Abstract
Insulin coordinates the complex response to feeding, affecting numerous metabolic and hormonal pathways. Forkhead box protein O1 (FoxO1) is one of several signaling molecules downstream of insulin; FoxO1 drives gluconeogenesis and is suppressed by insulin. To determine the role of FoxO1 in mediating other actions of insulin, we studied mice with hepatic deletion of the insulin receptor, FoxO1, or both. We found that mice with deletion of the insulin receptor alone showed not only hyperglycemia but also a 70% decrease in plasma insulin-like growth factor 1 and delayed growth during the first 2 months of life, a 24-fold increase in the soluble leptin receptor and a 19-fold increase in plasma leptin levels. Deletion of the insulin receptor also produced derangements in fatty acid metabolism, with a decrease in the expression of the lipogenic enzymes, hepatic diglycerides, and plasma triglycerides; in parallel, it increased expression of the fatty acid oxidation enzymes. Mice with deletion of both insulin receptor and FoxO1 showed a much more modest phenotype, with normal or near-normal glucose levels, growth, leptin levels, hepatic diglycerides, and fatty acid oxidation gene expression; however, lipogenic gene expression remained low. Taken together, these data reveal the pervasive role of FoxO1 in mediating the effects of insulin on not only glucose metabolism but also other hormonal signaling pathways and even some aspects of lipid metabolism.
Foxo1 regulates transcriptional programs to drive gluconeogenesis and fatty acid oxidation while modulating the IGF1 and leptin axes.
Insulin plays a central role in coordinating the transition from fasting to feeding. It suppresses the production of glucose and ketones, stimulates lipogenesis and cholesterol synthesis, and also appears to promote growth and fertility (1). Both insulin deficiency, which occurs in type 1 diabetes, and insulin resistance, which occurs in type 2 diabetes, result in profound metabolic defects that can produce severe morbidity and mortality.
Insulin exerts its effects by binding to the insulin receptor and activating its tyrosine kinase activity. This, in turn, leads to the activation of a complex signaling cascade with multiple branches (1). In the canonical pathway, insulin receptor phosphorylates the insulin receptor substrate (IRS) proteins, which activate phosphoinositide 3-kinase, which in turn, activates Akt. Akt activates mammalian target of rapamycin complex 1 and suppresses glycogen synthase kinase 3 and forkhead box protein O1 (FoxO1). Each of these signaling molecules has an important function: mammalian target of rapamycin complex 1 promotes protein and lipid synthesis, glycogen synthase kinase 3 inhibits glycogen synthesis, and FoxO1 drives gluconeogenesis. However, the full complement of targets downstream of each of these signals and the extent to which they overlap with one another are not clear.
Liver insulin receptor knockout (LIRKO) mice highlight the importance of hepatic insulin signaling in maintaining whole-body homeostasis (2–4). In addition to hyperglycemia and hyperinsulinemia (5, 6), LIRKO mice show derangements in other hormonal axes (2, 7), as well as reduced levels of lipogenic gene expression, lipogenesis, and plasma triglycerides (TGs) (4). FoxO1 is critical to the hyperglycemic phenotype of LIRKO mice, as the deletion of hepatic FoxO1 is sufficient to restore normal glucose tolerance in LIRKO mice (5, 6). Deletion of hepatic FoxO1 also normalizes glucose homeostasis in mice that are insulin resistant as a result of deletion of the IRS proteins (8), the Akt proteins (9), or other dietary and genetic insults (10). In addition to driving gluconeogenesis, FoxO1 has been reported to regulate lipid metabolism and growth pathways (5, 8, 11–14).
To explore further the role of FoxO1 in mediating the effects of insulin, we studied mice with deletion of the insulin receptor, FoxO1, or both in their livers. We found that FoxO1 is required for the development of not only hyperglycemia and hyperinsulinemia but also derangements in the leptin and insulin-like growth factor 1 (IGF1) axes, the hepatic lipidome, and the expression of the genes required for fatty acid oxidation. On the other hand, the ability of FoxO1 deletion to rescue lipogenic gene expression was modest.
Materials and Methods
Animals
Liver-specific knockout (KO) mice were generated by crossing mice harboring floxed alleles of the insulin receptor (Insr) (2) and/or FoxO1 (15) with mice harboring a transgene encoding the Cre recombinase under the albumin promoter. All mice were maintained on a C57BL/6 background. Unless otherwise indicated, we used male mice fed a standard chow diet ad libitum and euthanized in the nonfasted state at 1400 hours. All animal experiments were performed with the approval of the Institutional Animal Care and Research Advisory Committee at Boston Children’s Hospital.
Liver Western blotting
Livers were homogenized in radioimmunoprecipitation assay buffer, and 50 µg lysate was loaded onto sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels. Blots were blocked in SuperBlock buffer (Thermo Scientific), incubated overnight with primary antibody (Table 1), and detected with secondary antibody conjugated with horseradish peroxidase.
Table 1.
Antibodies Used for Western Blot Staining
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer Providing the Antibody, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Insulin receptor | Unknown | Insulin Rβ | Santa Cruz Biotechnology, sc-711 | Rabbit; polyclonal | 1:1000 | AB_631835 |
| Forkhead box protein O1 | Unknown | FOXO1 (C29H4) | Cell Signaling Technology, 2880 | Rabbit; monoclonal | 1:1000 | AB_2106495 |
| β-Actin | Unknown | β-Actin (C-4) | Santa Cruz Biotechnology, sc-47778 | Mouse; monoclonal | 1:1000 | AB_2714189 |
Abbreviation: RRID, Research Resource Identifier.
Glucose tolerance testing
Mice were fasted for 14 hours overnight and then given 1 g glucose/kg body weight by intraperitoneal injection. Blood glucose levels were monitored via tail nick at 0, 15, 30, 60, 90, and 120 minutes after glucose injection.
Gene expression analysis/real-time PCR
RNA was isolated from frozen livers using Trizol (Life Technologies). cDNA was synthesized using a reverse transcription kit (Applied Biosystems), and used for real-time polymerase chain reaction (PCR) analysis with SYBR Green (Life Technologies). Gene expression was normalized to TATA-box binding protein.
Plasma chemistry and metabolic measurements
Plasma samples collected at the time of euthanasia were used in the following enzyme-linked immunosorbent assays (ELISAs): insulin (Crystal Chem), leptin (Crystal Chem), C-peptide (Alpco), soluble leptin receptor (MyBioSource), or IGF1 (R&D Systems). Plasma TGs were measured using a colorimetric assay (Infinity), according to the manufacturer’s instructions.
Hepatic lipids
Livers were homogenized in 50 mM NaCl, and lipid was extracted with chloroform:methanol (2:1, volume to volume ratio). TGs were measured using a colorimetric assay (Infinity), as previously described (4).
Body length measurements
Mouse body length was recorded between 4 and 9 weeks of age by measuring the length from the snout to the anus.
Liquid chromatography–tandem mass spectometry analysis
Liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis was performed on a single cohort of male mice, using Orbitrap Q-Exactive (Thermo Fisher Scientific) coupled to UltiMate 3000 ultra-high-performance LC (Thermo Fisher Scientific). A 500-μg protein aliquot of liver homogenate was used to extract glycerolipids, glycerophospholipids, and sphingolipids (16). Lipid extracts, along with spiked-in internal standards (Supplemental Table 1 (279.5KB, pdf) ), were used for LC-MS/MS analysis, and individual peaks were identified by the algorithm LipidSearch 4.1sp (Thermo Fisher Scientific). The ceramide (Cer) species [Cer (d34:1)] was identified as a C16:0 Cer, based on the MS/MS fragmentation pattern. Normalization was performed using spiked-in standards for the specified lipid classes.
Statistical analysis
Data are represented by the mean ± standard error of the mean (SEM), unless otherwise indicated. Significance was assessed by a two-tailed Student t test with unequal variance. Comparisons between KOs and controls were defined as significant only if P < 0.05 for both the KO vs the pooled controls and the KO vs its specific littermate Cre-negative control, unless otherwise indicated. Data are representative of two or three independent cohorts, unless otherwise indicated.
Please see Supplemental Information (279.5KB, pdf) for additional methods.
Results
Mice were generated using a transgene encoding the Cre recombinase under the albumin promoter and floxed alleles of the insulin receptor (Insr) or FoxO1. FOXO1 protein was not detected in the livers of mice with hepatocyte-specific deletion of FoxO1 (LFKO) or both the insulin receptor and FoxO1 (LDKO); insulin receptor was not detected in the livers of LIRKO or LDKO mice [Fig. 1(a)]. We also studied the littermate floxed controls of the LFKO, LIRKO, and LDKO mice. As these mice were generally phenotypically similar, they were pooled for analysis and labeled as “controls.”
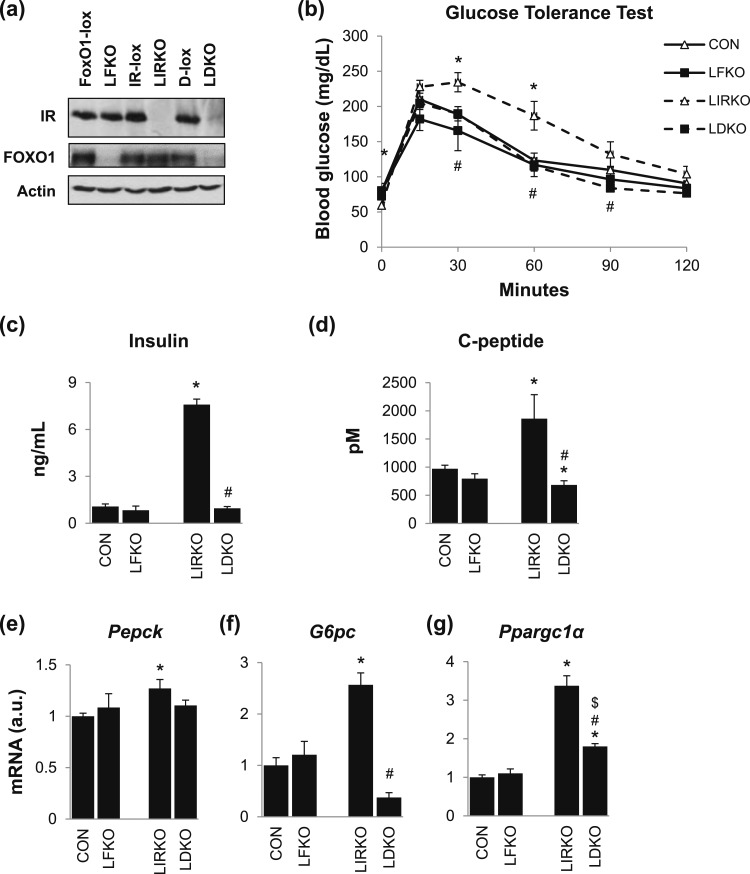
Figure 1.
FoxO1 is required for the hyperinsulinemia and hyperglycemia observed in LIRKO mice. Male LFKO, LIRKO, and LDKO mice and their Cre-negative littermate controls [FoxO1-lox, insulin receptor (IR)-lox, and D-lox or when pooled together, controls (CON)] were maintained on a chow diet and euthanized in the nonfasted state at 8 to 10 weeks of age. (a) Western blots were performed on liver homogenates. (b) Glucose tolerance testing was performed at 6 to 8 weeks of age. (c) Plasma insulin and (d) C-peptide levels were measured by ELISA in samples taken at the time of euthanasia. (e–g) Hepatic expression of gluconeogenic genes was measured using real-time PCR. Error bars represent SEM; n = 6 to 10. *P < 0.05 vs control mice; #P < 0.05 LIRKO vs LDKO; $P < 0.05 LFKO vs LDKO. G6pc, glucose 6-phosphatase; Pepck, phosphoenolpyruvate carboxykinase; Ppargc1α, peroxisome proliferator–activated receptor γ, coactivator 1 α.
LFKO mice showed no changes in glucose tolerance, plasma insulin and C-peptide levels, or hepatic gluconeogenic gene expression [Fig. 1(b)–1(g)]. LIRKO mice, on the other hand, showed profound derangements in glucose metabolism: severe hyperglycemia, hyperinsulinemia, increased levels of plasma C-peptide, increased hepatic levels of the gluconeogenic enzymes glucose 6-phosphatase (G6pc) and phosphoenolpyruvate carboxykinase (Pepck), and increased levels of the transcriptional coactivator peroxisome proliferator–activated receptor γ, coactivator 1 α (Ppargc1α). Interestingly, all of these parameters were normalized or nearly normalized in LDKO mice.
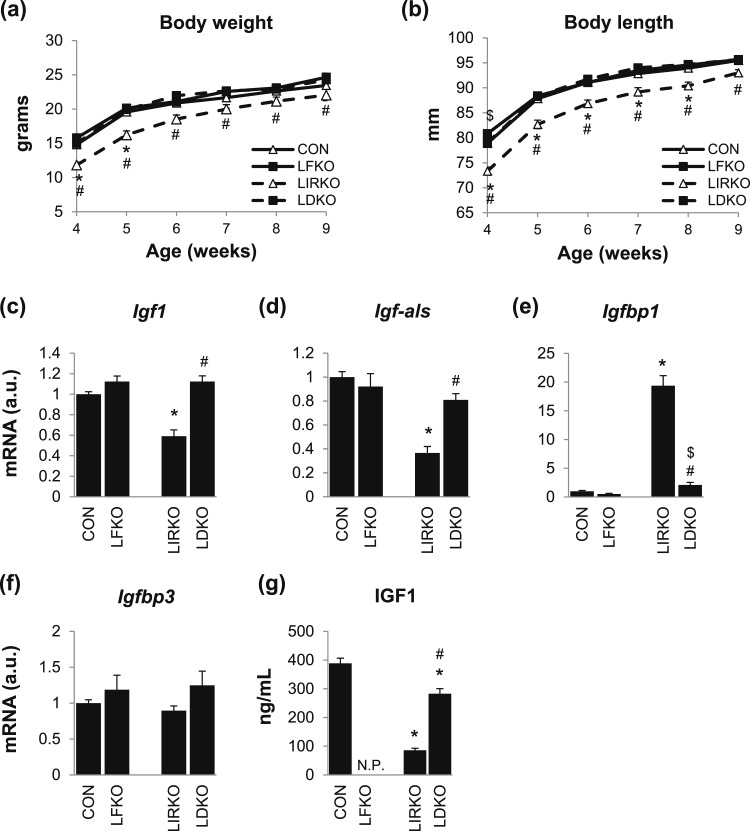
LIRKO mice showed a substantial decrease in body weight and length at 4 weeks of age [Fig. 2(a) and 2(b)]. By 9 weeks of age, these defects had resolved, but several derangements in the IGF1 axis were nonetheless observed: Igf1 and IGF-binding protein (IGFBP), acid labile subunit (Igf-als), which promote growth, were decreased 40% to 60%; Igfbp1, which inhibits IGF1 signaling, was increased 19-fold; and Igfbp3, which stabilizes IGF1, was unchanged [Fig. 2(c)–2(f)]. Plasma IGF1 levels were reduced >70% at both 4 (data not shown) and 9 weeks of age [Fig. 2(g)]. Again, all of these parameters were normalized or nearly normalized in LDKO mice.
Figure 2.
FoxO1 is required for the growth retardation observed in LIRKO mice. (a) Body weights and (b) body lengths of male LFKO, LIRKO, LDKO, and control mice were measured from 4 to 9 weeks of age. (c–f) Gene expression in the liver and (g) IGF1 in the plasma were measured in 8- to 10-week-old male mice using real-time PCR and an ELISA assay. Error bars represent SEM; n = 6 to 10. *P < 0.05 vs control mice; #P < 0.05 LIRKO vs LDKO; $P < 0.05 LFKO vs LDKO. N.P., not performed.
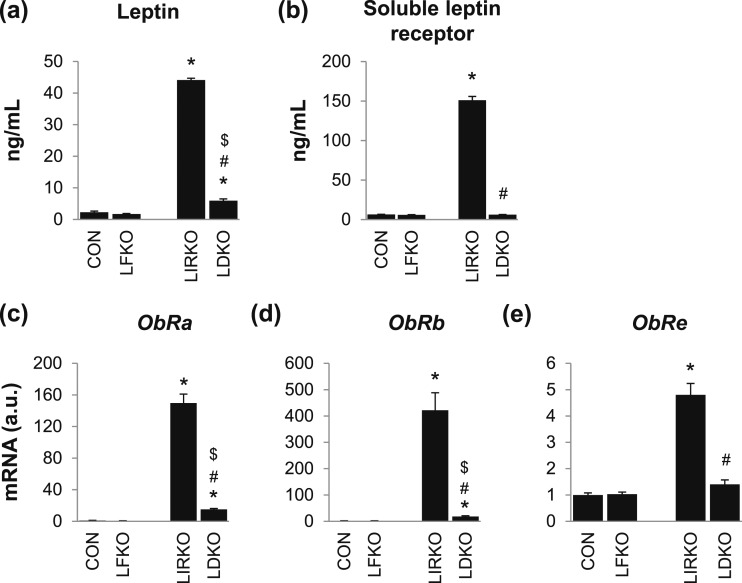
LIRKO mice showed a 20-fold increase in plasma leptin [Fig. 3(a)]. This was associated with a 20-fold increase in plasma levels of the soluble leptin receptor [Fig. 3(b)]. The soluble leptin receptor is the major leptin-binding protein in plasma and appears to regulate the bioavailability of leptin (17). The soluble leptin receptor contains the extracellular domain of the leptin receptor. It can be generated by ectodomain cleavage of either the long isoform of the leptin receptor (ObRb, which contains the extracellular domain, transmembrane domain, and cytoplasmic domain), or the short isoforms of the leptin receptor (such as ObRa, which contains the extracellular domain and transmembrane domain but not cytoplasmic domain) (18–20). The soluble leptin receptor can also arise from ObRe, which lacks both the cytoplasmic and transmembrane domains and is secreted directly into the plasma. LIRKO mice showed a 150-fold increase in ObRa, a 400-fold increase in ObRb, and a 5-fold increase in ObRe [Fig. 3(c)–3(e)]. In LDKO mice, plasma leptin and soluble leptin receptor as well as expression of ObRa, ObRb, and ObRe were significantly reduced compared with LIRKO mice, although plasma leptin, ObRa, and ObRb remained elevated compared with controls (Fig. 3). In contrast, LFKO mice showed no substantial derangements in the leptin axis.
Figure 3.
FoxO1 is required for the hyperleptinemia observed in LIRKO mice. Male mice were euthanized at 8 to 10 weeks of age. (a) Leptin and (b) the soluble leptin receptor were measured by ELISA in the plasma, and (c–e) gene expression was measured by real-time PCR in the liver. Error bars represent SEM; n = 6 to 10. *P < 0.05 vs control mice; #P < 0.05 LIRKO vs LDKO; $P < 0.05 LFKO vs LDKO. ObRa, ObRb, and ObRe, leptin receptor isoforms.
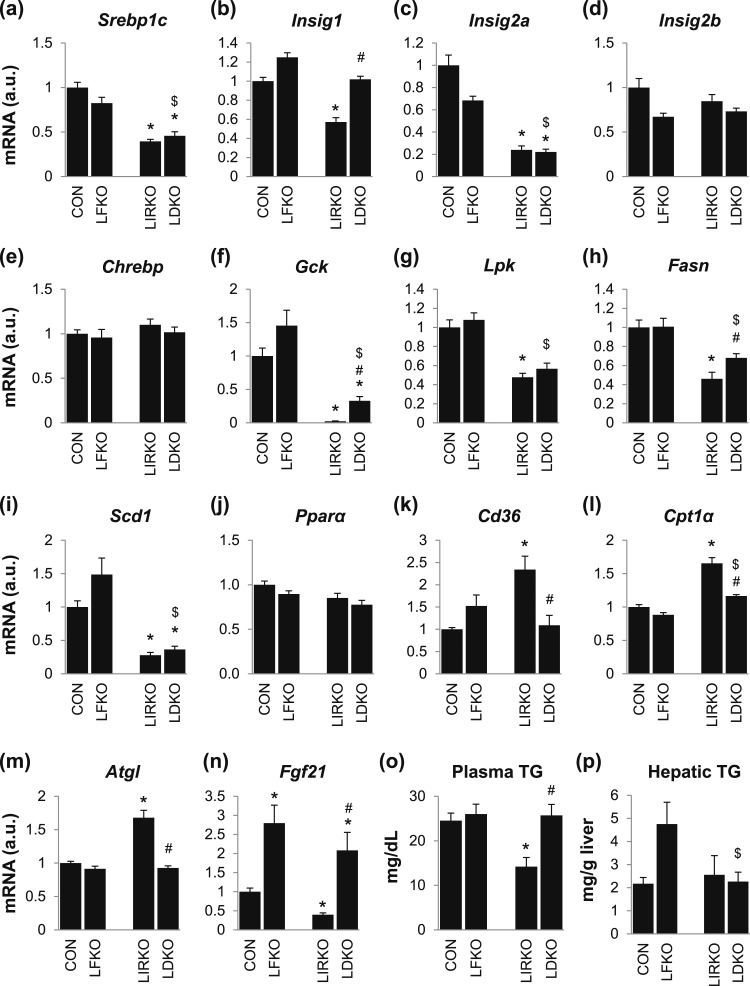
Insulin plays a key role in the regulation of lipid metabolism, stimulating lipogenesis and suppressing fatty acid oxidation. The insulin-stimulated lipogenic transcription factor, sterol regulatory element–binding protein 1c (Srebp1c), was reduced by 50% in LIRKO livers. However, the insulin-induced gene (Insig) transcripts, which encode proteins that inhibit the maturation of SREBP1C (21, 22), were suppressed, with Insig1 and Insig2a reduced by 40% and 80%, respectively, and Insig2b unchanged [Fig. 4(a)–4(d)]. The carbohydrate-stimulated lipogenic transcription factor, carbohydrate response element-binding protein (Chrebp), was not altered [Fig. 4(e)]. Nonetheless, the genes encoding the lipogenic enzymes glucokinase (Gck), pyruvate kinase (Lpk), fatty acid synthase (Fasn), and stearoyl-coenzyme A (CoA) desaturase 1 (Scd1) were reduced 50% to 90% [Fig. 4(f)–4(i)].
Figure 4.
FoxO1 is required for some of the defects in lipid metabolism observed in LIRKO mice. (a–n) Hepatic gene expression, (o) plasma TG, and (p) hepatic TG were measured in male mice euthanized at 8 to 10 weeks of age. Error bars represent SEM; n = 6 to 10. *P < 0.05 vs control mice; #P < 0.05 LIRKO vs LDKO; $P < 0.05 LFKO vs LDKO. Cd36, cluster of differentiation 36; Pparα, peroxisome proliferator–activated receptor α.
The genes involved in the uptake and oxidation of fatty acids—cluster of differentiation (Cd36), adipose TG lipase (Atgl), and carnitine palmitoyltransferase 1α (Cpt1α)—were increased in LIRKO livers, even though Pparα levels were not changed [Fig. 4(j)–4(m)]. On the other hand, fibroblast growth factor 21 (Fgf21) was suppressed by 60% in LIRKO livers [Fig. 4(n)]. These changes in gene expression were associated with a 50% reduction in TG secretion and a 40% reduction in plasma TGs but no change in hepatic TGs [Fig. 4(o) and 4(p); Supplemental Fig. 1 (279.5KB, pdf) ].
The deletion of FoxO1 differentially affected the genes involved in lipogenesis vs fatty acid oxidation. Although Gck and Fasn were increased in LDKO vs LIRKO, all of the lipogenic genes (Srebp1c, Lpk, and Scd1, as well as Gck and Fasn) remained 30% to 80% lower in LDKO mice than controls [Fig. 4(a)–4(i)]. On the other hand, Cd36, Atgl, and Cpt1α were normalized in the livers of LDKO mice, and Fgf21 was increased in the livers of both LDKO and LFKO mice compared with controls [Fig. 4(k)–4(n)]. These changes in gene expression were associated with a slight increase in TG secretion in LDKO compared with LIRKO mice, normalization of plasma TGs, and no change in hepatic TGs [Fig. 4(o) and 4(p); Supplemental Fig. 1 (279.5KB, pdf) ].
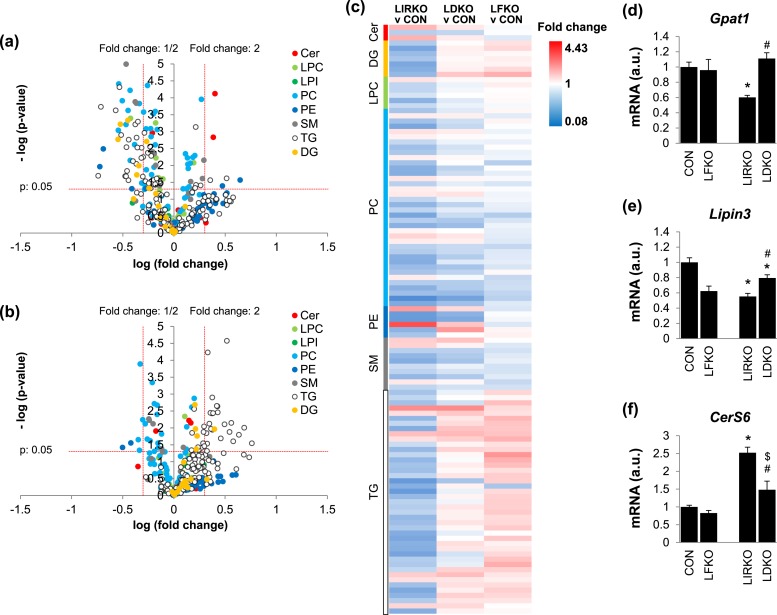
We next performed lipidomic analysis in LFKO, LIRKO, and LDKO mice. In LIRKO livers, the total TG, Cer, phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphingomyelin (SM) content was normal (data not shown). However, the diglyceride (DG) content was reduced by almost 50% (Supplemental Fig. 2 (279.5KB, pdf) ), with five DG species decreased by >50%: DG (34:3), DG (36:4), DG (40:8), DG (51:0), and DG (53:3) [Fig. 5(a) and 5(c); Supplemental Table 2 (279.5KB, pdf) ]. In addition, two Cer species were increased more than twofold: Cer (d34:1) and Cer (d41:1). Finally, there were marked changes in the lipid composition; for example, LIRKO livers were enriched in TG (52:1) and TG (54:2), even though total TG levels did not change [Fig. 5(c); Supplemental Table 2 (279.5KB, pdf) ].
Figure 5.
FoxO1 is required for many of the derangements in the hepatic lipidome observed in LIRKO mice. The livers of 8- to 10-week-old male mice were subjected to (a–c) lipidomics and (d–f) real-time PCR. Hepatic lipid species in (a) LIRKO and (b) LDKO compared with control mice. The horizontal dotted line represents P = 0.05. Vertical lines represent a fold change of 0.5 or 2. (c) Heat map showing fold change for each lipid species in LIRKO, LDKO, and LFKO livers relative to controls. Only species that were significantly different in LIRKO vs controls are shown (P < 0.05); n = 5 to 15. (d–f) Hepatic gene expression is shown. Error bars represent SEM; n = 6 to 10. *P < 0.05 vs control mice; #P < 0.05 LIRKO vs LDKO; $P < 0.05 LFKO vs LDKO. LPC, lysophosphatidylcholine.
Many of the changes in lipid content and composition observed in LIRKO mice were lost in LDKO mice [Fig. 5(b) and 5(c); Supplemental Table 2 (279.5KB, pdf) ]. First, none of the DG species measured was found to be significantly reduced in LDKO mice. Second, the increases in Cer (d34:1) and Cer (d41:1) observed in LIRKO livers were attenuated in LDKO livers. And, finally, most of the changes in PC species observed in LIRKO mice were restored toward normal in LDKO mice, with only PC (38:2), PC (42:10), and PC (42:6) still significantly suppressed by >50%. On the other hand, LDKO mice showed a marked change in TG species composition [Fig. 5(c); Supplemental Table 2 (279.5KB, pdf) ].
In parallel, we measured expression of 16 genes involved in TG, SM, Cer, PC, and PE metabolism in the livers of LIRKO mice and their floxed controls using real-time PCR (Supplemental Fig. 3 (279.5KB, pdf) ). Several enzymes were significantly decreased: glycerol-3-phosphate acyltransferase 1 (Gpat1), which catalyzes the first step in glycerolipid synthesis, was reduced 40%; Lipin3, which converts phosphatidic acid to DG, was reduced 45%; diacylglycerol O-acyltransferase 2 (Dgat2), which converts DG to TG, was decreased 38%; and PE N-methyltransferase (Pemt), which converts PE to PC, was reduced 27%. On the other hand, phosphate cytidyltransferase 1β (Pcyt1β), which catalyzes the rate-limiting step of PC synthesis, was increased 1.5-fold; choline/ethanolamine phosphotransferase 1 (Cept1), which generates PC from DG, was increased 1.2-fold; SM synthase 2 (Sgms2), which catalyzes SM synthesis, was increased 1.2-fold; and Cer synthase S6 (CerS6), which promotes the synthesis of long-chain C16:0 ceramides, such as Cer (d34:1), was increased 2.5-fold.
Of these, only Gpat1, Lipin3, and CerS6 were significantly and consistently changed in LDKO vs LIRKO mice: Gpat1 and CerS6 were largely restored to normal in LDKO livers, whereas Lipin3 levels were only partially restored [Fig. 5(d)–5(f)].
Discussion
Here, we show that FoxO1 is required for most of the phenotypic defects observed in LIRKO mice. These data are consistent with a role for FoxO1 as a key driver of the fasting response: it promotes gluconeogenesis and fatty acid oxidation, while interfering with growth and the IGF1 axis.
IGF1 is a major regulator of somatic growth. IGF1 is secreted by the liver in response to growth hormone. In the plasma, IGF1 circulates in a ternary complex with IGF-ALS and IGFBP3 before reaching its target cells (23). The ability of IGF1 to activate its receptor is further modulated by other IGFBPs, some of which appear to inhibit IGF1 action, such as IGFBP1. Insulin promotes growth by inducing IGF1 and IGF-ALS and suppressing IGFBP1. Thus, patients with type 1 diabetes in poor glycemic control show low serum IGF1, increased IGFBP1, and growth impairment (24–26).
At 4 weeks of age, LIRKO mice show reduced body length and weight. At 9 weeks of age, LIRKO mice show reduced Igf1 and Igf-als expression, increased Igfbp1 expression, and reduced plasma IGF1 levels. However, body weight and length are normal. The fact that LIRKO mice ultimately reach normal adult size, despite these derangements in the IGF1 axis, points to the existence of compensatory mechanisms (27).
In any case, the defects in IGF1 signaling and growth retardation observed in LIRKO mice are almost entirely normalized by the hepatic deletion of FoxO1. Likewise, mice with a deletion of the IRS signaling node show growth delay and defects in the IGF1 axis that resolve with concurrent deletion of FoxO1 (8). These data indicate that FoxO1 suppresses IGF1 and growth in the absence of normal insulin signaling.
Leptin is a major regulator of appetite, energy expenditure, and metabolism. Leptin’s primary effects are centrally mediated (28). The role of the soluble leptin receptor in modulating leptin’s effect is not clear: the soluble leptin receptor reduces leptin clearance from the circulation (17, 19), and overexpression of the soluble leptin receptor reduces body weight and increases energy expenditure in mice (19, 29); on the other hand, soluble leptin receptor reduces leptin transport across the blood-brain barrier (30) and impairs leptin signaling in vitro (31). LIRKO mice show a marked increase in the soluble leptin receptor and hyperleptinemia, which are largely restored in LDKO mice, consistent with the fact that the leptin receptor promoter contains FoxO1-binding sites (7).
Insulin also plays a fundamental role in the control of lipid and glucose metabolism. LIRKO livers show dramatic changes in the PC and TG species composition, a near 50% reduction in DG content, and an increase in CerS6 and Cer (d34:1). Interestingly, the deletion of FoxO1 in LIRKO mice normalized most of these changes, indicating that FoxO1 may have a broader effect on lipid metabolism than previously expected.
The derangements in Cer and DGs are of particular interest, as both are elevated in the livers of mice and humans with obesity and type 2 diabetes and closely correlated with insulin resistance (32). This has led some to suggest that these lipids impair insulin signaling and thereby produce insulin resistance and hyperglycemia (32–35). However, determining which of these molecules, if either, is a cause rather than a consequence of insulin resistance has been difficult.
Of the DG and Cer species previously reported to be increased in insulin-resistant mice and humans (35, 36), three were significantly changed in LIRKO livers: Cer (d34:1), DG (34:1), and DG (36:4). Cer (d34:1) was previously shown to be increased in the livers of obese mice, whereas DG (34:1) and DG (36:4) were shown to be increased in the livers of obese humans (35, 36). The fact that Cer (d34:1) is increased in LIRKO livers indicates that insulin suppresses Cer (d34:1) and suggests that the increase in Cer (d34:1) observed in obese livers is, at least in part, a consequence of insulin resistance. On the other hand, the fact that DG (34:1) and DG (36:4) are reduced in LIRKO livers indicates that the increased levels of DG (34:1) and DG (36:4) observed in obese livers are unlikely to be a consequence of defective insulin signaling. Instead, increased levels of these DG species must be causal to, and/or develop in parallel with, insulin resistance.
In the fasted state, adipocytes release free fatty acids, which are taken up by the liver and oxidized. The oxidation of fatty acids is necessary for ketone generation. It also drives gluconeogenesis, both by diverting pyruvate into the gluconeogenic pathway and by providing the ATP necessary to fuel gluconeogenesis. Insulin suppresses adipose tissue lipolysis and fatty acid oxidation. Consistent with this, LIRKO mice show an increase in the expression of the genes required for the uptake of fatty acids from the plasma (Cd36), the release of fatty acids from intracellular lipid droplets (Atgl), and the translocation of fatty acids into the mitochondria where they can be oxidized (Cpt1α). LIRKO mice also show a reduction in Gpat1. GPAT1 catalyzes the acylation of glycerol-3-phosphate, the first step in TG synthesis, and thereby competes with CPT1α for acyl-CoA (37, 38). In LDKO livers, the expression of all of these genes is normal. These data are consistent with a model in which FoxO1 triggers a transcriptional program to increase free fatty acid availability, as well as their channeling toward oxidation through CPT1α.
In contrast, Fgf21 shows a distinct pattern of regulation; it is increased in LFKO livers, consistent with prior reports (39), as well as in LDKO livers. This is interesting for several reasons. First, FGF21 is considered a fasting hormone and is induced by the transcription factor PPARα. However, in contrast to Cpt1α, another PPARα target, Fgf21 is decreased in LIRKO mice. Second, Fgf21 is the only gene in this study that was perturbed by the deletion of FoxO1 alone. These data reveal Fgf21 to be a unique target of FoxO1. An important remaining question is the physiological impact of this regulation. Although pharmacological doses of FGF21 stimulate lipolysis (40), the role of endogenous FGF21 on lipolysis is less clear, and at least under some conditions, FGF21 may suppress adipose tissue lipolysis (41–43). Thus, FoxO1 could potentially activate lipolysis by inhibiting FGF21.
The ability of hepatic FoxO1 to modulate adipose lipolysis may underlie its ability to control hepatic gluconeogenesis indirectly (5, 6, 44). Deletion of FoxO1 in the livers of mice with defective hepatic insulin signaling somehow restores the ability of insulin to control hepatic glucose production, revealing a noncanonical, indirect pathway by which FoxO1 may drive gluconeogenesis (9). It has been suggested that in this indirect pathway, FoxO1 in the liver promotes adipose tissue lipolysis and the flux of free fatty acids to the liver. The increase in free fatty acid flux would drive fatty acid oxidation and thereby, promote gluconeogenesis (5, 9, 44). How hepatic FoxO1 exerts this effect on the adipose tissue is not known. As discussed previously, FGF21 is one potential mediator. IGF1 and leptin are other potential mediators, as they appear to be normalized in LDKO mice and can cross talk with the insulin receptor at the level of the adipocyte to regulate lipolysis (45, 46); in addition, leptin can act centrally to improve insulin sensitivity.
In our studies, the only abnormality of LIRKO mice not corrected by deletion of FoxO1 is the defect in lipogenesis. LIRKO mice show a decrease in the lipogenic transcription factor Srebp1c and its targets, Gck, Fasn, and Scd1. Insulin induces SREBP1C by increasing its transcription and promoting its cleavage (47). That is, SREBP1C is synthesized as a membrane-bound precursor, which must undergo proteolytic cleavage to generate its active, nuclear form (47). This cleavage is inhibited by the INSIG proteins, which are encoded by two genes (21, 22). INSIG1 is under the control of the SREBPs: SREBP promotes transcription of Insig1 as part of an inhibitory feedback loop (48). INSIG2 is encoded by two transcripts (21, 22). Insig2a is the major Insig2 transcript in fasted livers and is suppressed by feeding and insulin treatment (21, 22). However, Insig2a is reduced in LIRKO and LDKO mice, as well as mice with deletion of the Akt signaling node (4, 49, 50). Taken together, these data indicate that Insig2a transcription is not directly regulated by hepatic insulin/Akt/Foxo1 signaling. However, the post-transcriptional regulation of INSIG2 does appear to require hepatic insulin signaling, as INSIG2 protein levels are increased in LIRKO livers (4).
Srebp1c, Gck, Fasn, and Scd1 remain low in LDKO mice. These data are consistent with the notion that insulin induces SREBP1C independently of FoxO1 (50). Recent studies have shown FoxO1 to be important for the induction of Gck transcription in the early refeeding period (51, 52). However, it should be noted that in the early refeeding period, SREBP1C, which is a key regulator of Gck, is not yet fully active (53). Our studies in nonfasted mice show that the deletion of FoxO1 in the context of normal insulin signaling (LFKO) has no effect on Gck and that the deletion of FoxO1 in LIRKO mice (LDKO) produces only a modest induction of Gck. These data suggest that in contrast to what is observed in early refeeding, the role of FoxO1 on Gck under normal fed conditions may be more limited.
We also find that although Chrebp levels are normal in LIRKO livers, the ChREBP target, Lpk, is reduced. This could be a result of the crosstalk between ChREBP and SREBP1C mediated by GCK: GCK is induced by SREBP1C and is, in turn, required for the generation of the glycolytic products necessary for the activation of ChREBP (54).
In summary, FoxO1 plays a unique, nonredundant role in mediating the effects of insulin. Mice with deletion of both insulin receptor and FoxO1 in their livers appear to manifest a particularly beneficial phenotype: these mice show normal or near-normal glucose homeostasis, hepatic lipid content, and IGF1 and leptin signaling. However, they are unable to induce hepatic Srebp1c, an important driver of lipogenesis and potentially hyperlipidemia and steatosis.
Acknowledgments
We thank Drs. Robert Farese, Jr. and Tobias Walther (Harvard T. H. Chan School of Public Health, Boston, MA) for support with the LC-MS/MS measurements and analysis.
Financial Support: This work was funded by an American Heart Association Predoctoral Fellowship and Established Investigator Award (to A.V.L. and S.B.B., respectively) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK094162 (to S.B.B.). Z.W.L. is supported by National Institutes of Health Grant R01 DK101579 and Mather’s Foundation.
Author Contributions: A.V.L., M.E.G., I.S., D-J.S., and R.C. performed the experiments and analyzed the data. Z.W.L. performed the MS run and helped with the lipid extraction and data analysis. I.S. and S.B.B. wrote and edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATGL
- adipose triglyceride lipase
- Cer
- ceramide
- CerS6
- ceramide synthase S6
- CHREBP
- carbohydrate response element-binding protein
- CoA
- coenzyme A
- CON
- pooled Cre-negative Flox controls
- CPT1α
- carnitine palmitoyltransferase 1α
- DG
- diglyceride
- ELISA
- enzyme-linked immunosorbent assay
- FASN
- fatty acid synthase
- FGF21
- fibroblast growth factor 21
- FOXO1
- forkhead box protein O1
- GCK
- glucokinase
- GPAT
- glycerol-3-phosphate acyltransferase
- IGF
- insulin-like growth factor
- IGF-ALS
- insulin-like growth factor-binding protein, acid labile subunit
- IGFBP
- insulin-like growth factor-binding protein
- INSIG
- insulin-induced gene
- IRS
- insulin receptor substrate
- KO
- knockout
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- LDKO
- liver double (forkhead box protein O1 and insulin receptor) knockout
- LFKO
- liver forkhead box protein O1 knockout
- LIRKO
- liver insulin receptor knockout
- LPK
- pyruvate kinase
- PC
- phosphatidylcholine
- PCR
- polymerase chain reaction
- PE
- phosphatidylethanolamine
- PPARGC1α
- peroxisome proliferator–activated receptor γ, coactivator 1α
- SCD1
- stearoyl-coenzyme A desaturase 1
- SEM
- standard error of the mean
- SM
- sphingomyelin
- SREBP1C
- sterol regulatory element–binding protein 1c
- TG
- triglyceride.
References
- 1.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68(1):123–158. [DOI] [PubMed] [Google Scholar]
- 2.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6(1):87–97. [PubMed] [Google Scholar]
- 3.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7(2):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas JT, Miao J, Chanda D, Wang Y, Zhao E, Haas ME, Hirschey M, Vaitheesvaran B, Farese RV Jr, Kurland IJ, Graham M, Crooke R, Foufelle F, Biddinger SB. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 2012;15(6):873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titchenell PM, Chu Q, Monks BR, Birnbaum MJ. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nat Commun. 2015;6:7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O-Sullivan I, Zhang W, Wasserman DH, Liew CW, Liu J, Paik J, DePinho RA, Stolz DB, Kahn CR, Schwartz MW, Unterman TG. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat Commun. 2015;6:7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen SE, Kokkotou E, Biddinger SB, Kondo T, Gebhardt R, Kratzsch J, Mantzoros CS, Kahn CR. High circulating leptin receptors with normal leptin sensitivity in liver-specific insulin receptor knock-out (LIRKO) mice. J Biol Chem. 2007; 282(32):23672–23678 [DOI] [PubMed] [Google Scholar]
- 8.Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueki K, Kahn CR, Birnbaum MJ. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18(3):388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel VT, Choi CS, Phillips TG, Romanelli AJ, Geisler JG, Bhanot S, McKay R, Monia B, Shutter JR, Lindberg RA, Shulman GI, Veniant MM. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes. 2006;55(7):2042–2050. [DOI] [PubMed] [Google Scholar]
- 11.Wan M, Leavens KF, Saleh D, Easton RM, Guertin DA, Peterson TR, Kaestner KH, Sabatini DM, Birnbaum MJ. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab. 2011;14(4):516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeusler RA, Kaestner KH, Accili D. FoxOs function synergistically to promote glucose production. J Biol Chem. 2010;285(46):35245–35248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haeusler RA, Pratt-Hyatt M, Welch CL, Klaassen CD, Accili D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 2012;15(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang K, Li L, Qi Y, Zhu X, Gan B, DePinho RA, Averitt T, Guo S. Hepatic suppression of Foxo1 and Foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology. 2012;153(2):631–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128(2):309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. [DOI] [PubMed] [Google Scholar]
- 17.Zastrow O, Seidel B, Kiess W, Thiery J, Keller E, Böttner A, Kratzsch J. The soluble leptin receptor is crucial for leptin action: evidence from clinical and experimental data. Int J Obes Relat Metab Disord. 2003;27(12):1472–1478. [DOI] [PubMed] [Google Scholar]
- 18.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–1271. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Wang Z, Li C. Modulation of circulating leptin levels by its soluble receptor. J Biol Chem. 2001;276(9):6343–6349. [DOI] [PubMed] [Google Scholar]
- 20.Maamra M, Bidlingmaier M, Postel-Vinay MC, Wu Z, Strasburger CJ, Ross RJ. Generation of human soluble leptin receptor by proteolytic cleavage of membrane-anchored receptors. Endocrinology. 2001;142(10):4389–4393. [DOI] [PubMed] [Google Scholar]
- 21.Yabe D, Brown MS, Goldstein JL. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc Natl Acad Sci USA. 2002;99(20):12753–12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Natl Acad Sci USA. 2003;100(6):3155–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166(2):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halldin MU, Hagenäs L, Tuvemo T, Gustafsson J. Profound changes in the GH-IGF-I system in adolescent girls with IDDM: can IGFBP1 be used to reflect overall glucose regulation? Pediatr Diabetes. 2000;1(3):121–130. [DOI] [PubMed] [Google Scholar]
- 25.Massa G, Dooms L, Bouillon R, Vanderschueren-Lodeweyckx M. Serum levels of growth hormone-binding protein and insulin-like growth factor I in children and adolescents with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993;36(3):239–243. [DOI] [PubMed] [Google Scholar]
- 26.Raisingani M, Preneet B, Kohn B, Yakar S. Skeletal growth and bone mineral acquisition in type 1 diabetic children; abnormalities of the GH/IGF-1 axis. Growth Horm IGF Res. 2017;34:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96(13):7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108(8):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lou PH, Yang G, Huang L, Cui Y, Pourbahrami T, Radda GK, Li C, Han W. Reduced body weight and increased energy expenditure in transgenic mice over-expressing soluble leptin receptor. PLoS One. 2010;5(7):e11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol. 2008;214(2):301–305. [DOI] [PubMed] [Google Scholar]
- 31.Yang G, Ge H, Boucher A, Yu X, Li C. Modulation of direct leptin signaling by soluble leptin receptor. Mol Endocrinol. 2004;18(6):1354–1362. [DOI] [PubMed] [Google Scholar]
- 32.Petersen MC, Shulman GI. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol Sci. 2017;38(7):649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luukkonen PK, Zhou Y, Sädevirta S, Leivonen M, Arola J, Orešič M, Hyötyläinen T, Yki-Järvinen H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64(5):1167–1175. [DOI] [PubMed] [Google Scholar]
- 34.Magkos F, Su X, Bradley D, Fabbrini E, Conte C, Eagon JC, Varela JE, Brunt EM, Patterson BW, Klein S. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology. 2012;142(7):1444–1446.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Brönneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Blüher M, Krönke M, Brüning JC. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20(4):678–686. [DOI] [PubMed] [Google Scholar]
- 36.Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, Petersen KF, Samuel VT, Shulman GI. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA. 2011;108(39):16381–16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wendel AA, Cooper DE, Ilkayeva OR, Muoio DM, Coleman RA. Glycerol-3-phosphate acyltransferase (GPAT)-1, but not GPAT4, incorporates newly synthesized fatty acids into triacylglycerol and diminishes fatty acid oxidation. J Biol Chem. 2013;288(38):27299–27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wendel AA, Lewin TM, Coleman RA. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim Biophys Acta. 2009;1791(6):501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haeusler RA, Han S, Accili D. Hepatic FoxO1 ablation exacerbates lipid abnormalities during hyperglycemia. J Biol Chem. 2010;285(35):26861–26868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415–425. [DOI] [PubMed] [Google Scholar]
- 41.Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150(10):4625–4633. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Ge H, Weiszmann J, Hecht R, Li YS, Véniant MM, Xu J, Wu X, Lindberg R, Li Y. Inhibition of lipolysis may contribute to the acute regulation of plasma FFA and glucose by FGF21 in ob/ob mice. FEBS Lett. 2009;583(19):3230–3234. [DOI] [PubMed] [Google Scholar]
- 43.Yang C, Wang C, Ye M, Jin C, He W, Wang F, McKeehan WL, Luo Y. Control of lipid metabolism by adipocyte FGFR1-mediated adipohepatic communication during hepatic stress. Nutr Metab (Lond). 2012;9(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, Ruan HB, Yang X, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolinder J, Lindblad A, Engfeldt P, Arner P. Studies of acute effects of insulin-like growth factors I and II in human fat cells. J Clin Endocrinol Metab. 1987;65(4):732–737. [DOI] [PubMed] [Google Scholar]
- 46.Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, Chin WW, Cusin I, Rohner-Jeanrenaud F, Burger AG, Zapf J, Meier CA. Direct effects of leptin on brown and white adipose tissue. J Clin Invest. 1997;100(11):2858–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horton JD. Sterol regulatory element-binding proteins: transcriptional activators of lipid synthesis. Biochem Soc Trans. 2002;30(6):1091–1095. [DOI] [PubMed] [Google Scholar]
- 48.Attie AD. Insig: a significant integrator of nutrient and hormonal signals. J Clin Invest. 2004;113(8):1112–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miao J, Haas JT, Manthena P, Wang Y, Zhao E, Vaitheesvaran B, Kurland IJ, Biddinger SB. Hepatic insulin receptor deficiency impairs the SREBP-2 response to feeding and statins. J Lipid Res. 2014;55(4):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Titchenell PM, Quinn WJ, Lu M, Chu Q, Lu W, Li C, Chen H, Monks BR, Chen J, Rabinowitz JD, Birnbaum MJ. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metab. 2016;23(6):1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haeusler RA, Hartil K, Vaitheesvaran B, Arrieta-Cruz I, Knight CM, Cook JR, Kammoun HL, Febbraio MA, Gutierrez-Juarez R, Kurland IJ, Accili D. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nat Commun. 2014;5:5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langlet F, Haeusler RA, Linden D, Ericson E, Norris T, Johansson A, Cook JR, Aizawa K, Wang L, Buettner C, Accili D. Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell. 2017;171(4):824–835.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci USA. 1998;95(11):5987–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dentin R, Pégorier JP, Benhamed F, Foufelle F, Ferré P, Fauveau V, Magnuson MA, Girard J, Postic C. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004;279(19):20314–20326. [DOI] [PubMed] [Google Scholar]