Abstract
Several studies demonstrated a correlation between green tea consumption and a reduced cancer risk. Among different components, green tea polyphenols have been identified as molecules responsible for the beneficial effects showed by the green tea against oxidative stress and cell invasiveness. In this study, we investigated the effects of green tea polyphenol extracts (GTPs) in human gastric MKN-28 cell line. To this aim, we have first evaluated the effect of GTPs on oxidative stress induced cell injury. The pre-treatment with 10−4 M catechin equivalents of GTPs exerts a protective effect on xanthine–xanthine oxidase induced cell cytotoxicity, thus confirming the anti-oxidant properties of GTPs. The effect of GTPs was also extended to the invasive ability of MKN-28 cells stimulated with TNF-α or LPS, as pro-inflammatory factors. Migration and matrigel invasion assays demonstrated that GTPs exposure (10−6 M) prevents the increase in cell invasiveness induced by TNF-α or LPS. Finally, we have analyzed the effect of GTPs on the levels of Matrix Metalloproteinases (MMP)-9/2, whose expression is up-regulated by TNF-α or LPS.
Our results indicated that the pre-treatment with GTPs was able to reduce MMP-9/2 expression at both protein and enzyme activity levels in the conditioned media of TNF-α or LPS stimulated MKN-28 cells.
In conclusion, our results demonstrated that green tea polyphenol extract reduces the invasiveness of gastric MKN-28 cancer cells through the reduction of TNF-α or LPS induced MMP-9/2 up-regulation. Therefore, these data support the hypothesis that GTPs could exert a protective role against the metastatic process in gastric cancer.
Keywords: Green tea polyphenols, Matrix Metalloproteinase-9 (MMP-9), Matrix Metalloproteinase-2 (MMP-2), MKN-28 gastric cancer cells, Cell migration, Cell invasion
Abbreviations: ECM, Extracellular matrix; MMP-, Matrix metalloproteinase; TNF-α, Tumor necrosis factor α; LPS, Lipopolysaccharide; GTPs, Green tea polyphenols extract; DMEM, Dulbecco's Modified Eagles's Medium; FBS, fetal bovine serum; PBS, Phosphate-buffer saline; DMSO, Dimethyl sulfoxide; ROS, Reactive Oxygen Species
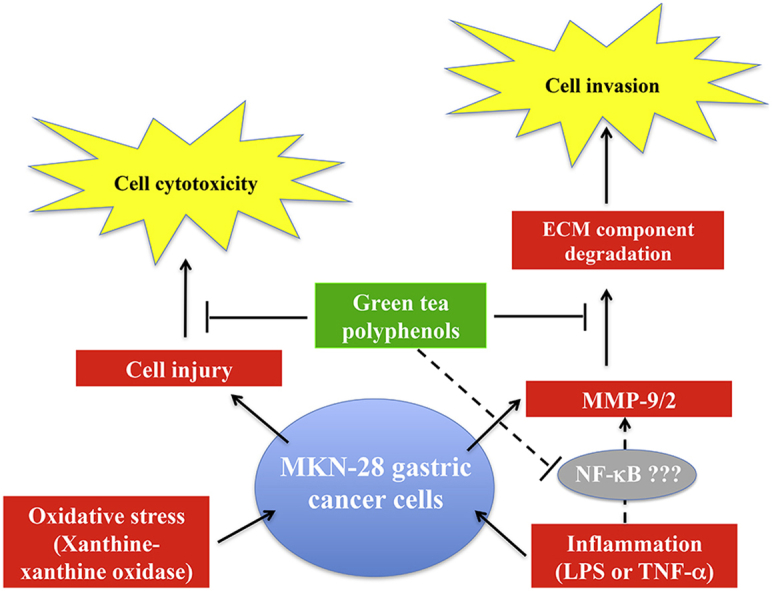
Graphical abstract
Highlights
-
•
Green tea polyphenols prevent oxidative stress induced cytotoxicity in MKN-28 cells.
-
•
Green tea polyphenols reduce invasiveness of MKN-28 cells.
-
•
Green tea polyphenols reduce MMP-9/2 levels induced by TNF-alpha or LPS.
-
•
Green tea polyphenols might prevent cell-invasiveness during inflammation.
1. Introduction
Gastric cancer (GC) represents one of the most common lethal tumors in the world [1], [2] as for many patients the poor prognosis is made at an advanced stage of the metastatic process. The decrease in the incident of gastric cancer is associated with standard of living and proper dietary habits [3], [4].
Tumor dissemination is based on the ability of cancer cells to invade the surrounding microenvironment through the degradation of extracellular matrix (ECM). Matrix Metalloproteinases (MMPs), are a family of zinc-dependent endopeptidases secreted as inactive zymogens by stromal and tumor cells; the cleavage of a prodomain yield their active form [5]. MMPs play a crucial role during tumor invasion and metastasis through the ability to degrade extracellular matrix proteins [6], [7]. Several studies have reported increased levels of different MMPs in several cancer tissues [8] and, in human cancer, their overexpression has been related to tumor progression as well as in its poor prognosis [9].
Among various MMPs involved in cancer, attention has been focused on the gelatinases MMP-2 and MMP-9 as they are overexpressed in a variety of malignant tumors and their expression and activity are often associated with tumor aggressiveness [10], [11] through their involvement in cell migration and invasiveness [6], [7].
Furthermore, in the early stage of the metastatic process a key role is played by MMPs through their up-regulation by TNF-α- [7], [12] and/or LPS- induced inflammatory process [13]. As inflammation is closely linked to tumor progression, it has been reported that polyphenol containing dietary substances with potent anti-inflammatory activities, exert chemopreventive effects on carcinogenesis [14], [15], [16]. In fact, besides having a cancer chemopreventive activity, polyphenols have been shown to inhibit tumor invasion, a crucial step for the development of metastasis of solid tumors [14], [17].
Among vegetables, green tea is an important dietary source of polyphenols, and tea consumption has been correlated to anti-carcinogenic and anti-proliferative effects [15], [18]. Majority of the green tea polyphenols (GTPs) are monomeric flavonols called also catechins, and the most abundant are (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epicatechin (EC) (Fig. S1) [19]. Most studies have suggested that the anti-inflammatory effects of polyphenols were attributable to their anti-oxidant properties acting against Reactive Oxygen Species (ROS) [20]. In addition, polyphenols have an important protective role not only in the early stages of carcinogenesis, but also in the development of metastasis, through interference with the activity of metalloproteinases [21], [22].
This study reports the effects of green tea polyphenols on i) the oxidative stress in a model of gastric cancer cells (MKN-28); ii) the levels of MMP-2/9 in TNF-α- or LPS-stimulated MKN-28 cells; iii) MKN-28 cell migration and invasion abilities.
2. Materials and methods
2.1. Reagents
Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), trypsin-EDTA and phosphate-buffered saline (PBS) pH 7.4 were obtained from Lonza (Basel, Switzerland); Xanthine and 3-(4,5dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide (MTT) were purchased from Applichem (Inc, USA); Xanthine oxidase and Protease inhibitor cocktail Kit from MP Biomedical (Germany); Matrigel and cell culture inserts were purchased from BD Biosciences (Bedford, MA); Tumor necrosis factor-α (TNF-α) and Lipopolysaccharide (LPS) were obtained from Sigma Chemical Co. (St. Louis, MO). Rabbit polyclonal antibodies against MMP-9 and MMP-2 was purchased from Epitomics (Burlingame, U.S.A.). Horseradish peroxidase-conjugated (HRP) goat anti-mouse and anti-rabbit IgG antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Western Chemiluminescent HRP substrate kit and Centrifugal filter Units (Amicon Ultra 10 K) was obtained from Millipore (Burlington, MA).
2.2. Preparation of green tea polyphenols extract
Green tea extract was prepared using an aqueous extraction simulating a normal brewing for a cup of tea. Two grams of commercially available green tea were placed in a 250 mL conical flask and 200 mL of boiling water was added. The extract was heated on a hot plate at 90 °C for 5 min with magnetic stirring. The extract was filtered through a 0.45 μm pore Millex HV filter (PVDF) from Millipore (Bedford, MA, USA), 5-fold diluted with distilled water and analyzed by high pressure liquid chromatography (HPLC). Phenolic compounds were separated by HPLC (Shimadzu-CLASS M10) configured with LC-10AD pumps, SPD-M10A diode array detector, and Rheodyne injector [14]. The sum of the concentration of all compounds present in the extract was performed and the molecular weight of the catechin (CAT) was considered to obtain the molar concentration of green tea polyphenol extract (GTPs), and therefore, GTPs amount corresponded to CAT equivalents. GTPs of 10 mmol/L CAT equivalents in DMSO was prepared, aliquoted, stored at −80 °C to maintain stability, and diluted appropriately just before cell treatments. All other reagents were from Sigma–Aldrich.
2.3. Cell cultures and treatments
The MKN-28 human gastric adenocarcinoma cell line, (American Type Culture Collection, ATCC Manassas, VA) [23] was cultured Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1.5 mM l-glutamine, 50 IU/ml penicillin and 50 μg/ml streptomycin. The cells were maintained at 37 °C, in a humidified atmosphere of 5% CO2 and sub-confluent cultures were splitted to 1:3 to 1:6 twice a week by 0.25% trypsin solution and then seeded at appropriate cell density with culture medium. Treatments of sub-confluent cells were performed replacing the culture medium with those containing the appropriate inductor.
Oxidative stress was induced by incubating MKN-28 cells with increasing concentration (25–100 U/L) of Xanthine Oxidase (XO) in the presence of its substrate Xanthine (X) to a final concentration of 1 mM for 2 h. To explore the protective effects of green tea polyphenols extracts on X/XO induced oxidative stress, MKN-28 cells were pre-treated with various concentrations of green tea extract (10−8 to 10−4 M) for 3 h, followed by the addition of 80 U/L Xanthine Oxidase Xanthine (1 mM) for 2 h.
The exposure to LPS or TNF-α (10 ng/ml for both) were performed for 24 h, following the pretreatments with various concentrations of GTPs (10−8 to 10−4 M) for 6 h. All the treatments were performed under serum-free conditions in the presence of 0.1% (v/v) DMSO used as vehicle for GTPs.
For preparation of conditioned medium, cells were grown to sub-confluence in 100-mm plates in DMEM supplemented with 10% FBS, washed three times with PBS and then subjected to the treatments. At the end of the treatment, medium was collected, centrifuged at 6000 × g for 10 min to remove cells and debris. The conditioned medium was concentrated approximately 20-fold by ultra-filtration using Amicon Ultra (Millipore) with a cut-off of 10 K at 4 °C. The concentrated medium was stored in aliquots at – 80 °C until used for western blotting analysis or gelatin zymography.
Whole protein cell extracts were prepared by lysing cells in 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1% Igepal, 1× protease inhibitor (cat. n. 11836153001, Roche Applied Science, Italy) and phosphatase inhibitor cocktail (cat. n. 524627, Calbiochem, Italy). Protein concentration was determined by the Bradford protein assay [24] using bovine serum albumin (BSA) as standard.
2.4. Cell viability assay
Cells were plated onto 96-well-plates (3–5 × 104 per well) in complete medium and after the treatments, their viability was evaluated as mitochondrial activity using the MTT assay [25]. Briefly, the medium was removed and cells incubated with 200 μl MTT solution (0.5 mg/ml) for 2 h in a humidified 5% CO2 incubator at 37 °C. Thereafter, the solution was removed, formazan solubilized in 200 μl of isopropanol containing 0.04 N HCl and the absorbance measured at 590 nm (background at 630 nm was subtracted) using a microplate reader (BP800 BIOHIT). Results were expressed as percentage of cell survival versus control cells cultured in serum-free medium with 0.1% DMSO (vehicle) which represent the 100% survival.
2.5. Western blotting
Aliquots of concentrated medium, corresponding to identical amount of cell proteins, were analyzed by Western blotting. After treatments, protein samples were mixed with SDS loading buffer (Tris HCl 0.5 M, pH 6.8; SDS 4%; glycerol 20%; 2-mercaptoethanol 10%; bromophenol blue 0.004%), boiled for 2 min and subjected to SDS-PAGE. After the electrophoresis run, proteins were transferred to a nitrocellulose membrane (BA85; Schleincher & Schull) and then incubated (1/5000 diluted) with a primary antibody against MMP-9 or MMP-2 (rabbit monoclonal, Epitomics, Italy, cat. n. EP1254, and P08253, respectively). After incubation with an appropriate peroxidase-linked secondary antibody (Santa Cruz Biotechnology Inc), detection was achieved using the Western Chemiluminescent HRP substrate kit (Millipore). Densitometric analysis of signals was performed using the free image-processing software ImageJ, version1.40q.
2.6. Gelatin zymography
Gelatinolytic activity was assayed by gelatin zymography as described previously [6]. In brief, concentrated conditioned media corresponding to equal amount of protein lysates, were analyzed under non reducing condition, by a 9% polyacrylamide gel co-polymerized with 1 mg/ml gelatin (Sigma–Aldrich). Electrophoresis was conducted at 35 mA constant current for 60–120 min at 4 °C. After the run, gels were washed in 2.5% Triton X-100 for 1 h and then incubated in 50 mM Tris–HCl, pH 7.5, 200 mM NaCl, 5 mM CaCl2 and 5 μM ZnCl2 at 37 °C for 48 h. Gels were fixed in 30% methanol and 10% acetic acid for 30 min, stained with 0.5% Coomassie Brilliant Blue R-250 and finally destained with 50% methanol and 5% acetic acid. Gelatinolytic activity was visualized in the zymogram as clear bands against blue background.
2.7. Migration and invasion assays
Cell migration activity was examined, as previously reported previously [6] by the three dimensional Boyden chamber assay (Cell Culture Inserts, BD Bioscience, 8-μm pore size) in 24-well culture plates. Cell invasion assay was performed using Matrigel Invasion Boyden Chambers (BD Bioscience, 8-μm pore size) matrigel coated membrane. The cell culture inserts were rehydrated and prepared as described in the manufacturer's instructions. Briefly, 2 × 104 cells in 500 μl were added to the upper chambers in a low serum medium (0.5% FBS) and 750 μl medium, supplemented with 5% FBS chemoattractant, was added to the bottom well. After 2 h cells were pretreated with GTPs or vehicle (0.1% DMSO) for 6 h, followed by stimulation with LPS (10 ng/ml) or TNF-α (10 ng/ml) for 18 h. After incubation, the non-invading cells were removed from the upper surface of the membrane with a cotton swab. The cells on the lower surface of the membrane were fixed in methanol, followed by staining in solutions II and III from the Diff-Quik Staining Kit (BIOMAP) for 2 min each. After two washes with water, the inserts were allowed to air dry and cells were observed by phase-contrast-microscope (Carl Zeiss HBO 50/AC, 40× objective) connected to a digital photocamera (Canon, PowerShot G9) with a suitable software (Remote Capture DC, Canon). Abilities of migration and invasion were quantified by counting cells in 5 randomly selected visual fields and the mean of the cells migrating through control insert membrane (migration) or the mean of the cells invading through Matrigel insert membrane (invasion), were determined, respectively.
2.8. Statistical analysis
All the treatments were performed in triplicate and the data derive from at least three independent experiments. The results are expressed as mean value with ± standard deviation (SD).
3. Results
3.1. Pretreatment with green tea polyphenols extract reduces xanthine–xanthine oxidase induced cytotoxicity in human MKN-28 gastric cancer cells
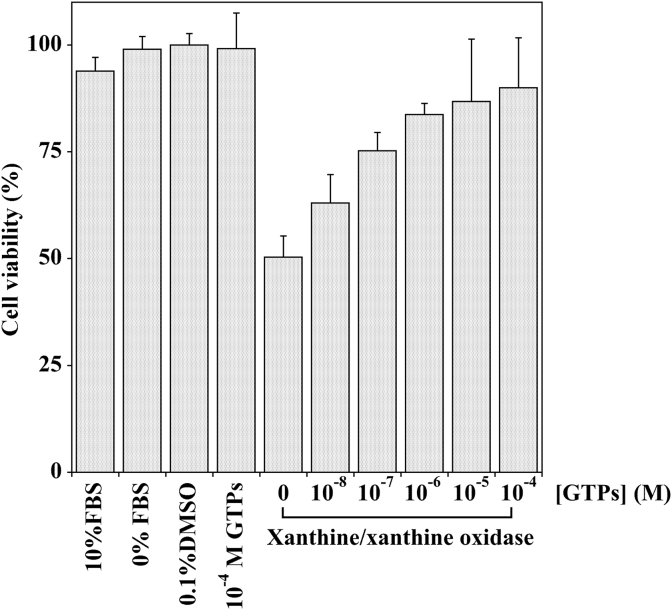
To examine whether green tea polyphenols extract counteracts Xanthine–Xanthine Oxidase induced cytotoxicity in MKN-28 cells, preliminary experiments were conducted to determine the appropriate oxidative stress conditions that induced cell toxicity. To this aim, MKN-28 cells were treated with increasing concentrations of Xanthine Oxidase in the presence of its substrate Xanthine. After incubation, cell viability was determined by MTT assay (Fig. S2, Panel A) which indicated a concentration–dependent reduction in cell viability; an IC50 value corresponding to 80 U/L was derived from a semilogarithmic plot (Fig. S2, Panel B). Therefore, this concentration of XO was chosen to evaluate GTPs protective effect on MKN-28 cells. Cells were then pretreated with various concentrations of GTPs for 3 h and then oxidative stress was induced as reported above. The result (Fig. 1) shows that the pretreatment with GTPs exerts a protective effect against XO/X induced cell cytotoxicity. This effect is concentration-dependent and the treatment with GTPs at the concentration of 10−4 M exhibited the highest cell viability as compared with that of cells exposed to XO/X alone. It has to be noted that either GTPs alone, at the highest concentration tested (10−4 M), or its vehicle (0.1% DMSO) did not affect cell viability.
Fig. 1.
Effect of the pretreatment with GTPs on Xanthine–Xanthine Oxidase induced cell cytotoxicity in MKN-28 cells. MKN-28 cells were pre-incubated with the indicated GTPs concentration in serum-free medium containing 0.1% (v/v) DMSO (vehicle) for three hours; the cells were then exposed to DMEM containing Xanthine (1 mM) and Xanthine oxidase (75 mU/ml) or with serum-free medium containing the vehicle alone for additional two hours. Control cells were incubated with DMEM in the presence (10%) or in the absence of FBS, as well as in the presence of 10−4 M GTPs. Cell viability was determined as reported in Section 2.3. The values represent the mean of three separate experiments performed in triplicate.
3.2. Effect of GTPs on cell migration and invasion in TNF-α or LPS-stimulated MKN-28 cells
The effect of GTPs on TNF-α or LPS treated MKN-28 cells, on migration and invasive ability was first evaluated.
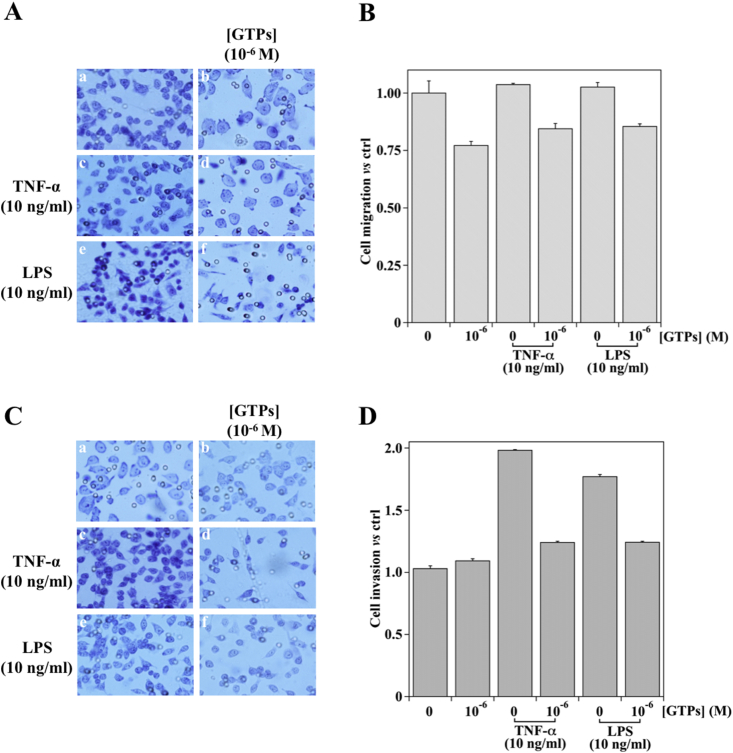
The treatment with TNF-α or LPS did not affect MKN-28 cell migration capability (Fig. 2, Panel A, pictures a, c, and e). The GTPs pre-treatment (10−6 M) did not significantly modify this behavior both in TNF-α and LPS-treated cells (Fig. 2, Panel A, pictures b, d, and f; Fig. 2, Panel B).
Fig. 2.
Effect of GTPs on cell migration or invasion in TNF-α or LPS stimulated MKN-28 cells. For migration (Panel A) or invasion assay (Panel C) MKN-28 cells were incubated in Boyden chambers or Matrigel coated membranes, respectively. Cells were treated with 10−6 M GTPs (images b, d, f) or vehicle alone (0.1% DMSO, images a) for six hours and then stimulated for 18 h with 10 ng/ml TNF-α (images c, d) or LPS (images e, f). Cells were then fixed and stained as detailed in Section 2.7. Each image shows a representative of five fields of three different experiment, visualized by a phase contrast microscope (40× objective). The quantification of the migrating (Panel B) or invading (Panel D) cells was calculated as the ratio versus the control unstimulated cells (representative images a, set as 1).
Vice versa, in MKN-28 cells exposed to TNF-α or LPS, a 2-fold increase in cell invasiveness was observed in both cases (Fig. 2, Panel C, pictures a, c, and e). Interestingly, the GTPs pre-treatment prevents the increase of cell invasiveness induced by TNF-α or LPS (Fig. 2, Panel C, pictures b, d, and f). In particular, at a concentration of 10−6 M GTPs the invasiveness of MKN-28 cells approached that observed in the absence of the inflammatory stimulus (Fig. 2, Panel D).
3.3. GTPs reduces the up-regulation of MMP-9/2 levels induced by TNF-α or LPS in MKN-28 cells
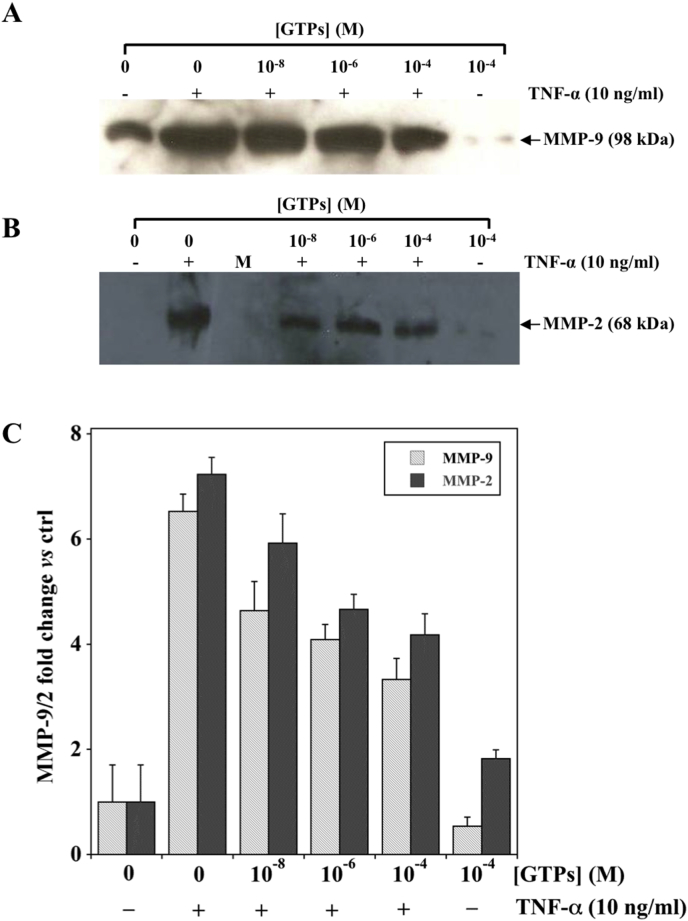
It has been reported that the stimulation with TNF-α or with LPS significantly increases the levels of MMP-9/2 in the cell conditioned medium of gastric cancer cells [12], [13]. To test whether GTPs could affect TNF-α or LPS induced MMP-9/2 secretion, western blotting analysis was performed on cell conditioned media from untreated or GTPs pre-treated MKN-28 cells. Under the experimental conditions used, TNF-α was able to induce an increase (about 7-fold) in MMP-9/2 secretion (Fig. 3, Panels A and B, respectively).
Fig. 3.
Concentration-dependent effect of GTPs on MMP-9/2 protein levels induced by TNF-α stimulation in MKN-28 cells. Cells were pre-incubated with the indicated concentration of GTPs for six hours and then exposed to 10 ng/ml TNF-α for additional 18 h. The concentrated conditioned media were analyzed by Western Blotting for MMP-9 (Panel A) and MMP-2 (Panel B), respectively for a representative experiment (Panel C). Densitometric analysis of MMP-9/2 protein expression levels. The data obtained from three different experiments were averaged and reported as MMP-9/2 fold change vs control cells (set as 1) cultured in the absence of GTPs and TNF-α.
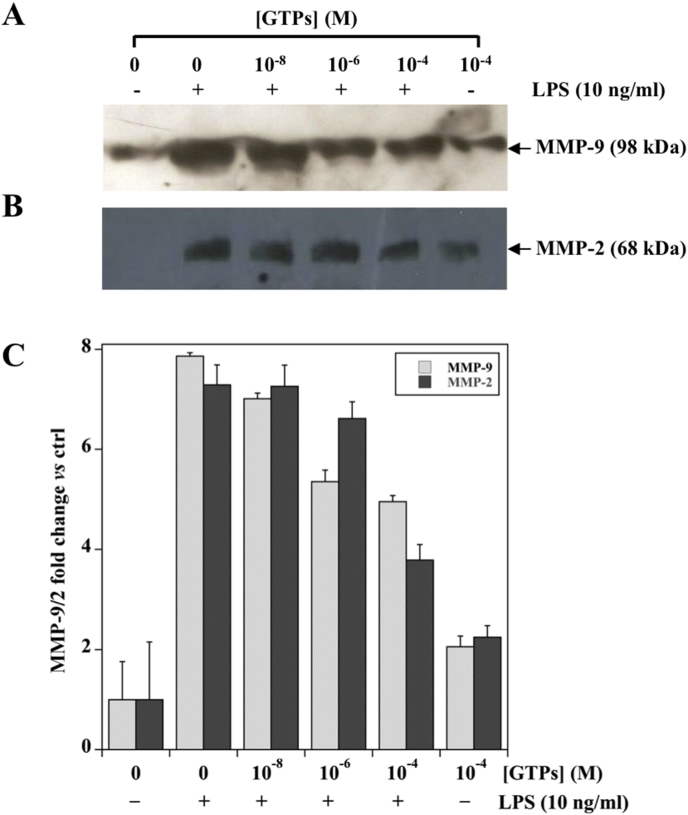
The pre-treatment with GTPs reduced the amount of both MMP-9 (Fig. 3, Panel A) and MMP-2 (Fig. 3, Panel B) in the culture medium. This effect was concentration dependent and the highest concentration of GTPs (10−4 M) induced about one half reduction in MMP-9/2 (Fig. 3, Panel C). A similar behavior was observed when the effect on the GTPs pre-treatment was evaluated on LPS induced MMP-9/2 protein expression level (Fig. 4).
Fig. 4.
Concentration-dependent effect of GTPs on MMP-9/2 protein levels induced by LPS stimulation in MKN-28 cells. Cells were pre-incubated with the indicated concentration of GTPs for six hours and then exposed to 10 ng/ml LPS for additional 18 h. The concentrated conditioned media were analyzed by Western Blotting for MMP-9 (Panel A) and MMP-2 (Panel B), respectively for a representative experiment. (Panel C) Densitometric analysis of MMP-9/2 protein expression levels. The data obtained from three different experiments were averaged and reported as MMP-9/2 fold change vs control cells (set as 1) cultured in the absence of GTPs and LPS.
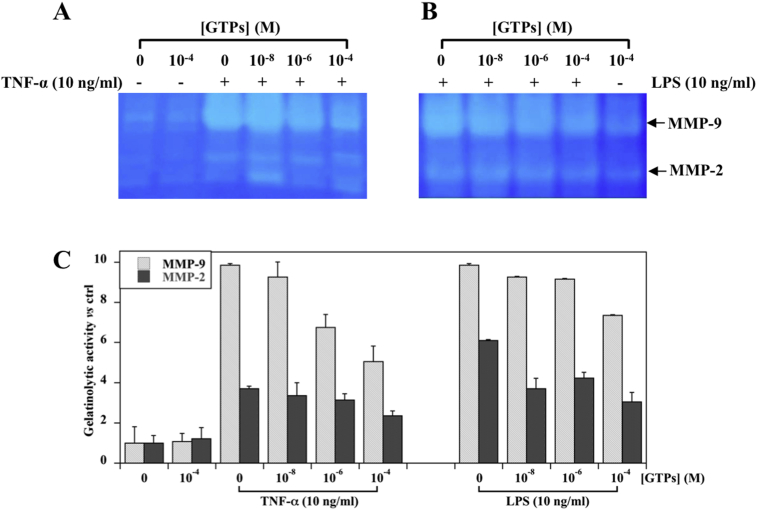
To examine whether GTPs affect also the gelatinolytic activity of TNF-α or LPS induced MMP-9/2 expression, gelatin zymography analysis was performed on conditioned media from MKN-28 cells. TNF-α treatments caused an increase of the MMP-9 and MMP-2 gelatinolytic activity (Fig. 5, Panel A), although the effect on the MMP-9 activity was approximately 2.5-fold higher than that observed for MMP-2 (Fig. 5, Panel C).
Fig. 5.
Concentration-dependent effect of GTPs on MMP-9/2 gelatinolytic activity levels induced by TNF-α or LPS stimulation in MKN-28 cells. Cells were pre-incubated with the indicated concentration of GTPs for six hours and then exposed to 10 ng/ml TNF-α (Panel A) or LPS (Panel B) for additional 18 h. The concentrated conditioned media were analyzed by gelatin zymography as detailed in Section 2.6. The position of the MMP-9/2 migrating band is indicated by an arrow on the right. The images show a representative experiment. (Panel C) Densitometric analysis of MMP-9/2 gelatinolytic activity levels. The data obtained from three different experiments were averaged and reported as MMP-9/2 fold change gelatinase activity vs control cells (set as 1) cultured in the absence of GTPs and TNF-α or LPS.
After a pretreatment of cells with various concentrations of GTPs for 6 h, the addition of TNF-α for 18 h, caused a concentration-dependent inhibition of both MMP-9/2 gelatinolytic activity (Fig. 5, Panel A). In particular, the pre-treatment with GTPs at the highest concentration (10−4 M), led to an approximately one half reduction of MMP-9 gelatinolytic activity. In the case of MMP-2, the pre-treatment with GTPs induced a less evident reduction in the gelatinolytic activity (Fig. 5, Panel A and C). A similar behavior, although to a lesser extent, was observed when the effect of the GTPs pre-treatment was evaluated on LPS induced MMP-9/2 gelatinolytic activity level (Fig. 5, Panels B and C).
It has to be pointed out that the pre-treatment with GTPs alone, at the highest concentration (10−4 M) and in the absence of LPS or TNF-α, did not interfere with MMP-9/2 gelatinolytic activity (Fig. 5, Panel C), as compared to control cells (0.1% DMSO).
All these results demonstrated that GTPs are able to counteract the secretion and the gelatinolytic activity of MMP-9/2 in MKN-28 cells upon stimulation by either TNF-α or LPS.
4. Discussion
Numerous in vitro and in vivo studies have shown that green tea has a chemo-preventive effect against gastric cancer [15], [17], [26]. Although the beneficial effects of green tea consumption on human health are widely recognized, the functions and the mechanisms through which specific components of green tea exert their effects still need to be clarified.
The human gastric cancer MKN-28 cell line represents an appropriate in vitro system for analyzing the antioxidant properties of compounds even derived from natural sources. For instance, it was demonstrated that apple polyphenol extracts prevent ROS induced injury in these gastric cancer cells, suggesting that a diet rich in polyphenols could be useful in the prevention of gastric diseases related to the generation of ROS [14].
In this study, we have first investigated whether green tea polyphenol extract prevented oxidative stress induced injury in MKN-28 cells. We found that the pretreatment with GTPs reduces the Xanthine/Xanthine oxidase ROS-cytotoxicity in MKN-28 cells. In particular, 10−4 M catechin equivalents of GTPs showed the maximal protection and the cell viability approached that observed in the absence of the oxidative stress injury.
It is well documented that the expression and activity of some MMPs increased in several type of human cancer cells, and their up-regulation correlated with an increase in cell migration and invasion. In the metastatic process, a key role is played by interleukins, interferons and growth factors [8], [27] which stimulate tumor stromal cells to product MMPs. In particular, MMP-2 and MMP-9 are involved in the invasive metastatic potential of tumor cells and both enzymes are expressed also in human gastric cancer cells [27]. Accumulating evidences have shown that TNF-α and LPS, key mediators of inflammation and cancer, stimulate the overproduction of MMP-9/2 [7], [12], [13]. Here, we found that the pre-treatment with GTPs is able to reduce MMP-9/2 at both the protein expression level and gelatinolytic activity in TNF-α or LPS-stimulated MKN-28 cells.
As matrix MMPs can regulate the tumor microenvironment through the degradation of ECM components [9], [12], [13], we also evaluated the effect of GTPs on the cell invasiveness. Our results suggest that GTPs can inhibit in vitro migration and invasion ability of TNF-α or LPS-stimulated MKN-28 cells, even though the inhibitory effect on the invasiveness was more evident.
Since in cancer cells the TNF-α and LPS induced inflammatory response involves the activation of the transcription factor NF-κB [28], that in turn induced the MMP-9 upregulation [29], we can hypothesize that GTPs exert their protective effect targeting the NF-κB signaling pathway in MKN-28 cell line, as already reported in other cancer cells [30], [31].
5. Conclusions
From the data presented it is clear that green tea polyphenol extracts have preciouses qualities because they interfere with molecular pathways which include metalloproteinases levels thus providing the biological background of the beneficial actions of green tea. These polyphenols extracts could be used in their natural form for the prevention therapy. These results suggest that an alimentary style rich in polyphenol extracts might contribute to the prevention of gastric cancer as they interfere with the levels of MMP-9/2, whose unbalance is involved in inflammatory process.
Conflict of interest
The Authors declare no conflict of interest.
Acknowledgments
This research was supported in part by the University of Naples Parthenope, “Bando per la ricerca individuale, annua-lità 2015″ to MM and RA.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.biopen.2016.10.002.
Contributor Information
Mariorosario Masullo, Email: mario.masullo@uniparthenope.it.
Gerardo Nardone, Email: gerardo.nardone@unina.it.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.González C.A., Sala N., Rokkas T. Gastric cancer: epidemiologic aspects. Helicobacter. 2013;18:34–38. doi: 10.1111/hel.12082. [DOI] [PubMed] [Google Scholar]
- 2.Venerito M., Nardone G., Selgrad M., Rokkas T., Malfertheiner P. Gastric cancer-epidemiologic and clinical aspects. Helicobacter. 2014;19:32–37. doi: 10.1111/hel.12164. [DOI] [PubMed] [Google Scholar]
- 3.Fang X., Wei J., He X., An P., Wang H., Jiang L., Shao D., Liang H., Li Y., Wang F., Min J. Landscape of dietary factors associated with risk of gastric cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Cancer. 2015;51:2820–2832. doi: 10.1016/j.ejca.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Abnet C.C., Corley D.A., Freedman N.D., Kamangar F. Gastroenterology. 2015;148:1234–1243. doi: 10.1053/j.gastro.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tallant C., Marrero A., Gomis-Rüth F.X. Matrix metalloproteinases: fold and function of their catalytic domains. Biochim. Biophys. Acta. 2010;1803:20–28. doi: 10.1016/j.bbamcr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Pagliara V., Adornetto A., Mammì M., Masullo M., Sarnataro D., Pietropaolo C., Arcone R. Protease Nexin-1 affects the migration and invasion of C6 glioma cells through the regulation of urokinase Plasminogen Activator and Matrix Metalloproteinase-9/2. Biochim. Biophys. Acta. 2014;1843:2631–2644. doi: 10.1016/j.bbamcr.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Shiozaki A., Shimizu H., Ichikawa D., Konishi H., Komatsu S., Kubota T., Fujiwara H., Okamoto K., Iitaka D., Nakashima S., Nako Y., Liu M., Otsuji E. Claudin 1 mediates tumor necrosis factor alpha-induced cell migration in human gastric cancer cells. World J. Gastroenterol. 2014;21:17863–17876. doi: 10.3748/wjg.v20.i47.17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 9.Kessenbrok K., Plaks V., Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu D., Zhang Z., Li Y., Zheng J., Dong G., Wang W., Ji G. Matrix metalloproteinase-9 is associated with disease-free survival and overall survival in patients with gastric cancer. Int. J. Cancer. 2011;129:887–895. doi: 10.1002/ijc.25734. [DOI] [PubMed] [Google Scholar]
- 11.Gerstein E.S., Sini L., Ryabov A.B., Dvorova E.K., Yurchenko A.A., Stilidi I.S., Kushlinskii N.E., Davydov M.I. Comparative enzyme immunoassay of matrix metalloproteinases-2, -7, -9 and their tissue inhibitor-2 in tumors and plasma of patients with gastric cancer. Bull. Exp. Biol. Med. 2009;148:899–902. doi: 10.1007/s10517-010-0847-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim S., Choi M.G., Lee H.S., Lee S.K., Kim S.H., Kim W.W., Hur S.M., Kim J.H., Choe J.H., Nam S.J., Yang J.H., Kim S., Lee J.E., Kim J.S. Silibinin suppresses TNF-alpha-induced MMP-9 expression in gastric cancer cells through inhibition of the MAPK pathway. Molecules. 2009;14:4300–4311. doi: 10.3390/molecules14114300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardoso A.P., Pinto M.L., Pinto A.T., Pinto M.T., Monteiro C., Oliveira M.I., Santos S.G., Relvas J.B., Seruca R., Mantovani A., Mareel M., Barbosa M.A., Oliveira M.J. Matrix metalloproteases as maestros for the dual role of LPS- and IL-10-stimulated macrophages in cancer cell behaviour. BMC Cancer. 2015;15:456–469. doi: 10.1186/s12885-015-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graziani G., D'Argenio G., Tuccillo C., Loguercio C., Ritieni A., Morisco F., Del Vecchio Blanco C., Fogliano V., Romano M. Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut. 2005;54:193–200. doi: 10.1136/gut.2004.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu F., Jin Z., Jiang H., Xiang C., Tang J., Li T., He J. Tea consumption and the risk of five major cancers: a dose-response meta-analysis of prospective studies. BMC Cancer. 2014;14:197–216. doi: 10.1186/1471-2407-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho Y.C., Yang S.F., Peng C.Y., Chou M.Y., Chang Y.C. Epigallocatechin-3-gallate inhibits the invasion of human oral cancer cells and decreases the productions of matrix metalloproteinases and urokinase-plasminogen activator. J. Oral. Pathol. Med. 2007;36:588–593. doi: 10.1111/j.1600-0714.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 17.Yang C.S., Wang X., Lu G., Picinich S.C. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adhami V.M., Ahmad N., Mukhtar H. Molecular targets for green tea in prostate cancer prevention. J. Nutr. 2003;133:2417S–2424S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- 19.Zuo Y., Chen H., Deng Y. Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta. 2002;57:307–316. doi: 10.1016/s0039-9140(02)00030-9. [DOI] [PubMed] [Google Scholar]
- 20.Wiseman H., Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda-Yamamoto M., Kawahara H., Tahara N., Tsuji K., Hara Y., Isemura M. Effects of tea polyphenols on the invasion and matrix metalloproteinases activities of human fibrosarcoma HT1080 cells. J. Agric. Food Chem. 1999;47:2350–2354. doi: 10.1021/jf9811525. [DOI] [PubMed] [Google Scholar]
- 22.Demeule M., Brossard M., Pagé M., Gingras D., Béliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim. Biophys. Acta. 2000;1478:51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 23.Romano M., Razandi M., Sekhon S., Krause W.J., Ivey K.J. Human cell line for study of damage to gastric epithelial cells in vitro. J. Lab. Clin. Med. 1988;111:430–440. [PubMed] [Google Scholar]
- 24.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Yang C.S., Lee M.J., Chen L., Yang G.Y. Polyphenols as inhibitors of carcinogenesis. Environ. Health Perspect. 1997;105:971–976. doi: 10.1289/ehp.97105s4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coussens L.M., Werb Z. Matrix metalloproteinases and the development of cancer. Chem. Biol. 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- 28.Pal S., Bhattacharjee A., Ali A., Mandal N.C., Mandal S.C., Pal M. Chronic inflammation and cancer: potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J. Inflamm. 2014;11:1–28. doi: 10.1186/1476-9255-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bond M., Fabunmi R.P., Baker A.H., Newby A.C. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kB. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad N., Gupta S., Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch. Biochem. Biophys. 2000;376:338–346. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 31.Zaveri N.T. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.