Abstract
Background
Symptoms in polycystic liver disease (PLD) are thought to be caused by compression of organs and structures by the enlarged liver.
Aim
The aim of this article is to assess the impact of liver volume on symptoms and quality of life (QoL) in PLD.
Methods
We included PLD patients from two prospective studies that used the PLD-questionnaire (PLD-Q) for symptom assessment. QoL was assessed through SF-36, summarized in a physical (PCS) and mental (MCS) component score. Liver volume was correlated with PLD-Q total scores. Patients were classified based on height-corrected liver volume in mild (<1600 ml), moderate (1600–3200 ml), and severe (>3200 ml) disease. PLD-Q and QoL (PCS and MCS) scores were compared across disease stages.
Results
We included 82 of 131 patients from the original studies (disease stages; mild n = 26, moderate n = 33, and severe n = 23). Patients with larger liver volume reported higher symptom burden (r = 0.516, p < 0.001). Symptom scores increased with disease progression, except for abdominal pain (p = 0.088). PCS decreased with advancing disease (p < 0.001), in contrast to MCS (p = 0.055). Moderate (p = 0.007) and severe (p < 0.001) PLD patients had lower PCS scores than the general population.
Conclusion
PLD with larger liver volume is more likely to be symptomatic and is associated with lower QoL.
Keywords: Autosomal dominant polycystic kidney disease (ADPKD), autosomal dominant polycystic liver disease (ADPLD), hepatomegaly, symptoms, quality of life
Introduction
Polycystic liver disease (PLD) is characterized by formation of multiple cysts that causes progressive liver enlargement.1 It occurs isolated in autosomal dominant polycystic liver disease (ADPLD) and in coexistence of kidney cysts in autosomal dominant polycystic kidney disease (ADPKD).1,2 As liver function impairment is absent in PLD, the contention is that PLD-related symptoms are caused by compression of adjacent organs and structures by the enlarged cystic liver.
Improving insight into mechanisms underlying the development of PLD-related symptoms is paramount to optimize treatment strategies, as indications for therapy in PLD are almost completely driven by symptoms. Knowledge on symptom patterns and quality of life (QoL) in different disease stages informs patients about the natural course of the disease.
A recent Korean study reported more abdominal symptoms in PLD patients with larger liver volumes. However, it is unknown whether these results can be translated to a Western population as anthropometric properties of Asians are different and culture can influence symptom presentation.3,4 Furthermore, this study used a general gastrointestinal symptom rating scale instead of a validated PLD-questionnaire (PLD-Q)5 that lacks PLD-related problems such as fear and anxiety about the future, limited mobility, tiredness and problems with intercourse.6 A complete assessment of PLD-related symptoms is important to fully comprehend the impact of liver volume on PLD. Finally, the impact of liver volume on QoL in different disease stages remains to be elucidated.
In this analysis we investigated the impact of liver volume on PLD-related symptoms and QoL in a Western PLD population using data from two prospective studies that reflect different PLD disease stages.
Methods
Study design
We pooled baseline data from two prospective studies that used the PLD-Q to assess symptoms in PLD: (1) the Developing Interventions to halt Progression of ADPKD (DIPAK) Observational Study and (2) the Controlled trial of URSOdeoxycholic acid to Reduce liver volume in polycystic liver disease (CURSOR) study. The DIPAK Observational study is a multicenter, longitudinal study to investigate disease progression in ADPKD. We included patients who were enrolled in Radboud University Nijmegen Medical Center, Nijmegen, the Netherlands, before March 2016. CURSOR is an international, multicenter, randomized, controlled clinical trial that assesses the efficacy of ursodeoxycholic acid as a volume-reducing treatment in symptomatic PLD.7 Patients were enrolled from May 2014 through February 2015 in Radboud University Nijmegen Medical Center, Nijmegen, the Netherlands; Academic Medical Center, Amsterdam, the Netherlands; and the Biodonostia Health Research Institute, Donostia-San Sebastián, Spain. Original studies were approved by local institutional review boards and were conducted according to the ethical guidelines of the 1975 Declaration of Helsinki. In case a patient participated in both studies, we used the most recent data.
Patients
Adult PLD patients who completed the PLD-Q and imaging (computed tomography (CT) in CURSOR and magnetic resonance imaging (MRI) in DIPAK patients) at baseline were eligible for this analysis. PLD was defined as ≥20 hepatic cysts on MRI or CT, which was judged independently by two investigators (MN and SV). Patients were categorized by a previously published disease classification for PLD.5 Mild disease was defined as <1600 ml height-corrected liver volume (htLV), moderate as 1600–3200 ml and >3200 ml as severe disease. We excluded patients with abdominal surgery six months prior to baseline and patients with more than one month between scan and PLD-Q to limit bias in the relation between symptoms and liver volume.
PLD-Q
Patients completed the PLD-Q (version 1) to assess frequency and discomfort of PLD-associated symptoms.6 The PLD-Q is an extensively validated patient-reported outcome measure including 13 disease-specific symptoms. Each individual symptom is assessed with a frequency (six-point Likert scale “never” to “always”) and discomfort (five-point Likert scale “not at all” to “a lot”) question. Severity scores of individual symptoms are the sum of the frequency and discomfort score (range 2–11). A severity score of 2–3 can be considered as no symptoms, 4–5 as mild, 6–7 as moderate, 8–9 as moderately severe and 10–11 as severe symptoms. The PLD-Q total score is the sum of all severity scores and was transformed to a score of 0–100, where a higher score represents a higher symptom burden. Total scores were missing when ≥2 severity scores were missing.
QoL
QoL was assessed with the Short-Form 36 (SF-36), a generic questionnaire that contains 36 questions that can be summarized in a physical (PCS) and mental (MCS) component scale.8 The scores of the PCS and MCS range from 8 to 73 and 10 to 74, respectively, whereas lower scores represent a lower QoL. Scores and missing data of the SF-36 were handled according to the user’s manual.9
Liver volumes
Liver and kidney volumes were measured with a segmentation technique, which involves manual tracing of the liver boundaries. A software program interpolates between CT or MRI slices and calculates the areas within the indicated circumference. For liver segmentation we included liver parenchyma and cysts, the gallbladder, vessels surrounded by liver parenchyma and vessels that are part of the liver hilum. Kidney segmentation included kidney tissue, kidney cysts, pyelum and main kidney vessels when fully surrounded by kidney tissue. Excluded were pyelum and kidney vessels when not surrounded by kidney tissue.
For CT scans we used Pinnacle3® volumetric software version 9.10 (Philips Radiation Oncology Systems; Fitchburg, WI, USA), which has been validated previously for PLD.10–12 MRI scans were measured with ITK-SNAP version 3.4.0 (Penn Image Computing and Science Laboratory, Philadelphia, PA, USA, and Scientific Computing and Imaging Institute, Salt Lake City, UT, USA). We validated ITK-SNAP to measure liver volumes in PLD for this study (Supplemental Appendix) and found a mean difference in liver volume of 1.8% ± 1.1 between ITK-SNAP measurements and Pinnacle3®. Variability between ITK-SNAP measurements by two independent investigators was −0.4% ± 1.4. These differences were considered acceptable for the purpose of our study.
Statistical analysis
Patient characteristics are presented across disease stages as median with interquartile range (IQR) or as proportion of total and compared with the Kruskal-Wallis test or chi-squared test. Liver (htLV) and kidney (htKV) volumes were height-corrected and log-transformed to get normally distributed variables for further analysis. Correlation between htLV and PLD-Q total score was calculated with Spearman’s correlation coefficient. As the CURSOR study included symptomatic patients with larger liver volumes, we performed a sensitivity analysis in DIPAK patients to assess whether selection bias has confounded this correlation. Subsequently, we assessed whether the relation between htLV and symptoms remained significant after adjustment for possible confounders with a multivariate analysis. We entered the PLD-Q total score as an independent variable and htLV, age, gender, diagnosis and original study (CURSOR or DIPAK) as dependent variables. In the subgroup of ADPKD patients, we also entered htKV and renal function (estimated glomerular filtration rate using the Modification of Diet in Renal Disease) in the multivariate model.
To assess whether individual symptoms and QoL (PCS and MCS) were distributed differently across disease stages, scores were compared with the Kruskal-Wallis test. A one-sample Wilcoxon rank test was performed to determine whether QoL scores (PCS and MCS) were different from general population norm scores. As PLD occurs predominantly in females, we used female reference norm scores (51.03 (41.10–55.86) and MCS: 52.80 (43.40–57.19)).9 Significant differences were compared with age-matched female reference values to assess whether the difference holds true.9 Statistical analyses were performed in SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The significance level was set to a p value < 0.05.
Results
Patient characteristics
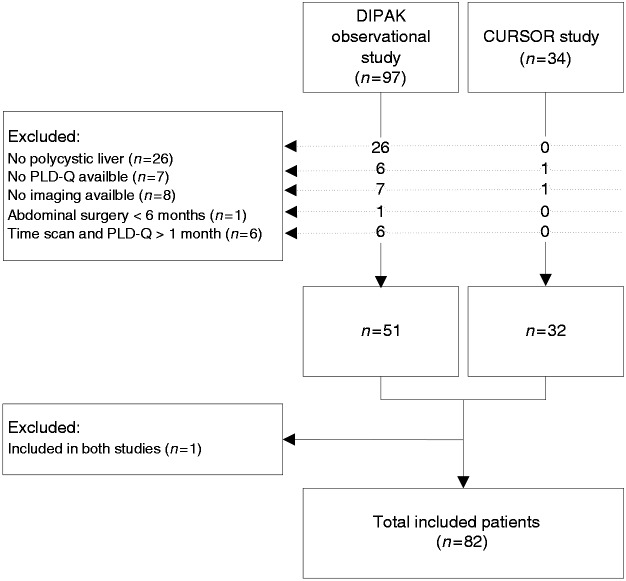
From the 131 patients included in the original studies, 82 patients were eligible for this study. Figure 1 depicts reasons for exclusion. Table 1 shows patient characteristics categorized by disease stage. We included 26 mild PLD patients, 33 patients with moderate disease, and 23 patients with severe PLD. Median age, gender, and underlying diagnosis (ADPKD or ADPLD) were not significantly different across the disease stages. In the ADPKD subgroup as well, no differences between renal function and kidney volume were found.
Figure 1.
Flowchart shows reasons for exclusion from original studies. Of the 131 patients assessed for eligibility, 82 were included in the study.
PLD-Q: polycystic liver disease questionnaire; DIPAK: Developing Interventions to halt Progression of autosomal dominant polycystic kidney disease (ADPKD); CURSOR: Controlled trial of URSOdeoxycholic acid to Reduce liver volume in polycystic liver disease
Table 1.
Characteristics of included patients categorized by disease stage.
| Mild < 1600 ml (n = 26) | Moderate 1600–3200 ml (n = 33) | Severe >3200 ml (n = 23) | p value | |
|---|---|---|---|---|
| Age, median (IQR), years | 48 (33–57) | 49 (46–57) | 43 (40–56) | 0.232b |
| Female, n (%) | 18 (69) | 28(85) | 21 (91) | 0. 114c |
| Diagnosis ADPKD, n (%) | 24 (92) | 24 (73) | 17 (52) | 0.139c |
| HtLV (ml/m), median (IQR) | 1087 (924–1306) | 2375 (1941–2754) | 4571 (3868–5411) | 0.002b |
| HtKV (ml/m), median (IQR)a | 801 (374–1191) | 836 (361–1275) | 614 (380–1480) | 0.258b |
| Renal function (eGFR), median (IQR)a | 73 (44–86) | 64 (46–77) | 71 (54–84) | 0.177b |
Kidney volumes and renal function of ADPKD patients only. P values tested with bKruskal-Wallis test or cChi-Square test. eGFR: estimated glomerular filtration rate using the Modification of Diet in Renal Disease formula; ADPKD: autosomal dominant polycystic kidney disease; htKV: height-corrected kidney volume; htLV: height-corrected liver volume; IQR: interquartile range.
Symptom distribution
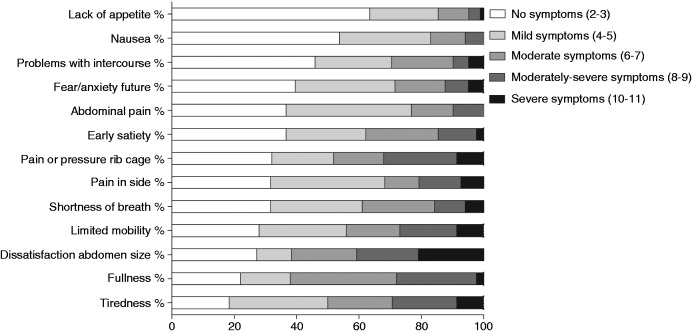
PLD-Q total score could be calculated in all patients. Figure 2 shows the distribution of symptoms in the total study population. The majority had at least mild symptoms of tiredness (81.7%), fullness (78.0%), dissatisfaction with abdomen size (72.8%) and limited mobility (72%). Only 36.6% experienced lack of appetite (mild or higher severity). The most burdensome symptom, scored as moderately severe or severe was dissatisfaction with abdomen size (40.8%), followed by pain and pressure in the rib cage (32.1%), tiredness (29.2%), fullness (28.0%) and limited mobility (26.8%). None of the patients experienced severe nausea or abdominal pain.
Figure 2.
Severity of individual symptoms (%) as scored on the polycystic liver disease questionnaire (PLD-Q) in the total study population. Scores are ranging from 2 to 11, whereas higher scores correspond with more severe symptoms (darker bars).
Correlation symptoms and htLV
Patients with larger htLV reported higher symptom burden measured with the PLD-Q (r = 0.532, p < 0.001). Sensitivity analysis in DIPAK patients showed also a significant correlation (r = 0.621, p < 0.001). In the multivariate model, the association between symptoms measured with the PLD-Q and htLV remained significant (p < 0.001) after adjustment for age (p = 0.50), gender (p = 0.02), diagnosis (p = 0.05) and the original study (p = 0.19) (Table 2). In the ADPKD subgroup, symptom burden was still associated with htLV (p < 0.001) after adjustment for age (p = 0.69), gender (p = 0.02), original study (p = 0.24), htKV (p = 0.27) and renal function (p = 0.46).
Table 2.
Multivariate regression analysis of PLD-Q score.
| All patients (n = 82) |
ADPKD patients (n = 65) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariable B (95% CI) | p value | Multivariable B (95% CI) | p value | Univariable B (95% CI) | p value | Multivariable B (95% CI) | p value | |
| Intercept | −106.82 (−153.38 to −60.26) | <0.001 | −117.93 (−174.60 to −61.26) | <0.001 | −116.36 (−161.96 to −70.76) | <0.001 | −109.39 (−187.17 to −31.61) | 0.007 |
| HtLVa | 18.15 (12.14 to 24.17) | <0.001 | 12.93 (6.66 to 19.21) | <0.001 | 18.97 (13.05 to 24.89) | <0.001 | 15.33 (8.56 to 22.11) | <0.001 |
| Age | 0.12 (−0.22 to 0.45) | 0.50 | −0.10 (−0.59 to 0.39) | 0.69 | ||||
| Female | 10.44 (1.56 to 19.31) | 0.02 | 11.84 (1.84 to 21.83) | 0.02 | ||||
| Diagnosis ADPKD | −10.68 (−21.21 to −0.15) | 0.05 | NA | NA | ||||
| Original study | 6.56 (−3.24 to 16.36) | 0.19 | 6.24 (−4.18 to 16.66) | 0.24 | ||||
| HtKVa | −3.46 (−9.63 to 2.71) | 0.27 | ||||||
| Renal function (eGFR) | −0.08 (−0.30 to 0.14) | 0.46 | ||||||
Liver and kidney volumes are height adjusted and log transformed. ADPKD: autosomal dominant polycystic kidney disease; PLD-Q: polycystic liver disease questionnaire; eGFR: estimated glomerular filtration rate using the Modification of Diet in Renal Disease formula; htKV: height-corrected kidney volume, htLV: height-corrected liver volume; CI: confidence interval.
Severity of individual symptoms across disease stages
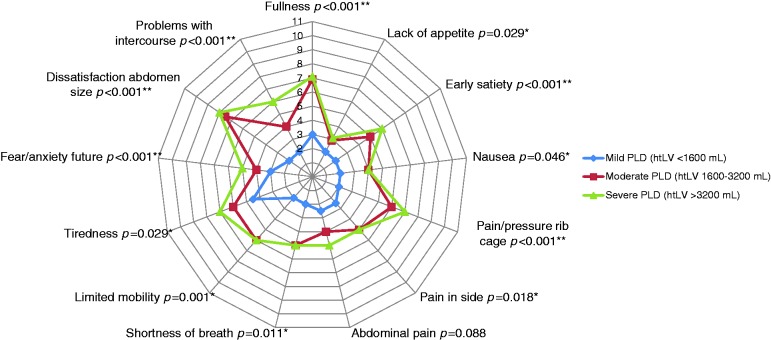
Figure 3 shows the median individual symptom scores per disease stage. All individual symptom scores were higher at more advanced disease stages, except for abdominal pain (p = 0.088). In mild PLD, only one symptom of a mild nature was present (tiredness). In moderate PLD, patients had five symptoms of at least moderate severity and this increased to seven symptoms of this nature in severe PLD. The most burdensome symptom with a moderately severe nature was dissatisfaction with abdomen size in moderate and severe stages.
Figure 3.
Median individual symptom scores per disease stage. A severity score of 2-3 can be considered as no symptoms (center), 4-5 as mild, 6-7 as moderate, 8-9, as moderately-severe, and 10-11 as severe symptoms (most outer lines).
PLD: polycystic liver disease; htLV: height-corrected liver volume.
Quality of life across disease stages
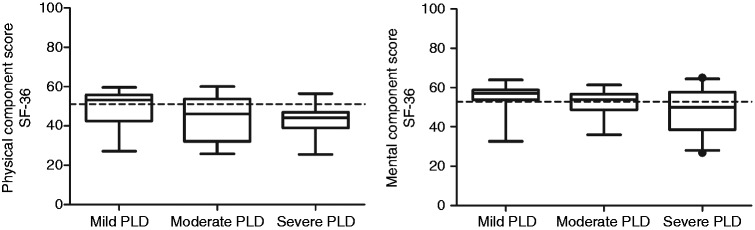
SF-36 data were available in 79 patients (mild n = 24; moderate n = 32; and severe disease n = 23). The PCS decreased significantly with advancing disease stage (p = 0.021), while there was no significant effect of disease stage on the MCS (p = 0.055) (Figure 4). PCS of mild PLD patients was similar to the female reference population (53.30 (42.47–55.86) vs. 51.03, p = 0.932). The PCS of moderate (46.15 (32.16–53.81) vs. 51.03, p = 0.007) and severe (44.09 (38.93–46.94) vs. 51.03, p < 0.001) PLD patients was significantly lower compared to the female reference population. This difference remained after comparing with an age-matched reference population (PCS 51.61, p = 0.004 and p < 0.001).
Figure 4.
Left panel: physical component scores (PCS) decreased significantly with advancing disease stages (p = 0.021). Right panel: no significant effect of disease stage on the mental component scale (MCS) (p = 0.055). The dotted line reflects the reference value of the general population. Disease stages are defined as: mild <1600 mL, moderate 1600–3200 mL; and severe >3200 mL height corrected liver volume.
PLD: polycystic liver disease; SF-36: Short-Form 36.
Discussion
We show that PLD patients with more severe hepatomegaly have a higher symptom burden, with increasing symptom severity across all components of the PLD-Q, except for abdominal pain. QoL, particularly the physical component, decreases with advancing disease and drops below levels seen in the general population in patients with htLV ≥ 1600 ml.
Liver volume affected symptom burden and QoL in those with moderate disease stage and beyond, suggesting that liver volume-reducing therapies are beneficial in these patients. As patients with htLV > 1600 ml also have a higher risk of pressure-related complications compared to patients with smaller livers,5 this liver volume can be used as evidence-based inclusion criteria for symptomatic disease in future studies.
There were no large differences in symptom patterns per disease stage between the Western and Asian population,5 indicating that possible differences in culture and anthropometric properties have no large effect on symptom development in PLD.
Surprisingly, abdominal pain was not associated with disease stage. Although frequency of abdominal pain seems to be increased in PLD compared to the general population,6,13 we found no correlation with liver volume. This accords with another large study in 1043 ADPKD patients that also did not establish a relation between abdominal pain and liver or kidney volume.14 It is possible that cyst growth rather than mere static liver volume is responsible for symptoms of abdominal pain.
The main strength of this study is that we were able to include the whole spectrum of liver volumes from rather normal volumes (htLV 834 ml) to very severe hepatomegaly with htLVs up to 10 times as large (htLV 8382 ml). Our cohort was highly enriched with severe PLD because inclusion mainly took place at a national referral center. In comparison, the prevalence of this stage in the general ADPKD population is approximately 5%, leading to similar numbers of patients with severe PLD in our study compared to large studies that include more than 450 patients.5,15 This enrichment enabled us to compare symptoms and QoL across different disease stages.
We used a reliable and extensively validated disease-specific questionnaire to assess symptom burden in PLD.6 This instrument allowed us to investigate the broad spectrum of symptoms in PLD, while generic gastrointestinal questionnaires under-represent some problems related to PLD.
Limitation of the cross-sectional design is that we could not assess the effect of liver volume growth on symptoms. Although the CURSOR trial has a longitudinal design, mean increase in liver volumes was only 4%, which is too small to conclude whether change in liver volume led to aggravation of symptoms.7 This longitudinal relationship should be a topic for future studies, for instance, when follow-up data of the DIPAK study become available.
Second, the CURSOR included symptomatic patients with (uncorrected) liver volumes > 2500 ml, while the DIPAK inclusion criteria are independent of symptoms and liver volume. The enrichment of symptomatic patients with larger livers may result in a spurious correlation between symptoms and liver volume. However, the fact that correlation between symptoms and liver volume was even stronger in the sensitivity analysis restricted to DIPAK-enrolled patients fuels the contention that this correlation is independent of inclusion criteria.
Finally, we pooled two studies with different imaging modalities. To validate pooling of MRI and CT images in our study, we compared the two different volumetric software packages and differences were small (1.8%). In addition, a randomized controlled trial that assesses changes in liver volume after somatostatin analog treatment also found excellent correlation between liver volumes measured from CT and MRI images.16 Therefore, we think that this has not influenced our results.
This study suggests that an alternative diagnosis than merely PLD should be considered in patients with mild PLD presenting with moderate to severe symptoms. In case of ADPKD, symptoms might be related to large polycystic kidneys rather than liver when PLD stage does not correlate with the experienced symptoms. Given that tiredness and dissatisfaction with abdomen size were respectively the most frequent and burdensome symptoms, clinicians should support adequate coping strategies to reduce this burden. We suggest that abdominal pain should not be the primary indication to start therapy, since no study so far has detected a relation with static liver volume or cyst growth.
In conclusion, larger liver volume has a negative impact on symptoms and QoL in PLD. Particularly, polycystic livers above twice the normal size (≥1600 ml htLV) are associated with symptomatic disease and QoL impairment. We suggest this volume as evidence-based inclusion criteria for future studies investigating new therapies for symptomatic PLD.
Supplementary Material
Acknowledgments
The DIPAK Consortium is a collaboration between four university hospitals established to study ADPKD and to develop treatment strategies for this disease. The DIPAK Consortium is sponsored by the Dutch Kidney Foundation (grant CP10.12). Principal investigators are (in alphabetical order): J.P.H. Drenth (Dept. of Gastroenterology and Hepatology, Radboud University Nijmegen Medical Center), J.W. de Fijter (Dept. Nephrology, Leiden University Medical Center), R.T. Gansevoort (Dept. of Nephrology, University Medical Center Groningen), D.J.M. Peters (Dept. of Human Genetics, Leiden University Medical Center), J. Wetzels (Dept. of Nephrology, Radboud University Nijmegen Medical Center), and R. Zietse (Dept. of Internal Medicine, Erasmus Medical Center Rotterdam).
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approval
Original studies were approved by local institutional review boards and were conducted according to the ethical guidelines of the 1975 Declaration of Helsinki.
Informed consent
Also stated in the study design section of the methods.
References
- 1.Gevers TJ, Drenth JP. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol 2013; 10: 101–108. [DOI] [PubMed] [Google Scholar]
- 2.Hoevenaren IA, Wester R, Schrier RW, et al. Polycystic liver: Clinical characteristics of patients with isolated polycystic liver disease compared with patients with polycystic liver and autosomal dominant polycystic kidney disease. Liver Int 2008; 28: 264–270. [DOI] [PubMed] [Google Scholar]
- 3.Rush EC, Freitas I, Plank LD. Body size, body composition and fat distribution: Comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr 2009; 102: 632–641. [DOI] [PubMed] [Google Scholar]
- 4.Fukuhara S, Bito S, Green J, et al. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol 1998; 51: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Park HC, Ryu H, et al. Clinical correlates of mass effect in autosomal dominant polycystic kidney disease. PloS One 2015; 10: e0144526–e0144526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neijenhuis MK, Gevers TJ, Hogan MC, et al. Development and validation of a disease-specific questionnaire to assess patient-reported symptoms in polycystic liver disease. Hepatology 2016; 64: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Agnolo HM, Kievit W, Takkenberg RB, et al. Ursodeoxycholic acid in advanced polycystic liver disease: An international multicenter randomized controlled phase 2 trial: CURSOR: Controlled trial of URSOdeoxycholic acid to Reduce liver volume in polycystic liver disease. J Hepatol 2016; 65: 601–607. [DOI] [PubMed] [Google Scholar]
- 8.Ware JE, Jr, Kosinski M. SF-36® Physical & Mental Health Summary scales: A manual for users of version 1, Lincoln, RI: QualityMetric Incorporated, 2001. [Google Scholar]
- 9.Ware JE KM, Keller SD. SF-36 Physical and Mental Health Summary scales: A user’s manual, Boston, MA: Health Institute, New England Medical Center, 1994. [Google Scholar]
- 10.Warner JD, Irazabal MV, Krishnamurthi G, et al. Supervised segmentation of polycystic kidneys: A new application for stereology data. J Digit Imaging 2014; 27: 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Keimpema L, Ruurda JP, Ernst MF, et al. Laparoscopic fenestration of liver cysts in polycystic liver disease results in a median volume reduction of 12.5%. J Gastrointest Surg 2008; 12: 477–482. [DOI] [PubMed] [Google Scholar]
- 12.van Keimpema L, Nevens F, Vanslembrouck R, et al. Lanreotide reduces the volume of polycystic liver: A randomized, double-blind, placebo-controlled trial. Gastroenterology 2009; 137: 1661–1668.e1-e2. [DOI] [PubMed] [Google Scholar]
- 13.Wijnands TF, Neijenhuis MK, Kievit W, et al. Evaluating health-related quality of life in patients with polycystic liver disease and determining the impact of symptoms and liver volume. Liver Int 2014; 34: 1578–1583. [DOI] [PubMed] [Google Scholar]
- 14.Miskulin DC, Abebe KZ, Chapman AB, et al. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1–4: A cross-sectional study. Am J Kidney Dis 2014; 63: 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan MC, Abebe K, Torres VE, et al. Liver involvement in early autosomal-dominant polycystic kidney disease. Clin Gastroenterol Hepatol 2015; 13: 155–164.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan MC, Masyuk TV, Page L, et al. Somatostatin analog therapy for severe polycystic liver disease: Results after 2 years. Nephrol Dial Transplant 2012; 27: 3532–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.