Abstract
Gut-microbiome provides the complementary metabolic potential to the human system. To understand the active participation and the performance of the microbial community in human health, the concept of gut as a plug-flow reactor with the fed-batch mode of operation can provide better insight. The concept suggests the virtual compartmentalized gut with sequential stratification of the microbial community in response to a typical host genotype. It also provides the analysis plan for gut microbiome; and its relevance in developing health management options under the identified clinical conditions.
Keywords: Gut microbiome, Plug-flow reactor, Microbial diversity, Human health, SNP
The advent of detailing in gut physiology, that gave an insight through gut microbiota, has now opened a new paradigm in understanding of human health. An individual with a typical lifestyle and socio-economic status has its own nutritional regime. With this concept, a correlation between human genetics and the type of nutrition has evolved as: nutrigenetics or nutriomics [1]. Detailed information and understanding of human gut microbiome has resulted in the emergence of a new concept of “superorganism”. The concept considers that human physiology is an aggregate action of the combined genetic intelligence of its genome and the gut microbiota. The genetic information participating through gut microbiota is enormous; and the ratio 1:1 for human cells to microbes in the gut is estimated [2–4]. The characteristic of gut biochemistry is the observed pH and oxygen level gradient, and the type of diet that contributes to the overall physiology of this ecosystem that includes the colonization of different microbes [5, 6]. For example, the polysaccharides as a major diet component have a direct influence on host–microbiome interaction. It not only results in dysbiosis but also increases the level of fucosylation of epithelial layer [7]. The microbiome and host interaction with given type of diet generate the required energy for this macro-ecosystem that is the superorganism [8]. The diet and typical host conditions were studied using various models including the germ-free animal model. The studies suggest that a typical diet plan can influence species diversity and their abundance profile in favor of delivering health benefits [6, 9]. The optimum functioning of gut physiology requires a desired microbial community. This is composed of keystone species, which bring out are essential functions and for its hierarchy in metabolism [10]. The overall metabolism of the gut ecosystem is the outcome of a collaborative effort between host housekeeping activity and survival of microbiome. The complex molecules are utilized for energy generation not only for the host through metabolites released by the host but also by the resultant secretome due to response to the microbial community. The final gut-milieu has a mixture of metabolite through inter-species interactions and due to biotransformation [8, 11]. There are different reports on physiological performance under the defined clinical scenarios that showed dysbiosis [12–14]; of which obesity is highly studied model [15]. To address the issue of dysbiosis, the different diet plans are reported [16–18]. In brief, this highly active surface area of the intestinal tract, which is about 200 m2 in human, provides the platform for host and microbial interspecies interaction, leading to different metabolic and key signaling events, which are linked to health [19–21].
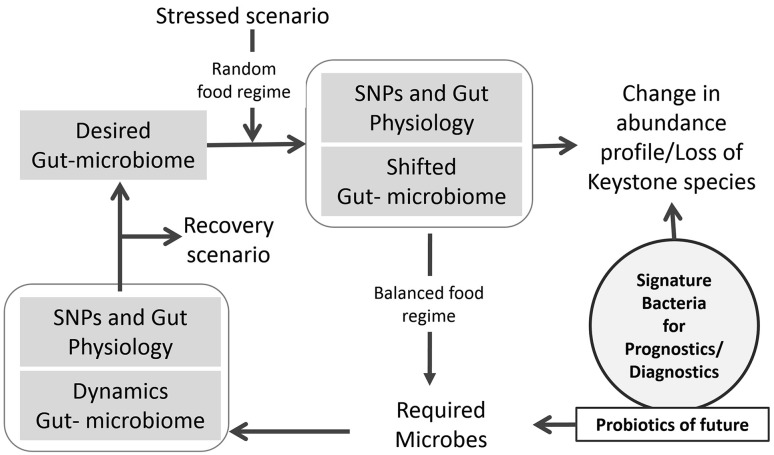
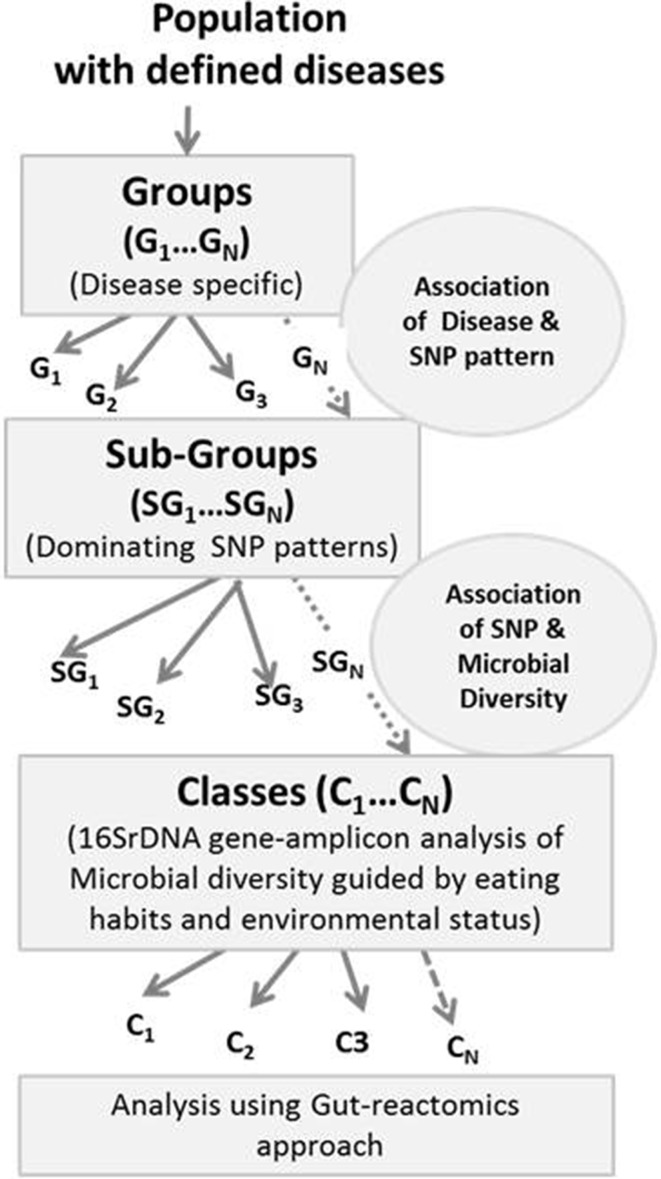
The human gut is essentially a bioreactor system, which requires regular feeding with a basic nutritional diet to ensure its optimum performance. If we take the analogy of conventional fed-batch reactor then its feed profile can be planned for desired performance using evolutionary algorithm [22]. The second important aspect of the gut ecosystem is the environment created by the host, which is decided and influenced by its genotype or its characteristics in terms of SNPs [23]. In a holistic approach, the performance of host genome with its complementary microbiome as a partner would result in series of metabolites and other secretory products with defined nutritional parameters. Hence, the gut-bioreactor has working options as shown in Fig. 1. Therefore, in principle, the gut-bioreactor with its pH and oxygen gradient, which is across the total length of the reactor, would create the fed-batch operational conditions with virtual plug-flow compartments. This scenario matches with a reactor operating in fed-batch mode on food and vegetable waste [24]. In this reactor also, there is pH gradient from 6.5 to 8.5 with almost anaerobic conditions. With the change in loading conditions of the same type of feed, the microbial diversity shifts but overall output remains same due to microbial community plasticity. However, in human gut-ecosystem, an additional variant is the active reactor surface, which directly interacts either with brain or some organs; and it provides the fluctuation in reactant biochemistry with in the reactor. Under the stressed conditions, although the genotype remains the same but the reactor’s expression changes through the active surface area, which affects the overall environment for residing community. Therefore, under the stressed condition, the reactor will observe a new enrichment plan that would selectively shift its microbial community structure [25]. The emerged scenario would identify new abundance profile for the dominating species with almost no keystone species that are otherwise linked with the healthy system. Considering the gut as a bioreactor, in principle, its desired performance could be realized back by altering the feed plan or quality with the fine-tuning of the surface secretions under the shifted gradient of pH and oxygen. It is quite possible with the analogy of virtual plug flow system; it will demand a specific feeding schedule this would gradually meet the required body function. This reprogramming of reactor would almost bring back the original microbial abundance data; and then with time, the microbial functional plasticity should support the desired reactor output or will require support with bioaugmentation [26]. It also suggests that there exists a correlation, which supports the healthy state of an individual; and it is a function of the microbial community and its typical SNP profile. With the changing efficiency of gut-bioreactor and stress scenario due to environmental variations, the different clinical conditions could emerge. Hence, we can bring a new equation wherein keeping the average genetic profile constant, the microbial diversity can be linked to a clinical scenario [27, 28] as shown in Fig. 2. The comparative analysis of stressed microbial community profile from a healthy gut system would suggest the probable keystone species. If the generated data remains statistically significant for gut samples derived from a group that represents same average dominating SNPs, then the identified keystone species would find its application in developing probiotic or monitoring tools.
Fig. 1.
Conceptual flow for gut-reactomics
Fig. 2.
Sample classification for gut-reactomics
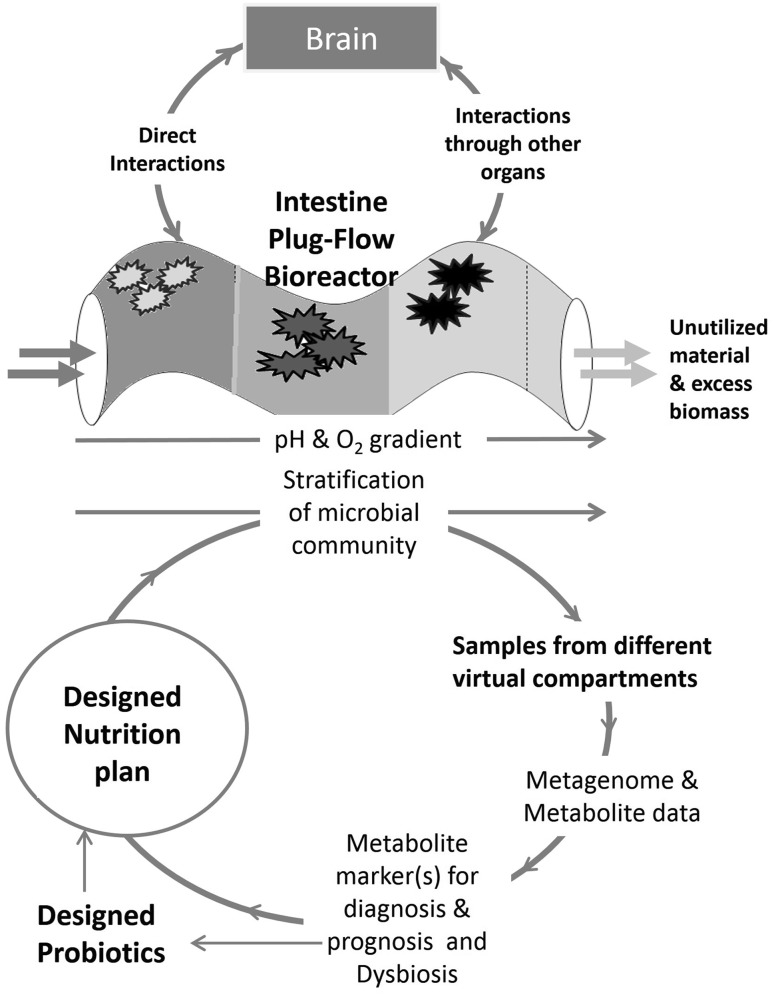
The functional nexus between different organs and gut is emerging that is linked even with diseases, cognitive performance and aging [29–33]. Nevertheless, relating all this with the concept of keystone species ignores the vast diversity data associated with the complexity of gut-bioreactor. It could be possible that the stressed condition could only affect the population dynamics by playing with the abundance data with no change in species diversity. The observed change in gut biochemistry could be due to changes in the ratio of the different functional genetic unit that might be affecting the different levels or flux flow of metabolites. The bias with measurements and analysis of abundance data also does not give us an access to significantly under-represented population or a key species. Such species might have relevance to shifted metabolism. To understand more about the functional biochemistry of this reactor, the support from mathematical models can help in evaluation of the efficiency of the plug-flow conditions with given loading and its schedule. By defining the experimental boundaries for community performance, the expected metabolite profile under dynamic conditions can be also predicted. The developed model can be validated using germ-free animals with designed microbial community structure. The studies are required to develop the linkages with metabolite profile that would provide a deeper insight of shifted function of this “superorganism” with dysbiosis. Such studies will yield biomarkers that could be either a metabolite or a microbial species, which would find applications in health management in dysbiosis as shown in Fig. 3 [21].
Fig. 3.
Experimental design and analysis for gut-reactomics
The multiomics approaches with different computational tools are emerging as an option to address the gut microbiome–host interactions [34]. However, the experimental design and analysis in gut-microbiome interactions need a paradigm shift. For this plug-flow reactor, the properties derived from nutriomics of the host with meta-omics of microbial communities could become individual specific. It could be explored under a different definition, the “Gut-reactomics” wherein the conditional performance of gut could be studied for its functional output. The study of gut microbiome should not be only restricted to taxonomical associations as shown in Fig. 3. Since, in this reactor, the output of the reactor is utilized by the host system through active reactor surface area whereas an unutilized material with excess microbial mass is discarded routinely. Hence, a metabolite profile in these virtual compartments of the plug-flow reactor could provide better insight of sequential stratification of microbial colonies. However, at present, in most of the cases, the gut microbiome analysis is represented by an average composite or grab sample for this reactor. To understand the performance of the microbial community in these virtual compartments, there is need to address the flux of metabolite through these virtual compartments. The sequential utilization of intake material would dictate the colonization of microbial abundance in each compartment, which would be in relation to the prevailing host response in that compartment. To understand the dynamics of this complex reactor, the support from modeling tools will help in better experimental design [22, 35, 36].
Different experimental model with the demographic and temporal data would finally define the working boundary for this plug-flow bioreactor. The analysis will identify the metabolites or a species, which are specific to a particular clinical scenario. The comparative microbial genomics of dysbiosis will help in designing the probiotics of future, which will require support from nutrigenomics for welfare, and intervention of stressed human physiology.
Acknowledgements
The authors thank the Director, CSIR-NEERI for providing constant support and infrastructure for the research work; and manuscript has institute’s publication reference number KRC\2017\Dec\EBGD\2.
References
- 1.Mutch DM, Wahli W, Williamson G. Nutrigenomics and nutrigenetics: the emerging faces of nutrition. FASEB. 2005;19:1602–1616. doi: 10.1096/fj.05-3911rev. [DOI] [PubMed] [Google Scholar]
- 2.Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segre JA, Salafsky N. Hominid superorganisms. Science. 2016;353:350–351. doi: 10.1126/science.aag2788. [DOI] [PubMed] [Google Scholar]
- 4.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 6.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalová L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E. Personalized nutrition by prediction of glycemic esponses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Porter NT, Martens EC. The critical roles of polysaccharides in gut microbial ecology and physiology. Annu Rev Microbiol. 2017;71:349–369. doi: 10.1146/annurev-micro-102215-095316. [DOI] [PubMed] [Google Scholar]
- 8.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cencič A, Langerholc T. Functional cell models of the gut and their applications in food microbiology—a review. Int J Food Microbiol. 2010;141:S4–S14. doi: 10.1016/j.ijfoodmicro.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flint HJ, Duncan SH, Louis P. The impact of nutrition on intestinal bacterial communities. Curr Opin Microbiol. 2017;38:59–65. doi: 10.1016/j.mib.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Wissenbach DK, Oliphant K, Rolle-Kampczyk U, Yen S, Höke H, Baumann S, Haange SB, Verdu EF, Allen-Vercoe E, von Bergen M. Optimization of metabolomics of defined in vitro gut microbial ecosystems. Int J Med Microbiol. 2016;306:280–900. doi: 10.1016/j.ijmm.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Gevers D, Kugathasan S, Knights D, Kostic AD, Knight R, Xavier RJ. A microbiome foundation for the study of Crohn’s disease. Cell Host Microbe. 2017;21:301–304. doi: 10.1016/j.chom.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D’Amato M, Bonfiglio F, McDonald D, Gonzalez A, McClure EE. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2:17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 15.Malaisé Y, Menard S, Cartier C, Gaultier E, Lasserre F, Lencina C, Harkat C, Geoffre N, Lakhal L, Castan I, Olier M. Gut dysbiosis and impairment of immune system homeostasis in perinatally-exposed mice to Bisphenol A precede obese phenotype development. Sci Rep. 2017;7:14472. doi: 10.1038/s41598-017-15196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu P, Hong F, Wang J, Wang J, Zhao X, Wang S, Xue T, Xu J, Zheng X, Zhai Y. DBZ is a putative PPARγ agonist that prevents high fat diet-induced obesity, insulin resistance and gut dysbiosis. Biochim Biophys Acta Gen Subj. 2017;1861:2690–2701. doi: 10.1016/j.bbagen.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Pindjakova J, Sartini C, Lo Re O, Rappa F, Coupe B, Lelouvier B, Pazienza V, Vinciguerra M. Gut dysbiosis and adaptive immune response in diet-induced obesity vs. systemic inflammation. Front Microbiol. 2017;8:1157. doi: 10.3389/fmicb.2017.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzutsev A, Badger JH, Perez-Chanona E, Roy S, Salcedo R, Smith CK, Trinchieri G. Microbes and cancer. Ann Rev Immunol. 2017;35:199–228. doi: 10.1146/annurev-immunol-051116-052133. [DOI] [PubMed] [Google Scholar]
- 19.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee WJ, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol. 2014;10:416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- 21.Kurilshikov A, Wijmenga C, Fu J, Zhernakova A. Host genetics and gut microbiome: challenges and perspectives. Trends Immunol. 2017;38:633–647. doi: 10.1016/j.it.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Ronen M, Shabtai Y, Guterman H. Optimization of feeding profile for a fed-batch bioreactor by an evolutionary algorithm. J Biotechnol. 2002;97:253–263. doi: 10.1016/S0168-1656(02)00106-2. [DOI] [PubMed] [Google Scholar]
- 23.Hu P, Hsieh MH, Lei MJ, Cui B, Chiu SK, Tzeng CM. A simple algorithm for population classification. Sci Rep. 2016;6:23491. doi: 10.1038/srep23491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulhane M, Pandit P, Khardenavis A, Singh D, Purohit H. Study of microbial community plasticity for anaerobic digestion of vegetable waste in anaerobic baffled reactor. Renew Energy. 2017;101:59–66. doi: 10.1016/j.renene.2016.08.021. [DOI] [Google Scholar]
- 25.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coyte KZ, Schluter J, Foster KR. The ecology of the microbiome: networks, competition, and stability. Science. 2015;350:663–666. doi: 10.1126/science.aad2602. [DOI] [PubMed] [Google Scholar]
- 27.Franzosa EA, Huang K, Meadow JF, Gevers D, Lemon KP, Bohannan BJ, Huttenhower C. Identifying personal microbiomes using metagenomic codes. Proc Natl Acad Sci. 2015;112:E2930–E2938. doi: 10.1073/pnas.1423854112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang K, Gaitsch H, Poon H, Cox NJ, Rzhetsky A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat Genet. 2017;49:1319–1325. doi: 10.1038/ng.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annalisa N, Alessio T, Claudette TD, Erald V, Antonino DL, Nicola DD. Gut microbioma population: an indicator really sensible to any change in age, diet, metabolic syndrome, and life-style. Mediat Inflamm. 2014 doi: 10.1155/2014/901308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luceri C, Bigagli E, Pitozzi V, Giovannelli L. A nutrigenomics approach for the study of anti-aging interventions: olive oil phenols and the modulation of gene and microRNA expression profiles in mouse brain. Eur J Nutr. 2017;56:865–877. doi: 10.1007/s00394-015-1134-4. [DOI] [PubMed] [Google Scholar]
- 31.Meng Q, Ying Z, Noble E, Zhao Y, Agrawal R, Mikhail A, Zhuang Y, Tyagi E, Zhang Q, Lee JH, Morselli M. Systems nutrigenomics reveals brain gene networks linking metabolic and brain disorders. EBioMedicine. 2016;7:157–166. doi: 10.1016/j.ebiom.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noble EE, Hsu TM, Kanoski SE. Gut to brain dysbiosis: mechanisms linking Western Diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci. 2017;11:9. doi: 10.3389/fnbeh.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallick H, Ma S, Franzosa EA, Vatanen T, Morgan XC, Huttenhower C. Experimental design and quantitative analysis of microbial community multiomics. Genome Biol. 2017;18:228. doi: 10.1186/s13059-017-1359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moorthy AS, Eberl HJ. compuGUT: an in silico platform for simulating intestinal fermentation. SoftwareX. 2017;6:237–242. doi: 10.1016/j.softx.2017.06.004. [DOI] [Google Scholar]
- 36.Liotta F, Chatellier P, Esposito G, Fabbricino M, Van Hullebusch ED, Lens PN. Hydrodynamic mathematical modelling of aerobic plug flow and non-ideal flow reactors: a critical and historical review. Crit Rev Environ Sci Technol. 2014;44:2642–2673. doi: 10.1080/10643389.2013.829768. [DOI] [Google Scholar]