Abstract
Background:
Three different tyrosine kinase inhibitors have been approved as first-line therapies for epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer with similar overall survival. This study determined dynamic changes in quality of life (QoL) for patients using these therapies after controlling for potential confounders.
Methods:
From 2011 to 2016, we prospectively assessed the utility values and QoL scores of patients using the EuroQol five-dimension and World Health Organization Quality-of-Life – Brief questionnaires. QoL functions after initiation of treatment were estimated using a kernel-smoothing method. Dynamic changes in major determinants were repeatedly assessed for constructing mixed models.
Results:
A total of 344 patients were enrolled, with 934 repeated assessments. After controlling for performance status, disease progression, EGFR mutation subtype and other confounders, the mixed models showed significantly lower QoL scores for afatinib versus gefitinib in the physical, psychological and social domains, and 10 facets. The differences seemed to appear 10 months after initiation of treatment. In contrast, there was no significant difference between erlotinib and gefitinib in the scores of all domains and facets.
Conclusion:
QoL in patients receiving afatinib seemed to be lower than in those receiving gefitinib. Since the sample sizes in this study were relatively small, more studies are warranted to corroborate these results.
Keywords: EGFR mutation, non-small-cell lung cancer, quality of life, tyrosine kinase inhibitor
Introduction
Recognition of driver mutations has had a great influence on the management of lung cancer. One of the most important molecular alterations is the epidermal growth factor receptor (EGFR) mutation. In first-line therapy for advanced tumors with sensitizing EGFR mutations, tyrosine kinase inhibitors (TKIs) significantly prolong progression-free survival (PFS) as compared with platinum doublet chemotherapy.1–3
More than half of non-small-cell lung cancer (NSCLC) patients tested for EGFR mutations in Taiwan have shown positive results.4 Three EGFR-TKIs – gefitinib, erlotinib and afatinib – have been approved for first-line therapies for these patients. In a randomized trial conducted by Yang and colleagues,5 erlotinib was found to be similar to gefitinib in terms of PFS and overall survival (OS). No significant difference in OS between afatinib and gefitinib was observed in another study.6 Although afatinib has been found to improve PFS in comparison to gefitinib,7 it seems to be associated with more treatment-related grade 3 diarrhea, rash or acne, mucositis and paronychia.7,8 To move beyond tests of efficacy in randomized trials and determine the comparative effectiveness with real-world evidence,9–11 a long-term follow-up study investigating the effects of these agents on quality of life (QoL) warrants further exploration.
Patients with NSCLC have been shown to experience reduced QoL and emotional functioning.12 To our knowledge, there has been only one study using the EuroQol five-dimension instrument (EQ-5D), a less sensitive questionnaire, to compare the QoL of different EGFR-TKIs.7 The results did not show a significant difference between gefitinib and afatinib. Based on our clinical observation, we hypothesized that dynamic changes in QoL among patients receiving different first-line EGFR-TKIs may differ from each other. By assessing QoL prospectively, this study aimed to compare the effects of three first-line therapies on the QoL of patients with EGFR mutation-positive advanced NSCLC. Moreover, we controlled for potential cofounders by constructing mixed models.
Patients and methods
This study was approved by the Institutional Review Board of National Cheng Kung University Hospital (NCKUH) before commencement (B-ER-105-402), and all participants provided written informed consent.
From May 2011 to December 2016, all EGFR mutation-positive advanced NSCLC patients who visited the outpatient departments of NCKUH were invited to join the study. We verified the diagnosis of EGFR mutation-positive NSCLC with histopathology, cytology and molecular biology. The QIAampTM DNA Mini Kit (Qiagen, Valencia, CA, USA) was used to analyze EGFR mutations of effusion cytology and tissue samples. The inclusion criterion was fully awake consciousness based on Glasgow Coma Scales. Patients with a malignancy at another site or tumor stages I, II and IIIA at the initiation of treatment were excluded, leaving only subjects with recurrent or newly diagnosed advanced NSCLC in the analysis. Each time participants visited the oncology clinics, they were invited to voluntarily complete the QoL questionnaires. Most of their QoL was measured in the first few months, because many patients were hesitant to be repeatedly assessed too many times using the same questionnaires. The repeated assessments were taken at least 2 weeks apart to avoid high collinearity. In general, the oncologists examined these patients every 2–4 weeks when they received EGFR-TKIs. Thus, the process governing the repeated assessments resembled quasi-random sampling.
QoL questionnaires
The study subjects were invited to complete the EQ-5D and World Health Organization Quality-of-Life – Brief (WHOQOL-BREF) questionnaires using tablet computers. An experienced research assistant was available to help the subjects if any questions arose. The EQ-5D questionnaire is a generic, preference-based instrument that is used to estimate the utility values of QoL.13 The five dimensions assessed by the EQ-5D include mobility, self-care, usual activities, pain/discomfort and anxiety/depression, each of which has three levels of severity. Using the scoring function from Taiwan,14 these health state parameters were transformed into a utility value ranging from 0 to 1, in which 0 represented death and 1 indicated full health.
The WHOQOL-BREF questionnaire is a generic psychometric instrument.15 It is sensitive to the QoL of lung cancer patients16 and has good psychometric properties.17 According to the scoring rules provided by WHO,18 each facet was scored from 1 to 5, where a higher score indicated a better QoL. By multiplying the average of the scores of all facets in the same domain by four, a domain score was also calculated. Each domain score ranged from 4 to 20. The score of an omitted facet was replaced with the average score of the other facets in the same domain. The domain score was not calculated when more than two facets were missing from the domain (the social domain only was calculated if ⩽1 facet was missing).
QoL function after therapy
Gefitinib, erlotinib and afatinib have been defined as the standard first-line therapies for EGFR mutation-positive advanced NSCLC.19 Clinically, it takes 5–10 times the half-life20 for an EGFR-TKI to be eliminated after discontinuing the medication. In addition, it takes several days of incubation for any adverse events to occur. Consequently, we assumed QoL assessed within 15 days after the last treatment was related to EGFR-TKIs. To avoid confounding, subjects who received other anti-cancer treatments in combination with first-line EGFR-TKIs were excluded.
For each QoL assessment, the time after treatment was defined as the period between the date of initiation of treatment and the date of assessment. To estimate the QoL function in terms of time, Gaussian kernel-smoothing was applied.21 Namely, for a particular time point t, the estimation of the mean QoL at this time point was the weighted average of QoL assessments, where the weights were determined by a parameter named bandwidth. The bandwidth was set at 0.1 in this study. A bootstrap approach was applied to construct the relevant confidence intervals for the mean function estimations of QoL. Rather than the assessment, the subject was the unit used for bootstrapping. At each time point, a 95% confidence interval was constructed using the 2.5 and 97.5 percentiles of 1000 mean QoL estimates from bootstrapping.
Dynamic changes of confounders
We recorded sex, education, employment, marital status and comorbidities at the initiation of treatment, whereas age was repeatedly calculated at each QoL assessment. The identified comorbidities include cerebrovascular disease, coronary artery disease, chronic obstructive pulmonary disease (COPD), diabetes mellitus and end-stage renal disease. We created a system that automatically abstracted the abovementioned characteristics. In addition, the Eastern Cooperative Oncology Group (ECOG) performance status and state of metastasis at the time of QoL assessment were evaluated from medical records and radiological reports, respectively. Disease progression at the time of QoL assessment was determined through radiographic evidence based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.22
Statistical analysis
For repeated assessments within individual subjects, the determinants of QoL were investigated using a linear mixed model. The utility value of QoL estimated with the EQ-5D and the score for each domain and facet in the WHOQOL-BREF were used as the dependent variables. We included the following variables as predictors/confounders in constructing the statistical models: sex (male versus female), education (⩾12 years versus <12 years), employment (employed versus unemployed), marital status (married versus single/divorced/widowed), comorbidities (without versus with comorbidity), recurrence (recurrent versus newly diagnosed cancer), EGFR mutation subtype (exon 19 deletions versus mutations other than exon 19 deletions), treatment (erlotinib or afatinib versus gefitinib), and age, ECOG performance status (0–1 versus 2–4), brain metastasis (with versus without metastasis), disease progression (with versus without progression) at the time of QoL assessment. A negative coefficient denoted that the variable predicted a worse QoL score, with the magnitude representing the effect.
To test the robustness of our results, we further performed subgroup analyses for participants with common EGFR mutations and participants with newly diagnosed lung cancer only. R version 3.2.3 and the Statistical Analysis System® software version 9.4 (SAS Institute, Cary, NC, USA) were used to perform the analyses. All p values reported were two-sided.
Results
From May 2011 to December 2016, a total of 344 patients receiving gefitinib, erlotinib and afatinib as first-line therapies for EGFR mutation-positive advanced NSCLC participated in the study, for whom 934 QoL assessments were performed. The frequency distribution of the number of QoL assessments per participant is summarized in Supplementary Table 1. Table 1 shows the characteristics of nonparticipants and participants stratified according to treatment. Participants receiving afatinib showed higher proportions with higher levels of education, and harbored exon 19 deletions; those receiving erlotinib had a higher proportion of brain metastases than those in the gefitinib group. The PFS among the three first-line treatments did not differ from one another (see Supplementary Figure 1). However, nonparticipants were older and had a higher proportion of comorbidities and poorer performance status compared with the participants. Their PFS was shorter than that of the participants.
Table 1.
Demographic and clinical characteristics of nonparticipants and participants stratified by treatment.
| Participants |
Non-participants | ||||
|---|---|---|---|---|---|
| Gefitinib | Erlotinib | Afatinib | p value | ||
| Number of subjects, n | 242 | 45 | 57 | 264 | |
| Number of assessments, n | 666 | 121 | 147 | NA | |
| Age,a mean (SD) years | 63.7 (11.2) | 61.9 (12.8) | 60.8 (10.2) | 0.173 | 69.3 (11.6) |
| Male, n (%) | 89 (36.8) | 20 (44.4) | 23 (40.4) | 0.590 | 89 (33.7) |
| Education, n (%) | |||||
| ⩾12 years | 42 (17.4) | 16 (35.6) | 20 (35.1) | 0.002 | NA |
| <12 years | 199 (82.2) | 29 (64.4) | 37 (64.9) | ||
| Missing | 1 (0.4) | 0 | 0 | ||
| Employment, n (%) | |||||
| Employed | 53 (21.9) | 13 (28.9) | 18 (31.6) | 0.187 | NA |
| Unemployed | 189 (78.1) | 32 (71.1) | 37 (64.9) | ||
| Missing | 0 | 0 | 2 (3.5) | ||
| Marital status, n (%) | |||||
| Married | 179 (74.0) | 37 (82.2) | 45 (79.0) | 0.414 | NA |
| Single/divorced/widowed | 63 (26.0) | 8 (17.8) | 12 (21.1) | ||
| Comorbidities, n (%) | |||||
| Cerebrovascular disease | 8 (3.3) | 4 (8.9) | 1 (1.8) | 0.134 | 17 (6.4) |
| Coronary artery disease | 11 (4.6) | 2 (4.4) | 2 (3.5) | 0.942 | 30 (11.4) |
| COPD | 13 (5.4) | 5 (11.1) | 2 (3.5) | 0.229 | 24 (9.1) |
| Diabetes mellitus | 28 (11.6) | 6 (13.3) | 3 (5.3) | 0.321 | 50 (18.9) |
| End-stage renal disease | 10 (4.1) | 1 (2.2) | 2 (3.5) | 0.821 | 12 (4.6) |
| Performance status,a n (%) | |||||
| ECOG: 0–1 | 220 (90.9) | 39 (86.7) | 52 (91.2) | 0.612 | 202 (76.5) |
| ECOG: 2–4 | 21 (8.7) | 6 (13.3) | 5 (8.8) | 52 (19.7) | |
| Missing | 1 (0.4) | 0 | 0 | 10 (3.8) | |
| Disease by recurrence, n (%) | |||||
| Recurrent lung cancer | 46 (19.0) | 9 (20.0) | 12 (21.1) | 0.936 | 31 (11.7) |
| Newly diagnosed cancer | 196 (81.0) | 36 (80.0) | 45 (79.0) | 233 (88.3) | |
| Mutation subtype, n (%) | |||||
| Exon 19 deletions | 98 (40.5) | 18 (40.0) | 30 (52.6) | 0.011 | 119 (45.1) |
| L858R substitution | 127 (52.5) | 26 (57.8) | 18 (31.6) | 119 (45.1) | |
| Other mutations | 17 (7.0) | 1 (2.2) | 9 (15.8) | 26 (9.9) | |
| Brain metastasis,a n (%) | 54 (22.3) | 22 (48.9) | 17 (29.8) | 0.001 | 68 (25.8) |
| PFS, median (IQR) months | 11.4 (7.4–21.7) | 12.8 (6.1–24.7) | 12.3 (7.8–37.1) | 0.541 | 10.0 (5.5–18.2) |
At the initiation of treatment.
COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; NA, not applicable; PFS, progression-free survival; SD, standard deviation.
QoL changes after different treatments
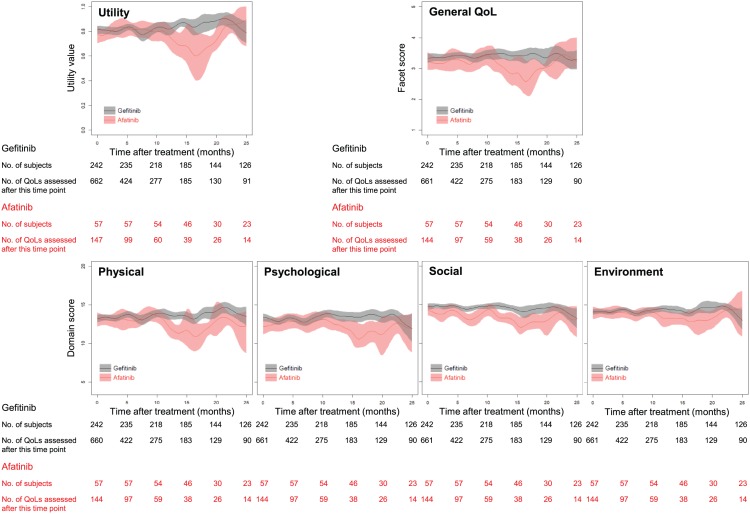
Figure 1 depicts fluctuations of utility values and QoL scores in the four domains after treatment with afatinib versus gefitinib. Compared with gefitinib, the utility value and QoL scores in the physical, psychological and social domains for afatinib were lower about 10 months after treatment. In contrast, the utility value and domain scores for erlotinib did not differ significantly from those for gefitinib (Supplementary Figure 2).
Figure 1.
Fluctuations in utility values and QoL scores in four domains after first-line treatment with afatinib versus gefitinib. The colored shadow illustrates a 95% confidence interval for each function.
QoL, quality of life.
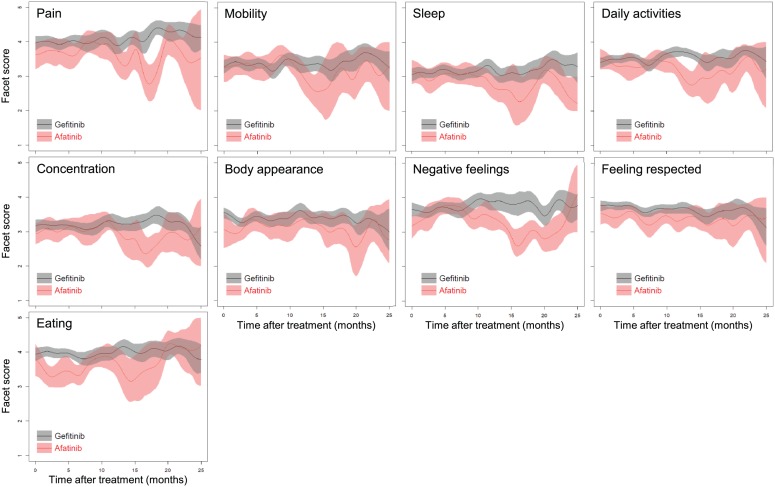
QoL scores in the nine facets after treatment with afatinib versus gefitinib are depicted in Figure 2. Similar to the findings in Figure 1, most facet scores for afatinib were lower than those for gefitinib about 10 months after treatment. The differences in the ‘pain’, ‘body appearance’ and ‘eating’ facets seemed to appear immediately after the initiation of treatment. Score changes after treatment with erlotinib versus gefitinib are shown in Supplementary Figure 3, where patients receiving erlotinib had QoL scores in the nine facets that were similar to those receiving gefitinib.
Figure 2.
Scores changes in nine facets after first-line treatment with afatinib versus gefitinib. The colored shadow illustrates a 95% confidence interval for each function.
Determinants of QoL
Linear mixed models were constructed to explore the determinants of QoL (Table 2). As expected, a good performance status was the most important predictor for increases in utility value and QoL scores in all domains and most facets, whereas disease progression was a predictor of lower utility value and scores. Similar to our prior findings,23 patients with exon 19 deletions had better utility values and QoL scores as compared with mutations other than exon 19 deletions. QoL scores for the psychological and environment domains were increased in male subjects and in those with education ⩾12 years, respectively; COPD and brain metastasis had a negative impact on scores in the physical domain. After controlling for age, sex, education, employment, marital status, comorbidities, performance status, recurrence, EGFR mutation subtype, brain metastasis and disease progression, we found that the QoL scores in the 3 domains and 10 facets related to afatinib were significantly lower than those for gefitinib, including scores for ‘pain’, ‘body appearance’ and ‘eating.’ However, the QoL scores for erlotinib and gefitinib did not differ.
Table 2.
Regression coefficients based on mixed model analyses of the EQ-5D and WHOQOL-BREF.
| Sex (male/female) |
Education (⩾12/<12years) |
COPD (no/yes) |
ECOG (0–1/2–4) |
EGFR (del19+/del19–) |
Brain metastasis (yes/no) |
Disease progression (yes/no) |
Erlotinib versus gefitinib |
Afatinib versus gefitinib |
|
|---|---|---|---|---|---|---|---|---|---|
| EQ-5D: | |||||||||
| Utility value | 0.26 (0.03)c | 0.03 (0.02)a | –0.08 (0.02)c | ||||||
| WHOQOL-BREF: | |||||||||
| General QoL | 0.24 (0.08)b | 0.23 (0.12)a | 0.45 (0.10)c | 0.15 (0.06)a | –0.35 (0.08)c | –0.24 (0.08)b | |||
| Physical | 2.47 (0.35)c | 0.54 (0.22)a | –0.80 (0.25)b | –0.73 (0.30)a | |||||
| Pain | 0.81 (0.14)c | –0.31 (0.12)b | –0.32 (0.11)b | ||||||
| Energy/fatigue | 0.36 (0.14)a | 0.45 (0.12)c | 0.18 (0.08)a | –0.24 (0.09)b | –0.33 (0.09)c | ||||
| Mobility | 1.23 (0.13)c | 0.18 (0.08)a | –0.17 (0.09)a | –0.26 (0.10)b | –0.26 (0.10)b | ||||
| Sleep | 0.21 (0.09)a | 0.46 (0.15)b | –0.23 (0.11)a | ||||||
| Daily activities | 0.87 (0.11)c | 0.20 (0.07)b | –0.25 (0.08)b | –0.23 (0.09)b | |||||
| Psychological | 0.57 (0.26)a | 1.79 (0.36)c | 0.93 (0.24)c | –0.83 (0.25)b | –0.98 (0.32)b | ||||
| Concentration | 0.29 (0.10)b | 0.29 (0.14)a | 0.49 (0.12)c | 0.22 (0.08)b | –0.20 (0.09)a | –0.29 (0.09)b | –0.24 (0.10)a | ||
| Body appearance | 0.26 (0.08)b | 0.28 (0.13)a | 0.20 (0.08)b | –0.24 (0.10)a | –0.33 (0.11)b | ||||
| Negative feelings | 0.36 (0.09)c | 0.40 (0.14)b | 0.33 (0.09)c | –0.22 (0.11)a | –0.30 (0.12)a | ||||
| Social | 0.99 (0.30)b | 0.40 (0.20)a | –0.51 (0.21)a | –1.13 (0.27)c | |||||
| Feeling respected | 0.16 (0.06)a | –0.17 (0.08)a | –0.27 (0.08)b | ||||||
| Environment | 0.67 (0.23)b | 1.11 (0.27)c | 0.41 (0.18)a | –0.62 (0.19)b | |||||
| Financial support | 0.56 (0.11)c | 0.18 (0.09)a | |||||||
| Leisure activities | 0.28 (0.12)a | 0.82 (0.15)c | –0.33 (0.11)b | ||||||
| Eating | 0.36 (0.13)b | –0.30 (0.11)b |
Mixed model analyses adjusted for age, sex, education, employment, marital status, comorbidities, performance status, recurrence, EGFR mutation subtype, brain metastasis, disease progression and treatment. Values in parentheses are standard errors. a p < 0.05; b p < 0.01; c p < 0.001; those with p ⩾ 0.05 are left blank.
COPD, chronic obstructive pulmonary disease; del19, exon 19 deletions; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; EQ-5D, EuroQol five-dimension questionnaire; QoL, quality of life; WHOQOL-BREF, World Health Organization Quality-of-Life – Brief version.
We excluded participants with uncommon EGFR mutations (Supplementary Table 2), and the effects of afatinib were almost the same as before (Supplementary Table 3). Supplementary Tables 4 and 5 show the subgroup analysis of participants with newly diagnosed lung cancer, where the effects became less significant, although a negative impact for afatinib was still observed.
Discussion
Similar to a previous report,7 a lower utility value measured with the EQ-5D for patients receiving afatinib as compared with gefitinib was not detected. However, we did find significantly lower QoL scores in 3 domains and 10 facets of the WHOQOL-BREF on patients treated with afatinib versus gefitinib after controlling for potential confounders using mixed models (Table 2). The dynamic changes in QoL functions after initiation of afatinib also revealed lower utility values and scores approximately 10 months after treatment (Figures 1 and 2). While these findings do not necessarily indicate a causal association, we have the following arguments to hypothesize that such a relationship may exist: first, this study was conducted prospectively, was limited to subjects with EGFR mutations, QoL was assessed prior to the elimination of EGFR-TKIs, and patients influenced by other therapies were excluded. Our findings of lower QoL scores in the afatinib group thus cannot be explained by the preceding factors. Second, the mixed models showed statistically significant effects of the following predictors on QoL scores and corroborated previous reports: performance status,24 disease progression,25 exon 19 deletions,23 sex,24 education,26 COPD27 and metastasis.25 These findings partially validate our statistical models. Third, participants receiving afatinib were generally younger in age, had higher educational levels, proportions of employment and exon 19 deletions, and had lower proportions of various comorbidities than those receiving gefitinib (Table 1). They were expected to show better QoL scores for most items, but Figures 1 and 2 appear to indicate the opposite. When we reanalyzed the data limited to assessments performed 10 months after treatment (n = 59 and 275 for afatinib and gefitinib, respectively, in Figure 1), the trends were even more consistent. In other words, the poorer QoL scores cannot be explained by factors other than afatinib. Finally, with a slightly smaller sample size of patients receiving erlotinib than that receiving afatinib, we were unable to detect any facet or domain with statistically lower QoL scores in patients receiving erlotinib as compared with gefitinib. Since the frequencies and severities of adverse events of afatinib were reported to be higher than those for other EGFR-TKIs in previous studies,28,29 we tentatively concluded that our follow-up studies consistently showed afatinib to be associated with poorer QoL.
A larger proportion of participants receiving afatinib harbored uncommon EGFR mutations (Table 1), which might have led to lower QoL scores in these patients and may thus have confounded the results. Nevertheless, after excluding participants with uncommon EGFR mutations (Supplementary Tables 2 and 3), the association between afatinib and poorer QoL scores still exists. We further performed a subgroup analysis by excluding recurrent lung cancer to test the robustness of our results (Supplementary Tables 4 and 5). Because of the reduction in sample size, some of the effects of afatinib on QoL scores became insignificant. However, the directions of the effects remained the same.
Why many QoL scores for afatinib were lower than those for gefitinib about 10 months after treatment remains unclear. Because afatinib is considered to be an irreversible tyrosine kinase blockade, its associated adverse events would tend to be more severe and persistent for a longer period of time than those associated with gefitinib. Figure 2 indicates that facet scores for pain, body appearance and eating appeared to be lower for patients receiving afatinib at the initiation of treatment, which were consistent with the more severe paronychia, folliculitis and mucositis related to afatinib in the first months. The lower QoL scores were followed by a persistent or aggravated trend after 10 months compared with gefitinib. Additional symptoms of insomnia accompanied with poor concentration, mobility and daily activities further affected negative psychological feelings. These findings appear to correspond well with clinical observations. Although many patients dropped out of the analysis after 10 months of treatment, 95% confidence intervals for QoL scores of afatinib still lie outside those for gefitinib. Namely, the effects still existed given a reduced amount of QoL assessments after this time point.
Several limitations must be acknowledged in this study: first, although the process governing the repeated QoL assessments resembled quasi-random sampling, each assessment was not taken at a predefined period. Nonetheless, because most patients were still taking pills at the time of the QoL assessments (919 of 934 assessments), the QoL differences among these three groups of patients should not be confounded by time after treatments. Future studies assessing QoL at predefined periods would be helpful to verify the exact time when QoL begins to deteriorate. Second, the EQ-5D and WHOQOL-BREF were applied to assess QoL, both of which are generic questionnaires and do not include lung cancer-specific items such as cough or dyspnea. Although a disease-specific instrument is capable of detecting more symptoms unique to lung cancer, our results showing worse QoL scores in 3 domains and 10 facets in participants receiving afatinib would not be biased. Instead, these tools provide an opportunity to detect common QoL impairments among lung cancer patients. Future studies exploring QoL changes by condition-specific instruments for different treatments are indicated to test the same hypothesis. Third, since this study was observational in nature, we were unable to control the dosage and frequency in each EGFR-TKI. Nonetheless, because the patients’ QoL scores were measured by a self-report, and there was no prior knowledge in patients receiving different treatments, this concern would not threaten the validity of this study. Finally, a limited number of subjects were recruited in this single-center study, so one still must be cautious before generalizing the results to all patients receiving different first-line EGFR-TKIs.
In conclusion, patients receiving afatinib as a first-line therapy for EGFR mutation-positive advanced NSCLC showed worse QoL scores in most domains and many facets in comparison with patients taking gefitinib. QoL scores for erlotinib did not differ significantly from those for gefitinib. The findings appear to correspond with clinically observable persistent adverse events resulting from afatinib as an irreversible tyrosine kinase blockade. We recommend more studies be performed to corroborate our results and hypothesis. Clinicians caring such patients might consider incorporating this piece of information into shared decision-making.
Supplemental Material
Supplemental material, Ther_Adv_Med_Oncol_revision_supplement_marked1 for Dynamic changes in quality of life after three first-line therapies for EGFR mutation-positive advanced non-small-cell lung cancer by Szu-Chun Yang, Chien-Chung Lin, Wu-Wei Lai, Sheng-Mao Chang, Jing-Shiang Hwang, Wu-Chou Su and Jung-Der Wang in Therapeutic Advances in Medical Oncology
Acknowledgments
We are indebted to Yau-Lin Tseng, Yi-Ting Yen, Wen-Ping Su, Shang-Yin Wu, Yu-Ming Yeh and Cheng-Hung Lee for their generous support with the recruitment of subjects. This study is based in part on data from the Cancer Data Bank of National Cheng Kung University Hospital.
Footnotes
Funding: This study was supported by grants from the National Cheng Kung University Hospital (NCKUH-10606015 to Szu-Chun Yang) and the Ministry of Health and Welfare (MOHW106-TDU-B-211-144004 to Wu-Chou Su).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplementary material accompanying this article can be found in the online version.
ORCID iD: Jung-Der Wang  https://orcid.org/0000-0002-3176-4500
https://orcid.org/0000-0002-3176-4500
Contributor Information
Szu-Chun Yang, Department of Internal Medicine, National Cheng Kung University Hospital and Department of Public Health, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Chien-Chung Lin, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Wu-Wei Lai, Department of Surgery, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Sheng-Mao Chang, Department of Statistics, College of Management, National Cheng Kung University, Tainan, Taiwan.
Jing-Shiang Hwang, Institute of Statistical Science, Academia Sinica, Taipei, Taiwan.
Wu-Chou Su, Department of Internal Medicine, National Cheng Kung University Hospital, 138 Sheng-Li Road, Tainan 704, Taiwan.
Jung-Der Wang, Department of Internal Medicine, National Cheng Kung University Hospital and Department of Public Health, College of Medicine, National Cheng Kung University, 1 University Road, Tainan 701, Taiwan.
References
- 1. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- 2. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 3. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 4. Yatabe Y, Kerr KM, Utomo A, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol 2015; 10: 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang JJ, Zhou Q, Yan HH, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer 2017; 116: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017; 28: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomized controlled trial. Lancet Oncol 2016; 17: 577–589. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Sheng J, Yang Y, et al. Optimized selection of three major EGFR-TKIs in advanced EGFR-positive non-small cell lung cancer: a network meta-analysis. Oncotarget 2016; 7: 20093–20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cahill J, Learner N. Managed care pharmacy sees potential of comparative effectiveness research to improve patient care and lower costs. Pharmacoeconomics 2010; 28: 931–944. [DOI] [PubMed] [Google Scholar]
- 10. Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf 2017; 26: 1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jarow JP, LaVange L, Woodcock J. Multidimensional evidence generation and FDA regulatory decision making: defining and using “real-world” data. JAMA 2017; 318: 703–704. [DOI] [PubMed] [Google Scholar]
- 12. Chabowski M, Polański J, Mazur G, et al. Sociodemographic and clinical determinants of quality of life of patients with non-small cell lung cancer. Adv Exp Med Biol. Epub ahead of print 2 June 2017. DOI: 10.1007/5584_2017_36. [DOI] [PubMed] [Google Scholar]
- 13. Szende A, Oppe M, Devlin N. EQ-5D value sets: inventory, comparative review and user guide. Dordrecht: Springer, 2007. [Google Scholar]
- 14. Lee HY, Hung MC, Hu FC, et al. Estimating quality weights for EQ-5D (EuroQol-5 dimensions) health states with the time trade-off method in Taiwan. J Formos Med Assoc 2013; 112: 699–706. [DOI] [PubMed] [Google Scholar]
- 15. Yao G, Chung CW, Yu CF, et al. Development and verification of validity and reliability of the WHOQOL-BREF Taiwan version. J Formos Med Assoc 2002; 101: 342–351. [PubMed] [Google Scholar]
- 16. Yang SC, Lai WW, Hsiue TR, et al. Health-related quality of life after first-line anti-cancer treatments for advanced non-small cell lung cancer in clinical practice. Qual Life Res 2016; 25: 1441–1449. [DOI] [PubMed] [Google Scholar]
- 17. Lin CY, Yang SC, Lai WW, et al. Rasch models suggested the satisfactory psychometric properties of the World Health Organization Quality of Life-Brief among lung cancer patients. J Health Psychol 2017; 22: 1–12. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. WHOQOL-BREF: introduction, administration, scoring, and generic version of the assessment, field trial version. Geneva: World Health Organization, 1996. [Google Scholar]
- 19. Greenhalgh J, Dwan K, Boland A, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev 2016; 25: CD010383. [DOI] [PubMed] [Google Scholar]
- 20. Birkett DJ. Pocket guide: pharmacokinetics made easy. 2nd ed. New York: McGraw-Hill, 2011. [Google Scholar]
- 21. Hwang JS, Wang JD. Integrating health profile with survival for quality of life assessment. Qual Life Res 2004; 13: 1–10. [DOI] [PubMed] [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 23. Yang SC, Wang JD. Better quality-of-life in patients with exon 19 deletion receiving first-line tyrosine kinase inhibitors for advanced non-small-cell lung cancer. Eur Respir J 2017; 50: PA4235. [Google Scholar]
- 24. Zimmermann C, Burman D, Swami N, et al. Determinants of quality of life in patients with advanced cancer. Support Care Cancer 2011; 19: 621–629. [DOI] [PubMed] [Google Scholar]
- 25. Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol 2013; 8: 997–1003. [DOI] [PubMed] [Google Scholar]
- 26. Lee LJ, Chung CW, Chang YY, et al. Comparison of the quality of life between patients with non-small-cell lung cancer and healthy controls. Qual Life Res 2011; 20: 415–423. [DOI] [PubMed] [Google Scholar]
- 27. Wang JW, Gong XH, Ding N, et al. The influence of comorbid chronic diseases and physical activity on quality of life in lung cancer survivors. Support Care Cancer 2015; 23: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 28. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2015; 88: 74–79. [DOI] [PubMed] [Google Scholar]
- 29. Passaro A, Di Maio M, Del Signore E, et al. Management of nonhematologic toxicities associated with different EGFR-TKIs in advanced NSCLC: a comparison analysis. Clin Lung Cancer 2014; 15: 307–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Ther_Adv_Med_Oncol_revision_supplement_marked1 for Dynamic changes in quality of life after three first-line therapies for EGFR mutation-positive advanced non-small-cell lung cancer by Szu-Chun Yang, Chien-Chung Lin, Wu-Wei Lai, Sheng-Mao Chang, Jing-Shiang Hwang, Wu-Chou Su and Jung-Der Wang in Therapeutic Advances in Medical Oncology