Abstract
Background
Hepatitis B vaccine administered shortly after birth is highly effective in preventing mother to child transmission (MTCT) of infection. While hepatitis B vaccine was introduced in Haiti as part of a combined pentavalent vaccine in 2012, a birth dose is not yet included in the immunization schedule.
Objectives
Determine the seroprevalence of hepatitis B virus (HBV) infection among pregnant women to evaluate the risk of MTCT.
Study design
We selected 1364 residual serum specimens collected during a 2012 human immunodeficiency virus (HIV) sentinel serosurvey among pregnant women attending antenatal care clinics. Haiti was stratified into two regions: West, which includes metropolitan Port-au-Prince, and non-West, which includes all other departments. We evaluated the association between demographic and socioeconomic characteristics and HIV infection with HBV infection.
Results
Of 1364 selected specimens, 1307 (96%) were available for testing. A total of 422 specimens (32.7%) tested positive for total anti-HBc (38.2% in West vs. 27% in non-West, p < 0.001), and 33 specimens (2.5%) were HBsAg positive (2.1% in West vs. 3% in non-West, p = 0.4). Of HBsAg positive specimens, 79% had detectable HBV DNA. Women aged 30 and older had more than double the odds of positive total anti-HBc than women aged 15–19 years (p < 0.001). Women with secondary (adjusted odds ratio (aOR) = 0.54; 95% CI: 0.36–0.81) and post-secondary education (aOR = 0.40, 95% CI: 0.19–0.79) had lower odds of total anti-HBc positivity compared with women with no education. HIV-status was not associated with HBV infection.
Conclusions
Haiti has an intermediate endemicity of chronic HBV infection with high prevalence of positive HBV DNA among chronically infected women. Introduction of a universal birth dose of hepatitis B vaccine might help prevent perinatal HBV transmission.
Keywords: Hepatitis B, Pregnant women, Haiti, Hepatitis B vaccines, Serosurvey
1. Background
Hepatitis B virus (HBV) infection is a leading cause of morbidity and mortality due to hepatocellular carcinoma and liver cirrhosis worldwide. In the absence of vaccination, more than 50% of infection is attributable to infection during childhood and adolescence, and more than 20% of HBV-related deaths are attributable to perinatal infection [1,2]. The risk of progression to chronic infection is inversely related to the age of acquisition of infection; chronic infection develops in 80–90% of infants infected in the first year of life; this risk declines to 30–50% for children infected between 1 and 4 years of age [2].
In October 2012, Haiti became the last country in the Americas to introduce hepatitis B vaccine. The vaccine is provided at 6, 10, and 14 weeks of age, and is given as part of a combined pentavalent vaccine that protects against diphtheria, pertussis, tetanus, Hemophilus influenza type b (Hib) and hepatitis B. Despite recommendations by the World Health Organization (WHO) [2], a birth dose of hepatitis B vaccine is not included in the present vaccination schedule in Haiti. While childhood vaccination is effective in preventing horizontal HBV infection, it does not prevent perinatal transmission. In addition, pregnant women in Haiti are not routinely screened for HBV infection.
Since 2012, the Pan American Health Organization (PAHO) has been in the process of developing a regional strategy to address viral hepatitis. One of the components of this strategy is to establish baseline country estimates of the burden of hepatitis. Data on the hepatitis B disease burden in Haiti are outdated and limited. A study conducted in 2006 among pregnant women reported an overall seroprevalence of hepatitis B surface antigen (HBsAg) of 5% with wide variations ranging from 1.0% to 8.5%, depending on the sampling clinic [3]. However, no data were available on hepatitis B envelope antigen (HBeAg) or HBV DNA levels which are important predictors for risk of mother to child transmission (MTCT) of hepatitis B. Small studies conducted in the 1980s and among Haitian immigrants in the United States and Canada reported almost 50% prevalence of total antibody to hepatitis B core antigen (total anti-HBc) and a prevalence of HBsAg varying from 4% to 6% [4–7], indicating past or present infection. In a review of the 2013 blood donation screening data from the national blood transfusion program in Haiti, the prevalence of HBsAg was 3.5% with wide variability by department (2–8%) (Ernst Noel, personal communication).
2. Objectives
We conducted this study among pregnant women to assess the seroprevalence of HBV infection in this population and subsequent risk of MTCT. These results are essential to build evidence for introduction of a hepatitis B vaccine birth dose in Haiti.
3. Study design
3.1. Sample selection
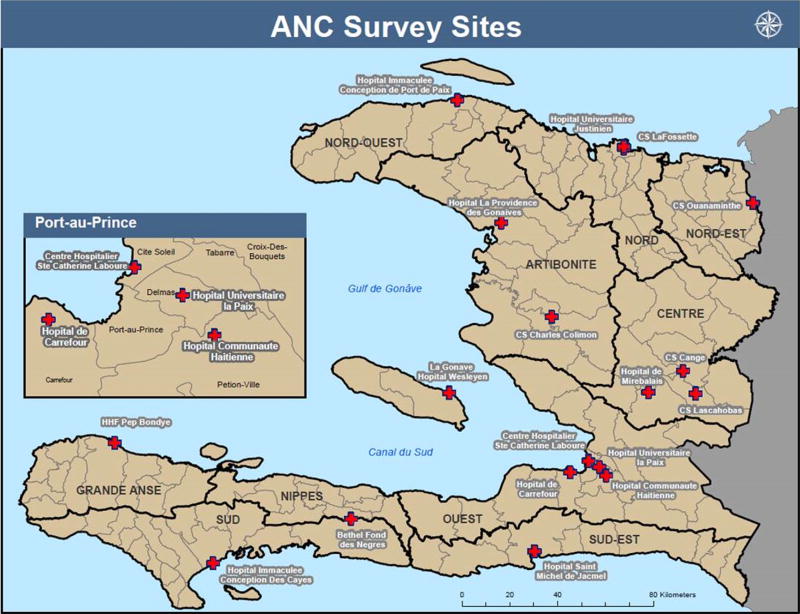
We selected 1364 specimens from 6241 de-linked, residual serum specimens collected for the 2012 Biannual Sentinel Serosurvey for HIV among Pregnant Women. In summary, the sentinel HIV serosurvey included 18 antenatal care (ANC) sites, which were chosen to be geographically representative, with at least one site selected per department and representing urban and rural areas (Fig. 1). The ANC sites provide services for the prevention of MTCT of HIV.
Fig. 1.
Distribution of the hospitals included in the antenatal care clinic serosurvey—Haiti, 2012.
Haiti is divided into 10 departments including Metropolitan Port-au-Prince. For this survey, we stratified Haiti into 2 regions: (1) the West region which includes the Ouest department along with metropolitan Port-au-Prince, and houses almost one third of the Haitian population, and (2) the non-West region which includes the remaining nine departments (Nord, Nord-Est, Nord-Ouest, Artibonite, Centre, Sud-Est, Sud, Nippes, Grande Anse). All 164HIV-positive samples from the total sample of 6241 specimens were included in this study to test for co-infection rates for hepatitis B and HIV. For the HIV negative specimens, we randomly selected 600 specimens in each region by using an expected HBsAg prevalence of 5%, a desired precision of 2%, a 95% probability of achieving that precision, a design effect of 1.1 and an estimated 15% inadequate specimens. Age, education level, marital status, and job status information were available for each selected specimen. Education level was classified as no education (never entered school), primary (1–8 years of school), secondary (9–12 years of school), and post-secondary (any school years after secondary). The protocol was approved by the Haiti national bioethics committee and by the CDC human subject research office.
3.2. Laboratory testing
All specimens were first tested for total anti-HBc. If total anti-HBc was positive, then specimens were tested for HBsAg. Serology for hepatitis B markers was performed on an automated platform using chemiluminescent immunoassays (VITROS ECi, Ortho Clinical Diagnostics, Rochester, NY). Samples positive for HBsAg were tested for HBV DNA by in-house quantitative PCR assays as previously described in Ref. [8]. Briefly, total nucleic acids (TNA) were extracted using 200 µL of serum with the Roche MagNA Pure Isolation Kit I (Roche Applied Science, Rochester, NY) with a final elution volume of 50 µL followed by Taqman quantitative PCR (qPCR) for HBV DNA. The limit of detection of HBV DNA by PCR was 50 IU/mL.
3.3. Statistical analysis
We adjusted for weights considering the selection probability in each region and the non-response rate based on site and HIV status. Seroprevalence of HBV infection was estimated by demographic and socio-economic characteristics for each region. We compared results for West vs. non-West region using p-values based on chi-square tests for homogeneity. In addition, we evaluated the association between demographic and socio-economic characteristics and HIV status with HBsAg and total anti-HBc in each region using chi-square tests for independence to evaluate the effects of covariates. A multivariable logistic regression model was fit to assess the factors associated with total anti-HBc positivity. All reported percentages are weighted. Analysis was conducted using the statistical software R v. 3.0.2.
4. Results
Of the 1200 HIV-negative and 164 HIV-positive selected serum specimens, 1184 (98.7%) and 123 (75%) had enough volume to be tested for all markers, respectively. Therefore a total of 1307 specimens (634 in West and 673 in non-West region) were tested for seromarkers of HBV infection. More women completed secondary and higher education in the West compared with non-West region (p < 0.001), and there were more HIV-positive women in the non-West (12.6%) compared with the West region (6%) (p < 0.001) (Table 1).
Table 1.
General characteristics of survey sample of pregnant women attending antenatal care clinics—Haiti, 2012.
| West region N = 634 |
Non-West region N = 673 |
p-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | |||||
| 15–19 years | 88 | 13.9 | 95 | 14.0 | 0.6 |
| 20–24 years | 177 | 27.9 | 175 | 25.7 | |
| 25–29 years | 173 | 27.3 | 183 | 26.8 | |
| 30–34 years | 118 | 18.6 | 123 | 18.0 | |
| 35–39 years | 60 | 9.5 | 70 | 11.6 | |
| ≥40 years | 18 | 2.8 | 27 | 3.9 | |
| Education level | |||||
| None | 50 | 7.9 | 74 | 11.0 | <0.001 |
| Primary | 190 | 30.0 | 273 | 41.0 | |
| Secondary | 359 | 56.6 | 300 | 44.1 | |
| Post-secondary | 35 | 5.5 | 26 | 3.9 | |
| Marital status | |||||
| Married | 146 | 23.0 | 166 | 24.4 | 0.6 |
| Not-married | 488 | 77.0 | 507 | 75.6 | |
| Job status | |||||
| Employed | 349 | 55.0 | 409 | 59.9 | 0.08 |
| Unemployed | 285 | 45.0 | 264 | 40.1 | |
| HIV status | |||||
| Positive | 38 | 6.0 | 85 | 12.6 | <0.001 |
| Negative | 596 | 94.0 | 588 | 87.4 | |
Percentages are weighted to account for the study design.
Of the 1307 tested women, 422 (32.7%, 95% CI: 29.2–36.2) were total anti-HBc positive. Anti-HBc prevalence was higher in the non-West compared with West region (38.2%, 95% CI: 34.5–41.9 vs. 27.0%, 95% CI: 23.5–30.4; p < 0.001). Overall 33 women (2.5%, 95% CI: 1.7–3.4) were positive for HBsAg, with 13 women (2.1%, 95% CI: 1.0–3.2) and 20 women (3.0%, 95% CI: 1.7–4.3) testing HBsAg positive in the West and non-West region, respectively (Table 2).
Table 2.
Hepatitis B seroprevalence by demographic and socio-economic characteristics, and HIV status among pregnant women attending antenatal care clinics—Haiti, 2012.
| HBsAg positive
|
Total anti-HBc positive
|
||||||
|---|---|---|---|---|---|---|---|
| N | n | % | p-value | n | % | p-value | |
| Total | 1307 | 33 | 2.5 | – | 422 | 32.7 | – |
| Department | |||||||
| West | 634 | 13 | 2.1 | 0.4 | 171 | 27.0 | <0.001 |
| Non-West | 673 | 20 | 3.0 | 251 | 38.2 | ||
| Age | |||||||
| 15–19 years | 183 | 5 | 2.7 | 0.1 | 40 | 21.3 | <0.001 |
| 20–24 years | 352 | 4 | 1.1 | 104 | 29.3 | ||
| 25–29 years | 356 | 7 | 2.0 | 110 | 30.6 | ||
| 30–34 years | 241 | 9 | 3.7 | 92 | 38.2 | ||
| 35–39 years | 130 | 5 | 3.8 | 56 | 50.0 | ||
| ≥40 years | 45 | 3 | 6.7 | 20 | 44.4 | ||
| Education level | |||||||
| None | 124 | 5 | 4.0 | 0.4 | 59 | 47.6 | <0.001 |
| Primary | 463 | 14 | 3.0 | 163 | 36.5 | ||
| Secondary | 659 | 13 | 2.0 | 186 | 28.1 | ||
| Post-secondary | 61 | 1 | 1.6 | 14 | 23.0 | ||
| Marital status | |||||||
| Married | 312 | 8 | 2.6 | 1.0 | 92 | 29.2 | 0.2 |
| Other | 995 | 25 | 2.5 | 330 | 33.9 | ||
| Job status | |||||||
| Employed | 758 | 20 | 2.5 | 0.9 | 266 | 34.7 | 0.04 |
| Unemployed | 549 | 13 | 2.4 | 156 | 30.1 | ||
| HIV status | |||||||
| Positive | 123 | 3 | 2.4 | 1.0 | 48 | 43.9 | 0.006 |
| Negative | 1184 | 30 | 2.5 | 374 | 31.6 | ||
Percentages were weighted to account for the study design
The prevalence of HBsAg generally increased with age from 2.7% among women aged 15–19 years to 6.7% among those aged 40 and older, but this did not reach statistical significance (Table 2). Total anti-HBc prevalence increased with age ranging from 21% to 50% (p < 0.001). Similarly, HBsAg and total anti-HBc seroprevalence decreased with higher educational level but the decrease was only significant for total anti-HBc (Table 2). HIV-positive women had higher prevalence of positive total anti-HBc than HIV-negative women (44% vs. 32%, p = 0.006), but a similar HBsAg prevalence [2.4% (3/123)] as HIV-negative women [2.5% (30/1184)]. None of the HIV-positive women in the West region were HBsAg positive while three women were co-infected with HBV in the non-West region.
Among the 33HBsAg positive women, 26 (79%; 85% in West region, 75% in non-West region) had detectable HBV DNA levels: six had HBV DNA <1000 IU/mL, five had HBV DNA = 1000–4999 IU/mL, and 15 (46%; 46% in West region, 45% in non-West region) had HBV DNA levels ≥5000 IU/mL. Nine HBsAg positive women had HBV DNA levels >200,000 IU/mL, among whom one was HIV-positive.
After adjusting for all covariates, the risk of anti-HBc positivity increased with age; women older than 30 years had more than double the odds of being total anti-HBc positive than those aged 15–19 years (Table 3). Women with secondary and post-secondary education had 46% and 60% lower odds for total anti-HBc positivity compared to those with no education, respectively. Women who were not married had 1.4 times higher odds for total anti-HBc positivity compared with married women (p = 0.04). Job status and HIV-status were not associated with total anti-HBc positivity after controlling for the other variables.
Table 3.
Factors associated with anti-HBc positivity among pregnant women attending ante-natal care clinics—Haiti, 2012.
| Characteristics | Total anti-HBc positive
|
||
|---|---|---|---|
| Adjusted OR | 95% CI | p-value | |
| Age (years) | |||
| 15–19 | 1.00 | – | |
| 20–24 | 1.58 | 1.03–2.45 | 0.03 |
| 25–29 | 1.71 | 1.11–2.68 | 0.01 |
| 30–34 | 2.43 | 1.53–3.91 | <0.001 |
| 35–39 | 3.26 | 1.94–5.53 | <0.001 |
| ≥40 | 2.77 | 1.33–5.75 | 0.006 |
| Education level | |||
| None | 1.00 | – | |
| Primary | 0.73 | 0.48–1.10 | 0.1 |
| Secondary | 0.54 | 0.36–0.81 | 0.003 |
| Post-secondary | 0.40 | 0.19–0.79 | 0.01 |
| Marital status | |||
| Married | 1.00 | ||
| Not-married | 1.37 | 1.01–1.87 | 0.04 |
| Job status | |||
| Employed | 1.00 | – | |
| Unemployed | 0.91 | 0.70–1.17 | 0.4 |
| HIV status | |||
| Negative | 1.00 | – | |
| Positive | 1.37 | 0.92–2.02 | 0.1 |
95% CI: 95% confidence interval; analysis was adjusted for all variables in this table.
5. Discussion and conclusions
This study is the first to assess the prevalence of HBV infection and measure HBV DNA levels among pregnant women in Haiti. We found that 2.5% of pregnant women were HBsAg positive, of whom about three-fourths had detectable HBV DNA, including almost half with HBV DNA levels ≥5000 IU/mL, and about a quarter with HBV DNA levels >200,000 IU/mL. HBV DNA levels are the most important predictors of MTCT of HBV infection [9–12]. These findings highlight the need for an introduction of a hepatitis B birth dose to prevent MTCT of HBV infection in Haiti.
Based on findings in this serosurvey and using the model developed by Goldstein et al., introduction of a hepatitis B birth dose with an assumption of achieving hepatitis B vaccine birth dose coverage within the range of 50–80% could prevent 38–61% of perinatal infections and HBV-related deaths due to perinatal infection in Haiti [13]. In 2012, PAHO recommended introduction of a hepatitis B vaccine birth dose in all countries in the Americas [14]. As of 2013, only 17 of 35 (48%) member states of PAHO have introduced a hepatitis B vaccine birth dose in the routine immunization schedule [15].
Given that Haiti is a low-income country with an intermediate endemicity of chronic HBV infection defined as a population HBsAg prevalence of 2–7% [2], the most operational intervention for prevention of perinatal HBV transmission would be universal introduction of hepatitis B vaccine within 24 h of birth without screening of pregnant women [16–19]. With Haiti’s limited resources and weak infrastructure, it might not be feasible or cost-effective to screen all pregnant women for HBsAg or to include hepatitis B immunoglobulin (HBIG) in the prophylaxis protocol. In addition, infants who need post-exposure prophylaxis at birth might be missed with a targeted vaccination strategy. For example, in Greenland, a high proportion of infants born to HBsAg positive mothers did not receive hepatitis B vaccine at birth, and were not followed up after birth as a result of healthcare staff turnover and administrative failures to screen all pregnant women [20].
Introduction of a hepatitis B vaccine birth dose is most feasible when deliveries occur in healthcare settings. This is not the case in Haiti where only 36% of deliveries occur in a healthcare setting [21]. However, experience from countries in the Western Pacific Region (WPR), which had a high prevalence of chronic HBV infection prior to hepatitis B vaccine introduction, and where delivery in healthcare settings is also low, suggests that it is feasible to successfully introduce a birth dose of hepatitis B vaccine in home-delivery settings [22]. Innovative interventions to increase birth dose coverage have been implemented in WPR countries, including promoting facility-based deliveries in conjunction with birth dose introduction, which helped improve timely birth dose administration and prevented perinatal infections. Promotion of facility-based delivery also contributed to decreasing maternal and neonatal mortality to meet the millennium development goals 4 and 5. In addition, home birth attendants were trained on vaccine administration and reporting so that infants delivered at home receive the birth dose in a timely manner [22,23]. Availability of good microplans was essential for successful administration of the birth dose at home [23]. Moreover, the use of monovalent hepatitis B vaccine in a controlled-temperature-chain (outside of the cold chain) and availability of Uniject could facilitate the logistics of using this vaccine in home-settings [22,24–27]. Although not currently licensed or pre-qualified for this use, several studies have demonstrated that hepatitis B vaccine can be kept up to one month in a controlled-temperature chain at temperatures up to 37 °C without losing its potency [25,26]. Testing the effectiveness of using the vaccine in a controlled-temperature chain in Haiti would help inform policy makers and perhaps facilitate the introduction of this strategy.
The prevalence of chronic HBV infection in Haiti among pregnant women has decreased compared to previous years. Prevalence in 2012 was half the prevalence reported in 2006 in the same population [3]. The prevalence of total anti-HBc in younger age groups was lower than among older women in 2012. In addition, unlike studies in the United States, Europe, and Ghana, which reported higher prevalence of chronic HBV infection among HIV-infected patients [28–32], we did not find a significant association between HIV and HBV infection in this study which is similar to findings reported among pregnant women in Uganda, Cameroon, and India [33–35]. Given that HIV and HBV have similar modes of transmission, preventive interventions targeting HIV infection, such as HIV counseling, testing, and treatment services available at those ANC site, and screening of blood for HIV and hepatitis B in Haiti, might have led to the decrease in HBV infection rates among pregnant women in Haiti [36,37].
This study has three main limitations. First, results cannot be generalized to the Haitian population as findings are only applicable to pregnant women with access to prenatal healthcare. According to the Demographic and Health Survey (DHS) conducted in 2012, 90% of women who were pregnant received prenatal care which makes our results generalizable to pregnant women in Haiti [21]. Second, we were unable to determine the risk factors for HBV infection among pregnant women given that the sentinel serosurvey of pregnant women did not collect data on risk factors. However, it would be difficult to assess hepatitis B risk factors by self-reporting as infection could have occurred several years prior. Finally, we had a small sample of HBsAg positive women, which might have contributed to the inability to identify factors significantly associated with HBsAg positivity among pregnant women in Haiti.
In conclusion, Haiti has an intermediate endemicity for chronic HBV infection and the prevalence of chronic HBV infection among pregnant women has been decreasing over time. However, the high prevalence of elevated viral loads among pregnant women with chronic infection indicates the potential high risk of MTCT of HBV. The introduction of pentavalent vaccine in 2012, and future addition of hepatitis B vaccination at birth could further help decrease childhood and perinatal HBV infection. However, ensuring access to the hepatitis B birth dose and achieving good vaccination coverage with all doses of hepatitis B-containing vaccines are essential. Serosurveys for HBV infection among children are needed to provide additional data to support the introduction of a hepatitis B birth dose in Haiti.
Acknowledgments
Jacquecius Compère; Natacha Louis Jeune, Josiane Buteau, Nicole Freeman LNSP; Yves Gaston Deslouches, Roc Magloire, MSPP; Institut Haïtien de l’Enfance; Barbara Roussel, NASTAD; Alina Choudhury, Emory University; Barbara Marston, Jan Drobeniuc, Francisco Averhoff, Yves Frantz Jean-Louis, Chong-Gee Teo, Kathleen Wannemuehler, CDC.
Funding
Testing of the specimens was supported by the Centers for Disease Control and Prevention.
Footnotes
Competing interests
None declared.
Ethical approval
The protocol was approved by the Haiti national bioethics committee and by the CDC human subject research office.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Findings in this manuscript were partially presented at IDWeek 2015, October 7–11, 2015 in San Diego, CA.
References
- 1.World Health Organization Documenting the impact of hepatitis Bimmunization: best practices for conducting a serosurvey WHO. 2011 < http://whqlibdoc.who.int/hq/2011/WHO_IVB_11.08_eng.pdf./>.
- 2.World Health Organization Hepatitis B vaccines. WHO Position Paper, Weekly Epidemiological Record. 2009;40(84):405–420. < http://www.who.int/wer/2009/wer8440.pdf/>. [Google Scholar]
- 3.Andernach IE, Nolte C, Pape JW, Muller CP. Slave trade and hepatitis B virus genotypes and subgenotypes in Haiti and Africa. Emerg. Infect. Dis. 2009;15:1222–1228. doi: 10.3201/eid1508.081642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulos R, Ruff AJ, Nahmias A, Holt E, Harrison L, Magder L, et al. Herpes simplex virus type 2 infection, syphilis, and hepatitis B virus infection in Haitian women with human immunodeficiency virus type 1 and human T lymphotropic virus type I infections. J. Infect. Dis. 1992;166:418–420. doi: 10.1093/infdis/166.2.418. [DOI] [PubMed] [Google Scholar]
- 5.Delage G, Montplaisir S, Remy-Prince S, Pierri E. Hepatitis B virus immunization study group: prevalence of hepatitis B virus infection in pregnant women in the Montreal area. Can. Med. Assoc. J. 1986;134:897–901. [PMC free article] [PubMed] [Google Scholar]
- 6.Malison MD, Kane MA, Johnson JM, Schable CA, Gridley MJ, Polkowski J. A seroprevalence survey of hepatitis B markers among Haitians in a Southwest Florida farming community. Am. J. Public Health. 1985;75:1094–1095. doi: 10.2105/ajph.75.9.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskowitz LB, Kory P, Chan JC, Haverkos HW, Conley FK, Hensley GT. Unusual causes of death in Haitians residing in Miami. JAMA. 1983;250:1187–1191. [PubMed] [Google Scholar]
- 8.Mixson-Hayden T, Lee D, Ganova-Raeva L, Drobeniuc J, Stauffer WM, Teshale E, Kamili S. Hepatitis B virus and hepatitis C virus infections in United States-bound refugees from Asia and Africa. Am. J. Trop. Med. Hyg. 2014;90(6):1014–1020. doi: 10.4269/ajtmh.14-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleich LM, Swenson ES. Prevention of neonatal hepatitis B virus transmission. J. Clin. Gastroenterol. 2014;48:765–772. doi: 10.1097/MCG.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 10.Wiseman E, Fraser MA, Holden S, Glass A, Kidson BL, Heron LG, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med. J. Aust. 2009;190:489–492. doi: 10.5694/j.1326-5377.2009.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 11.Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J. Viral Hepat. 2012;19:e18–e25. doi: 10.1111/j.1365-2893.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 12.Singh AE, Plitt SS, Osiowy C, Surynicz K, Kouadjo E, Preiksaitis J, Lee B. Factors associated with vaccine failure and vertical transmission of hepatitis Bamong a cohort of Canadian mothers and infants. J. Viral Hepat. 2011;18(7):468–473. doi: 10.1111/j.1365-2893.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int. J. Epidemiol. 2005;34:1329–1339. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 14.Pan American Health Organization. [accessed 8.11.15];World Hepatitis Day Hepatitis vaccination in Latin America and the Caribbean. 2012 < http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=18239&Itemid/>.
- 15.World Health organization. [accessed 10.11.15];Global policy report on prevention and control of viral hepatitis in WHO member states. < http://apps.who.int/iris/bitstream/10665/85397/1/9789241564632_eng.pdf?ua=1/>.
- 16.Komastsu H. Hepatitis B virus: where do we stand and what is the next step for eradication. World J. Gastroenterol. 2014;20:8998–9016. doi: 10.3748/wjg.v20.i27.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu HA, Woerdenbag HJ, Kane S, Riewpaiboon A, van Hulst M, Postma MJ. Economic evaluations of hepatitis B vaccination for developing countries. Expert Rev. Vaccines. 2009;8(7):907–920. doi: 10.1586/erv.09.53. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal R, Ghoshal UC, Naik SR. Assessment of cost-effectiveness of universal hepatitis B immunization in a low-income country with intermediate endemicity using a Markov model. J. Hepatol. 2003;38:215–222. doi: 10.1016/s0168-8278(02)00382-3. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Salomon JA, Goldie SJ. Economic evaluation of hepatitis B vaccination in low-income countries: using cost-effectiveness affordability curves. Bull. World Health Organ. 2007;85:833–842. doi: 10.2471/BLT.06.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Børresen ML, Koch A, Biggar RJ, Ladefoged K, Melbye M, Wohlfahrt J, Krause TG. Effectiveness of the targeted hepatitis B vaccination program in Greenland. Am. J. Public Health. 2012;102:277–284. doi: 10.2105/AJPH.2011.300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cayemittes M, Busangu MF, Bizimana JD, Barrère B, Sévère B, Cayemittes V, Charles E. Enquête Mortalité, Morbidité et Utilisation des Services, Haïti. Calverton, Maryland, USA: MSPP, IHE et ICF International; 2012. [accessed 8.11.15]. < http://mspp.gouv.ht/site/downloads/EMMUS%20V%20document%20final.pdf./> 2013. [Google Scholar]
- 22.Hennessey K, Mendoza-Aldana J, Bayutas B, Lorenzo-Mariano KM, Diorditsa S. Hepatitis B control in the World Health Organization’s Western Pacific Region: targets, strategies, status. Vaccine. 2013;31S:J85–J92. doi: 10.1016/j.vaccine.2012.10.082. [DOI] [PubMed] [Google Scholar]
- 23.Hutin Y, Hennessey K, Cairns L, Zhang Y, Li H, Zhao L, et al. Improving hepatitis B vaccine timely birth dose coverage: lessons from five demonstration projects in China, 2005–2009. Vaccine. 2013;31S:J49–J55. doi: 10.1016/j.vaccine.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Li J, Chen H, Li F, Armstrong GL, Nelson C, et al. Hepatitis B vaccination of newborn infants in rural China: evaluation of a village-based, out-of-cold-chain delivery strategy. Bull. World Health Organ. 2007;85:688–694. doi: 10.2471/BLT.06.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun LTJ, Jezek J, Peterson S, Tyagi A, Perkins S, Sylvester D, et al. Characterization of a thermostable hepatitis B vaccine formulation. Vaccine. 2009;27:4609–4614. doi: 10.1016/j.vaccine.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 26.Hipgrave DB, Maynard JE, Biggs BA. Improving birth dose coverage of hepatitis B vaccine. Bull. World Health Org. 2006;84(1):65–71. doi: 10.2471/blt.04.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutanto A, Suarnawa IM, Nelson CM, Stewart T, Soewarso TI. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull. World Health Organ. 1999;77:119–126. [PMC free article] [PubMed] [Google Scholar]
- 28.Spradling P, Richardson JT, Buchacz K, Moorman AC, Brooks JT. HIV Outpatient Study (HOPS) Investigators. Prevalence of chronic hepatitis B virus infection among patients in the HIV Outpatient Study 1996–2007. J. Viral Hepat. 2010;17:879–886. doi: 10.1111/j.1365-2893.2009.01249.x. [DOI] [PubMed] [Google Scholar]
- 29.Konopnicki D, Mocroft A, de Wit S, Antunes F, Ledergerber B, Katlama C, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [DOI] [PubMed] [Google Scholar]
- 30.Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B in human immunodeficiency virus-infected subjects. J. Infect. Dis. 2003;188:571–577. doi: 10.1086/377135. [DOI] [PubMed] [Google Scholar]
- 31.Thio CL, Seaberg EC, Skolasky R, Jr, Phair J, Visscher B, Muñoz A, et al. Multicenter AIDS cohort study HIV-1, hepatitis B virus and risk of liver related mortality in the Multicenter cohort study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 32.Cho Y, Bonsu G, Akoto-Ampaw A, Nkrumah-Mills G, Nimo JJA, Park JK, Ki M. The prevalence and risk factors for hepatitis B surface Ag positivity in pregnant women in Eastern Region of Ghana. Gut Liver. 2012;6:235–240. doi: 10.5009/gnl.2012.6.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayo P, Ochola E, Oleo C, Mwaka AD. High prevalence of hepatitis B virus infection among pregnant women attending antenatal care: a cross-sectional study in two hospitals in northern Uganda. BMJ Open. 2014;4:e005889. doi: 10.1136/bmjopen-2014-005889. http://dx.doi.org/10.1136/bmjopen-2014-005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fomulu NJ, Morfaw FL, Torimiro JN, Nana P, Koh MV, William T. Prevalence, correlates and pattern of Hepatitis B among antenatal clinic attenders in Yaounde-Cameroon: is perinatal transmission of HBV neglected in Cameroon? BMC Pregnancy Childbirth. 2013;13:158. doi: 10.1186/1471-2393-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta KD, Antala S, Mistry M, Goswami Y. Seropositivity of hepatitis B, hepatitis C, syphilis, and HIV in antenatal women in India. J. Infect. Dev. Ctries. 2013;7:832–837. doi: 10.3855/jidc.2764. [DOI] [PubMed] [Google Scholar]
- 36.Gaillard EM, Boulos LM, Cayemittes MPA, Eustache L, Van Onacker JD, Duval N, et al. Understanding the reasons for decline of HIV prevalence in Haiti. Sex Transm. Infect. 2006;82(Suppl. I):i14–i20. doi: 10.1136/sti.2005.018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hallett TB, Aberle-Grasse J, Bello G, Boulos LM, Cayemittes MPA, Cheluget B, et al. Declines in HIV prevalence can be associated with changing sexual behaviour in Uganda, urban Kenya, Zimbabwe, and urban Haiti. Sex Transm. Infect. 2006;82(Suppl. I):i1–i8. doi: 10.1136/sti.2005.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]