Abstract
Sleep is a recurrent physiologic and fundamental process in every human being, regardless of ethnicity, gender, birthplace, or occupation; however, the features of sleep are swayed by genetic background and environmental influences. All these factors have an intricate relationship, and arise from a complex and assorted genetic repertoire in the alleles that promote a higher genetic variation in human populations.
Sleep disorders have become an uprising public health problem in the modern society; in addition, the correlation between sleep disorders and the development of late chronic diseases has been extensively studied, finding an important causality between them. Therefore, an adequate evaluation of the current situation in a developing continent such as Africa is essential to develop satisfactory health policies.
In this review, we will reprise several aspects that influence the sleep-wake cycle in individuals with African heritage (including African Americans and sub-Saharan Africans), such as genetic background, HIV infection, tropical diseases, immunological markers, cultural aspects, and place them into Africa's context in order to have a better comprehension of its situation.
Abbreviations: CNS, Central nervous system; CRP, C-reactive protein; Cry, Cryptochrome; CSF, Cerebrospinal fluid; HAT, Human African trypanosomiasis; IL-6, Interleukin 6; IL1R2, Interleukin 1 receptor type 2; OSA, Obstructive sleep apnea; PER, Period; PLEK, Plekstrin; SNP, Single nucleotide polymorphism; SES, Socioeconomic status
Keywords: Sleep disorders, African heritage, Obstructive sleep apnea, Tropical diseases, Inflammatory markers
Highlights
-
•
African genes predispose to obstructive sleep apnea and short sleep.
-
•
CRP and IL-6 induce a pro-inflammatory state and sleep disturbances.
1. Introduction
The importance of non-transmittable diseases in Africa is becoming increasingly recognizable, given the lifestyle changes and rapid urbanization; however, few epidemiological studies of the incidence, prevalence, and cause of these diseases have been done. Sleep disorders are among these diseases, and they have important consequences on patients, given that they have a great impact on health, work, and quality of life [1], [2].
Although there are numerous cross-sectional studies examining the prevalence of sleep disorders and their consequences in America and Europe [3], little is known about their occurrence, natural history, and implications on preexisting conditions in Africa.
Even though we lack such information, we can employ data taken from African Americans and some sub-Saharan countries and extrapolate it. This will help us to have a better understanding of the current situation of sleep disorders in Africa and their impact on its inhabitants.
2. African genes and sleep
The notion that sleep may be orchestrated by conserved genetic mechanisms has not yet led to a cohesive understanding of it; however, it is clear that circadian genes affect the timing of sleep. In mammals and invertebrates, the circadian system involves molecular feedback loops within cells that maintain a 24-hour rhythm. The main components of this system are divided into positive and negative regulators. In humans, BMAL1 and NPAS2/CLOCK are the positive regulators that drive the transcription of Per (Period) and Cry (Cryptochrome), which feedback and inhibit the transcription of BMAL1 and NPAS2/CLOCK, thereby forming the negative regulators. Following degradation of the negative regulators, a new cycle begins [4]. This biological clock regulates the timing of sleep and physiological processes that are of fundamental importance to human health, performance, and well-being. Nowadays, there is a global tendency towards decline in sleep duration, mainly due to extrinsic factors. This has been highly documented in America since the 1960s, and it shows a decrease from 8.5 hours to an average of 6 hours in the 2000s [5].
In a study that included individuals with African heritage, allele variations of Per2, Per3, Clock, and AANAT were found. Given that these genes are involved in timing of sleep and activity, it is very possible that the light input pathway has been optimized by natural selection for specific latitudes [3], [6], [7]. This could explain why individuals with African ancestry show different sleep patterns when compared to other ethnic groups in the U.S., having a higher prevalence in both short sleep (< 5 hours), as well as long sleep (> 9 hours) [6], [8], [9], [10]. Interestingly, they also present different sleep architecture, having less Stage 3 non-REM sleep than any other ethnicity in the U.S. [9], [10]. This is particularly important when we take into consideration that there are several studies that correlate a higher cardiovascular risk and a higher incidence of chronic diseases in short sleepers [8], [9], [10]. This can be explained by the circadian activation of multiple genes that are associated with lipid metabolism such as SREBF1 and CPT1A [10]. Furthermore, these two sleep conducts are also correlated to a higher risk of developing hypertension and type 2 diabetes [10]. There are even some studies that found a higher over-all mortality in short sleepers [9], [10].
Obstructive sleep apnea (OSA) is one of the most common and serious sleep disorders. It is characterized by complete or partial obstructions of the upper airway. This condition predisposes to cardiovascular disease, aortic disease, hypertension, stroke, diabetes, clinical depression, and obesity.
There are several genes and single nucleotide polymorphisms (SNPs) that are linked to the development of OSA in African descendants. The presence and activation of certain genes like PPARGC1B (peroxisome proliferator-activated receptor gamma, co-activator 1 beta) and SNP rs6888451 are highly associated with OSA [11]. Another study associated the presence of SNP rs9526240 within serotonin receptor 2a as being a common finding in African descendants with OSA [12]. There are several other genes linked to OSA like SNP rs11126184 in the pleckstrin (PLEK) gene and SNP rs7030789 in the lysophosphatidic acid receptor 1 (LPAR1) gene, which were found to be highly associated to a high Apnea–Hypopnea Index. Moreover, ARNTL (aryl hydrocarbon receptor nuclear translocator-like) and SNP rs10766071 were associated with polysomnographic sleep shortness in these individuals [12].
Regarding African phenotypic features (i.e. anthropometric), which are naturally inherited and are gene-dependent, craneofacial variations like a narrower upper airway, micrognatia, a lower implanted hyoid bone, among others, are associated to a higher risk of developing OSA [13], [14]. The heritability of these features suggests the role of different genes and alleles, and justifies the higher prevalence of OSA in African descendants [15].
3. The immune system and sleep
Sleep and the circadian system exert a strong regulatory influence on immune functions and vice versa. Studies have revealed a selectively enhancing influence of sleep on cytokines that maintain a functioning immune system.
Molecules like IL1-β and TNF-α have been shown to increase non-REM sleep. When inhibited, the amount of spontaneous non-REM sleep is reduced [16], [17]. It has been observed that African descendants have higher levels of TNF-α and IL1-β [16]. This could explain the shortness of sleep in some of these individuals.
It has been described that C-reactive protein (CRP) rises significantly during an inflammatory state. This molecule is an acute phase reactant; it is released to the bloodstream within a few hours after tissue injury, infection, or other causes of inflammation. High levels of CRP are predictive of future cardiovascular morbidity. In epidemiologic studies, short sleep duration and sleep complaints have also been associated with increased cardiovascular morbidity. African descendants have the swiftest elevation of CRP during sleep [16]. In addition, CRP and IL-6 are related to a high body mass index (BMI); this is particularly important in patients with OSA, which is directly proportional to the severity of the disease [18]. Another study found that women with African heritage produce higher amounts (43%) of IL-6 after 120 minutes of a stress-induced inflammatory response [19], [20]. These traits could contribute to the association of short sleep duration and cardiovascular risk observed in these persons.
4. Infections and sleep
Africa is the continent with the highest prevalence of HIV infection in the world, with an estimate of ~ 25,000,000 people in the Sub-Saharan Africa region living with HIV infection. HIV/AIDS is the main cause of mortality in Sub-Saharan Africa, with estimates ~ 12% of all deaths [21].
Individuals that are HIV-positive are prone to develop neurocognitive sleep disorders; it is estimated that 73% of HIV-positive patients suffer from a bad sleep quality [22]. A study found that 45% of HIV patients sleep less than 6 hours per night; additionally, 56% of the sample reported fragmented sleeping. It was also described that HIV-positive patients with African heritage had a higher sleep apnea index in comparison to healthy controls [23].
Sleep analysis through polysomnography in HIV patients revealed that they develop alterations in sleep architecture; they had longer sleep-onset latency and a shorter total sleep time; however, REM sleep and non-REM sleep were within the normal parameters [23], [24]. A study performed in Nigeria that applied the Pittsburgh Sleep Quality Index (PSQI) reported that 59.3% of the subjects with HIV described having poor quality sleep [25]. Likewise, poor sleep maintenance is a common complaint in HIV-positive patients, with 56% of them experiencing waking after sleep onset (WASO) with 15% of their total sleep period. A set of cytokine gene variations were studied among these patients, the main finding was that polymorphisms in IL1R2 and TNF-α genes were significantly associated with WASO, and hence, HIV [26].
Africa has the highest incidence of human African trypanosomiasis (HAT), also called “sleep sickness” or Chagas disease. This is caused by several species of trypanosome, which have been suspected to infect the human species since the dawn of man, which gives us the incentive to ponder that it played a major role in the adaptation of the human species to the African environment [27]. HAT has had a huge cultural impact in sub-Saharan African countries, as of the economic implication of having a waned performance, hence the Zulu term of Nagana (N'gana) to name this disease, which means powerless/useless. A study found that hypocretin (excitatory neuropeptide) levels in the cerebrospinal fluid (CSF) of patients with HAT were lower than in controls (421.5 ± 123.4 pg/ml vs 517.32 ± 194.5 pg/ml, respectively) [28]. This could explain the sleepiness found in these patients; however, the mechanism by which this decline in hypocretin levels occurs still remains to be determined.
HAT is an inflammatory state, and more so, it mainly affects the CNS. It has been described that inflammatory molecules such as IL-6, TNF-α, IFN-γ, and CRP are closely related to the occurrence of sleep disorders [29]. Taking the latter into consideration, a study measured the levels of IL-6, IL-8, IL-10, TNF, and IFN-γ in the CSF and serum of patients with HAT in the Democratic Republic of Congo, before and after treatment. They found a rise of IL-6, IL-8, and IL-10 in intermediate and late stages of the disease. All of them decreased after treatment, although keeping above-average concentrations months following treatment. [30].
This inflammatory state and the persistent production of pro-inflammatory cytokines have detrimental effects on the sleep-wake cycle of these patients and could alter the idiosyncratic duration of sleep observed in some of these individuals.
5. Cultural aspects and sleep
Worldwide, insomnia and insomnia-related sleep disorders are the most prevalent, followed by OSA [1]. Africa is believed to have an underdiagnosed incidence and prevalence of OSA, as well as other sleep disorders [31].
In the U.S., African descendants are more prone to develop insomnia than the rest of the population; this is often related to a lower socioeconomic status (SES), rather than to a racial/ethnic feature [31], [32], [33], [34]. A study in the U.S. found that a low SES was the main aspect that provoked sleep complaints among American minorities, including African descendants, rather than the racial characteristics per se [34]. Furthermore, a study comparing other SES indicators such as employment, education, and marital status, among others, found that all these factors have a negative impact on sleep in all ethnicities, being strikingly higher in African descendants [32]. Another study brings the socioeconomic factor in relation to insomnia, finding that individuals with less official education had worse sleep quality and a higher incidence of insomnia [33], [35], [36].
These facts are of particularly importance, given that the major part of the African population is currently living in developing countries with high poverty rates with a development index below the international average [37].
6. Discussion
Sleep disorders comprise ~ 80 different entities, and most of them can be effectively treated; however, the prevalence, burden, and management of sleep disorders are often ignored or overlooked. This often leads to an under appreciation and poor management, making this group of illnesses a serious health concern. This is particularly evident in Africa, a continent with a population that has a unique genetic repertoire and cultural background.
If we take into consideration what was previously discussed, the genetic background deeply influences the architecture of sleep. In the case of the individuals with an African ancestry, the variations that strike the most are SNPs that negatively affect the duration and quality of sleep, such as rs6888451, rs9526240 within the HTR2A gene, and rs11126184 in the PLEK gene [11], [12], which predispose to OSA. Population-based studies suggest that OSA prevalence is higher in African Americans compared with Caucasians; unfortunately, there are no data about OSA prevalence in African countries for comparison. Obtaining such data is imperative, given that OSA is an important risk factor for developing hypertension and cardiovascular diseases, independent of excess weight and other potentially confounding factors.
From the immunological standpoint, there is evidence that some inflammatory mediators like TNF-α and IL1β, which increase non-REM sleep, are elevated in these individuals, as well as in HIV patients [38]. Another interesting fact, is the rapid elevation of CRP in African descendants when their total sleep is either < 5 or > 9 hours; this is the ethnic group with the swiftest elevation in CRP levels when compared to all other ethnicities [19], and may be the cause of the different sleep pattern observed in this group. Studies have shown that African descendants have a higher prevalence of short sleep (< 5 hours) and long sleep (> 9 hours) than any other ethnicity, heightening the rise in CRP levels [39].
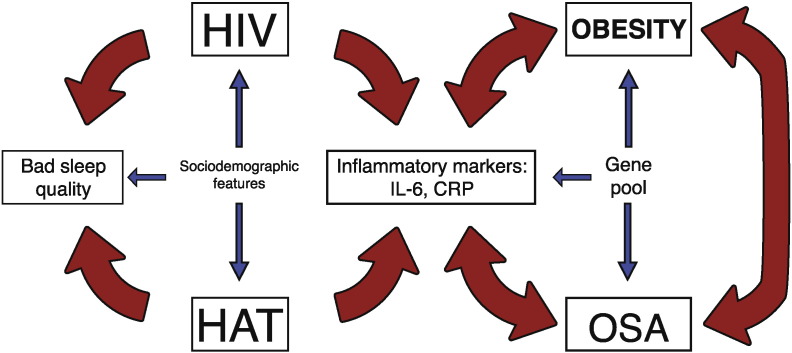
The contemporary rise in obesity has also been observed in sub-Sahara Africa. Obesity itself results in an inflammatory state that results in an increase of IL-6 and CRP. In addition, obesity exponentially increases the risk of suffering OSA. Moreover, there are studies that directly correlate OSA with the elevation of CRP and IL-6 [18], [40]. This derives in a positive feedback loop between OSA, CRP/IL-6, and obesity, which ultimately ends in a severe sleep and homeostatic disarray, as well as predisposing to OSA (Fig. 1). This correlation is of particular importance, given that the prevalence of overweight and obesity has drastically increased in developing countries, especially in South Africa [41].
Fig. 1.
Interactions between OSA, infectious diseases, and African genes.
Obesity in an inflammatory state that results in an increase of IL-6 and CRP, both conditions increase the risk of suffering OSA. In turn, OSA induces an increase of CRP and IL-6. This derives in a positive feedback loop between OSA, CRP/IL-6, and obesity, which ultimately ends in a severe sleep and homeostatic disarray. In addition, infectious diseases like HIV and HAT also lead to an increase of pro-inflammatory cytokines that favor the development of OSA.
CRP, C-reactive protein; HAT, human African trypanosomiasis; HIV, human immunodeficiency virus; OSA, obstructive sleep apnea;
Africa is the continent with the highest prevalence for HIV and HAT infection in the world. These conditions favor the predisposition of developing neurocognitive and sleep disorders in the African population. These include bad sleep quality (estimated in 73% of HIV + patients), short periods of sleep (< 6 hours in 45% of patients), and alterations in the architecture of the circadian cycle [23], [24], [25], [26]. The presence of WASO has been described in 56% of HIV + patients, correlating a narrow relationship between WASO and polymorphisms in IL-1R2 and TNF-α genes [26]. In patients with HAT, hypocretin levels in CSF are lower than in non-infected patients, which can explain the sleepiness [28]. These infected patients also presented elevated levels of IL-6, IL-8, IL-10, TNF-α, and IFN-γ in CSF and plasma in intermediate and late stages, but decrease after treatment [30]. Alterations of these inflammatory mediators are highly predisposing to sleep disorders.
With growing urbanization, changing lifestyles, and poor surveillance, the incidence of sleep disorders might increase rapidly in Africa even while the region still stands a great burden from infectious diseases like HIV and HAT. These trends will further stretch already weak health systems and could result in a reversal of recent improvements in health. As the HIV/HAT epidemic develops, countries in Africa will need to evaluate how to address these diseases and its repercussions on sleep. This is of utmost importance, given that these individuals are particularly predisposed to alterations in sleep duration, polymorphisms that alter the levels of pro-inflammatory cytokines, and the development of OSA (Fig. 1). This combination of factors results in a higher incidence of cardiovascular disease, in addition to other long-term health consequences.
OSA and short sleep are probably the leading sleep disorders in Africa; if we sum these conditions to the epidemic of infectious diseases, we could infer that African inhabitants are prone to develop a pro-inflammatory state that results in a homeostatic disarray that favors the development of conditions that account for the leading causes of mortality in adults, such as hypertension and cardiovascular disease (Fig. 1). Interestingly, an adequate management of these sleep disorders through public health initiatives could taper these conditions.
The impact of sleep on health and well-being is under-recognized. But the growing body of knowledge about the complex structure, function, and mechanisms of sleep, as well as the consequences when sleep is disturbed, should serve as a reminder for making sleep a public health priority [42]. The need for thorough and systematic epidemiological studies in Africa to prevent further aggravation of sleep disorders and its impact on well-being is imperative to establish adequate public policies that help improve the health of its inhabitants.
References
- 1.Kirsch Douglas. Wiley-Blackwell; October 2013. Sleep Medicine in Neurology. (First Chapter) [Google Scholar]
- 2.Trenell M.I., Marshall N.S., Rogers N.L. Sleep and metabolic control: waking to a problem? Clin. Exp. Pharmacol. Physiol. 2007;34:1–9. doi: 10.1111/j.1440-1681.2007.04541.x. [DOI] [PubMed] [Google Scholar]
- 3.Adenekan B., Pandey A., Mckenzie S. Sleep in America: role of racial/ethnic differences. Sleep Med. Rev. 2013;17(4):255–262. doi: 10.1016/j.smrv.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciarleglio C.M., Ryckman K., Servick S.V. Genetic Differences in human circadian clock genes among worldwide populations. J. Biol. Rhythm. 2008;23(4):330–340. doi: 10.1177/0748730408320284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budhiraja R., Roth T., Hudgel D.W. Prevalence and Polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34(7):859–867. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia G., Patterson N., Pasaniuc B. Genome-wide comparison of African-ancestry populations from CARe and other cohorts reveals signals of natural selection. Am. J. Hum. Genet. 2011;89(3):368–381. doi: 10.1016/j.ajhg.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnardottir E.S., Nikonova E.V., Shockley K.R. Blood-gene expression reveals reduced circadian rhythmicity in individuals resistant to sleep deprivation. Sleep. 2014;37(10):1589–1600. doi: 10.5665/sleep.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kripke D.F., Simons R.N., Garfinkel L. Short and long sleep and sleeping pills. Is increased mortality associated? Arch. Gen. Psychiatry. 1979;36:103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 9.Gallicchio L., Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J. Sleep Res. 2009;18:148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 10.Cappuccio F.P., D’Elia L., Strazzullo P. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larkin E.K., Patel S.R., Goodloe R.J. A candidate gene study of obstructive sleep apnea in European Americans and African Americans. Am. J. Respir. Crit. Care Med. 2010;182(7):947–953. doi: 10.1164/rccm.201002-0192OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kripke D.F., Kline L.E., Nievergelt C.M. Genetic variants associated with sleep disorders. Sleep Med. 2015;16(2):217–224. doi: 10.1016/j.sleep.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larrabee Wayne F. second ed. Lippincott Williams & Wilkins; 2004. Surgical Anatomy of the Face; pp. 27–32. (Chapter 3) [Google Scholar]
- 14.Chi L., Comyn F.-L., Keenan B.T. Heritability of craniofacial structures in normal subjects and patients with sleep apnea. Sleep. 2014;37(10):1689–1698. doi: 10.5665/sleep.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiter M.E., Decoster J., Jacobs L. Normal sleep in African-Americans and Caucasian-Americans: a meta-analysis. Sleep Med. 2011 Mar;12(3):209–214. doi: 10.1016/j.sleep.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Krueger J.M. The role of cytokines in sleep regulation. Curr. Pharm. Des. 2008;14(32):3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krueger J.M., Clinton J.M., Winters B.D. ). Involvement of cytokines in slow wave sleep. Prog. Brain Res. 2011;193:39–47. doi: 10.1016/B978-0-444-53839-0.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnardottir E.S., Maislin G., Schwab R.J. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the icelandic sleep apnea cohort. Sleep. 2012;35(7):921–932. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandner M.A., Buxton O.M., Jackson N. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36(5):769–779. doi: 10.5665/sleep.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christian L.M., Glaser R., Porter K. Stress-induced inflammatory responses in women: effects of race and pregnancy. Psychosom. Med. 2013;75(7):658–669. doi: 10.1097/PSY.0b013e31829bbc89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Library Cataloguing-in-Publication Data . UNAIDS / JC2502/1/E”- Revised and Reissued, November 2013. 2013. Global report: UNAIDS report on the global AIDS epidemic. [Google Scholar]
- 22.Maschke M. Incidence and prevalence of neurological disorders associated with HIV since the introduction of Highly Active Antiretroviral Therapy (HAART) J. Neurol. Neurosurg. Psychiatry. 2000;69(3):376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamaldo C.E., Spira A.P., Hock R.S. Sleep, function & HIV: a multi-method assessment. AIDS Behav. 2013;17(8):2808–2815. doi: 10.1007/s10461-012-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiegand M., Möller A.A., Schreiber W. Alterations of nocturnal sleep in patients with HIV infection. Acta Neurol. Scand. 1991;83:141–142. doi: 10.1111/j.1600-0404.1991.tb04664.x. [DOI] [PubMed] [Google Scholar]
- 25.Oshinaike O., Akinbami A., Ojelabi O. Quality of sleep in an HIV population on antiretroviral therapy at an urban tertiary centre in Lagos, Nigeria. Neurol. Res. Int. 2014;2014:1–6. doi: 10.1155/2014/298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee K.A., Gay C., Pullinger C.R. Cytokine polymorphisms are associated with poor sleep maintenance in adults living with human immunodeficiency virus/acquired immunodeficiency syndrome. Sleep. 2014;37(3):453–463. doi: 10.5665/sleep.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steverding D. The history of African trypanosomiasis. Parasites Vectors. 2008;1(3):1–8. doi: 10.1186/1756-3305-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dauvilliers Y., Bisser S., Chapotot F. Hypocretin and Human African trypanosomiasis. Sleep. 2008;31(3):348–354. doi: 10.1093/sleep/31.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullington Janet M. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24.5:775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lejon V., Lardon J., Kenis G. Interleukin (IL)-6, IL-8 and IL-10 in serum and CSF of Trypanosoma brucei gambiense sleeping sickness patients before and after treatment. Trans. R. Soc. Trop. Med. Hyg. May-Jun 2002;96(3):329–333. doi: 10.1016/s0035-9203(02)90115-x. [DOI] [PubMed] [Google Scholar]
- 31.Grandner M.A., Patel N.P., Gehrman P.R. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med. 2010;11(5):470–478. doi: 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandner M.A., Petrov M.E.R., Rattanaumpawan P. Sleep symptoms, race/ethnicity, and socioeconomic position. J. Clin. Sleep Med. 2013;9(9):897–905. doi: 10.5664/jcsm.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hale L., Do D.P. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mezick E.J., Matthews K.A., Hall M. Influence of race and socioeconomic status on sleep: Pittsburgh Sleep SCORE project. Psychosom. Med. 2008;70(4):410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gellis Les A., Lichstein Kenneth L., Scarinci Isabel C. Socioeconomic status and insomnia. J. Abnorm. Psychol. Feb 2005;114(1):111–118. doi: 10.1037/0021-843X.114.1.111. [DOI] [PubMed] [Google Scholar]
- 36.Patel N.P., Grandner M.A., Xie D. “Sleep disparity” in the population: poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health. 2010;10:475. doi: 10.1186/1471-2458-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barthélémy Kuate Defo. Demographic, epidemiological, and health transitions: are they relevant to population health patterns in Africa? Glob. Health Action. 2014;7 doi: 10.3402/gha.v7.22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bélec L., Meillet D., Hernvann A. Differential elevation of circulating interleukin-1 beta, tumor necrosis factor alpha, and interleukin-6 in AIDS-associated cachectic states. Clin. Diagn. Lab. Immunol. 1994;1(1):117–120. doi: 10.1128/cdli.1.1.117-120.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buxton O.M., Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc. Sci. Med. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 40.Casale M. Obstructive sleep apnea syndrome: from phenotype to genetic basis. Curr. Genomics. 2009;10(2):119–126. doi: 10.2174/138920209787846998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The GBD 2013 Obesity Collaboration et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. Aug 30 2014; 384(9945): 766–781. [DOI] [PMC free article] [PubMed]
- 42.Ferrie J.E., Kumari M., Salo P. Sleep epidemiology--a rapidly growing field. Int. J. Epidemiol. 2011;40(6):1431–1437. doi: 10.1093/ije/dyr203. [DOI] [PMC free article] [PubMed] [Google Scholar]