Abstract
Background
The pathogenesis of non-alcoholic fatty liver disease (NAFLD) has not been well recognized yet.
Objective
This study aimed to investigate the association between serum magnesium concentration and NAFLD.
Methods
Study participants were healthy individuals who had undergone liver biopsies between January 2012 and August 2015 as a routine pre-transplant check-up before living donor liver transplantation. Liver biopsy specimens were evaluated by an expert pathologist regarding presence of hepatic steatosis and steatohepatitis. Serum magnesium concentration was measured and compared in those with normal liver biopsy and those with steatosis and steatohepatitis.
Results
A total of 226 individuals were included. Eighty-two individuals (36.2%) had hepatic steatosis and 22 (9.7%) individuals had steatohepatitis and steatosis in their liver histology. Lower serum magnesium concentration was independently associated with hepatic steatosis (OR: 0.059; 95% CI: 0.011–0.325, p = 0.001). Serum magnesium concentration was independently associated with steatohepatitis compared to those without steatohepatitis (1.80 ± 0.48 mg/dl and 2.18 ± 0.31 mg/dl) (OR: 0.11; 95% CI: 0.02–0.41, p = 0.001). Serum magnesium concentration was significantly lower in individuals with steatohepatitis (1.80 ± 0.48 mg/dl) compared to individuals without steatosis (2.23 ± 0.31 mg/dl, p < 0.001) and individuals with only steatosis (2.07 ± 0.29 mg/dl, p = 0.017).
Conclusion
Serum magnesium concentration is independently associated with hepatic steatosis and steatohepatitis in our study population.
Keywords: Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, magnesium, liver biopsy
Key point summary
The pathogenesis of non-alcoholic fatty liver disease (NAFLD) has not been well recognized yet.
Decreased serum magnesium has been associated with insulin resistance, metabolic syndrome and its components, including diabetes mellitus in previous studies. This study aimed to investigate the association between NAFLD and serum magnesium concentration.
Lower serum magnesium concentration was independently associated with biopsy-proven hepatic steatosis and steatohepatitis.
Serum magnesium could discriminate between hepatic steatosis and steatohepatitis.
Introduction
The prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing and is now considered the most common cause of abnormal liver enzymes worldwide.1 The highest prevalence of NAFLD has been reported in Middle Eastern and South American countries.1,2 The spectrum of NAFLD ranges from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH) that may eventuate in liver cirrhosis.3 Patients with NAFLD also have substantial risk for development of hepatocellular carcinoma (HCC) compared to the general population.4 NAFLD is supposed to be correlated with metabolic syndrome and its components, and some consider NAFLD as hepatic manifestation of metabolic syndrome.5 However, up to 30% of NAFLD patients are non-obese and only 20% to 80% of NAFLD patients fulfill criteria for metabolic syndrome.6,7
The pathogenesis of NAFLD has not been well recognized, although several mechanisms have been proposed. Abnormalities of hormones and micronutrients have been reported in pathogenesis of NAFLD.8 Thyroid hormone abnormalities, alterations of adipocytokines such as adiponectin and leptin, have been proposed.9–11 Among vitamins and micronutrients, vitamin D deficiency has been reported to be associated with NAFLD.12 Vitamin D deficiency is also an independent risk factor for development of NASH and is associated with its histological severity.13
Magnesium (Mg++) is an abundant cation in the human body that is known to be involved in multiple physiological pathways like cellular energy metabolism, DNA transcription, protein synthesis and electrolyte balance.14 Magnesium is also a vital cation in neuromuscular function and bone formation.14 Serum magnesium concentration is tightly controlled; however, hypomagnesemia may occur as a consequence of increased renal excretion or decreased gastrointestinal absorption of magnesium.15 Hypomagnesemia is therefore associated with osteoporosis, seizure, depression and several neuromuscular abnormalities.16 Hypomagnesemia is also associated with diabetes mellitus, hypertension and dyslipidemia.17,18 This study aimed to investigate the association between hepatic steatosis and serum magnesium concentration in a young lean population without metabolic syndrome.
Methods and materials
Study participants
This retrospective study was conducted at Namazi University Hospital affiliated with Shiraz University of Medical Sciences, Shiraz, Iran. All of the study participants were first-degree relatives of pediatric patients in our liver transplant waiting list due to liver cirrhosis or other indications for transplantation. These participants were apparently healthy individuals who had undergone liver biopsy for evaluation of liver histology between January 2012 and August 2015. Liver biopsies were performed as a routine pre-transplant check-up before living donor liver transplantation. Liver function tests, age, gender, weight, height, fasting plasma glucose, serum magnesium and lipid profile were recorded. Individuals with a history of chronic liver diseases such as autoimmune hepatitis, hepatitis B or C virus-induced hepatitis, hepato-billiary cancers, Wilson’s disease, those with >10 g/day alcohol consumption, and individuals receiving some specific medications known to cause hepatic steatosis (amiodarone, valporic acid, etc.) were excluded from the study. NAFLD was defined as presence of steatosis in ≥ 5% of hepatocytes19 in addition to the above-mentioned criteria. All donor candidates underwent ultrasound-guided liver biopsy using standard Tru-Cut needles. Tissue slides were prepared and stained with the hematoxylin and eosin staining method. Liver biopsy specimens were evaluated by one expert pathologist regarding presence and degree of hepatic steatosis and steatohepatitis. Hepatic steatosis was defined as presence of intracytoplasmic fat droplets displacing the nucleus to the cell periphery. Steatohepatitis was defined as presence of clues for hepatocyte injury such as ballooning and lobular inflammation in addition to steatosis. Individuals were divided into those without hepatic macrovesicular steatosis (grade 0), individuals with ≤5% hepatic macrovesicular steatosis (grade 1) and those with > 5% macrovesicular hepatic steatosis (grade 2). Fibrosis score-4 (FIB-4) has been used for the noninvasive estimation of hepatic fibrosis in study participants. In NASH, a FIB-4 score < 1.30 is consistent with F0–F1 fibrosis and a FIB-4 score > 2.67 is consistent with F3–F4 fibrosis.20
Ethics and consent
The study protocol including benefits and harms was explained for study participants and written informed consents were obtained. The study was approved by the ethical committee of Shiraz University of Medical Sciences. The study protocol was carried out in accordance with the Declaration of Helsinki as revised in Seoul 2008.
Statistical analysis
Student’s t-test was used for comparisons of continuous variables, Chi-square test was used for comparison of categorical variables. Data were presented using means ± standard deviation for numeric variables, and percentages and counts for categorical variables. Logistic regression analysis was used to evaluate association of different risk factors with liver steatosis and steatohepatitis in liver biopsies. A one-way analysis of variance (ANOVA) and post-hoc Tukey test were used to evaluate the differences of mean serum magnesium between those without hepatic macrovesicular steatosis (grade 0), individuals with ≤5% hepatic macrovesicular steatosis (grade 1) and those with > 5% macrovesicular hepatic steatosis (grade 2). ANOVA and post-hoc Tukey test were also used to evaluate the differences of mean serum magnesium between those with normal liver biopsy, those with only steatosis and those with steatohepatitis in liver biopsy. The optimal cutoffs of serum magnesium in association with hepatic steatosis and steatohepatitis in liver biopsies were calculated based on receiver operating characteristics (ROC) curve analysis using area under the curve (AUC). Statistical analysis was performed with SPSS 18.0 (SPSS Inc, Chicago, IL, USA). A p value of <0.05 was considered statistically significant.
Results
A total of 226 individuals (143 female and 83 male) were included. Eighty-two individuals (36.2%) had hepatic steatosis, among them 22 (9.7%) individuals had steatohepatitis and steatosis in their liver histology. Fifty-five individuals (24.3%) had NAFLD (presence of steatosis in ≥ 5% of hepatocytes). None of the participants had diabetes mellitus and hypertension. Mean age of individuals with and without hepatic steatosis was 33.28 ± 7.55 and 31.72 ± 6.56 years, respectively (p = 0.11). In univariate analysis, higher weight (70.80 ± 10.79 vs 63.44 ± 9.57 kg, p < 0.001), increased cholesterol (179.50 ± 35.35 vs 166.04 ± 36.50 mg/dl, p = 0.009), triglycerides (TG) (132.90 ± 79.68 vs 93.10 ± 46.78 mg/dl, p < 0.001), fasting plasma glucose (FPG) (92.12 ± 11.21 vs 87.02 ± 10.21 mg/dl, p = 0.001), alanine aminotransferase (ALT) (22.59 ± 12.01 vs 17.69 ± 11.16 IU/l, p = 0.002), alkaline phosphatase (213.19 ± 73.31 vs 183.22 ± 62.65 IU/l, p = 0.001) and lower serum magnesium (2.01 ± 0.35 vs. 2.23 ± 0.31 mg/dl, p = 0.001) were associated with hepatic steatosis (Table 1). In multivariate logistic regression analysis, higher FPG, higher alkaline phosphatase and lower serum magnesium concentration were independently associated with hepatic steatosis (Table 2). Participants were divided as to dose without steatosis, individuals with ≤5% steatosis and those with >5% steatosis. In one-way ANOVA analysis, there was a statistically significant difference between these three groups in terms of serum magnesium concentration (F (2,147) = 8.923, p < 0.001). A post hoc Tukey test revealed that serum magnesium concentration was significantly lower in individuals with ≤5% steatosis (2.04 ± 0.29 mg/dl) compared to individuals without steatosis (2.23 ± 0.31 mg/dl) (p = 0.006). Serum magnesium concentration was significantly lower in individuals with > 5% steatosis (1.91 ± 0.51 mg/dl) compared to individuals without steatosis (2.23 ± 0.31 mg/dl) (p = 0.002) (Figure 1).
Table 1.
Baseline characteristics of patients.
| With steatosis | Without steatosis | p value | |
|---|---|---|---|
| Age (years) | 33.28 ± 7.55 | 31.72 ± 6.56 | 0.11 |
| Weight (kg) | 70.80 ± 10.79 | 63.44 ± 9.57 | <0.001 |
| Triglyceride (mg/dl) | 132.90 ± 79.68 | 93.10 ± 46.78 | <0.001 |
| Cholesterol (mg/dl) | 179.50 ± 35.35 | 166.04 ± 36.50 | 0.009 |
| LDL (mg/dl) | 105.62 ± 33.91 | 98.54 ± 29.02 | 0.129 |
| HDL (mg/dl) | 45.72 ± 11.54 | 46.86 ± 10.85 | 0.496 |
| AST (IU/l) | 20.98 ± 8.33 | 19.80 ± 7.86 | 0.288 |
| ALT (IU/l) | 22.59 ± 12.01 | 17.69 ± 11.16 | 0.002 |
| Alk pho (IU/l) | 213.19 ± 73.31 | 183.22 ± 62.65 | 0.001 |
| FPG (mg/dl) | 92.12 ± 11.21 | 87.02 ± 10.21 | 0.001 |
| Mg (mg/dl) | 2.01 ± 0.35 | 2.23 ± 0.31 | 0.001 |
M: male; F: female; LDL: low-density lipoprotein; HDL: high-density lipoprotein; AST: aspartate aminotransferase; ALT: alanine aminotransferase; Alk pho: alkaline phosphatase; FPG: fasting plasma glucose; Mg: magnesium.
Table 2.
Logistic regression analysis of risk factors of hepatic steatosis.
| OR | 95% CI | p value | |
|---|---|---|---|
| Weight (kg) | 1.033 | 0.991–1.077 | 0.122 |
| Triglyceride (mg/dl) | 1.006 | 0.999–1.013 | 0.089 |
| Cholesterol (mg/dl) | 0.998 | 0.986–1.011 | 0.768 |
| ALT (IU/l) | 1.009 | 0.975–1.045 | 0.608 |
| Alk pho (IU/l) | 1.011 | 1.003–1.018 | 0.004 |
| FPG (mg/dl) | 1.056 | 1.012–1.102 | 0.012 |
| Mg (mg/dl) | 0.059 | 0.011–0.325 | 0.001 |
ALT: alanine aminotransferase; Alk pho: alkaline phosphatase; FPG: fasting plasma glucose; Mg: magnesium; OR: odds ratio; CI: confidence interval.
Figure 1.
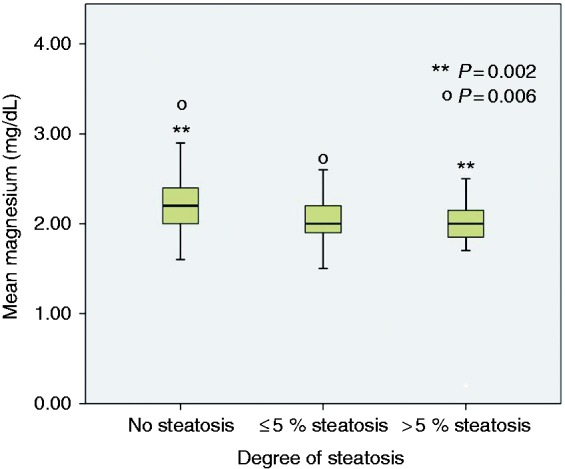
Comparison of serum magnesium between those with normal liver biopsy, those with ≤ 5 % hepatic steatosis and those with > 5% hepatic steatosis.
In one-way ANOVA analysis, there was a statistically significant difference between those without steatosis, those with only steatosis and those with steatohepatitis in terms of serum magnesium concentration (F (2,147) = 8.923, p < 0.001). A post hoc Tukey test revealed that serum magnesium concentration was significantly lower in individuals with steatosis (2.07 ± 0.29 mg/dl) compared to individuals without steatosis (2.23 ± 0.31 mg/dl) (p = 0.020). Serum magnesium concentration was significantly lower in individuals with steatohepatitis (1.80 ± 0.48 mg/dl) compared to individuals without steatosis (2.23 ± 0.31 mg/dl, p < 0.001) and individuals with steatosis (2.07 ± 0.29 mg/dl, p = 0.017) (Figure 2).
Figure 2.
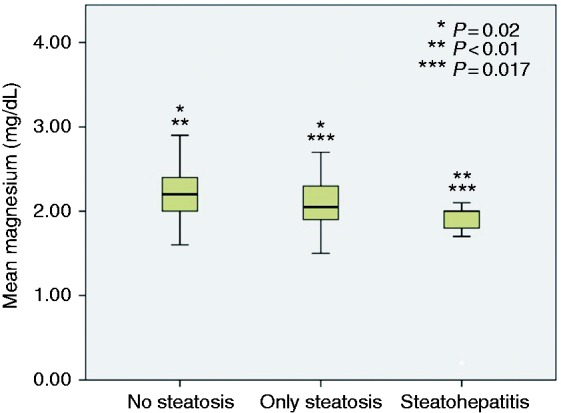
Comparison of serum magnesium between those with normal liver biopsy, those with hepatic steatosis and those with steatohepatitis.
The univariate analysis showing the association of different factors with steatohepatitis is outlined in Table 3. In regression analysis, higher TG level (146.09 ± 76.90 vs 103.82 ± 61.28) and lower serum magnesium concentration were independently associated with steatohepatitis compared to those without steatohepatitis (1.80 ± 0.48 mg/dl and 2.18 ± 0.31 mg/dl) (odds ratio (OR): 0.11; 95% confidence interval (CI): 0.02–0.41, p = 0.001) (Table 4).
Table 3.
Univariate analysis of patients with and without steatohepatitis in liver biopsies.
| With steatohepatitis | Without steatohepatitis | p value | |
|---|---|---|---|
| Sex (M/F) | 10/12 | 73/131 | 0.35 |
| Age (years) | 32.95 ± 7.15 | 32.30 ± 6.97 | 0.973 |
| Weight (kg) | 73.59 ± 11.41 | 65.34 ± 10.26 | 0.001 |
| Triglyceride (mg/dl) | 146.09 ± 76.90 | 103.82 ± 61.28 | 0.003 |
| Cholesterol (mg/dl) | 185.81 ± 35.88 | 169.38 ± 36.37 | 0.046 |
| LDL (mg/dl) | 112.89 ± 41.39 | 99.84 ± 29.51 | 0.082 |
| HDL (mg/dl) | 46.05 ± 9.10 | 46.49 ± 11.31 | 0.870 |
| AST (IU/l) | 22.90 ± 12.01 | 19.96 ± 7.47 | 0.103 |
| ALT (IU/l) | 26.18 ± 12.64 | 18.80 ± 11.40 | 0.005 |
| Alk pho (IU/l) | 200.00 ± 73.21 | 193.91 ± 67.88 | 0.692 |
| FPG (mg/dl) | 90.42 ± 10.63 | 88.85 ± 10.92 | 0.533 |
| Mg (mg/dl) | 1.80 ± 0.48 | 2.18 ± 0.31 | <0.001 |
M: male; F: female; LDL: low-density lipoprotein; HDL: high-density lipoprotein; AST: aspartate aminotransferase; ALT: alanine aminotransferase; Alk pho: alkaline phosphatase; FPG: fasting plasma glucose; Mg: magnesium.
Table 4.
Logistic regression analysis showing independent risk factors predicting steatohepatitis.
| OR | 95% CI | p value | |
|---|---|---|---|
| Weight (kg) | 1.034 | 0.994–1.076 | 0.097 |
| Triglyceride (mg/dl) | 1.007 | 1.001–1.014 | 0.025 |
| ALT (IU/l) | 1.014 | 0.981–1.049 | 0.410 |
| Cholesterol (mg/dl) | 1.000 | 0.988–1.012 | 0.988 |
| Mg | 0.11 | 0.029–0.418 | 0.001 |
ALT: alanine aminotransferase; Mg: magnesium; OR: odds ratio; CI: confidence interval.
Mean FIB-4 was 0.67 ± 0.3 in individuals with steatosis and 0.69 ± 0.30 in individuals without steatosis (p = 0.676) (Figure 3). Stage F0–F1 fibrosis (cutoff value of 1.30 for FIB-4) was detected in one individual in the steatosis group and five individuals without steatosis (p = 0.25). On liver biopsy, only one individual was reported to have mild fibrosis. None of the study participants had stage F3–F4 fibrosis (cutoff value of >2.67 for FIB-4).
Figure 3.
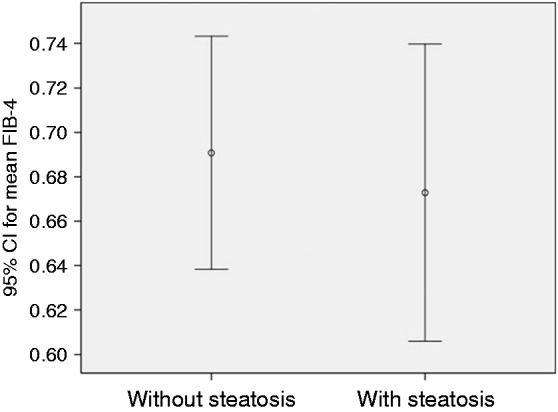
Comparison of fibrosis score-4 (FIB-4) in those with and without hepatic steatosis; p > 0.05.
CI: confidence interval.
A cutoff value of 2.05 mg/dl for serum magnesium was a predictor of the presence of steatohepatitis in liver biopsies (sensitivity = 61%; specificity = 86%; AUC = 0.773; p = 0.001).
Discussion
Results of the present study showed that lower serum magnesium concentration was independently associated with biopsy-proven hepatic steatosis and steatohepatitis. While comparing those with only steatosis and those with steatohepatitis in liver biopsies, serum magnesium was significantly lower in participants with steatohepatitis. Therefore, serum magnesium could discriminate between hepatic steatosis and steatohepatitis. A cutoff value of 2.05 mg/dl for serum magnesium could predict the presence of steatohepatitis in liver histology with a rather good specificity and fair sensitivity. The other important finding was the high prevalence of biopsy-proven hepatic steatosis and NAFLD among our study population that consisted of asymptomatic, non-diabetic, young, healthy individuals. Although the majority had only mild degrees of hepatic steatosis, this signifies high burden of hepatic steatosis and steatohepatitis even in a young, healthy population. Whether these individuals will progress to higher degrees of steatosis or remain at the same stage is an interesting topic for future studies.
Insulin resistance is supposed to be the main underlying mechanism in pathogenesis of NAFLD.21 Magnesium plays a central role in insulin action and insulin regulates cellular magnesium concentration.22 Insulin receptors are glycoproteins composed of two alpha-subunits and two beta-subunits with intrinsic tyrosine kinase activity. The initial step in insulin action on target cells is its binding to these receptors and activation of protein kinase.23 Activation of protein kinase results in initiation of a signaling cascade leading to increased glucose uptake by several tissues. A decreased magnesium level has been suggested to cause decreased tyrosine kinase activity and subsequent decreased ability of insulin to stimulate glucose uptake in adipose tissue and skeletal muscle.24 It seems that magnesium is involved in glucose transport in the insulin signaling pathway.25 Decreased magnesium level may also lead to insulin resistance by decreased cellular glucose utilization.
Epidemiological evidence supports an association between decreased serum magnesium level and diabetes/metabolic syndrome. A cross-sectional study in Brazil showed that serum and intracellular magnesium levels were lower in patients with components of metabolic syndrome like obesity, insulin resistance and patients with moderate to severe hepatic steatosis on ultrasound.26 A strong inverse association has been also observed between serum magnesium and glycemic indices and insulin resistance in the pre-diabetes stage.27 Magnesium deficiency was also seen in association with insulin resistance in obese children.28 A large Canadian cohort study showed that higher dietary magnesium intake is associated with low insulin resistance measured by homeostatic model assessment model for insulin resistance (HOMA-IR).29 Results of another large cohort were in favor of a protective role of dietary magnesium against metabolic syndrome.30 Based on these findings, some studies have investigated whether magnesium supplementation will improve the insulin resistance component of metabolic syndrome including glycemic indices and lipid profile. A recent meta-analysis of randomized clinical trials reported beneficial effects of magnesium supplementation on glucose metabolism. This review revealed that magnesium treatment reduced FPG in patients with diabetes mellitus and improved insulin resistance and two hours post-prandial glucose in those at risk of diabetes mellites.31
As described previously, a significant proportion of NAFLD patients do not fulfill criteria for metabolic syndrome. These patients are usually non-obese and called metabolically obese but normal weight (MONW). The main underlying mechanism in this subset of patients is again supposed to be insulin resistance as reflected by HOMA-IR.32 These patients may show the phenotype of insulin resistance regardless of the presence of other components of metabolic syndrome.32 Therefore, magnesium may have a pivotal role in hepatic steatosis in this subgroup of patients because of its role in the insulin signaling pathway and insulin resistance. These patients have higher visceral adiposity and higher body fat mass compared to non-NAFLD healthy individuals.33 Weight gain, higher cholesterol and fructose intake have been also reported in the pathogenesis of these patients.34,35 Our results showed that higher FPG and higher serum TG levels were independent predictors of hepatic steatosis and steatohepatitis, respectively. These findings are in consistent with previous studies demonstrating higher serum TG and FPG levels as risk factors of lean NAFLD.36
The association of serum magnesium with hepatic steatosis has been shown previously in a study with a limited number of patients.37 In the mentioned study a group of patients with hepatic steatosis with alcoholic liver disease and NAFLD were compared to healthy individuals. Patients with hepatic steatosis had lower magnesium levels compared to the healthy controls. Patients with alcoholic liver disease are usually malnourished and hypomagnesemia is anticipated in these patients.38 Therefore, the major caveat in the mentioned study is including patients with alcoholic liver disease with NAFLD patients. Furthermore, hepatic steatosis was confirmed using ultrasound in the mentioned study.37 Another study investigated the association of hypomagnesaemia with insulin resistance in obese patients. In a sub-group of patients undergoing liver biopsy, lower serum magnesium was associated with NASH.39 The mentioned study was conducted among obese individuals with insulin resistance and only 33 patients had liver biopsy. Results of a recent in vitro study supported the role of magnesium in pathogenesis and treatment of NAFLD. In this study magnesium isoglycyrrhizinate treatment of the hepatic L02 cell line inhibited lipid accumulation and apoptosis in these cells via suppression of unfolded protein response and inhibition of nuclear factor-κB (NF-κB) in hepatic cells overloaded with lipid.40
To the best of our knowledge, the present study is the first that reports an association of serum magnesium concentration with hepatic steatosis diagnosed with liver biopsy in a normal population. This study suggests lower serum magnesium may be involved in the pathogenesis of NAFLD. Our study also reported prevalence of biopsy-proven NAFLD in a population of apparently healthy non-diabetic individuals. Although most of our study participants had mild degrees of steatosis and steatohepatitis, this is an important issue showing high prevalence of hepatic steatosis in a normal population. The most important limitation of the study is the impact of nutritional status on serum nutrients including magnesium. However, none of the study participants were malnourished and lower serum magnesium cannot be attributed to malnourishment.
In conclusion, our results suggested that decreased serum magnesium is associated with hepatic steatosis and steatohepatitis in normal non-diabetic individuals. However, few data are still available about the impact of hypomagnesemia on NAFLD and these results should be validated in larger studies.
Acknowledgments
Author contributions are as follows: AE: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and statistical analysis; SN: acquisition of data, analysis and interpretation of data and critical revision of the manuscript for important intellectual content; BG: acquisition of data, analysis and interpretation of data and critical revision of the manuscript for important intellectual content; and SM: acquisition of data, analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the ethical committee of Shiraz University of Medical Sciences. The study protocol was carried out in accordance with the Declaration of Helsinki as revised in Seoul 2008.
Informed consent
The study protocol including benefits and harms was explained for study participants and written informed consents were obtained.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 2.Eshraghian A. High prevalence of non-alcoholic fatty liver disease in the Middle East: Lifestyle and dietary habits. Hepatology 2017; 65: 1077–1077. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34: 274–285. [DOI] [PubMed] [Google Scholar]
- 4.Mohamad B, Shah V, Onyshchenko M, et al. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int 2016; 10: 632–639. [DOI] [PubMed] [Google Scholar]
- 5.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005; 143: 722–728. [DOI] [PubMed] [Google Scholar]
- 6.Wattacheril J, Sanyal AJ. Lean NAFLD: An underrecognized outlier. Curr Hepatol Rep 2016; 15: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012; 91: 319–327. [DOI] [PubMed] [Google Scholar]
- 8.Eshraghian A, Taghavi SA. Systematic review: Endocrine abnormalities in patients with liver cirrhosis. Arch Iran Med 2014; 17: 713–721. [PubMed] [Google Scholar]
- 9.Eshraghian A, Hamidian Jahromi A. Non-alcoholic fatty liver disease and thyroid dysfunction: A systematic review. World J Gastroenterol 2014; 20: 8102–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stojsavljević S, Gomerčić Palčić M, Virović Jukić L, et al. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 2014; 20: 18070–18091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polyzos SA, Aronis KN, Kountouras J, et al. Circulating leptin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Diabetologia 2016; 59: 30–43. [DOI] [PubMed] [Google Scholar]
- 12.Dasarathy J, Periyalwar P, Allampati S, et al. Hypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver disease. Liver Int 2014; 34: e118–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson JE, Roth CL, Wilson LA, et al. Vitamin D deficiency is associated with increased risk of non-alcoholic steatohepatitis in adults with non-alcoholic fatty liver disease: Possible role for MAPK and NF-κB? Am J Gastroenterol 2016; 111: 852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musso CG. Magnesium metabolism in health and disease. Int Urol Nephrol 2009; 41: 357–362. [DOI] [PubMed] [Google Scholar]
- 15.Quamme GA. Recent developments in intestinal magnesium absorption. Curr Opin Gastroenterol 2008; 24: 230–235. [DOI] [PubMed] [Google Scholar]
- 16.Touyz RM. Magnesium in clinical medicine. Front Biosci 2004; 9: 1278–1293. [DOI] [PubMed] [Google Scholar]
- 17.Guerrero-Romero F, Rodríguez-Morán M. Low serum magnesium levels and metabolic syndrome. Acta Diabetol 2002; 39: 209–213. [DOI] [PubMed] [Google Scholar]
- 18.Sarrafzadegan N, Khosravi-Boroujeni H, Lotfizadeh M, et al. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition 2016; 32: 409–417. [DOI] [PubMed] [Google Scholar]
- 19.Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int 2017; 37(Suppl 1): 85–89. [DOI] [PubMed] [Google Scholar]
- 20.Martínez SM, Crespo G, Navasa M, et al. Noninvasive assessment of liver fibrosis. Hepatology 2011; 53: 325–335. [DOI] [PubMed] [Google Scholar]
- 21.Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 2014; 59: 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang DL, Yen CF, Nadler JL. Insulin increases intracellular magnesium transport in human platelets. J Clin Endocrinol Metab 1993; 76: 549–553. [DOI] [PubMed] [Google Scholar]
- 23.Farese RV. Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. Am J Physiol Endocrinol Metab 2002; 283: E1–E11. [DOI] [PubMed] [Google Scholar]
- 24.Kandeel FR, Balon E, Scott S, et al. Magnesium deficiency and glucose metabolism in rat adipocytes. Metabolism 1996; 45: 838–843. [DOI] [PubMed] [Google Scholar]
- 25.Whitelaw DC, Gilbey SG. Insulin resistance. Ann Clin Biochem 1998; 35: 567–583. [DOI] [PubMed] [Google Scholar]
- 26.Lima Mde L, Cruz T, Rodrigues LE, et al. Serum and intracellular magnesium deficiency in patients with metabolic syndrome—evidences for its relation to insulin resistance. Diabetes Res Clin Pract 2009; 83: 257–262. [DOI] [PubMed] [Google Scholar]
- 27.Yadav C, Manjrekar PA, Agarwal A, et al. Association of serum selenium, zinc and magnesium levels with glycemic indices and insulin resistance in pre-diabetes: A cross-sectional study from South India. Biol Trace Elem Res 2017; 175: 65–71. [DOI] [PubMed] [Google Scholar]
- 28.Huerta MG, Roemmich JN, Kington ML, et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care 2005; 28: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 29.Cahill F, Shahidi M, Shea J, et al. High dietary magnesium intake is associated with low insulin resistance in the Newfoundland population. PLoS One 2013; 8: e58278–e58278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore-Schiltz L, Albert JM, Singer ME, et al. Dietary intake of calcium and magnesium and the metabolic syndrome in the National Health and Nutrition Examination (NHANES) 2001–2010 data. Br J Nutr 2015; 114: 924–935. [DOI] [PubMed] [Google Scholar]
- 31.Veronese N, Watutantrige-Fernando S, Luchini C, et al. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur J Clin Nutr 2016; 70: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 32.Sinn DH, Gwak GY, Park HN, et al. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in nonobese, non-diabetic middle-aged Asian adults. Am J Gastroenterol 2012; 107: 561–567. [DOI] [PubMed] [Google Scholar]
- 33.Katsuki A, Sumida Y, Urakawa H, et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care 2003; 26: 2341–2344. [DOI] [PubMed] [Google Scholar]
- 34.Chang Y, Ryu S, Sung E, et al. Weight gain within the normal weight range predicts ultrasonographically detected fatty liver in healthy Korean men. Gut 2009; 58: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 35.Mosca A, Nobili V, De Vito R, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. Epub ahead of print 6 February 2017. DOI: 10.1016/j.jhep.2016. [DOI] [PubMed]
- 36.Omagari K, Kadokawa Y, Masuda J, et al. Fatty liver in nonalcoholic non-overweight Japanese adults: Incidence and clinical characteristics. J Gastroenterol Hepatol 2002; 17: 1098–1105. [DOI] [PubMed] [Google Scholar]
- 37.Turecky L, Kupcova V, Szantova M, et al. Serum magnesium levels in patients with alcoholic and non-alcoholic fatty liver. Bratisl Lek Listy 2006; 107: 58–61. [PubMed] [Google Scholar]
- 38.Rossi RE, Conte D, Massironi S. Diagnosis and treatment of nutritional deficiencies in alcoholic liver disease: Overview of available evidence and open issues. Dig Liver Dis 2015; 47: 819–825. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez-Hernández H, Gonzalez JL, Rodríguez-Morán M, et al. Hypomagnesemia, insulin resistance, and non-alcoholic steatohepatitis in obese subjects. Arch Med Res 2005; 36: 362–366. [DOI] [PubMed] [Google Scholar]
- 40.Xu Q, Wang J, Chen F, et al. Protective role of magnesium isoglycyrrhizinate in non-alcoholic fatty liver disease and the associated molecular mechanisms. Int J Mol Med 2016; 38: 275–282. [DOI] [PubMed] [Google Scholar]


