of pro-inflammatory and degrading enzymes, thus suggesting that MVs can contribute to OA development not only through ECM mineralization but also by participating in cell-cell communication [184]. However, the lack of well-developed techniques to purify single types of EVs makes it difficult to establish the exact role of MVs, EXs and other microvesicles in the complex network of events that lead to the development of OA.
Finally, we described the use of MV-mimicking proteoliposomes as a model system to understand the role of single MV components during normal and pathological mineralization as well as to screen small therapeutic molecules. The development of MV-mimicking proteoliposomes may have an additional advantage. Recent research has been focused on the development of systems able to transport drugs to a specific location with high spatial and temporal accuracy by using carriers conjugated to targeting moieties [231, 232]. In this regard, proteoliposomes decorated with AnxA5, or other collagen-binding moieties, and loaded with anti-inflammatory drugs can be developed to enter the cartilage of OA patients following intra-articular injection and efficiently block further OA progression. Similarly, proteoliposomes can be designed to target vascular tissues and slowly release the payload to block mineralization. It is clear that further investigations on the properties of MVs may lead not only to a better understanding of the pathways regulating the MVs’ cellular origin and mineral propagation, but also the possibility to develop novel therapeutic strategies.
General significance.
MVs have been extensively investigated owing to their roles in skeletal and ectopic mineralization. MVs serve as a model system for lipid raft structures, and for the mechanisms of genesis and release of extracellular vesicles.
Highlights.
This review addresses a series of questions about matrix vesicles and biomimetic models for matrix vesicles.
Matrix vesicles are roughly spherical extracellular vesicles of 100–300 nm in diameter.
Preparation of hydrated samples is necessary for preserving matrix vesicles in their native state.
Mechanisms of the biogenesis of matrix vesicles are still unclear.
Proteoliposomes can serve as a model to understand matrix vesicle-induced mineralization.
Acknowledgments
The authors thank National Institute of General Medical Sciences grants (R01CA179087, R01GM115972); FAPESP (2016/21236-0, 2014/11941-3 and 2014/00371-1), CAPES and CNPq for the financial support to the laboratory. MB and AMSS received a FAPESP and CAPES scholarship, respectively. PC also acknowledges CNPq for research fellowships. Polonium grant N°33540RG is gratefully acknowledged.
Abbreviations
- ABs
apoptotic bodies
- ACP
amorphous calcium phosphate
- ACVs
articular cartilage vesicles
- AFM
atomic force microscopy
- ANK
ankyrin
- AnxA1, AnxA2, AnxA4, AnxA5, AnxA6, AnxA7
vertebrate annexins 1, 2, 4, 5, 6, 7
- BCP
basic calcium phosphate
- BMP
bone morphogenetic protein
- CHOL
cholesterol
- CPPD
calcium pyrophosphate dihydrate
- Cryo-EM
cryo-electron microscopy
- DAG
diacylgycerol
- DOPC
dioleoylphosphatidylcholine
- DPPC
dipalmitoylphosphatidylcholine
- DPPS
dipalmitoylphosphatidylserine
- E
enrichment
- ECM
extracellular matrix
- EM
electron microscopy
- ENPP1 (or NPP1)
ectonucleotide pyrophosphatase/phosphodiesterase 1
- EVs
extracellular vesicles
- EXs
exosomes
- FFA
free fatty acid
- GPI
glycosylphosphatidylinositol
- IIAC
idiopathic infantile arterial calcification
- IL-1beta
interleukin-1 beta
- IVs
intracellular vesicles
- MAG
monoacylglycerol
- miRNA
microRNA
- MMPs
matrix metalloproteinases
- MVs
matrix vesicles
- NC
nucleation core
- ND
not determined
- OA
osteoarthritis
- OCP
octacalcium phosphate
- OMFs
organelle membrane fractions
- OPN
osteopontin
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PHOSPHO1
orphan phosphatase 1
- PI
phosphatidylinositol
- Pi
inorganic phosphate
- PiT-1
phosphate transporter 1
- PMVs
plasma membrane vesicles
- PPi
inorganic pyrophosphate
- PS
phosphatidylserine
- RA
rheumatoid arthritis
- SD
standard deviation
- SM
sphingomyelin
- SMPD3
sphingomyelin phosphodiesterase 3
- TAG
triacylglycerol
- TEM
transmission electron microscopy
- TLR
toll-like receptor
- TNAP
tissue-nonspecific alkaline phosphatase
- TSG101
tumor susceptibility gene 101
- UCC
unidentified calcium carrier
- VSMCs
vascular smooth muscle cells
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 2.Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Invest. 2010;90:1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed) 2011;3:711–35. doi: 10.2741/e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stratmann U, Schaarschmidt K, Wiesmann HP, Plate U, Höhling HJ. Mineralization during matrix-vesicle-mediated mantle dentine formation in molars of albino rats: a microanalytical and ultrastructural study. Cell Tissue Res. 1996;284:223–30. doi: 10.1007/s004410050582. [DOI] [PubMed] [Google Scholar]
- 5.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen NX, O’Neill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 2008;23:1798–1805. doi: 10.1359/JBMR.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shroff RC, Shanahan CM. The vascular biology of calcification. Semin Dial. 2007;20:103–109. doi: 10.1111/j.1525-139X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 8.Golub EE. Biomineralization and matrix vesicles in biology and pathology. Semin Immunopathol. 2011;33:409–417. doi: 10.1007/s00281-010-0230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui L, Houston DA, Farquharson C, MacRae VE. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone. 2016;87:147–158. doi: 10.1016/j.bone.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Wuthier RE, Lipscomb GF. Matrix vesicles: structure, composition, formation and function in calcification. Front Biosci. 2011;16:2812–2902. doi: 10.2741/3887. [DOI] [PubMed] [Google Scholar]
- 11.Golub EE. Role of matrix vesicles in biomineralization. Biochim Biophys Acta. 2009;1790:1592–1598. doi: 10.1016/j.bbagen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro IM, Landis WJ, Risbud MV. Matrix vesicles: Are they anchored exosomes? Bone. 2015;79:29–36. doi: 10.1016/j.bone.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, Buzás EI, Lötvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu LN, Sauer GR, Genge BR, Wuthier RE. Induction of mineral deposition by primary cultures of chicken growth plate chondrocytes in ascorbate-containing media. Evidence of an association between matrix vesicles and collagen. J Biol Chem. 1989;264:21346–21355. [PubMed] [Google Scholar]
- 15.Wu LN, Genge BR, Lloyd GC, Wuthier RE. Collagen-binding proteins in collagenase-released matrix vesicles from cartilage. Interaction between matrix vesicle proteins and different types of collagen. J Biol Chem. 1991;266:1195–1203. [PubMed] [Google Scholar]
- 16.Kirsch T, Wuthier RE. Stimulation of calcification of growth plate cartilage matrix vesicles by binding to type II and X collagens. J Biol Chem. 1994;269:11462–11469. [PubMed] [Google Scholar]
- 17.Kirsch T, Harrison G, Golub EE, Nah HD. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem. 2000;275:35577–35583. doi: 10.1074/jbc.M005648200. [DOI] [PubMed] [Google Scholar]
- 18.Wu LN, Genge BR, Dunkelberger DG, LeGeros RZ, Concannon B, Wuthier RE. Physicochemical characterization of the nucleational core of matrix vesicles. J Biol Chem. 1997;272:4404–4411. doi: 10.1074/jbc.272.7.4404. [DOI] [PubMed] [Google Scholar]
- 19.Bechkoff G, Radisson J, Bessueille L, Bouchekioua-Bouzaghou K, Buchet R. Distinct actions of strontium on mineral formation in matrix vesicles. Biochem Biophys Res Commun. 2008;373:378–381. doi: 10.1016/j.bbrc.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Thouverey C, Bechkoff G, Pikula S, Buchet R. Inorganic pyrophosphate as a regulator of hydroxyapatite or calcium pyrophosphate dihydrate mineral deposition by matrix vesicles. Osteoarthritis Cartilage. 2009;17:64–72. doi: 10.1016/j.joca.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KA, Hessle L, Vaingankar S, Wennberg C, Mauro S, Narisawa S, Goding JW, Sano K, Millán JL, Terkeltaub R. Osteoblast tissue-nonspecific alkaline phosphatase antagonizes and regulates PC-1. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1365–R1377. doi: 10.1152/ajpregu.2000.279.4.R1365. [DOI] [PubMed] [Google Scholar]
- 22.Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millán JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav MC, Simão AM, Narisawa S, Huesa C, McKee MD, Farquharson C, Millán JL. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J Bone Miner Res. 2011;26:286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X, Cui Y, Han J. Phosphate/pyrophosphate and MV-related proteins in mineralisation: discoveries from mouse models. Int Biol Sci. 2012;8:778–790. doi: 10.7150/ijbs.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciancaglini P, Yadav MC, Simão AM, Narisawa S, Pizauro JM, Farquharson C, Hoylaerts MF, Millán JL. Kinetic analysis of substrate utilization by native and TNAP-, NPP1-, or PHOSPHO1-deficient matrix vesicles. J Bone Miner Res. 2010;25:716–723. doi: 10.1359/jbmr.091023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts S, Narisawa S, Harmey D, Millán JL, Farquharson C. Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization. J Bone Miner Res. 2007;22:617–627. doi: 10.1359/jbmr.070108. [DOI] [PubMed] [Google Scholar]
- 27.Millán JL. The role of phosphatases in the initiation of skeletal mineralization. Calcif Tissue Int. 2013;93:299–306. doi: 10.1007/s00223-012-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guicheux J, Palmer G, Shukunami C, Hiraki Y, Bonjour JP, Caverzasio J. A novel in vitro culture system for analysis of functional role of phosphate transport in endochondral ossification. Bone. 2000;27:69–74. doi: 10.1016/s8756-3282(00)00302-1. [DOI] [PubMed] [Google Scholar]
- 29.Yadav MC, Bottini M, Cory E, Bhattacharya K, Kuss P, Narisawa S, Sah RL, Beck L, Fadeel B, Farquharson C, Millán JL. Skeletal mineralization deficits and impaired biogenesis and function of chondrocyte-derived matrix vesicles in Phospho1(−/−) and Phospho1/Pi t1 double-knockout mice. J Bone Miner Res. 2016;31:1275–1286. doi: 10.1002/jbmr.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Xu J, Kirsch T. Annexin-mediated Ca2+ influx regulates growth plate chondrocyte maturation and apoptosis. J Biol Chem. 2003;278:3762–3769. doi: 10.1074/jbc.M208868200. [DOI] [PubMed] [Google Scholar]
- 31.Balcerzak M, Malinowska A, Thouverey C, Sekrecka A, Dadlez M, Buchet R, Pikula S. Proteome analysis of matrix vesicles isolated from femurs of chicken embryo. Proteomics. 2008;8:192–205. doi: 10.1002/pmic.200700612. [DOI] [PubMed] [Google Scholar]
- 32.Thouverey C, Malinowska A, Balcerzak M, Strzelecka-Kiliszek A, Buchet R, Dadlez M, Pikula S. Proteomic characterization of biogenesis and functions of matrix vesicles released from mineralizing human osteoblast-like cells. J Proteomics. 2011;74:1123–1134. doi: 10.1016/j.jprot.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Bolean M, Simão AM, Kiffer-Moreira T, Hoylaerts MF, Millán JL, Itri R, Ciancaglini P. Proteoliposomes with the ability to transport Ca(2+) into the vesicles and hydrolyze phosphosubstrates on their surface. Arch Biochem Biophys. 2015;584:79–89. doi: 10.1016/j.abb.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirsch T, Nah HD, Demuth DR, Harrison G, Golub EE, Adams SL, Pacifici M. Annexin V-mediated calcium flux across membranes is dependent on the lipid composition: implications for cartilage mineralization. Biochemistry. 1997;36:3359–3367. doi: 10.1021/bi9626867. [DOI] [PubMed] [Google Scholar]
- 35.Kirsch T, Pfäffle M. Selective binding of anchorin CII (annexin V) to type II and X collagen and to chondrocalcin (C-propeptide of type II collagen). Implications for anchoring function between matrix vesicles and matrix proteins. FEBS Lett. 1992;310:143–147. doi: 10.1016/0014-5793(92)81316-e. [DOI] [PubMed] [Google Scholar]
- 36.Bossi M, Hoylaerts MF, Millán JL. Modifications in a flexible surface loop modulate the isozyme-specific properties of mammalian alkaline phosphatases. J Biol Chem. 1993;268:25409–25416. [PubMed] [Google Scholar]
- 37.Jahnen-Dechent W, Schäfer C, Ketteler M, McKee MD. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med (Berl) 2008;86:379–89. doi: 10.1007/s00109-007-0294-y. [DOI] [PubMed] [Google Scholar]
- 38.Brylka L, Jahnen-Dechent W. The role of fetuin-A in physiological and pathological mineralization. Calcif Tissue Int. 2013;93:355–64. doi: 10.1007/s00223-012-9690-6. [DOI] [PubMed] [Google Scholar]
- 39.Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. 1999;84:166–78. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 40.Yuan Q, Jiang Y, Zhao X, Sato T, Densmore M, Schüler C, Erben RG, McKee MD, Lanske B. Increased osteopontin contributes to inhibition of bone mineralization in FGF23-deficient mice. J Bone Miner Res. 2014;29:693–704. doi: 10.1002/jbmr.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoac B, Nelea V, Jiang W, Kaartinen MT, McKee MD. Mineralization-inhibiting effects of transglutaminase-crosslinked polymeric osteopontin. Bone. 2017;101:37–48. doi: 10.1016/j.bone.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Price PA, Toroian D, Lim JE. Mineralization by inhibitor exclusion: the calcification of collagen with fetuin. J Biol Chem. 2009;284:17092–101. doi: 10.1074/jbc.M109.007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watkins EL, Stillo JV, Wuthier RE. Subcellular fractionation of epiphyseal cartilage: isolation of matrix vesicles and profiles of enzymes, phospholipids, calcium and phosphate. Biochim Biophys Acta. 1980;631:289–304. doi: 10.1016/0304-4165(80)90303-7. [DOI] [PubMed] [Google Scholar]
- 44.Warner GP, Hubbard HL, Lloyd GC, Wuthier RE. 32Pi- and 45Ca-metabolism by matrix vesicle-enriched microsomes prepared from chicken epiphyseal cartilage by isosmotic Percoll density-gradient fractionation. Calcif Tissue Int. 1983;35:327–338. doi: 10.1007/BF02405054. [DOI] [PubMed] [Google Scholar]
- 45.Balcerzak M, Radisson J, Azzar G, Farlay D, Boivin G, Pikula S, Buchet R. A comparative analysis of strategies for isolation of matrix vesicles. Anal Biochem. 2007;361:176–182. doi: 10.1016/j.ab.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa Y, Chin JE, Schalk EM, Wuthier RE. Effect of amino acid levels on matrix vesicle formation by epiphyseal growth plate chondrocytes in primary culture. J Cell Physiol. 1986;126:399–406. doi: 10.1002/jcp.1041260310. [DOI] [PubMed] [Google Scholar]
- 47.Hale JE, Wuthier RE. The mechanism of matrix vesicle formation. Studies on the composition of chondrocyte microvilli and on the effects of microfilament-perturbing agents on cellular vesiculation. J Biol Chem. 1987;262:1916–1925. [PubMed] [Google Scholar]
- 48.Genge BR, Sauer GR, Wu LN, McLean FM, Wuthier RE. Correlation between loss of alkaline phosphatase activity and accumulation of calcium during matrix vesicle-mediated mineralization. J Biol Chem. 1988;263:18513–18519. [PubMed] [Google Scholar]
- 49.Boyan BD, Schwartz Z, Swain LD, Carnes DL, Zislis T. Differential expression of phenotype by resting zone and growth region costochondral chondrocytes in vitro. Bone. 1988;9:185–194. doi: 10.1016/8756-3282(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 50.Anderson HC. Molecular biology of matrix vesicles. Clin Orthop Relat Res. 1995:266–280. [PubMed] [Google Scholar]
- 51.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garimella R, Bi X, Camacho N, Sipe JB, Anderson HC. Primary culture of rat growth plate chondrocytes: an in vitro model of growth plate histotype, matrix vesicle biogenesis and mineralization. Bone. 2004;34:961–970. doi: 10.1016/j.bone.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Kirsch T, von der Mark K. Remodelling of collagen types I, II and X and calcification of human fetal cartilage. Bone Miner. 1992;18:107–117. doi: 10.1016/0169-6009(92)90851-4. [DOI] [PubMed] [Google Scholar]
- 54.Kirsch T, Nah HD, Shapiro IM, Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Descalzi Cancedda F, Gentili C, Manduca P, Cancedda R. Hypertrophic chondrocytes undergo further differentiation in culture. J Cell Biol. 1992;117:427–435. doi: 10.1083/jcb.117.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gentili C, Bianco P, Neri M, Malpeli M, Campanile G, Castagnola P, Cancedda R, Cancedda FD. Cell proliferation, extracellular matrix mineralization, and ovotransferrin transient expression during in vitro differentiation of chick hypertrophic chondrocytes into osteoblast-like cells. J Cell Biol. 1993;122:703–712. doi: 10.1083/jcb.122.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roach HI, Erenpreisa J, Aigner T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol. 1995;131:483–494. doi: 10.1083/jcb.131.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dean DD, Schwartz Z, Bonewald L, Muniz OE, Morales S, Gomez R, Brooks BP, Qiao M, Howell DS, Boyan BD. Matrix vesicles produced by osteoblast-like cells in culture become significantly enriched in proteoglycan-degrading metalloproteinases after addition of beta-glycerophosphate and ascorbic acid. Calcif Tissue Int. 1994;54:399–408. doi: 10.1007/BF00305527. [DOI] [PubMed] [Google Scholar]
- 59.Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- 60.Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res. 1994;9:843–854. doi: 10.1002/jbmr.5650090610. [DOI] [PubMed] [Google Scholar]
- 61.Kardos TB, Hubbard MJ. Are matrix vesicles apoptotic bodies? Prog Clin Biol Res. 1982;101:45–60. [PubMed] [Google Scholar]
- 62.Kirsch T, Wang W, Pfander D. Functional differences between growth plate apoptotic bodies and matrix vesicles. J Bone Miner Res. 2003;18:1872–1881. doi: 10.1359/jbmr.2003.18.10.1872. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto S, Ochs RL, Rosen F, Quach J, McCabe G, Solan J, Seegmiller JE, Terkeltaub R, Lotz M. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc Natl Acad Sci USA. 1998;95:3094–3099. doi: 10.1073/pnas.95.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirsch T. Physiological and pathological mineralizations: a complex multifactorial process. Curr Opin Orthop. 2007;18:425–427. [Google Scholar]
- 65.Magne D, Bluteau G, Faucheux C, Palmer G, Vignes-Colombeix C, Pilet P, Rouillon T, Caverzasio J, Weiss P, Daculsi G, Guicheux J. Phosphate is a specific signal for ATDC5 chondrocyte maturation and apoptosis-associated mineralization: possible implication of apoptosis in the regulation of endochondral ossification. J Bone Miner Res. 2003;18:1430–1442. doi: 10.1359/jbmr.2003.18.8.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 67.Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA, Aronin N, Khvorova A. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. doi: 10.3402/jev.v5.32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Register TC, Warner GP, Wuthier RE. Effect of L- and D-tetramisole on 32Pi and 45Ca uptake and mineralization by matrix vesicle-enriched fractions from chicken epiphyseal cartilage. J Biol Chem. 1984;259:922–928. [PubMed] [Google Scholar]
- 69.Bohn WW, Stein RM, Hsu HH, Morris DC, Anderson HC. Isolation of a plasma membrane-enriched fraction from collagenase-suspended rachitic rat growth plate chondrocytes. J Orthop Res. 1984;1:319–324. doi: 10.1002/jor.1100010312. [DOI] [PubMed] [Google Scholar]
- 70.Wuthier RE, Chin JE, Hale JE, Register TC, Hale LV, Ishikawa Y. Isolation and characterization of calcium-accumulating matrix vesicles from chondrocytes of chicken epiphyseal growth plate cartilage in primary culture. J Biol Chem. 1985;260:15972–15979. [PubMed] [Google Scholar]
- 71.Register TC, McLean FM, Low MG, Wuthier RE. Roles of alkaline phosphatase and labile internal mineral in matrix vesicle-mediated calcification. Effect of selective release of membrane-bound alkaline phosphatase and treatment with isosmotic pH 6 buffer. J Biol Chem. 1986;261:9354–9360. [PubMed] [Google Scholar]
- 72.Wu LN, Yoshimori T, Genge BR, Sauer GR, Kirsch T, Ishikawa Y, Wuthier RE. Characterization of the nucleational core complex responsible for mineral induction by growth plate cartilage matrix vesicles. J Biol Chem. 1993;268:25084–25094. [PubMed] [Google Scholar]
- 73.Buchet R, Pikula S, Magne D, Mebarek S. Isolation and characteristics of matrix vesicles. Methods Mol Biol. 2013;1053:115–124. doi: 10.1007/978-1-62703-562-0_7. [DOI] [PubMed] [Google Scholar]
- 74.Rabinovitch AL, Anderson HC. Biogenesis of matrix vesicles in cartilage growth plates. Fed Proc. 1976;35:112–116. [PubMed] [Google Scholar]
- 75.Cecil RN, Anderson HC. Freeze-fracture studies of matrix vesicle calcification in epiphyseal growth plate. Metabolic Bone Disease & Related Research. 1978;1:89–95. doi: 10.1016/0221-8747(83)90014-0. [DOI] [PubMed] [Google Scholar]
- 76.Borg TK, Runyan R, Wuthier RE. A freeze-fracture study of avian epiphyseal cartilage differentiation. Anat Rec. 1981;199:449–457. doi: 10.1002/ar.1091990402. [DOI] [PubMed] [Google Scholar]
- 77.Akisaka T, Shigenaga Y. Ultrastructure of growing epiphyseal cartilage processed by rapid freezing and freeze-substitution. J Electron Microsc (Tokyo) 1983;32:305–320. [PubMed] [Google Scholar]
- 78.Akisaka T, Kawaguchi H, Subita GP, Shigenaga Y, Gay CV. Ultrastructure of matrix vesicles in chick growth plate as revealed by quick freezing and freeze substitution. Calcif Tissue Int. 1988;42:383–393. doi: 10.1007/BF02556357. [DOI] [PubMed] [Google Scholar]
- 79.Abdallah D, Hamade E, Merhi RA, Bassam B, Buchet R, Mebarek S. Fatty acid composition in matrix vesicles and in microvilli from femurs of chicken embryos revealed selective recruitment of fatty acids. Biochem Biophys Res Commun. 2014;446:1161–1164. doi: 10.1016/j.bbrc.2014.03.069. [DOI] [PubMed] [Google Scholar]
- 80.Wuthier RE. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochim Biophys Acta. 1975;409:128–143. doi: 10.1016/0005-2760(75)90087-9. [DOI] [PubMed] [Google Scholar]
- 81.Thouverey C, Strzelecka-Kiliszek A, Balcerzak M, Buchet R, Pikula S. Matrix vesicles originate from apical membrane microvilli of mineralizing osteoblast-like Saos-2 cells. J Cell Biochem. 2009;106:127–138. doi: 10.1002/jcb.21992. [DOI] [PubMed] [Google Scholar]
- 82.Jiang L, Cui Y, Luan J, Zhou X, Han J. A comparative proteomics study on matrix vesicles of osteoblast-like Saos-2 and U2-OS cells. Intractable Rare Dis Res. 2013;2:59–62. doi: 10.5582/irdr.2013.v2.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao Z, Camalier CE, Nagashima K, Chan KC, Lucas DA, de la Cruz MJ, Gignac M, Lockett S, Issaq HJ, Veenstra TD, Conrads TP, Beck GR. Analysis of the extracellular matrix vesicle proteome in mineralizing osteoblasts. J Cell Physiol. 2007;210:325–335. doi: 10.1002/jcp.20826. [DOI] [PubMed] [Google Scholar]
- 84.Xiao Z, Conrads TP, Beck GR, Veenstra TD. Analysis of the extracellular matrix and secreted vesicle proteomes by mass spectrometry. Methods Mol Biol. 2008;428:231–244. doi: 10.1007/978-1-59745-117-8_13. [DOI] [PubMed] [Google Scholar]
- 85.Xiao Z, Blonder J, Zhou M, Veenstra TD. Proteomic analysis of extracellular matrix and vesicles. J Proteomics. 2009;72:34–45. doi: 10.1016/j.jprot.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 86.Morris DC, Masuhara K, Takaoka K, Ono K, Anderson HC. Immunolocalization of alkaline phosphatase in osteoblasts and matrix vesicles of human fetal bone. Bone Miner. 1992;19:287–298. doi: 10.1016/0169-6009(92)90877-g. [DOI] [PubMed] [Google Scholar]
- 87.Katchburian E, Severs NJ. Matrix constituents of early developing bone examined by freeze fracture. Cell Biol Int Rep. 1983;7:1063–1070. doi: 10.1016/0309-1651(83)90012-7. [DOI] [PubMed] [Google Scholar]
- 88.Nogales E, Scheres SH. Cryo-EM: A Unique tool for the visualization of macromolecular complexity. Mol Cell. 2015;58:677–689. doi: 10.1016/j.molcel.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kühlbrandt W. Cryo-EM enters a new era. Elife. 2014;3:e03678. doi: 10.7554/eLife.03678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Asano S, Engel BD, Baumeister W. In situ cryo-electron tomography: A post-reductionist approach to structural biology. J Mol Biol. 2016;428:332–343. doi: 10.1016/j.jmb.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 91.Peress NS, Anderson HC, Sajdera SW. The lipids of matrix vesicles from bovine fetal epiphyseal cartilage. Calcif Tissue Res. 1974;14:275–281. doi: 10.1007/BF02060301. [DOI] [PubMed] [Google Scholar]
- 92.Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McKee MD, Yadav MC, Foster BL, Somerman MJ, Farquharson C, Millán JL. Compounded PHOSPHO1/ALPL deficiencies reduce dentin mineralization. J Dent Res. 2013;92:721–727. doi: 10.1177/0022034513490958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuzynski M, Goss M, Bottini M, Yadav MC, Mobley C, Winters T, Poliard A, Kellermann O, Lee B, Millán JL, Napierala D. Dual role of the Trps1 transcription factor in dentin mineralization. J Biol Chem. 2014;289:27481–27493. doi: 10.1074/jbc.M114.550129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RT, Alvarez-Hernandez D, Shroff R, Yin X, Muller K, Skepper JN, Mayr M, Reutelingsperger CP, Chester A, Bertazzo S, Schurgers LJ, Shanahan CM. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312–1323. doi: 10.1161/CIRCRESAHA.116.305012. [DOI] [PubMed] [Google Scholar]
- 96.Lorent JH, Levental I. Structural determinants of protein partitioning into ordered membrane domains and lipid rafts. Chem Phys Lipids. 2015;192:23–32. doi: 10.1016/j.chemphyslip.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 97.Róg T, Vattulainen I. Cholesterol, sphingolipids, and glycolipids: what do we know about their role in raft-like membranes? Chem Phys Lipids. 2014;184:82–104. doi: 10.1016/j.chemphyslip.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 98.Zimmermann B, Wachtel HC, Noppe C. Patterns of mineralization in vitro. Cell Tissue Res. 1991;263:483–493. doi: 10.1007/BF00327281. [DOI] [PubMed] [Google Scholar]
- 99.Takeuchi A, Ohtsuki C, Miyazaki T, Kamitakahara M, Ogata S, Yamazaki M, Furutani Y, Kinoshita H, Tanihara M. Heterogeneous nucleation of hydroxyapatite on protein: structural effect of silk sericin. J R Soc Interface. 2005;2:373–378. doi: 10.1098/rsif.2005.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dey A, Bomans PH, Müller FA, Will J, Frederik PM, de With G, Sommerdijk NA. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat Mater. 2010;9:1010–1014. doi: 10.1038/nmat2900. [DOI] [PubMed] [Google Scholar]
- 101.Hortells L, Sosa C, Millán Á, Sorribas V. Critical parameters of the in vitro method of vascular smooth muscle cell calcification. PLoS One. 2015;10:e0141751. doi: 10.1371/journal.pone.0141751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orriss IR, Arnett TR, Russell RG. Pyrophosphate: a key inhibitor of mineralisation. Curr Opin Pharmacol. 2016;28:57–68. doi: 10.1016/j.coph.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Hunter GK. Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int. 2013;93:348–354. doi: 10.1007/s00223-013-9698-6. [DOI] [PubMed] [Google Scholar]
- 104.O’Young J, Liao Y, Xiao Y, Jalkanen J, Lajoie G, Karttunen M, Goldberg HA, Hunter GK. Matrix Gla protein inhibits ectopic calcification by a direct interaction with hydroxyapatite crystals. J Am Chem Soc. 2011;133:18406–18412. doi: 10.1021/ja207628k. [DOI] [PubMed] [Google Scholar]
- 105.Lomashvili KA, Wang X, Wallin R, O’Neill WC. Matrix Gla protein metabolism in vascular smooth muscle and role in uremic vascular calcification. J Biol Chem. 2011;286:28715–28722. doi: 10.1074/jbc.M111.251462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cai MM, Smith ER, Holt SG. The role of fetuin-A in mineral trafficking and deposition. Bonekey Rep. 2015;4:672. doi: 10.1038/bonekey.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang H, Wang Y. Morphology control of hydroxyapatite microcrystals: synergistic effects of citrate and CTAB, Mater. Sci Eng C Mater Biol Appl. 2016;62:160–165. doi: 10.1016/j.msec.2016.01.052. [DOI] [PubMed] [Google Scholar]
- 108.Wuthier RE, Bisaz S, Russell RG, Fleisch H. Relationship between pyrophosphate, amorphous calcium phosphate and other factors in the sequence of calcification in vivo. Calcif Tissue Res. 1972;10:198–206. doi: 10.1007/BF02012549. [DOI] [PubMed] [Google Scholar]
- 109.Sauer GR, Wuthier RE. Fourier transform infrared characterization of mineral phases formed during induction of mineralization by collagenase-released matrix vesicles in vitro. J Biol Chem. 1988;263:13718–13724. [PubMed] [Google Scholar]
- 110.Wu Y, Tseng YH, Chan JCC. Morphology control of fluoroapatite crystallites by citrate ions. Cryst Growth Des. 2010;10:4240–4242. [Google Scholar]
- 111.Hu YY, Rawal A, Schmidt-Rohr K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc Natl Acad Sci USA. 2010;107:22425–22429. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li L, Buchet R, Wu Y. Dimethyl sulfoxide-induced hydroxyapatite formation: a biological model of matrix vesicle nucleation to screen inhibitors of mineralization. Anal Biochem. 2008;381:123–128. doi: 10.1016/j.ab.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 113.Tavafoghi M, Cerruti M. The role of amino acids in hydroxyapatite mineralization. J R Soc Interface. 2016;13 doi: 10.1098/rsif.2016.0462. pii: 20160462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sarig S. Aspartic acid nucleates the apatite crystallites of bone: a hypothesis. Bone. 2004;35:108–113. doi: 10.1016/j.bone.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 115.Veis A. Phosphoproteins from teeth and bone. Ciba Found Symp. 1988;136:161–177. doi: 10.1002/9780470513637.ch11. [DOI] [PubMed] [Google Scholar]
- 116.Gorski JP. Acidic phosphoproteins from bone matrix: a structural rationalization of their role in biomineralization. Calcif Tissue Int. 1992;50:391–396. doi: 10.1007/BF00296767. [DOI] [PubMed] [Google Scholar]
- 117.Fujisawa R, Tamura M. Acidic bone matrix proteins and their roles in calcification. Front Biosci (Landmark Ed) 2012;17:1891–1903. doi: 10.2741/4026. [DOI] [PubMed] [Google Scholar]
- 118.Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- 119.Robinson C, Brookes SJ, Shore RC, Kirkham J. The developing enamel matrix: nature and function. Eur J Oral Sci. 1998;106(Suppl 1):282–291. doi: 10.1111/j.1600-0722.1998.tb02188.x. [DOI] [PubMed] [Google Scholar]
- 120.Melcher M, Facey SJ, Henkes TM, Subkowski T, Hauer B. Accelerated nucleation of xydroxyapatite using an engineered hydrophobin fusion protein. Biomacromolecules. 2016;17:1716–1726. doi: 10.1021/acs.biomac.6b00135. [DOI] [PubMed] [Google Scholar]
- 121.Boskey AL, Bullough PG, Posner AS. Calcium-acidic phospholipid-phosphate complexes in diseased and normal human bone. Metab Bone Dis Relat Res. 1982;4:151–156. doi: 10.1016/0221-8747(82)90029-7. [DOI] [PubMed] [Google Scholar]
- 122.Nancollas GH, Tsortos A, Zieba A. The nucleation and growth of calcium phosphate crystals at protein and phosphatidylserine liposome surfaces. Scanning Microsc. 1996;10:499–507. [PubMed] [Google Scholar]
- 123.Laird DF, Mucalo MR, Yokogawa Y. Growth of calcium hydroxyapatite (Ca-HAp) on cholesterol and cholestanol crystals from a simulated body fluid: A possible insight into the pathological calcifications associated with atherosclerosis. J Colloid Interface Sci. 2006;295:348–363. doi: 10.1016/j.jcis.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 124.Wang HP, Feng XJ, Gou BD, Zhang TL, Xu SJ, Wang K. Effects of LDL, cholesterol, and their oxidized forms on the precipitation kinetics of calcium phosphates. Clin Chem. 2003;49:2027–2036. doi: 10.1373/clinchem.2003.024513. [DOI] [PubMed] [Google Scholar]
- 125.Ali SY, Sajdera SW, Anderson HC. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci USA. 1970;67:1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ali SY, Anderson HC, Sajdera SW. Enzymic and electron-microscopic analysis of extracellular matrix vesicles associated with calcification in cartilage. Biochem J. 1971;122:56P. doi: 10.1042/bj1220056pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garimella R, Bi X, Anderson HC, Camacho NP. Nature of phosphate substrate as a major determinant of mineral type formed in matrix vesicle-mediated in vitro mineralization: An FTIR imaging study. Bone. 2006;38:811–817. doi: 10.1016/j.bone.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 128.Cheng PT, Pritzker KP. Pyrophosphate, phosphate ion interaction: effects on calcium pyrophosphate and calcium hydroxyapatite crystal formation in aqueous solutions. J Rheumatol. 1983;10:769–777. [PubMed] [Google Scholar]
- 129.Williams CJ. Familial calcium pyrophosphate dihydrate deposition disease and the ANKH gene. Curr Opin Rheumatol. 2003;15:326–331. doi: 10.1097/00002281-200305000-00023. [DOI] [PubMed] [Google Scholar]
- 130.Zhang W, Doherty M, Bardin T, Barskova V, Guerne PA, Jansen TL, Leeb BF, Perez-Ruiz F, Pimentao J, Punzi L, Richette P, Sivera F, Uhlig T, Watt I, Pascual E. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70:563–570. doi: 10.1136/ard.2010.139105. [DOI] [PubMed] [Google Scholar]
- 131.Richette P, Bardin T, Doherty M. An update on the epidemiology of calcium pyrophosphate dihydrate crystal deposition disease. Rheumatology (Oxford) 2009;48:711–715. doi: 10.1093/rheumatology/kep081. [DOI] [PubMed] [Google Scholar]
- 132.Foster BL, Nagatomo KJ, Tso HW, Tran AB, Nociti FH, Narisawa S, Yadav MC, McKee MD, Millán JI, Somerman MJ. Tooth root dentin mineralization defects in a mouse model of hypophosphatasia. J Bone Miner Res. 2013;28:271–282. doi: 10.1002/jbmr.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Landis WJ, Arsenault AL. Vesicle- and collagen-mediated calcification in the turkey leg tendon. Connect Tissue Res. 1989;22:35–42. doi: 10.3109/03008208909114118. [DOI] [PubMed] [Google Scholar]
- 134.Murshed M, Harmey D, Millán JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vittur F, Stagni N, Moro L, de Bernard B. Alkaline phosphatase binds to collagen; a hypothesis on the mechanism of extravesicular mineralization in epiphyseal cartilage. Experientia. 1984;40:836–837. doi: 10.1007/BF01951980. [DOI] [PubMed] [Google Scholar]
- 136.von der Mark K, Mollenhauer J. Annexin V interactions with collagen. Cell Mol Life Sci. 1997;53:539–545. doi: 10.1007/s000180050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Belluoccio D, Grskovic I, Niehoff A, Schlötzer-Schrehardt U, Rosenbaum S, Etich J, Frie C, Pausch F, Moss SE, Pöschl E, Bateman JF, Brachvogel B. Deficiency of annexins A5 and A6 induces complex changes in the transcriptome of growth plate cartilage but does not inhibit the induction of mineralization. J Bone Miner Res. 2010;25:141–153. doi: 10.1359/jbmr.090710. [DOI] [PubMed] [Google Scholar]
- 138.Belluoccio D, Etich J, Rosenbaum S, Frie C, Grskovic I, Stermann J, Ehlen H, Vogel S, Zaucke F, von der Mark K, Bateman JF, Brachvogel B. Sorting of growth plate chondrocytes allows the isolation and characterization of cells of a defined differentiation status. J Bone Miner Res. 2010;25:1267–1281. doi: 10.1002/jbmr.30. [DOI] [PubMed] [Google Scholar]
- 139.Grskovic I, Kutsch A, Frie C, Groma G, Stermann J, Schlötzer-Schrehardt U, Niehoff A, Moss SE, Rosenbaum S, Pöschl E, Chmielewski M, Rappl G, Abken H, Bateman JF, Cheah KS, Paulsson M, Brachvogel B. Depletion of annexin A5, annexin A6, and collagen X causes no gross changes in matrix vesicle-mediated mineralization, but lack of collagen X affects hematopoiesis and the Th1/Th2 response. J Bone Miner Res. 2012;27:2399–2412. doi: 10.1002/jbmr.1682. [DOI] [PubMed] [Google Scholar]
- 140.Brachvogel B, Dikschas J, Moch H, Welzel H, von der Mark K, Hofmann C, Pöschl E. Annexin A5 is not essential for skeletal development. Mol Cell Biol. 2003;23:2907–2913. doi: 10.1128/MCB.23.8.2907-2913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Minashima T, Small W, Moss SE, Kirsch T. Intracellular modulation of signaling pathways by annexin A6 regulates terminal differentiation of chondrocytes. J Biol Chem. 2012;287:14803–14815. doi: 10.1074/jbc.M111.297861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cmoch A, Strzelecka-Kiliszek A, Palczewska M, Groves P, Pikula S. Matrix vesicles isolated from mineralization-competent Saos-2 cells are selectively enriched with annexins and S100 proteins. Biochem Biophys Res Commun. 2011;412:683–687. doi: 10.1016/j.bbrc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 143.Nahar NN, Missana LR, Garimella R, Tague SE, Anderson HC. Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and noncollagenous matrix proteins. J Bone Miner Metab. 2008;26:514–519. doi: 10.1007/s00774-008-0859-z. [DOI] [PubMed] [Google Scholar]
- 144.Lin Z, Rodriguez NE, Zhao J, Ramey AN, Hyzy SL, Boyan BD, Schwartz Z. Selective enrichment of microRNAs in extracellular matrix vesicles produced by growth plate chondrocytes. Bone. 2016;88:47–55. doi: 10.1016/j.bone.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morhayim J, Baroncelli M, van Leeuwen JP. Extracellular vesicles: specialized bone messengers. Arch Biochem Biophys. 2014;561:38–45. doi: 10.1016/j.abb.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 146.Withrow J, Murphy C, Liu Y, Hunter M, Fulzele S, Hamrick MV. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18:286. doi: 10.1186/s13075-016-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Holliday LS, McHugh KP, Zuo J, Aguirre JI, Neubert JK, Rody WJ., Jr Exosomes: novel regulators of bone remodelling and potential therapeutic agents for orthodontics. Orthod Craniofac Res. 2017;20(Suppl 1):95–99. doi: 10.1111/ocr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu LN, Ishikawa Y, Sauer GR, Genge BR, Mwale F, Mishima H, Wuthier RE. Morphological and biochemical characterization of mineralizing primary cultures of avian growth plate chondrocytes: evidence for cellular processing of Ca2+ and Pi prior to matrix mineralization. J Cell Biochem. 1995;57:218–237. doi: 10.1002/jcb.240570206. [DOI] [PubMed] [Google Scholar]
- 149.Strzelecka-Kiliszek A, Bozycki L, Mebarek S, Buchet R, Pikula S. Characteristics of minerals in vesicles produced by human osteoblasts hFOB 1.19 and osteosarcoma Saos-2 cells stimulated for mineralization. J Inorg Biochem. 2017;171:100–107. doi: 10.1016/j.jinorgbio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 150.Hasegawa T, Yamamoto T, Tsuchiya E, Hongo H, Tsuboi K, Kudo A, Abe M, Yoshida T, Nagai T, Khadiza N, Yokoyama A, Oda K, Ozawa H, de Freitas PHL, Li M, Amizuka N. Ultrastructural and biochemical aspects of matrix vesicle-mediated mineralization. Jpn Dent Sci Rev. 2017;53:34–45. doi: 10.1016/j.jdsr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Genge BR, Wu LN, Wuthier RE. In vitro modeling of matrix vesicle nucleation: synergistic stimulation of mineral formation by annexin A5 and phosphatidylserine. J Biol Chem. 2007;282:26035–26045. doi: 10.1074/jbc.M701057200. [DOI] [PubMed] [Google Scholar]
- 152.Boonrungsiman S, Gentleman E, Carzaniga R, Evans ND, McComb DW, Porter AE, Stevens MM. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci USA. 2012;109:14170–14175. doi: 10.1073/pnas.1208916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu LN, Genge BR, Kang MW, Arsenault AL, Wuthier RE. Changes in phospholipid extractability and composition accompany mineralization of chicken growth plate cartilage matrix vesicles. J Biol Chem. 2002;277:5126–5133. doi: 10.1074/jbc.M107899200. [DOI] [PubMed] [Google Scholar]
- 154.Hosoya H, Kobayashi R, Tsukita S, Matsumura F. Ca(2+) -regulated actin and phospholipid binding protein (68 kD-protein) from bovine liver: identification as a homologue for annexin VI and intracellular localization. Cell Motil Cytoskeleton. 1992;22:200–210. doi: 10.1002/cm.970220307. [DOI] [PubMed] [Google Scholar]
- 155.Ma AS, Bystol ME, Tranvan A. In vitro modulation of filament bundling in F-actin and keratins by annexin II and calcium. In Vitro Cell Dev Biol Anim. 1994;30A:329–335. doi: 10.1007/BF02631454. [DOI] [PubMed] [Google Scholar]
- 156.Alvarez-Martinez MT, Porte F, Liautard JP, Sri Widada J. Effects of profilin-annexin I association on some properties of both profilin and annexin I: modification of the inhibitory activity of profilin on actin polymerization and inhibition of the self-association of annexin I and its interactions with liposomes. Biochim Biophys Acta. 1997;1339:331–340. doi: 10.1016/s0167-4838(97)00018-6. [DOI] [PubMed] [Google Scholar]
- 157.Mortimer JC, Laohavisit A, Macpherson N, Webb A, Brownlee C, Battey NH, Davies JM. Annexins: multifunctional components of growth and adaptation. J Exp Bot. 2008;59:533–544. doi: 10.1093/jxb/erm344. [DOI] [PubMed] [Google Scholar]
- 158.Cornely R, Rentero C, Enrich C, Grewal T, Gaus K. Annexin A6 is an organizer of membrane microdomains to regulate receptor localization and signalling. IUBMB Life. 2011;63:1009–1017. doi: 10.1002/iub.540. [DOI] [PubMed] [Google Scholar]
- 159.Millán JL, Whyte MP. Alkaline phosphatase and hypophosphatasia. Calcif Tissue Int. 2016;98:398–416. doi: 10.1007/s00223-015-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Anderson HC, Hsu HH, Morris DC, Fedde KN, Whyte MP. Matrix vesicles in osteomalacic hypophosphatasia bone contain apatite-like mineral crystals. Am J Pathol. 1997;151:1555–1561. [PMC free article] [PubMed] [Google Scholar]
- 161.Anderson HC, Sipe JB, Hessle L, Dhanyamraju R, Atti E, Camacho NP, Millán JL, Dhamyamraju R. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841–847. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kirsch T. Biomineralization–an active or passive process? Connect Tissue Res. 2012;53:438–445. doi: 10.3109/03008207.2012.730081. [DOI] [PubMed] [Google Scholar]
- 163.Mebarek S, Hamade E, Thouverey C, Bandorowicz-Pikula J, Pikula S, Magne D, Buchet R. Ankylosing spondylitis, late osteoarthritis, vascular calcification, chondrocalcinosis and pseudo gout: toward a possible drug therapy. Curr Med Chem. 2011;18:2196–2203. doi: 10.2174/092986711795656153. [DOI] [PubMed] [Google Scholar]
- 164.Sampson HW, Davis RW, Dufner DC. Spondyloarthropathy in progressive ankylosis mice: ultrastructural features of the intervertebral disk. Acta Anat (Basel) 1991;141:36–41. doi: 10.1159/000147096. [DOI] [PubMed] [Google Scholar]
- 165.Magne D, Julien M, Vinatier C, Merhi-Soussi F, Weiss P, Guicheux J. Cartilage formation in growth plate and arteries: from physiology to pathology. Bioessays. 2005;27:708–716. doi: 10.1002/bies.20254. [DOI] [PubMed] [Google Scholar]
- 166.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta, Arterioscler. Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 167.New SE, Aikawa E. Role of extracellular vesicles in de novo mineralization: an additional novel mechanism of cardiovascular calcification. Arterioscler Thromb Vasc Biol. 2013;33:1753–1758. doi: 10.1161/ATVBAHA.112.300128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 169.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus, Arterioscler. Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 170.Mintz GS, Kovach JA, Javier SP, Pichard AD, Kent KM, Popma JJ, Salter LF, Leon MB. Mechanisms of lumen enlargement after excimer laser coronary angioplasty. An intravascular ultrasound study. Circulation. 1995;92:3408–3414. doi: 10.1161/01.cir.92.12.3408. [DOI] [PubMed] [Google Scholar]
- 171.Ali S, Wisby A. Apatite crystal nodules in arthritic cartilage. Eur J Rheumatol Inflam. 1978;1:115–119. [Google Scholar]
- 172.Derfus BA, Kurian JB, Butler JJ, Daft LJ, Carrera GF, Ryan LM, Rosenthal AK. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29:570–574. [PubMed] [Google Scholar]
- 173.Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, Rutsch F, Schäfer SF, Niggemeyer O, Steinhagen J, Lohmann CH, Pap T, Rüther W. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60:2694–2703. doi: 10.1002/art.24774. [DOI] [PubMed] [Google Scholar]
- 174.Hirose J. Clinical presentation and diagnosis of calcium deposition diseases. Int J Clin Rheumatol. 2010;5:117–128. [Google Scholar]
- 175.Sun Y, Hanley EN. Calcium-containing crystals and osteoarthritis. Curr Opin Rheumatol. 2007;18:472–478. [Google Scholar]
- 176.Ali SY. Apatite-type crystal deposition in arthritic cartilage. Scan Electron Microsc Pt. 1985;4:1555–1566. [PubMed] [Google Scholar]
- 177.Derfus B, Kranendonk S, Camacho N, Mandel N, Kushnaryov V, Lynch K, Ryan L. Human osteoarthritic cartilage matrix vesicles generate both calcium pyrophosphate dihydrate and apatite in vitro. Calcif Tissue Int. 1998;63:258–262. doi: 10.1007/s002239900523. [DOI] [PubMed] [Google Scholar]
- 178.Derfus BA, Kurtin SM, Camacho NP, Kurup I, Ryan LM. Comparison of matrix vesicles derived from normal and osteoarthritic human articular cartilage. Connect Tissue Res. 1996;35:337–342. doi: 10.3109/03008209609029209. [DOI] [PubMed] [Google Scholar]
- 179.Rosenthal AK. Articular cartilage vesicles and calcium crystal deposition diseases. Curr Opin Rheumatol. 2016;28:127–132. doi: 10.1097/BOR.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, Grewal R, Ninomiya J, Coyne CB, Jackson WT. Autophagy modulates articular cartilage vesicle formation in primary articular chondrocytes. J Biol Chem. 2015;290:13028–13038. doi: 10.1074/jbc.M114.630558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pritzker KPH. Counterpoint: hydroxyapatite crystal deposition is not intimately involved in the pathogenesis and progression of human osteoarthritis. Curr Rheumatol Rep. 2009;11:148–153. doi: 10.1007/s11926-009-0021-5. [DOI] [PubMed] [Google Scholar]
- 183.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millán JL. Tissue non-specific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol. 2005;174:5016–5023. doi: 10.4049/jimmunol.174.8.5016. [DOI] [PubMed] [Google Scholar]
- 185.Messer L, Alsaleh G, Freyssinet JM, Zobairi F, Leray I, Gottenberg JE, Sibilia J, Toti-Orfanoudakis F, Wachsmann D. Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Arthritis Res Ther. 2009;11:R40. doi: 10.1186/ar2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jubeck B, Gohr C, Fahey M, Muth E, Matthews M, Mattson E, Hirschmugl C, Rosenthal AK. Promotion of articular cartilage matrix vesicle mineralization by type I collagen. Arthritis Rheum. 2008;58:2809–2817. doi: 10.1002/art.23762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ferreira E, Porter RM, Wehling N, O’Sullivan RP, Liu F, Boskey A, Estok DM, Harris MB, Vrahas MS, Evans CH, Wells JW. Inflammatory cytokines induce a unique mineralizing phenotype in mesenchymal stem cells derived from human bone marrow. J Biol Chem. 2013;288:29494–29505. doi: 10.1074/jbc.M113.471268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ding J, Ghali O, Lencel P, Broux O, Chauveau C, Devedjian JC, Hardouin P, Magne D. TNF-alpha and IL-1beta inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009;84:499–504. doi: 10.1016/j.lfs.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 189.Schor AM, Allen TD, Canfield AE, Sloan P, Schor SL. Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci. 1990;97:449–461. doi: 10.1242/jcs.97.3.449. [DOI] [PubMed] [Google Scholar]
- 190.Boström K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114(1 Suppl):I547–I552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 192.Hsu HH, Camacho NP. Isolation of calcifiable vesicles from human atherosclerotic aortas. Atherosclerosis. 1999;143:353–362. doi: 10.1016/s0021-9150(98)00322-0. [DOI] [PubMed] [Google Scholar]
- 193.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 194.Shroff RC, McNai R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;21:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 195.Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, Jahnen-Dechent W, Shanahan CM. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–2930. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 196.Schurgers LJ, Spronk HM, Skepper JN, Hackeng TM, Shanahan CM, Vermeer C, Weissberg PL, Proudfoot D. Post-translational modifications regulate matrix Gla protein function: importance for inhibition of vascular smooth muscle cell calcification. J Thromb Haemost. 2007;12:2503–2511. doi: 10.1111/j.1538-7836.2007.02758.x. [DOI] [PubMed] [Google Scholar]
- 197.Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Viegas CS, Rafael MS, Enriquez JL, Teixeira A, Vitorino R, Luís IM, Costa RM, Santos S, Cavaco S, Neves J, Macedo AL, Willems BA, Vermeer C, Simes DC. Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system. Arterioscler Thromb Vasc Biol. 2015;35:399–408. doi: 10.1161/ATVBAHA.114.304823. [DOI] [PubMed] [Google Scholar]
- 199.Murshed M, McKee MD. Molecular determinants of extracellular matrix mineralization in bone and blood vessels. Curr Opin Nephrol Hypertens. 2010;19:359–365. doi: 10.1097/MNH.0b013e3283393a2b. [DOI] [PubMed] [Google Scholar]
- 200.Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert WA, Superti-Furga A, Terkeltaub R. PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fakhry M, Roszkowska M, Briolay A, Bougault C, Guignandon A, Diaz-Hernandez JL, Diaz-Hernandez M, Pikula S, Buchet R, Hamade E, Badran B, Bessueille L, Magne D. TNAP stimulates vascular smooth muscle cell trans-differentiation into chondrocytes through calcium deposition and BMP-2 activation: Possible implication in atherosclerotic plaque stability. Biochim Biophys Acta. 2017;1863:643–653. doi: 10.1016/j.bbadis.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 202.Kiffer-Moreira T, Yadav MC, Zhu D, Narisawa S, Sheen C, Stec B, Cosford ND, Dahl R, Farquharson C, Hoylaerts MF, Macrae VE, Millán JL. Pharmacological inhibition of PHOSPHO1 suppresses vascular smooth muscle cell calcification. J Bone Miner Res. 2013;28:81–91. doi: 10.1002/jbmr.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dai XY, Zhao MM, Cai Y, Guan QC, Zhao Y, Guan Y, Kong W, Zhu WG, Xu MJ, Wang X. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 2013;83:1042–1051. doi: 10.1038/ki.2012.482. [DOI] [PubMed] [Google Scholar]
- 204.New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113:72–77. doi: 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ciancaglini P, Siamo A, Bolean M, Millán J, Rigos C, Yoneda J, Colhone M, Stabeli R. Proteoliposomes in nanobiotechnology. Biophys Rev. 2012;4:67–81. doi: 10.1007/s12551-011-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Simao A, Bolean M, Cury T, Stabeli R, Itri R, Ciancaglini P. Liposomal systems as carriers for bioactive compounds. Biophys Rev. 2015;4:391–397. doi: 10.1007/s12551-015-0180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Blandford NR, Sauer GR, Genge BR, Wu LN, Wuthier RE. Modeling of matrix vesicle biomineralization using large unilamellar vesicles. J Inorg Biochem. 2003;94:14–27. doi: 10.1016/s0162-0134(02)00629-3. [DOI] [PubMed] [Google Scholar]
- 208.Rigos C, Nobre T, MED Z, Ward R, Ciancaglini P. Circular dichroism associated with surface tension and dilatation elasticity to study the association of Na, K-ATPase subnits. J Coll Interf Sci. 2008:478–484. doi: 10.1016/j.jcis.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 209.Rigos CF, de Lima Santos H, Yoneda JS, Montich G, Maggio B, Ciancaglini P. Cytoplasmatic domain of Na,K-ATPase alpha-subunit is responsible for the aggregation of the enzyme in proteoliposomes. Biophys Chem. 2010;146:36–41. doi: 10.1016/j.bpc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 210.Ciancaglini P, Simão AM, Camolezi FL, Millán JL, Pizauro JM. Contribution of matrix vesicles and alkaline phosphatase to ectopic bone formation. Braz J Med Biol Res. 2006;39:603–610. doi: 10.1590/s0100-879x2006000500006. [DOI] [PubMed] [Google Scholar]
- 211.Simão AM, Yadav MC, Narisawa S, Bolean M, Pizauro JM, Hoylaerts MF, Ciancaglini P, Millán JL. Proteoliposomes harboring alkaline phosphatase and nucleotide pyrophosphatase as matrix vesicle biomimetics. J Biol Chem. 2010;285:7598–7609. doi: 10.1074/jbc.M109.079830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Simão AM, Bolean M, Hoylaerts MF, Millán JL, Ciancaglini P. Effects of pH on the production of phosphate and pyrophosphate by matrix vesicles’ biomimetics. Calcif Tissue Int. 2013;93:222–232. doi: 10.1007/s00223-013-9745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bolean M, Simão AM, Favarin BZ, Millán JL, Ciancaglini P. The effect of cholesterol on the reconstitution of alkaline phosphatase into liposomes. Biophys Chem. 2010;152:74–79. doi: 10.1016/j.bpc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 214.Bolean M, Simão AM, Favarin BZ, Millán JL, Ciancaglini P. Thermodynamic properties and characterization of proteoliposomes rich in microdomains carrying alkaline phosphatase. Biophys Chem. 2011;158:111–118. doi: 10.1016/j.bpc.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Camolezi FL, Daghastanli KR, Magalhães PP, Pizauro JM, Ciancaglini P. Construction of an alkaline phosphatase-liposome system: a tool for biomineralization study. Int J Biochem Cell Biol. 2002;34:1091–1101. doi: 10.1016/s1357-2725(02)00029-8. [DOI] [PubMed] [Google Scholar]
- 216.Ierardi DF, Pizauro JM, Ciancaglini P. Erythrocyte ghost cell-alkaline phosphatase: construction and characterization of a vesicular system for use in biomineralization studies. Biochim Biophys Acta. 2002;1567:183–192. doi: 10.1016/s0005-2736(02)00615-6. [DOI] [PubMed] [Google Scholar]
- 217.Benatti CR, Epand RM, Lamy MT. Low cholesterol solubility in DODAB liposomes. Chem Phys Lipids. 2007;145:27–36. doi: 10.1016/j.chemphyslip.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 218.Sesana S, Re F, Bulbarelli A, Salerno D, Cazzaniga E, Masserini M. Membrane features and activity of GPI-anchored enzymes: alkaline phosphatase reconstituted in model membranes. Biochemistry. 2008;47:5433–5440. doi: 10.1021/bi800005s. [DOI] [PubMed] [Google Scholar]
- 219.Simão AM, Yadav MC, Ciancaglini P, Millán JL. Proteoliposomes as matrix vesicles’ biomimetics to study the initiation of skeletal mineralization. Braz J Med Biol Res. 2010;43:234–241. doi: 10.1590/s0100-879x2010007500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Garcia AF, Simão AM, Bolean M, Hoylaerts MF, Millán JL, Ciancaglini P, Costa-Filho AJ. Effects of GPI-anchored TNAP on the dynamic structure of model membranes. Phys Chem Chem Phys. 2015;17:26295–26301. doi: 10.1039/c5cp02377g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Eanes ED, Hailer AW, Costa JL. Calcium phosphate formation in aqueous suspensions of multilamellar liposomes. Calcif Tissue Int. 1984;36:421–430. doi: 10.1007/BF02405354. [DOI] [PubMed] [Google Scholar]
- 222.Eanes ED, Hailer AW. Liposome-mediated calcium phosphate formation in metastable solutions. Calcif Tissue Int. 1985;37:390–394. doi: 10.1007/BF02553708. [DOI] [PubMed] [Google Scholar]
- 223.Eanes E, Hailer A, Costa J. Calcium phosphate precipitation in aqueous suspensions of phosphatidylserine-containing anionic liposomes. Calcif Tissue Int. 1987:43–48. doi: 10.1007/BF02555727. [DOI] [PubMed] [Google Scholar]
- 224.Eanes ED. Biophysical aspects of lipid interaction with mineral: liposome model studies. Anat Rec. 1989;224:220–225. doi: 10.1002/ar.1092240211. [DOI] [PubMed] [Google Scholar]
- 225.Lanier M, Sergienko E, Simão AM, Su Y, Chung T, Millán JL, Cashman JR. Design and synthesis of selective inhibitors of placental alkaline phosphatase. Bioorg Med Chem. 2010;18:573–579. doi: 10.1016/j.bmc.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Genge BR, Wu LN, Wuthier RE. Kinetic analysis of mineral formation during in vitro modeling of matrix vesicle mineralization: effect of annexin A5, phosphatidylserine, and type II collagen. Anal Biochem. 2007;367:159–166. doi: 10.1016/j.ab.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 227.Genge BR, Wu LN, Wuthier RE. Mineralization of annexin-5-containing lipid-calcium-phosphate complexes: modulation by varying lipid composition and incubation with cartilage collagens. J Biol Chem. 2008;283:9737–9748. doi: 10.1074/jbc.M706523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bolean M, Borin IA, Simao AMS, Bottini M, Bagotolli LA, Hoylaerts MF, Millán JL, Ciancaglini P. Topographic analysis by atomic force microscopy of proteoliposomes matrix vesicle mimetics harboring TNAP and AnxA5. Biochim Biophys Acta. 2017;1859:1911–1920. doi: 10.1016/j.bbamem.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Di Mauro S, Manes T, Hessle L, Kozlenkov A, Pizauro JM, Hoylaerts MF, Millán JL. Kinetic characterization of hypophosphatasia mutations with physiological substrates. J Bone Miner Res. 2002;17:1383–1391. doi: 10.1359/jbmr.2002.17.8.1383. [DOI] [PubMed] [Google Scholar]
- 230.Sergienko E, Su Y, Chan X, Brown B, Hurder A, Narisawa S, Millán JL. Identification and characterization of novel tissue-nonspecific alkaline phosphatase inhibitors with diverse modes of action. J Biomol Screen. 2009;14:824–837. doi: 10.1177/1087057109338517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bottini M, Sacchetti C, Pietroiusti A, Bellucci S, Magrini A, Rosato N, Bottini N. Targeted nanodrugs for cancer therapy: prospects and challenges. J Nanosci Nanotechnol. 2014;14:98–114. doi: 10.1166/jnn.2014.9010. [DOI] [PubMed] [Google Scholar]
- 232.Bottini M, Bhattacharya K, Fadeel B, Magrini A, Bottini N, Rosato N. Nanodrugs to target articular cartilage: An emerging platform for osteoarthritis therapy. Nanomedicine. 2016;12:255–68. doi: 10.1016/j.nano.2015.09.013. [DOI] [PubMed] [Google Scholar]


