Abstract
Retrograde signaling is essential for neuronal growth, function and survival; however, we know little about how signaling endosomes might be directed from synaptic terminals onto retrograde axonal pathways. We have identified Khc-73, a plus-end directed microtubule motor protein, as a regulator of sorting of endosomes in Drosophila larval motor neurons. The number of synaptic boutons and the amount of neurotransmitter release at the Khc-73 mutant larval neuromuscular junction (NMJ) are normal, but we find a significant decrease in the number of presynaptic release sites. This defect in Khc-73 mutant larvae can be genetically enhanced by a partial genetic loss of Bone Morphogenic Protein (BMP) signaling or suppressed by activation of BMP signaling in motoneurons. Consistently, activation of BMP signaling that normally enhances the accumulation of phosphorylated form of BMP transcription factor Mad in the nuclei, can be suppressed by genetic removal of Khc-73. Using a number of assays including live imaging in larval motor neurons, we show that loss of Khc-73 curbs the ability of retrograde-bound endosomes to leave the synaptic area and join the retrograde axonal pathway. Our findings identify Khc-73 as a regulator of endosomal traffic at the synapse and modulator of retrograde BMP signaling in motoneurons.
Author summary
Retrograde axonal transport is essential for normal synaptic function and neuronal survival. How endosomes are specifically sorted from the synaptic terminal for retrograde axonal transport is currently not known. At the Drosophila neuromuscular junction, receptors for the Bone Morphogenic Protein signaling pathway are transported from the synapse to the neuron cell body for the proper establishment of synaptic growth and function of motoneurons. Using this system we demonstrate that a kinesin motor protein, Khc-73, is necessary for the efficient sorting of retrograde bound vesicles to the retrograde transport route.
Introduction
Bidirectional communication between the neuronal cell body and distant synaptic terminals is essential for synapse formation, plasticity and neuronal survival [1, 2]. This is achieved primarily through highly regulated axonal transport. Anterograde transport is mediated by plus-end directed kinesin motor proteins that deliver synaptic vesicles and newly synthesized proteins to the synapse, while retrograde transport of cargo destined for the cell body, such as activated receptor complexes, is accomplished by dynein protein complexes [1, 3–5]. Kinesin and dynein motors are also required for endosomal traffic within the cell. The coordinated action of anterograde and retrograde motors ensures the proper sorting and delivery of signaling complexes, proteins and organelles [6]. Although defects in endosomal traffic and axonal transport have been associated with a number of nervous system diseases including Charcot-Marie-Tooth disease, Amyotrophic Lateral Sclerosis, Huntington’s disease and Parkinson’s disease, we know little about how signaling endosomes are routed from the synapse to the retrograde pathway [7–13]. Retrograde signaling has been extensively studied at the Drosophila larval neuromuscular junction (NMJ). In particular the Bone Morphogenic Protein signaling pathway (BMP) has been identified as a major regulator of synaptic growth and function. As such, many regulators of synaptic endosomal sorting have been identified in the regulation of BMP signals at synaptic terminals. Nevertheless, how activated receptors are preferentially sorted to travel to the nucleus is currently unknown.
The movement of endosomes within the cytoplasm is directed through the actions of microtubule binding proteins such as minus end dynein motors, and plus end directed kinesins. Co-ordination and competition between these opposing motors for endosome cargoes regulates the transport of proteins to their correct targets [1, 4, 14–18]. In this study, we have discovered a surprising role for the plus-end directed microtubule motor protein Khc-73 in retrograde sorting of signaling vesicles at the Drosophila larval NMJ.
Khc-73 and its vertebrate homolog KIF13B/GAKIN are kinesin 3 motor protein family members with multiple protein domains and diverse roles in both vertebrates and invertebrates [15, 19–32]. At its N-terminal, Khc-73 contains a kinesin motor necessary for its association with microtubules and plus-end directed transport to synaptic terminals, and at its C-terminal, a Cytoskeletal Associated Protein GLYcine rich (CAP-GLY) domain that provides microtubule association properties [30, 32]. In the nervous system, through microtubule cytoskeleton interactions, both KIF13B and Khc-73 have been shown to participate in mechanisms that control neuronal polarity: Khc-73 has a role in spindle orientation in neuroblasts [30], and KIF13B is involved in the establishment of axonal structures in post-mitotic neurons [20]. Interestingly, KIF13B/Khc-73 has been implicated in the regulation of endosomal dynamics [33, 34] and axonal transport [15] through interaction with Rab5-GTPases. Our previous findings suggested that Khc-73, under strong inhibitory control of the microRNA miR-310-313 cluster in motoneurons at the Drosophila NMJ, plays an important role in the regulation of synaptic function by influencing presynaptic neurotransmitter release [35].
In order to investigate the mechanism of action of Khc-73, we have generated loss of function deletions in Khc-73 gene in D. melanogaster and examined the motoneurons of third instar larvae. While the number of synaptic boutons at the NMJ and the amount of neurotransmitter release per action potential are unaffected in Khc-73 mutant larvae, we find a small but significant decrease in the number of presynaptic release sites. Our experiments indicate the presence of Khc-73 function in BMP signaling by demonstrating a strong genetic interaction between Khc-73 and members of the BMP signaling pathway. We further show that activation of retrograde BMP signaling that normally leads to accumulation of pMad in the nuclei of motoneurons is significantly suppressed when Khc-73 is genetically removed. Our findings suggest that Khc-73 exerts its function by influencing the sorting of endosomes at the NMJ and promoting retrograde routing of endosomes. Our findings identify, for the first time, a plus-end directed microtubule motor protein as a regulator of retrograde signaling in motoneurons.
Results
Khc-73 loss of function mutant
Khc-73 is a member of the KIF superfamily of kinesin motor proteins and the homologue of the vertebrate KIF13B/GAKIN (S1A Fig) [30, 35]. We have previously shown that Khc-73 is a target of the micro RNA miR-310-313 cluster in motoneurons. We found that loss of the miR-310-313 cluster led to abnormally enhanced neurotransmitter release at the NMJ; this enhancement was fully reversed to wild type levels as a result of neuronal knock down of Khc-73 [35]. In order to investigate the role of Khc-73 in more detail, we generated deletions in the Khc-73 gene by imprecise excision of a P-element transposon insert in the vicinity of the 5’ UTR of Khc-73 (S1B Fig). We isolated two deletion flies Khc-73149 and Khc-73193 missing portions of the Khc-73 5’UTR and the ATG start (S1B Fig); we also isolated a fly where the P-element was excised precisely leaving the entire genetic region of Khc-73 intact (Khc-73100) (S1B Fig). Our western blot analysis with an antibody against the C-terminal end of Khc-73 indicates that both Khc-73149 and Khc-73193 are protein null alleles (S1C Fig). Khc-73 is maternally expressed and is expressed in the embryo [32]. We examined the expression pattern of Khc-73 protein in motoneurons with transgenic overexpression, since we were not able to detect a specific signal using our antibody against Khc-73 in larval preparations. We overexpressed HA-tagged Khc-73 transgene in motor neurons and detected punctate accumulation of HA-Khc-73 both in axons and in synaptic boutons at the NMJ (S1D and S1E Fig). In addition, we tested transcriptional activity of Khc-73 by generating a Khc-73-Gal4 fly (containing 4kb of Khc-73 genomic sequence driving Gal4 expression, see methods). Crossing this fly to UAS-mCD8-GFP transgene led to the expression of GFP in nearly all neurons including motor neurons, suggesting that Khc-73 transcription is active in all motor neurons in third instar larvae (S1F and S1G Fig). We also found Khc-73 transcription widely expressed in the brain of adult flies (S1H Fig).
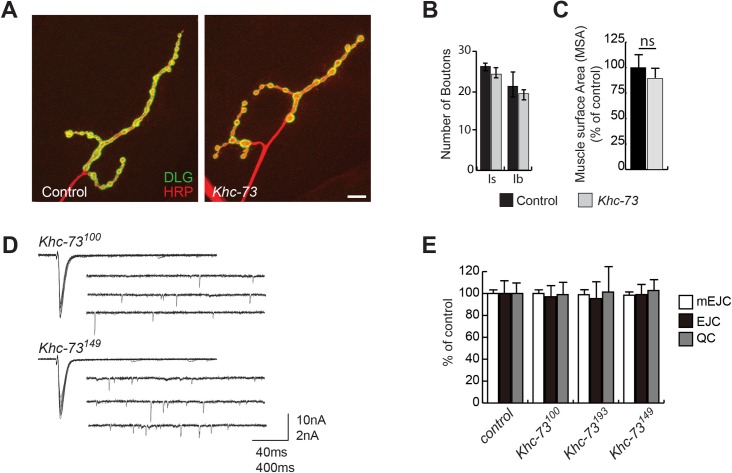
Based on the previously published roles for Khc-73 in neuroblasts, endosomal sorting, axon morphology and synaptic function [21, 29, 30, 34–36], we expected loss of Khc-73 to cause significant defects in the normal synaptic function and/or structure. To our surprise, we found only mild defects (S2E and S2F Fig) in Khc-73 mutant larvae in our assessment of gross synaptic structure at the larval NMJ. The number of synaptic boutons and the muscle surface area (MSA) at NMJs were not significantly different comparing Khc-73 mutant and control larvae; this was true for muscle 4 NMJs (Fig 1A–1C) as well as muscle 6/7 NMJs (S1I and S1J Fig).
Fig 1. Khc-73 mutants have normal synaptic structure and function.
(A) Khc-73 synapse structure at muscle 4. Postsynaptic Dlg stain (green) and presynaptic neuron HRP stain (red). Control (Khc-73100). Scale bar is 10μm. (B) Quantification of bouton number in Control (Khc-73100) and Khc-73 (Khc-73149) mutants at muscle 4 n = 17, 18 NMJs. (C) Muscle surface area of muscle 4 in Control (Khc-73100) and Khc-73 (Khc-73149). Muscle 4 n = 17, 18. (D) Representative traces of EJCs and mEJCs from third instar larval NMJ in precise excision Khc-73100 (top) and Khc-73 mutant (Khc-73149) (bottom). (E) Quantification of mEJC, EJC and QC for control w1118, Khc-73100, Khc-73193 and Khc-73149. N = 9, 14, 7 and 11 NMJs. Error Bars are SEM. Student’s t-test. ns-no statistical significance.
To test whether loss of Khc-73 might affect synaptic function, we examined the baseline electrophysiological properties including miniature excitatory postsynaptic currents (mEPSCs), evoked excitatory postsynaptic currents (EPSCs) and quantal content (QC) and found no differences between Khc-73 mutants and wild type larvae (Fig 1D and 1E). Similarly, we tested synaptic vesicle recycling dynamics in Khc-73 mutant NMJs with high frequency stimulation and found no significant difference in the decay of the synaptic response compared to controls (S1K and S1L Fig). Consistent with the lack of defects in baseline synaptic function, we found no significant changes in the fluorescent intensity of the synaptic vesicle calcium sensor synaptotagmin (SYT), synaptic vesicle marker cysteine string protein (CSP) or synaptic vesicle recycling protein Epidermal growth factor receptor pathway substrate clone 15 (EPS15) in Khc-73 mutant larvae (S2A–S2D Fig). We found a mild reduction in the staining intensity for the postsynaptic marker Discs large (Dlg) (S2E and S2F Fig) but no differences in the expression level of postsynaptic glutamate receptor subunit A (GluRIIA) (S2G Fig).
Altogether these findings indicate that synaptic growth and function are largely normal in Khc-73 mutant.
Khc-73 mutant larvae have reduced number of presynaptic release sites
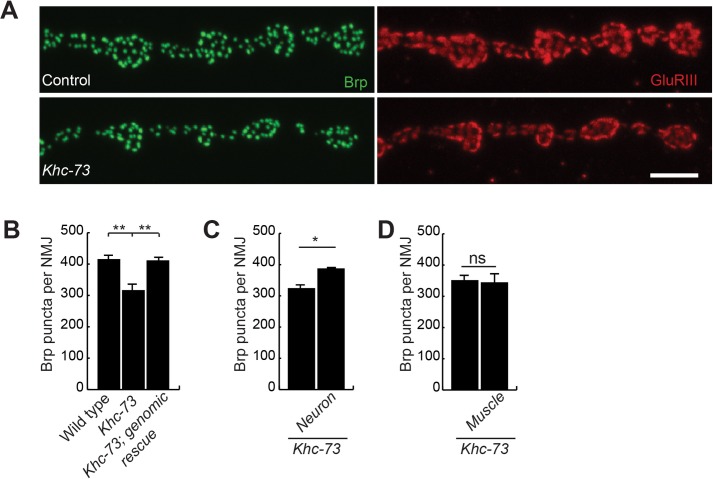
We have previously reported an increase in the accumulation of the active zone protein Bruchpilot (Brp) in miR-310-313 cluster mutant larvae that could be reduced by transgenic knockdown of Khc-73 [35]. Therefore, we set out to conduct a deeper examination of Khc-73 mutants to understand the mechanism of action of Khc-73 in motoneurons. As our previous data would predict, we found a significant decrease in the number of presynaptic release sites per NMJ in Khc-73 mutant larvae, as indicated by a reduction of the number of Brp Puncta (Fig 2A and 2B). Inclusion of a genomic fragment containing the entire genetic region of Khc-73 gene restored synaptic defects in Khc-73 mutant larvae, indicating that this defect is related to loss of Khc-73 (Fig 2B). Previously we showed that Khc-73 is under control of the microRNA cluster miR-310-313 [35]. To maintain this relationship in our tissue specific rescue, we used a Khc-73 transgene Khc-73-3’UTR(K014) [35] that retains this negative regulatory control. We found that transgenic expression of Khc-73 in presynaptic motoneurons, but not in postsynaptic muscles was sufficient to establish a normal number of presynaptic release sites (Fig 2C and 2D). This result indicates that Khc-73 function in motoneurons is required for normal maturation of synaptic release sites.
Fig 2. Khc-73 mutants have fewer active zones.
(A) Terminal boutons on muscle 4 NMJs stained with anti-nc82 (Brp) (green) and anti-GluRIII (red) in control (Khc-73100) and Khc-73(Khc-73149) third instar larva. Scale Bar is 5μm. (B) Quantification of active zones at muscle 4 NMJ with neuron and muscle rescue. Wild-type (Khc-73100), Khc-73 (Khc-73149), Khc-73; genomic rescue (Khc-73149; CH321-36I16/+). N = 36, 19, and 17 NMJs. (C) Khc-73 neuron rescue of Brp puncta. Khc-73 control (OK371-Gal4, Khc-73149/Khc-73149) and Neuron rescue (OK371-Gal4, Khc-73149/Khc-73149; UAS-HA-Khc-73(K014)/+). N = 20, 18 NMJs. (D) Khc-73 muscle rescue of Brp puncta. Khc-73 control (Khc-73149; MHC-Gal4/+), and Khc-73 muscle rescue (Khc-73149; MHC-Gal4/UAS-HA-Khc-73(K014)). N = 6 and 6 NMJs. Error bars are SEM. One-Way ANOVA and Student’s t-test. *P<0.05, **P<0.01, ns-no significance.
Khc-73 is required for efficient BMP signaling in motor neurons
During larval development, both the coordinated growth of synaptic boutons and the establishment of synaptic strength at the NMJ are largely dependent on a retrograde signaling cascade that is initiated by the release of the Bone morphogenic protein Glass bottom boat (Gbb) in postsynaptic muscles. Gbb signals through type I and type II BMP receptors, leading to phosphorylation of and subsequent accumulation of the BMP transcription factor Mad (Mothers against decapentaplegic) in the nuclei of motor neurons [37–41]. Through this signaling cascade, genes that control synaptic growth and function are transcriptionally regulated [42–44]. The decrease in the number of presynaptic release sites in Khc-73 mutant larvae, therefore, prompted us to examine the state of BMP signaling in these mutants.
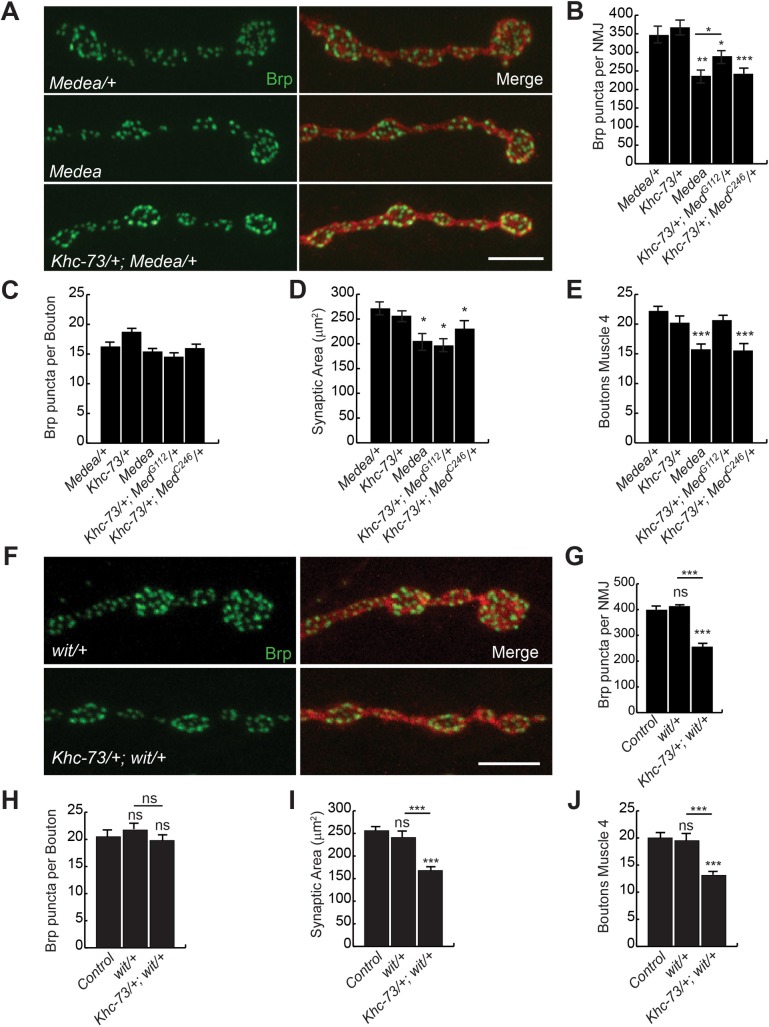
The first indication of Khc-73 involvement with BMP signaling came from genetic interaction experiments between Khc-73 and the Drosophila homolog of vertebrate SMAD4, Medea. Medea is a transcriptional co-factor that is required for normal BMP signaling in motor neurons [45]. We found that a combination of previously published alleles MedeaG112 and MedeaC246 resulted in a very small reduction in the number of boutons at the NMJ compared to MedeaC246 homozygous loss of function mutant [45], suggesting that G112 is a hypomorphic allele (Fig 3A and 3E). Interestingly, in transheterozygous combinations of Khc-73 and Medea, we found a significant reduction in the number of presynaptic release sites per NMJ (Fig 3A and 3B), no change in Brp puncta per bouton (Fig 3C), a significant reduction in synaptic area (Fig 3D) and a significant reduction in bouton number with MedC246 allele but not MedG112 (Fig 3E), as compared to heterozygous MedeaG112 controls. This transheterozygous genetic interaction suggested that Khc-73, while having a mild influence on baseline BMP signaling, becomes critical when BMP signaling is compromised. In support of these results, we also found a strong genetic interaction between Khc-73 mutants and a mutation in the BMP type II receptor wishful thinking (wit): transheterozygous combination between Khc-73 and wit A12 mutants led to a significant reduction in the number of presynaptic release sites (Fig 3F and 3G), synaptic area (Fig 3I) and bouton number (Fig 3J) but no change in Brp puncta per bouton (Fig 3H).
Fig 3. Khc-73 genetic interaction with BMP pathway.
(A) Terminal boutons on muscle 4 of third instar larva in Medea/+ (MedG112/+), Medea (MedC246/MedG112) and Khc-73/+; Medea/+ (Khc-73193/+; MedG112/+). Brp (green) and HRP (red). Scale bar is 5μm. (B-E) Quantification of (B) Brp puncta per NMJ, (C) Brp puncta per bouton, (D) HRP synaptic area, (E) Number of boutons at muscle 4 NMJ for Medea/+ (MedG112/+), Khc-73/+ (Khc-73149/+), Medea (MedC246/MedG112), Khc-73/+; Medea/+ (Khc-73193/+; MedG112/+) and Khc-73/+; Medea/+ (Khc-73149/+; MedC246/+), N = 31, 17, 17, 21 and 14 NMJs. (F) Terminal boutons on muscle 4 in wit/+ (witA12 /+) and Khc-73/+; wit/+ (Khc-73149/+; witA12/+) mutant larva. (G-J) Quantification of (G) Brp puncta per NMJ, (H) Brp puncta per bouton, (I) HRP Synaptic area, (J) Number of boutons at muscle 4 NMJs for Control (Khc-73100/+), wit/+ (witA12/+) and Khc-73/+; wit/+ (Khc-73149/+; witA12/+). N = 11, 8 and 10. Error bars are SEM. One-Way ANOVA and Student’s t-test. *P<0.05, **P<0.01, ***<0.001.
To further explore the functional link between Khc-73 and BMP signaling, we generated double mutant combinations of Khc-73 and Medea. We assessed these double mutant combinations for defects in Brp puncta number at the NMJ and for accumulation of Brp in axons (as previously reported [46]). We found that defects in active zone number and abnormal Brp accumulation in axons in the transallelic combination of MedeaC246/MedeaG112 were not further enhanced upon removal of Khc-73 (S3A–S3F Fig), indicating that Khc-73 and Med likely function in the same and not parallel pathways with respect to these phenotypes.
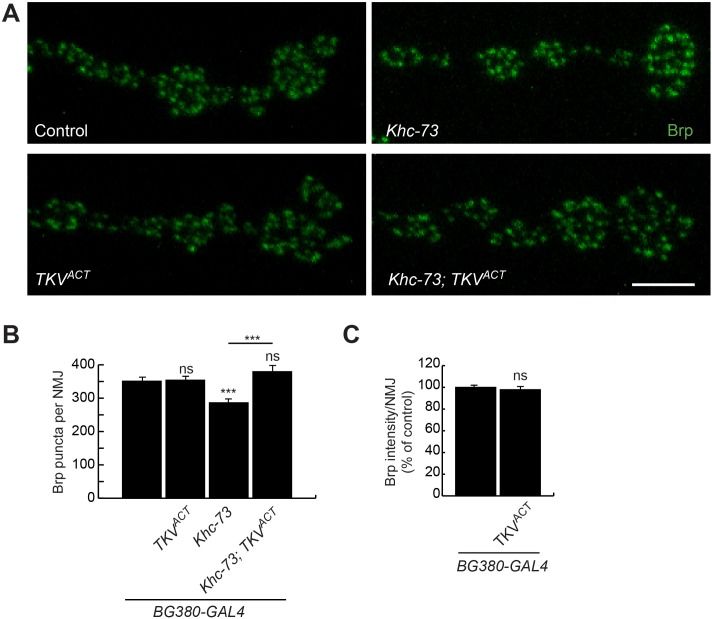
We then tested whether defects in Brp puncta number in Khc-73 mutants can be restored by overexpressing BMP signaling in motoneurons. Indeed, overexpression of a constitutively active form of BMP type I receptor Thick veins (TKVACT) in motoneurons was capable of reversing the reduction in Brp puncta defect in Khc-73 mutant larvae (Fig 4A–4C).
Fig 4. Enhanced BMP signaling suppresses loss of Brp puncta in Khc-73 mutants.
(A) Brp puncta in terminal boutons of muscle 4 NMJs in Control (BG380-Gal4/+; UAS-luciferase/+), TKVACT (BG380-Gal4/+; UAS-TKVACT/+), Khc-73 (BG380-Gal4/+; Khc-73149/Khc-73149), Khc-73; TKVACT (BG380-Gal4/+; Khc-73149; UAS-TKVACT/+) larvae. (B) Quantification of BRP puncta per NMJ in genotypes in (A). N = 18, 17, 13, 12 NMJs. (C) Overexpression of activated form of TKV in motoneurons does not enhance BRP intensity at the NMJ. Control (BG380-Gal4/+; UAS-luciferase/+); TKVACT (BG380-Gal4/+; UAS-TKVACT). N = 19, 17 NMJs. Error bars are SEM. Student’s t-test. ***<0.001. ns-no significance. Scale bar is 5μm.
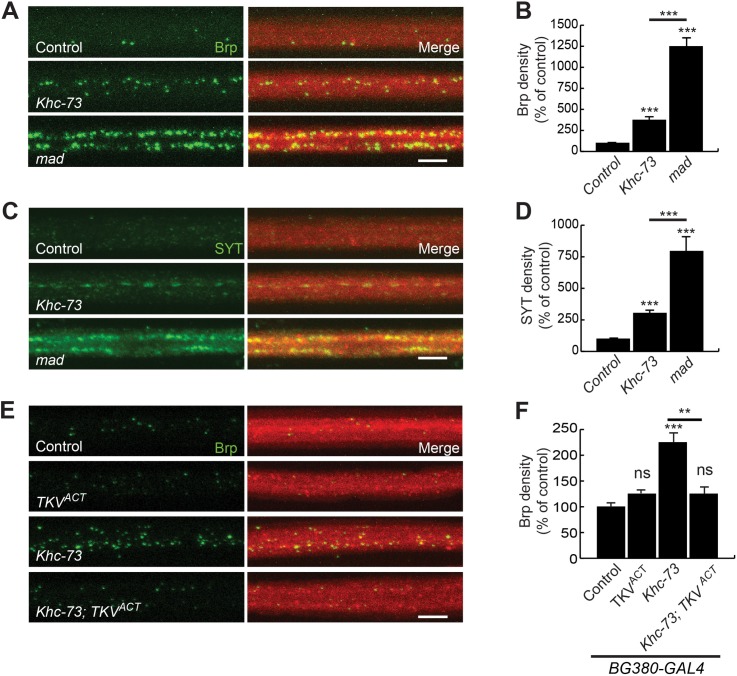
These results prompted us to compare the degree of axonal accumulation of Brp and another synaptic marker, synaptotagmin (SYT) between Khc-73 and Mad mutants. For this we used a Mad mutant allele (MadK00237) that is known to show a strong reduction of synaptic growth and function at the NMJ and exhibit defects in axonal transport of synaptic markers [41]. Both endogenous Brp and SYT accumulated in large aggregates in axons of Mad mutant larvae compared to wild type or Khc-73 mutant larvae, highlighting the fact that Khc-73 related axonal defects would be comparable to a hypomorphic loss of function of BMP signaling (Fig 5A–5D). In support of this interpretation, the increase in Brp accumulation in axons of Khc-73 mutant larvae was fully reversed as a result of overexpression of TKVACT (Fig 5E and 5F).
Fig 5. Synaptic proteins Brp and synaptotagmin accumulate in axons of Khc-73 and mad mutants.
(A) Brp puncta in axons of Control (Khc-73100/+), Khc-73 (Khc-73149) and mad (madK00237). (B) Quantification of Brp density (Number of Brp puncta/μm3) expressed as a percentage of control. N = 19, 10, 10. (C) SYT puncta in axons of Control (Khc-73100/+), Khc-73 (Khc-73149) and mad (madK00237). (D) Quantification of SYT density (Number of Brp puncta/μm3) expressed as a percentage of control. N = 19, 10, 10. (E) Brp puncta in axons of Control (BG380-Gal4/+), TKVACT (BG380-Gal4/+; UAS-TKVACT/+), Khc-73 (BG380-Gal4/+; Khc-73149), Khc-73; TKVACT (BG380-Gal4/+; Khc-73149; UAS-TKVACT/+). (F) Quantification of Brp puncta axon density for genotypes in (E). N = 10, 5, 10 and 9 larvae. Error Bars are SEM. Student’s t-test. **P<0.01, ***P<0.001. ns-no statistical significance. Scale bar is 5μm.
We also tested whether abnormal axonal accumulation of Brp in Khc-73 mutant larvae could be due to changes in microtubule structures; however, we found no significant changes in the expression of acetylated tubulin in axons or terminals in Khc-73 mutant larvae when compared to wild type counterparts (S3G and S3H Fig). These results further support that the defects associated with active zone numbers in Khc-73 mutant larvae are most likely related to defects in BMP signaling.
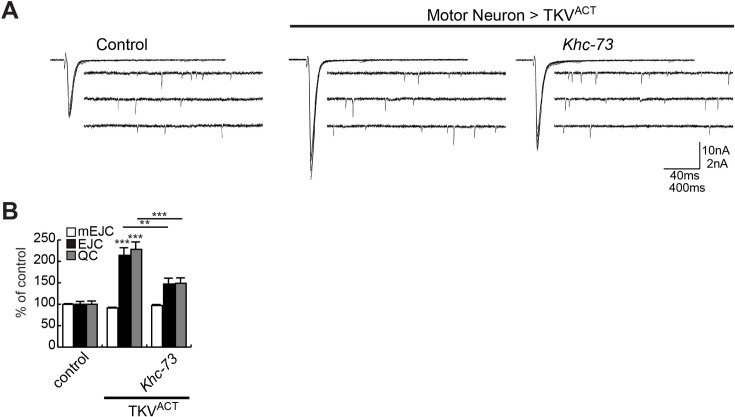
While we did not detect measurable changes in the accumulation of pMad in response to loss of Khc-73 (S4A and S4B Fig), or overexpression of Khc-73 transgene (S4C and S4D Fig), the genetic interactions described above provide strong evidence for a functional link between Khc-73 and BMP signaling. In order to strengthen this link and extend it to the regulation of synaptic function, we conducted a number of electrophysiological examinations. Mild to moderate overexpression of TKVACT in motor neurons can lead to an enhancement in synaptic release without significantly affecting the number of synaptic boutons [44, 47]. We found that loss of Khc-73 could significantly block the ability of TKVACT to enhance synaptic strength (Fig 6A and 6B).
Fig 6. Khc-73 suppression of BMP pathway activation at the NMJ.
(A) Representative traces of EJC and mEJCs of control (BG380-Gal4/+), Motor Neuron>TKVACT (BG380-Gal4/+; UAS-TKVACT/+) and Motor Neuron>TKVACT, Khc-73 (BG380-Gal4/+; Khc-73149; UAS-TKVACT/+). (B) Quantification of mEJC, EJC and QC of genotypes shown in (A). n = 18, 18 and 20. Error bars are SEM. One-Way ANOVA and Student’s t-test. **P<0.01, ***<0.001.
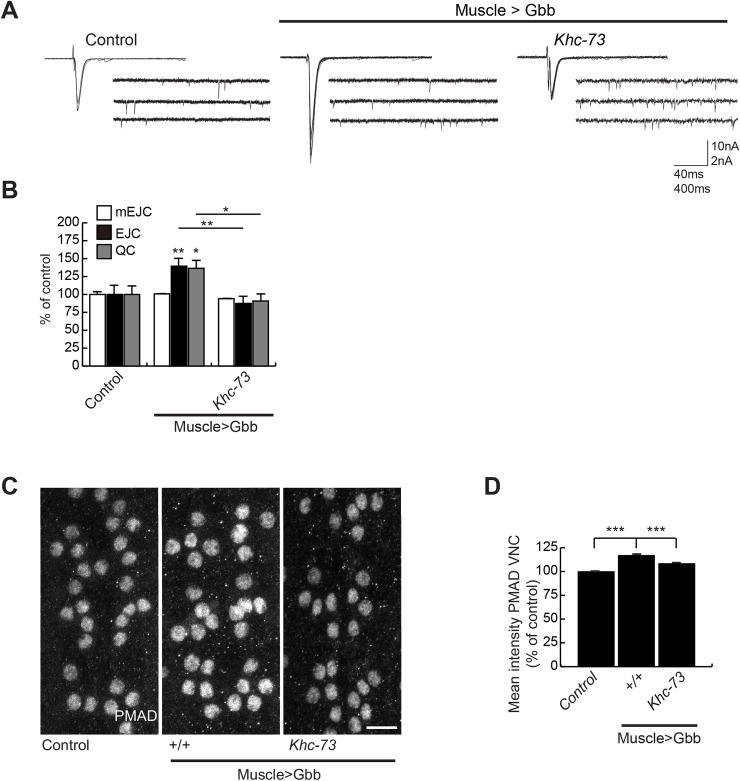
Similarly, we found that overexpression of the BMP ligand Gbb in postsynaptic muscles led to a significant enhancement in quantal content (Fig 7A and 7B). As in the case of TKVACT induced enhancement in neurotransmitter release, loss of Khc-73 led to a significant suppression of Gbb-induced enhancement in neurotransmitter release (Fig 7A and 7B).
Fig 7. Khc-73 is required for retrograde BMP signaling at the NMJ.
(A) Representative traces of EJC and mEJCs of Control (MHC-Gal4/+), Muscle>Gbb (UAS-Gbb99/+; MHC-Gal4/+) and Muscle>Gbb, Khc-73 (Khc-73193, UAS-Gbb99/Khc-73149, +; MHC-Gal4/+). (B) Quantification of mEJC, EJC and QC for genotypes shown in (A). n = 10, 10 and 7. (C) pMad staining in the motoneuron nuclei of ventral nerve cord in MHC Control (MHC-GAL4/+), MHC>Gbb (UAS-Gbb99/+; MHC-GAL4/+) and MHC>Gbb, Khc-73 (Khc-73193, UAS-Gbb99/Khc-73149, +; MHC-GAL4/+) larvae. (D) Quantification of the mean fluorescence intensity of nuclei for genotypes in (C). n = 302(7), 372(9) and 5(210), Nuclei (larvae) respectively. Error Bars are SEM. One way ANOVA. Student’s t-test. *P<0.05, **P<0.01, ***P<0.001.
In addition, we quantified the accumulation of pMad in motoneuron nuclei in the ventral nerve cord (VNC) as a result of postsynaptic overexpression of Gbb. It is well accepted that the accumulation of pMad in the nuclei of motor neurons is a reliable readout of the strength and efficiency of retrograde BMP signaling in motor neurons and is essential for BMP-dependent transcriptional regulation as well as regulation of synaptic function [38, 40, 44, 48, 49]. Muscle overexpression of Gbb led to a statistically significant increase in pMad in the nuclei of motoneurons, which was fully reversed as a result of loss of Khc-73 (Fig 7C and 7D).
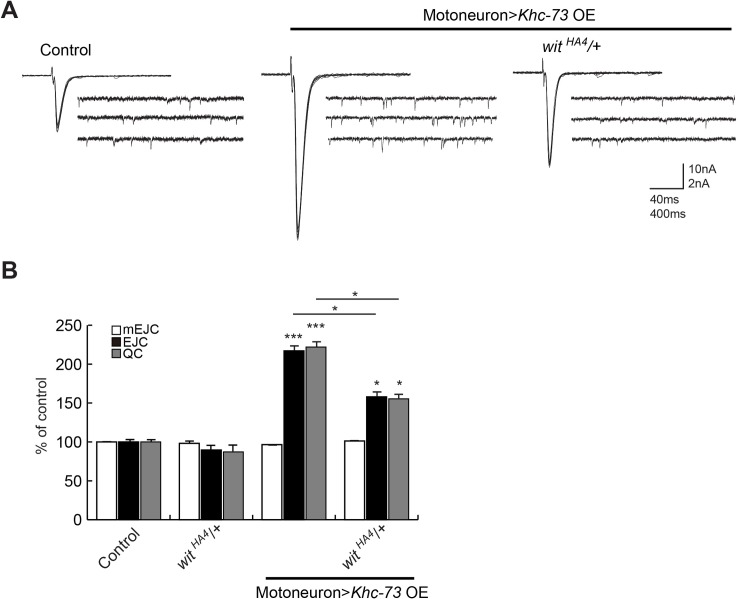
Finally, we tested whether Khc-73 gain-of-function would be dependent on normal BMP signaling in motoneurons. We have previously shown that Khc-73 overexpression in motoneurons leads to an enhancement of neurotransmitter release [35]. We found that heterozygosity for the BMP type II receptor wishful thinking (wit) was sufficient to suppress this enhancement to a large extent (Fig 8A and 8B), further supporting the presence of a functional link between Khc-73 and BMP signaling.
Fig 8. Khc-73 induced enhancement of synaptic release is suppressed by heterozygosity in BMP receptor wit.
(A) Representative traces for EJCs and mEJCs in control (OK371-Gal4/+; UAS-luciferase/+), Khc-73 OE (OK371-Gal4/UAS-Khc-73) and Khc-73 OE; witHA4/+ (OK371-Gal4/UAS-Khc-73; witHA4/+). (B) Quantification of mEJC, EJC and QC for genotypes in control (OK371-Gal4/+; UAS-luciferase/+), witHA4/+ (OK371-Gal4/+; witHA4/+), Khc-73 OE (OK371-Gal4/UAS-Khc-73) and Khc-73 OE; witHA4/+ (OK371-Gal4/UAS-Khc-73; witHA4/+). N = 10, 10, 10 and 10. Error Bars are SEM. Student’s t-test. *P<0.05, **P<0.01, ***P<0.001. ns-no statistical significance.
From these results a picture emerges, indicating a strong functional link between Khc-73 and BMP signaling in motor neurons. But how does Khc-73 interact with BMP signaling? BMP signaling in motoneurons depends on tightly regulated endosomal traffic. For example, pMad accumulation in motoneuron nuclei in response to activation of BMP signaling at the synapse is dependent on retrograde routing of signaling endosomes containing BMP receptor complexes from the nerve terminal along axons to the cell body [5]. Conversely, routing of BMP receptor complexes to lysosomal pathways appears as one of the mechanisms that attenuates BMP signaling in motor neurons [50–52]. Therefore, we considered a role for Khc-73 in both retrograde routing as well as lysosomal sorting of BMP receptor complexes.
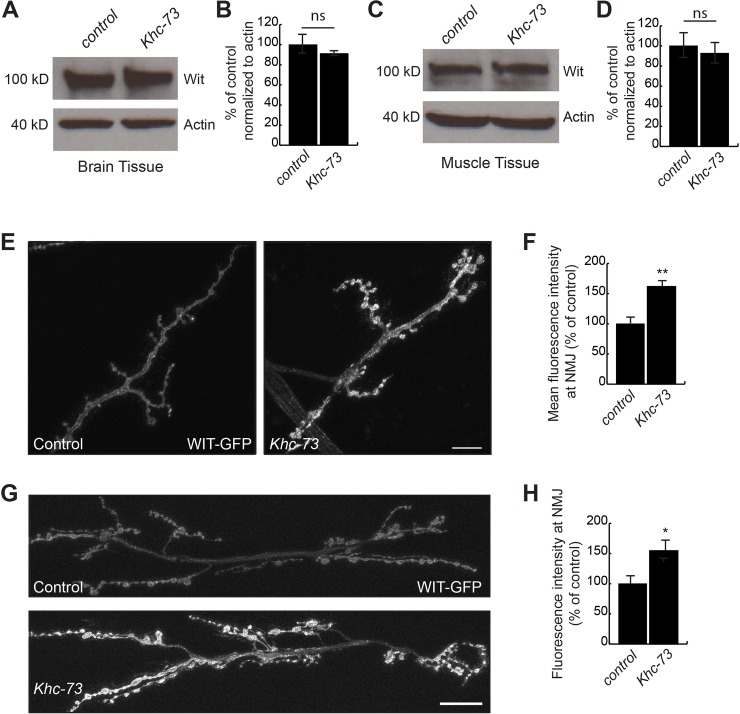
To test these possibilities, we assessed the level of BMP receptors Wit and TKV using a combination of Western blot analysis and immunohistochemistry. Western blot analysis of CNS and body wall muscle tissue (containing NMJ terminals) revealed no change in the level of endogenous Wit protein as a result of genetic removal of Khc-73 (Fig 9A–9D). The available antibody to Wit does not detect endogenous Wit in immunohistochemistry. Thus we turned to transgenic Wit and Tkv to visualize their localization at the synapse. Static images of the boutons in live preps of WIT-GFP revealed punctate accumulations at the NMJ and an increase of Wit receptor intensity in Khc-73 mutants at muscle 4 and muscles 6/7 (Fig 9E–9H). Similarly, TKV:YFP transgene expression appeared more punctate at muscle 4 (S5A Fig) and muscles 6/7 (S5C Fig), trending towards increased intensity at muscle 4 (S5B Fig), while significantly increasing in intensity at muscles 6/7 (S5D Fig) in Khc-73 mutants. We ruled out changes in TKV:YFP transgene transcription by quantitative PCR (S5E Fig) and did not observe obvious changes in axonal traffic of TKV:YFP in motoneurons (S5F Fig).
Fig 9. BMP receptors Wit and TKV accumulate at Khc-73 mutant NMJs.
(A) Representative western blot of Wit levels in brain tissue from control (w1118) and Khc-73 (Khc-73149) mutants. Actin loading control. (B) Quantification of Wit protein band intensity normalized to actin of (A) and shown as percentage of control. n = 3 blots. (C) Representative western blot of Wit levels in muscle tissue from control (w1118) and Khc-73 (Khc-73149) mutants. Actin loading control. (D) Quantification of Wit protein band intensity normalized to actin for (C) and shown as percentage of control. n = 3 blots. (E) Live image of muscle 4 NMJs in unfixed larvae for Control (BG380-Gal4/+; OK371-Gal4/ UAS-Wit-GFP) and Khc-73 (BG380-Gal4/+; Khc-73149, OK371-Gal4 / Khc-73149, UAS-Wit-GFP). Scale bar is 10μm. (F) Quantification of mean fluorescence intensity as percentage of control for genotypes in (E). N = 11, 6. (G) Live image of muscle 6/7 NMJs in unfixed larvae for Control (BG380-Gal4/+; OK371-Gal4/UAS-Wit-GFP) and Khc-73 (BG380-Gal4/+; Khc-73149, OK371-Gal4/ Khc-73149, UAS-Wit-GFP). Scale bar is 20μm. (H) Quantification of mean fluorescence intensity as percentage of control for genotypes in (G). N = 10, 9 NMJs. Error Bars are SEM. Student’s t-test. *P<0.05, **P<0.01. ns-no statistical significance.
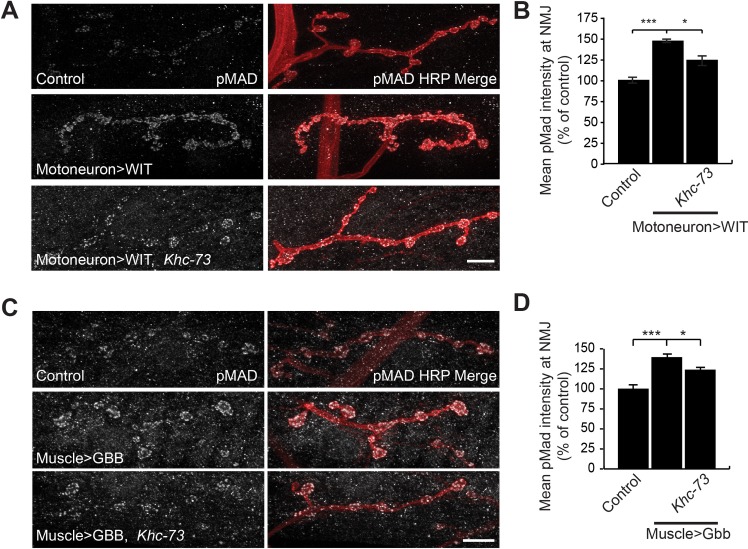
We next tested our model that Khc-73 loss can suppress BMP signaling by examining pMAD levels in larvae overexpressing the Wit receptor in presynaptic neurons and in larvae overexpressing the Gbb ligand from postsynaptic muscle. Overexpression of Wit enhanced presynaptic pMad levels (Fig 10A and 10B). In Khc-73 mutants, this enhancement was significantly suppressed (Fig 10A and 10B). Similarly, muscle overexpression of Gbb enhanced pMAD levels in presynaptic boutons. Khc-73 loss also suppressed this increase (Fig 10C and 10D).
Fig 10. Activation of BMP signaling is suppressed in Khc-73 mutant larvae.
(A) pMad staining at the NMJ for Control (BG380-GAL4/+), Motoneuron>WIT (BG380-GAL4/+; UAS-Wit/+) and Motoneuron>Wit, Khc-73 (BG380-GAL4/+; UAS-Wit, Khc-73149/+, Khc-73149). (B) Quantification of mean pMad fluorescence intensity for genotypes in (A). N = 22, 44 and 10 NMJs. (C) pMad staining at the NMJ for Control (MHC-Gal4/+), Muscle>Gbb (UAS-Gbb99/+; MHC-Gal4/+) and Muscle>Gbb, Khc-73 (Khc-73193, UAS-Gbb99/Khc-73149, +; MHC-Gal4/+) (D) Quantification of mean pMad fluorescence intensity for genotypes in (C). N = 12, 12, and 15 NMJs. Error bars are S.E.M. Student’s t-test. *P<0.05, ***P<0.001. Scale bar is 10μm.
It has been demonstrated that BMP receptor activity can be dampened when trapped inside the lumen of multivesicular bodies (MVBs) at the NMJ [53]. Generally, MVBs are intracellular vesicles that contain one or more smaller vesicles within their lumen and play an important role in signal transduction and endosomal sorting [54, 55]. Current evidence suggests that MVBs may be at the crossroads for endosomal cargo joining the lysosomal pathway, the retrograde pathway or the exosomal secretory pathway [55, 56]. We find that fluorescence intensity of the MVB localized protein Hrs (hepatocyte growth factor related tyrosine kinase substrate) is increased by 20% at the NMJ in Khc-73 mutant larvae overexpressing the BMP receptor Wit (S6A and S6B Fig). Suggesting that there are more MVBs in Khc-73 mutants in this Wit overexpressing background. Therefore, a scenario can be considered in which retrograde bound BMP receptors are encapsulated in multivesicular bodies and may be stalled at the NMJ in Khc-73 mutants.
Together, these results suggest that degradation of BMP receptors is not a likely explanation for the inhibition of BMP signaling in Khc-73 mutant larvae. Secondly, our findings suggest that while BMP receptors appear to accumulate at the NMJs in Khc-73 mutants, they are in an endosomal state that prevents these receptors from signaling.
Khc-73 is necessary for proper endosomal sorting at synaptic terminals
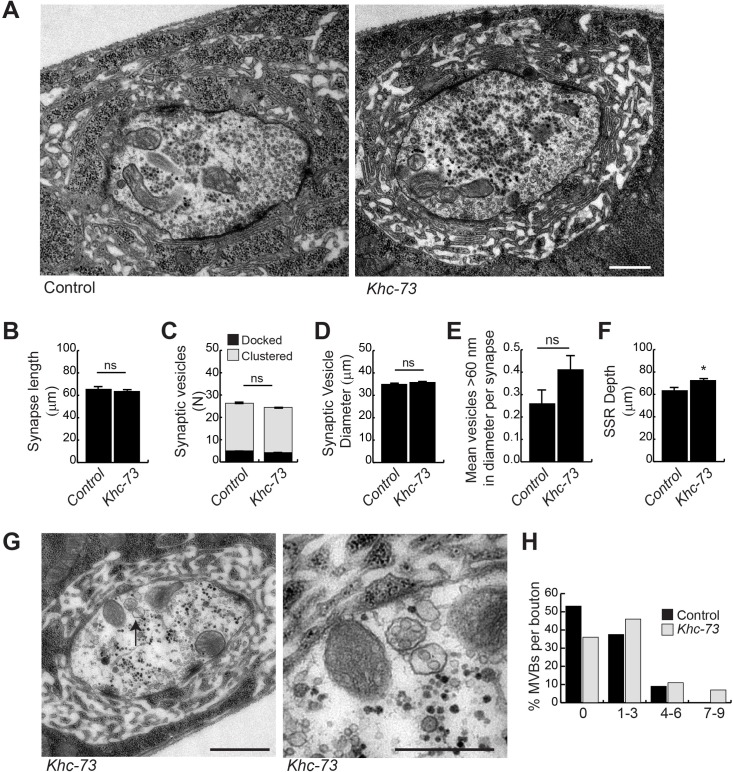
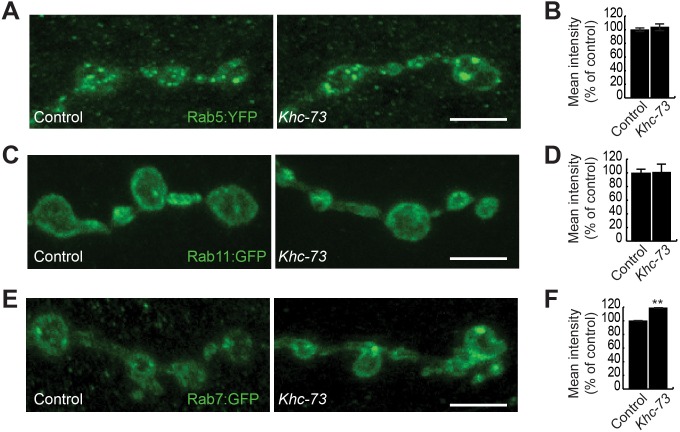
Previous studies on Khc-73/KIF13B have identified endosomal sorting roles for this protein [15, 21, 22, 27, 28, 34, 57]. In order to gain additional insight into the role of Khc-73 in the regulation of endosomal traffic, we conducted an ultrastructural analysis of NMJ synapses in Khc-73 mutant larva. Our analysis revealed no gross abnormalities in presynaptic boutons (Fig 11A–11F): different morphometric measures of active zones and synaptic vesicles appeared normal in Khc-73 mutant larvae (Fig 11B–11E); however, we did detect a small increase in the depth of the subsynaptic reticulum (SSR) (Fig 11F). Interestingly, although we find no statistical difference in the mean MVBs per bouton (1.28±0.29 control and 1.68±0.41 Khc-73), we found a proportion of boutons with an abnormally higher number of MVBs (7–9 MVBs per bouton) in Khc-73 mutant larvae (Fig 11G and 11H). The trend towards more MVBs in Khc-73 mutant boutons suggested a role for Khc-73 in endosomal sorting. Therefore, we turned to exploring a possible role for Khc-73 in the regulation of endosomal dynamics by examining the expression of transgenic Rab-GTPases at the synapse. Rab-GTPases are small GTPases that associate with endocytic vesicles and are known to mediate many aspects of endosomal traffic in all eukaryotes [58]. Based on previous reports on interaction between Khc-73 with the early endosome associated Rab5 in vitro [34], we tested the expression pattern of Rab5 at the NMJ in Khc-73 mutant larvae with a UAS-Rab5:YFP transgene. However, we found that in Khc-73 mutants the punctate appearance of Rab5:YFP was unaffected in terms of fluorescence intensity or localization (Fig 12A and 12B). Similarly, we did not detect any effect on the expression level of the recycling endosomal marker Rab11 (Fig 12C and 12D). In most eukaryotic cells Rab5 positive internalized vesicles become associated with Rab7 along their path of maturation [59–62]; Rab7 containing late endosomes are then either routed to the lysosomal pathway or the recycling pathway [58, 63]. In neurons, the transition from Rab5 to Rab7 is also necessary for routing late endosomes onto the retrograde pathway [64]. The retrograde pathway is necessary for transporting signaling complexes, neurotrophic factors and other cellular proteins from nerve endings to the cell body [2]. Interestingly unlike the case of Rab5, we found an abnormal increase in Rab7 accumulation at synaptic boutons in Khc-73 mutants (Fig 12E and 12F). These results suggested to us that the normal dynamics of Rab7 positive vesicles and by extension those of BMP receptors are disrupted in Khc-73 mutant larvae.
Fig 11. EM analysis of Khc-73 mutant larvae.
(A) EM micrographs of Control (Khc-73100) and Khc-73 (Khc-73193). Magnification 25000x. Scale bar is 0.5 μm. (B) Quantification of synapse length in Control (Khc-73100) and Khc-73 (Khc-73193). N = 79, 153 synapses. (C) Quantification of docked and clustered synaptic vesicles at each synapse for Control (Khc-73100) and Khc-73 (Khc-73193). N = 79, 153 synapses. (D) Quantification of synaptic vesicle diameter for Control (Khc-73100) and Khc-73 (Khc-73193). N = 2082, 3750 synaptic vesicles. (E) Mean number of vesicles >60nm in diameter at the synapse. N = 79, 153 synapses. (F) Quantification of subsynaptic reticulum (SSR) depth at the synapse. N = 79, 153 synapses. (G) EM micrographs of multivesicular bodies in Khc-73 mutant. Magnification of MVB denoted by arrow in left panel (right panel). Scale bar 1 μm. (H) Proportion of boutons with indicated number of multivesicular bodies for control (Khc-73100) and Khc-73 (Khc-73193). N = 33, 28 boutons. Error bars are S.E.M. Student’s t-test. *P<0.05, ns-no statistical significance.
Fig 12. Endosomal markers in Khc-73 mutant larvae.
(A) RAB5:YFP at muscle 4 NMJ terminal boutons stained with anti-GFP in control (VGlut-Gal4/+; UAS-RAB5:YFP/+) and Khc-73 (VGlut-Gal4/+; Khc-73149/Khc-73149; UAS-RAB5:YFP). (B) Quantification of RAB5:YFP fluorescence intensity at muscle 4 NMJ for genotypes in (A). n = 8, 12 NMJs. (C) RAB11:GFP at muscle 4 NMJ terminal boutons stained with anti-GFP in control (OK371-GAL4/ UAS-RAB11:GFP) and Khc-73 (OK371-GAL4, Khc-73149/Khc-73149; UAS-RAB11:GFP). (D) Quantification of RAB11:GFP puncta fluorescence intensity at muscle 4 NMJs for genotypes in (C). N = 10, 10 NMJs. (E) RAB7:GFP at muscle 4 NMJ terminal boutons stained with anti-GFP in control (OK371-GAL4/+; UAS-RAB7:GFP/+) and Khc-73 (OK371-GAL4, Khc-73149/+, Khc-73149; UAS-RAB7:GFP/+). (F) Quantification of RAB7:GFP puncta fluorescence intensity at muscle 4 NMJs for genotypes in (E). N = 20, 17 NMJs. Scale bar is 5μm. Error Bars are SEM. Student’s t-test. **P<0.01.
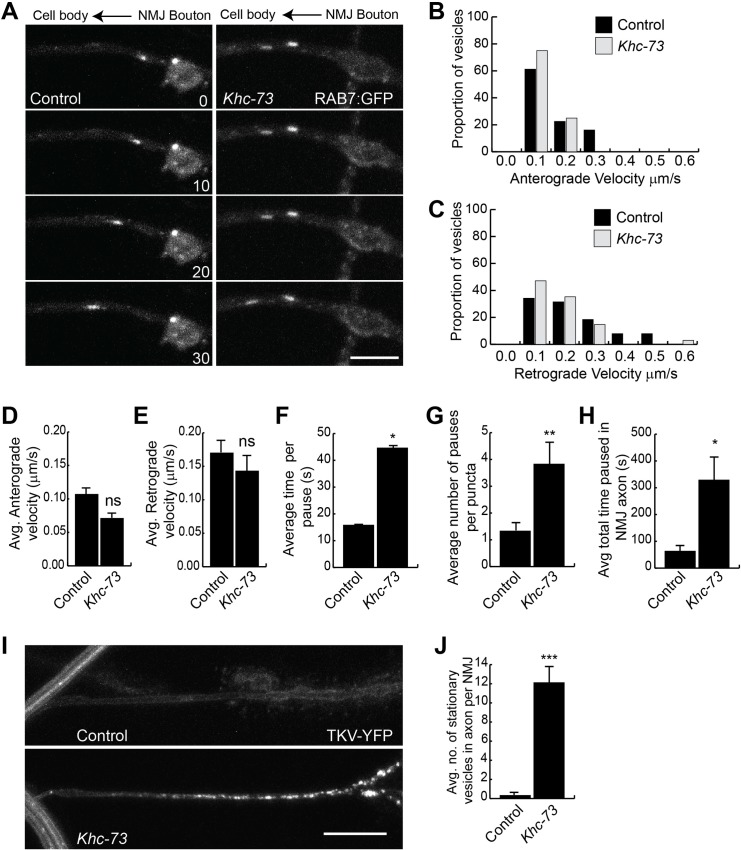
In order to examine the dynamics of late endosomal traffic in more detail, we set out to conduct live imaging in dissected larvae expressing Rab7:GFP. To see if our observations of Rab7:GFP would be relevant to the dynamics of Wit/Tkv complexes, we confirmed in fixed samples that Wit and Rab7:GFP colocalized when expressed simultaneously (S7A Fig, Pearson’s r coefficient 0.68). We also confirmed that Tkv and Wit colocalized at the NMJ (S7B Fig, Pearson’s r coefficient 0.60). In live dissected larval preparations, Rab7:GFP showed dynamic movement within synaptic boutons in both wild type and Khc-73 mutants (Fig 13A–13C and S1 Movie and S2 Movie). We noticed that occasionally a Rab7 marked vesicle left the synaptic area and moved retrograde towards the shaft of the axon. Vesicles entering the axon moved, paused and continued moving out of the NMJ. We measured the velocity of these vesicles when in motion and calculated the mean velocity in the anterograde and retrograde directions (Fig 13B–13E, S8A–S8D Fig and S3 Movie and S4 Movie) and found no statistical difference in their velocities. We also recorded the time spent paused in a single spot (Fig 13F), the number of pauses for each spot (Fig 13G) and summed the total time paused in the axon (Fig 13H). Here, our assessment of Rab7 dynamics revealed a significant difference between control and Khc-73 mutant larvae. We recorded long periods of pausing or stalling of Rab7 positive vesicles in Khc-73 mutants, which showed statistical difference compared to our recordings in control larvae (Fig 13F and 13H, S1 Movie and S2 Movie). This pausing phenotype provides one explanation for the increase in Rab7:GFP in Khc-73 NMJs, however alternative explanations related to Rab7:GFP protein turnover are also possible.
Fig 13. Live imaging of Rab7:GFP at synaptic terminals.
(A) Montage of RAB7:GFP retrograde movement at muscle 4 NMJ in control (OK371-GAL4/+; UAS-RAB7:GFP/+) and Khc-73 (OK371-GAL4, Khc-73149/+, Khc-73149; UAS-RAB7:GFP/+) larvae. Scale bar is 5μm. Time is seconds. (B) Histogram of Anterograde velocities of RAB7:GFP puncta in (A). N = 18(42), 12(36) NMJs(puncta). (C) Histogram of Retrograde velocities of RAB7:GFP puncta in (A). N = 18(42), 12(36) NMJs(puncta). (D) Average anterograde velocity of RAB7:GFP puncta for genotypes in (A). N = 18(42), 12(36) NMJs(puncta). (E) Average retrograde velocity of RAB7:GFP puncta for genotypes in (A). N = 18(42), 12(36) NMJs(puncta). (F) Average time RAB7:GFP puncta spent in each pause event. For genotypes in (A). N = 18(42), 12(36) NMJs(puncta). (G) Average number of pauses per RAB7:GFP puncta.for genotypes in (A). N = 18(42), 12(36) NMJs(puncta). (H) Total time RAB7:GFP puncta remained paused within proximal axon for genotypes in (A). N = 18(42), 12(36) NMJs(puncta). (I) TKV-YFP expression in control (BG380-Gal4/+; UAS-TKV-YFP/+) and Khc-73 (BG380-GAL4/+; Khc-73149; UAS-TKV-YFP/+) larvae in the NMJ axon of muscle 4. (J) Quantification of the number of stationary puncta observed within the axon from time lapse movies of genotypes in (I). N = 6, 8 NMJs. Scale bar is 5 μm. Error Bars are SEM. Student’s t-test. *P<0.05, **P<0.01, ***P<0.001. ns-no statistical significance.
We next performed time lapse imaging on TKV-YFP expressing Khc-73 mutant larvae focusing on the axon shaft near the synapse. Here we observed a similar stalling phenotype of TKV-YFP puncta in Khc-73 mutants whereas in control larvae the axonal shaft was devoid of stalled puncta (Fig 13I and 13J, S5 Movie and S6 Movie)).
As an additional test for axonal retrograde transport, we used a peripheral axon injury model developed by Collins and colleagues for activating Jun-N-terminal kinase (JNK) signaling in motor neurons [65]. In this model, crushing peripheral axons in larvae leads to a strong transcriptional upregulation of the JNK phosphatase puckered (puc) in the injured motoneurons [65]. The puc transcriptional response to axon injury is dependent on axonal retrograde transport [65]. Using a puc-LacZ transcriptional reporter line, we assessed JNK activation in motoneurons in response to nerve crush. In Khc-73 larvae, we found that puc transcriptional upregulation as a result of axonal injury was indistinguishable from that of control larvae (S8E–S8H Fig). Thus we can rule out a defect in retrograde axonal transport in Khc-73 mutants. Similarly, we did not find any significant changes in axonal transport of mitochondria in Khc-73 mutant larvae (S7 Movie and S8 Movie). These results provided strong evidence for a model in which Khc-73 is required primarily in synaptic terminals for efficient routing of retrograde vesicles onto the retrograde path with little influence on bidirectional axonal transport.
Discussion
Khc-73 is a novel regulator of synaptic endosomal sorting and a modulator of BMP signaling in motoneurons
Khc-73 function plays a supporting role in retrograde BMP signaling under basal conditions. However under conditions of enhanced BMP signaling, this endosomal coordination by Khc-73 becomes critical to transmit the retrograde signal from the synapse to the neuronal cell body.
Efficient retrograde signaling from synaptic terminals back to the neuronal soma is critical for appropriate neuronal function and survival [2, 7–11]. Nevertheless, we know very little about the molecular steps that facilitate the routing of synaptic endosomes destined for retrograde axonal pathways. Here we describe several lines of evidence for a potential role for Khc-73 in this process. Khc-73 mutant larvae develop grossly normal synaptic structure and function at the Drosophila larval neuromuscular junction (NMJ), but we find a reduction in the number of presynaptic release sites. Through genetic interaction experiments, we show that this defect is most likely the result of abnormal BMP signaling in motoneurons: transheterozygous combinations of Khc-73 and Medea or wit mutants show a significant loss of presynaptic release sites compared to control. Khc-73 becomes even more critical, when higher demand is put on the motoneuron by activating BMP signaling: loss of Khc-73 largely blocks the retrograde enhancement in synaptic release in response to activation of BMP pathway in motor neurons. Consistently we have previously shown that transgenic knock down of Khc-73 in motoneurons blocks the ability of the NMJ to undergo retrograde synaptic homeostatic compensation [35]. Our findings show that when BMP signaling is activated, loss of Khc-73 reduces the accumulation of pMad in motoneuron nuclei, suggesting a role for Khc-73 in the regulation of retrograde signaling. Our immunohistochemical assessment and live imaging analysis of Khc-73 mutant larvae provide evidence for involvement of Khc-73 in at least two steps in endosomal dynamics in motoneurons. On the one hand, Khc-73 is required for normal dynamics of internalized endosomes through late endosomal and multivesicular stages, and on the other Khc-73 plays a role in facilitating the routing of endosomes onto the retrograde pathway (see Fig 14A for model). These defects have two main consequences: first, we find an accumulation of BMP receptors at the NMJ (possibly in multivesicular bodies) without increased local signaling, suggesting that these receptor containing endosomes might be trapped in a state between late endosomal and lysosomal stage (see Fig 14B for model). Second, we see a dampening of the ability of retrograde bound Rab7:GFP tagged endosomes to join the retrograde pathway, illustrating a defect in retrograde movement of vesicles and possibly providing an underlying explanation for the reduction in pMAD when retrograde BMP signaling is activated in Khc-73 mutants. These results together present Khc-73, a plus-end microtubule motor, in the unexpected role of regulation of endosomal traffic from synapse to the soma in motoneurons with a role for ensuring the efficiency of retrograde BMP signaling.
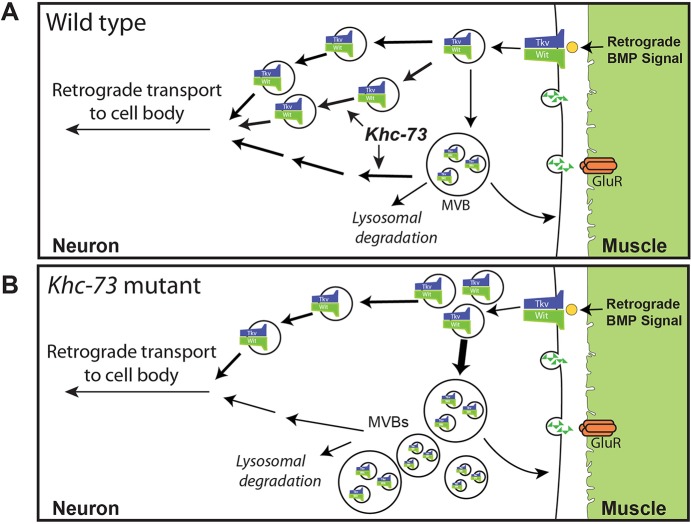
Fig 14. Model slide for Khc-73 function at the synaptic terminal.
(A) In wild-type neuromuscular junctions, Khc-73 mediates the efficient transit of retrograde bound endosomes to the retrograde transport machinery. Its function could include enhancement of sorting to retrograde pathways and/or exit of endosomes from the MVB. (B) In the absence of Khc-73, retrograde bound endosomes do not efficiently engage with the retrograde machinery and accumulate in late endosome compartments. This inefficient level of routing is sufficient for basal levels of BMP retrograde signaling. However, when BMP signaling is enhanced, Khc-73 function is needed to mediate the exit of retrograde endosomes from the synapse. In its absence, retrograde bound signaling complexes are slowed in their exit from the synapse, they accumulate and signaling to the cell body is inhibited.
While our findings provide compelling evidence for the proposed model above, we cannot, at this time, rule out the possibility that the abnormal accumulation of BMP receptors at the NMJ and the slowing of retrograde movement of Rab7 positive endosome in Khc-73 mutant larvae could be due to a defect in an intermediate molecule, whose anterograde transport is dependent on Khc-73. In support of such model, we do report an abnormal accumulation of Brp and SYT (two synaptic proteins) in axons. While our data suggests that this abnormal accumulation can be remedied by transgenic activation of BMP signaling in Khc-73 mutants, we cannot rule out the possibility that an anterograde transport defect might exist for other proteins independent of the interaction between Khc-73 and BMP signaling.
Khc-73 interactions with dynein, microtubules and endosomes
Our findings point to a model in which Khc-73 facilitates the routing of retrograde bound vesicles onto the retrograde axonal pathway. This model predicts coordination between endosomes, dynein motors and kinesin Khc-73. The coordinated involvement of dynein and kinesin motor proteins in the transport and sorting of endosomes has been previously proposed and examples supporting this model are mounting [14, 66, 67]. Previously published data for Khc-73 and KIF13B have provided evidence that interaction between early endosomes, dynein motors and microtubules are possible. Khc-73/KIF13B is capable of binding to the GTPase Rab5 (found on early endosomes), thus allowing Khc-73 to localize directly to Rab5 endosomes [15, 34, 68]. As a kinesin motor protein, Khc-73 could then transport these endosomes to the retrograde pathway by moving along the microtubule network in the synapse.
Compelling evidence for a dynein interaction with Khc-73 has been previously demonstrated during mitotic spindle formation [24]. The Khc-73/KIF13B stalk domain is phosphorylated by Par1b and this creates a 14-3-3 adapter protein binding motif [29]. It has been proposed that physical interaction between Khc-73 stalk domain and the dynein interacting protein NudE via 14-3-3 ε/ζ might underlie the interaction between Khc-73 and dynein that is necessary for appropriate mitotic spindle formation [24]. Interestingly, transgenic knock down of NudE in Drosophila larval motoneurons leads to a reduction in the number of presynaptic release sites, a phenotype reminiscent of Khc-73 loss of function [69]. Thus, Khc-73 contains domains and protein-protein interactions that are capable of coordinating endosomes, microtubules and dynein. We propose that Khc-73 is necessary for the normal endosomal sorting and exit of endosomes from the NMJ to support efficient retrograde BMP signaling.
Methods
Fly stocks
Flies were cultured at 25°C on standard medium except for Gene Switch experiments where RU486 was added to the media (50μM). The following stocks were used: MedC246 (Y324term mutation)[45] and MedG112 (mutation in splice donor site of exon 4)[45] from Herman Aberle [45]. witA12 [37, 39, 70]. witHA4 [37, 45, 49]. UAS-TKVACT and UAS-Gbb99 [40] provided by M.B. O’Connor (University of Minnesota, Minneapolis, MN), UAS-Wit [39], UAS-HA-Khc-73 and UAS-HA-Khc-73-3’UTR(K014) [35], UAS-Wit-GFP, UAS-TKV-YFP [5], BG380-Gal4 [71], Elav-Gal4 [72], OK371-Gal4[73], MHC-Gal4 [74]. Bloomington stocks used were P{y[+m8] = Mae-UAS.6.11}Khc-73[DP00530] (RRID:BDSC_22058), UAS-Rab5:YFP (RRID:BDSC_9775), UAS-Rab7:GFP(RRID:BDSC_42706), UAS-Rab11:GFP (RRID:BDSC_50782), VGlut-Gal4 (RRID:BDSC_24635), MadK00237 (RRID:BDSC_10474), UAS-Mito-HA-GFP (RRID:BDSC_8442), nSyb-Gal4 (RRID:BDSC_51635). UAS-luciferase (RRID:BDSC_35788). puckered LacZ insertion pucE69 [75]. Wild type stock used was w1118.
Deletion of Khc-73 by P-element excision
Khc-73 deletions were created by mobilizing the P-element from y[1] w[67c23]; P{y[+m8] = Mae-UAS.6.11}Khc-73[DP00530]. Virgin y[1] w[67c23]; P{y[+m8] = Mae-UAS.6.11}Khc-73[DP00530] female flies were mated to Cyo/+; Δ2–3, Sb/TM6b males. Male progenies of y[1] w[67c23]; P{y[+m8] = Mae-UAS.6.11}Khc-73[DP00530]/Cyo; Δ2–3, Sb/+ were mated to virgin y, w; CyoGFP/Adv females. Yellow, non Sb, yellow eyed progeny were singly mated to y, w; Adv/CyoGFP virgins and individual stocks were established. P-element excisions were screened with the following primers: OED91: CTGACGGCGCTGTTGCTTG and OED96: GATCTAGAGATGATTCTGCATCACTAG TAAAAATT.
DNA plasmids
Khc-73 promoter GAL4 construct was generated by cloning a 4kb fragment upstream of the translational start site with primers OED453: CAG GTA CCG CCG AGG AAC CGC TAA CG and OED452:CAG GTA CCC GCG GAT GTG GAT GCA GC. Vector pW+SN attB was modified with a GAL4 sequence cloned as a KpnI/NotI fragment.Khc-73 promoter was subsequently inserted into the unique KpnI site. Genomic Khc-73 is from BACPAC clone CH321-36I16 (BACPAC Resources Center).
Transgenic strains
Transgenic fly CH321-36I16 was made by standard embryo injection of BACPAC clone CH321-36I16 (BACPAC Resources Center) with ΦC31 –mediated integration into attP site at position 86F of chromosome III.
Electron microscopy
Wandering third instar larvae were dissected, prepared and embedded as described in [76]. Ultra-thin serial sections of 50 nm thickness were cut from muscle 6, 7 and 12 of hemisegment A3. Electron micrographs were taken at a magnification of 25,000x for measurements, 25,000x and 40,000x for figures. Serial Reconstruction and analysis was conducted on FIJI (Fiji is Just ImageJ) (NIH) [77] and Reconstruct v.1.1.0.0 Software [78].
Immunostaining
Wandering third instar larvae were dissected as previously described [74]. Third Instar larvae were dissected in cold HL3 and fixed with 4% Paraformaldehyde for 10 min or 5min ice cold Methanol for GluRIIA staining. Larvae were washed with PBS (Phosphate buffered saline), permeabilized with PBT (PBS with 0.1% Triton X-100), blocked with 5% Normal Goat Serum (NGS) in PBT and placed in primary antibody overnight at 4°C. The larvae were then washed three times for 15min in PBT, placed in secondary antibody for 2 hrs, washed three times for 15min with PBT and mounted in Vectashield (Vector labs). Antibodies used are as follows: anti-GluRIII (1:500) (gift from A. DiAntonio, Washington Univ. St. Louis, MO), anti-Hrs (1:200), anti-SYT (1:1000) (gift from H. Bellen, Baylor College of Medicine, Houston, TX), anti-pMAD (PS1)(1:200) (gift from M.B. O’Connor, University of Minnesota, Minneapolis, MN). anti-Dlg (1:500), anti-nc82 (1:500), anti-GluRIIA(1:500), anti-CSP(1:500), anti-EPS15 (1:50), anti-LacZ (1:100) and anti-Wit (1:10) (Developmental Studies Hybridoma Bank(DSHB)), anti-HA (1:500) (HA.11 clone 16B12) (Covance Research Products), anti-GFP (1:500) (A6455) (Molecular Probes), anti-GFP (1:500) (Rat IgG2a, GF090R)(Nacalai Tesque Inc.), anti-HRP conjugated Alexa 647(1:250) (Jackson ImmunoResearch), anti-acetylated tubulin (1:500) (T7451, clone 6-11B-1 Sigma-Aldrich) and anti-pSmad3 (EP823Y)(Epitomics).
Western blot analysis
Western blots were performed as previously described [41]. Muscle tissue (without the nervous system and motor axons or imaginal discs) or Brain tissue (VNC and axons) were isolated from wandering third instar larvae dissected in cold HL3. Western blot analysis was performed according to manufacturer’s protocols. Antibodies used: anti-Khc-73 (1:2000)[35], anti-Wit (1:10) (DSHB), anti-actin (Millipore, MAB1501). Gel images were scanned and band intensities were quantified using FIJI (Fiji is just ImageJ software) (NIH) [77].
Confocal imaging and image analysis
Synapses were imaged using a ConfoCor LSM710 and Zeiss LSM 780 on an Axiovert 200M inverted microscope (Carl Zeiss, Inc.) with 63x/1.4 oil objective. Image analysis was performed with ImageJ 1.46j (NIH) [79], Imaris (Bitplane Scientific Software), Image Analyst MKII (Image Analyst Software, Novato, CA) and Metamorph (Molecular Devices).
Live imaging
Wandering third instar larvae were dissected in room temperature HL3 to remove the guts and fat bodies. The larval filet was then inverted and stretched onto a coverslip using magnetic dissection pins inside a chamber consisting of a coverslip surrounded by magnet strips. Larval prep was maintained at room temperature in an HL3 bath during imaging. NMJs at hemisegment A3 and A4, muscles 6/7 and 4 were imaged. Axons were imaged at hemisegment A3 to A4. Larvae were imaged for a maximum of 30 minutes after dissection. Axons and NMJs were imaged with 63x 1.4NA oil objective on Axiovert 200 inverted microscope with Zeiss LSM780 confocal (Carl Zeiss, Inc.).
Nerve crush assay
The nerve crush assay was performed as previously described [65]. Briefly, third instar larvae were anaesthetized with carbon dioxide. The segmental nerves at the midbody were then pinched with Dumostar number 5 forceps for five seconds. The larvae were then recovered on standard media for 25 hours at 25°C after which time they were dissected and stained for LacZ.
Electrophysiology
Wandering third instar larvae were dissected in cold HL3 solution following standard protocol [80]. The spontaneous (mEJC) and evoked (EJC) membrane currents were recorded from muscle 6 in abdominal segment A3 with standard two-electrode voltage-clamp technique [41]. All the recordings were performed at room temperature in HL3 solution containing 0.5mM Ca2+ unless otherwise indicated. The current recordings were collected with AxoClamp2B amplifier (Molecular Devices Inc.) using Clampex 9.2 software (Molecular Devices Inc.). The nerve stimulation was delivered through a suction electrode which held the cut nerve terminal cord. In all voltage clamp recordings, muscles were held at -80 mV. The holding current was less than 5 nA for 90% of the recordings and we rejected any recording that required more than 10 nA current to maintain the holding potential.
The amplitudes of mEJC and EJC were measured using Mini Analysis 6.0.3 software (Synaptosoft) and verified by eye. QC was calculated by dividing the mean EJC amplitude by mean mEJC amplitude. The recording traces were generated with Origin 7.5 software (Origin Lab).Spontaneous and evoked potentials were measured as previously described [49]. Standard two-electrode voltage-clamp technique was used as described in [44].
Supporting information
(A) Khc-73 protein domain structure in D. melanogaster and H. sapiens. K (Kinesin motor), FHA (Forkhead Associated), CC (coiled coil) and CAP-GLY (Cytoskeletal Associated Proteins–Glycine Rich) domains. (B) Khc-73 gene structure and location of DP00530 insert. Untranslated regions (UTR) are in solid black. Exons in grey. Deletions Khc-73193 and Khc-73149 are indicated by solid lines. (C) Western blot of Control (Khc-73100), Khc-73 heterozygotes (Khc-73149/+), Khc-73 mutants (Khc-73149) (left) and Control (Khc-73100), Khc-73193 (right) with anti-Khc-73 (top) and actin loading control (bottom).
(D) Localization of HA-Khc-73 in axons (BG380-Gal4/+; UAS-HA-Khc-73). Scale bar is 5μm.
(E) Localization of HA-Khc-73 in muscle 4 NMJ terminal boutons (BG380-Gal4/+; UAS-HA-Khc-73). Scale bar is 5μm. (F) Expression of Khc-73-GAL4 visualized with mCD8:GFP in ventral nerve cord (Khc-73-Gal4/UAS-mCD8:GFP). Scale bar is 100μm. (G) Expression of Khc-73-GAL4 visualized with mCD8:GFP in muscle 4 NMJ (Khc-73-Gal4/UAS-mCD8:GFP). Scale bar is 5μm. (H) Expression pattern of Khc-73-GAL4 visualized with mCD8:GFP in the adult CNS and VNC. (I) Quantification of bouton number in Control (Khc-73100) and Khc-73 (Khc-73149) mutants at muscle 6/7. N = 17, 18 NMJs. Error Bars are SEM. (J) Muscle surface area of muscle 6/7 in Control (Khc-73100) and Khc-73 (Khc-73149). Muscle 6/7 normalized to control. n = 17, 18. Error Bars are SEM. Student’s t-test. ns-no statistical significance. (K) High Frequency Stimulation trace for Control (Khc-73100) and Khc-73 (Khc-73193). N = 11, 13 NMJs. (L) Quantification of EJP amplitudes for genotypes in (K). Recording duration was 600 seconds at 10Hz. EJPs were binned per 30 seconds and amplitudes were averaged per bin. Relative EJP amplitudes were normalized to the average amplitude for the first 15seconds of recording for each genotype. Error bars are SEM. N = 9,8.
(TIF)
(A) Muscle 4 terminal boutons stained with anti-synaptotagmin (SYT). Scale is 5μm. (B) Quantification of SYT mean fluorescence intensity normalized to HRP signal and expressed as a percentage of control for muscle 4 boutons in control (Khc-73193/+) and Khc-73 (Khc-73193/Khc-73193). N = 10, 8 NMJs. (C) Quantification of Cysteine String Protein (CSP) mean fluorescence intensity, normalized to HRP intensity and expressed as a percentage of control, of muscle 4 NMJs. Control (Khc-73100) and Khc-73 (Khc-73193), N = 9, 10. (D) Quantification of EPS-15 staining in control (w1118) and Khc-73 (Khc-73149) larval NMJs. N = 6, 6. (E) Muscle 4 terminal boutons stained with Dlg in control (Khc-73100) (top) and Khc-73 (Khc-73193) (bottom) third instar larvae. Scale bar is 5μm. (F) Quantification of Dlg fluorescence intensity normalized to HRP intensity and expressed as a percentage of control.
(G) Quantification of GluRIIA fluorescence intensity normalized to HRP intensity and expressed as a percentage of control for Control (Khc-73100) and Khc-73 (Khc-73149) mutants. Error Bars are SEM. Student’s t-test. ***P<0.001, ns-no statistical significance.
(TIF)
(A) Brp puncta in terminal boutons of muscle 4 NMJs in Control (Khc-73100/+), Medea (MedeaC246/MedeaG112) and Khc-73; Medea (Khc-73149; MedeaC246 / MedeaG112) larvae. (B) Quantification of BRP puncta number for genotypes in (A). N = 19,20,20 NMJs. (C) Quantification of synaptic area identified with HRP staining for genotypes in (A). N = 19,20,20 NMJs. (D) Quantification of bouton number per NMJ for genotypes in (A). N = 19,20,20 NMJs. (E) Brp puncta in axons of Control (Khc-73100/+), Medea (MedeaC246/MedeaG112) and Khc-73; Medea (Khc-73149; MedeaC246 / MedeaG112) larvae. Green channel–Brp, Red channel–HRP. (F) Quantification of Brp puncta axon density for genotypes in (E) normalized to control. N = 10,10 and 10 Larvae. (G) Acetylated tubulin staining in muscle 4 NMJs of Control (Khc-73100/+) and Khc-73 (Khc-73149), Dashed line–Quantified region. Acetylated-tubulin (green) and HRP (red). Scale bar is 10μm. (H) Quantification of Acetylated-tubulin intensity in axon region indicated by dashed line in (G) N = 9,8. Error Bars are SEM. Student’s t-test. *P<0.05, **P<0.01, ***P<0.001. ns-no statistical significance. Scale bar is 5μm.
(TIF)
(A) Quantification of pMad intensity at muscle 4 NMJs for control (Khc-73100), Khc-73149 and Khc-73193. N = 10, 12 and 15 NMJs. (B) Quantification of pMad intensity in VNC neurons for control (w1118) and Khc-73 mutants (Khc-73149). N = 6(217), 6(234), VNCs(nuclei). (C) Quantification of pMAD intensity at the NMJ in larvae overexpressing Khc-73 in motoneurons. Control (OK371-Gal4/+) and Khc-73 OE (OK371-Gal4/UAS-Khc-73). N = 20, 19. (D) Quantification of pMAD intensity in the ventral nerve cord in larvae overexpressing Khc-73 in motoneurons. Control (OK371-Gal4/+) and Khc-73 OE (OK371-Gal4/UAS-Khc-73) N = 4(100), 3(143), larvae (nuclei).Error Bars are SEM. Student’s t-test. ns-no statistical significance.
(TIF)
(A) Image of muscle 4 NMJs in live, unfixed larvae for Control (BG380-Gal4/+; OK371-Gal4/+; UAS-TKV-YFP/+) and Khc-73 (BG380-Gal4/+; Khc-73149,OK371-Gal4/ Khc-73149; UAS-TKV-YFP/+). Scale bar is 10μm. (B) Quantification of mean fluorescence intensity as percentage of control for genotypes in (A). N = 14, 12 NMJs. (C) Live image of muscle 6/7 NMJs in live, unfixed larvae for Control (BG380-Gal4/+; OK371-Gal4/+; UAS-TKV-YFP/+) and Khc-73 (BG380-Gal4/+; Khc-73149,OK371-Gal4/ Khc-73149; UAS-TKV-YFP/+). Scale bar is 10μm. (D) Quantification of mean fluorescence intensity as percentage of control for genotypes in (C). N = 15, 15 NMJs.
(E) Quantitative PCR analysis of UAS-TKV-YFP mRNA expression levels in Khc-73 mutants. N = 3 technical replicates. (F) Kymograph of TKV-YFP live imaging of axons in third instar larvae in control (BG380-Gal4/+; OK371-Gal4/+; UAS-TKV-YFP/+) and Khc-73 mutants (BG380-Gal4/+; Khc-73149 /Khc-73149, OK371-Gal4; UAS-TKV-YFP/+) larvae. Scale bar is 10μm. Error Bars are SEM. Student’s t-test. ***P<0.001. ns-not statistically significant.
(TIF)
(A) Muscle 4 terminal bouton in Wit OE Control (OK371-Gal4, Khc-73149/UAS-Wit) and Wit OE, Khc-73 larvae (OK371-Gal4, Khc-73149/UAS-Wit, Khc-73149) stained for Hrs (white) and Hrp (red). Scale bar is 5μm. (B) Quantification of Hrs staining in (A). N = 20, 20 NMJs. Error Bars are SEM. *P<0.05. Student’s t-test.
(TIF)
(A) Muscle 4 terminal boutons in motoneurons overexpressing Wit and Rab7:GFP (nSyb-Gal4, UAS-TKV-YFP/UAS-Rab7:GFP) stained with Wit (Green) and GFP (red) antibodies. Scale bar is 5μm. (B) Muscle 4 terminal boutons in motoneurons overexpressing Wit and TKV-YFP (UAS-Wit/+; nSyb-Gal4, UAS-TKV-YFP/+) stained with Wit (Green) and GFP (red) antibodies. Scale bar is 5μm.
(TIF)
(A) RAB7:GFP expressed in live third instar larval axons of Control (BG380-Gal4/+; UAS-RAB7:GFP/+) and Khc-73 mutants (BG380-Gal4/+; Khc-73149; UAS-RAB7:GFP/+). Scale bar is 5 μm. (B) Kymographs from live imaging of RAB7:GFP in axons of Control (BG380-Gal4/+; UAS-RAB7:GFP/+) and Khc-73 mutants (BG380-Gal4/+; Khc-73149; UAS-RAB7:GFP/+). (C) Histogram of Retrograde velocities of RAB7:GFP puncta in Control (BG380-Gal4/+; UAS-RAB7:GFP/+) and Khc-73 mutants (BG380-Gal4/+; Khc-73149; UAS-RAB7:GFP/+). n = 5, 8 axons and n = 64, 49 puncta respectively.
(D) Histogram of anterograde velocities of RAB7:GFP puncta in Control and Khc-73 mutants. n = 5, 8 axons and n = 49, 27 puncta respectively. (E) LacZ staining in control (Khc73100; pucE69 /+) and Khc-73 mutant (Khc-73149; pucE69 /+) larval motor neuron nuclei in the ventral nerve cord. Scale bar is 10μm. (F) Quantification of mean LacZ fluorescence staining in nuclei for genotypes in (E). N = 279, (6) for control (Khc73100; pucE69 /+) and 535, (11) for Khc-73 mutant (Khc-73149; pucE69 /+), Nuclei, (Ventral Nerve Cords), respectively. (G) LacZ staining in control (Khc73100; pucE69 /+) and Khc-73 mutant (Khc-73149; pucE69 /+) larval motor neuron nuclei in the ventral nerve cord after nerve crush assay. Scale bar is 10μm. (H) Quantification of Anterior and Posterior mean fluorescence of nuclei in (G) expressed as Posterior/Anterior ratio. N = 95, 113, (4) for Khc-73100; pucE69 /+. 219, 300, (10) for Khc-73149; pucE69 /+. Anterior nuclei, posterior nuclei, (Ventral Nerve Cords), respectively. Error Bars are SEM. Student’s t-test. ns-no statistical significance.
(TIF)
Live imaging of Rab7:GFP in third instar larva, muscle 4 NMJ in control (OK371-GAL4/+; UAS-RAB7:GFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 5 μm.
(GIF)
Live imaging of Rab7:GFP in third instar larva, muscle 4 NMJ in Khc-73 mutant (OK371-GAL4, Khc-73149/+, Khc-73149; UAS-RAB7:GFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 5 μm.
(GIF)
Live imaging of Rab7:GFP in third instar larva axon in control (OK371-GAL4/+; UAS-RAB7:GFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 10 μm.
(GIF)
Live imaging of Rab7:GFP in third instar larva axon in Khc-73 mutant (OK371-GAL4, Khc-73149/+, Khc-73149; UAS-RAB7:GFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 10 μm.
(GIF)
Live imaging of TKV:YFP in third instar larva, muscle 4 NMJ in control (BG380-Gal4/+; OK371-Gal4/+; UAS-TKV-YFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 5 μm.
(GIF)
Live imaging of TKV:YFP in third instar larva, muscle 4 NMJ in Khc-73 mutant (BG380-Gal4/+; Khc-73149,OK371-Gal4/ Khc-73149; UAS-TKV-YFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 5 μm.
(GIF)
Live imaging of axons in third instar larva of control (OK371-GAL4/UAS-mito:GFP). Retrograde direction is to the left. Scale bar is 10μm.
(GIF)
Live imaging of axons in third instar larva of Khc-73 mutant (OK371-GAL4, Khc-73149/UAS-mito:GFP, Khc-73149). Retrograde direction is to the left. Scale bar is 10μm.
(GIF)
Acknowledgments
We thank M.B. O’Connor, H. Aberle, A. DiAntonio, A. Chishti, H. Bellen, and C. Doe for providing us with reagents and fly stocks. We thank E. Maksoud, M. Mori, H. Cifuentes and N. Chicoine of the Haghighi lab. Electron microscopy was performed with J. Ouellette of the McGill Pharmacology and Therapeutics EM Facility. Images were collected at the McGill Advanced BioImaging Facility (ABIF) and the Buck Institute Imaging and Morphology Core. We thank the Bloomington Stock Center for fly stocks.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was initiated through support from a CIHR grant to A.P.H. who was a Canada Research Chair holder in Drosophila Neurobiology. The initial observations for this work were made while APH was supported by a CIHR grant. This work was supported by an NIH grant (R01NS082793) to A.P.H. E.H.L was supported by the McLaughlin Post Doctoral Fellowship, McGill University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hirokawa N., Niwa S., and Tanaka Y., Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron, 2010. 68(4): 610–38. doi: 10.1016/j.neuron.2010.09.039 [DOI] [PubMed] [Google Scholar]
- 2.Perlson E., Maday S., Fu M.M., Moughamian A.J., and Holzbaur E.L., Retrograde axonal transport: pathways to cell death? Trends Neurosci, 2010. 33(7): 335–44. doi: 10.1016/j.tins.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kardon J.R. and Vale R.D., Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol, 2009. 10(12): 854–65. doi: 10.1038/nrm2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panayotis N., Karpova A., Kreutz M.R., and Fainzilber M., Macromolecular transport in synapse to nucleus communication. Trends Neurosci, 2015. 38(2): 108–16. doi: 10.1016/j.tins.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Smith R.B., Machamer J.B., Kim N.C., Hays T.S., and Marques G., Relay of retrograde synaptogenic signals through axonal transport of BMP receptors. J Cell Sci, 2012. 125(Pt 16): 3752–64. doi: 10.1242/jcs.094292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soldati T. and Schliwa M., Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol, 2006. 7(12): 897–908. doi: 10.1038/nrm2060 [DOI] [PubMed] [Google Scholar]
- 7.BasuRay S., Mukherjee S., Romero E.G., Seaman M.N., and Wandinger-Ness A., Rab7 mutants associated with Charcot-Marie-Tooth disease cause delayed growth factor receptor transport and altered endosomal and nuclear signaling. J Biol Chem, 2013. 288(2): 1135–49. doi: 10.1074/jbc.M112.417766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White J.A. 2nd, Anderson E., Zimmerman K., Zheng K.H., Rouhani R., and Gunawardena S., Huntingtin differentially regulates the axonal transport of a sub-set of Rab-containing vesicles in vivo. Hum Mol Genet, 2015. 24(25): 7182–95. doi: 10.1093/hmg/ddv415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y., Zhou B., Lin M.Y., Wang S., Foust K.D., and Sheng Z.H., Endolysosomal Deficits Augment Mitochondria Pathology in Spinal Motor Neurons of Asymptomatic fALS Mice. Neuron, 2015. 87(2): 355–70. doi: 10.1016/j.neuron.2015.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha A.R., Hill J., Utton M.A., Asuni A.A., Ackerley S., Grierson A.J., et al. , Parkinson's disease alpha-synuclein mutations exhibit defective axonal transport in cultured neurons. J Cell Sci, 2004. 117(Pt 7): 1017–24. doi: 10.1242/jcs.00967 [DOI] [PubMed] [Google Scholar]
- 11.Gunawardena S., Her L.S., Brusch R.G., Laymon R.A., Niesman I.R., Gordesky-Gold B., et al. , Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron, 2003. 40(1): 25–40. [DOI] [PubMed] [Google Scholar]
- 12.Clague M.J. and Rochin L., Parkinson's Disease: A Traffic Jam? Curr Biol, 2016. 26(8): R332–4. doi: 10.1016/j.cub.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 13.Esposito G., Ana Clara F., and Verstreken P., Synaptic vesicle trafficking and Parkinson's disease. Dev Neurobiol, 2012. 72(1): 134–44. doi: 10.1002/dneu.20916 [DOI] [PubMed] [Google Scholar]
- 14.Bielska E., Schuster M., Roger Y., Berepiki A., Soanes D.M., Talbot N.J., et al. , Hook is an adapter that coordinates kinesin-3 and dynein cargo attachment on early endosomes. J Cell Biol, 2014. 204(6): 989–1007. doi: 10.1083/jcb.201309022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey S., Banker G., and Ray K., Anterograde Transport of Rab4-Associated Vesicles Regulates Synapse Organization in Drosophila. Cell Rep, 2017. 18(10): 2452–2463. doi: 10.1016/j.celrep.2017.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendricks A.G., Perlson E., Ross J.L., Schroeder H.W. 3rd, Tokito M., and Holzbaur E.L., Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr Biol, 2010. 20(8): 697–702. doi: 10.1016/j.cub.2010.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller M.J., Klumpp S., and Lipowsky R., Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc Natl Acad Sci U S A, 2008. 105(12): 4609–14. doi: 10.1073/pnas.0706825105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soppina V., Rai A.K., Ramaiya A.J., Barak P., and Mallik R., Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc Natl Acad Sci U S A, 2009. 106(46): 19381–6. doi: 10.1073/pnas.0906524106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanada T., Lin L., Tibaldi E.V., Reinherz E.L., and Chishti A.H., GAKIN, a novel kinesin-like protein associates with the human homologue of the Drosophila discs large tumor suppressor in T lymphocytes. J Biol Chem, 2000. 275(37): 28774–84. doi: 10.1074/jbc.M000715200 [DOI] [PubMed] [Google Scholar]
- 20.Horiguchi K., Hanada T., Fukui Y., and Chishti A.H., Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J Cell Biol, 2006. 174(3): 425–36. doi: 10.1083/jcb.200604031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanai Y., Wang D., and Hirokawa N., KIF13B enhances the endocytosis of LRP1 by recruiting LRP1 to caveolae. J Cell Biol, 2014. 204(3): 395–408. doi: 10.1083/jcb.201309066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanamarlapudi V., Centaurin-alpha1 and KIF13B kinesin motor protein interaction in ARF6 signalling. Biochem Soc Trans, 2005. 33(Pt 6): 1279–81. doi: 10.1042/BST20051279 [DOI] [PubMed] [Google Scholar]
- 23.Lamason R.L., Kupfer A., and Pomerantz J.L., The dynamic distribution of CARD11 at the immunological synapse is regulated by the inhibitory kinesin GAKIN. Mol Cell, 2010. 40(5): 798–809. doi: 10.1016/j.molcel.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu M.S. and Prehoda K.E., A NudE/14-3-3 pathway coordinates dynein and the kinesin Khc73 to position the mitotic spindle. Dev Cell, 2013. 26(4): 369–80. doi: 10.1016/j.devcel.2013.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noseda R., Guerrero-Valero M., Alberizzi V., Previtali S.C., Sherman D.L., Palmisano M., et al. , Kif13b Regulates PNS and CNS Myelination through the Dlg1 Scaffold. PLoS Biol, 2016. 14(4): e1002440 doi: 10.1371/journal.pbio.1002440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarbashevich K., Dzementsei A., and Pieler T., A novel function for KIF13B in germ cell migration. Dev Biol, 2011. 349(2): 169–78. doi: 10.1016/j.ydbio.2010.10.016 [DOI] [PubMed] [Google Scholar]
- 27.Xing B.M., Yang Y.R., Du J.X., Chen H.J., Qi C., Huang Z.H., et al. , Cyclin-dependent kinase 5 controls TRPV1 membrane trafficking and the heat sensitivity of nociceptors through KIF13B. J Neurosci, 2012. 32(42): 14709–21. doi: 10.1523/JNEUROSCI.1634-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada K.H., Nakajima Y., Geyer M., Wary K.K., Ushio-Fukai M., Komarova Y., et al. , KIF13B regulates angiogenesis through Golgi to plasma membrane trafficking of VEGFR2. J Cell Sci, 2014. 127(Pt 20): 4518–30. doi: 10.1242/jcs.156109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimura Y., Terabayashi T., and Miki H., Par1b/MARK2 phosphorylates kinesin-like motor protein GAKIN/KIF13B to regulate axon formation. Mol Cell Biol, 2010. 30(9): 2206–19. doi: 10.1128/MCB.01181-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegrist S.E. and Doe C.Q., Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell, 2005. 123(7): 1323–35. doi: 10.1016/j.cell.2005.09.043 [DOI] [PubMed] [Google Scholar]
- 31.Yamada K.H., Kang H., and Malik A.B., Antiangiogenic Therapeutic Potential of Peptides Derived from the Molecular Motor KIF13B that Transports VEGFR2 to Plasmalemma in Endothelial Cells. Am J Pathol, 2017. 187(1): 214–224. doi: 10.1016/j.ajpath.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H.P., Liu Z.M., and Nirenberg M., Kinesin-73 in the nervous system of Drosophila embryos. Proc Natl Acad Sci U S A, 1997. 94(4): 1086–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bentley M., Decker H., Luisi J., and Banker G., A novel assay reveals preferential binding between Rabs, kinesins, and specific endosomal subpopulations. J Cell Biol, 2015. 208(3): 273–81. doi: 10.1083/jcb.201408056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huckaba T.M., Gennerich A., Wilhelm J.E., Chishti A.H., and Vale R.D., Kinesin-73 is a processive motor that localizes to Rab5-containing organelles. J Biol Chem, 2011. 286(9): 7457–67. doi: 10.1074/jbc.M110.167023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsurudome K., Tsang K., Liao E.H., Ball R., Penney J., Yang J.S., et al. , The Drosophila miR-310 cluster negatively regulates synaptic strength at the neuromuscular junction. Neuron, 2010. 68(5): 879–93. doi: 10.1016/j.neuron.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkateswarlu K., Hanada T., and Chishti A.H., Centaurin-alpha1 interacts directly with kinesin motor protein KIF13B. J Cell Sci, 2005. 118(Pt 11): 2471–84. doi: 10.1242/jcs.02369 [DOI] [PubMed] [Google Scholar]
- 37.Aberle H., Haghighi A.P., Fetter R.D., McCabe B.D., Magalhaes T.R., and Goodman C.S., wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron, 2002. 33(4): 545–58. [DOI] [PubMed] [Google Scholar]
- 38.Keshishian H. and Kim Y.S., Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends Neurosci, 2004. 27(3): 143–7. doi: 10.1016/j.tins.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 39.Marques G., Bao H., Haerry T.E., Shimell M.J., Duchek P., Zhang B., et al. , The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron, 2002. 33(4): 529–43. [DOI] [PubMed] [Google Scholar]
- 40.McCabe B.D., Marques G., Haghighi A.P., Fetter R.D., Crotty M.L., Haerry T.E., et al. , The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron, 2003. 39(2): 241–54. [DOI] [PubMed] [Google Scholar]
- 41.Merino C., Penney J., Gonzalez M., Tsurudome K., Moujahidine M., O'Connor M.B., et al. , Nemo kinase interacts with Mad to coordinate synaptic growth at the Drosophila neuromuscular junction. J Cell Biol, 2009. 185(4): 713–25. doi: 10.1083/jcb.200809127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim N.C. and Marques G., Identification of downstream targets of the bone morphogenetic protein pathway in the Drosophila nervous system. Dev Dyn, 2010. 239(9): 2413–25. doi: 10.1002/dvdy.22368 [DOI] [PubMed] [Google Scholar]
- 43.Kim N.C. and Marques G., The Ly6 neurotoxin-like molecule target of wit regulates spontaneous neurotransmitter release at the developing neuromuscular junction in Drosophila. Dev Neurobiol, 2012. 72(12): 1541–58. doi: 10.1002/dneu.22021 [DOI] [PubMed] [Google Scholar]
- 44.Ball R.W., Warren-Paquin M., Tsurudome K., Liao E.H., Elazzouzi F., Cavanagh C., et al. , Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron, 2010. 66(4): 536–49. doi: 10.1016/j.neuron.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 45.McCabe B.D., Hom S., Aberle H., Fetter R.D., Marques G., Haerry T.E., et al. , Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron, 2004. 41(6): 891–905. [DOI] [PubMed] [Google Scholar]
- 46.Berke B., Wittnam J., McNeill E., Van Vactor D.L., and Keshishian H., Retrograde BMP signaling at the synapse: a permissive signal for synapse maturation and activity-dependent plasticity. J Neurosci, 2013. 33(45): 17937–50. doi: 10.1523/JNEUROSCI.6075-11.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Connor-Giles K.M., Ho L.L., and Ganetzky B., Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth. Neuron, 2008. 58(4): 507–18. doi: 10.1016/j.neuron.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goold C.P. and Davis G.W., The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron, 2007. 56(1): 109–23. doi: 10.1016/j.neuron.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haghighi A.P., McCabe B.D., Fetter R.D., Palmer J.E., Hom S., and Goodman C.S., Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron, 2003. 39(2): 255–67. [DOI] [PubMed] [Google Scholar]
- 50.Nahm M., Lee M.J., Parkinson W., Lee M., Kim H., Kim Y.J., et al. , Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron, 2013. 77(4): 680–95. doi: 10.1016/j.neuron.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweeney S.T. and Davis G.W., Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron, 2002. 36(3): 403–16. [DOI] [PubMed] [Google Scholar]
- 52.Tsang H.T., Edwards T.L., Wang X., Connell J.W., Davies R.J., Durrington H.J., et al. , The hereditary spastic paraplegia proteins NIPA1, spastin and spartin are inhibitors of mammalian BMP signalling. Hum Mol Genet, 2009. 18(20): 3805–21. doi: 10.1093/hmg/ddp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodal A.A., Blunk A.D., Akbergenova Y., Jorquera R.A., Buhl L.K., and Littleton J.T., A presynaptic endosomal trafficking pathway controls synaptic growth signaling. J Cell Biol, 2011. 193(1): 201–17. doi: 10.1083/jcb.201009052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dobrowolski R. and De Robertis E.M., Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat Rev Mol Cell Biol, 2012. 13(1): 53–60. https://doi.org/10.1038/nrm3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanson P.I. and Cashikar A., Multivesicular body morphogenesis. Annu Rev Cell Dev Biol, 2012. 28: 337–62. doi: 10.1146/annurev-cellbio-092910-154152 [DOI] [PubMed] [Google Scholar]
- 56.Von Bartheld C.S. and Altick A.L., Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog Neurobiol, 2011. 93(3): 313–40. doi: 10.1016/j.pneurobio.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schou K.B., Mogensen J.B., Morthorst S.K., Nielsen B.S., Aleliunaite A., Serra-Marques A., et al. , KIF13B establishes a CAV1-enriched microdomain at the ciliary transition zone to promote Sonic hedgehog signalling. Nat Commun, 2017. 8: 14177 doi: 10.1038/ncomms14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wandinger-Ness A. and Zerial M., Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol, 2014. 6(11): a022616 doi: 10.1101/cshperspect.a022616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rink J., Ghigo E., Kalaidzidis Y., and Zerial M., Rab conversion as a mechanism of progression from early to late endosomes. Cell, 2005. 122(5): 735–49. doi: 10.1016/j.cell.2005.06.043 [DOI] [PubMed] [Google Scholar]
- 60.Vonderheit A. and Helenius A., Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol, 2005. 3(7): e233 doi: 10.1371/journal.pbio.0030233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poteryaev D., Datta S., Ackema K., Zerial M., and Spang A., Identification of the switch in early-to-late endosome transition. Cell, 2010. 141(3): 497–508. doi: 10.1016/j.cell.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 62.Rojas R., van Vlijmen T., Mardones G.A., Prabhu Y., Rojas A.L., Mohammed S., et al. , Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol, 2008. 183(3): 513–26. doi: 10.1083/jcb.200804048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Priya A., Kalaidzidis I.V., Kalaidzidis Y., Lambright D., and Datta S., Molecular insights into Rab7-mediated endosomal recruitment of core retromer: deciphering the role of Vps26 and Vps35. Traffic, 2015. 16(1): 68–84. doi: 10.1111/tra.12237 [DOI] [PubMed] [Google Scholar]
- 64.Deinhardt K., Salinas S., Verastegui C., Watson R., Worth D., Hanrahan S., et al. , Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron, 2006. 52(2): 293–305. doi: 10.1016/j.neuron.2006.08.018 [DOI] [PubMed] [Google Scholar]
- 65.Xiong X., Wang X., Ewanek R., Bhat P., Diantonio A., and Collins C.A., Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol, 2010. 191(1): 211–23. doi: 10.1083/jcb.201006039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu M.M. and Holzbaur E.L., JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. J Cell Biol, 2013. 202(3): 495–508. doi: 10.1083/jcb.201302078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barkus R.V., Klyachko O., Horiuchi D., Dickson B.J., and Saxton W.M., Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol Biol Cell, 2008. 19(1): 274–83. doi: 10.1091/mbc.E07-03-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jenkins B., Decker H., Bentley M., Luisi J., and Banker G., A novel split kinesin assay identifies motor proteins that interact with distinct vesicle populations. J Cell Biol, 2012. 198(4): 749–61. doi: 10.1083/jcb.201205070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valakh V., Naylor S.A., Berns D.S., and DiAntonio A., A large-scale RNAi screen identifies functional classes of genes shaping synaptic development and maintenance. Dev Biol, 2012. 366(2): 163–71. doi: 10.1016/j.ydbio.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison S.D., Broadie K., van de Goor J., and Rubin G.M., Mutations in the Drosophila Rop gene suggest a function in general secretion and synaptic transmission. Neuron, 1994. 13(3): 555–66. [DOI] [PubMed] [Google Scholar]
- 71.Budnik V., Koh Y.H., Guan B., Hartmann B., Hough C., Woods D., et al. , Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron, 1996. 17(4): 627–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin D.M. and Goodman C.S., Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron, 1994. 13(3): 507–23. [DOI] [PubMed] [Google Scholar]
- 73.Mahr A. and Aberle H., The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr Patterns, 2006. 6(3): 299–309. doi: 10.1016/j.modgep.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 74.Schuster C.M., Davis G.W., Fetter R.D., and Goodman C.S., Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron, 1996. 17(4): 641–54. [DOI] [PubMed] [Google Scholar]
- 75.Ring J.M. and Martinez Arias A., puckered, a gene involved in position-specific cell differentiation in the dorsal epidermis of the Drosophila larva. Dev Suppl, 1993: 251–9. [PubMed] [Google Scholar]
- 76.Jia X.X., Gorczyca M., and Budnik V., Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J Neurobiol, 1993. 24(8): 1025–44. doi: 10.1002/neu.480240804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., et al. , Fiji: an open-source platform for biological-image analysis. Nat Methods, 2012. 9(7): 676–82. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fiala J.C., Reconstruct: a free editor for serial section microscopy. J Microsc, 2005. 218(Pt 1): 52–61. doi: 10.1111/j.1365-2818.2005.01466.x [DOI] [PubMed] [Google Scholar]
- 79.Schneider C.A., Rasband W.S., and Eliceiri K.W., NIH Image to ImageJ: 25 years of image analysis. Nat Methods, 2012. 9(7): 671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stewart B.A., Atwood H.L., Renger J.J., Wang J., and Wu C.F., Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A, 1994. 175(2): 179–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Khc-73 protein domain structure in D. melanogaster and H. sapiens. K (Kinesin motor), FHA (Forkhead Associated), CC (coiled coil) and CAP-GLY (Cytoskeletal Associated Proteins–Glycine Rich) domains. (B) Khc-73 gene structure and location of DP00530 insert. Untranslated regions (UTR) are in solid black. Exons in grey. Deletions Khc-73193 and Khc-73149 are indicated by solid lines. (C) Western blot of Control (Khc-73100), Khc-73 heterozygotes (Khc-73149/+), Khc-73 mutants (Khc-73149) (left) and Control (Khc-73100), Khc-73193 (right) with anti-Khc-73 (top) and actin loading control (bottom).
(D) Localization of HA-Khc-73 in axons (BG380-Gal4/+; UAS-HA-Khc-73). Scale bar is 5μm.
(E) Localization of HA-Khc-73 in muscle 4 NMJ terminal boutons (BG380-Gal4/+; UAS-HA-Khc-73). Scale bar is 5μm. (F) Expression of Khc-73-GAL4 visualized with mCD8:GFP in ventral nerve cord (Khc-73-Gal4/UAS-mCD8:GFP). Scale bar is 100μm. (G) Expression of Khc-73-GAL4 visualized with mCD8:GFP in muscle 4 NMJ (Khc-73-Gal4/UAS-mCD8:GFP). Scale bar is 5μm. (H) Expression pattern of Khc-73-GAL4 visualized with mCD8:GFP in the adult CNS and VNC. (I) Quantification of bouton number in Control (Khc-73100) and Khc-73 (Khc-73149) mutants at muscle 6/7. N = 17, 18 NMJs. Error Bars are SEM. (J) Muscle surface area of muscle 6/7 in Control (Khc-73100) and Khc-73 (Khc-73149). Muscle 6/7 normalized to control. n = 17, 18. Error Bars are SEM. Student’s t-test. ns-no statistical significance. (K) High Frequency Stimulation trace for Control (Khc-73100) and Khc-73 (Khc-73193). N = 11, 13 NMJs. (L) Quantification of EJP amplitudes for genotypes in (K). Recording duration was 600 seconds at 10Hz. EJPs were binned per 30 seconds and amplitudes were averaged per bin. Relative EJP amplitudes were normalized to the average amplitude for the first 15seconds of recording for each genotype. Error bars are SEM. N = 9,8.
(TIF)
(A) Muscle 4 terminal boutons stained with anti-synaptotagmin (SYT). Scale is 5μm. (B) Quantification of SYT mean fluorescence intensity normalized to HRP signal and expressed as a percentage of control for muscle 4 boutons in control (Khc-73193/+) and Khc-73 (Khc-73193/Khc-73193). N = 10, 8 NMJs. (C) Quantification of Cysteine String Protein (CSP) mean fluorescence intensity, normalized to HRP intensity and expressed as a percentage of control, of muscle 4 NMJs. Control (Khc-73100) and Khc-73 (Khc-73193), N = 9, 10. (D) Quantification of EPS-15 staining in control (w1118) and Khc-73 (Khc-73149) larval NMJs. N = 6, 6. (E) Muscle 4 terminal boutons stained with Dlg in control (Khc-73100) (top) and Khc-73 (Khc-73193) (bottom) third instar larvae. Scale bar is 5μm. (F) Quantification of Dlg fluorescence intensity normalized to HRP intensity and expressed as a percentage of control.
(G) Quantification of GluRIIA fluorescence intensity normalized to HRP intensity and expressed as a percentage of control for Control (Khc-73100) and Khc-73 (Khc-73149) mutants. Error Bars are SEM. Student’s t-test. ***P<0.001, ns-no statistical significance.
(TIF)
(A) Brp puncta in terminal boutons of muscle 4 NMJs in Control (Khc-73100/+), Medea (MedeaC246/MedeaG112) and Khc-73; Medea (Khc-73149; MedeaC246 / MedeaG112) larvae. (B) Quantification of BRP puncta number for genotypes in (A). N = 19,20,20 NMJs. (C) Quantification of synaptic area identified with HRP staining for genotypes in (A). N = 19,20,20 NMJs. (D) Quantification of bouton number per NMJ for genotypes in (A). N = 19,20,20 NMJs. (E) Brp puncta in axons of Control (Khc-73100/+), Medea (MedeaC246/MedeaG112) and Khc-73; Medea (Khc-73149; MedeaC246 / MedeaG112) larvae. Green channel–Brp, Red channel–HRP. (F) Quantification of Brp puncta axon density for genotypes in (E) normalized to control. N = 10,10 and 10 Larvae. (G) Acetylated tubulin staining in muscle 4 NMJs of Control (Khc-73100/+) and Khc-73 (Khc-73149), Dashed line–Quantified region. Acetylated-tubulin (green) and HRP (red). Scale bar is 10μm. (H) Quantification of Acetylated-tubulin intensity in axon region indicated by dashed line in (G) N = 9,8. Error Bars are SEM. Student’s t-test. *P<0.05, **P<0.01, ***P<0.001. ns-no statistical significance. Scale bar is 5μm.
(TIF)
(A) Quantification of pMad intensity at muscle 4 NMJs for control (Khc-73100), Khc-73149 and Khc-73193. N = 10, 12 and 15 NMJs. (B) Quantification of pMad intensity in VNC neurons for control (w1118) and Khc-73 mutants (Khc-73149). N = 6(217), 6(234), VNCs(nuclei). (C) Quantification of pMAD intensity at the NMJ in larvae overexpressing Khc-73 in motoneurons. Control (OK371-Gal4/+) and Khc-73 OE (OK371-Gal4/UAS-Khc-73). N = 20, 19. (D) Quantification of pMAD intensity in the ventral nerve cord in larvae overexpressing Khc-73 in motoneurons. Control (OK371-Gal4/+) and Khc-73 OE (OK371-Gal4/UAS-Khc-73) N = 4(100), 3(143), larvae (nuclei).Error Bars are SEM. Student’s t-test. ns-no statistical significance.
(TIF)
(A) Image of muscle 4 NMJs in live, unfixed larvae for Control (BG380-Gal4/+; OK371-Gal4/+; UAS-TKV-YFP/+) and Khc-73 (BG380-Gal4/+; Khc-73149,OK371-Gal4/ Khc-73149; UAS-TKV-YFP/+). Scale bar is 10μm. (B) Quantification of mean fluorescence intensity as percentage of control for genotypes in (A). N = 14, 12 NMJs. (C) Live image of muscle 6/7 NMJs in live, unfixed larvae for Control (BG380-Gal4/+; OK371-Gal4/+; UAS-TKV-YFP/+) and Khc-73 (BG380-Gal4/+; Khc-73149,OK371-Gal4/ Khc-73149; UAS-TKV-YFP/+). Scale bar is 10μm. (D) Quantification of mean fluorescence intensity as percentage of control for genotypes in (C). N = 15, 15 NMJs.
(E) Quantitative PCR analysis of UAS-TKV-YFP mRNA expression levels in Khc-73 mutants. N = 3 technical replicates. (F) Kymograph of TKV-YFP live imaging of axons in third instar larvae in control (BG380-Gal4/+; OK371-Gal4/+; UAS-TKV-YFP/+) and Khc-73 mutants (BG380-Gal4/+; Khc-73149 /Khc-73149, OK371-Gal4; UAS-TKV-YFP/+) larvae. Scale bar is 10μm. Error Bars are SEM. Student’s t-test. ***P<0.001. ns-not statistically significant.
(TIF)
(A) Muscle 4 terminal bouton in Wit OE Control (OK371-Gal4, Khc-73149/UAS-Wit) and Wit OE, Khc-73 larvae (OK371-Gal4, Khc-73149/UAS-Wit, Khc-73149) stained for Hrs (white) and Hrp (red). Scale bar is 5μm. (B) Quantification of Hrs staining in (A). N = 20, 20 NMJs. Error Bars are SEM. *P<0.05. Student’s t-test.
(TIF)
(A) Muscle 4 terminal boutons in motoneurons overexpressing Wit and Rab7:GFP (nSyb-Gal4, UAS-TKV-YFP/UAS-Rab7:GFP) stained with Wit (Green) and GFP (red) antibodies. Scale bar is 5μm. (B) Muscle 4 terminal boutons in motoneurons overexpressing Wit and TKV-YFP (UAS-Wit/+; nSyb-Gal4, UAS-TKV-YFP/+) stained with Wit (Green) and GFP (red) antibodies. Scale bar is 5μm.
(TIF)
(A) RAB7:GFP expressed in live third instar larval axons of Control (BG380-Gal4/+; UAS-RAB7:GFP/+) and Khc-73 mutants (BG380-Gal4/+; Khc-73149; UAS-RAB7:GFP/+). Scale bar is 5 μm. (B) Kymographs from live imaging of RAB7:GFP in axons of Control (BG380-Gal4/+; UAS-RAB7:GFP/+) and Khc-73 mutants (BG380-Gal4/+; Khc-73149; UAS-RAB7:GFP/+). (C) Histogram of Retrograde velocities of RAB7:GFP puncta in Control (BG380-Gal4/+; UAS-RAB7:GFP/+) and Khc-73 mutants (BG380-Gal4/+; Khc-73149; UAS-RAB7:GFP/+). n = 5, 8 axons and n = 64, 49 puncta respectively.
(D) Histogram of anterograde velocities of RAB7:GFP puncta in Control and Khc-73 mutants. n = 5, 8 axons and n = 49, 27 puncta respectively. (E) LacZ staining in control (Khc73100; pucE69 /+) and Khc-73 mutant (Khc-73149; pucE69 /+) larval motor neuron nuclei in the ventral nerve cord. Scale bar is 10μm. (F) Quantification of mean LacZ fluorescence staining in nuclei for genotypes in (E). N = 279, (6) for control (Khc73100; pucE69 /+) and 535, (11) for Khc-73 mutant (Khc-73149; pucE69 /+), Nuclei, (Ventral Nerve Cords), respectively. (G) LacZ staining in control (Khc73100; pucE69 /+) and Khc-73 mutant (Khc-73149; pucE69 /+) larval motor neuron nuclei in the ventral nerve cord after nerve crush assay. Scale bar is 10μm. (H) Quantification of Anterior and Posterior mean fluorescence of nuclei in (G) expressed as Posterior/Anterior ratio. N = 95, 113, (4) for Khc-73100; pucE69 /+. 219, 300, (10) for Khc-73149; pucE69 /+. Anterior nuclei, posterior nuclei, (Ventral Nerve Cords), respectively. Error Bars are SEM. Student’s t-test. ns-no statistical significance.
(TIF)
Live imaging of Rab7:GFP in third instar larva, muscle 4 NMJ in control (OK371-GAL4/+; UAS-RAB7:GFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 5 μm.
(GIF)
Live imaging of Rab7:GFP in third instar larva, muscle 4 NMJ in Khc-73 mutant (OK371-GAL4, Khc-73149/+, Khc-73149; UAS-RAB7:GFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 5 μm.
(GIF)
Live imaging of Rab7:GFP in third instar larva axon in control (OK371-GAL4/+; UAS-RAB7:GFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 10 μm.
(GIF)
Live imaging of Rab7:GFP in third instar larva axon in Khc-73 mutant (OK371-GAL4, Khc-73149/+, Khc-73149; UAS-RAB7:GFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 10 μm.
(GIF)
Live imaging of TKV:YFP in third instar larva, muscle 4 NMJ in control (BG380-Gal4/+; OK371-Gal4/+; UAS-TKV-YFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 5 μm.
(GIF)
Live imaging of TKV:YFP in third instar larva, muscle 4 NMJ in Khc-73 mutant (BG380-Gal4/+; Khc-73149,OK371-Gal4/ Khc-73149; UAS-TKV-YFP/+). Retrograde direction (toward motoneuron cell body) is to the left. Scale bar is 5 μm.
(GIF)
Live imaging of axons in third instar larva of control (OK371-GAL4/UAS-mito:GFP). Retrograde direction is to the left. Scale bar is 10μm.
(GIF)
Live imaging of axons in third instar larva of Khc-73 mutant (OK371-GAL4, Khc-73149/UAS-mito:GFP, Khc-73149). Retrograde direction is to the left. Scale bar is 10μm.
(GIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.