Abstract
Heterotopic ossification (HO), a serious disorder of extra-skeletal bone formation, occurs as a common complication of trauma or in rare genetic disorders. Many conserved signaling pathways have been implicated in HO; however, the exact underlying molecular mechanisms for many forms of HO are still unclear. The emerging picture is that dysregulation of bone morphogenetic protein (BMP) signaling plays a central role in the process, but that other conserved signaling pathways, such as Hedgehog (HH), Wnt/β-catenin and Fibroblast growth factors (FGF), are also involved, either through cross-talk with BMP signaling or through other independent mechanisms. Deep understanding of the conserved signaling pathways is necessary for the effective prevention and treatment of HO. In this review, we update and integrate recent progress in this area. Hopefully, our discussion will point to novel promising, druggable loci for further translational research and successful clinical applications.
Keywords: Heterotopic ossification (HO), Fibrodysplasia ossificans progressiva (FOP), Conserved signaling pathways, Transforming growth factor β (TGF-β), Hedgehog (HH), Fibroblast growth factor (FGF), Bone morphogenetic protein (BMP), Wnt/β-catenin
1. Introduction
Heterotopic ossification (HO) is extra-skeletal bone formation within soft tissue. Acquired HO (aHO), the most common form, was first described in World War I among soldiers and was frequently reported following traumatic injuries [1]. Unfortunately, the exact causes and underlying mechanisms of aHO are still unclear. Hereditary forms of HO, such as Fibrodysplasia Ossificans Progressiva (FOP) [2] and Progressive Osseous Heteroplasia (POH) [3], are rare but life-threatening genetic disorders. FOP is caused by gain-of-function mutation of the bone morphogenetic protein (BMP) type I receptor, ACVR1 (also called ALK2) whereas loss-of-function mutation of the imprinted gene, GNAS, is responsible for POH [4–6]. However, understanding of how the BMP and GNAS pathways regulate ectopic bone formation remains limited. Although different types of HO each have unique features, the hypothesis underlying this review is that there must be some common fundamental mechanisms that are responsible for the core HO phenotype. We therefore focus primarily on conserved signaling pathways, including BMP/TGF-β, Wnt/β-catenin, and Hedgehog (HH), since they are more likely to be part of the final common pathways leading to HO. The assumption that there are such convergent pathways leading to HO suggests that lessons learned from rare diseases might also be applicable to the more common forms of HO. This review will integrate knowledge of the different conserved signaling pathways involved in HO into a common testable working model that summarizes our current understanding of the disorder.
2. BMP/TGF-β signaling
BMP signaling is the most extensively studied pathway involved in HO. BMPs, members of the TGF-β superfamily, play central roles in bone regeneration and repair [7]. BMPs signal through cell surface receptor complexes that consist of a two type I and two type II subunits. BMP ligands bind with high affinity to the type I subunits, followed by heterodimerization with the type II subunits (BMPRII) and activation of canonical SMAD and/or non-SMAD pathways that regulate expression of target genes. During normal development of skeletal long bones, BMP signaling induces proliferation and differentiation of condensing mesenchymal stem cells (MSC) towards chondrocytes and osteocytes [7]. Similarly, BMP signaling appears to play a pivotal role in both in hereditary and acquired HO [8].
2.1. BMP/TGF-β signaling in FOP
Gain-of-function mutations of ACVR1 in patients with FOP cause dysregulation of BMP signaling with resultant progressive ectopic endochondral ossification [9]. Several animal models recapitulate the features of FOP. Conditional expression of ACVR1 in the mesodermal cell lineage, using the nuclear factor associated T-cell c1 (Nfatc1)-cre, causes formation of ectopic cartilage and bone at the distal joints of the extremities in mice [10]. Knockin mice carrying mutant ALK2 (R206H) develop a phenotype that mimics FOP [11]. Our mouse line (Nse-BMP4), that overexpresses BMP4 under the control of the neuron specific enolase (NSE) promoter, similarly recapitulates the characteristic features of FOP, indicating that increased BMP signaling is critical for HO [12].
To further understand whether constitutively active (ca) mutant type I (ALK2) receptors can function independently of signaling complexes, Bagarova et al. knocked down the type II receptors (BMPRII or ActRII) and found that this abrogated caALK2-mediated signaling and activation of transcription and disrupted caALK2-induced HO [13]. Thus caALK2 does not signal independent of receptor subunit complexes in HO. More specifically, signaling by mutant ALK2 is enhanced by BMP type II receptors via phosphorylation of a threonine residue (T203), that is essential for intracellular signal transduction [14]. However, the abnormalities in BMP signaling in FOP are nuanced and complicated. For example, Shore et al. investigated lymphocytes from FOP patients and found increased expression of BMPR1A, BMP4, ID1 and ID3 and, more interestingly, activation of the non-canonical P38 MAPK pathway [15]. Billing et al. also found that BMP signals are transmitted through both MAPK and SMAD pathways in FOP [16].
Surprisingly, Hatsell et al. found that activin A, another member of the TGF-β superfamily, triggers ectopic ossification in an animal model of FOP [17]. Based on this finding, the authors proposed that mutant ACVR1 abnormally transduces BMP signaling in response to activin-A rather than a BMP [18]. Further, pharmacologic inhibition of TGF-β signaling decreases osteogenic differentiation of FOP fibroblasts [19]. Finally, Smad transcriptional activation is also regulated by binding of the transcriptional coactivator, Yes-associated protein (YAP), to Smad through YAP’s ww1 domain. Chen et al. recently described design of peptide aptamers to disrupt this Yap-Smad interaction thereby providing a new potential therapeutic approach to HO [20].
2.2. BMP/TGF-β signaling in acquired HO
Acquired HO (aHO) is a serious complication of traumatic tissue damage [21,22]. aHO is a particularly difficult clinical challenge, since 1) the underlying mechanisms of aHO are likely more variable than in the genetic disorders, and 2) few commonly accepted animal models are available for study of aHO. Nevertheless, substantial clinical and experimental evidence suggests that BMP signaling plays a central role in aHO. For example, high levels of BMP2 were observed in the serum of patients following traumatic brain injury (TBI), a common pre-condition for aHO [23]. In a traumatic injury model, expression of various osteogenic-related gene transcripts, including ALP, BMP-2, BMP-3, COLL10A1, COL2A1, COL11A, COMP, CSF2, CSF3, MMP8, MMP9, SMAD1 and VEGFa, was increased in wound tissue. Expression of BMPRIA, a type I high affinity BMP receptor was also increased [24]. Similarly, expression of BMP2, BMP4, BMP7 and BMP9 was significantly enhanced in another aHO animal model (spinal cord injury, SCI). BMPR1a and BMPR2 also were up-regulated seven days after SCI, and phosphorylation and translocation of Smad to nuclei was increased [25]. Similarly, in a burn/tenotomy model, Bmp-Smad signaling was highly increased in the fibroproliferative and immature stage of HO, but not in mature ectopic bone in both primary and recurrent HO [26]. Peterson et al. found that BMPRI/BMP/SMAD signaling was increased in inguinal derived MSCs after burn injury, and LDN, a small molecule inhibitor of type I BMP receptors, inhibited osteogenic differentiation of MSCs. They further found that adenosine triphosphate (ATP) hydrolysis reduced BMP signaling in MSCs in vitro and decreased HO formation in vivo [27]. Finally, direct injection of BMP2 after trauma led to BMPRIA-mediated ectopic bone formation seven days following severe injury, but BMPRII expression was not changed [28].
By contrast, expression of BMP2/BMP4 was significantly reduced while BMP1/TGF-β1 expression was elevated in traumatically injured muscle derived from patients that developed HO within one year, suggesting that TGF-β signaling may also contribute to aHO [29]. In HO patients following total hip athroplasty (THA) surgery, immunohistochemistry demonstrated TGF-β2 expression in the bone-forming zones of HO [30]. Further, Suutre et al. showed that TGF-β2 was critical for HO formation after THA; TGF-β2 was increased threefold in immature compared to mature HO but other TGF-β isoforms (TGF-β1 and TGF-β3) did not differ significantly [31].
In addition, Activin A also may play a key role in HO after TBI or SCI, since Activin A up-regulation is commonly seen after these injuries [32–34]. Notably, Khallaf et al. found that long bone fractures heal more quickly in SCI patients and implicated Activin A as one of the mediators of this accelerated osteogenesis [35].
Thus, while it is commonly accepted that dysregulation of BMP signaling leads to HO, this appears to be an oversimplification, especially in aHO, and more detailed studies will be necessary to more clearly define the precise role of BMP signaling.
3. Hedgehog (HH) signaling
HH signaling is important for normal skeletal development, i.e., Indian HH (IHH) is responsible for chondrocyte differentiation and sonic HH (SHH) induces the formation of osteoblasts [36–38]. HH signaling is tightly associated with and/or regulated by multiple factors, including the aforementioned BMP signaling, both in normal and heterotopic bone formation. HH signaling also plays an important role in osteogenesis during the healing of stress fractures by directly regulating bone formation and angiogenesis [39]. However, studies of normal skeletogenesis have also generated conflicting data. For example, in metatarsal organ cultures, activation of BMP signaling enhanced bone collar formation cooperatively with HH input. However single-cell quantitative RT-PCR analyses showed heterogeneity of perichondrial cells with HH signaling suppressed BMP-induced chondrogenic differentiation in some cells, at least partially by inhibiting the expression of Sox5, Sox6, and Sox9 [40]. Other studies indicate that HH-Gli1 itself can induce early osteoblast differentiation, that there is a redundant role for Gli2, and that HH is involved in the repressor function of Gli3 in osteogenesis [41].
3.1. HH signaling in HO
HH signaling plays important roles in pathological contexts, especially in Progressive Osseous Heteroplasia (POH), which is caused by heterozygous null mutation in GNAS that encodes Gαs [42,43]. Enhanced HH signaling mediates the downstream effects of GNAS mutation, since Gαs is an activator of PKA, which is an inhibitor of HH signaling. Regard et al. found that HH signaling was increased in Gnas−/− subcutaneous mesenchymal progenitors (SMPs) and bone marrow stromal cells (BMSCs), while wnt-β-catenin level was decreased [44,45]. Consistent with this idea, Gli1 and Gli2 were up-regulated in lesions of POH patients. Similarly, Gli2 and Gli3A levels were enhanced in limb buds of an animal model of POH (Prx1-cre, Gnasf/− mice). These observations suggest that HH inhibitors or Gli inhibitors could be a new avenue for POH prevention and treatment.
HH signaling was also critical for chondrocyte differentiation in HO in the BMP2-induced ectopic bone mouse model established by Stoege et al. [46]. Specifically, Indian HH (IHH) was expressed in prehypertrophic and hypertrophic chondrocytes in a pattern that overlapped with parathyroid hormone related protein (PTHrp) receptor; furthermore, Ptc, a receptor of IHH, was also expressed in HO. Similarly, Sugita et al. showed that in patients with ossification of the posterior longitudinal ligament in the neck, IHH promotes abnormal chondrocyte differentiation in endochondral ossification and enhanced bone formation [47]. Finally, enhanced IHH signaling in mildly affected Ndst (Golgi-associated N-sulfotransferase 1)−/− mouse embryos triggered excessive proliferation of chondrogenic progenitors and ectopic bone formation [48].
Collectively these observations suggest that HH signaling plays crucial roles in various form of HO, but the exact nature of the crosstalk between HH and BMP or other signaling pathways is still largely unknown.
3.2. WNT/β-catenin signaling
The canonical Wnt pathway is initiated by Wnt ligands binding to a complex receptor composed of frizzled and lipoprotein receptor-related proteins (LRP5 and LRP6), which activates a β-catenin signaling cascade that regulates target gene expression. Wnt/β-catenin was commonly proposed as a mediator of HH or BMP signaling, both in the context of normotopic bone formation and HO. In normotopic bone formation, BMP signaling can both activate or inhibit Wnt signaling [49]. BMP induces Dikkopf-related protein 1 (DKK1) and Sost expression through MAPK and SMAD signaling, respectively, which inhibit Wnt in osteoblasts. Thus ablation of SMAD4 in osteoblasts results in increased canonical Wnt signaling. However, BMP2-induced LRP-5 expression with the resultant increase in Wnt signaling contributes to chondrocyte hypertrophy, and wnt3a or overexpression of β-catenin conversely promotes BMP signaling.
In the context of HO, Wnt signaling is critical for chondrocyte maturation and osteoblast differentiation. Immunohistochemical analysis of HO lesions from elbow trauma patients showed increased expression of β-catenin as well as Runx2, a downstream effector of BMP signaling. Mir203 targeting of Runx2 inhibits β-catenin and effectively inhibits traumatic HO formation [50]. Moreover, Mitsui et al. found that aberrant Wnt/β-catenin signaling increased BMP2 expression, and that the two pathways contributed to HO following adrenal myelolipoma [51]. Similarly, Noh et al. showed that BMP9 coordinated with β-catenin to cause ectopic bone in colon cancer [52].
Wnt signaling also can be downstream of HH signaling. For example, Yang et al. found that there is ectopic bone formation in ptch1 mutant mice whereas bone formation was inhibited in β-catenin mutants. In β-catenin/ptch1 double mutant mice HH signaling was still upregulated, but bone formation was blocked. This suggests that β-catenin signaling acts downstream of HH signaling [53]. In the context of POH, further studies found that β-catenin protein and Wnt/β-catenin target gene expression inversely correlates with Ptch1, Gli1 and Hip1. Thus disruption of the balance between Wnt/β-catenin and HH signaling may contribute to POH [44].
BMP and Wnt signaling also coordinately regulate osteogenic differentiation of MSCs. For example, Gu et al. found that Ginkgo biloba extract, a traditional herbal medicine, promotes osteogenic differentiation of bone marrow derived mesenchymal stem cells (BMMSCs) via upregulation of both BMP and Wnt signaling, and that loss-of-function of either BMP or Wnt decreased the osteogenic capacity of BMMSCs. These data indicated the critical role of both pathways in osteogenic differentiation of MSCs [54]. Guerrero et al. also showed that high phosphate could activate SMAD1/5/8 and promote osteogenic differentiation of rat BMMSCs and also increase expression of Lrp5, a ligand of Wnt. Inhibiting the Wnt pathway with DKK1 reduced BMP2 expression, and activation of the Wnt pathway enhanced BMP2 expression [55]. However, other studies suggest that BMP and Wnt might play different roles in osteogenic differentiation depending upon the source of the MSCs. For example, Su et al. reported that Wnt was the major factor to promote osteogenesis in BMMSCs, whereas BMP signaling was the major factor for adipose derived mesenchymal stem cells (ADMSCs) [56]. In general, most studies suggest that Wnt signaling is less important than BMP signaling in osteogenic differentiation of MSCs.
3.3. Other potentially important signaling pathways
There are a number of other conserved signaling pathways that are likely involved in HO including FGF, Notch, and many others. We will briefly highlight some of the most important studies of these pathways:
Extracellular Signal-regulated Kinases (ERK), are widely expressed protein intracellular signaling molecules that are associated with cell differentiation and proliferation. Though ERK signaling seems to be unchanged in FOP patients, it is activated in trauma-induced HO [57] and OPLL patients [58]. Furthermore, Yang et al. found that connexin 43 promotes osteogenic differentiation of the posterior longitudinal ligament cells via regulation of ERK activity by stabilizing Runx2 [59].
The FGF family of growth factors is critically important for normal bone development, and FGF signaling may also play a pivotal role in HO. Freeman et al. proposed that hypoxia-associated oxidative stress initiates mast cell proliferation and FGF secretion, which promote fibroproliferation, that, in turn, causes HO [60]. In vitro studies also indicated that low doses of FGF2 appeared to enhance BMP2-induced ectopic bone formation via increased expression of BMPRIb and phosphorylated SMAD1. However, high doses of FGF2 inhibited osteogenic differentiation of mesenchymal lineage cells [61].
Notch signaling also plays a role in differentiation of MSCs into osteocytes. Notch can either promote or inhibit BMP induced osteoblastgenesis [62]. Xu et al. reported that expression of Notch1 and hairy and enhancer of split (Hes), both at mRNA and protein levels, is increased in patients with ligament ossification of hip joints, indicating that abnormal notch signaling may contribute to HO [63].
Hypoxia-inducible factor1-alpha (HIF1-α) is a key transcriptional regulator of the cellular response to hypoxia. Dysregulation and overexpression of HIF1-α has been strongly implicated in many different physiological and pathophysiological processes including HO. For example, Lin et al. showed that HIF1-α was significantly up-regulated in proliferative and chondrogenic stages in an aHO rat model [64]. Wang et al. further confirmed that cellular hypoxia promotes heterotopic endochondral ossification by amplifying BMP signaling in FOP patients [65, 66]. Agarwal et al. also indicated that HIF1-α was critical for both trauma-induced and genetic HO, especially in immature HO stages. Further, several HIF1-α inhibitors were found to be promising drugs to prevent and treat HO [67,68].
Extracellular matrix (ECM) is a collection of secreted extracellular molecules, such as collagen, laminin, vitronectin, fibronectin and glycosaminoglycans (GAG), that provide structural and biochemical support to the surrounding cells. Recently, accumulating evidence suggests that ECM may play key biochemical and biophysical roles in HO. For example, Rodenberg et al. showed that levels of matrix metalloproteinase-9 (MMP9) are elevated in HO, both in vivo and in vitro. They suggested the MMP9 could be used as an in vivo diagnostic marker of HO [69]. Kundu et al. documented that vitronectin, an adhesion molecule in the integrin family, enhanced focal adhesion formation by activating the focal adhesion kinase (FAK) and paxillin and eventually diminished activation of the extracellular signal-regulated kinase (ERK) and phosphatidylinositide 3-kinases (PI3K) pathways in osteogenic differentiation of MSC. In contrast, type-I collagen exhibited reduced focal adhesion formation but increased activation of ERK and PI3K. Surprisingly, both substrates (type-I collagen and vitronectin) promoted osteogenic differentiation of MSCs. In addition, exogenous type II collagen directly activated FAK-JNK signaling and resulted in the phophorylation of Runx2 [70], and type II collagen-HA/TCP-implanted rats also showed significant callus formation in vivo [71]. Glycosaminoglycans (GAG) also potentiate osteogenic differentiation of rat MSCs, and GAG mimetics (OTR4120) have similar effects on MSC [72]. Hwang et al. also highlighted the biophysical roles of ECM in HO by demonstrating that a stiff ECM stimulates ERK and JNK to activate TAZ (transcriptional coactivator with PDZ-binding mottif) and induce osteogenic differentiation of MSCs [73,74]. Similarly, integrins, transmembrane receptors that are the bridges for cell-cell and cell-ECM interactions, were demonstrated to be involved in chondrogenic differentiation of MSCs, and blocking the effects of integrins significantly increased BMP-Smad signaling [75].
4. A working model
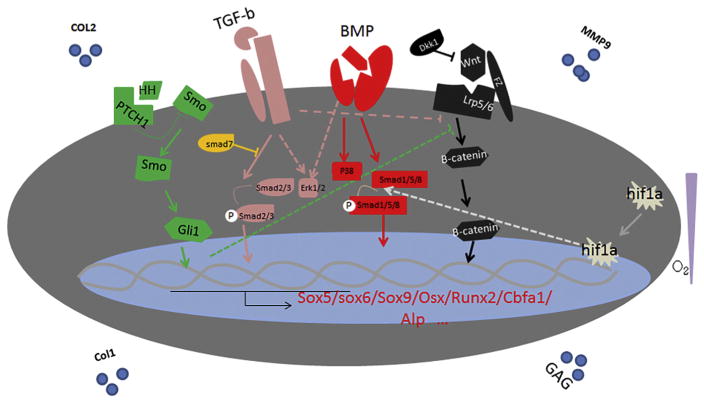
Many signal pathways are involved in HO, and integrating all of these candidate signal pathways into a readily testable working model is important to provide a guideline for future HO prevention or treatment. Although these pathways summate to form a signaling network, not all signaling pathways contribute equally, and the dominate mechanisms are likely to differ at different stages of HO. For example, traumatic injury initiates the process of aHO, and abnormal injury responses (AIR) are crucial at that stage, while at the later stages, osteogenic signaling plays key roles. Further, at any given moment the relevant signaling pathways may not play equal roles. For example, at the stage of pathological activation of tissue-resident MSCs and subsequent osteogenic differentiation, all microenviromental cues, including hypoxia, various cytokines such as FGF, integrins, and the TGF-β/BMP signaling cascade, converge on MSCs, and interactions among the different pathways may augment or ameliorate the actions of other pathways. Despite this complexity, some generalizations can be drawn about the overarching signaling systems that are most involved. Thus BMP signaling through both Smad and Mapk appears to play key roles throughout the process of HO, and the impact of other signaling pathways, such as HH and Wnt, appears to occur indirectly by modifying BMP signaling through a variety of different feedback mechanisms or by cross-regulations between the pathways (Fig. 1).
Fig. 1.
Intergrated working model. We propose that BMP signaling cascade through Smad and Mapk pathways is the major regulator of the pathological osteogenic process. Nevertheless, cross-talking at different levels between BMP, other conserved signal pathways could lead to synegetic effects, competitive inhibition, and/or feedback regulations. Together, they form an overwhelmingly complicate network.
5. Conclusion
HO is a dynamic pathologic process in which a variety of different conserved signal transduction pathways regulate each stage of ectopic bone formation. Based on the available literature and our own observations, we proposed an overall working model that integrates the major conserved signaling pathways. In this model, BMP/TGF-β signaling takes the central role in the overall HO process, while others, such as HIF-1α, FGF, ECM, and Wnt signaling, either help to set up the pre-conditions for HO, or regulate the whole process through a variety of different feedback mechanisms. More detailed studies are warranted to further confirm or clarify the details of this model.
Acknowledgments
We appreciate the help from many members of the Kessler lab. LK is supported in part by National Nature Science Foundation of China (81472087) and Nature Science Foundation of Anhui Province, China (1508085MC45).
References
- 1.Forsberg JA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg. 2009;91:1084–1091. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 2.Frederick SA. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191–205. doi: 10.1016/j.berh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shore EM. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346:99–106. doi: 10.1056/NEJMoa011262. [DOI] [PubMed] [Google Scholar]
- 4.Feldman G, Li M, Martin S, et al. Fibrodysplasia ossificans progressiva, a heritable disorder of severe heterotopic ossification, maps to human chromosome 4q27-31. Am J Hum Genet. 2000;66:128–135. doi: 10.1086/302724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culbert AL, Chakkalakal SA, Theosmy EG, et al. Alk2 regulates early chondrogenic fate in fibrodysplasia ossificans progressiva heterotopic endochondral ossification. Stem Cells (Dayton, Ohio) 2014;32:1289–1300. doi: 10.1002/stem.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regard JB. Activation of hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med. 2014;19:1505–1512. doi: 10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-duffhues G, Hiepen C, Knaus P, et al. Bone morphogenetic protein signaling in bone homeostasis. Bone. 2015;80:43–59. doi: 10.1016/j.bone.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Bouvard B, Masson C. Fibrodysplasia ossificans progressiva. A case report and focus on the BMP signaling pathway. Morphologie. 2016;100:250. doi: 10.1016/j.morpho.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Loder SJ, Cameron B, et al. BMP signaling mediated by constitutively active Activin type 1 receptor (ACVR1) results in ectopic bone formation localized to distal extremity joints. Dev Biol. 2015;400:202–209. doi: 10.1016/j.ydbio.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto M, Ohte S, Masashi S, et al. Establishment of a novel model of chondrogenesis using murine embryonic stem cells carrying fibrodysplasia ossificans progressiva-associated mutant ALK2. Biochem Biophys Res Commun. 2014;455:347–352. doi: 10.1016/j.bbrc.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Kan L, Hu M, Gomes WA, et al. Transgenic Mice overexpressing BMP4 develop fibrodysplasia ossificans progressiva(FOP)-like phenotype. Am J Pathol. 2004;165:1107–1115. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jana B, Vonner AJ, Armstrong KA, et al. Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol. 2013;33:2413–2424. doi: 10.1128/MCB.01595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto M. Mutant activin-like kinase 2 in fibrodysplasia ossificans progressiva are activated via T203 by bmp type II receptors. Mol Endocrinol. 2015;29:140–152. doi: 10.1210/me.2014-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiori JL, Billings PC, de la Peña LS, et al. Dysregulation of the BMP-p38 MAPK signaling pathway in cells from patients with fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 2006;21:902–909. doi: 10.1359/jbmr.060215. [DOI] [PubMed] [Google Scholar]
- 16.Bobick BE, et al. Regulation of cartilage formation and maturation by mitogenactivated protein kinase signaling. Birth Defects Res C Embryo Today. 2008;84:131–154. doi: 10.1002/bdrc.20126. [DOI] [PubMed] [Google Scholar]
- 17.Hatsell SJ. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Genetic Disorders. 2015;7:303ra137. doi: 10.1126/scitranslmed.aac4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hildebrand L, Stange K, Deichsel A, et al. The Fibrodysplasia Ossificans Progressiva (FOP) mutation p.R206H in ACVR1 confers an altered ligand response. Cell Signal. 2017;29:23–30. doi: 10.1016/j.cellsig.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Micha D. Inhibition of TGFβ signaling decreases osteogenic differentiation of fibrodysplasia ossificans progressiva fibroblasts in a novel in vitro model of the disease. Bone. 2016;84:169–180. doi: 10.1016/j.bone.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Chen D. Rational design of YAP WW1 domain-binding peptides to target TGFβ/BMP/Smad–YAP interaction in heterotopic ossification. J Pept Sci. 2015 doi: 10.1002/psc.2824. http://dx.doi.org/10.1002/psc.2824. [DOI] [PubMed]
- 21.Winkler S, Craiovan B, Wagner F, et al. Pathogenesis and prevention strategies of heterotopic ossification in total hip arthroplasty: a narrative literature review and results of a survey in Germany. Arch Orthop Trauma Surg. 2015;135:481–489. doi: 10.1007/s00402-015-2174-1. [DOI] [PubMed] [Google Scholar]
- 22.Kavitha R, Peterson J, Agarwal S, et al. Role of gender in burn-induced heterotopic ossification and mesenchymal cell osteogenic differentiation. Plast Reconstr Surg. 2015;135:1631–1641. doi: 10.1097/PRS.0000000000001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yk W. Comparative study of serum levels of BMP-2 and heterotopic ossification in traumatic brain injury and fractures patients. Zhongguo GU Shang. 2011;24:399–403. [PubMed] [Google Scholar]
- 24.Evans KN, Potter BK, Brown TS, et al. Osteogenic gene expression correlates with development of heterotopic ossification in war wounds. Clin Orthop Relat Res. 2014;472:396–404. doi: 10.1007/s11999-013-3325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang H. Novel mouse model of spinal cord injury-induced heterotopic ossification. J Rehabil Res Dev. 2014;51:1109–1118. doi: 10.1682/JRRD.2014.01.0019. [DOI] [PubMed] [Google Scholar]
- 26.Peterson JR, Agarwal S, Brownley RC, et al. Direct mouse Trauma/Burn model of heterotopic ossification. JOVE-JOURNAL of Visualized Experiments. 2015;102:e52880. doi: 10.3791/52880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonathan RP. Treatment of heterotopic ossification through remote ATP hydrolysis. Cancer. 2014;6:255ra132. doi: 10.1126/scitranslmed.3008810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu XH, Heejae K, Mohammad S, et al. A novel mouse model of trauma induced heterotopic ossification. J Orthop Res. 2014;32:183–188. doi: 10.1002/jor.22500. [DOI] [PubMed] [Google Scholar]
- 29.Jackson WM, Aragon AB, Onodera J, et al. Cytokine expression in muscle following traumatic injury. J Orthop Res. 2011;29:1613–1620. doi: 10.1002/jor.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toom A, Arend A, Gunnarsson D, et al. Bone formation zones in heterotopic ossifications: histologic findings and increased expression of bone morphogenetic protein 2 and transforming growth factors beta2 and beta3. Calcif Tissue Int. 2007;80:259–267. doi: 10.1007/s00223-007-9000-x. [DOI] [PubMed] [Google Scholar]
- 31.Suutre S, Toom A, Arend A. Bone tissue content of TGF-beta2 changes with time in human heterotopic ossification after total hip arthroplasty. Growth Factors. 2009;27:114–120. doi: 10.1080/08977190802703976. [DOI] [PubMed] [Google Scholar]
- 32.Phillips DJ, Nguyen P, Adamides AA, et al. Activin a release into cerebrospinal fluid in a subset of patients with severe traumatic brain injury. J Neurotrauma. 2006;23:1283–1294. doi: 10.1089/neu.2006.23.1283. [DOI] [PubMed] [Google Scholar]
- 33.Tretter YP, Hertel M, Munz B, et al. Induction of activin A is essential for the neuroprotective action of basic fibroblast growth factor in vivo. Nat Med. 2006;6:812–815. doi: 10.1038/77548. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YW, Denham J. Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem Cells Dev. 2006;2006(15):943–952. doi: 10.1089/scd.2006.15.943. [DOI] [PubMed] [Google Scholar]
- 35.Khallaf FG, Kehinde EO. Growth factors and cytokines in patients with long bone fractures and associated spinal cord injury. J Orthop. 2016;13:69–75. doi: 10.1016/j.jor.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Day TF, et al. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90(Suppl 1):19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]
- 37.Alman BA, et al. The role of hedgehog signalling in skeletal health and disease. Nat Rev Rheumatol. 2015;11:552–560. doi: 10.1038/nrrheum.2015.84. [DOI] [PubMed] [Google Scholar]
- 38.Briscoe J, et al. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 39.Kazmers NH, McKenzie JA, Shen TS, Long F, Silva MJ. Hedgehog signaling mediates woven bone formation and vascularization during stress fracture healing. Bone. 2015;81:524–532. doi: 10.1016/j.bone.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hojo H, Ohba S, Taniguchi K, Shirai M, Yano F, Saito T, et al. Hedgehog-Gli activators direct osteochondrogenic function of bone morphogenetic protein toward osteogenesis in the perichondrium. J Biol Chem. 2013;288(14):9924–9932. doi: 10.1074/jbc.M112.409342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hojo H, Ohba S, Yano F, Saito T, Ikeda T, Nakajima K, et al. Gli1 protein participates in Hedgehog-mediated specification of osteoblast lineage during endochondral ossification. J Biol Chem. 2012;287(21):17860–17869. doi: 10.1074/jbc.M112.347716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pignolo RJ, Ramaswamy G, Fong JT, et al. Progressive osseous heteroplasia: diagnosis, treatment, and prognosis. Appl Clin Genet. 2015;8:37–48. doi: 10.2147/TACG.S51064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cairns DM, Pignolo RJ, Uchimura T, et al. Somitic disruption of GNAS in chick embryos mimics progressive osseous heteroplasia. J Clin Invest. 2013;123:3624–3633. doi: 10.1172/JCI69746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regard JB, Malhotra D, Gvozdenovic-Jeremic J, et al. Activation of hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med. 2013;19:1505–1512. doi: 10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Kaplan FS. Different roles of GNAS and cAMP signaling during early and late stages of osteogenic differentiation. Horm Metab Res. 2012;44:724–731. doi: 10.1055/s-0032-1321845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoeger T, Proetzel GE, Welzel H, et al. In situ gene expression analysis during BMP2-induced ectopic bone formation in mice shows simultaneous endochondral and intramembranous ossification. Growth Factors. 2002;20:197–210. doi: 10.1080/0897719021000069579. [DOI] [PubMed] [Google Scholar]
- 47.Sugita D, Yayama T, Uchida K, et al. Indian hedgehog signaling promotes chondrocyte differentiation in enchondral ossification in human cervical ossification of the posterior longitudinal ligament. Spine. 2013;38:E1388–96. doi: 10.1097/BRS.0b013e3182a40489. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 48.Yasuda T, Mundy C, Kinumatsu T, et al. Sulfotransferase Ndst1 is needed for mandibular and TMJ development. J Dent Res. 2010;89:1111–1116. doi: 10.1177/0022034510373766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin EJ, Lee SY, Choi YA, et al. BMP-2-enhanced chondrogenesis involves p38 MAPK-mediated down-regulation of Wnt-7a pathway. Mol Cells. 2006;22:353–359. [PubMed] [Google Scholar]
- 50.Tu B, Liu S, Yu B, et al. miR-203 inhibits the traumatic heterotopic ossification by targeting Runx2. Cell Death Dis. 2016;7:e2436. doi: 10.1038/cddis.2016.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitsui Y, Yasumoto H, Hiraki M, et al. Coordination of bone morphogenetic protein 2 (BMP2) and aberrant canonical Wnt/β-catenin signaling for heterotopic bone formation in adrenal myelolipoma: a case report. Can Urol Assoc J. 2014;8:E104–E107. doi: 10.5489/cuaj.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noh BJ, Kim YW. A rare colon cancer with ossification: pathogenetic analysis of bone formation. Ann Clin Lab Sci. 2016;46:428–432. [PubMed] [Google Scholar]
- 53.Mak KK, Chen MH, Day TF, et al. Wnt/beta-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development. 2006;133(18):3695–3707. doi: 10.1242/dev.02546. [DOI] [PubMed] [Google Scholar]
- 54.Gu Q, Chen C, Zhang Z, et al. Ginkgo biloba extract promotes osteogenic differentiation of human bone marrow mesenchymal stem cells in a pathway involving Wnt/β-catenin signaling. Pharmacol Res. 2015;97:70–78. doi: 10.1016/j.phrs.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Guerrero F, Herencia C, Almadén Y, Martínez-Moreno JM, Montes de Oca A, et al. TGF-b prevents phosphate-induced osteogenesis through inhibition of BMP and Wnt/b-catenin pathways. PLoS ONE. 2014;9(2):e89179. doi: 10.1371/journal.pone.0089179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su X, Liao L, Shuai Y, et al. MiR-26a functions oppositely in osteogenic differentiation of BMSCs and ADSCs depending on distinct activation and roles of Wnt and BMP signaling pathway. Cell Death Dis. 2015;6:e1851. doi: 10.1038/cddis.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tu B, Liu S, Liu G, et al. Inhibition of connexin 43 prevents trauma-induced heterotopic ossification. Sci Rep. 2016;6:37184. doi: 10.1038/srep37184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen D, Liu Y, Yang H, et al. Connexin 43 promotes ossification of the posterior longitudinal ligament through activation of the ERK1/2 and p38 MAPK pathways. Cell Tissue Res. 2016;363:765–773. doi: 10.1007/s00441-015-2277-6. [DOI] [PubMed] [Google Scholar]
- 59.Yang H, Shi L, Shi G, et al. Connexin 43 affects osteogenic differentiation of the posterior longitudinal ligament cells via regulation of ERK activity by stabilizing Runx2 in ossification. Cell Physiol Biochem. 2016;38:237–247. doi: 10.1159/000438625. [DOI] [PubMed] [Google Scholar]
- 60.Freeman TA, Parvizi J, Dela Valle CJ. Mast cells and hypoxia drive tissue metaplasia and heterotopic ossification in idiopathic arthrofibrosis after total knee arthroplasty. Fibrogenesis Tissue Repair. 2010;3(17) doi: 10.1186/1755-1536-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakamura Y, Tensho K, Nakaya H, et al. Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone. 2005;36:399–407. doi: 10.1016/j.bone.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 62.Lin GL. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112:3491–3501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu W, Liang CG, Li YF, et al. Involvement of Notch1/Hes signaling pathway in ankylosing spondylitis. Int J Clin Exp Pathol. 2015;8:2737–2745. [PMC free article] [PubMed] [Google Scholar]
- 64.Lin L, Shen Q, Xue T. Heterotopic ossification induced by Achilles tenotomy via endochondral bone formation: expression of bone and cartilage related genes. Bone. 2010;46:425–431. doi: 10.1016/j.bone.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Lindborg C, Lounev V, et al. Cellular hypoxia promotes heterotopic ossification by amplifying BMP signaling. J Bone Miner Res. 2016;31:1652–1665. doi: 10.1002/jbmr.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkler S, Niedermair T, Füchtmeier B, et al. The impact of hypoxia on mesenchymal progenitor cells of human skeletal tissue in the pathogenesis of heterotopic ossification. Int Orthop. 2015;39:2495–2501. doi: 10.1007/s00264-015-2995-0. [DOI] [PubMed] [Google Scholar]
- 67.Agarwal S, Loder S, Brownley C, et al. Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. Proc Natl Acad Sci U S A. 2016;113:E338–47. doi: 10.1073/pnas.1515397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimmermann SM, Würgler-Hauri CC, Wanner GA, et al. Echinomycin in the prevention of heterotopic ossification — an experimental antibiotic agent shows promising results in a murine model. Injury. 2013;44:570–575. doi: 10.1016/j.injury.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 69.Rodenberg E, Azhdarinia A, Lazard ZW, et al. Matrix metalloproteinase-9 is a diagnostic marker of heterotopic ossification in a murine model. Tissue Eng Part A. 2011;17:2487–2496. doi: 10.1089/ten.TEA.2011.0007. [DOI] [PubMed] [Google Scholar]
- 70.Kundu AK. Vitronectin and collagen I differentially regulate osteogenesis in mesenchymal stem cells. Biochem Biophys Res Commun. 2006;347:347–357. doi: 10.1016/j.bbrc.2006.06.110. [DOI] [PubMed] [Google Scholar]
- 71.Chiu LH, Lai WF, Chang SF, et al. The effect of type II collagen on MSC osteogenic differentiation and bone defect repair. Biomaterials. 2014;35:2680–2691. doi: 10.1016/j.biomaterials.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Chiu LH, Lai WF, Chang SF, et al. The effect of type II collagen on MSC osteogenic differentiation and bone defect repair. Biomaterials. 2014;35(9):2680–2691. doi: 10.1016/j.biomaterials.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Hwang JH, Byun MR, Kim AR, et al. Extracellular Matrix Stiffness Regulates Osteogenic Differentiation through MAPK Activation. PLoS One. 2015;10:e0135519. doi: 10.1371/journal.pone.0135519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy KC, Hoch AI, Harvestine JN, et al. Mesenchymal stem cell spheroids retain osteogenic phenotype through α2β1 signaling. Stem Cells Transl Med. 2016;5:1229–1237. doi: 10.5966/sctm.2015-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang T, Wen F, Wu Y, et al. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials. 2015;38:72–85. doi: 10.1016/j.biomaterials.2014.10.010. [DOI] [PubMed] [Google Scholar]