Abstract
Drug addiction is a chronic, devastating, but treatable disorder. A core principle of drug addiction treatment states that no single treatment is appropriate for everyone (NIDA, 2012); treatments need to adjust based on patient characteristics and response in order to be maximally effective. For cocaine use disorders (CUD), specifically, the most potent intervention currently available for initiating abstinence is behavior therapy using contingency management (CM) procedures, with early cessation being a robust predictor of future abstinence. This raises two key questions for treatment development research: First, can we significantly improve initial CM response rates with targeted adjunctive interventions? Second, for individuals who fail to achieve initial abstinence with CM, is pharmacotherapy an effective augmentation strategy? This paper describes how a sequential, multiple assignment, randomized trial (SMART) design has advantages over a fixed-intervention approach when it comes to collecting data needed to answer both questions. The first aim will examine whether Acceptance and Commitment Therapy (ACT) in combination with CM increases initial abstinence response rates (i.e., 2 consecutive weeks of cocaine-negative urine screens). The second aim will examine whether ACT+CM in combination with modafinil promotes abstinence achievement in initial non-responders. Results are expected to inform how we tailor treatment of CUD to maximize outcomes.
Keywords: cocaine use disorder, sequential, multiple assignment, randomized trial (SMART), contingency management (CM), Acceptance and Commitment Therapy (ACT), modafinil, Bayesian approach
1. Introduction
Cocaine use disorders (CUD) comprise a public health problem in need of new treatment approaches. Cocaine affects multiple brain circuits, with prolonged exposure to cocaine compromising cognitive and behavioral processes associated with reward, motivation, learning, and inhibitory control (1–3). The complexity of the disorder has presented numerous treatment challenges. Controlled studies have demonstrated effectiveness for several types of behavioral therapies, including cognitive-behavioral therapy (CBT), motivational interviewing (MI), and contingency management (CM)(4, 5), along with promising pharmacotherapies (6, 7). Given the growing armamentarium of CUD interventions available, the treatment development research field has called for use of newer design methodologies that will lead to greater individualization or “tailoring” of interventions to the unique needs of the patient (e.g., PA-13-077; NIDA Principles of Drug Addiction Treatment, 2012)(8).
The sequential, multiple assignment, randomized trial (SMART) is an experimental design used for constructing empirically-supported adaptive treatment interventions (ATIs). For treatment of CUD, an ATI would present a sequence of interventions that work best for an individual patient across the stages of addiction treatment, from abstinence initiation to relapse prevention, dependent upon treatment response. The first decision stage of the SMART provides data for identifying the best initial treatment. The second decision stage of the SMART compares additional treatment options for initial treatment responders versus on-responders. Below we present the rationale for a two-staged SMART design that, compared to traditional fixed-design clinical trials, adapts treatment based on patient response, much like actual clinical practice.
1.1. Rationale for the study
This two stage SMART design will evaluate the impact of a sequence of treatment combinations for CUD, including CM, Acceptance and Commitment Therapy (ACT) and modafinil (a stimulant medication with low abuse potential that has been shown to facilitate cocaine abstinence). Presently, CM is the most reliably effective treatment for producing initial abstinence in patients with CUD (9). Based on operant learning principles, CM involves the systematic reinforcement of desired or therapeutic behaviors and the withholding of reinforcement of undesired behaviors. An extensive literature of controlled-studies documents the success of these interventions (10). We (11) and others (12–14) have implemented high-magnitude CM interventions during initial weeks of CUD treatment to produce abstinence rates as high as 40%. Given the robustness of initial abstinence in predicting long-term abstinence (e.g.15, 16), it behooves practitioners and treatment researchers to identify and develop creative approaches to increase the number of CM “responders”.
Adding acceptance and mindfulness-based treatment strategies, such as ACT, to CM may lead to improved abstinence outcomes. Broadly, ACT has demonstrated larger effects than treatment as usual, drug counseling, and methadone maintenance alone (relative risk [RR] range: 1.58–4.17; odds ratio [OR] = 2.32), with emerging research also favoring ACT over CBT or intensive 12-step facilitation (RR range: 0.64 to 1.76)(17). Studies of ACT for drug abuse have enrolled patients with opiate use disorder (Hayes et al., 2004;Stotts et al., 2012) or a mix of drug use disorders (Luoma et al., 2012; Lanza & Menendez, 2013), but not cocaine use disorder in particular. One study of ACT for stimulant use disorder focused on methamphetamine specifically (Smout et al., 2010). ACT is transdiagnostic, however, meaning that the key therapeutic processes apply broadly and beyond a single disorder or symptom (Dindo et al., 2017).
ACT targets psychological inflexibility, including experiential avoidance, fusion with unhelpful thoughts and emotions, lack of present moment focus, attachments to rigid ideas about oneself, and detachment from values. Experiential avoidance (EA), or the tendency to engage in escape or avoidance responses (e.g., substance use) in the presence of negative affect, is a significant and potentially modifiable predictor of response to CM (18). Therefore, providing ACT + CM early in CUD treatment may improve abstinence outcomes. For example, in a sample of 99 patients with CUD who received 4 weeks of CM treatment targeting abstinence initiation (18), post-hoc comparisons showed that the non-responder subgroup (i.e., patients who failed to achieve initial abstinence) had higher levels of EA, as measured by the Avoidance Inflexibility Scale (19). ACT applies mindfulness and experiential exercises to reduce EA, increase tolerance of negative or aversive emotional and physical states (i.e., distress tolerance), and increase responding in adaptive ways according to relevant contingencies despite negative internal experiences, suggesting synergistic mechanisms of action for combining ACT with CM as a way to improve response. Thus, supplementing CM with ACT may be especially effective for CUD patients who exhibit high levels of EA and relatively low sensitivity to reward contingencies.
Patients who do not respond to initial treatment may arguably be most in need of adjunctive pharmacotherapy as a second treatment. Studies investigating the neurochemistry of CUD have shown that low dopamine transmission is associated with poor response to CM treatment (20), suggesting that fundamental biological differences in the functioning of the brain reward system explain the inability of some patients to respond to alternative, non-drug reinforcers (21). It follows that pharmacological interventions that target striatal dopamine signaling might serve as a therapeutic adjunct for enhancing CM responding (i.e., responsivity to rewards) in this subset of patients. Modafinil has both dopaminergic and glutamatergic activity that may be useful for CUD. In human laboratory studies, modafinil has been shown to reduce cocaine-induced euphoria (22–24) and cocaine self-administration (23). In an initial outpatient clinical trial of 62 cocaine-dependent patients, modafinil was superior to placebo in facilitating abstinence and reducing cocaine-positive urines (25); however, subsequent trials have found this benefit limited to subsets of patients, including male participants (26) and those without a history of alcohol dependence (27). Kampman recently presented data showing that modafinil-treated subjects were significantly more likely than placebo-treated subjects to be cocaine abstinent throughout the entire clinical trial period, and to be continuously abstinent from cocaine by self-report during the last 3-weeks of the trial (28). Thus, of the numerous candidate medications evaluated to promote cessation of cocaine use, modafinil appears to be the most promising.
For patients who achieve early cocaine abstinence, ACT-based strategies may play an essential role in the maintenance of behavior change by shifting patients’ motivation from external (e.g., CM) to internal incentives or sources of motivation. As described above, ACT teaches skills for managing stress and other aversive emotional and physical experiences that commonly trigger relapse while, at the same time, helping the patient develop sustainable, value driven, goal-directed approach behaviors (29, 30). Thus, we predict that continuing ACT during the second treatment phase when high-magnitude CM is discontinued will be effective in maintaining cocaine abstinence in initial responders.
1.2. Study aims and hypotheses
We propose a SMART design to inform the development of an ATI for cocaine cessation and relapse prevention. Specifically, the design will provide data useful for addressing three primary questions. First, which treatment should be provided initially? Second, which second treatment should be provided to initial responders? Third, which second treatment should be provided to initial non-responders?
Specifically, we will test the following hypotheses: (1) initial treatment (4 weeks) with ACT and CM (ACT+CM) will produce higher response (abstinence) rates than initial treatment that combines standard Drug Counseling with CM (DC+CM); (2) for initial responders, continued ACT+CM will be more effective (higher abstinence rates) than continued DC+CM; (3) for initial non-responders, continued ACT+CM treatment with pharmacotherapy (modafinil) augmentation will be most effective in promoting abstinence relative to treatment combinations involving DC and/or placebo.
In the context of comparing first and second treatments, we will assess additional information concerning potential moderators and mediators of treatment response. Two secondary hypotheses are specified: (1) the benefit of ACT+CM over DC+CM on initial response rates will be greater in the subgroup of individuals with higher pretreatment EA scores and higher distress tolerance scores; and (2) the effects of ACT+CM will be mediated by changes in EA and reward sensitivity as measured by behavior economic (e.g., delay discounting) tasks.
2. Methods
2.1. Trial design overview
As described above and shown in Figure 1, the SMART design starts with a comparison of two initial treatments (ACT+CM versus DC+CM). Responding and non-responding participants will receive parallel second treatments according to the same sequence of decision rules. Only non-responding participants in each adaptive intervention will be re-randomized to a second treatment.
Figure 1.
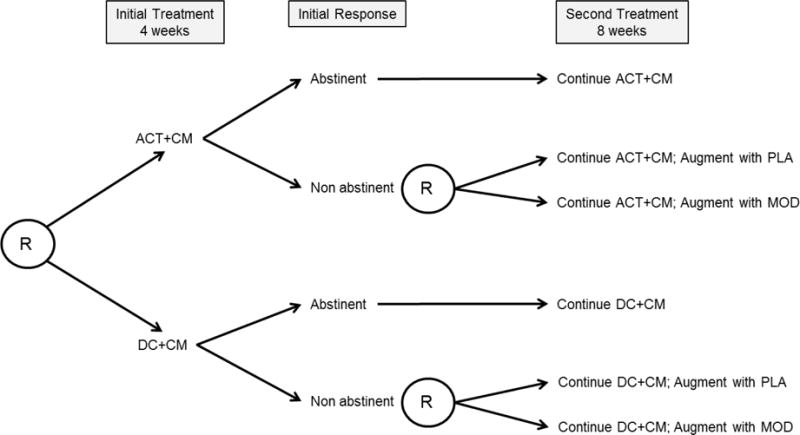
Overview of Study Design. Note: R=randomization; ACT=Acceptance and Commitment Therapy; DC=Drug Counseling; CM=Contingency Management; PLA=placebo; MOD=modafinil. Abstinent=6 consecutive (2 wks) cocaine-negative (BE<150 ng/ml) urines samples.
Eligible participants who complete a 1-week intake evaluation and pre-treatment assessment phase will be randomly assigned to one of the two 4-week initial treatments, ACT+CM or DC+CM, using urn randomization to ensure balance between groups on baseline EA level. Study visits will be thrice weekly (MWF) and will include urine drug screening at each visit and therapy (ACT or DC) sessions on two visits per week. Following initial treatment, the primary outcome of response/non-response will be determined. Subjects who submit 6 consecutive (2 weeks) cocaine negative urine samples by week 4 will be classified as responders. Those who fail to meet response criteria will be classified as non-responders. Using similar procedures, we (11) and others (12) have obtained a 40/60% split of responders/non-responders that we expect to replicate here.
During the second phase of treatment, responders will continue to receive their assigned initial treatment. Non-responders in each initial treatment arm will be re-randomized to a second, adjunctive treatment consisting of pharmacotherapy augmentation with either modafinil (MOD) or placebo (PLA). All subjects will continue attending three clinic visits per week, including two psychotherapy sessions per week, during the 8-week second treatment period.
2.2. Recruitment and Eligibility
The study will enroll treatment-seeking individuals, 18 to 60 years old, who meet current DSM-5 criteria for CUD of at least moderate severity (≥4 symptoms). Eligible subjects must submit at least one positive urine toxicology screen for the cocaine metabolite, benzoylecgonine (BE ≥ 150 ng/mL) during intake to ensure enrollment of individuals actively using cocaine. Subjects meeting moderate or severe diagnostic criteria for substance use disorders other than cocaine, marijuana, or nicotine will be excluded. Other exclusion criteria will include having a significant and unstable medical/psychiatric disorder or taking medications (e.g., propranolol, phenytoin, warfarin, or diazepam), which are contraindicated for modafinil pharmacotherapy. No pregnant women will be permitted in the study. Females of child-bearing potential must agree to use an acceptable method of birth control during study participation and for one month after discontinuation of the study medication.
2.3. Treatments
2.3.1. Acceptance and Commitment Therapy (ACT)
The overarching goal of ACT is to decrease EA while increasing acceptance and willingness to experience unpleasant thoughts, feelings, and physical symptoms (31–33). More specifically, ACT will assist patients with CUD to notice their internal cravings and triggers, to abandon their attempts to manage these triggers via active avoidance, suppression or other control-based strategies, and to make commitments to engage in behaviors consistent with their chosen values or goals. ACT encourages clients to experience thoughts and feelings from an observer perspective, and helps them not to believe distressing thoughts and feelings as if they are literally true and in need of action. ACT treatment for this trial is based on a manual developed and tested previously as a methadone detoxification therapy (34). Using the same targeted processes, the manual has been adapted so that the goals of ACT apply to cocaine abstinence. For example, choosing a valued direction, i.e., commitment to methadone detoxification has been re-written to apply to cocaine cessation. Acceptance rather than avoidance of opiate withdrawal symptoms has been re-written to apply to craving for cocaine. Experienced ACT therapists will be taught to use the manual as a map with flexibility, while ensuring that core processes such as acceptance, mindfulness, defusion, values, and committed action are thoroughly covered. Mindfulness and acceptance exercises and metaphors will be used to pursue therapeutic goals, more so than didactic skills training and instruction.
2.3.2. Drug Counseling (DC)
Manual-guided individual DC is modeled after the NIDA Collaborative Cocaine Treatment Study (35). DC approximates clinical practice as it is considered the most common type of evidence-based treatment in the community for patients actively using cocaine. DC educates patients about important concepts in addiction recovery based on the underlying philosophy that physical, emotional, spiritual, and interpersonal needs must all be addressed to support recovery. In the DC model, patients are encouraged to attend self-help programs (e.g., Alcoholics Anonymous) and to subscribe to a disease model of addiction while navigating through a series of topics designed to provide psychoeducation and support for abstinence and recovery (e.g., planning recovery, identifying ‘people, places, and things,’ establishing a support system, relationships in recovery). Thus, putative mechanisms of DC effects (i.e., knowledge and support) are distinct from ACT mechanisms, making DC an appropriate treatment comparison condition.
2.3.3. Contingency Management (CM)
During the initial phase of treatment, we will use the same high-magnitude CM schedule shown previously to be feasible and effective in facilitating initial cocaine abstinence (11). Subjects will earn vouchers for cocaine-negative urine samples collected at scheduled clinic visits (MWF) each week. Under an escalating reinforcement schedule, voucher values will begin at $15 and increase by $10 for each consecutive negative urine. Bonus vouchers ($10) will be given for three consecutive negative urines. Provision of a cocaine-positive urine or failure to provide a scheduled sample will result in no vouchers earned and will reset the schedule to the initial value ($15). Voucher earnings will be electronically loaded on study debit cards that can be used at any establishment that accepts debit cards. The total amount of CM vouchers that can be earned with 100% abstinence in the first 4 weeks is $630. CM during the second phase of treatment will continue to offer the opportunity to earn rewards for engaging in targeted behaviors but will switch to the standard, lower-cost, prize bowl method described by Petry (14). Prizes range in value from $0 (50%), $5 (41.9%), $20 (8.0%) to $100 (.2%).
2.3.4. Pharmacotherapy augmentation
We chose modafinil as the pharmacotherapy augmentation strategy for non-responders based on growing and encouraging evidence from numerous clinical trials in cocaine dependent treatment-seeking patients (25, 27, 28, 36). Its dopaminergic and glutamatergic activity makes sense theoretically for non-responders who likely represent a subgroup with greater biological/neurochemical impairment and for whom behavioral interventions alone may not sufficiently change dopamine transmission (20). Modafinil will start at 200 mg (day 1) and increase to the fixed dose of 300 mg (day 2). Placebo capsules will be identical in size, color, coating and shape. Participants in both conditions will be instructed to take two capsules at the same scheduled time (morning) per day throughout treatment. Capsules will be packaged in blister cards with emergency replacement cards provided in the event that a subject forgets to bring their weekly card to the clinic or to replace lost cards. Each medication dose will contain 50 mg of the biochemical tracer riboflavin to assess medication compliance.
2.4. Measures
2.4.1. Screening and eligibility
All consenting subjects will receive a comprehensive medical and psychiatric evaluation during a 1-week intake period, including a physical examination, laboratory chemistries, and electrocardiogram. Trained clinicians will administer the Structured Clinical Interview for DSM-5 (37), Addiction Severity Index (38), and Timeline Followback (39) interview assessments.
2.4.2. Treatment mechanisms
EA will be measured with the Avoidance and Inflexibility Scale (AIS), a 13-item self-report measure, on which respondents indicate, using a 5-point Likert-style scale, their level of avoidance and inflexibility with regard to internal experiences. Higher scores indicate more avoidant and inflexible responses to internal states associated with cocaine use. AIS scores at pretreatment will be used as the stratification variable for initial randomization using ≥/< 45 as the cutoff score shown in our previous study (18) to distinguish CM responders from non-responders with good sensitivity and specificity indices. Two measures of distress tolerance, the Distress Tolerance Scale (40) and the Cold Pressor Task (41), will be used to assess tolerance of subjective emotional distress and objective physical distress, respectively.
Drug reward sensitivity will be assessed using two behavioral economic measures, Delay Discounting (DD) and the Cocaine-Purchasing Task (CPT). DD describes how a reward loses value as a function of increasing delay to its receipt (42–45), with steeper discounting being positively associated with vulnerability to substance use disorders, including cocaine (46–50). The CPT simulates changes in price and consumption of drug in order to assess demand curves associated with drug consumption (51–55). The slope of the curve indicates the responsiveness of demand (elasticity) associated with changes in drug cost.
2.4.3. Cocaine use outcomes
The primary outcome measure of cocaine use/nonuse will be based on qualitative urine drug screens (UDS) coded as “positive” for cocaine use if BE ≥ 150 ng/mL. Urine samples will be collected at each clinic visit (MWF) and tested on-site with immediate results used to determine CM rewards. Additionally, the Timeline Followback (39) method will be used to record self-reported daily cocaine use during the study.
2.4.4. Medication monitoring
On thrice weekly clinic visits, the morning medication dose on the blister card will be taken by the subject at the dispensing window under observation by study staff. Additional methods for monitoring medication compliance will be followed, including pill counts and analysis of riboflavin in urine with UV detection.
3. Statistical analysis plan and power
SMART designs lend themselves to the flexibility and interpretability of Bayesian approaches. For each primary study question, Bayesian analyses provide results that are straightforward to interpret in terms of probabilistic statements. First, what is the probability that ACT+CM confers benefit relative to DC+CM on abstinence at end of initial treatment? Second, among non-responders at end of initial treatment, what are the relative probabilities that pharmacotherapy augmentation confers benefit at end of second treatment for those initially receiving ACT+CM versus DC+CM? Third, among responders at end of initial treatment, what is the probability that continued ACT+CM confers benefit relative to continued DC+CM at end of second treatment? By estimating the probability that such effects exist, we are assessing the probability that the alternative hypothesis is true, a probability that is, by definition, not accessible to Frequentist methods (which simply rejects or fails to reject the null hypothesis). Indeed, the Food and Drug Administration (FDA) has discussed the use of Bayesian statistical methods to make decisions regarding the efficacy of new treatments as an alternative to Frequentist methods in developing clinical applications (56–61). The current proposal will provide the best, unbiased estimates for the benefit conferred across treatment sequences, conditional upon initial response, while also estimating the probability that such effects exist. Posterior distributions can then be used as informative priors for continued monitoring in expansions of treatments and treatment strategies exhibiting initial promise.
Broadly, the analytic strategy will use generalized linear modeling with logistic models for evaluation of abstinence. Intention-to-treat principles will be used such that all participants undergoing randomization will be included in the primary analysis. We will use the conservative single imputation procedure for handling intermittent missing data, as described by McPherson (62). For participants who fail to provide urine for analysis, the imputation procedure replaces the missing value with a positive score on the UDS. Logistic regression coefficient priors will take the form ~N (mean = 0, var = 1 × 106) in the log (odds) scale. Sensitivity analyses using optimistic and pessimistic, skeptical priors will evaluate prior assumptions (63, 64). Inverse probability weight will permit unbiased effect size estimates in the context of re-randomization.
Effect size estimates for the primary hypotheses were derived from our previous study (11) and pooled data across cocaine clinical trials (25–28, 65, 66). Given the uncertainty inherent in existing empirical evidence and even greater uncertainty for conditional probabilities of response, prudence dictates that robust trial planning relies upon probability distributions of plausible effects that take into account the uncertainty of our predictions of effect. Figure 2 and Table 1 depict the probability point-estimate distributions and average parameter estimates, respectively, associated with each treatment effect, derived from logistic regression models with vague neutral priors, averaged across K = 500 Monte Carlo trials with a simulation sample size N=160. For Aim 1 hypothesis testing, simulation results predict an average difference of 20% in the probabilities of response (abstinence) favoring ACT+CM over DC+CM. For initial non-responders who receive ACT+CM or DC+CM during second treatment (Aim 2), adding modafinil pharmacotherapy (vs. placebo) is expected to increase the probability of response by 21% and 17%, respectively. Finally, for initial responders (Aim 3), simulated results predict an average 15% difference in the probability of response at end of second treatment, favoring continued ACT+CM over DC+CM. Applying this simulation model to go/no go decision making criteria, we stipulate a priori that a > 80% chance (posterior probability) of treatment conferring benefit (i.e., Pr (Pr θ > 0|data) > 0.80) constitutes sufficient evidence for testing the full adaptive intervention in a larger confirmatory trial.
Figure 2.
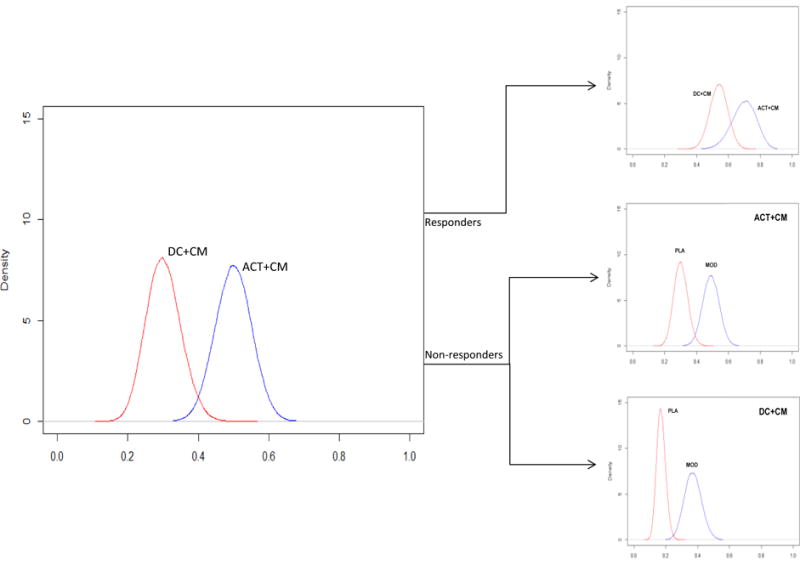
Probability point-estimate distributions associated with each treatment effect. Note. DC=Drug Counseling; CM=Contingency Management; ACT=Acceptance and Commitment Therapy; PLA=placebo; MOD=modafinil.
Table 1.
Parameter point-estimates and 95% Credible Intervals associated with each treatment effect averaging over K = 500 simulations.
| Effect | Average Point Estimate | Average Interval (95% C.I.) Estimate |
|---|---|---|
| Effect of Initial Treatment | ||
| Hypothesis Test Aim 1: | ||
| ACT+CM > DC+CM | 0.20 | 0.05–0.34 |
| Effect of Second Treatment (Initial non-responders) | ||
| Hypothesis Test Aim 2 | ||
| ACT+CM | ||
| MOD > PLA | 0.21 | 0.11–0.31 |
| DC+CM | ||
| MOD > PLA | 0.17 | 0.09–0.26 |
| Effect of Second Treatment (Initial responders) | ||
| Hypothesis Test Aim 3: | ||
| ACT+CM > DC+CM | 0.15 | 0.06–0.24 |
4. Conclusions
This paper describes a SMART design being used to inform the development of an ATI that has the potential for answering the ultimate clinical question of what first and second treatments work best for each individual across the stages of recovery, from abstinence initiation to relapse prevention. Cocaine use disorders are complex neurobiological and behavioral diseases. This complexity gives rise to treatment challenges, in particular, the challenge of addressing substantial heterogeneity in patient presentation and treatment response. The addiction field has essentially abandoned the “one size fits all” notion, recognizing instead that “no single treatment is appropriate for everyone” (8). Now, with a growing menu of evidence-based treatment interventions, along with SMART and other innovative clinical trial designs, the field is well-positioned to advance progress toward more personalization of treatment.
Initial observations from patients enrolled in this ongoing trial indicate good acceptability of the adaptive interventions. As recommended (67), participants are informed during the consent process of the possible treatment sequences to which they might be randomized. With few exceptions, participants seem to understand the approach and react positively to the notion of changing interventions based on their response, suggesting good acceptability with the adaptive design, as noted by others (67, 68).
If successful, the proposed trial will identify an individualized sequence of treatments for CUD that maximizes treatment efficacy/response and boosts cessation rates above their current levels. Positive findings of the trial will support the use of ACT in combination with CM and will support modafinil as an effective second treatment for initial non-responders, whereas unexpected results will inform protocol revisions and methodological refinements to continue to improve the delivery of evidence-based interventions for CUD. By directly addressing heterogeneity of treatment response, this type of treatment development research holds promise for having a real-world impact on the treatment of cocaine addiction and other chronic substance use disorders.
Acknowledgments
Funding provided by the National Institute on Drug Abuse (NIDA) Grant R01 DA039125.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience. 2011;12(11):652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. The American journal of psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. The Journal of clinical investigation. 2003;111(10):1444–51. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll KM. Recent advances in the psychotherapy of addictive disorders. Current psychiatry reports. 2005;7(5):329–36. doi: 10.1007/s11920-005-0032-5. [DOI] [PubMed] [Google Scholar]
- 5.Carroll KM, Onken LS. Behavioral therapies for drug abuse. The American journal of psychiatry. 2005;162(8):1452–60. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karila L. Pharmacological treatments of alcohol and drug addiction: what’s new? Current pharmaceutical design. 2011;17(14):1320. doi: 10.2174/138161211796150882. [DOI] [PubMed] [Google Scholar]
- 7.Mariani JJ, Levin FR. Psychostimulant treatment of cocaine dependence. The Psychiatric clinics of North America. 2012;35(2):425–39. doi: 10.1016/j.psc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NIDA. Principles of Drug Addiction Treatment: A research-based guide. 3rd. U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 9.Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, et al. Prize-based contingency management for the treatment of substance abusers: a meta-analysis. Addiction. 2014;109(9):1426–36. doi: 10.1111/add.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–60. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz JM, Green CE, Stotts AL, Lindsay JA, Rathnayaka NS, Grabowski J, et al. A two-phased screening paradigm for evaluating candidate medications for cocaine cessation or relapse prevention: Modafinil, levodopa-carbidopa, naltrexone. Drug Alcohol Depend. 2014;136:100–7. doi: 10.1016/j.drugalcdep.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisaga A, Aharonovich E, Cheng WY, Levin FR, Mariani JJ, Raby WN, et al. A placebo-controlled trial of memantine for cocaine dependence with high-value voucher incentives during a pre-randomization lead-in period. Drug Alcohol Depend. 2010;111(1–2):97–104. doi: 10.1016/j.drugalcdep.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandrey R, Bigelow GE, Stitzer ML. Contingency management in cocaine abusers: a dose-effect comparison of goods-based versus cash-based incentives. Exp Clin Psychopharmacol. 2007;15(4):338–43. doi: 10.1037/1064-1297.15.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol. 2012;80(2):276–85. doi: 10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brensilver M, Heinzerling KG, Swanson AN, Shoptaw SJ. Placebo-group responders in methamphetamine pharmacotherapy trials: the role of immediate establishment of abstinence. Exp Clin Psychopharmacol. 2012;20(5):430–5. doi: 10.1037/a0029210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plebani JG, Kampman KM, Lynch KG. Early abstinence in cocaine pharmacotherapy trials predicts successful treatment outcomes. J Subst Abuse Treat. 2009;37(3):313–7. doi: 10.1016/j.jsat.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stotts AL, Northrup TF. The Promise of Third-Wave Behavioral Therapies in the Treatment of Substance Use Disorders. Curr Opin Psychol. 2015;2:75–81. doi: 10.1016/j.copsyc.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stotts AL, Vujanovic A, Heads A, Suchting R, Green CE, Schmitz JM. The role of avoidance and inflexibility in characterizing response to contingency management for cocaine use disorders: A secondary profile analysis. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2015;29(2):408–13. doi: 10.1037/adb0000011. [DOI] [PubMed] [Google Scholar]
- 19.Gifford EV, Lillis J. Avoidance and inflexibility as a common clinical pathway in obesity and smoking treatment. Journal of health psychology. 2009;14(7):992–6. doi: 10.1177/1359105309342304. [DOI] [PubMed] [Google Scholar]
- 20.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. The American journal of psychiatry. 2011;168(6):634–41. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. The American journal of psychiatry. 2007;164(4):622–9. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 22.Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, et al. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70(1):29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 23.Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33(4):761–8. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- 24.Malcolm R, Swayngim K, Donovan JL, DeVane CL, Elkashef A, Chiang N, et al. Modafinil and cocaine interactions. Am J Drug Alcohol Abuse. 2006;32(4):577–87. doi: 10.1080/00952990600920425. [DOI] [PubMed] [Google Scholar]
- 25.Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30(1):205–11. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 26.Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, et al. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat. 2012;43(3):303–12. doi: 10.1016/j.jsat.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, et al. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104(1–2):133–9. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kampman KM, Plebani J, Lynch KG, Pettinati HM, Mahoney E, Slome M, et al. Modafinil for the treatment of cocaine dependence. American College of Neuropsychopharmacology Annual Meeting; Hollywood Florida. 2013. [Google Scholar]
- 29.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanter JW, Baruch DE, Gaynor ST. Acceptance and commitment therapy and behavioral activation for the treatment of depression: description and comparison. The Behavior analyst/MABA. 2006;29(2):161–85. doi: 10.1007/BF03392129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luoma JB, Hayes SC, Walsher RD. Learning ACT: An acceptance and Commitment therapy skills-training manual for therapists. Oakland, CA: New Harbinger Publications, Inc.; 2007. [Google Scholar]
- 32.Strosahi KD, Hayes SC. A practical guide to Acceptance and Commitment Therapy. Springer; 2010. [Google Scholar]
- 33.Read K. Acceptance and Commitment Therapy: Cognitive Defusion. Mill Valley, CA: Psychotherapy.net, LLC.; 2013. [Google Scholar]
- 34.Stotts AL, Green C, Masuda A, Grabowski J, Wilson K, Northrup TF, et al. A stage I pilot study of acceptance and commitment therapy for methadone detoxification. Drug Alcohol Depend. 2012;125(3):215–22. doi: 10.1016/j.drugalcdep.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, et al. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Archives of general psychiatry. 1999;56(6):493–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- 36.Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O’Brien CP. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94–9. doi: 10.1016/j.drugalcdep.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.First MB, William JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5 (Scid-5-cv): Clinician Version. Amer Psychiatric Pub Incorporated; 2015. [Google Scholar]
- 38.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 39.Sobbell LC, Sobell MB. Alcohol Timeline Followback Users’ Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- 40.Simons JS, Gaher RM. The Distress Tolerance Scale: Development and validation of a self-report measure. Motivation and Emotion. 2005;29(2):83–109. [Google Scholar]
- 41.Hayes SC, Bissett R, Korn Z, Zettle RD, Rosenfarb I, Cooper I, et al. The impact of acceptance versus control rationales on pain tolerance. Psychological Record. 1999;49:33–47. [Google Scholar]
- 42.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96(1):73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- 43.MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216(3):305–21. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200(1):1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17(8):651–67. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Rodriguez O, Secades-Villa R, Weidberg S, Yoon JH. A systematic assessment of delay discounting in relation to cocaine and nicotine dependence. Behavioural processes. 2013;99:100–5. doi: 10.1016/j.beproc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Secades-Villa R, Weidberg S, Garcia-Rodriguez O, Fernandez-Hermida JR, Yoon JH. Decreased delay discounting in former cigarette smokers at one year after treatment. Addict Behav. 2014;39(6):1087–93. doi: 10.1016/j.addbeh.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Yoon JH, Higgins ST. Turning k on its head: comments on use of an ED50 in delay discounting research. Drug Alcohol Depend. 2008;95(1–2):169–72. doi: 10.1016/j.drugalcdep.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon JH, Higgins ST, Bradstreet MP, Badger GJ, Thomas CS. Changes in the relative reinforcing effects of cigarette smoking as a function of initial abstinence. Psychopharmacology (Berl) 2009;205(2):305–18. doi: 10.1007/s00213-009-1541-4. [DOI] [PubMed] [Google Scholar]
- 50.Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol. 2007;15(2):176–86. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]
- 51.Acker J, MacKillop J. Behavioral economic analysis of cue-elicited craving for tobacco: a virtual reality study. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2013;15(8):1409–16. doi: 10.1093/ntr/nts341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amlung MT, Acker J, Stojek MK, Murphy JG, MacKillop J. Is talk "cheap"? An initial investigation of the equivalence of alcohol purchase task performance for hypothetical and actual rewards. Alcoholism, clinical and experimental research. 2012;36(4):716–24. doi: 10.1111/j.1530-0277.2011.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruner NR, Johnson MW. Demand curves for hypothetical cocaine in cocaine-dependent individuals. Psychopharmacology (Berl) 2014;231(5):889–97. doi: 10.1007/s00213-013-3312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobs EA, Bickel WK. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp Clin Psychopharmacol. 1999;7(4):412–26. doi: 10.1037//1064-1297.7.4.412. [DOI] [PubMed] [Google Scholar]
- 55.Johnson MW, Bickel WK. Replacing relative reinforcing efficacy with behavioral economic demand curves. Journal of the experimental analysis of behavior. 2006;85(1):73–93. doi: 10.1901/jeab.2006.102-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berry DA. Introduction to Bayesian methods III: use and interpretation of Bayesian tools in design and analysis. Clin Trials. 2005;2(4):295–300. doi: 10.1191/1740774505cn100oa. discussion 1–4, 64–78. [DOI] [PubMed] [Google Scholar]
- 57.FDA. Innovation/Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products. http://www.fda.gov/oc/initiatives/criticalpath/whitepaper.html[Online]. Available: http://www.fda.gov/oc/initiatives/criticalpath/whitepaper.html.
- 58.Goodman SN. Introduction to Bayesian methods I: measuring the strength of evidence. Clin Trials. 2005;2(4):282–90. doi: 10.1191/1740774505cn098oa. discussion 301–4, 64–78. [DOI] [PubMed] [Google Scholar]
- 59.Lipscomb B, Ma G, Berry DA. Bayesian predictions of final outcomes: regulatory approval of a spinal implant. Clin Trials. 2005;2(4):325–33. doi: 10.1191/1740774505cn104oa. discussion 34–9, 64–78. [DOI] [PubMed] [Google Scholar]
- 60.O’Neill RT. FDA’s critical path initiative: a perspective on contributions of biostatistics. Biometrical journal Biometrische Zeitschrift. 2006;48(4):559–64. doi: 10.1002/bimj.200510237. [DOI] [PubMed] [Google Scholar]
- 61.Temple R. How FDA currently makes decisions on clinical studies. Clin Trials. 2005;2(4):276–81. doi: 10.1191/1740774505cn097oa. discussion 364–78. [DOI] [PubMed] [Google Scholar]
- 62.McPherson S, Barbosa-Leiker C, Burns GL, Howell D, Roll J. Missing data in substance abuse treatment research: current methods and modern approaches. Exp Clin Psychopharmacol. 2012;20(3):243–50. doi: 10.1037/a0027146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spiegelhalter DJ, Best NG. Bayesian approaches to multiple sources of evidence and uncertainty in complex cost-effectiveness modelling. Statistics in medicine. 2003;22(23):3687–709. doi: 10.1002/sim.1586. [DOI] [PubMed] [Google Scholar]
- 64.Spiegelhalter DJ, Best NG, Carlin BP, Linde A, Van der Klei IJ. Bayesian measures of model complexity and fit. Journal of Royal Statistical Society. 2002;64(4):583–639. [Google Scholar]
- 65.Kampman KM, Lynch KG, Pettinati HM, Spratt K, Wierzbicki MR, Dackis C, et al. A double blind, placebo controlled trial of modafinil for the treatment of cocaine dependence without comorbid alcohol dependence. Drug Alcohol Depend. 2015;155:105–10. doi: 10.1016/j.drugalcdep.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmitz JM, Rathnayaka N, Green CE, Moeller FG, Dougherty AE, Grabowski J. Combination of Modafinil and d-amphetamine for the Treatment of Cocaine Dependence: A Preliminary Investigation. Front Psychiatry. 2012;3:77. doi: 10.3389/fpsyt.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Translational behavioral medicine. 2014;4(3):260–74. doi: 10.1007/s13142-014-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Almirall D, Compton SN, Gunlicks-Stoessel M, Duan N, Murphy SA. Designing a pilot sequential multiple assignment randomized trial for developing an adaptive treatment strategy. Statistics in medicine. 2012;31(17):1887–902. doi: 10.1002/sim.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]


