Abstract
Epilepsy is the most common neurological disorder which significantly affects the quality of life and poses a health as well as economic burden on society. Epilepsy affects approximately 70 million people in the world. The present article reviews the scientific rationale, brief pathophysiology of epilepsy and newer antiepileptic drugs which are presently under clinical development. We have searched the investigational drugs using the key words ‘antiepileptic drugs,’ ‘epilepsy,’ ‘Phase I,’ ‘Phase II’ and ‘Phase III’ in American clinical trial registers (clinicaltrials.gov), the relevant published articles using National Library of Medicine's PubMed database, company websites and supplemented results with a manual search of cross-references and conference abstracts. This review provides a brief description about the antiepileptic drugs which are targeting different mechanisms and the clinical development status of these drugs. Besides the presence of old as well as new AEDs, still there is a need of new drugs or the modified version of old drugs in order to make affected people free of seizures. An optimistic approach should be used to translate the success of preclinical testing to clinical practice. There is an urgent need to improve animal models and to explore new targets with better understanding in order to develop the novel drugs with more efficacy and safety.
Keywords: Seizures, Epilepsy, Drug development, Neuroprotection, Clinical trial, Antiepileptic
Highlights
-
•
This review primarily focused on antiepileptic drugs under clinical development.
-
•
The more realistic approach is needed to discover and develop the novel antiepileptic drugs.
-
•
Modification of conventional drugs or search of newer targets can lead to development of promising antiepileptic drugs.
-
•
To develop more efficacious and safe drugs for treatment of epilepsy and refractory seizures
-
•
There are a number of novel antiepileptic compounds which are under various stages of drug development.
1. Introduction
Epilepsy is the most common neurological disorder which significantly affects the quality of life and poses a health as well as economic burden on society. Epilepsy affects an approximately 70 million people in the world [72]. In the United States, more than 300,000 people with epilepsy are younger than 14 and more than 500,000 are older than 65. With age, the incidence rate of epilepsy is fluctuating as high levels in childhood followed by decreasing order in early adult life which precedes by second high rate at the age of more than 65 years old [62]. Epilepsy diminishes health related quality of people as there is increased risk of injuries during seizures and higher mortality as compared to normal people. Epilepsy affects an estimated 1.5 million women in the United States [77]. The estimation of the corresponding rates is higher in low and middle income countries [72]. In India, the prevalence of epilepsy is 6–10 per 1000 people [92]. Sudden unexpected death in epilepsy (SUDEP) is the most common and important cause of death which is directly related to epilepsy and is the major cause of mortality in chronic uncontrolled epileptic patients [102]. With the help of new and improved approaches, many new AEDs and modified version of older drugs have been available to treat epilepsy. Of these new AEDs, few are rarely prescribed because of their serious side effects.
Despite availability of several AEDs, one-third of patients still have intolerable condition. Currently there is an urgent need to create new opportunities and improve the existing drugs to relieve patients. A review was conducted to find articles reporting on antiepileptic drugs which are under developmental phase in partial onset seizures, refractory partial seizures, generalized tonic clonic seizures, and resistant partial onset seizures in the year of 2015 and 2016. A literature search using the keywords seizures, epilepsy, drug development, clinical trials, and antiepileptic has been accomplished. Multiple databases were searched including ClinicalTrial.gov, Pubmed, database, company websites and supplemented results with a manual search of cross-references and conference abstracts. This review covers briefly introduction, pathophysiology and recent ongoing clinical trials of epilepsy.
2. Pathophysiology
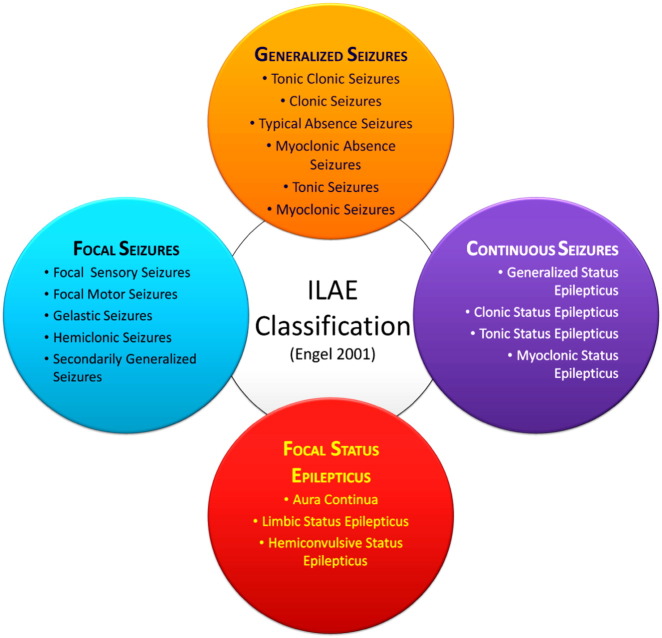
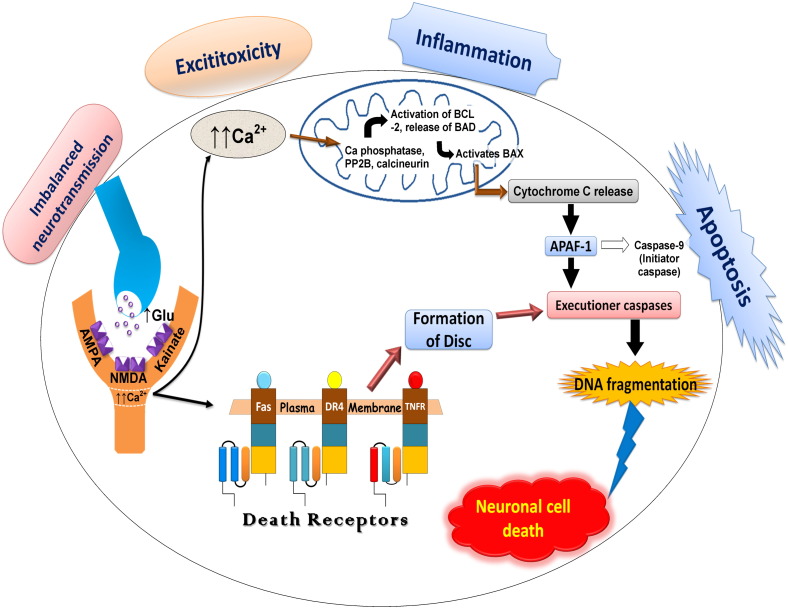
In 2015, according to ILAE the conceptual definition of seizures or epilepsy includes “a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain” and epilepsy as “a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures, and by the neurobiologic, cognitive, psychological, and social consequences of this condition”. For this, at least one episode of epilepsy is needed. The ILAE and the international Bureau for epilepsy (IBE) in 2014 have advised to regard epilepsy as a disease. The new clinical definition of epilepsy as a disease includes one of the following: (A) at least two unprovoked (or reflex) seizures occurring more than 24 h apart; or (B) one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years; or (C) diagnosis of an epilepsy syndrome [86] (Fig. 1). The hyper synchronous, abnormal discharge of neurons begins in discrete region of cortex and then spread to neighbouring regions. These activated neurons emit excessive bursts of action potential or electrical energy. The pathophysiology of epilepsy involves conversion of a normal network into a hyper excitable network. It is associated with a group of processes which disturb extracellular ion homeostasis, alter energy metabolism, change receptor function and alter transmitter uptake. In CNS, the brain consists of nerve cells and these nerve cells communicate and interact with each other through axons by discharging tiny electrical impulses. The brain along with nerve cells works on the phenomenon of electricity. The output of these electrical impulses is the release of chemicals called neurotransmitters from the axon end which in turn interacts with the next cell. These chemicals (neurotransmitters) can be excitatory or inhibitory. The balance of these excitatory and inhibitory impulses is very important to maintain the action potential of neurons [36], [62]. Release of excessive excitatory glutamate overactivates NMDA receptors resulting in excessive influx of Ca2 + ions. The overflow of Ca2 + levels caused deteriorate condition which activates cytoplasmic proteases (such as calpain I), which proteolysis cytoskeletal and other proteins [100], neuronal nitric oxide synthase (nNOS), which increases nitric oxide production in turn generating the free radical peroxynitrite that damages DNA [31] which ultimately lead to neuronal cell death (Fig. 2).
Fig. 1.
Epilepsy classification.
Fig. 2.
Basic cascade events of pathophysiology of epilepsy.
3. Antiepileptic drugs under clinical development
During the last three decades, the antiepileptic drug development has been focused on the specific therapeutic targets concerning the neurobiology of epilepsy but most of the available AEDs have not shown efficacy in treatment of patients with refractory epilepsy. Despite the apparent success of the AED discovery process, there is a need of developing more efficacious and safe antiepileptic drugs particularly for the treatment of refractory seizures. The primary goal of newer AED research should be to develop novel compounds for controlling seizures with more therapeutic efficacy and less adverse effects. Three main strategies are currently adopted by the researchers and pharmaceutical industry in order to develop novel compounds for treatment of epilepsy: (a) modification of existing drugs, (b) development of compounds against novel therapeutic targets or new hypothesis, and (c) non-mechanism based drug screening in conventional and newer animal models [81], [93]. Few of newer drugs which are presently under the clinical development phases (Table 1) are discussed below.
Table 1.
Current developmental status of new drugs for treatment of epilepsy.
| S. no. | Name of intervention | Sponsor | Structure | Therapeutic target | Condition | Clinical development phase |
|---|---|---|---|---|---|---|
| 1 | Muscimol | National Institute of Neurological Disorders and Stroke (NINDS) | 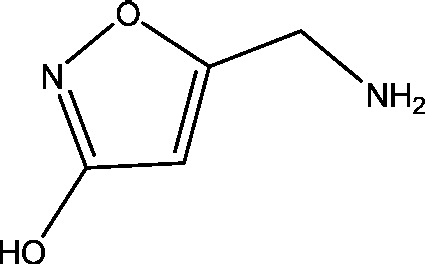 |
GABAA receptor | Epilepsy | Phase 1 |
| 2 | Bumetanide | – | 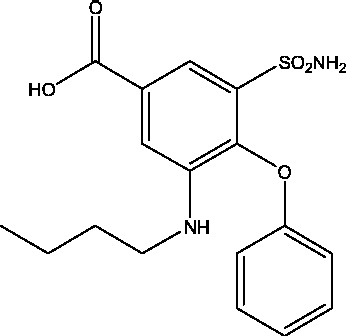 |
NKCC1 inhibition | Neonatal seizures | Phase 1 |
| 3 | BGG492 (Selurampanel) | Novartis Pharmaceuticals | 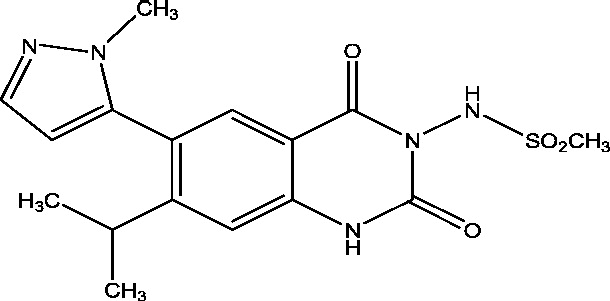 |
AMPA/kainate receptor antagonism | Refractory partial seizures | Phase 2 |
| 4 | Ganaxolone | Marinus Pharmaceuticals | 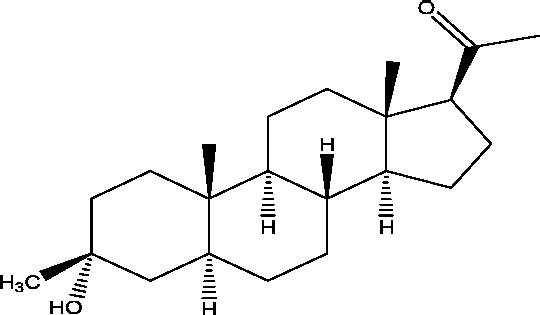 |
Positive allosteric modulator of GABAA receptors | Uncontrolled partial epilepsy; catamenial epilepsy | Phase 2 |
| 5 | Buspirone | NINDS | 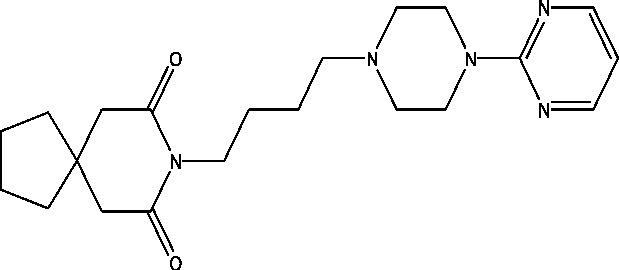 |
5-HT1A receptor partial agonist | Localized epilepsy | Phase 2 |
| 6 | YKP3089 | SK Life Sciences |  |
Sodium channel modulation, ↑ GABA release | Resistant partial onset seizures | Phase 2 |
| 7 | PRX-00023 | NINDS | 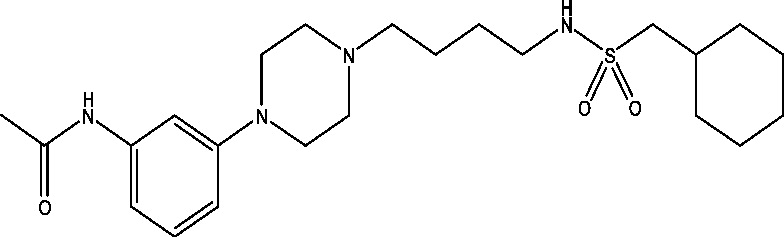 |
5-HT receptors | TLE; partial epilepsy | Phase 2 |
| 8 | GWP42003-P (Cannabidiol) | GW Research Ltd. INSYS Therapeutics Inc. |
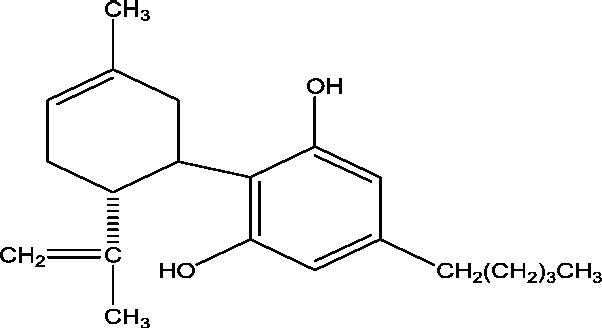 |
– | Dravet syndrome Lennox Gastaut syndrome Myoclonic epilepsy |
Phase 2 Phase 3 |
| 9 | Verapamil | Beverly S. Wical, Gillette Children's Specialty Healthcare | 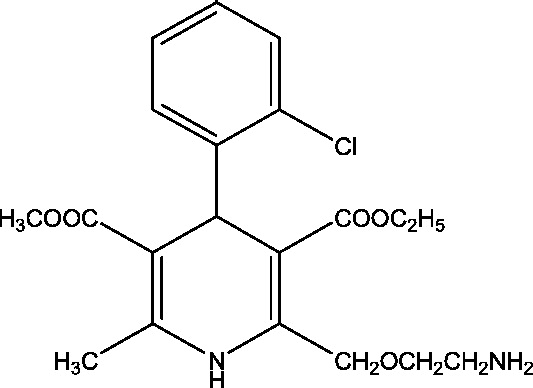 |
Calcium channel modulation | Dravet syndrome | Phase 2 |
| 10 | RWJ-333369 (carisbamate) | SK Life Science | 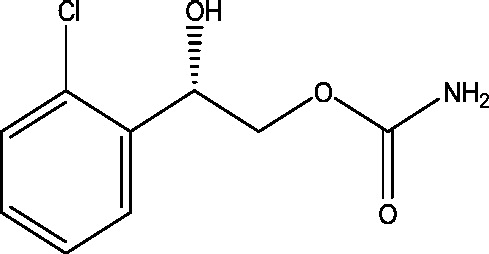 |
Not elucidated | Partial epilepsy | Phase 3 |
| 11 | Thalidomide | National Institute of Neurology and Neurosurgery, Mexico | 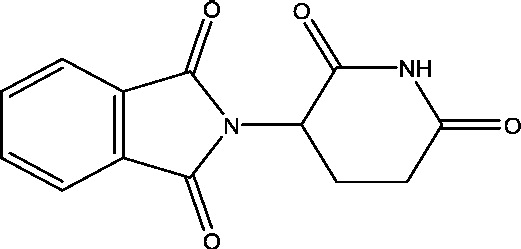 |
Refractory epilepsy | Phase 2 | |
| 12 | VX-765 | Vertex Pharmaceuticals | 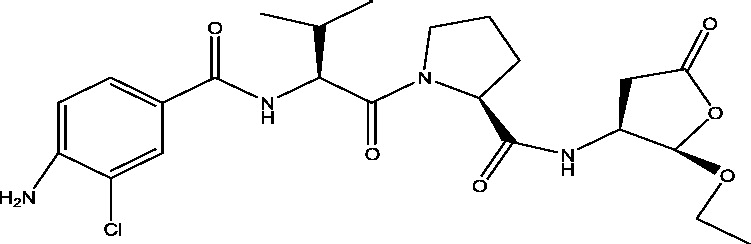 |
Caspase-1 inhibitor | Resistant partial epilepsy | Phase 2 |
| 13 | Eslicarbazepine acetate | Sunovion Pharmaceuticals Inc. Bial-Portela USA |
 |
Sodium channel inhibition | Epilepsy with partial onset seizures Refractory partial seizures |
Phase 3 |
| 14 | Docosahexaenoic acid | Canadian College of Naturopathic Medicine (CCNM) | 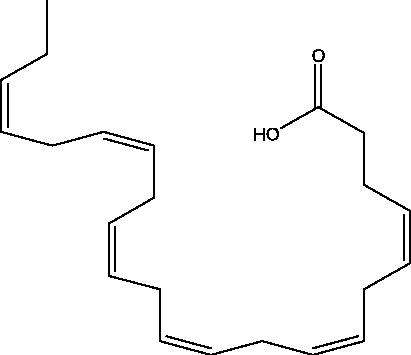 |
Refractory Seizures | Phase 3 | |
| 15 | Seletracetam (ucb 44212) | UCB, Inc. | 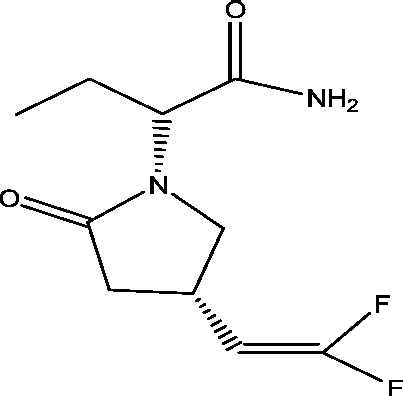 |
SV2A; presynaptic calcium channels inhibition | Partial onset seizures | Phase 3 |
| 16 | USL255 | Upsher-Smith Laboratories | 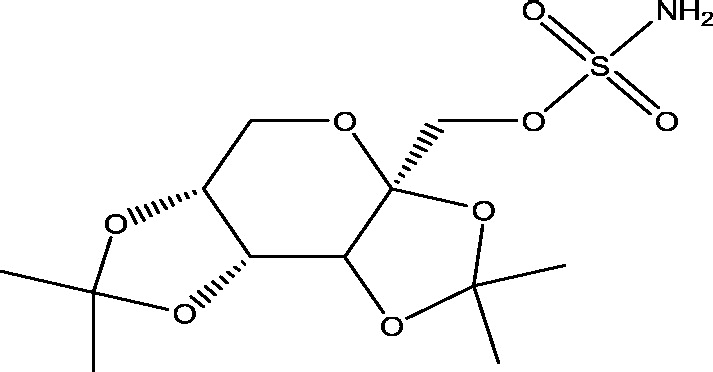 |
Sodium and calcium channels modulation | Refractory partial-onset seizures | Phase 3 |
| 17 | Brivaracetam (ucb 34714) | UCB Inc. | 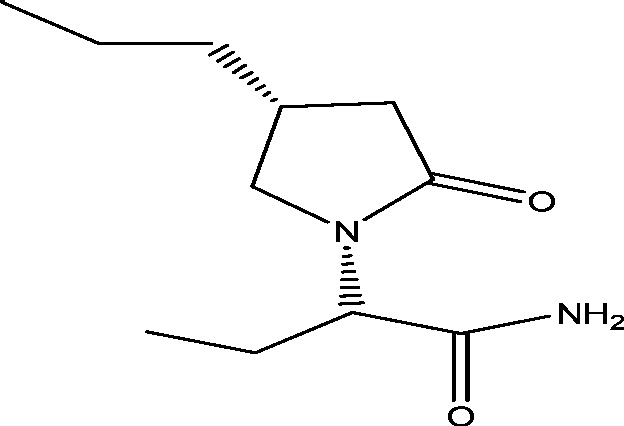 |
Same as of levetiracetam | Epilepsy | Phase 3 |
| 18 | Pregabalin | Pfizer | 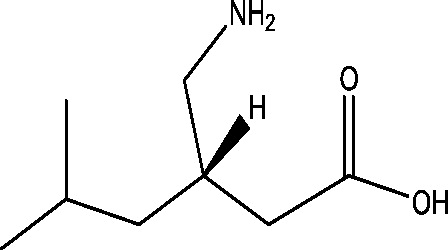 |
Calcium channel modulation | Partial onset seizures; primary GTC seizures | Phase 3 |
| 19 | RAD001 (Everolimus) | Novartis Pharmaceuticals Baylor College of Medicine New York University School of Medicine |
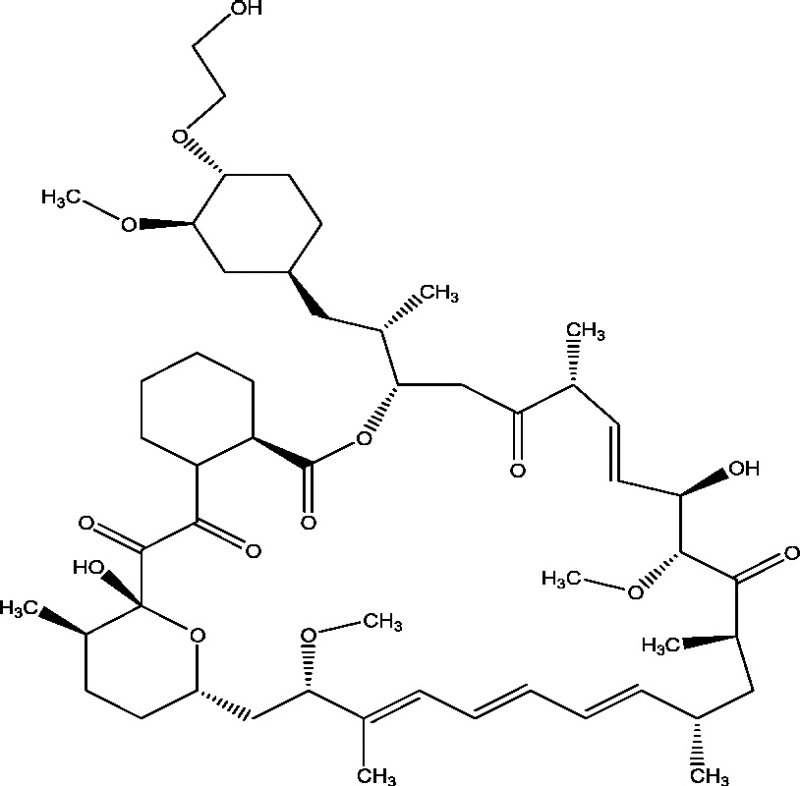 |
mTOR inhibition | Tuberous sclerosis complex-associated refractory seizures | Phase 3 |
| 20 | E2007 (Perampanel) | Eisai Inc. | 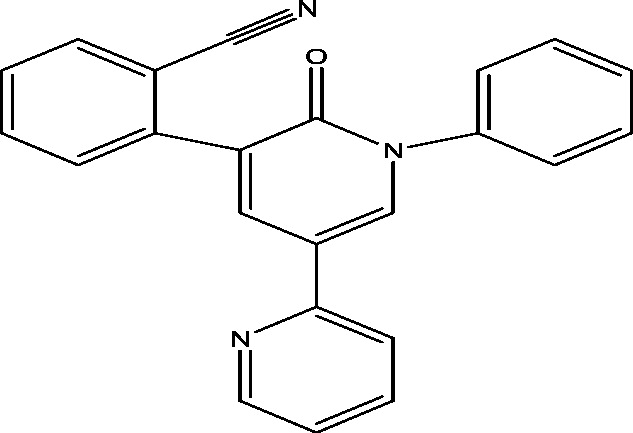 |
Non-competitive AMPA antagonism | Refractory partial seizures; primary generalized tonic clonic seizures | Phase 3 |
| 21 | TRI476 | Novartis Pharmaceuticals | 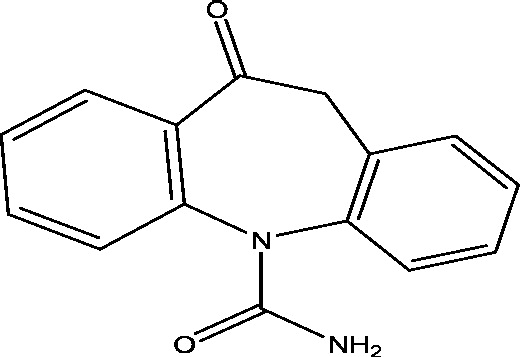 |
Sodium channel modulation | Partial onset seizures | Phase 3 |
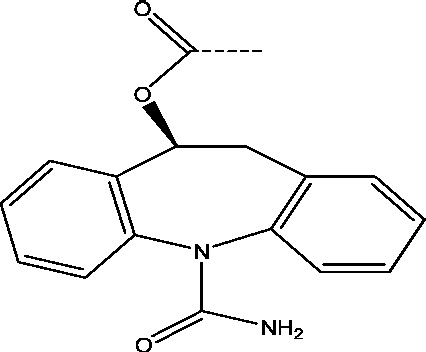
| 22 | Retigabine/ezogabine | GlaxoSmithKline | 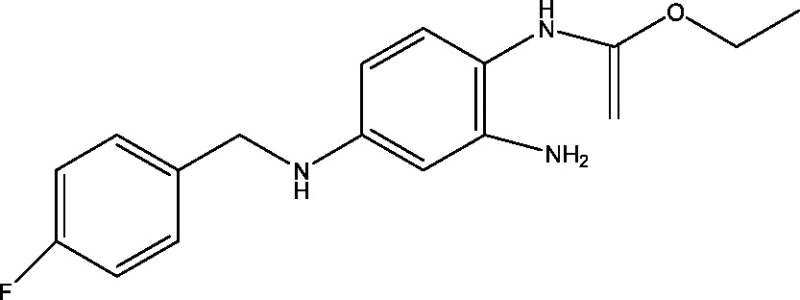 |
Potassium channel modulation | Partial onset refractory seizures | Phases 3, 4 |
3.1. Seletracetam and brivaracetam
The modification of the levetiracetam led to the development of the compounds, seletracetam and brivaracetam, which have greater affinity towards SV2A [57]. Seletracetam is a structural analogue of the levetiracetam, sharing the same mechanism of action by binding to synaptic vesicle protein 2A (SV2A). It possesses a 10-fold greater affinity towards SV2A which is further involved in synaptic neurotransmitter release [68]. Like levetiracetam, seletracetam did not show any anticonvulsant activity in the two classical screening models for AEDs: the maximal electroshock test (MES) and the pentylenetetrazol (PTZ) test [71]. However, it showed potent activity in other animal models of partial (kindled models) and generalized epilepsy (audiogenic seizures and genetic absence epilepsy rats from Strasbourg) [24]. On the other side, brivaracetam also possesses sodium channel blocking property which may account for its activity in other preclinical screening models, including MES and PTZ seizure models [70]. In placebo controlled Phase II trials, both brivaracetam and seletracetam have found to be effective in patients with photosensitive epilepsy [25], [56]. Positive results from stage III trials implicated that brivaracetam at the dose of 50 mg when compared to 5 mg/day and 20 mg/day [28] and at the dose of 100 mg/day as compared to 20 mg/day and 50 mg/day [89] significantly improved seizure frequency in adults (16 to 70 years) with uncontrolled partial onset seizures and adjunctive brivaracetam in adults with uncontrolled focal and generalized seizures [61] along with evidence that it is around 10 times more potent for the prevention of certain types of seizure in mouse models than levetiracetam, of which it is an analogue [71].
Recently, brivaracetam was approved on Jan 14, 2016 by European Medicine Agency (EMA) and on Feb 18, 2016 by the U.S. Food and Drug Administration (FDA). It was approved under the trade name Briviact (developed and marketed by UCB) as an add-on treatment to other medications to treat partial onset seizures in patients age 16 years and older with epilepsy. Briviact is available in three formulations, including film-coated tablets, oral solution and solution for injection/infusion [1].
3.2. Bumetanide
Bumetanide is an inhibitor of Na-K-Cl cotransporter (NKCC). Two isoforms of NKCC are present in the body: NKCC1 is expressed in the brain and NKCC2 is present in the kidney. It has a potential use in patients with temporal lobe epilepsy having defective chloride homeostasis which is caused by increased NKCC1 expression and depolarizing GABA responses [55]. There is evidence that bumetanide application suppressed the seizure-like activity in human TLE slices through GABAergic responses [53]. Bumetanide administration in patients with temporal lobe epilepsy caused a significant reduction in number of seizures and epileptiform discharges in brain [42]. A pilot study is already underway to explore the pharmacokinetic and safety profile of bumetanide in newborns with refractory seizures [80], [37].
3.3. BGG492 (Selurampanel)
The novel idea of AMPA-glutamate receptor antagonism has gained a considerable interest for the development of new antiepileptic drugs targeting this novel mechanism of action. BGG492 (Selurampanel) is an orally active competitive AMPA antagonist. It has good oral bioavailability and blood–brain barrier penetration [46]. In preclinical development, BGG492 has shown anticonvulsant activity in several rodent models of epilepsy including maximal electroshock seizure, audiogenic seizure, amygdala kindling model, and genetic models [51], [58]. BGG-492 is currently in Phase II development for the treatment of patients (Table 1) with partial epilepsy [38].
3.4. Ganaxolone
Ganaxolone is the only neurosteroid in clinical development for treatment of partial onset seizures. It possesses GABA modulatory activity via acting through GABAA receptors. Several Phase II studies have been conducted in adults and pediatric patients with partial-onset seizures, refractory epilepsy and infantile spasms [26], [79], [9]. It was also found to be safe and effective as adjunctive therapy in adults with partial seizures [8], [74]. Ganaxolone is currently in Phase III development for epilepsy (Table 1).
3.5. YKP3089
YKP-3089 is a novel compound showing a broad spectrum of efficacy in various animal models of seizures and epilepsy [26]. It is well tolerated and showed to be effective in patients with photosensitive epilepsy [41]. A Phase IIb Trial of YKP3089 is ongoing in patients with partial onset seizures [4], [27].
3.6. PRX-00023
PRX-0023 (Naluzotan) is a serotonergic drug which acts via 5-HT1A receptor. Currently, the researchers are investigating the effects of the experimental medication PRX-00023 on seizure frequency in patients with focal epilepsy [85].
3.7. GWP42003-P (Cannabidiol)
GWP42003-P (Cannabidiol) is a biologically active compound obtained from cannabis plant. Recently, this compound is being investigated for treatment of epilepsy. A Phase II study of safety and pharmacokinetics of multiple doses of GWP42003-P compared with placebo in children with Dravet syndrome has been completed in 2015 but results not posted yet [3]. Phase III trials have been initiated to explore the efficacy and safety of Cannabidiol (GWP42003P) in children and young adults with Dravet syndromes [22], [23], [16] and in children and young adults with Dravet or Lennox Gastaut syndromes [19], [17]. Phase 3 study of long-term safety and efficacy of Cannabidiol Oral Solution as adjunctive therapy for pediatric and adult subjects with treatment resistant seizure disorders, including Lennox Gastaut syndrome (LGS) or Dravet syndrome (DS) is going on. These studies will be approximately completed in 2016 and 2017.
3.8. RWJ-333369 (carisbamate)
Carisbamate (RWJ-333369) is a novel neuromodulator drug and is currently under the clinical development for the treatment of epilepsy. It has shown the efficacy and tolerability in a Phase II clinical trial in patients with epilepsy [45], [73]. 3 Phase III clinical trials of carisbamate as treatment in partial onset seizures have been completed. Among them only one randomized double blind Phase III study showed significant positive efficacy outcome at the dose of 400 mg/day in adults with refractory partial onset seizures [50], [94], [14], [15], [12].
3.9. Thalidomide
Thalidomide has shown strong anticonvulsant properties in various animal models of epilepsy. In an open label study, it has shown strong therapeutic efficacy in patients with refractory epilepsy [76].
3.10. VX-765
VX-765 (Belnacasan) is an oral compound that inhibits Caspase-1 which is involved in IL-1beta production and a wide range of immune and inflammatory processes. It is found to be effective in animal models of both acute and chronic partial seizures [69]. In Phase II study of clinical development, VX765 has been found to be well tolerated, safe and efficacious (Table 1) in treatment of patients with drug-resistant partial onset epilepsy [99].
3.11. Eslicarbazepine acetate
Eslicarbazepine acetate is a new compound with anticonvulsant activity whose mechanism of action is by blocking the voltage-gated sodium channel. In 2013, eslicarbazepine acetate under the trade name of Aptiom was approved by the U.S. Food and Drug Administration (FDA), an antiepileptic drug (AED), for use as adjunctive treatment of partial-onset seizures with or without secondary generalization, developed by Sunovion Pharmaceuticals Inc. (Sunovion). The efficacy and safety of eslicarbazepine acetate (BIA 2–093) as monotherapy are being investigated in patients with newly diagnosed partial-onset seizures [40]. In Phase III double blind, randomized, multicentre study in subjects with partial seizures which were not controlled by current AEDs, ESL as monotherapy showed effective results as significantly improved seizure outcome and well tolerated [54], [95]. ESL as monotherapy long term Phase III study in subjects with partial onset seizures and similarly a Phase III study of ESL as adjunctive therapy for refractory partial seizures are going and will be completed in 2016 [39], [44], [91]. Results of a one year ESLIBASE retrospective study have revealed that eslicarbazepine acetate was well tolerated and effective in patients with focal seizures [105].
3.12. Retigabine/ezogabine
Retigabine has been shown to be a novel AED that acts by a unique mechanism, activating potassium channels and GABA receptors [67]. Retigabine has shown efficacy in several animal models of epilepsy [84]. The safety and efficacy of retigabine have been evaluated in Phase III studies in refractory epilepsy patients with partial-onset seizures and were found to be well tolerated and efficacious as adjunctive therapy in these groups of patients [29], [82]. Currently, a multicentric, long-term, Phase III study is ongoing to assess the long-term safety, tolerability and efficacy of retigabine immediate-release (IR) as adjunctive therapy (Table 1) in treatment of patients with drug-resistant partial-onset seizures, results are expected during 2016 [5].
Retigabine is also in Phase IV trial to examine its effect on urinary voiding function in subjects with partial onset seizures in addition to existing anti-epileptic drugs [20]. A Phase IV adjunctive treatment dose optimization study evaluating the efficacy, safety, and health outcomes of ezogabine/retigabine immediate release (IR) (GW582892) in adult subjects with partial onset seizures (POS) was terminated in 2013 after enrolling 6 subjects as decision was taken by sponsor (GlaxoSmithkline) [64]. GSK considered the early adjunctive treatment study population is irrelevant after reassessing the benefit and risk profile of retigabine [35]. Apart from this, one more Phase II study of pharmacokinetics, safety and tolerability of ezogabine/retigabine in subjects aged 12 years to less than 18 years with uncontrolled partial onset seizures or Lennox Gastaut syndrome was terminated as warned by FDA [98].
3.13. Docosahexaenoic acid
Omega-3 polyunsaturated fatty acids (PUFAs) are dietary fatty acids that are involved in several physiological processes of brain. Docosahexaenoic acid (DHA) which is an omega-3 fatty acid has been found to increase seizure thresholds in animals [101]. Subcutaneous administration of DHA increased the resistance to seizures in rodent PTZ model of epilepsy [103]. A double blind placebo controlled Phase II trial is ongoing to evaluate the effects of DHA in fish oil for the treatment of seizure disorders [34].
3.14. USL255
Topiramate Immediate Release (TPM-IR) is a broad-spectrum, well-established AED with multiple mechanisms of action including GABAA agonist, glutamate antagonist, and sodium channel antagonist [83]. TPM-IR is approved in many countries as an adjunctive treatment for patients with partial-onset seizures or primary generalized tonic–clonic seizures. USL255 is an extended release topiramate (TPM-XR) formulation that was developed to deliver consistent drug release over a 24 h dosing interval [33]. A double-blind, randomized, placebo controlled PREVAIL Phase III clinical study (Table 1) demonstrated that once-daily administration of USL255 is an effective adjunctive therapy for reducing the seizure frequency in patients with refractory partial onset seizures [32].
3.15. Pregabalin
Pregabalin is one of the newer antiepileptic drugs developed for the treatment of partial epilepsy. The primary mechanism of action of pregabalin is the inhibition of depolarization at voltage gated calcium channels resulting in decreased release of excitatory neurotransmitters (glutamate) at nerve terminals [90]. It showed a potent anticonvulsant activity in various experimental models of seizures and epilepsy such as maximal electroshock (MES), pentylenetetrazol induced seizures, audiogenic seizures in genetically seizure susceptible DBA/2 mice and kindled seizure in rats [65], [106]. The results of three randomized placebo controlled trials indicate that pregabalin is highly effective in the treatment of patients with partial seizures with or without secondary generalization [30]. The efficacy and safety of pregabalin as an add-on therapy in treatment of refractory partial epilepsy have been evaluated in six randomized, placebo-controlled trials involving 2009 participants. It was found to be significantly effective in reducing seizure frequency and increasing seizure freedom rates in patients with drug-resistant partial epilepsy [66]. Currently, four Phase III, double-blind, placebo-controlled, multicentric studies are ongoing in order to assess the efficacy and safety of pregabalin as adjunctive therapy in pediatric patients (Table 1) with partial onset seizures and in pediatric and adult subjects with primary generalized tonic clonic seizures which are estimated to be completed in 2016, 2017 and 2018 respectively [13], [11], [2], [10].
3.16. RAD001 (Everolimus)
Everolimus is a mammalian target of rapamycin (mTOR) complex 1 inhibitor with demonstrated benefit in several manifestations of tuberous sclerosis complex. In a multicentric, open-label, Phase II clinical trial, Everolimus treatment demonstrated a significant reduction in the frequency as well as duration of seizures in TSC refractory epilepsy patients [60]. A Phase II study of adjunctive Everolimus (RAD 001) therapy for epilepsy in children with Sturge–Weber Syndrome is going on and expected to be completed in 2018 [18]. Another Phase II study to measure effect of Everolimus on mTOR signaling in patients with tuberous sclerosis complex (TSC) and Focal Cortical Dysplasia (FCD) is going on with estimated enrollment of 30 patients is currently going on and will be finished in 2017 [6]. The safety and efficacy of two trough ranges of Everolimus as adjunctive therapy in a randomized, placebo-controlled Phase III study in TSC patients with refractory partial-onset seizures have been completed and results are expected during 2016 [7].
3.17. E2007 (Perampanel)
E2007 (Perampanel) is an orally administered, highly selective non-competitive AMPA-glutamate receptor antagonist, discovered and under development by Eisai Laboratories [52]. Perampanel has shown a broad spectrum of activity in several animal models of epilepsy including MES seizure, PTZ induced seizures, audiogenic seizures and amygdala kindled model of epilepsy [51], [87]. The safety, efficacy and tolerability of Perampanel as adjunctive therapy in patients with refractory partial-onset seizures have been demonstrated in many Phase III, randomized, double blind, placebo-controlled trials (Table 1) and several extension studies [21], [48], [59], [96], [97]. All these studies build a strong evidence to support the efficacy of Perampanel, as adjunctive therapy in treatment of refractory partial-onset seizures.
A Phase III study showed that administration of Perampanel continued the recovery of seizure score and seizure frequency in terms of safety and efficacy in adolescent patients with drug resistant partial seizures [88]. Another Phase III study showed the positive outcome of adjunctive Perampanel in primary generalized tonic clonic seizures (PGTC) in adolescent patients as it significantly attenuated the frequency of seizures in patients with drug resistant PGTC seizures [49]. Recently, 2 Phase 4 trial for the rational polytherapy using Perampanel dual therapy anticonvulsant combination treatments of adults with refractory focal epilepsy and the evaluation of the efficacy of Perampanel added to monotherapy for partial onset seizures with or without secondarily generalized seizures and has been started in 2015/2016 and expected time for completion is 2018 [78], [104].
3.18. TRI476
Oxcarbazepine (TRI476) is a second generation AED approved as initial or add-on therapy for partial epilepsy patients. In a randomized controlled trial, adjunctive once-daily SPN-804 (extended release formulation of Oxcarbazepine) has been shown to improve seizure control in patients with refractory partial-onset seizures [47]. Currently, the long term safety and efficacy of TRI476 are being evaluated in a multicentric Phase III trial in children with inadequately controlled partial onset seizures, to completed in May 2016 [63].
4. Discussion & conclusion
In the past few decades, several new antiepileptic drugs have been developed for the treatment of seizures and epilepsy but most of the available antiepileptic drugs (AEDs) are not showing any efficacy in patients with refractory epilepsy. Thus, there is a need for discovery and development of some novel antiepileptic agents with more efficacy and safety in treating the refractory seizures. Currently, the research should be focused on the newer strategies to develop the novel compounds by targeting the mechanisms involved in epileptogenesis and pharmacoresistance. In this review, we discussed about the current scenario in the development of investigational drugs possessing antiepileptic potential. Most of the researchers and pharmaceutical industries have adopted three main approaches for developing new antiepileptic drugs including, i) structural modification of existing drugs, ii) compounds targeting the novel mechanisms, and iii) non-mechanism based screening of drug compounds in conventional and newer animal models of epilepsy.
Seletracetam and brivaracetam are developed from structural modification of already approved antiepileptic drug, levetiracetam. These both act by the similar mechanism, binding with synaptic vesicle 2 protein (SV2A). Seletracetam possessed the greater affinity towards SV2a as compared to levetiracetam. Both analogs (seletracetam & brivaracetam) are currently under the various stages of clinical development [25], [56], [75]. Another drug, bumetanide, has a potential use in treatment of patients with temporal lobe epilepsy (TLE) having defective chloride homeostasis because of its novel mechanism of action by inhibiting Na-K-Cl co-transporter (NKCC). In a clinical study, bumetanide has shown a significant efficacy by reducing the number of seizures and epileptiform discharges in patients with TLE [42]. Selurampanel, a competitive AMPA antagonist, has gained a considerable interest of pharmaceutical industry. It has shown a potential anticonvulsant activity in several rodent models of epilepsy. Selurampanel is currently being in Phase-II of clinical development for treatment of patients with partial epilepsy. Another AMPA receptor antagonist, Perampanel, has shown adequate evidence supporting its efficacy and tolerability as adjunctive therapy in partial onset refractory seizures and in primary generalized tonic clonic seizures [49], [59], [96]. Similarly, Ganaxolone (GABA modulator), Naluzotam (serotonergic drug) and Cannabidiol are in pipeline of clinical development for treatment of focal seizures and related syndromes. Carisbamate (RWJ-333369), a novel neuromodulator, is currently under the clinical development for treatment of epilepsy. Its efficacy has been confirmed in a Phase II clinical study and currently, only one Phase III clinical trial showed its antiepileptic effect. A produg of oxcarbazepine, eslicarbazepine, has been approved as adjunctive treatment for refractory partial onset seizures [43]. In an ESLIBASE retrospective study, eslicarbazepine was found to be well tolerated and effective in patients with focal seizures [105]. Retigabine has shown the anticonvulsant activity in animal model of epilepsy and acts by a distinct mechanism through potassium channels and GABA receptor modulation. It has shown efficacy and tolerability in patients with refractory epilepsy. Everolimus (mTOR complex I inhibitor) has also demonstrated a significant benefits in TSC refractory epilepsy patients. It is currently being studied to confirm its efficacy and tolerability in large number of patients.
There is a significant need for the development of newer antiepileptic drugs targeting the novel mechanism, for treatment of refractory seizures and intractable epilepsy and thus, help the patients to lead a normal life. Drugs targeting epileptogenesis (development of seizures) are urgently needed. The advances in preclinical and clinical drug development provide a hope towards the discovery and development of new molecular entities with more efficacious and well tolerated antiepileptic potential.
About 70% patients with epilepsy can be treated with drugs which make epilepsy the best treatable neurological condition. No doubt, new AEDs are available in market but still 30% of patients resistant to available therapy. In such cases, patients are subjected to combination therapy/polytherapy which in turn results in severe side effects and complex pharmacokinetic drug interactions. Besides the presence of old as well as new AEDs, still there is a need of new drugs or modified version of old drugs to make patients, free of seizures. A better knowledge of pathophysiology of epilepsy will help in understanding and development of new drug targets and this in turn can be aimed to design and develop new therapeutics entities/options in order to prevent or suppress seizures refractory to conventional drug therapy. Patients with epilepsy become dependent, sympathetic and miserable. It affects their quality and comfort of life. The side effects or adverse effects associated with AED therapy further rise to a negative impact on the life of epileptic patients. The development of safe and effective treatments for epilepsy is a major challenge to experimental and clinical neuroscience. From the various systematic reviews and analyses of data from experimental studies, it has been concluded that the experimental data which are valid and precise should be considered to translate to clinical trial.
Available animal models are not only contributing to preclinical screening of new antiepileptic drugs but also providing a useful platform for improving our understanding about the process of epileptogenesis. There should be an optimistic approach to translate the success of preclinical testing to clinical practice. Preclinical research should be refined so that animal models mimic the human condition perfectly and convert it into positive clinical output. In recent years there is a tremendous achievement in AED development but still epilepsy remains a major therapeutic challenge and effective, better tolerated, improving quality of life, the trio which are the ideal characteristics of AED still far away. The challenge for developing side effect free AED is by being more specific to the targets and by exploring the underlying mechanism of pathophysiology in depth. This will lead us novel and ideal compounds from bedside to bench translation. For the past 15 years, new generation of AED has been coming to the market. Many of them were the structural modification of old existing AED holding promises of lesser side effects and improved stability. Some of them used either as alone or in combination therapy. But their use is limited in combination therapy due to drug–drug interactions and also restricted to specific conditions of epilepsy. There are a number of novel antiepileptic compounds which are under various stages of drug development. These drugs are expected to provide better treatment options for epilepsy in terms of improved pharmacokinetics, safety and efficacy. So, the major concern is to reduce the economic as well as social burden on patients. There is an urgent need to improve animal models and explore new targets with better knowledge in order to develop the novel drugs for treatment of patients with refractory epilepsy.
References
- 1.http://www.fda.gov
- 2.Pfizer. NIH/National Library of Medicine; 2015. A 12-month Study to Evaluate the Safety and Tolerability of Pregabalin as Add-on Therapy in Pediatric Subjects 1 month to 16 years of age With Partial Onset Seizures and Pediatric and Adult Subjects 5 to 65 years of age With Primary Generalized Tonic-Clonic Seizures. (Available at: http://clinicaltrials.gov/ct2/show/NCT01463306 [Last accessed 08 June 2015]) [Google Scholar]
- 3.GW Research Ltd. NIH/National Library of Medicine; 2016. A Dose-ranging Pharmacokinetics and Safety Study of GWP42003-P in Children With Dravet Syndrome. (Available at: https://clinicaltrials.gov/ct2/show/NCT02091206 [Last accessed 3 April 2016]) [Google Scholar]
- 4.SK Life Science. NIH/National Library of Medicine; 2014. A Double-blind, Randomized, Placebo-controlled, Phase 2 Trial of YKP3089 as Adjunctive Therapy in Subjects With Partial Onset Seizures. (Available at: http://clinicaltrials.gov/ct2/show/NCT01866111 [Last accessed 08 June 2015]) [Google Scholar]
- 5.GlaxoSmithKline. NIH/National Library of Medicine; 2014. A Long-term, Safety, Tolerability, and Efficacy Study of Retigabine Immediate-release (IR) in Asian Adults With Partial Onset Seizures. (Available at: http://clinicaltrials.gov/ct2/show/NCT01777139 [Last accessed 08 June 2015]) [Google Scholar]
- 6.New York University School of Medicine. NIH/National Library of medicine; 2016. A Pilot Study to Evaluate the Effects of Everolimus on Brain mTOR Activity and Cortical Hyperexcitability in TSC and FCD. (Available at: https://clinicaltrials.gov/ct2/show/NCT02451696 [Last accessed 25 March 2016]) [Google Scholar]
- 7.Novartis Pharmaceuticals. NIH/National Library of Medicine; 2014. A Placebo-controlled Study of Efficacy & Safety of 2 Trough-ranges of Everolimus as Adjunctive Therapy in Patients With Tuberous Sclerosis Complex (TSC) & Refractory Partial-Onset Seizures (EXIST-3) (Available at: http://clinicaltrials.gov/ct2/show/NCT01713946 [Last accessed 14 March 2015]) [Google Scholar]
- 8.Marinus Pharmaceuticals. NIH/National Library of Medicine; 2009. A Randomized, Controlled Trial of Ganaxolone in Adult Uncontrolled Partial-Onset Seizures. (Available at: http://clinicaltrials.gov/ct2/show/NCT00465517 [Last accessed 20 December 2014]) [Google Scholar]
- 9.Marinus Pharmaceuticals. NIH/National Library of Medicine; 2009. A Randomized, Controlled Trial of Ganaxolone in Patients With Infantile Spasms. (Available at: http://clinicaltrials.gov/ct2/show/NCT00441896 [Last accessed 21 February 2015]) [Google Scholar]
- 10.Pfizer. NIH/National Library of Medicine; 2015. A Safety, Efficacy and Tolerability Trial of Pregabalin as Add-on Treatment in Pediatric and Adult Subjects With Primary Generalized Tonic-Clonic (i.e., Grand Mal) Seizures. (Available at: http://clinicaltrials.gov/ct2/show/NCT01747915 [Last accessed 14 April 2015]) [Google Scholar]
- 11.Pfizer. NIH/National Library of Medicine; 2015. A Safety, Efficacy, and Tolerability Trial of Pregabalin as Add-on Treatment in Pediatric Subjects < 4 years of age With Partial Onset Seizures. (Available at: http://clinicaltrials.gov/ct2/show/NCT02072824 [Last accessed 14 May 2015]) [Google Scholar]
- 12.SK Life Science. NIH/National Library of Medicine; 2013. A Study of the Effectiveness, Safety, and Tolerability of Carisbamate as Add-on Therapy in Patients With Partial Onset Seizures. (Available at: https://clinicaltrials.gov/ct2/show/NCT00740623 [Last accessed 1April 2016]) [Google Scholar]
- 13.Pfizer. NIH/National Library of Medicine; 2015. A Study of the Efficacy and Safety of Pregabalin as Add-on Therapy for Partial Onset Seizures in Children Ages 4–16 years (PERIWINKLE) (Available at: http://clinicaltrials.gov/ct2/show/NCT01389596 [Last accessed 13 May 2015]) [Google Scholar]
- 14.SK Life Science. NIH/National Library of Medicine; 2012. A Study of the Efficacy and Safety of RWJ-333369 as Add-on Therapy in the Treatment of Partial Onset Seizures. (Available at: https://clinicaltrials.gov/ct2/show/NCT00425282 [Last accessed 1 April 2016]) [Google Scholar]
- 15.SK Life Science. NIH/National Library of Medicine; 2012. A Study of the Efficacy and Safety of RWJ-333369 as Add-on Therapy in the Treatment of Partial Onset Seizures. (Available at: https://clinicaltrials.gov/ct2/show/NCT00433667 [Last accessed 1April 2016]) [Google Scholar]
- 16.GW Research Ltd. NIH/National Library of Medicine; 2015. A Study to Investigate the Efficacy and Safety of Cannabidiol (GWP42003-P) in Children and Young Adults With Dravet Syndrome. (Available at: https://clinicaltrials.gov/ct2/show/NCT02224703. [Last accessed 30 March 2016]) [Google Scholar]
- 17.GW Research Ltd. NIH/National Library of Medicine; 2015. A Study to Investigate the Efficacy and Safety of Cannabidiol (GWP42003-P; CBD) as Adjunctive Treatment for Seizures Associated With Lennox-Gastaut Syndrome in Children and Adults. (Available at: https://clinicaltrials.gov/ct2/show/NCT02224560 [Last accessed 30 March 2016]) [Google Scholar]
- 18.Baylor College of Medicine. NIH/National Library of medicine; 2015. Adjunctive Everolimus (RAD 001) Therapy for Epilepsy in Children With Sturge-Weber Syndrome (SWS) (Available at: https://clinicaltrials.gov/ct2/show/NCT01997255 [Last accessed 25 March 2016]) [Google Scholar]
- 19.GW Research Ltd. NIH/National Library of Medicine; 2016. An Open Label Extension Study of Cannabidiol (GWP42003-P) in Children and Young Adults With Dravet or Lennox-Gastaut Syndromes. (Available at: https://clinicaltrials.gov/ct2/show/NCT02224573 [Last accessed 30 March 2016]) [Google Scholar]
- 20.GlaxoSmithKline. NIH/National Library of Medicine; 2016. An Open Label Study to Evaluate the Effects of Ezogabine/Retigabine Added to Existing Anti-epileptic Drug(s) on Urinary Voiding Function in Subjects With Partial Onset Seizures. (Available at: https://clinicaltrials.gov/ct2/show/NCT01607346 [Last accessed 2 April 2016]) [Google Scholar]
- 21.Aneja S., Sharma S. Newer anti-epileptic drugs. Indian Pediatr. 2013;50:1033–1040. doi: 10.1007/s13312-013-0284-9. [DOI] [PubMed] [Google Scholar]
- 22.GW Research Ltd. NIH/National Library of Medicine; 2015. Antiepileptic Efficacy Study of GWP42003-P in Children and Young Adults With Dravet Syndrome. (Available at: http://clinicaltrials.gov/ct2/show/NCT02091375 [Last accessed 12 May 2015]) [Google Scholar]
- 23.GW Research Ltd. NIH/National Library of Medicine; 2015. Antiepileptic Efficacy Study of GWP42003-P in Children and Young Adults With Dravet Syndrome. (Available at: https://clinicaltrials.gov/ct2/show/NCT02091375 [Last accessed 30 March 2016]) [Google Scholar]
- 24.Bennett B., Matagne A., Michel P. Seletracetam (UCB 44212) Neurotherapeutics. 2007;4:117–122. doi: 10.1016/j.nurt.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bialer M., Johannessen S.I., Kupferberg H.J. Progress report on new antiepileptic drugs: a summary of the Eighth Eilat Conference (EILAT VIII) Epilepsy Res. 2007;73:1–52. doi: 10.1016/j.eplepsyres.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Bialer M., Johannessen S.I., Levy R.H. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X) Epilepsy Res. 2010;92:89–124. doi: 10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Bialer M., Johannessen S.I., Levy R.H. Progress report on new antiepileptic drugs: a summary of the Twelfth Eilat Conference (EILAT XII) Epilepsy Res. 2015;111:85–141. doi: 10.1016/j.eplepsyres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Biton V, Berkovic SF, AbouKhalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double blind, placebo controlled trial. Epilepsia. Jan 2014;55(1):5766. http://dx.doi.org/10.1111/epi.12433. [DOI] [PubMed]
- 29.Biton V., Gil-Nagel A., Brodie M.J. Safety and tolerability of different titration rates of retigabine (ezogabine) in patients with partial-onset seizures. Epilepsy Res. 2013;107:138–145. doi: 10.1016/j.eplepsyres.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Brodie M.J. Pregabalin as adjunctive therapy for partial seizures. Epilepsia. 2004;45:19–27. doi: 10.1111/j.0013-9580.2004.455004.x. [DOI] [PubMed] [Google Scholar]
- 31.Chuang Y.C. Mitochondrial dysfunction and oxidative stress in seizure-induced neuronal cell death. Acta Neurol Taiwan. 2010;19:3–15. [PubMed] [Google Scholar]
- 32.Chung S.S., Fakhoury T.A., Hogan R.E. Once-daily USL255 as adjunctive treatment of partial-onset seizures: randomized phase III study. Epilepsia. 2014;55:1077–1087. doi: 10.1111/epi.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark A.M., Halvorsen M.B., Braun T.L. USL255 extended-release topiramate: dose-proportional pharmacokinetics and tolerability in healthy volunteers. Epilepsia. 2014;55:1069–1076. doi: 10.1111/epi.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Canadian College of Naturopathic Medicine. NIH/National Library of Medicine; 2013. Clinical Trial of the Effects of DHA in the Treatment of Seizure Disorders. (Available at: http://clinicaltrials.gov/ct2/show/NCT01769092 [Last accessed 22 March 2015]) [Google Scholar]
- 35.GlaxoSmithKline. NIH/National Library of Medicine; 2014. Dose-optimization, Adjunctive Treatment Study of Ezogabine/Retigabine Immediate Release in Partial-Onset Seizures. (Available at: https://clinicaltrials.gov/ct2/show/NCT01721317 [Last accessed 30 March 2016]) [Google Scholar]
- 36.Duncan J.S., Sander J.W., Sisodiya S.M. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 37.Dzhala V.I., Brumback A.C., Staley K.J. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann Neurol. 2008;63:222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- 38.Novartis. NIH/National Library of Medicine; 2013. Efficacy and Safety of BGG492 as Adjunctive Treatment in Patients With Partial Onset Seizures. (Available at: http://clinicaltrials.gov/ct2/show/NCT01147003 [Last accessed 21 March 2015]) [Google Scholar]
- 39.Bial-Portela C S A. NIH/National Library of Medicine; 2015. Efficacy and Safety of Eslicarbazepine Acetate (BIA 2–093) as Adjunctive Therapy for Refractory Partial Seizures. (Available at: https://clinicaltrials.gov/ct2/show/NCT00988429 [Last accessed 2 April 2016]) [Google Scholar]
- 40.Bial-Portela C S.A. NIH/National Library of Medicine; 2014. Efficacy and Safety of Eslicarbazepine Acetate as Monotherapy for Patients With Newly Diagnosed Partial-Onset Seizures. (Available at: http://clinicaltrials.gov/ct2/show/NCT01162460 [Last accessed 23 January 2015]) [Google Scholar]
- 41.SK Life Science. NIH/National Library of Medicine; 2013. Efficacy of YKP3089 in Patients With Photosensitive Epilepsy. (Available at: http://clinicaltrials.gov/ct2/show/NCT00616148 [Last accessed 21 December 2014]) [Google Scholar]
- 42.Eftekhari S., Mehvari Habibabadi J., Najafi Ziarani M. Bumetanide reduces seizure frequency in patients with temporal lobe epilepsy. Epilepsia. 2013;54:e9–12. doi: 10.1111/j.1528-1167.2012.03654.x. [DOI] [PubMed] [Google Scholar]
- 43.Elger C., Halász P., Maia J. Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures: a randomized, double-blind, placebo-controlled, parallel-group phase III study. Epilepsia. 2009;50:454–463. doi: 10.1111/j.1528-1167.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- 44.Sunovion. NIH/National Library of Medicine; 2015. Eslicarbazepine Acetate Monotherapy Long Term Study. (Available at: https://clinicaltrials.gov/ct2/show/NCT00910247 [Last accessed 2 April 2016]) [Google Scholar]
- 45.Faught E., Holmes G.L., Rosenfeld W.E. Randomized, controlled, dose-ranging trial of carisbamate for partial-onset seizures. Neurology. 2008;71:1586–1593. doi: 10.1212/01.wnl.0000334751.89859.7f. [DOI] [PubMed] [Google Scholar]
- 46.Faught E. BGG492 (selurampanel), an AMPA/kainate receptor antagonist drug for epilepsy. Expert Opin Investig Drugs. 2014;23:107–113. doi: 10.1517/13543784.2014.848854. [DOI] [PubMed] [Google Scholar]
- 47.French J.A., Baroldi P., Brittain S.T. Efficacy and safety of extended-release oxcarbazepine (Oxtellar XR™) as adjunctive therapy in patients with refractory partial-onset seizures: a randomized controlled trial. Acta Neurol Scand. 2014;129:143–153. doi: 10.1111/ane.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.French J.A., Krauss G.L., Steinhoff B.J. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54:117–125. doi: 10.1111/j.1528-1167.2012.03638.x. [DOI] [PubMed] [Google Scholar]
- 49.French JA, Krauss GL, Wechsler RT, Wang XF, DiVentura B, Brandt C, Trinka E, O'Brien TJ, Laurenza A, Patten A, Bibbiani F. Perampanel for tonicclonic seizures in idiopathic generalized epilepsy: a randomized trial. Neurology. Sep 15 2015;85(11):9507. [DOI] [PMC free article] [PubMed]
- 50.Halford JJ, BenMenachem E, Kwan P, Ness S, Schmitt J, Eerdekens M, Novak G. A randomized, double-blind, placebo controlled study of the efficacy, safety, and tolerability of adjunctive carisbamate treatment in patients with partial-onset seizures. Epilepsia. Apr 2011;52(4):81625. http://dx.doi.org/10.1111/j.15281167.2010.02960. [DOI] [PubMed]
- 51.Hanada T., Hashizume Y., Tokuhara N. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. 2011;52:1331–1340. doi: 10.1111/j.1528-1167.2011.03109.x. [DOI] [PubMed] [Google Scholar]
- 52.Hibi S., Ueno K., Nagato S. Discovery of 2-(2-oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl) benzonitrile (perampanel): a novel, noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid (AMPA) receptor antagonist. J Med Chem. 2012;55:10584–10600. doi: 10.1021/jm301268u. [DOI] [PubMed] [Google Scholar]
- 53.Huberfeld G., Wittner L., Clemenceau S. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobson M.P., Pazdera L., Bhatia P. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a historical-control phase III study. BMC Neurol. Mar 28 2015;15:46. doi: 10.1186/s12883-015-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kahle K.T., Staley K.J. The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures. Neurosurg Focus. 2008;25 doi: 10.3171/FOC/2008/25/9/E22. [DOI] [PubMed] [Google Scholar]
- 56.Kasteleijn-Nolst Trenité D.G., Genton P., Parain D. Evaluation of brivaracetam, a novel SV2A ligand, in the photosensitivity model. Neurology. 2007;69:1027–1034. doi: 10.1212/01.wnl.0000271385.85302.55. [DOI] [PubMed] [Google Scholar]
- 57.Kenda B.M., Matagne A.C., Talaga P.E. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem. 2004;47:530–549. doi: 10.1021/jm030913e. [DOI] [PubMed] [Google Scholar]
- 58.Koller M., Lingenhoehl K., Schmutz M. Quinazolinedione sulfonamides: a novel class of competitive AMPA receptor antagonists with oral activity. Bioorg Med Chem Lett. 2011;21:3358–3361. doi: 10.1016/j.bmcl.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Krauss G.L., Perucca E., Ben-Menachem E. Long term safety of perampanel and seizure outcomes in refractory partial-onset seizures and secondarily generalized seizures: results from phase III, extension study 307. Epilepsia. 2014;55:1058–1068. doi: 10.1111/epi.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krueger D.A., Wilfong A.A., Holland-Bouley K. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74:679–687. doi: 10.1002/ana.23960. [DOI] [PubMed] [Google Scholar]
- 61.Kwan P, Trinka E, Van Paesschen W, Rektor I, Johnson ME, Lu S. Adjunctive brivaracetam for uncontrolled focal and generalized epilepsies: results of a phase III, double blind, randomized, placebo controlled, flexible dose trial. Epilepsia Jan 2014;55(1):3846. http://dx.doi.org/10.1111/epi.12391. [DOI] [PubMed]
- 62.Kwan P., Brodie M.J. Emerging drugs for epilepsy. Expert Opin Emerg Drugs. 2007;12:407–422. doi: 10.1517/14728214.12.3.407. [DOI] [PubMed] [Google Scholar]
- 63.Novartis Pharmaceuticals. NIH/National Library of Medicine; 2013. Long-term Safety and Tolerability of TRI476 (Oxcarbazepine) in Children With Inadequately Controlled Partial Onset Seizures. (Available at: http://clinicaltrials.gov/ct2/show/NCT01051193 [Last accessed 14 March 2015]) [Google Scholar]
- 64.GlaxoSmithKline. NIH/National Library of Medicine; 2014. Long-term Safety Study of Retigabine Immediate Release (IR) as Adjunctive Therapy in the Treatment of Adults With Partial-Onset Seizures (POS) (Available at: http://clinicaltrials.gov/ct2/show/NCT01336621 [Last accessed 22 March 2015]) [Google Scholar]
- 65.Lotarski S., Hain H., Peterson J. Anticonvulsant activity of pregabalin in the maximal electroshock-induced seizure assay in α2δ1 (R217A) and α2δ2 (R279A) mouse mutants. Epilepsy Res. 2014;108:833–842. doi: 10.1016/j.eplepsyres.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Lozsadi D., Hemming K., Marson A.G. Pregabalin add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev. 2008;1 doi: 10.1002/14651858.CD005612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luszczki J.J. Third-generation antiepileptic drugs: mechanisms of action, pharmacokinetics, and interactions. Pharmacol Rep. 2009;61:197–216. doi: 10.1016/s1734-1140(09)70024-6. [DOI] [PubMed] [Google Scholar]
- 68.Lynch B.A., Lambeng N., Nocka K. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maroso M., Balosso S., Ravizza T. Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8:304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matagne A., Margineanu D.G., Kenda B. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol. 2008;154:1662–1671. doi: 10.1038/bjp.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matagne A., Margineanu D.G., Potschka H. Profile of the new pyrrolidone derivative seletracetam (ucb 44212) in animal models of epilepsy. Eur J Pharmacol. 2009;614:30–37. doi: 10.1016/j.ejphar.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 72.Ngugi A.K., Bottomley C., Kleinschmidt I. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Novak G.P., Kelley M., Zannikos P. Carisbamate (RWJ-333369) Neurotherapeutics. 2007;4:106–109. doi: 10.1016/j.nurt.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marinus Pharmaceuticals. NIH/National Library of Medicine; 2013. Open-label Extension to Protocol 1042–0600. (Available at: http://clinicaltrials.gov/ct2/show/NCT00512317 [Last accessed 20 May 2015]) [Google Scholar]
- 75.UCB Pharma. NIH/National Library of Medicine; 2014. Open-label Follow up Trial to Evaluate Long-term Safety and Efficacy of Brivaracetam in Subjects Aged 16 years or Older Suffering From Epilepsy. (Available at: https://clinicaltrials.gov/ct2/show/NCT00150800 [Last accessed 20 March 2015]) [Google Scholar]
- 76.Palencia G., Martinez-Juarez I.E., Calderon A. Thalidomide for treatment of refractory epilepsy. Epilepsy Res. 2010;92:253–257. doi: 10.1016/j.eplepsyres.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Pennell P.B. Antiepileptic drugs during pregnancy: what is known and which AEDs seem to be safest. Epilepsia. 2008;49:43–55. doi: 10.1111/j.1528-1167.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mid-Atlantic Epilepsy and Sleep Center, LLC. NIH/National Library of Medicine; 2016. Perampanel for Treatment of Adults With Refractory Focal Epilepsy: A Pilot Study. (Available at: https://clinicaltrials.gov/ct2/show/NCT02727101 [Last accessed 5 April 2016]) [Google Scholar]
- 79.Pieribone V.A., Tsai J., Soufflet C. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia. 2007;48:1870–1874. doi: 10.1111/j.1528-1167.2007.01182.x. [DOI] [PubMed] [Google Scholar]
- 80.NINDS. NIH/National Library of Medicine; 2014. Pilot Study of Bumetanide for Newborn Seizures. (Available at: http://clinicaltrials.gov/ct2/show/NCT00830531 [Last accessed 22 April 2015]) [Google Scholar]
- 81.Pollard J.R., French J. Antiepileptic drugs in development. Lancet Neurol. 2006;5:1064–1067. doi: 10.1016/S1474-4422(06)70627-5. [DOI] [PubMed] [Google Scholar]
- 82.Porter R.J., Burdette D.E., Gil-Nagel A. Retigabine as adjunctive therapy in adults with partial-onset seizures: integrated analysis of three pivotal controlled trials. Epilepsy Res. 2012;101:103–112. doi: 10.1016/j.eplepsyres.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 83.Porter R.J., Dhir A., Macdonald R.L. Mechanisms of action of antiseizure drugs. In: Stefan H., Theodore W.H., editors. Handbook of Clinical Neurology. Elsevier; 2012. pp. 663–681. (Epilepsy, Part II). [DOI] [PubMed] [Google Scholar]
- 84.Porter R.J., Nohria V., Rundfeldt C. Retigabine. Neurotherapeutics. 2007;4:149–154. doi: 10.1016/j.nurt.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.NINDS. NIH/National Library of Medicine; 2014. PRX-00023 Therapy in Localization-related Epilepsy. (Available at: http://clinicaltrials.gov/ct2/show/NCT01281956 [Last accessed 22 January 2015]) [Google Scholar]
- 86.Fisher R.S., Acevedo C., Arzimanoglou A. A practical clinical definition of epilepsy. Epilepsia. 2014 doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 87.Rogawski M.A., Hanada T. Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol Scand Suppl. 2013;127:19–24. doi: 10.1111/ane.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosenfeld W, Conry J, Lagae L, Rozentals G, Yang H, Fain R, Williams B, Kumar D, Zhu J, Laurenza A. Efficacy and safety of perampanel in adolescent patients with drug-resistant partial seizures in three double-blind, placebo-controlled, phase III randomized clinical studies and a combined extension study. Eur J Paediatr Neurol Jul 2015;19(4):43545. http://dx.doi.org/10.1016/j.ejpn.2015.02.008. [DOI] [PubMed]
- 89.Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double blind, randomized, placebo controlled trial. Epilepsia. Jan 2014;55(1):4756. http://dx.doi.org/10.1111/epi.12432. [DOI] [PubMed]
- 90.Ryvlin P., Perucca E., Rheims S. Pregabalin for the management of partial epilepsy. Neuropsychiatr Dis Treat. 2008;4:1211–1224. doi: 10.2147/ndt.s4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bial-Portela C S.A. NIH/National Library of Medicine; 2014. Safety and Efficacy of Eslicarbazepine Acetate as Adjunctive Therapy for Partial Seizures in Elderly Patients. (Available at: http://clinicaltrials.gov/ct2/show/NCT01422720 [Last accessed 23 December 2014]) [Google Scholar]
- 92.Santhosh N.S., Sinha S., Satishchandra P. Epilepsy: Indian perspective. Ann Indian Acad Neurol. 2014;17:S3–S11. doi: 10.4103/0972-2327.128643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simonato M., French J.A., Galanopoulou A.S. Issues for new antiepilepsy drug development. Curr Opin Neurol. 2013;26:195–200. doi: 10.1097/WCO.0b013e32835efe29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sperling M.R., Greenspan A., Cramer J.A. Carisbamate as adjunctive treatment of partial onset seizures in adults in two randomized, placebo-controlled trials. Epilepsia. 2010;51:333–343. doi: 10.1111/j.1528-1167.2009.02318.x. [DOI] [PubMed] [Google Scholar]
- 95.Sperling M.R., Harvey J., Grinnell T. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a randomized historical-control phase III study based in North America. Epilepsia. Apr 2015;56(4):546–555. doi: 10.1111/epi.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Steinhoff B.J. Efficacy of perampanel: a review of pooled data. Epilepsia. 2014;55:9–12. doi: 10.1111/epi.12493. [DOI] [PubMed] [Google Scholar]
- 97.Steinhoff B.J., Ben-Menachem E., Ryvlin P. Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: a pooled analysis of three phase III studies. Epilepsia. 2013;54:1481–1489. doi: 10.1111/epi.12212. [DOI] [PubMed] [Google Scholar]
- 98.GlaxoSmithKline. NIH/National Library of Medicine; 2014. Study in Pediatric Subjects With Epilepsy. (Available at: https://clinicaltrials.gov/ct2/show/NCT01494584 [Last accessed 30 March 2016]) [Google Scholar]
- 99.Vertex Pharmaceuticals Incorporated. NIH/National Library of Medicine; 2013. Study of VX-765 in Subjects With Treatment-resistant Partial Epilepsy. (Available at: http://clinicaltrials.gov/ct2/show/NCT01048255 [Last accessed 23 December 2014]) [Google Scholar]
- 100.Syntichaki P., Tavernarakis N. The biochemistry of neuronal necrosis: rogue biology? Nat Rev Neurosci. 2003;4:672–684. doi: 10.1038/nrn1174. [DOI] [PubMed] [Google Scholar]
- 101.Taha A.Y., Burnham W.M., Auvin S. Polyunsaturated fatty acids and epilepsy. Epilepsia. 2010;51:1348–1358. doi: 10.1111/j.1528-1167.2010.02654.x. [DOI] [PubMed] [Google Scholar]
- 102.Tomson T., Walczak T., Sillanpaa M. Sudden unexpected death in epilepsy: a review of incidence and risk factors. Epilepsia. 2005;46:54–61. doi: 10.1111/j.1528-1167.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 103.Trépanier M.O., Lim J., Lai T.K. Intraperitoneal administration of docosahexaenoic acid for 14 days increases serum unesterified DHA and seizure latency in the maximal pentylenetetrazol model. Epilepsy Behav. 2014;33:138–143. doi: 10.1016/j.yebeh.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 104.Eisai Korea Inc. NIH/National Library of Medicine; 2016. Trial Evaluating the Efficacy and Safety of Perampanel Added to Monotherapy in Participants With Partial Onset Seizures. (Available at: https://clinicaltrials.gov/ct2/show/NCT02726074 [Last accessed 5 April, 2016]) [Google Scholar]
- 105.Villanueva V., Serratosa J.M., Guillamón E. Long-term safety and efficacy of eslicarbazepine acetate in patients with focal seizures: results of the 1-year ESLIBASE retrospective study. Epilepsy Res. 2014;108:1243–1252. doi: 10.1016/j.eplepsyres.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 106.Warner G., Figgit D.P. Pregabalin as adjunctive treatment of partial seizures. CNS Drugs. 2005;19:265–272. doi: 10.2165/00023210-200519030-00007. [DOI] [PubMed] [Google Scholar]