Abstract
We analyze the performance of a mammographically-configured, automated breast ultrasound (McABUS) scanner combined with a digital breast tomosynthesis (DBT) system. The GE Invenia ultrasound system was modified for integration with GE DBT systems. The ultrasound and DBT imaging were performed in the same mammographic compression. Our small preliminary study included thirteen cases, six of which contained invasive cancers. From analysis of these cases, current limitations and corresponding potential improvements of the system were determined. A registration analysis was performed to compare the ease of McABUS to DBT registration for this system with that of two systems designed previously. It was observed that, in comparison to data from an earlier study, the McABUS to DBT registration alignment errors for both this system and for a previously built combined system were smaller than for a previously built standalone McABUS system.
Keywords: Automated breast ultrasound, Digital breast tomosynthesis, Mammography, 3-D imaging, Compression, Combined system
Introduction
Several studies (Berg et al. 2008; Giger et al. 2016; Weigert and Steenbergen 2015; Wilczek et al. 2016) have shown significant increases in cancer detection rates with the addition of ultrasound (US) screening to mammography in dense breasts. Digital breast tomosynthesis (DBT) is more sensitive to breast cancer masses in the dense breasts than digital mammography (Sharpe et al. 2016). One recent study (Tagliafico et al. 2016) compared DBT with US for screening in mammographically negative dense breasts, and found that US detected almost double the number of cancers as the DBT, and had a comparable rate of false-positive recall for biopsy. Conventional breast ultrasound screening is highly dependent on skill and experience of the operator, and requires skillful probe manipulation. It is typically performed freehand in an uncompressed supine geometry. A significant source of the uncertainty, and hence recalls, in the reading of handheld ultrasound or automated breast ultrasound (ABUS) images is the difficulty in translating supine imaging to the upright compressed imaging of DBT or mammography (Brem and Gatewood 1992; Conway et al. 1991). ABUS to DBT registration is easier if the ABUS is instead performed in same geometry as the DBT imaging. We will refer to this as mammographically configured ABUS (McABUS).
To explore the potential of McABUS, two systems were previously designed to provide proof-of-concept for 1) combined McABUS-DBT (Padilla et al. 2013) and 2) standalone dual-sided McABUS (Carson et al. 2011; Larson et al. 2016). These two systems will be referred to as the first generation combined system and the standalone system, respectively. Both systems used GE LOGIQ 9 ultrasound scanners with M12L transducers (GE Healthcare, Milwaukee, WI, USA). A third system, referred to as the second generation combined system, is the focus of this study. This system is a prototype combined McABUS-DBT system in which our non-FDA approved, prototype research DBT unit was combined with the FDA approved supine screening ABUS system, the Invenia (GE Healthcare, Sunnyvale, CA, USA). While all the equipment mentioned above was made by, or in cooperation with GE, we note that this is not the only company to have made such equipment.
Our purpose was to examine the mass detection of the system to determine limitations and potential improvements, and also to compare the ease of McABUS to DBT registration for this system with that of the two previously designed systems. Comparable data is not available on other systems for automated breast ultrasound in the mammographic geometry (Dines et al. 2005; Leproux et al. 2010; Richter et al. 1997; Smith 2014; Vaughan et al. 2016).
Materials and Methods
The transducer transport and compression frame of the Invenia was modified and integrated into a mammography compression paddle which could be inserted into the prototype DBT system at the University of Michigan (Eberhard et al. 2006; Goodsitt et al. 2014) or on a FDA approved commercial DBT system, the SenoClaire (GE Healthcare, Chicago, IL, USA). In this system, referred to as the second generation combined system, the Invenia automatically scans the breast using a large (15.4 cm), 6-15 MHz bandwidth linear-array transducer at 10 MHz center frequency and produces a 3D B-scan ultrasound image volume measuring 15 cm × 10 cm × 5 cm. The probe’s unique concave shape gives it improved contact to the breast and better patient comfort. The Invenia was modified in several ways for this study, and the configuration used for this study is not FDA approved. The Invenia was given a new operator interface, and the transducer was mounted on a hinge that allowed it to be lifted up and out of the way of the x-ray beam path. The Invenia transducer is shown mounted on the prototype DBT and on the SenoClaire in Figures. 1A and 1B, respectively.
Figure 1. Modification of the Invenia for the mammographic geometry.
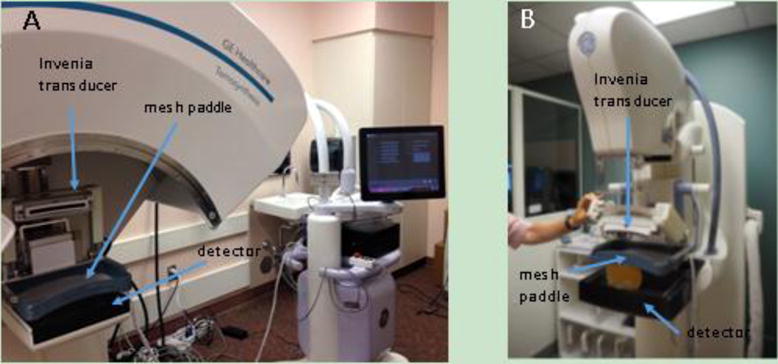
(A) Second generation combined system of prototype DBT and Invenia, using custom Invenia-compatible compression paddle. (B) GE SenoClaire DBT system with Invenia transducer and Invenia-compatible compression paddle.
The prototype DBT system was used as part of the second generation combined system for the cases presented here because the image quality from the prototype is equivalent to the SenoClaire and we encountered scheduling logistical issues with the SenoClaire system. That prototype DBT used the same detector design and the same x-ray tube as the Senographe Essential (GE Healthcare, Chicago, Il, USA). For the prototype DBT, multiple scanning modes are possible. For this study, nine X-ray projections were acquired from an angular range of 24 degrees, to match the SenoClaire system, which acquires nine projections from an angular range of 25 degrees. A Simultaneous Algebraic Reconstruction Technique (SART) algorithm (Zhang et al. 2006) was used to compute the DBT images, which are typically presented as a set of slices parallel to the detector. The resolution of these slices was 0.1 mm × 0.1 mm, and the spacing between each slice was 1 mm.
The curved dual modality curved compression paddle shown in Fig. 1A was specially designed to match the curved Invenia transducer. That paddle is composed of the same polyester chiffon material, with sub-millimeter size filaments and spacing, as that which is used in the inserts for the standard Invenia ABUS system. The mesh is clear enough to allow visual inspection of the breast position through the material and porous enough to allow ultrasound coupling lotion through the weave, eliminating most air bubbles between the transducer and the breast tissue. The mesh was tightly stretched across a thin, composite material frame and glued. The mesh and composite material paddle were then inserted into an aluminum frame that matches the exact dimensions of a standard tomosynthesis compression paddle for the SenoClaire system. A comparison between the 15.4 cm Invenia transducer and the 3.8 cm GE M12L transducer used in the first generation combined system is shown in Figure 2.
Figure 2. Comparison between ultrasound probes in first and second generation combined systems.
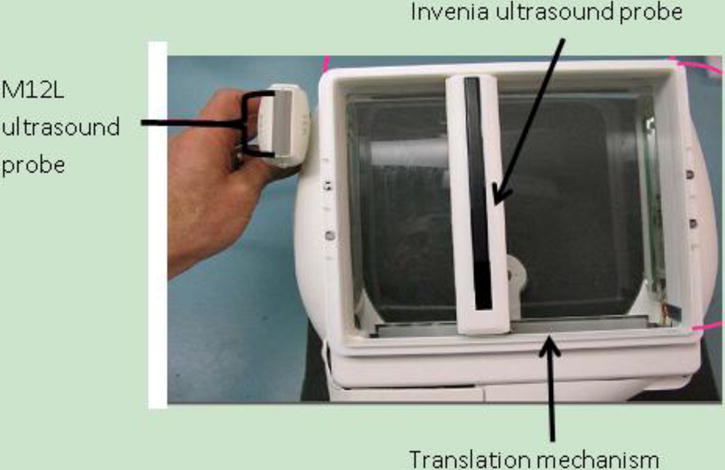
Comparison between the 3.8 cm (192 element) M12L transducer used in the first generation combined system and the 15.4 cm (768 element) Invenia probe used in the second generation combined system, shown within the commercial Invenia scanning frame.
The human subject study of thirteen patients imaged with the second generation combined system was conducted under IRB (HUM00069294), approved by the University of Michigan IRB. Informed consent was received from each subject in this study. Patients between the ages of 30 and 80 satisfying one of the following two criteria were recruited for this study: 1) Recalled for an ultrasound-guided biopsy of a suspicious mass detected either on their screening mammogram or through palpation, or 2) had a benign mass that had been previously diagnosed. Priority was given to patients with a higher probability of cancer. For all masses, the ground truth for the identity of the mass was either ultrasound guided biopsy, as was the case for all the cancers, or clinical image examination, rendered in the radiology report.
The patients had the standard two-view, cephalocaudal and mediolateral oblique examination of the affected breast with DBT, and a cephalocaudal image taken with the Invenia McABUS. Some cases, especially when the mass was not well visualized in the cephalocaudal view, had a mediolateral oblique image taken with the Invenia as well. To change geometries, the imaging modalities themselves were rotated, while the patient positioning remained the same. After imaging the breast with the DBT, polysonic ultrasound lotion (Parker Laboratories, Inc., Fairfield, NJ) was applied directly on the superior side of the breast through the chiffon mesh without changing the breast compression. For larger breasts, additional gel was added to the breast periphery with a gel containment method used in our previous studies (Li et al. 2010). The technologist then applied the ultrasound gel, lowered the Invenia transducer onto the mesh surface, checked the coupling, and initiated the ultrasound scan. After the ultrasound scan and release of the compression and removal of residual gel, the mediolateral oblique DBT, and possibly McABUS as well, were performed. The entire procedure for acquiring one McABUS and two DBT volumes required approximately 15 minutes per patient, depending on the ease of gel application. The scanning procedure for this study was simplified by the ability of the Invenia to image the whole breast in one, 30 second sweep.
A registration analysis was performed for both the second generation combined system and for the two systems previously designed. Using the two freely-distributed image viewing software packages ImageJ (U.S. National Institutes of Health, Bethesda, Maryland U.S.A.) and 3D Slicer (Fedorov et al. 2012), the first author determined visually the registration offsets to optimally align homologous masses in the McABUS and corresponding DBT images. Cases were only included in the analysis if the images from both modalities had obvious masses, and then the visually-determined centers of these masses were used as homologous points to align the images.
Because the acquired fields of view of the McABUS and DBT modalities are not the same, there is a built-in offset between the origin corners of images from the two different modalities. From the start and end coordinates of the probe output by our probe mover software, this calibration offset can be calculated for each case. In principle, if no breast motion occurs between the McABUS and DBT imaging, the calibration offset should align the McABUS and DBT images quite well. The only remaining sources of error would be the intrinsic inaccuracy of pulse-echo ultrasound imaging due to variable speed of sound in the breast (.i.e. refraction), and any vertical displacement of the probe due to upward force by the breast. However, in practice there is always some minimal breast motion, if only due to the transducer passage over the mesh and breast.
The relevant quantity that we then determined for each case was the alignment error, which is the registration offset needed to align the homologous masses in the images, with the calibration offset already accounted for. The alignment error is therefore a measure of relative breast misplacement and distortion between the two modalities.
Results
I. Preliminary Clinical Results
Thirteen patients were imaged for this preliminary analysis by the second generation combined system, and the images were first sequentially analyzed by a radiologist and then reviewed by the first author. The mass visibility was determined by the first author with aid from a radiologist. Six of these cases contained a malignant cancer, and 5 out of 6 of these cancers were found by at least one of the modalities in this combined system. The cancer that was missed by both modalities was unseen in the DBT due to its small size, and it is unknown whether its location was outside the ultrasound field of view. This 6 mm cancer was palpable and therefore still detectable clinically. In total, the DBT found 5 out of 6 cancers, and the McABUS found 3 out of 6 cancers. Two cancers were visible in the DBT but located too far inferior in the breast to be visible in the McABUS.
There were eight benign masses seen on at least one of the DBT or the McABUS. Two of the benign masses were not seen by the DBT, which was attributed to high breast density. Four of the benign masses were not seen on the McABUS. Two of these four masses were not imaged by the McABUS because they were positioned too far inferior to the transducer and the high frequency transducer used in this study was unable to image to the needed depth. The other two were not imaged because they were too close to the chest wall to be reached by the Invenia transducer, due to excess width in the transducer housing included for patient comfort in the intended supine imaging geometry. The visibility results for all of the masses are summarized in Table 1.
Table 1. Summary of visibility of all masses.
A summary of the visibility results for all fourteen masses from the thirteen cases in this study. In column 1, the grade listed for carcinomas is the Bloom Richardson grade. The breast density in column 2 was determined by radiologist assessment. Columns 3 & 4 give the visibility of the mass in the digital breast tomosynthesis (DBT) and mammographically configured automated breast ultrasound (McABUS) modalities of our new combined system.
| Mass type | Breast density | Visibile in DBT? | Visibile in McABUS? |
|---|---|---|---|
| Ductal carcinoma (grade 3) |
Scattered fibroglandular densities | Yes | Yes |
| Ductal carcinoma (grade 2) |
Scattered fibroglandular densities | Yes | Yes |
| Lobular carcinoma (grade 2) |
Scattered fibroglandular densities | Yes | No: mass was positioned too far inferior |
| Lobular carcinoma (grade 2) |
Scattered fibroglandular densities | Yes | Yes |
| Lobular carcinoma (grade 1) |
Heterogeneously dense | Yes | No: mass was positioned too far inferior |
| Lobular carcinoma (grade 1) |
Heterogeneously dense | No: mass was too small | No: mass either too small or out of the field of view |
| Fibroadenoma | Heterogeneously dense | No: breast was too dense | Yes |
| Cyst | Heterogeneously dense | Yes | No: mass was positioned too far inferior |
| Cyst | Scattered fibroglandular densities | Yes | Yes |
| Cyst | Heterogeneously dense | Yes | Yes |
| Cyst | Heterogeneously dense | No: breast was too dense | Yes |
| Cyst | Scattered fibroglandular densities | Yes | No: mass was positioned too far inferior |
| Cyst | Almost entirely fatty | Yes | No: mass was out of the field of view; too close to the chest wall |
| Cyst | Scattered fibroglandular densities | Yes | No: mass was out of the field of view; too close to the chest wall |
II. Illustrative Cases
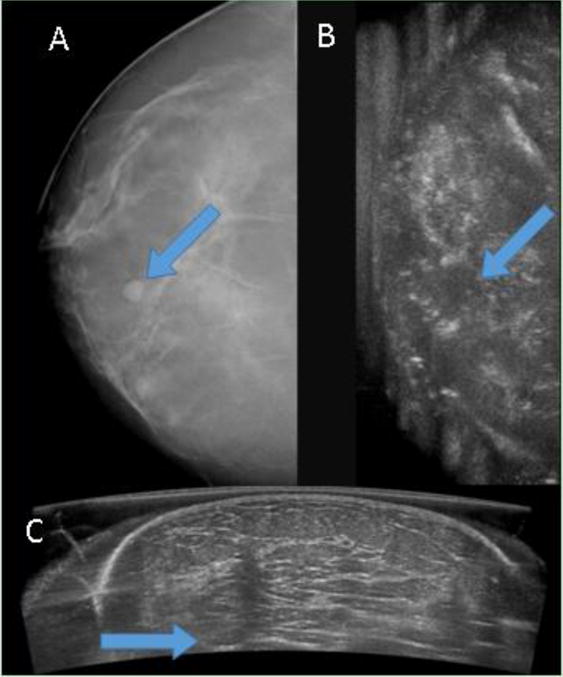
In one illustrative case (Fig. 3), a ductal carcinoma was identifiable in both the McABUS and the DBT. In the McABUS acquired view, the cancer is particularly obvious because of the spiculated hypoechoic region and the shadowing beneath it. In another case (Fig. 4), a benign cyst was seen in the DBT, but not in the McABUS, as the mass was positioned too deep (inferior) in the breast. This is an example of the current limited penetration of the single-sided McABUS, at least at the 10 MHz center frequency that was used. In a third case (Fig. 5), a fibroadenoma is visible in the McABUS, but not in the DB because it was obscured by the high breast density.
Figure 3. Invasive ductal carcinoma.
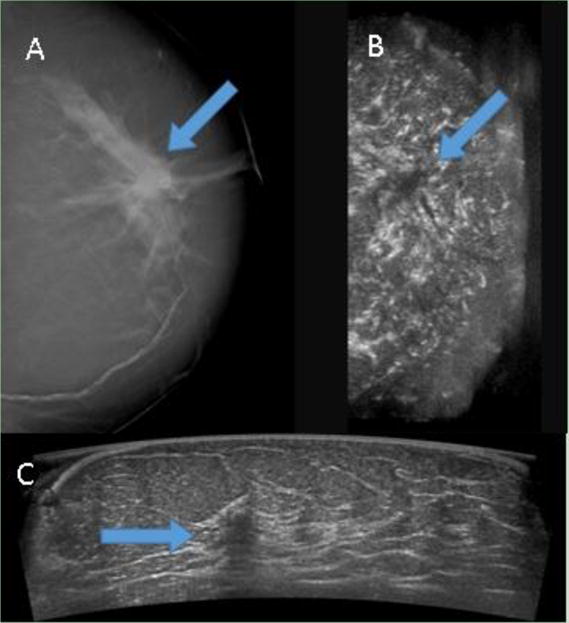
(A) digital breast tomosynthesis and (B) coronal reconstruction of automated breast ultrasound (ABUS) are shown side-by-side with the arrows marking the location of the carcinoma in both modalities. (C) ABUS acquired view is shown, with a hypoechoic region above a shadowed area clearly marking the presence of the cancer. The depth of the mass was about 27 mm, and the patient age was 72.
Figure 4. Benign cyst missed by Invenia.
(A) digital breast tomosynthesis (DBT) and (B) coronal reconstruction of automated breast ultrasound (ABUS) are shown side-by-side with the arrows marking the location of the cyst in both modalities. The cyst is clearly visible in the DBT, but unseen in the ABUS, since at this depth the image is blurry and only the strongest echoes are seen. (C) ABUS acquired view is shown, with arrow marking the probable location of the cyst. At this depth, the mass is blurry and blends in to the surrounding tissue. The depth of the mass was about 50 mm, and the patient age was 43.
Figure 5. Fibroadenoma missed by DBT.
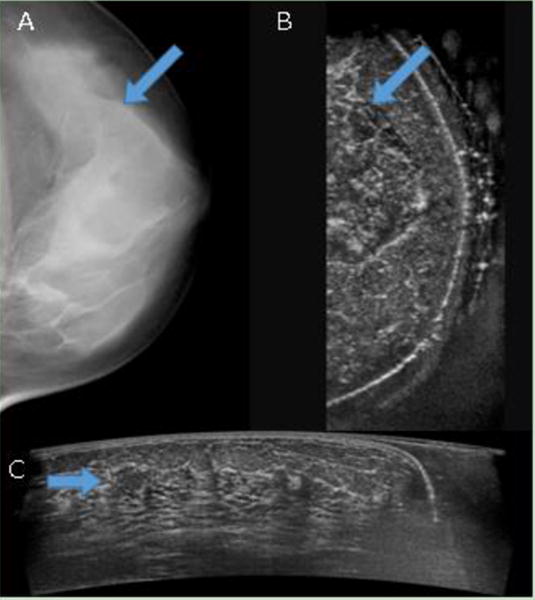
(A) digital breast tomosynthesis (DBT) and (B) coronal reconstruction of automated breast ultrasound (ABUS) are shown side-by-side, with the arrows marking the location of the fibroadenoma in both modalities. It can be seen that the fibroadenoma is indistinguishable in the DBT, but somewhat visible in the ABUS reconstructed coronal view. (C) ABUS acquired view is shown, and the clearly visible fibroadenoma is marked with an arrow. The depth of the mass was about 16 mm, and the patient age was 48.
III. Registration Analysis
The registration analysis discussed earlier is performed both for the cases imaged by the second generation combined system and for a different set of cases imaged previously by the two systems designed previously: The first generation combined system and the standalone system.
The means and standard deviations of the absolute values of the alignment errors for all three systems are shown in Figure 6, and the statistical significance of the results are given in Table 2. It should be noted that the standalone system was recalibrated at times and the physical location of the origin of the XY coordinate system moved by a few millimeters along the posterior-anterior axis, and the exact times at which this was done were not always kept track of. Thus, our results for the standalone system may have errors of a few millimeters (we estimate ~5 mm) in the posterior-anterior directions for some of the cases used in the analysis.
Figure 6. Absolute-value alignment errors.
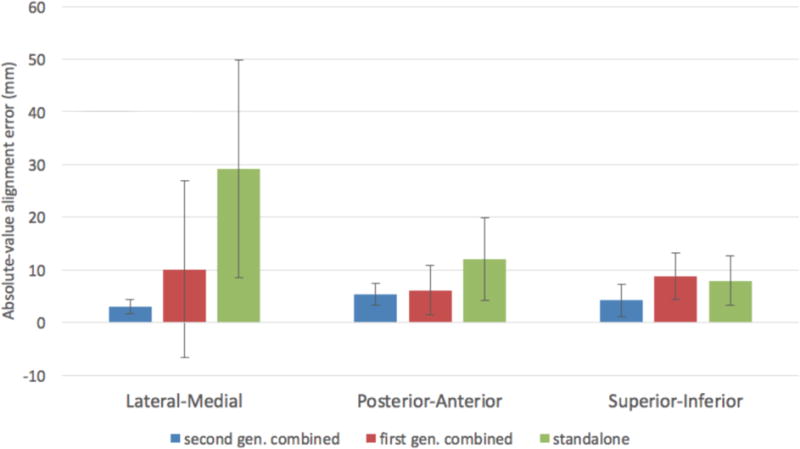
The mean and standard deviations of the absolute-value alignment errors in mm along all three axes are given for first and second generation combined systems, and the standalone system.
Table 2. Statistical significance of absolute-value alignment error between systems.
A two-tailed, unequal variance t-test was performed between all three possible pairs of the three systems, for the absolute-value alignment errors along all three axes. All p-values less than 0.05 are bolded. The sample sizes are as follows: Second generation combined (N=5), first generation combined (N=9), standalone (N=11).
| t-test absolute-value alignment error sample 1 | t-test absolute value alignment error sample 2 | Lateral-Medial p-value | Posterior-Anterior p-value | Superior-Inferior p-value |
|---|---|---|---|---|
| First gen. combined | Standalone | 0.044 | 0.065 | 0.693 |
| Second gen. combined | Standalone | 0.002 | 0.028 | 0.114 |
| First gen. combined | Second gen. combined | 0.268 | 0.723 | 0.064 |
Discussion
From this imaging study we have determined two major limitations and potential improvements to our system. One limitation is the excess width of the transducer housing of the Invenia transducer, included for patient comfort in the supine geometry of the commercial Invenia system, which prevented the transducer from getting near enough to the chest wall to see two benign masses. The second limitation is the limited depth penetration of the McABUS at the 10 MHz center frequency used, which prevented the McABUS from seeing two cancers, and also two benign masses. There are no such obvious improvements that could be made concerning the DBT modality in our system, however the DBT did miss two benign masses due to high breast density. With a lower center frequency and a thinner transducer housing, the McABUS would have improved detection and be better able to catch masses that are missed by the DBT for such reasons.
The depth of penetration of the McABUS could be improved by imaging at a center frequency lower than 10 MHz, at the cost of a lower spatial resolution. Sacrificing spatial resolution for increased coverage could be a good strategy if the system were to be used for screening, although an increase in false positives could be problematic. Another option would be to use dual-sided McABUS imaging, or to image from both the superior and the inferior sides of the breast. One could also get better posterior-anterior coverage by tilting the transducer or using 2D or 1¾D beam steering to image closer to the chest wall and more normal to the skin around the areolar region.
For the first and second generation combined systems, the breast was in the same compression for both the DBT and McABUS imaging. However, the breast was compressed separately for the ultrasound imaging for the standalone system. Thus, it was expected that the McABUS to DBT alignment error for the standalone system would be greater than for the first or second generation combined systems because of inevitable differences in the breast positioning for the two compressions. Our results were consistent with these expectations. Between the first and second generation combined systems, there was no statistically significant difference between the absolute-value alignment error along any of the three axes.
The lower alignment error with the combined systems as compared to the standalone system could be used as an argument in favor of combined McABUS-DBT imaging over McABUS and DBT imaging on separate systems. However, the ease of registration of images from a standalone McABUS system with the DBT could potentially be improved with increased training of the technologist for positioning of the breast along the lateral-medial axis, or with an automated skin line detection algorithm or registration marker beads. The second generation combined system retains several benefits over the first generation system, such as substantially reduced scan time, increased transverse coverage, and the absence of the need for image splicing.
A patient scan on the first generation system required about thirty minutes, while the second generation system required only about fifteen minutes. The difference in time came entirely from the change in the McABUS modality of the system. In the first generation system, the total ultrasound probe sweep time was five to eight minutes due to the required multiple later-medial sweeps of the transducer, whereas in the second generation system it is a single thirty-second sweep. An additional benefit of the single probe sweep was easier application of the ultrasound gel. In the second generation system, the gel could be applied to the probe and in front of the probe and the wider Invenia probe would spread the gel as it moved.
The ultrasound coverage was increased in the second generation system from that of the first generation system. The sweep length of the ultrasound modality in the first generation system was variable, but the sweep length was typically kept below 15 cm due to time constraints. Thus, this lateral-medial coverage was less in the first generation system than in the second generation system, which has a lateral-medial coverage of about 15 cm because that is roughly the width of the Invenia transducer. In the first generation system, there were not always enough lateral-medial sweeps taken to cover from near the posterior mesh paddle edge all the way out to the nipple, again due to time constraints. In the second generation system, the transducer sweeps posterior to anterior (chest wall to nipple) rather than lateral to medial as in the first generation system, and the probe sweeps from near this mesh paddle edge to out far past the nipple. Thus, the posterior-anterior coverage of the second generation system is greater than for the first generation system. Lastly, the imaging depth of the probes used in both systems is about 5cm, and the center frequency for both the M12L transducer used in the first generation system and the Invenia transducer was 10 MHz, thus we expect similar depth penetration and overall comparable superior-inferior coverage between the ultrasound modalities of the two systems.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) Grant ROI CA91713 and a contract N019172 from GE Global Research, Niskayuna, NY. Contributors from the University of Michigan included Heang-Ping Chan, Ph.D., who performed the digital breast tomosynthesis reconstructions used in this study, and Mary Burton, M.S., who was the human studies IRB liaison and data coordinator. Contributors from GE were Ying Mao, Ph.D., and Weston Griffin, Ph.D., who developed the paddle and transport mechanism used in this study with the Invenia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, Pisano ED, Jong RA, Evans WP, Morton MJ, Mahoney MC. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(19):2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem RF, Gatewood OM. Template-guided breast US. Radiology. 1992;184(3):872–874. doi: 10.1148/radiology.184.3.1509083. [DOI] [PubMed] [Google Scholar]
- Carson PL, Zafar F, Verweij SA, Lee WM, Goodsitt MM, LeCarpentier GL, Sinha S, Hooi FM, Roubidoux M, Fowlkes JB. Dual-sided automated ultrasound system in the mammographic geometry. Int Ultrasonics Symp (IUS), 2011 IEEE International. 2011:2134–2137. [Google Scholar]
- Conway WF, Hayes CW, Brewer WH. Occult breast masses: use of a mammographic localizing grid for US evaluation. Radiology. 1991;18(1):143–146. doi: 10.1148/radiology.181.1.1653441. [DOI] [PubMed] [Google Scholar]
- Dines KA, Kelly-Fry E, Romilly AP. Mammography method and apparatus. US 6,876879. U.S. Patent. 2005 Apr 05;
- Eberhard JW, Staudinger P, Smolenski J, Ding J, Schmitz A, McCoy J, Rumsey M, Al-Khalidy A, Ross W, Landberg CE, Claus BE. High speed, large angle mammography tomosynthesis system. Medical Imaging. 2006:61420C–61420C. [Google Scholar]
- Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy FM, Sonka M, Buatti J, Aylward SR, Miller JV, Pieper S, Kikinis R. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012 Nov;30(9):1323–41. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger ML, Inciardi MF, Edwards A, Papaioannou J, Drukker K, Jiang Y, Brem R, Brown JB. Automated Breast Ultrasound in Breast Cancer Screening of Women With Dense Breasts: Reader Study of Mammography-Negative and Mammography-Positive Cancers. AJR Am J Roentgenol. 2016:1–10. doi: 10.2214/AJR.15.15367. [DOI] [PubMed] [Google Scholar]
- Goodsitt MM, Chan HP, Schmitz A, Zelakiewicz S, Telang S, Hadjiiski L, Watcharotone K, Helvie MA, Paramagul C, Neal C, Christodoulou E. Digital breast tomosynthesis: studies of the effects of acquisition geometry on contrast-to-noise ratio and observer preference of low-contrast objects in breast phantom images. Phys Med Biol. 2014;59(19):5883–5902. doi: 10.1088/0031-9155/59/19/5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ED, Lee WM, Roubidoux MA, Goodsitt MM, Lashbrook C, Zafar F, Kripfgans OD, Thomenius K, Carson PL. Automated breast ultrasound: dual-sided compared with single-sided imaging. Ultrasound Med Biol. 2016 doi: 10.1016/j.ultrasmedbio.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproux A, Van Beek M, De Vries U, Wasser M, Bakker L, Cuisenaire O, Van Der Mark M, Entrekin R. Procs Progress in Biomedical Optics and Imaging - Proceedings of SPIE. 2010. Automated 3D whole-breast ultrasound imaging: Results of a clinical pilot study; p. 7629. [Google Scholar]
- Li J, Goodsitt MM, Padilla F, Fowlkes JB, Hooi FM, Lashbrook CR, Thomenius KE, Carson PL. Effect of Gel Retainment Dam on Automated Ultrasound Coverage in a Dual-Modality Breast Imaging System. J Ultrasound Med. 2010;29(7):1075–1081. doi: 10.7863/jum.2010.29.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla F, Roubidoux MA, Paramagul C, Sinha SP, Goodsitt MM, Le Carpentier GL, Chan HP, Hadjiiski LM, Fowlkes JB, Joe AD, Klein KA. Breast mass characterization using 3D automated ultrasound as an adjunct to digital breast tomosynthesis: A pilot study. J Ultras Med. 2013;32(1):93–104. doi: 10.7863/jum.2013.32.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Heywang-Köbrunner SH, Winzer KJ, Schmitt KJ, Prihoda H, Frohberg HD, Guski H, Gregor P, Blohmer JU, Fobbe F, Döinghaus K. Detection of malignant and benign breast lesions with an automated US system: Results in 120 cases. Radiology. 1997;205(3):823–830. doi: 10.1148/radiology.205.3.9393543. [DOI] [PubMed] [Google Scholar]
- Sharpe RE, Jr, Venkataraman S, Phillips J, Dialani V, Fein-Zachary VJ, Prakash S, Slanetz PJ, Mehta TS. Increased cancer detection rate and variations in the recall rate resulting from implementation of 3D digital breast tomosynthesis into a population-based screening program. Radiology. 2016;278(3):698–706. doi: 10.1148/radiol.2015142036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RV. The design, contsruction and testing of a hermetically sealed breast platform for dual-modality mammography. MSc (Med), University fo Cape Town. 2014 [Google Scholar]
- Tagliafico AS, Calabrese M, Mariscotti G, Durando M, Tosto S, Monetti F, Airaldi S, Bignotti B, Nori J, Bagni A, Signori A. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial. Journal of Clinical Oncology. 2016;34(16):1882–18888. doi: 10.1200/JCO.2015.63.4147. [DOI] [PubMed] [Google Scholar]
- Vaughan CL, Douglas TS, Said-Hartley Q, Baasch RV, Boonzaier JA, Goemans BC, Harverson J, Mingay MW, Omar S, Smith RV, Venter NC. Testing a dual-modality system that combines full-field digital mammography and automated breast ultrasound. Clin Imaging. 2016;40(3):498–505. doi: 10.1016/j.clinimag.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert J, Steenbergen S. The Connecticut experiments second year: ultrasound in the screening of women with dense breasts. The Breast Journal. 2015;21(2):175–180. doi: 10.1111/tbj.12386. [DOI] [PubMed] [Google Scholar]
- Wilczek B, Wilczek HE, Rasouliyan L, Leifland K. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur J Radiol. 2016;85(9):1554–1563. doi: 10.1016/j.ejrad.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chan HP, Sahiner B, Wei J, Goodsitt MM, Hadjiiski LM, Ge J, Zhou C. A comparative study of limited-angle cone-beam reconstruction methods for breast tomosynthesis Med. Phys. 2006;33(37):81–95. doi: 10.1118/1.223754. [DOI] [PMC free article] [PubMed] [Google Scholar]


