Abstract
Several studies have indicated a relationship between melatonin and idiopathic scoliosis, including our previous work which demonstrated that melatonin can inhibit osteoblast proliferation; however, the mechanism remains unclear. Here, we utilized a MTT assay to show that melatonin significantly reduces osteoblast proliferation in a concentration-and time-dependent manner. Through a combination of techniques, including real-time PCR, MTT assays, immunofluorescence, and luciferase assays, we confirmed that melatonin-induced changes in phosphorylated cAMP response element-binding protein (CREB) reduced transcriptional activity in a melatonin receptor-dependent manner. Surprisingly, treatment of osteoblasts with the mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) inhibitor PD98059 up-regulated other cascades upstream of CREB. We next treated cells with PKA and Src inhibitors and observed that melatonin can also activate the protein kinase A (PKA) and Src pathways. To examine whether Src is upstream from the cAMP-PKA pathway, we measured cAMP levels in response to melatonin with and without a Src inhibitor (PP2) and found that PP2 had no additional effect. Therefore, the transcription-dependent mechanisms involved in CREB phosphorylation, along with melatonin, activated Src via a parallel signaling pathway that was separate from that of PKA. Finally, we transfected osteoblasts with lentiviral CREB short hairpin (sh) RNAs and found a decrease in the expression of proliferating cell nuclear antigen (PCNA) and osteoblast proliferation. These results suggest that CREB and PCNA are downstream targets of melatonin signaling, and that the down-regulation of CREB, which is regulated via PKA and Src pathways, contributes to the melatonin-induced inhibition of osteoblast proliferation.
Keywords: Melatonin, osteoblast proliferation, CRE-dependent gene transcription, ERK pathway, PKA pathway, SRC pathway
Introduction
Idiopathic scoliosis (IS), the most common form of scoliosis, is characterized by a three-dimensional spinal deformity accompanied by rotation of the vertebrae. The pathogenesis of IS is not yet fully understood [1,2]; however, several studies have indicated that melatonin may play a role. Melatonin, a hormone produced by the pineal gland, is entrained by the light/dark cycle and performs important functions in the biological regulation of circadian rhythms, antioxidant protection, bone physiology, and aging [3-6]. Interestingly, basic research studies have found that excision of the pineal gland in double-foot rats results in a spinal deformity similar to human IS, and intraperitoneal injection of melatonin (at concentrations greater than the physiological secretion of melatonin in plasma) can prevent the development of experimental IS [7,8]. However, an alternative study found that intraperitoneal injections of melatonin, at concentrations that were close to the physiological levels of melatonin in the plasma, did not prevent IS. Conversely, the results of clinical studies have shown that low levels of melatonin can prevent the progression of partial IS [9]. Taken together, we speculate that melatonin may be associated with IS in a concentration-specific manner: a hypothesis that has not yet been fully investigated.
We have previously shown that melatonin has a dual effect on osteoblast proliferation that is dependent on concentration [10]. Specifically, concentrations of melatonin ranging from 1 nM to 100 μM promote osteoblast proliferation, whereas higher concentrations (1 mM) can induce G1 and G2/M phase arrest and inhibit osteoblast proliferation. This dose-dependent relationship between melatonin and osteoblasts could explain the differential effects of melatonin observed in the experiments described above, as certain concentrations of melatonin may decrease osteoblast proliferation, thereby restoring normal bone growth and preventing the progression of deformity.
Melatonin exerts its actions primarily through specific receptors, which has been described in different tissues and species [11,12]. Therefore, the regulation of osteoblast proliferation by melatonin could be related to changes in the melatonin receptors. Three types of mammalian melatonin receptors have been identified so far: MT1, MT2, and MT3, although the MT3 receptor is only expressed in birds and rodents [13]. Melatonin receptors act primarily through G-protein uncoupling, receptor downregulation, internalization, and phosphorylation, which could contribute to regulation of melatonin receptors [14,15].
The primary aim of this study is to explore the mechanism underlying the inhibitory effect of melatonin on osteoblast proliferation. Here, we examine the dose-dependent relationship between melatonin and osteoblast proliferation, and investigate the possibility that melatonin affects CRE-dependent gene transcription via a regulation system composed of multiple molecules. A better understanding of the regulation of osteoblasts by melatonin could be critical to the utilization of melatonin as a therapeutic option for IS, as improper melatonin supplementation could fail to prevent the progression of IS deformity.
Materials and methods
Reagents and cell culture
Melatonin, chemical inhibitors (PD98059, H89, PP2 and luzindole) and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium (MTT) were obtained from Sigma (St. Louis, MO, USA), the TRIzol Reagent and LipofectamineTM2000 Reagent from Invitrogen (Carlsbad, CA, USA), the Reverse-Transcription Kit and SYBR Premix Ex TaqTM II from TaKaRa (Dalian, China), the Cyclic AMP EIA Kit from Cayman (Ann Arbor, Michigan, USA), Luciferase Kit from Promega (Madison, WI, USA) and Fluoroshield Mounting Medium from Abcam Technology (Cambridge, US).
The primary antibodies for total-ERK1/2 (t-ERK1/2), phospho-ERK1/2 (p-ERK1/2), total-CREB (t-CREB), phospho-CREBser133 (p-CREB), total-p90RSK (t-p90RSK), phospho-p90RSK (p-p90RSK) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were obtained from Cell Signaling Technology (Danvers, MA, USA), the antibodies for PCNA and phospho-Src (p-Src) from Abcam Technology (Cambridge, US), and the fluorescent secondary antibodies from Cell Signaling Technology.
The human fetal osteoblastic cell line hFOB 1.19, kindly provided by Dr. Mamayannan [16]. Subramaniam, was maintained in a 1:1 mixture of Ham’s F-12 Medium/Dulbecco’s modified Eagle’s Medium without phenol red (Gibco, Life Technologies, Carlsbad, USA), supplemented with 10% fetal bovine serum (FBS) (HyClone, Thermo, Fremont, USA), in a humidified 5% CO2 atmosphere at 37°C, and the medium was changed every other day. The cells were utilized in Passages 8-12 and plated at 104 cells/cm2 for 4, 24 or 48 h before treatment. Cells were treated with melatonin, which was dissolved in 0.2% dimethyl sulfoxide (DMSO) or vehicle (0.2% DMSO in culture medium only) media containing 10% FBS.
Cell proliferation assay
A MTT proliferation assay was performed to determine the effect of melatonin on osteoblast proliferation, and was performed as previously described [10]. Briefly, human osteoblast cells (hFOB 1.19) were seeded onto 96-well plates (6000 cells/well) for 24 h. The medium was then replaced with 10% serum medium containing different concentrations of melatonin (range: 1-4 mm) for 0, 4, 24, or 48 h. To investigate the signaling pathway, cells were exposed to melatonin (2 mM) or one of the following inhibitors: the MEK inhibitor PD98059 (50 μM), the PKA inhibitor (H89; 50 μM), the Src inhibitor PP2 (20 μM), the melatonin receptor antagonist luzindole (10 μM), CREB-shRNA, or lentivirus Ctrl-shRNA alone, or in combination. After treatment, culture media was changed for serum-free culture media. MTT dissolved in phosphate buffer saline (PBS) was added to each well and then incubated for 4 h at 37°C. After this interval, the serum-free culture media containing MTT was discarded, and dimethyl sulfoxide was added to each well to dissolve the precipitate. The optical densities were measured at 490 nm spectral wavelength using a microplate reader (Thermo, Switzerland). Cell proliferation results are expressed as percentages. The absorbency measured from untreated cells was taken to be 100%.
Immunofluorescence
Cells were seeded in 24-well plates and either treated with vehicle or melatonin (2 mM) for 24 h. Following incubation, the subconfluent cells were rinsed three times with PBS, fixed for 15 min in 4% paraformaldehyde, permeated for 5 min with PBS/0.5% Triton X-100, and then treated for 30 min in PBS/4% bovine serum albumin (BSA) to block non-specific binding. All of the above procedures were carried out at room temperature, and cells were rinsed with PBS between each step. Next, cells were incubated overnight at 4°C with the p-CREB antibody, washed three times with PBS with Tween 20 and incubated for 1 h with a fluorescent secondary antibody. Finally, cells were washed in PBS, mounted with a Fluoroshield Mounting Medium, and visualized using a fluorescent microscope (Olympus, Japan). All steps were carried out in the darkroom, starting with the addition of the fluorescent secondary antibody.
Luciferase assay
The pGL3-CRE, pGL3-luc and phRL-TK were purchased from Genechem (China), and using the LipofectamineTM2000 reagent, cells transiently cotransfected with pGL3-CRE-luc or with pGL3 as the corresponding control, and with phRL-TK as an internal control to normalize the observed values cultured in 24-well plates on the basis of the instructions. After 24 h, cells were pretreated with melatonin (2 mM) or PD98059 (50 μM) alone, or in combination for 24 h, luciferase activity was measured using a Dual Luciferase Kit, per manufacturer’s instructions.
Real-time RT-PCR
RNA extraction and reverse-transcriotion PCR were performed as described previously [10]. Real-time PCR was performed on ABI Prism 7900HT Fast System (Applied Biosystems, Life technologies, Foster, CA, USA) using SYBR Premix Ex TaqTM II. Real-time PCR data were calculated using 2-ΔΔCt Method by the SDS 2.4 software package (Applied Biosystems). The sequences of all primers can be found in Table 1, and GAPDH was used as an endogenous control. Amplified products were resolved by electrophoresis on 1.8% agarose gels in TAE buffer. The products of the reaction were visualized by ethidium bromide staining.
Table 1.
Primer sequences used in real-time PCR experiments
Immunoblotting
Whole-cell lysates were lysed in radioimmunoprecipitation assay (RIPA) buffer on ice for 30 min, the protein fractions were centrifuged for 15 min at 4°C at 12000 g, and supernatants containing total protein were harvested. Then, the proteins were separated using 12% SDS-PAGE gels and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% BSA for 2 h incubated with the primary antibody (t-ERK1/2, p-ERK1/2, t-CREB, p-CREB, t-p90RSK, p-p90RSK, PCNA, p-Src and GAPDH overnight at 4°C. Following incubation, a horseradish peroxidase-conjugated secondary antibody was applied for 2 h at 4°C, and blots were visualized with an enhanced chemiluminescence (ECL) system (UVP Inc., Upland, CA, USA). Protein levels were normalized to the corresponding glyceraldehyde 3-phosphate dehydrogenase (GAPDH) band, and the optical density was quantified using Image J Software (NIH, Bethesda, MD, USA).
Infection with lentivirus
The CREB-shRNA lentiviral and ctrl-shRNA lentiviral were purchased from Genechem (China). Osteoblasts were infected with CREB-inducible lentivirus particles (5’-GTGGATAGTGTAACTGATT-3’) or negative control lentiviral particles (5’-TTCTCCGAACGTGTCACGT-3’) at multiplicity of infection (40) with ENi.S and 5 μg/ml Polybrene to obtain stably transfected CREB-shRNA and ctrl-shRNA cells. At 3 days after transfection, cells were stimulated with or without melatonin. The expression of p-CREB or PCNA was examined by Western blot, and cell proliferation was tested by MTT.
Quantification of cAMP levels
Cellular cAMP levels were quantified using Cyclic AMP EIA Kit. Cells was seeded in 12-well plates and cultured for 18-24 h at 37°C. Then treated for 30 min with 2 mM melatonin and/or 20 μM PP2. The cAMP quantitated according to the manufacturer’s instructions.
Statistical analyses
All data are expressed as the mean ± S.E.M. Two-way analysis of variance (ANOVA) was performed to analyze osteoblast proliferation. Statistical analysis among groups was performed using one-way ANOVA with Tukey’s multiple comparison test for the analysis of CRE-dependent gene transcription. All analysis was performed by R 3.3.1 software. The level of significance was set at P < 0.05. Results are representative examples of three independent experiments.
Results
Effects of melatonin on osteoblast proliferation
We first performed a MTT assay to analyze the dose-dependent effects of melatonin on the proliferation of hFOB 1.19 cells, which is a homogeneous and rapidly proliferating normal human mature osteoblastic cell line. As shown in Figure 1, all examined concentrations of melatonin (1, 2, 3, 4 mM) inhibited hFOB 1.19 proliferation in a concentration- and time-dependent manner, compared with untreated controls. In addition, the results of the MTT assay also showed that, At the 2 mM concentration, this inhibition was most prominent. (One-way ANOVA: 4 h, P < 0.05; 24 h, P < 0.01; 48 h, P < 0.01).
Figure 1.
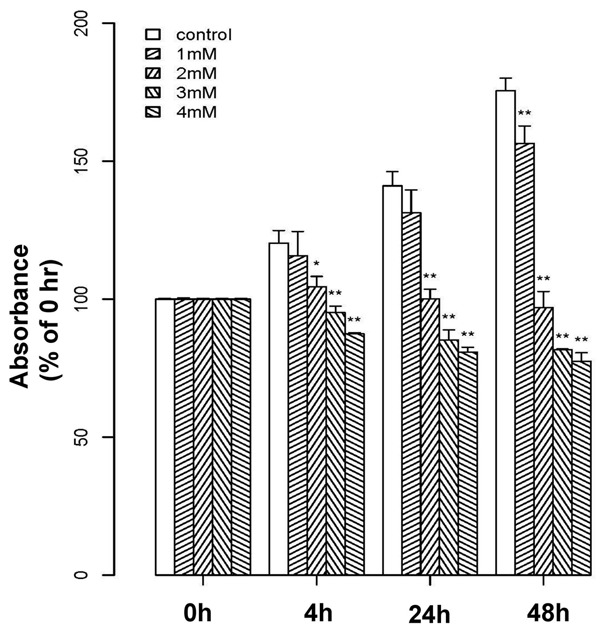
Effects of melatonin on osteoblast proliferation. Melatonin inhibited osteoblast proliferation in a concentration- and time-dependent manner, as determined by MTT assay. Cells were treated with melatonin at various concentrations (1, 2, 3, or 4 mM) or vehicle for 0, 4, 24 or 48 h. Results are displayed as the percent absorbance at 490 nm relative to untreated cells at 0 h. Experiments were performed in triplicate, and data are shown as the mean ± S.E.M. *P < 0.05 or **P < 0.01 compared with control cells.
Effects of melatonin receptor on osteoblast proliferation
Melatonin may act by binding to the MT1 and MT2 receptors, which are both G-protein-coupled, PTX-sensitive membrane receptors. Given the known role of melatonin receptors in cell proliferation, we examined whether melatonin inhibits osteoblast proliferation in a melatonin receptor-dependent manner. An examination via PCR revealed that both the MT1 (104 bp) and MT2 (198 bp) receptors were present in hFOB 1.19 cells, although the level of MT1 was higher than that of MT2 (Figure 2A). To further investigate whether melatonin inhibits proliferation through the melatonin receptors, a MTT assay was performed in the presence of a melatonin receptor antagonist (luzindole; 10 μM). Results showed that luzindole could block the inhibitory effect of melatonin on osteoblast proliferation, suggesting that cell growth inhibition is dependent on the melatonin receptors (Figure 2B).
Figure 2.
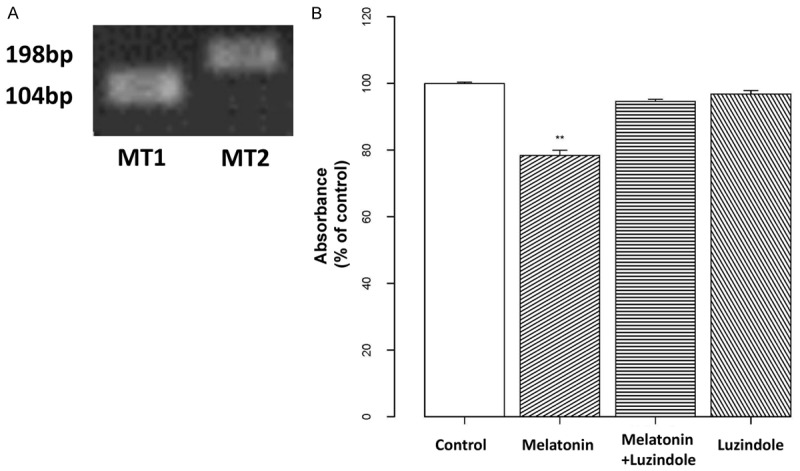
Effects of melatonin on cell proliferation are melatonin receptor-dependent. A: Total RNA was isolated from hFOB 1.19 cells and subjected to real-time RT-PCR using specific primers as shown in Table 1. GAPDH was used as an internal control. B: Osteoblast cells were pre-incubated with 10 μM of a melatonin receptor antagonist (luzindole) for 2 h, then treated with 10 μM melatonin for 24 h. Cells receiving only melatonin treatment or only luzindole were included for comparison. Cell proliferation was assessed with a MTT assay, and the results are displayed as the percent absorbance at 490 nm relative to controls (untreated). All experiments were performed in triplicate, and the data are shown as the mean ± S.E.M. *P < 0.05 or **P < 0.01, compared to controls.
Effects of melatonin on CREB phosphorylation and transcriptional activity
To examine a potential role of CREB in the inhibitory effects of melatonin, we analyzed the expression of p90 ribosomal S6 kinase (p90RSK) and CREB at 24 and 48 h with 2 mM melatonin. As shown in Figure 3A, melatonin inhibited the phosphorylation of p90RSK and CREB, as compared to controls (P < 0.01), with stronger suppression observed at 48 h; however, neither total p90RSK nor CREB levels exhibited a marked change. Next, an immunofluorescence assay was used to investigate p-CREB expression and nuclear localization. The results showed that the melatonin-treated group had less fluorescent staining than controls, especially with respect to nuclear localization (Figure 3B). This suggests that melatonin might influence gene transcription via p-CREB. Therefore, we used a cAMP responsive element (CRE)-luciferase assay to assess if melatonin-induced p-CREB inhibition leads to changes in CRE-dependent gene transcription, after transfection with a CRE-luc plasmid. The results of this assay showed that melatonin reduces the activity of CRE-luciferase (P < 0.01) and confirmed that melatonin can down-regulate CRE-dependent gene expression.
Figure 3.
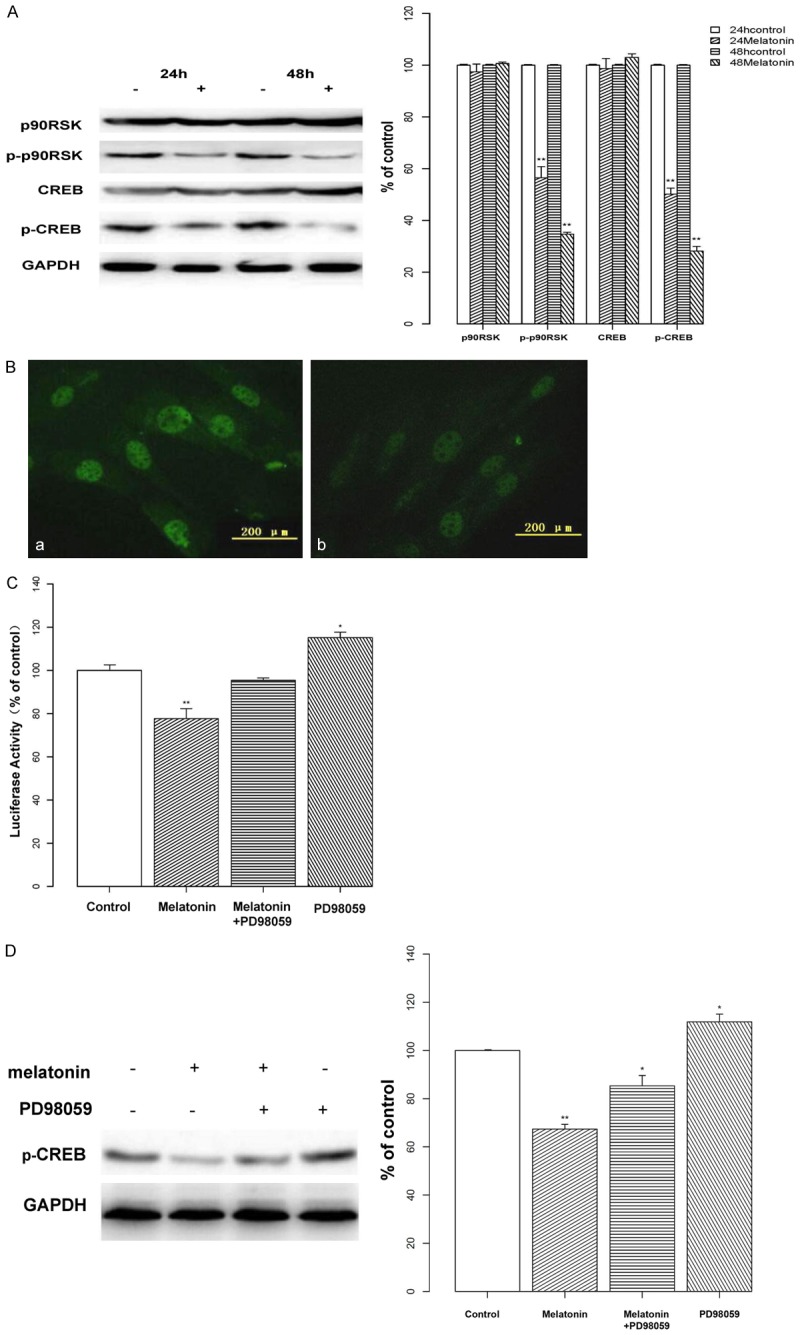
Effects of melatonin on CREB phosphorylation & transcriptional activity. A: The phosphorylation of p90RSK and CREB were investigated by Western blotting following treatment with melatonin (2 mM; indicated by “+”) or vehicle (indicated by “-”) for 24 or 48 h. Melatonin decreased the phosphorylated expression of p90RSK and CREB in osteoblast cells. Protein levels were analyzed and normalized to the corresponding GAPDH band. Data are displayed as the percent optical density relative to the respective controls. B: Osteoblast cells were treated with vehicle (a) or melatonin (2 mM; b) and stained with a p-CREB antibody. Visualization of the immunofluorescence assay confirmed that treatment with melatonin reduced fluorescent staining, especially nuclear localization. C: Osteoblasts were transiently co-transfected with the luciferase reporter vector pGL3-CRE-luc or with pGL3, and with phRL-TK. After 24 h, cells were treated with melatonin (2 mM) or a MEK inhibitor (PD98059; 50 μM) alone, or in combination for 24 h. CRE-luciferase activity was measured by CRE-luciferase assay. Melatonin treatment reduced the activity of the CRE-luciferase, whereas activity was elevated by PD98059 treatment. D: Osteoblasts were treated with melatonin (2 mM; indicated by “+”) or PD98059 (50 μM; indicated by “+”) alone, or in combination. Protein levels were analyzed by Western blotting and normalized to their respective GAPDH bands. Data are displayed as the percent optical density relative to each protein’s untreated control. Treatment with PD98059 increased the level of CREB phosphorylation. All experiments were performed in triplicate and data are shown as the mean ± S.E.M. *P < 0.05 or **P < 0.01, compared to controls.
To test whether there is a specific effect of MEK on CREB-induced transcription, cells were treated with the MEK inhibitor PD98059. Unexpectedly, treatment with both melatonin and PD98059 did not cause an obvious inhibition of CREB-induced transcriptional activity and was promoted simply with PD98059 (P < 0.05, Figure 3C). In addition, our results showed that the level of CREB phosphorylation was also elevated in the PD98059 group (P < 0.05, Figure 3D).
Relationship between PKA and the ERK/p90RSK/CREB pathway
We speculated that PD98059 was up-regulating other cascades upstream from CREB through a feedback mechanism. Many phosphorylation pathways relevant to melatonin can affect CREB activity; therefore, CREB inhibition, as observed in osteoblasts, could be the result of cooperation between multiple upstream factors. Since PKA is known to phosphorylate CREB at serine133, to examine the relationship between PKA and the ERK1/2 pathway we utilized a PKA inhibitor (H89). As shown in Figure 4A, cells pretreated with H89 and melatonin had only a slight reduction in expression of the p-ERK1/2/p-p90RSK/p-CREB pathway. In contrast, treatment with H89 alone induced an up-regulation (P < 0.05), suggesting that PKA has a significant inhibitory effect on the ERK1/2 cascade. Finally, we tested the effect of H89 on cell proliferation using a MTT assay and observed similar results (P < 0.05, Figure 4B).
Figure 4.
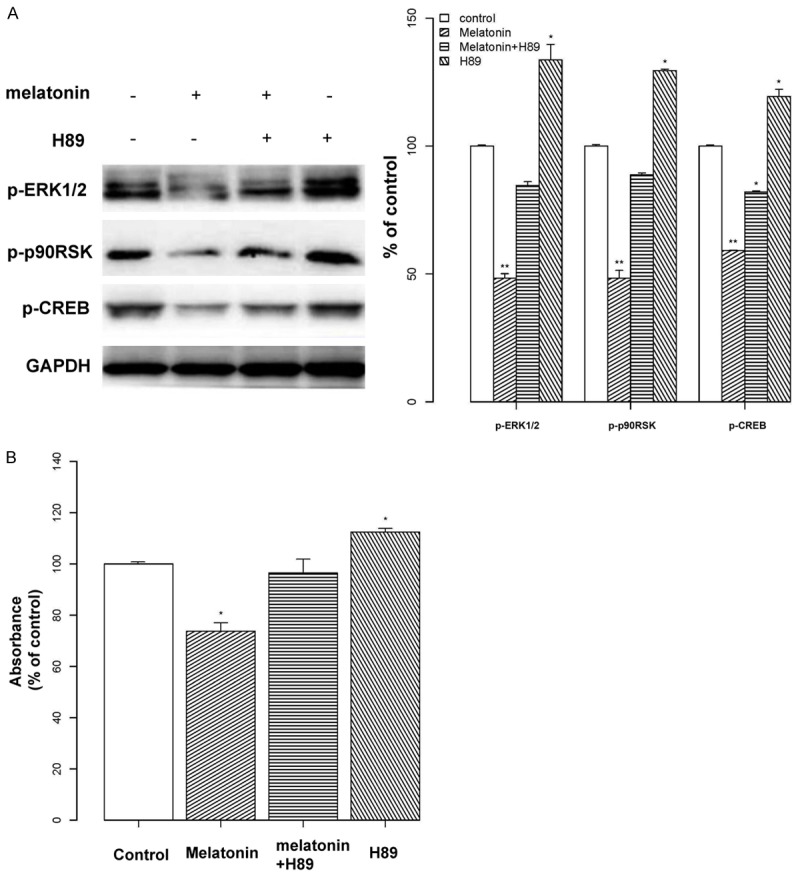
Relationship between PKA and the ERK/p90RSK/CREB pathway. A: Osteoblast cells were exposed to melatonin (2 mM; indicated by “+”) or the PKA inhibitor H89 (50 μM; indicated by “+”) alone, or in combination for 24 h. Then, the expression of proteins in the p-ERK1/2/p-p90RSK/p-CREB pathway was measured by Western blotting. Protein levels were normalized to the corresponding GAPDH band. Results are shown as the percent optical density relative to each protein’s untreated control. Co-treatment of cells with H89 and melatonin only slightly reduced the expression of proteins in the p-ERK1/2/p-p90RSK/p-CREB pathway. Treatment with H89 alone resulted in an up-regulation. B: Osteoblast cells were treated with melatonin (2 mM) and/or H89 (50 μM), for 24 h, or with H89 for 1 h. Cell proliferation was assessed with a MTT assay and results are displayed as the percent absorbance at 490 nm relative to controls (untreated). All experiments were performed in triplicate and data are shown as the mean ± S.E.M. *P < 0.05 or **P < 0.01, compared to controls.
Relationship between Src and the ERK/CREB pathway
To further investigate the regulation mechanism occurring upstream of CREB, we explored the Src protein. As shown in Figure 5A, we found that melatonin inhibited the expression of phosphorylated Src, compared with controls (P < 0.05), although no significant change was observed between 24 and 48 h. Next, we used the Src inhibitor PP2 to examine the effect of Src on p-ERK1/2 and p-CREB (Figure 5B). We compared the effect of PP2 on melatonin-treated cells and untreated controls, and found that the expression of phosphorylated ERK was remarkably reduced in the melatonin group (P < 0.01), as was the basal p-ERK1/2 level. A similar result was observed in p-CREB expression (P < 0.01). The results of a MTT assay showed the same trends occurred for cell proliferation (Figure 5C). These observations indicate that melatonin mediates a signal transduction cascade involving Src, as a bridge that connects upstream of the CREB pathway.
Figure 5.
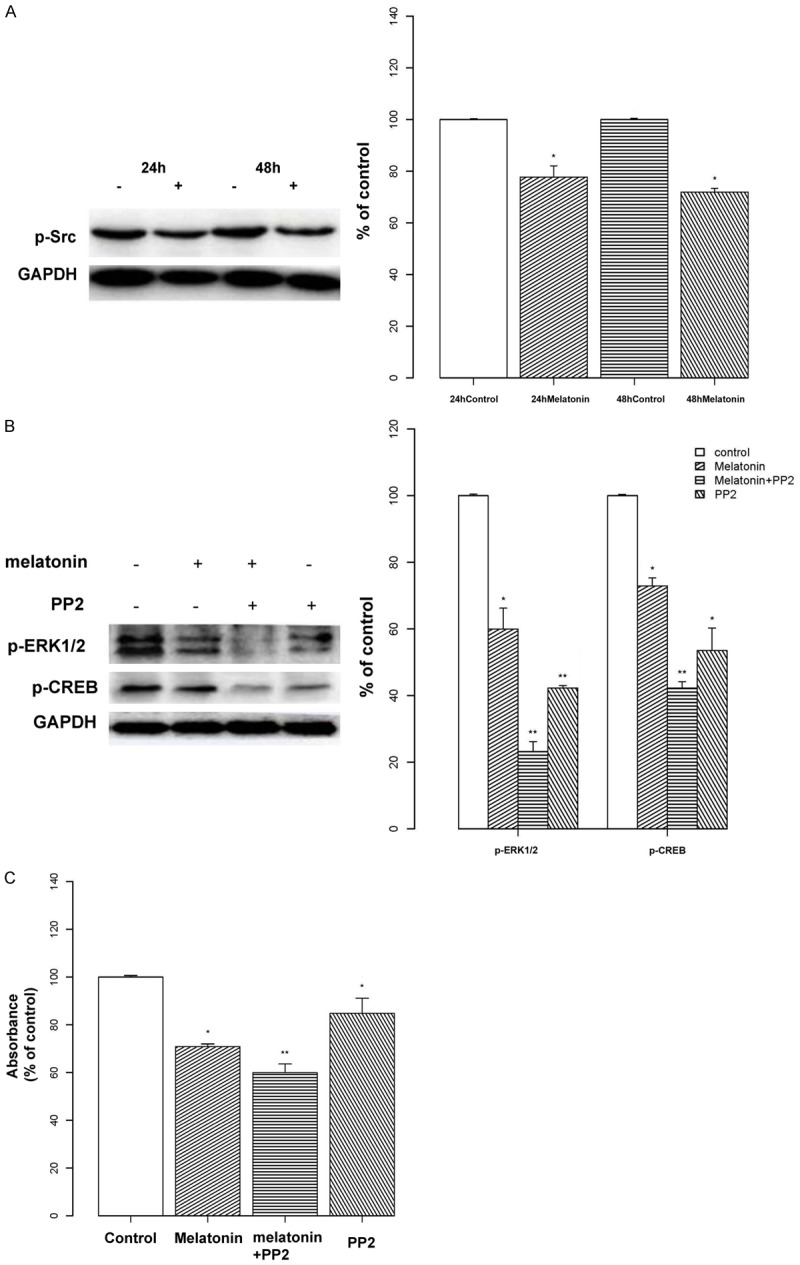
Relationship between Src and the ERK/CREB Pathway. A: Osteoblasts were treated with melatonin (2 mM) for 24 or 48 h and the expression of phosphorylated Src was analyzed by Western blotting. Protein levels were analyzed and normalized to the corresponding GAPDH band. Results are shown as the percent optical density relative to the respective control time point (24 or 48 h). Melatonin decreased the expression of phosphorylated Src in osteoblast cells. Protein levels were normalized to the corresponding GAPDH band. B: Osteoblast cells were treated with melatonin (2 mM; indicated by “+”) or the Src inhibitor PP2 (20 μM; indicated by “+”) alone, or in combination. Expression levels of p-ERK and p-CREB were analyzed by Western blotting. Protein levels were analyzed and normalized to the corresponding GAPDH band, and the results are shown as the percent optical density relative to the respective control (untreated) for each protein. Expression levels of p-ERK and p-CREB were remarkably reduced in the melatonin-treated group, and this effect was more pronounced in the melatonin + PP2 group. C: Osteoblast cells were treated with melatonin (2 mM) and/or PP2 (20 μM) for 24 h. Cell proliferation was assessed with a MTT assay, and the results are displayed as the percent absorbance at 490 nm relative to controls (untreated). All experiments were performed in triplicate and data are shown as the mean ± S.E.M. *P < 0.05 or **P < 0.01, compared to controls.
Relationship between cAMP-PKA and the Src pathway
To further examine whether Src is upstream from the cAMP-PKA pathway, we measured cAMP levels in response to melatonin with and without PP2. However, while melatonin decreased cAMP levels in osteoblasts, co-treatment with PP2 had no further effect (Figure 6).
Figure 6.
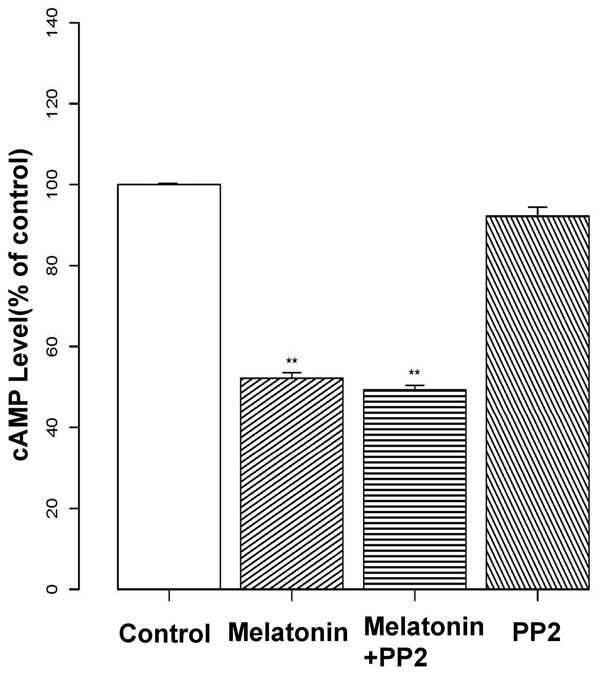
Relationship between cAMP-PKA and the Src pathway. Osteoblasts were pre-treated with melatonin (2 mM) and/or the Src inhibitor PP2 (20 μM) for 24 h. Levels of cAMP were then assessed by ELISA, and data are presented as the percent optical density relative to controls (untreated). Melatonin decreased cAMP, but treatment with PP2 had no additional effects. Experiments were performed in triplicate, and data are shown as the mean ± S.E.M. **P < 0.01, compared to control cells.
Effects of melatonin on the expression of PCNA
Finally, we analyzed the expression of PCNA, a CREB-related gene associated with proliferation. We performed a Western blot to determine the effect of CREB on PCNA at the protein level. Our analysis revealed that cells treated with melatonin exhibited reduced PCNA expression compared to controls (P < 0.01) and that no significant change was observed between 24 and 48 h (Figure 7A). To further understand the relationship between CREB, PCNA and cell proliferation in osteoblasts, cells were transfected with lentiviral CREB shRNAs to knock down CREB expression. Our results showed that cells transfected with lentiviral CREB shRNAs had decreased expression of PCNA and osteoblast proliferation, and in cells that received melatonin in addition to CREB shRNAs the effect was more pronounced (P < 0.01, Figure 7B and 7C).
Figure 7.
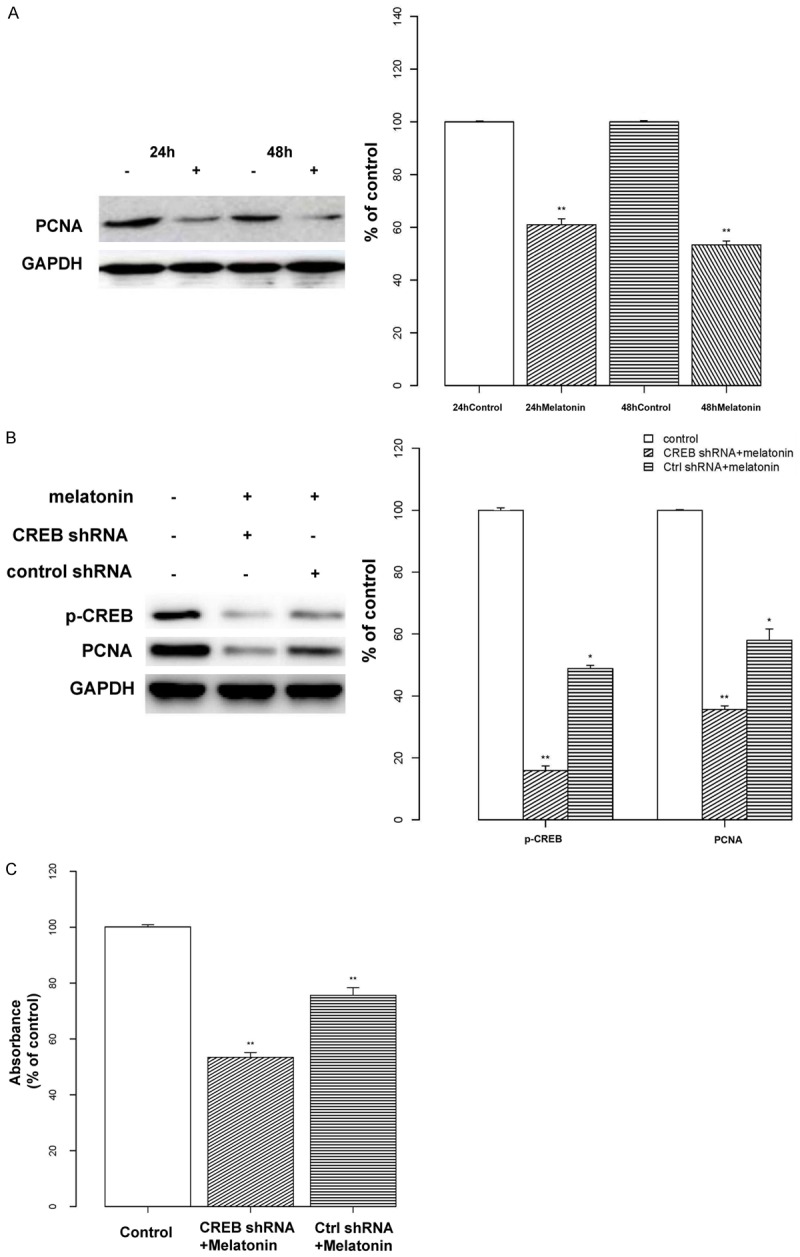
Relationship between CREB and PCNA expression and cell proliferation. A: Osteoblasts were treated with melatonin (2 mM; indicated by “+”) or vehicle for 24 or 48 h. The expression of proliferating cell nuclear antigen (PCNA) was assessed by Western blotting. Protein levels were normalized to the respective GAPDH band, and data are displayed as the percent optical density relative to the corresponding control (24 or 48 h). The analysis revealed that cells treated with melatonin exhibited weaker PCNA expression. B: Osteoblasts were treated with melatonin (2 mM; indicated by “+”) or vehicle and transfected with either lentiviral CREB shRNA or a control shRNA. The expression levels of p-CREB and PCNA were assessed by Western blotting. Protein levels were analyzed and normalized to the corresponding GAPDH band, and the results are shown as the percent optical density relative to the respective control (untreated) for each protein. Transfection with lentiviral CREB shRNAs decreased the expression of PCNA, and this effect was more pronounced by treatment with melatonin. C: Osteoblast cells were pre-treated with melatonin (2 mM) and transfected with either lentiviral CREB shRNA or a control shRNA. Cell proliferation was assessed with a MTT assay, and the results are displayed as the percent absorbance at 490 nm relative to controls (untreated). All experiments were performed in triplicate and data are shown as the mean ± S.E.M. *P < 0.05 or **P < 0.01, compared to controls.
Discussion
Discerning the pathogenesis of IS, along with developing effective preventions and therapeutics, remains a challenge for orthopedic experts [17-19]. Pharmaceutical treatments for IS may serve an auxiliary role alongside surgical options in the future. Here, we investigated the mechanism by which melatonin exerts inhibitory effects on osteoblast proliferation. Multiple studies have previously shown that melatonin levels are closely associated with IS [20,21], whereas studies investigating the effect of melatonin on osteoblasts are limited. In a prior study, we confirmed that melatonin exerts a dose-dependent effect on osteoblast proliferation [10]. In this work, we found that this inhibitory effect is most pronounced at a concentration of 2 mM. Accordingly, we conducted the following experiments utilizing 2 mM of melatonin. A growing number of studies have demonstrated that IS patients may have abnormalities in the melatonin pathway [22-24]. Recently, Moreau and colleagues found that abnormalities in melatonin signaling were 100% found in osteoblasts isolated from a group of IS patients undergoing surgical treatment [25]. In our study, we identified CREB and PCNA as novel downstream targets of melatonin signaling, and demonstrated that melatonin-induced changes in phosphorylated CREB can reduce transcriptional activity in a melatonin receptor-dependent manner. In addition, we confirmed that melatonin exerts its inhibitory effect on osteoblast proliferation through both the Src and PKA pathways, thereby inducing transcription-dependent mechanisms involved in down-regulation of CREB phosphorylation and PCNA expression. These findings suggest a possible clinical application for melatonin in the prevention and reversal of IS progression.
Melatonin membrane receptors have been reported to be widely expressed in a number of different tissues. A recent study found that melatonin protects cardiac progenitor cells against premature senescence in a melatonin membrane receptor-dependent manner [26]. However, in human leukemia cells, it has been reported that melatonin induces apoptosis through a pathway independent of melatonin membrane receptor activation [27]. These data confirm that melatonin can exert its biological functions through both melatonin membrane receptor-dependent and independent mechanisms. In the present study, we demonstrated that both MT1 and MT2 are present in hFOB 1.19 cells, and that the non-selective MT receptor antagonist luzindole can block the effect of melatonin on osteoblast proliferation. These results indicate that melatonin promotes osteoblast proliferation via a melatonin membrane receptor-dependent pathway.
Previous studies, using other cell types, have reported that activation of the mitogen-activated protein kinase (MAPK) pathway is associated with melatonin-mediated antiproliferative effects [28]. The MAPK family mainly consists of ERK, p38, and c-Jun N-terminal kinase (JNK). Once activated, the resulting signaling cascade activates downstream response molecules through phosphorylation, thereby regulating gene expression, cell proliferation, and differentiation [29,30]. We have previously demonstrated that melatonin has an inhibitory effect on osteoblast proliferation and that this is potentially mediated via the ERK pathway [31,32]. The kinase p90RSK is a main, proximal, intracellular, downstream target of ERK; p90RSK is activated and phosphorylated by ERK, which leads to the phosphorylation of CREB or c-Fos following nuclear translocation [33,34]. Other studies have shown that CREB expression is related to the transcriptional activity of cyclin D1 [35], which may be involved in the regulation of cell proliferation. To identify the downstream effectors that mediate the inhibitory actions of melatonin, we chose to focus on the mechanisms described above, particularly the expression of p90RSK and CREB.
Our data demonstrated that melatonin depressed the phosphorylation of p90RSK and CREB, as well as the nuclear localization of CREB. CREB is an important nuclear transcription factor that belongs to the CREB family, and its roles in the regulation of transcriptional functions, ranging from learning and memory to cell proliferation and differentiation to neuronal regeneration, have been emphasized [36]. Within the promoter regions of eukaryotic cells, CREB binds to DNA sequences known as CRE. CREB activation requires phosphorylation of the Ser-133 region of the kinase inducible domain (KID), which results in histone acetylation at the promoter sequence of CRE and further promotes gene transcription [37]. To determine if the decline in CREB phosphorylation, mediated by melatonin, leads to a change in CRE-dependent gene transcription, we performed a CRE-luciferase assay. Our results showed that melatonin treatment reduced CRE-luciferase activity, after transfection with a CRE-luc plasmid. We also observed that melatonin notably decreased the expression of CRE-dependent genes and CREB transcriptional activity in osteoblasts.
To better understand the role of MEK/ERK in CREB-induced transcriptional activity, we treated osteoblasts with the MEK inhibitor PD98059. Since CREB is a downstream receptor of ERK, we expected to observe an inhibition of CREB activation in response to PD98059. However, not only did we find that PD98059 did not block melatonin-mediated CREB phosphorylation, but levels of both CREB phosphorylation and CREB transcriptional activity were elevated. This suggests that PD98059 up-regulates other cascades upstream from CREB, and we therefore hypothesized that CREB activation is regulated by a complex process.
The cAMP content of bone tissue has been implicated in orthopedic diseases through the cAMP/PKA pathway [38]. CREB is the principal mediator in response to increased cAMP production, following phosphorylation by PKA, and activation of G-protein-coupled melatonin receptors could elevate levels of intracellular cAMP. Since CREB is a common substrate in the MEK/ERK and cAMP-PKA pathways, we assumed that the physiological process connecting melatonin-induced activation to CREB phosphorylation possibly involved the ERK and PKA pathways. To examine this possibility, we investigated the effect of cAMP-dependent protein kinase inhibitor H89 on CREB activation. Our results showed that H89 activated CREB phosphorylation, along with substantial increases in p-ERK1/2 and p-p90RSK expression. This suggests that, following melatonin treatment in osteoblasts, the PKA pathway exerts an inhibitory effect on the ERK1/2 pathway. It is also possible that a more extensive relationship exists, as multiple levels of cross-talk between the MEK/ERK1/2 pathway and cAMP-PKA cascade have been described [39]. However, the effects observed in CREB cannot be fully attributed to PKA, and the possibility exists that other transduction cascades contribute to the regulation of CREB expression. Therefore, we investigated Src family kinases (SFKs) as a potential alternative candidate associated with melatonin’s effects.
The Src protein is upstream of the MAPK pathway. Src regulates the activity of downstream kinases such as Ras, c-Raf, or ERK and contributes to numerous biological effects [40]. In addition, certain pathways can be phosphorylated by Src, leading to the activation of gene transcription [41]. Furthermore, Src activation can promote the expression of the cyclin A, D3/cyclin-dependent kinase (CDK) 4/6 complex, accelerate cell cycle transitions, and cause cell proliferation [42]. We previously demonstrated that melatonin regulates cell prolif eration via the regulation of cell cycle proteins [10], suggesting a potential role for Src in the inhibition of osteoblasts by melatonin. The results of this study showed that melatonin reduces the expression of phosphorylated Src protein. In addition, treatment of osteoblasts with the Src family kinase inhibitor PP2 further confirmed that Src plays a role in this process upstream of p-ERK/p-CREB. Taken together, we have shown that cAMP-PKA and Src pathways are involved in the melatonin-induced regulation of osteoblast proliferation. As PKA and ERK are downstream of cAMP, it is possible that Src is either upstream of the cAMP pathway, or works in parallel. To test this, we measured cAMP levels in response to melatonin, with and without PP2. Our results showed that the Src inhibitor had no effect on cAMP levels in osteoblasts in response to melatonin, suggesting that activation of the Src and cAMP-PKA pathways occurs in parallel, rather than sequentially.
The results of our CRE-luciferase assay proved that melatonin mediates CRE-dependent gene transcription in osteoblasts. To investigate potential CREB target genes associated with proliferation, we focused on PCNA, which is a CREB-related gene. In addition, PCNA is a CRE-dependent gene that is expressed at high levels particularly in the early G1 phase and is indispensable for the initiation of cell proliferation. Different complexes formed by the interactions between cyclin, CDK, and cyclin-dependent kinase inhibitor (CKI) regulate transformations of the cell cycle during each phase, while PCNA and CDK/cyclin compose an active complex in the nucleus, regulating cell proliferation [43]. In this study, we used RNA interference to investigate PCNA expression and found that cells transfected with lentiviral CREB shRNAs had decreased PCNA expression and osteoblast proliferation. Furthermore, co-treatment with melatonin and CREB shRNAs increased this effect, which confirmed that PCNA expression and osteoblast proliferation are dependent on CREB.
In summary, our results establish CREB and PCNA as novel downstream targets of melatonin signaling, and show that the down-regulation of CREB is regulated via the PKA and Src pathways, which contributes to the melatonin-induced inhibition of osteoblast proliferation. In addition, we show that melatonin activates Src via a parallel signaling pathway, separate from that of PKA. These results provide important insight into the mechanisms underlying melatonin supplementation, and this knowledge could be applied to using these supplements to prevent IS progression. Our data provide evidence for further research regarding the efficacious treatment of IS with melatonin.
Acknowledgements
This work was supported by two National Natural Science Foundation of China Grants (81271939 and 81472044). We also thanked Dr. Subramaniam M (Department of Biochemistry and Molecular Biology, Mayo Clinic) [47] for providing osteoblasts friendly, and Dr. Reiter RJ (Department of Cellular & Structural Biology, The UT Health Science Center) [10] for technical guidance and support.
Disclosure of conflict of interest
None.
References
- 1.Liu L, Zhu Y, Han X, Wu Y. The creation of scoliosis by scapula-to-contralateral ilium tethering procedure in bipedal rats: a kyphoscoliosis model. Spine. 2011;36:1340–1349. doi: 10.1097/BRS.0b013e3181f3d164. [DOI] [PubMed] [Google Scholar]
- 2.Kono H, Machida M, Saito M, Nishiwaki Y, Kato H, Hosogane N, Chiba K, Miyamoto T, Matsumoto M, Toyama Y. Mechanism of osteoporosis in adolescent idiopathic scoliosis: experimental scoliosis in pinealectomized chickens. J Pineal Res. 2011;51:387–393. doi: 10.1111/j.1600-079X.2011.00901.x. [DOI] [PubMed] [Google Scholar]
- 3.Song R, Ren L, Ma H, Hu R, Gao H, Wang L, Chen X, Zhao Z, Liu J. Melatonin promotes diabetic wound healing in vitro by regulating keratinocyte activity. Am J Transl Res. 2016;8:4682–4693. [PMC free article] [PubMed] [Google Scholar]
- 4.Li JG, Lin JJ, Wang ZL, Cai WK, Wang PN, Jia Q, Zhang AS, Wu GY, Zhu GX, Ni LX. Melatonin attenuates inflammation of acute pulpitis subjected to dental pulp injury. Am J Transl Res. 2015;7:66–78. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HH, Chang CL, Lin KC, Sung PH, Chai HT, Zhen YY, Chen YC, Wu YC, Leu S, Tsai TH, Chen CH, Chang HW, Yip HK. Melatonin augments apoptotic adipose-derived mesenchymal stem cell treatment against sepsis-induced acute lung injury. Am J Transl Res. 2014;6:439–458. [PMC free article] [PubMed] [Google Scholar]
- 6.Amstrup AK, Sikjaer T, Heickendorff L, Mosekilde L, Rejnmark L. Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: a randomized controlled trial. J Pineal Res. 2015;59:221–229. doi: 10.1111/jpi.12252. [DOI] [PubMed] [Google Scholar]
- 7.Man GC, Wang WW, Yim AP, Wong JH, Ng TB, Lam TP, Lee SK, Ng BK, Wang CC, Qiu Y, Cheng CY. A review of pinealectomy-induced melatonin-deficient animal models for the study of etiopathogenesis of adolescent idiopathic scoliosis. Int J Mol Sci. 2014;15:16484–99. doi: 10.3390/ijms150916484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akel I, Demirkiran G, Alanay A, Karahan S, Marcucio R, Acaroglu E. The effect of calmodulin antagonists on scoliosis: bipedal C57BL/6 mice model. Eur Spine J. 2009;18:499–505. doi: 10.1007/s00586-009-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machida M, Dubousset J, Yamada T, Kimura J. Serum melatonin levels in adolescent idiopathic scoliosis prediction and prevention for curve progression--a prospective study. J Pineal Res. 2009;46:344–348. doi: 10.1111/j.1600-079X.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Zhu Y, Xu Y, Reiter RJ. Melatonin delays cell proliferation by inducing G1 and G2/M phase arrest in a human osteoblastic cell line hFOB 1.19. J Pineal Res. 2011;50:222–231. doi: 10.1111/j.1600-079X.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 11.He C, Ma T, Shi J, Zhang Z, Wang J, Zhu K, Li Y, Yang M, Song Y, Liu G. Melatonin and its receptor MT1 are involved in the downstream reaction to luteinizing hormone and participate in the regulation of luteinization in different species. J Pineal Res. 2016;61:279–290. doi: 10.1111/jpi.12345. [DOI] [PubMed] [Google Scholar]
- 12.Benleulmi-Chaachoua A, Chen L, Sokolina K, Wong V, Jurisica I, Emerit MB, Darmon M, Espin A, Stagljar I, Tafelmeyer P, Zamponi GW, Delagrange P, Maurice P, Jockers R. Protein interactome mining defines melatonin MT1 receptors as integral component of presynaptic protein complexes of neurons. J Pineal Res. 2016;60:95–108. doi: 10.1111/jpi.12294. [DOI] [PubMed] [Google Scholar]
- 13.Hou SW, Zheng P, Sun FY. Melatonin inhibits outward delayed rectifier potassium currents in hippocampal CA1 pyramidal neuron via intracellular indole-related domains. J Pineal Res. 2004;36:242–249. doi: 10.1111/j.1600-079X.2004.00123.x. [DOI] [PubMed] [Google Scholar]
- 14.Nduhirabandi F, Lamont K, Albertyn Z, Opie LH, Lecour S. Role of toll-like receptor 4 in melatonin-induced cardioprotection. J Pineal Res. 2016;60:39–47. doi: 10.1111/jpi.12286. [DOI] [PubMed] [Google Scholar]
- 15.Espino J, Rodriguez AB, Pariente JA. The inhibition of TNF-alpha-induced leucocyte apoptosis by melatonin involves membrane receptor MT1/MT2 interaction. J Pineal Res. 2013;54:442–452. doi: 10.1111/jpi.12042. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniam M, Jalal SM, Rickard DJ, Harris SA, Bolander ME, Spelsberg TC. Further characterization of human fetal osteoblastic hFOB 1.19 and hFOB/ER alpha cells: bone formation in vivo and karyotype analysis using multicolor fluorescent in situ hybridization. J Cell Biochem. 2002;87:9–15. doi: 10.1002/jcb.10259. [DOI] [PubMed] [Google Scholar]
- 17.Chiu CK, Chan CYW, Aziz I, Hasan MS, Kwan MK. Assessment of intraoperative blood loss at different surgical stages during posterior spinal fusion surgery in the treatment of adolescent idiopathic scoliosis. Spine. 2016;41:E566–E573. doi: 10.1097/BRS.0000000000001304. [DOI] [PubMed] [Google Scholar]
- 18.Wang WW, Man GC, Wong JH, Ng TB, Lee KM, Ng BK, Yeung HY, Qiu Y, Cheng JC. Abnormal response of the proliferation and differentiation of growth plate chondrocytes to melatonin in adolescent idiopathic scoliosis. Int J Mol Sci. 2014;15:17100–17114. doi: 10.3390/ijms150917100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rushton PR, Elmalky M, Tikoo A, Basu S, Cole AA, Grevitt MP. The effect of metal density in thoracic adolescent idiopathic scoliosis. Eur Spine J. 2016;25:3324–3330. doi: 10.1007/s00586-015-4335-x. [DOI] [PubMed] [Google Scholar]
- 20.Man GC, Wong JH, Wang WW, Sun GQ, Yeung BH, Ng TB, Lee SK, Ng BK, Qiu Y, Cheng JC. Abnormal melatonin receptor 1B expression in osteoblasts from girls with adolescent idiopathic scoliosis. J Pineal Res. 2011;50:395–402. doi: 10.1111/j.1600-079X.2011.00857.x. [DOI] [PubMed] [Google Scholar]
- 21.Machida M, Dubousset J, Yamada T, Kimura J. Serum melatonin levels in adolescent idiopathic scoliosis prediction and prevention for curve progression-a prospective study. J Pineal Res. 2009;46:344–348. doi: 10.1111/j.1600-079X.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- 22.Girardo M, Bettini N, Dema E, Cervellati S. The role of melatonin in the pathogenesis of adolescent idiopathic scoliosis (AIS) Eur Spine J. 2011;20:68–74. doi: 10.1007/s00586-011-1750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yim AP, Yeung HY, Sun GQ, Lee KM, Ng TB, Lam TP, Ng BK, Qiu Y, Moreau A, Cheng JC. Abnormal skeletal growth in adolescent idiopathic scoliosis is associated with abnormal quantitative expression of melatonin receptor, MT2. Int J Mol Sci. 2013;14:6345–6358. doi: 10.3390/ijms14036345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shangguan L, Fan X, Luo Z. The association between melatonin signaling dysfunction and idiopathic scoliosis. Med Hypotheses. 2009;72:228–229. doi: 10.1016/j.mehy.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Moreau A, Wang DS, Forget S, Azeddine B, Angeloni D, Fraschini F, Labelle H, Poitras B, Rivard CH, Grimard G. Melatonin signaling dysfunction in adolescent idiopathic scoliosis. Spine. 2004;29:1772–1781. doi: 10.1097/01.brs.0000134567.52303.1a. [DOI] [PubMed] [Google Scholar]
- 26.Cai BZ, Ma WY, Bi CW, Yang F, Zhang L, Han ZB, Huang Q, Ding FZ, Li Y, Yan GG, Pan ZW, Yang BF, Lu YJ. Long noncoding RNA H19 mediates melatonin inhibition of premature senescence of c-kit(+) cardiac progenitor cells by promoting miR-675. J Pineal Res. 2016;61:82–95. doi: 10.1111/jpi.12331. [DOI] [PubMed] [Google Scholar]
- 27.Perdomo J, Cabrera J, Estevez F, Loro J, Reiter RJ, Quintana J. Melatonin induces apoptosis through a caspase-dependent but reactive oxygen species-independent mechanism in human leukemia Molt-3 cells. J Pineal Res. 2013;55:195–206. doi: 10.1111/jpi.12062. [DOI] [PubMed] [Google Scholar]
- 28.Liu XW, Zi Y, Liu YE, Zhang YB, Xiang LB, Hou MX. Melatonin exerts protective effect on N2a cells under hypoxia conditions through Zip1/ERK pathway. Neurosci Lett. 2015;595:74–80. doi: 10.1016/j.neulet.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 30.Kimura H, Okubo N, Chosa N, Kyakumoto S, Kamo M, Miura H, Ishisaki A. EGF positively regulates the proliferation and migration, and negatively regulates the myofibroblast differentiation of periodontal ligament-derived endothelial progenitor cells through MEK/ERKand JNK-dependent signals. Cell Physiol Biochem. 2013;32:899–914. doi: 10.1159/000354493. [DOI] [PubMed] [Google Scholar]
- 31.Liu LF, Zhu Y, Xu Y, Reiter RJ. Prevention of ERK activation involves melatonin-induced G1 and G2/M phase arrest in the human osteoblastic cell line hFOB 1.19. J Pineal Res. 2012;53:60–66. doi: 10.1111/j.1600-079X.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 32.Xiong XC, Zhu Y, Ge R, Liu LF, Yuan W. Effect of melatonin on the extracellular-regulated kinase signal pathway activation and human osteoblastic cell line hFOB 1.19 proliferation. Int J Mol Sci. 2015;16:10337–10353. doi: 10.3390/ijms160510337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh PO. Ferulic acid attenuates the down-regulation of MEK/ERK/p90RSK signaling pathway in focal cerebral ischemic injury. Neurosci Lett. 2015;588:18–23. doi: 10.1016/j.neulet.2014.12.047. [DOI] [PubMed] [Google Scholar]
- 34.Mizutani T, Fukushi S, Saijo M, Kurane I, Morikawa S. Regulation of p90RSK phosphorylation by SARS-CoV infection in Vero E6 cells. Febs Lett. 2006;580:1417–1424. doi: 10.1016/j.febslet.2006.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nubile M, Curcio C, Lanzini M, Calienno R, Lezzi M, Mastropasqua A, Di Nicola M, Mastropasqua L. Expression of CREB in primary pterygium and correlation with Cyclin D1, ki-67, MMP7, p53, p63, survivin and vimentin. Ophthalmic Res. 2013;50:99–107. doi: 10.1159/000347124. [DOI] [PubMed] [Google Scholar]
- 36.Casanova JR, Nishimura M, Swann JW. The effects of early-life seizures on hippocampal dendrite development and later-life learning and memory. Brain Res Bull. 2014;103:39–48. doi: 10.1016/j.brainresbull.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park HJ, Baek K, Baek JH, Kim HR. The cooperation of CREB and NFAT is required for PTHrP-induced RANKL expression in mouse osteoblastic cells. J Cell Physiol. 2015;230:667–679. doi: 10.1002/jcp.24790. [DOI] [PubMed] [Google Scholar]
- 38.Kim JM, Choi JS, Kim YH, Jin SH, Lim S, Jang HJ, Kim KT, Ryu SH, Suh PG. An activator of the cAMP/PKA/CREB pathway promotes osteogenesis from human mesenchymal stem cells. J Cell Physiol. 2013;228:617–626. doi: 10.1002/jcp.24171. [DOI] [PubMed] [Google Scholar]
- 39.Lausson S, Cressent M. Signal transduction pathways mediating the effect of adrenomedullin on osteoblast survival. J Cell Biochem. 2011;112:3807–3815. doi: 10.1002/jcb.23311. [DOI] [PubMed] [Google Scholar]
- 40.Montiel M, Quesada J, Jimenez E. Activation of calcium-dependent kinases and epidermal growth factor receptor regulate muscarinic acetylcholine receptor-mediated MAPK/ERK activation in thyroid epithelial cells. Cell Signal. 2007;19:2138–2146. doi: 10.1016/j.cellsig.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Kamiguti AS, Harris RJ, Slupsky JR, Baker PK, Cawley JC, Zuzel M. Regulation of hairy-cell survival through constitutive activation of mitogen-activated protein kinase pathways. Oncogene. 2003;22:2272–2284. doi: 10.1038/sj.onc.1206398. [DOI] [PubMed] [Google Scholar]
- 42.Lausson S, Cressent M. Signal transduction pathways mediating the effect of adrenomedullin on osteoblast survival. J Cell Biochem. 2011;112:3807–3815. doi: 10.1002/jcb.23311. [DOI] [PubMed] [Google Scholar]
- 43.Chae HD, Mitton B, Lacayo NJ, Sakamoto KM. Replication factor C3 is a CREB target gene that regulates cell cycle progression through the modulation of chromatin loading of PCNA. Leukemia. 2015;29:1379–1389. doi: 10.1038/leu.2014.350. [DOI] [PMC free article] [PubMed] [Google Scholar]


