Abstract
DEPDC5 is a newly identified epilepsy-related gene implicated in focal epilepsy, brain malformations, and Sudden Unexplained Death in Epilepsy (SUDEP). In vitro, DEPDC5 negatively regulates amino acid sensing by the mTOR complex 1 (mTORC1) pathway, but the role of DEPDC5 in neurodevelopment and epilepsy has not been described. No animal model of DEPDC5-related epilepsy has recapitulated the neurological phenotypes seen in patients, and germline knockout rodent models are embryonic lethal. Here, we establish a neuron-specific Depdc5 conditional knockout mouse by cre-recombination under the Synapsin1 promotor. Depdc5flox/flox-Syn1Cre (Depdc5cc+) mice survive to adulthood with a progressive neurologic phenotype that includes motor abnormalities (i.e., hind limb clasping) and reduced survival compared to littermate control mice. Depdc5cc+ mice have larger brains with increased cortical neuron size and dysplastic neurons throughout the cortex, comparable to the abnormal neurons seen in human focal cortical dysplasia specimens. Depdc5 results in constitutive mTORC1 hyperactivation exclusively in neurons as measured by the increased phosphorylation of the downstream ribosomal protein S6. Despite a lack of increased mTORC1 signaling within astrocytes, Depdc5cc+ brains show reactive astrogliosis. We observed two Depdc5cc+ mice to have spontaneous seizures, including a terminal seizure. We demonstrate that as a group Depdc5cc+ mice have lowered seizure thresholds, as evidenced by decreased latency to seizures after chemoconvulsant injection and increased mortality from pentylenetetrazole-induced seizures. In summary, our neuron-specific Depdc5 knockout mouse model recapitulates clinical, pathological, and biochemical features of human DEPDC5-related epilepsy and brain malformations. We thereby present an important model in which to study targeted therapeutic strategies for DEPDC5-related conditions.
Keywords: DEPDC5, Focal cortical dysplasia, mTOR, Familial focal epilepsy, Seizures, Conditional knockout, Megalencephaly
1. Introduction
DEPDC5 is a gene that has been recently associated with familial focal epilepsy as well as sporadic epilepsy (Dibbens et al., 2013; Epi4K and Epilepsy Phenome/Genome, 2017; Ishida et al., 2013; Lal et al., 2014; Picard et al., 2014). The list of epilepsy syndromes associated with pathogenic variants in DEPDC5 is expanding, with the severe early onset epilepsy syndrome known as infantile spasms recently included (Carvill et al., 2015). DEPDC5 variants may also confer an increased risk for Sudden Unexplained Death in Epilepsy (SUDEP) (Bagnall et al., 2016; Nascimento et al., 2015). In addition to a role in non-lesional focal epilepsy, DEPDC5 has also been associated with epileptogenic structural brain malformations, from focal cortical dysplasia (FCD) (Baulac et al., 2015; D’Gama et al., 2015a; Scheffer et al., 2014) to large cortical malformations, such as hemimegalencephaly (D’Gama et al., 2015b; Ricos et al., 2016; Scerri et al., 2015). Pathological examination of resected human brain tissue from patients with pathogenic variants in DEPDC5 reveals dysplastic neurons and evidence of mTOR pathway disruption (Scerri et al., 2015).
Altered mTOR complex 1 (mTORC1) signaling is implicated in many neurologic conditions including epilepsy, brain malformations, and autism (Lipton and Sahin, 2014). At the cellular level, the mTORC1 pathway is activated by pro-growth factors such as neurotrophins, growth factors, and amino acids, and inhibited by metabolic stress such as nutrient starvation and endoplasmic reticulum stress. The product of DEPDC5, pleckstrin (DEP) domain-containing protein 5 (DEPDC5) is a key member of the amino acid sensing machinery and negatively regulates the mTORC1 pathway (Bar-Peled et al., 2013). DEPDC5 is ubiquitously expressed in the developing and adult brain, with high levels of expression in neurons (Dibbens et al., 2013).
DEPDC5 is a component of the GATOR1 complex along with NPRL2 and NPRL3. Collectively, the GATOR1 complex inhibits RagA/B- and RagC/D-mediated mTORC1 recruitment to lysosomal membranes and inhibits downstream mTORC1-mediated phosphorylation of S6 kinase and its substrate S6 (Saxton and Sabatini, 2017; Wolfson et al., 2017). The GATOR1 complex is a key regulator of cellular amino acid and nutrient detection in non-neuronal cell lines. These signaling pathways are unique to the GATOR complex and function independently of TSC1/TSC2 signaling (Shimobayashi and Hall, 2016). Given the relatively recent recognition of their role in epilepsy and brain development, the role of DEPDC5 and GATOR1 signaling in neuronal function remains largely unexplored.
Recently, Baulac and colleagues demonstrated a post-zygotic, somatic mutation of DEPDC5 in the brain lesion of a patient with a germline DEPDC5 mutation (Baulac et al., 2015). This provides evidence that a “two-hit” functional knockout of DEPDC5 may underlie brain malformations in some patients, particularly those with MRI-evident brain lesions (Scheffer et al., 2014). No animal model of DEPDC5-related epilepsy exists, and many animal models of GATOR1 complex genes are embryonic lethal, including a Depdc5−/− mouse (Dickinson et al., 2016). A Depdc5−/− rat model is embryonic lethal, and the Depdc5+/− rats do not display spontaneous seizures and seizure thresholds were not evaluated (Marsan et al., 2016). Animal models of other mTOR pathway regulators are lethal in the embryonic period, including Tsc1 and Tsc2 (Han and Sahin, 2011) and Pten (Di Cristofano et al., 1998). The synapsin promoter driving Cre-recombinase expression starting at embryonic day 12–13 has been successfully utilized to generate neuron-specific inactivation models of other mTORopathies (Meikle et al., 2007; Yuan et al., 2012; Zhu et al., 2001).
Here, we report the generation and characterization of a neuron-specific Depdc5 mouse model, Depdc5flox/flox-Syn1Cre (Depdc5cc+), which displays a larger brain size, early mortality, lowered seizure threshold, evidence of mTOR hyperactivation, ectopic neurons in the hippocampus, and dysplastic neurons in the cortex. Taken together, we present the first mammalian model that recapitulates many features of the human conditions associated with pathogenic variants in DEPDC5.
2. Methods
2.1. Mouse alleles, breeding strategy, and phenotyping
Mouse experiments were performed in a mixed-strain background using equal numbers of male and female mice. All mice were housed in a 12-hr light-dark cycle, climate controlled room, with access to food and water ad lib. Depdc5tm1c(EUCOMM)Hmgu conditional mice (referred to as Depdc5c/c) contain loxP sites flanking exon 5 of the Depdc5 gene. Germline loss of Depdc5 exon 5 results in embryonic lethality (Dickinson et al., 2016). To generate the neuron-specific Depdc5 conditional knockout mice, the homozygous Depdc5 conditional mice (Depdc5c/c) were bred with female neuron-specific synapsin I cre (SynI-Cre) allele mice from our existing colony (Yuan et al., 2012). Depdc5c/w-SynICre (Depdc5c/w+) females were bred with Depdc5c/c male mice to generate litters of homozygous neuronal knockout Depdc5c/c-SynCre+ (referred to herein as Depdc5cc+), heterozygous Depdc5c/w-SynCre+ (referred to herein as Depdc5cw+), and littermate control Depdc5c/w-SynCre− and Depdc5c/c-SynCre− mice. Mice were monitored daily for survival and weighed at weaning, 30 days, 60–75 days, and 90 days of age.14 days, 28 days, 60 days and 90 days. During routine handling, animals were monitored for spontaneous convulsive seizures by visual inspection. Phenotypic evaluation for hunchback posture, Straub tail positioning, and tremors was performed as previously described (Yuan et al., 2012). As a test of progressive neurology dysfunction, hind limb strain test was applied to mice >90 day old, and scored as follows: 1, normal lateral spread of hind limbs; 2, hind limb shaking and out-of-plane movement; 3, hind limb movement toward midline; 4, clasping of hind limbs. At time of sacrifice, body weights and dissected brain weights (without olfactory bulbs) were recorded. All mouse procedures were performed in accordance with the Guide for the Humane Use and Care of Laboratory Animals, and the study was approved by the Animal Care and Use Committee of Boston Children’s Hospital.
2.2. DNA analysis
DNA was prepared from mouse toes/tails by standard procedures. Genotyping at the Depdc5 gene was performed using a primer pair that allows simultaneous analysis of both conditional and wild-type alleles (forward: 5′-CATAGACATCTTGATAAGGTCTTAGCC-3′ and reverse: 5′-TCAAGTGCAAGATCTTAAGTGATTGGC-3′), followed by agarose gel electrophoresis. An 852 base pair (bp) band was detected for the wild-type allele, and a 1069 bp band was detected for the conditional allele with the flanking loxP sites. Primers that amplify a 300 bp portion of the Cre recombinase were used to assess the presence of the SynI-Cre allele (Zhu et al., 2001).
2.3. RT-PCR analysis
RNA was extracted from adult mouse cortical and liver samples using a RNEasy Mini Kit (Qiagen). We used 1 μg of each RNA sample for reverse transcription with the iScript cDNA synthesis kit (Biogen). Quantitative PCR was performed using iScript Reverse Transcriptase Supermix (Biogen) on an Applied Biosystems 7300 Real-Time PCR System (Life Technologies). Depdc5 expression was normalized to Gapdh and expressed as a relative quantity. Parallel reactions containing no reverse transcriptase were used as negative controls to confirm the removal of all genomic DNA. The oligonucleotide sequences of the primers used are as follow: primer set 1 for Depdc5 forward GTGTGGACCAGACTGTGACTC and reverse GCACAGGTGCTCACCAAACTT, primer set 2 for Depdc5 forward CCCCAATGATGAGTACAGTCCTT and reverse CCACCAGGTCAAGAGTCACA, and Gapdh forward TGCGACTTCAACAGCAACTC and reverse ATGTAGGCCATGAGGTCCAC. RT-PCR product from Depdc5 primer set 2 was run on an acrylamide gel to evaluate product size. Results were estimated as cycle threshold (Ct) values; the Ct was calculated as the mean Ct for the target gene minus the mean Ct for the control gene. The fold differential expression in the target gene of KO compared with control cortical tissue was expressed as 2−ΔΔCt. Data analysis and graphics were performed using GraphPad Prism 5 software and represent the results of 5 mice per group for cortex and 3 mice per group for liver. Each sample was run in triplicate for each gene.
2.4. Immunohistochemistry
Coronal brain sections of 87–111 day-old adult mice were collected, fixed, and vibrotome-sectioned at 35 μm using standard methods. Floating sections were immunolabeled using the following primary antibodies: mouse anti-NeuN (AB_2298772), mouse anti-GFAP (AB_561049), rabbit-phospho-S6 Ribosomal Protein (Ser 240/244) (AB_10694233), rabbit-DEPDC5 (AB_2010354; AB_2010353); SMI-311 (AB_2565383). Primary antibodies were detected with the following secondary antibodies: goat anti-mouse 488 (AB_2534069), goat anti-rabbit 555 (AB_2535849). Nuclei were stained with Hoescht 33342 (ThermoFisher). Images were acquired using a Zeiss LSM700 Laser Scanning Confocal Microscope equipped with Plan Apochromat ×10/0.3 and x25/0.8 objectives. Neuronal density was analyzed by counting on average 100 NeuN-positive nuclei in each cortical layer per animal using Optical Fractionator in StereoInvestigator (MBF Bioscience). The remainder of images were analyzed using the ImageJ software. Blinded cortical thickness measurements were taken a minimum of 6 locations per section. Area measurements of Layer V NeuN-positive neurons were performed from at least 25 cells per section. A minimum of 3 anatomically match sections per animal were used from three control mice and four Depdc5cc+ mice. Data are expressed as mean ± S.E.M.
2.5. Western Blotting
To evaluate protein levels, protein extracts were prepared from dissected cortical tissue of adult control and Depdc5cc+ mice between 92–122 days old. Western blotting was performed using similar amounts of protein extract per lane in 8–12% SDS polyacrylamide precast gels (BIO-RAD Laboratories). Western blots were done using the following primary antibodies: mouse anti-GFAP (AB_561049), rabbit-phospho-S6 Ribosomal Protein (Ser 240/244) (AB_10694233), mouse-Ribosomal Protein S6 (AB_1129205), mouse-TSC1 (AB_2533292), rabbit-TSC2 (AB_10547134), rabbit-AKT (AB_329827), rabbit-phospho-AKT(Ser473) (AB_2315049), rabbit-DEPDC5 (AB_2010354; AB_2010353); mouse-alpha-tubulin (AB_2688039). IRDye 800CW- or 680LT-conjugated secondary antibodies (1:1500; LI-COR) were used. To visualize bands, Odyssey Fc Imager was used and densitometry analysis was performed using Image Studio Software (LI-COR). Each band was normalized to the alpha-tubulin signal per sample.
2.6. EEG telemetry and PTZ-induced Seizure Threshold
EEG recording was performed as previously described with implanted wireless telemetry transmitters (PhysioTel ETA-F10; DSI, Data Sciences International) (Dhamne et al., 2017). One-channel video-EEG was recorded differentially between the reference (right olfactory bulb) and active (left occipital lobe) electrodes, with data collected over 3–14 days, which included day and night cycles. The EEG was sampled at 1000 Hz. Prior to implanted electrodes, all mice were also monitored in their home cages by direct observation for at least 1 hour per week throughout development for evidence of spontaneous convulsive seizures, in addition to reviewing the video-EEG data. At the end of the recording period, animals were challenged to exposed to proconvulsant to evaluate seizure threshold: adult Depdc5cc+ and littermate control mice were challenged with a convulsive dose (40 mg/kg; intraperitoneally, i.p.) of pentylenetetrazole (PTZ; Sigma-Aldrich, Co), a GABAA receptor antagonist (Dhamne et al., 2015; Dhamne et al., 2017). Latency to generalized tonic-clonic seizures (GTCs), number of seizures and, total duration of GTCs were recorded per mouse; animals without seizures were assigned a time of 20 minutes at the end of the PTZ challenge observation period.
2.7. EEG analysis
The first 24 hours of recording were considered an acclimation period, and data from this period were not analyzed. Video-EEGs were reviewed offline for behavioral or electrographic seizures. A 1 hour segment of daytime (between 11am and 3pm) and a 1 hour nighttime (between 11pm – 3am) baseline EEG was analyzed for epileptiform spikes and spectral power band analysis. An epileptiform discharge was defined as a run of continuous spikes ≥ 5 s in duration on the EEG (Dhamne et al., 2017). Power in frequency bands of the baseline EEG was calculated by transforming the raw EEG signal to frequency domain using the fast Fourier transform (FFT) technique. The power frequency bands were analyzed as a ratio of its absolute power to the total absolute power (1–80 Hz), to compensate for inter-subject variability and artifacts.
2.8. Statistical Analysis
Statistical analysis was performed by GraphPad Prism 7 software. The results are presented as mean ± SEM. Normality was assessed by Kolmogorov–Smirnov normality test. Comparisons between two groups were performed by unpaired two-tailed Student’s t-test for parametric data and by Mann–Whitney test for non-parametric data. Comparisons between multiple groups were performed by 1-way ANOVA with Tukey’s multiple comparison test as post-hoc analysis for parametric data and by a Kruskal–Wallis test for non-parametric data. A log-rank (Mantel-Cox) test was performed to compare the Kaplan–Meier analysis for mortality and seizure latency.
3. Results
3.1. Conditional neuron-specific Depdc5 knockout mice survive to adulthood
We generated a conditional neuron-specific Depdc5 knockout mouse model to evaluate DEPDC5 function in the brain in vivo. Depdc5c/c mice contain loxP sites flanking exon 5 of the Depdc5 gene and are embryonic lethal when Cre is constitutively expressed (Dickinson et al., 2016). We crossed Depdc5c/c mice with a line containing the SynapsinI promoter-driven cre recombinase allele (SynICre) that leads to recombination of loxP sites in neurons beginning at E12.5 (Zhu et al., 2001). We generated litters of conditional neuron-specific Depdc5 knockouts (Depdc5cc+), heterozygous (Depdc5cw+), and littermate controls (Depdc5cc-, Depdc5cw-) in Mendelian ratios (Table 1).
Table 1. Litters of conditional neuron-specific Depdc5 knockout mice survive in expected Mendelian ratios.
Percentage (number of pups) are shown for each genotype for the 5 litters of crosses between homozygous Depdc5 conditional (Depdc5c/c) males and female Depdc5c/w-SynICre (Depdc5c/w+) mice.
| Litters | Depdc5cc+ (KO) | Depdc5cw+ (Het) | Depdc5cc− (Ctl) | Depdc5cw− (Ctl) | Total |
|---|---|---|---|---|---|
| 1 | 11% (1) | 33% (3) | 22% (2) | 22% (2) | 9 |
| 2 | 33% (3) | 22% (2) | 11% (1) | 33% (3) | 9 |
| 3 | 27% (3) | 36% (4) | 18% (2) | 18% (2) | 11 |
| 4 | 40% (4) | 20% (2) | 20% (2) | 20% (2) | 10 |
| 5 | 22% (2) | 22% (2) | 22% (2) | 33% (3) | 9 |
| Total | 27% | 27% | 19% | 25% | 48 |
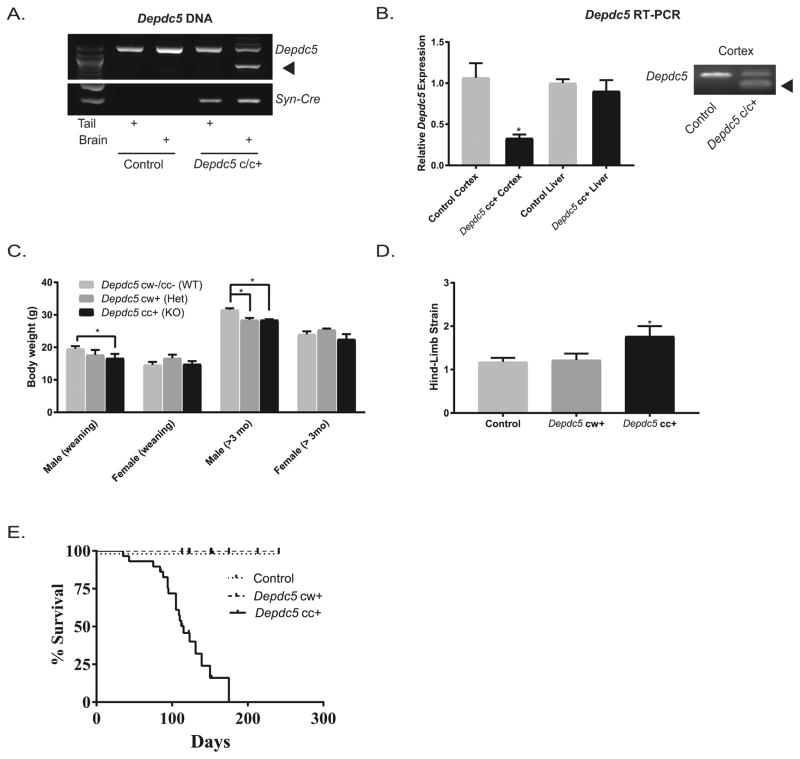
PCR amplification of DNA isolated from tails and brains of Depdc5cc+ and control mice were performed to evaluate for specificity of Cre-mediated recombination. We confirmed that Cre recombination resulted in truncation of the Depdc5 PCR product only in brain tissue of Depdc5cc+ mice (Fig. 1A). No reliable antibodies against mouse DEPDC5 exist currently. Although sequence homology with human DEPDC5 is 96%, several antibodies against human DEPDC5 did not reliably detect DEPDC5 in control mouse tissue by immunofluorescence or immunoblotting (data not shown). To address if the truncated Depdc5 gene resulted in a reduction in mRNA transcript, we evaluated Depdc5 mRNA transcripts from cortical samples of Depdc5cc+ mice and controls by RT-PCR. Depdc5 mRNA from Depdc5cc+ cortex was 33% of littermate control levels and truncated in length, while liver samples were unchanged across the genotypes (Fig. 1B). A complete loss would not be expected in whole cortex tissue since recombination is restricted to neurons, as previously shown (Zhu et al., 2001). Together these results demonstrate the neuron-specific SynICre expression in Depdc5cc+ mice leads to a truncation of the Depdc5 gene and loss of full-length Depdc5 transcript.
Figure 1. Conditional neuron-specific Depdc5 knockout (Depdc5cc+) mice have minimal weight loss but evidence of a progressive neurologic phenotype and reduced survival.
(A) Depdc5 PCR product is truncated (arrow) in brain tissue from Depdc5f/f mice with a copy of the Syn-Cre allele (Depdc5cc+), but not from tail or control mice samples. (B) Left: Quantitative analysis of RT-PCR of Depdc5 transcript is reduced in Depdc5cc+ cortical tissue (0.33 ± 0.04; p = 0.002) but not liver tissue (0.90 ± 0.13) compared to controls (cortex 1± 0.18; liver 1± 0.05). Right: Gel from Depdc5 RT-PCR product with a truncated product (arrow) only in Depdc5cc+ cortical tissue homogenate. (C) Compared to littermate controls, Depdc5cc+ male mice had reduced body weight in weanlings, P21–30, (1 ± 1.3 gram reduction; p = 0.04) and aged mice (3.1 ± 1.2 gram reduction; p = 0.03), whereas Depdc5cw+ males showed a significant weight reduction only in aged mice (3.2 ± 1.3 gram reduction; p = 0.046). No differences in female mice or between Depdc5cc+ and Depdc5cw+ were seen, n > 6 per sex within each genotype. (D) As a test of neurologic dysfunction, aged, >90 days old, Depdc5cc+ mice had evidence of hind limb strain (n=13; score = 1.8 ± 0.2) unlike control (n=17; score = 1.18 ± 0.1) and Depdc5cw+ (n=9; score = 1.22 ± 0.15) mice. (E) Monitoring survival of littermate mice revealed a markedly shortened lifespan of Depdc5cc+ (n=29; median survival = 115 days) compared to Depdc5cw+ (n=25) and control (n=30) mice. Error bars represent mean ± SEM. *p < 0.05 (2-way ANOVA and Tukey’s multiple comparison test).
Unlike the constitutive Depdc5 knockout mice, which are embryonic lethal, Depdc5cc+ and heterozygous Depdc5cw+ mice were viable and indistinguishable from littermate controls with exception of a mild reduction in weight gain (Fig. 1C). A reduction in weight of male Depdc5cc+ was evident at time of weaning and persisted through adulthood, whereas a reduction in weight of Depdc5cw+ mice compared to littermate controls only became evident after 3 months of age. There was no difference in the weight of female mice at any age. Subsequent experiments used equal numbers of males and females within groups, and groups with odd numbers used an extra female to decrease the effect of weight-loss in the male knockout mice. Unlike the constitutive Depdc5 knockout mice, Depdc5cc+ mice do not exhibit a hunchback phenotype at any age; however, Depdc5cc+ mice over 60 days old demonstrate evidence of neurologic dysfunction by limb-clasping behavior (hind limb strain) compared to Depdc5cw+ and littermate controls (Fig. 1D). This finding was equally distributed between both male and female Depdc5cc+ mice.
3.2. Conditional neuron-specific Depdc5 knockout mice exhibit mortality in adulthood
Despite a lack of systemic illness or overt signs of neurologic deficit, Depdc5cc+ have decreased survival (median 115 days) with no mice surviving past 175 days. Littermate control and Depdc5cw+ mice have normal survival (Fig. 1E). Depdc5cc+ mice were often found dead <24 h after being observed to be normal. There was no sex difference in age of death. No wounds or major pathology was seen on the animals to explain sudden death.
During normal handling circumstances, we observed spontaneous seizures in three mice, including one Depdc5cc+ mouse that died at 105 days of age during a generalized tonic-clonic seizure at the time of cage changing. Another two Depdc5cc+ mice exhibited tonic seizures, characterized by freezing (abrupt activity arrest) with arching of the tail and lack of response to stimulation that lasted approximately one minute before returning to baseline; both mice died within 2 weeks of the witnessed seizure, at 100 and 123 days of age.
3.3. Neuropathological effects in Depdc5cc+ mice
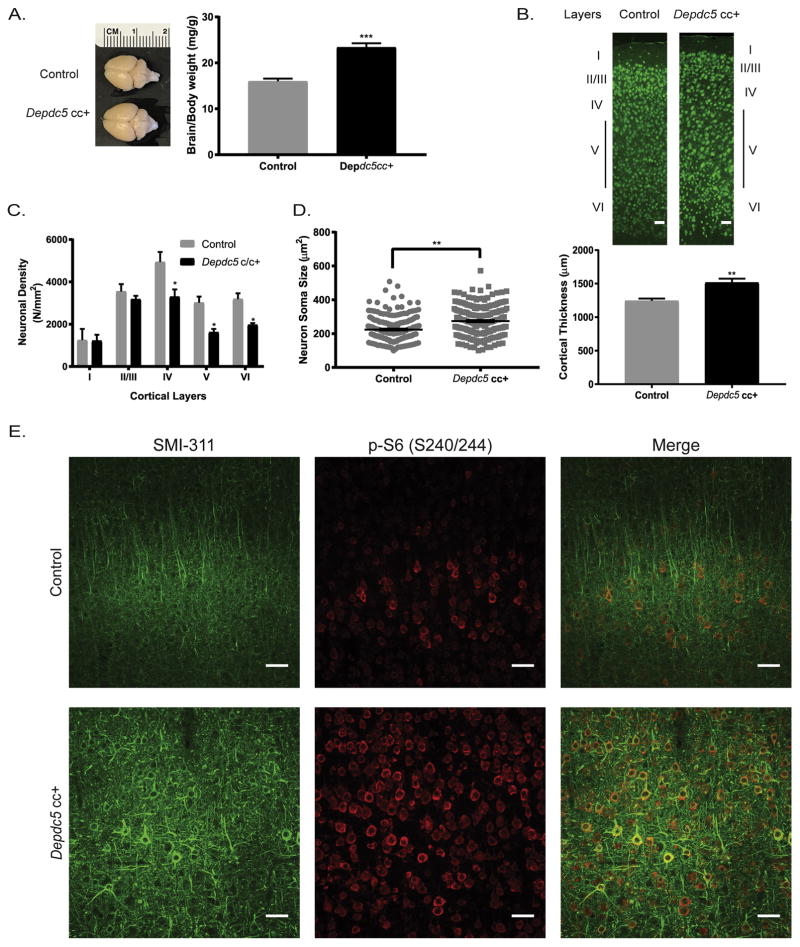
We assessed the neuropathological effects of the reduction of Depdc5 in the Depdc5cc+ mice. In adult mice, the Depdc5cc+ brains were markedly larger than littermate controls on gross inspection (Fig. 2A). After controlling for body weight, Depdc5cc+ brains weights were significantly greater than littermate controls after 60 days of age (p < 0.05) but not prior to 30 days of age (p = 0.46) (Fig. 2A). Analysis of sectioned brains, revealed an increase in cortical thickness in Depdc5cc+ mice compared to littermate controls (Fig. 2B). Neuronal density was not increased in Depdc5cc+ mice, but rather deeper layers had a decrease in neuronal density (p < 0.05) (Fig. 2C). In the deeper layers, neuronal enlargement was most evident in layer V neurons. To quantify the neuronal enlargement, blinded measurements of neuronal soma area was performed on NeuN-positive neurons in layer V of the motor cortex, as previously described (Meikle et al., 2008). We confirmed that neurons in Depdc5cc+ mice are significantly larger than littermate controls (p < 0.001) (Fig. 2D). Taken together these findings indicate that Depdc5 loss in our neuron-specific conditional mouse model leads to macrocephaly and increased neuron size, which are common features of models with mTORC1 hyperactivity (Switon et al., 2017).
Figure 2. Neuropathological defects in conditional neuron-specific Depdc5 knockout (Depdc5cc+) mice.
(A) Left: Representative images of adult dissected brains show increased brain size of Depdc5cc+ compared to littermate controls. Right: Brain weight is increased in Depdc5cc+ (n=10) mice compared to littermate control (n=13) adult >60 day old mice. (B) Increased cortical thickness with less distinct layers in Depdc5cc+ adult cortex compared to littermate controls. Top: Representative NeuN stained coronal sections, scale bars: 100μm. Bottom: Cortical thickness measurements at 6 paired sites in at least 3 sections per brain. (C) Neuronal density was unchanged in layers I–III and decreased in layers IV–V in Depdc5cc+ mice compared to matched controls; measured from on average 100 NeuN+ neurons per layer in each animal. (D) Neuron soma size is larger in Depdc5cc+ layers IV–V of M1 region of cortex compared to controls, measured from at least 175 NeuN-positive neurons per genotype. (E) Dysplastic neurons within Depdc5cc+ brains are evident by SMI 311 staining. Top panels in control mouse cortex with linear SMI 311 staining and minimal p-S6 staining. Bottom panels in Depdc5cc+ cortex with strong, disorganized SMI 311 staining colocalizes with p-S6 staining. Scale bars: 50μm. Sections from n=4 Depdc5cc+ and n=3 control brains. Graph error bars represent mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001 (Student’s t-test).
3.4 Depdc5cc+ mice have dysplastic neurons
Dysplastic neurons are often seen in models of mTOR hyperactivity and have been identified in humans with DEPDC5 variants (D’Gama et al., 2015b; Scheffer et al., 2014). Dysplastic neurons exhibit cytoskeleton abnormalities such as abnormal cytoplasmic accumulation of argyrophilic fibrils and increased expression of neurofilament proteins visualized by SMI 311 staining (Tassi et al., 2002). Coronal brain sections from control mice exhibited minimal SMI 311 staining limited to sparse III, V, and nonpyramidal layer VI neurons with projections aligned towards the cortical surface as expected (Ulfig et al., 1998) (Fig. 2E, top). In contrast, Depdc5cc+ cortical tissue exhibited strong SMI 311 staining throughout the cortex (Fig. 2D, bottom). Many SMI 311-positive layer V neurons in Depdc5cc+ mice had clear pyramidal morphology, but dendritic arbors were thicker, and projections appeared disorganized with abnormal orientation (Fig. 2E, bottom right).
3.5 mTOR hyperactivation in Depdc5cc+ neurons
Abnormal mTOR activity underlies many aspects of abnormal cortical development including dysplastic neurons within focal cortical dysplasia (Barkovich et al., 2015). A reliable measure of mTORC1 activity is increased phosphorylation of downstream ribosomal S6 (pS6 on Ser240/244). We found pS6 staining to be significantly increased in SMI 311-positive neurons in Depdc5cc+ mice compared to controls (Fig. 2E). Our findings are thus consistent with those in Depdc5+/− rats (Marsan et al., 2016) and humans with FCDs and DEPDC5 variants (Scerri et al., 2015), both of which show evidence of increased pS6 staining in dysplastic neurons.
We performed an evaluation of the cellular specificity of Depdc5 loss on mTORC1 activation. We assessed the extent of pS6 staining in neurons, astrocytes, and oligodendrocytes using the markers NeuN, GFAP, and Olig2, respectively. Relatively sparse and weak pS6 staining was seen in normal-appearing neurons throughout the cortex of control mice (Fig. 3A–C). Depdc5cc+ cortical sections demonstrated intense pS6 staining colocalized with NeuN-positive neurons throughout the cortical layers (Fig. 3D–F). These data indicate Depdc5 loss in cortical neurons results in increased neuronal soma size and mTORC1 hyperactivation.
Figure 3. Widespread architectural abnormalities and evidence of mTORC1 hyperactivation in adult conditional neuron-specific Depdc5 knockout (Depdc5cc+) mice.
A–F: Increased pS6 expression in NeuN+ neurons throughout the RSG region of the motor cortex of Depdc5cc+ brains (D–F) compared to controls (A–C). G–L: Occasional large pS6+ neurons within the CA1 pyramidal cell layer of the hippocampus of Depdc5cc+ mice (J–L) and also ectopically in the stratum oriens (L; arrowheads). M–R: In the CA3 region of the hippocampus, nearly all neurons are pS6+ including those outside the pyramidal cell layer (arrowheads) in Depdc5cc+ mice (P–R). S–X: In the dentate gyrus, robust pS6 positive neurons in the granule cell layer and hilus of Depdc5cc+ mice (V–X) with a few ectopic neurons (arrowheads). Sections from Depdc5cc+ (n=4) and control (n=3) brains. Scale bars: 100μm.
We evaluated other brain regions to determine if increased pS6-stained NeuN-positive neurons were isolated to the cortex of Depdc5cc+ mice. Increased pS6 expression was seen throughout the hippocampus of Depdc5cc+ mice compared to littermate controls. A fraction of CA1 pyramidal neurons highly expressed pS6, and many of these neurons were ectopically placed in the stratum oriens (Fig. 3J–L arrows, controls G–I). Nearly all neurons in the CA3 had increased pS6 staining [Fig. 3, Depdc5cc+ (P–R), control (M–O)], and similar to CA1 there was an increase in enlarged ectopic neurons in the Depdc5cc+ CA3 hippocampus (Fig. 3L arrows). The dentate gyrus showed increased pS6 staining in the granular cell layer and the hilum of Depdc5cc+ hippocampus compared to controls[Fig. 3, Depdc5cc+ (V–X), control (S–U)]. We also observed enlarged, strongly pS6-positive neurons in other brain regions including the thalamus and amygdala (Supplemental Fig. 1), but not in the caudate and putamen due to relatively sparse Cre expression in the striatum, as previously shown (Yamasaki et al., 2017).These findings strongly indicate that Depdc5 loss in neurons throughout the majority of the brain results in mTORC1 hyperactivation.
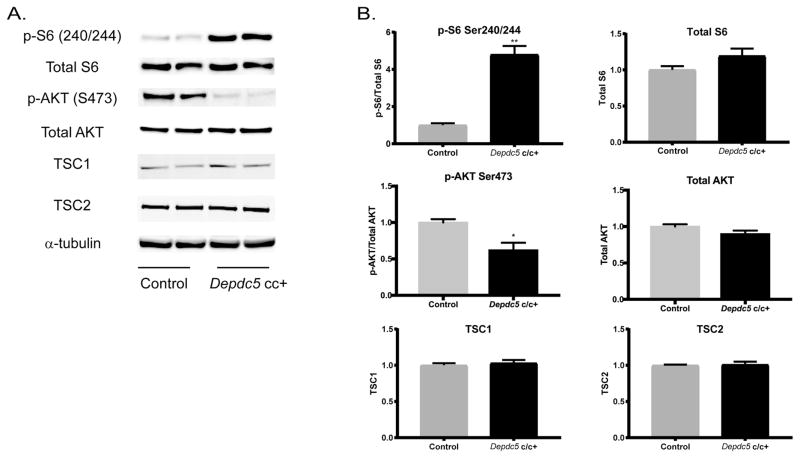
Western blot analyses of cortical lysates were performed to assess semi-quantitively mTOR activation in adult Depdc5cc+ mice. We confirmed a 4.8-fold increase (p<0.001) in pS6 in Depdc5cc+ cortical lysates compared to control lysates after normalizing to total S6 levels and alpha-tubulin as a loading control (Fig. 4). Total S6 levels were not significantly changed across genotypes. Decreased AKT phosphorylation (pAKT on Ser473) is expected from feedback signaling by hyperactive mTORC1 and has been reported in Tsc models (Meikle et al., 2008). We found a similar reduction of pAKT levels in Depdc5cc+ cortical lysates (0.62+/−0.1 of controls; p<0.05). No changes in TSC1 or TSC2 protein levels were seen, which indicates alterations in mTORC1 activity are not due to reductions in TSC1/TSC2 complex rather the loss of DEPDC5 regulation.
Figure 4. mTORC1-signaling is increased in conditional neuron-specific Depdc5 knockout (Depdc5cc+) cortical lysates.
(A) Immunoblots and (B) quantitative analysis of cortical brain lysates from adult (92–122 day old) Depdc5cc+ and littermate control mice demonstrate altered regulation of mTOR1C by increased p-S6(S240/244) and decreased p–AKT(S473) in Depdc5cc+ brains, which is independent of TSC1 and TSC2. Analysis from ≥3 samples per genotype. Expression of levels were normalized to alpha-tubulin, and p-S6(S240/244) and p–AKT(S473) were normalized to total levels of S6 and AKT, respectively. All ratios for the control samples are normalized to 1 with mean ± SEM. *p < 0.05; **p < 0.01 (Student’s t-test).
3.6 Reactive astrogliosis in Depdc5cc+ mice
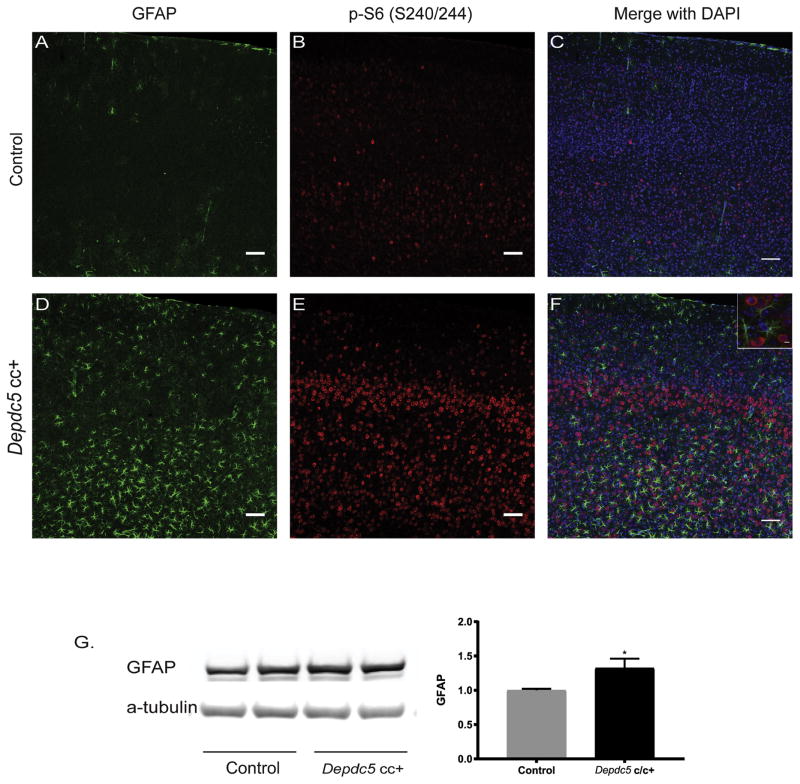
Double labeling for pS6 and glial markers was performed. There was no colocalization of pS6 and GFAP staining for astrocytes in Depdc5cc+ cortex (Fig. 5D–F). Interestingly, increased GFAP staining and evidence of reactive astrogliosis was present in Depdc5cc+ cortical sections (Fig. 5D–F) and was absent in control sections (Fig. 5A–C). Quantification by western blot revealed an increase in GFAP expression in Depdc5cc+ cortical lysate compared to controls (Fig. 5G; p<0.05). We assessed for oligodendrocytes by Olig2 staining, and found no colocalization with pS6 staining in the white or grey matter (Supplemental Fig. 2). Taken together, these data indicate that Depdc5cc+ mice have evidence of reactive gliosis, but the increased mTOR activation is specific to neurons.
Figure 5. Evidence of reactive astrogliosis in conditional neuron-specific Depdc5 knockout (Depdc5cc+) mice.
Astrogliosis was absent in control cortical sections (n=3 mice) (Fig. 5A–C) and present in adult Depdc5cc+ cortical sections (n=4 mice) (Fig. 5D–F). Scale bars: 50 μm. (F) Inset demonstrates lack of GFAP and pS6 colocalization, scale bar = 10 μm. (G) Immunoblots and quantitative analysis of cortical brain lysates for GFAP from adult Depdc5cc+ and littermate control mice (n=8 per genotype). Expression of levels were normalized to alpha-tubulin and control samples are normalized to 1 with mean ± SEM. *p < 0.05; **p < 0.01 (Student’s t-test).
3.7 Electrophysiologic effects in Depdc5cc+ mice
Video-EEG was recorded by wireless telemetry from a total of 7 Depdc5cc+ and 5 littermate control mice. Eight mice were recorded for 60 hours of which 2 control and 2 Depdc5cc+ mice were 65 days of age, and 1 control and 3 Depdc5cc+ mice were between 96 – 115 days old. We also evaluated longer-term recordings in 2 control and 2 Depdc5cc+ mice for a total of 10 recording days in mice between 89 – 115 days old. Despite witnessing three Depdc5cc+ mice having clear clinical seizures, including a terminal GTC seizure, we did not capture an electroclinical seizure in the 7 mice recorded on video-EEG. All mice had an expected 4–6 Hz background activity at rest with appropriate reactivity to salient stimuli. Rare and short spike trains were seen in Depdc5cc+ and control mice (Fig. 6A). Spike trains in Depdc5cc+ mice were not sufficiently sustained to qualify as electrographic seizures (Rensing et al., 2012). Similar to Depdc5+/− rats, the Depdc5cc+ mice did not exhibit significantly different interictal activity compared to control mice (Marsan et al., 2016). EEG frequency bands analyzed over a 1 hour period during the day and at night showed no significant difference in power in individual frequency bands, or power ratio frequencies across genotypes (data not shown).
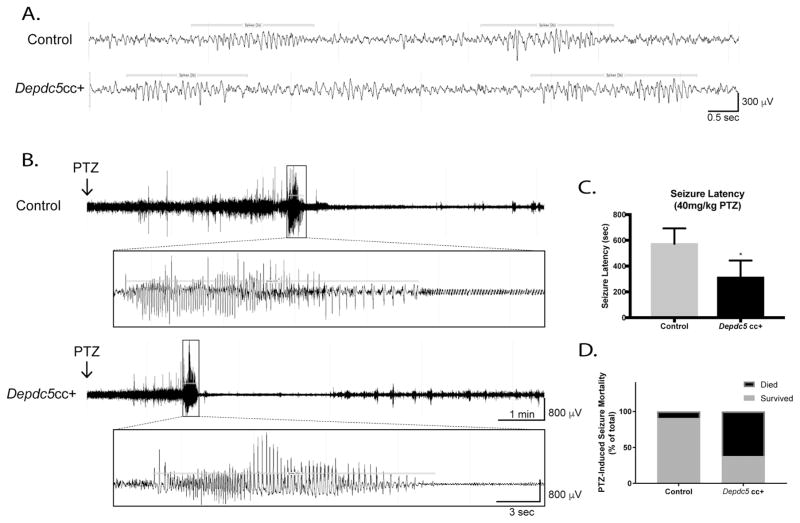
Figure 6. Electrophysiologic defects are evident in conditional neuron-specific Depdc5 knockout (Depdc5cc+) mice.
(A) Representative baseline EEG data from control and Depdc5cc+ mice demonstrates 2-second spike trains (horizontal bars; a normal rodent EEG finding) intermixed with a normal background EEG pattern. (B) Representative EEG tracings after PTZ injection demonstrate a shorter latency to GTC seizures in Depdc5cc+ mice. Clinical seizure onset corresponds to left box margin. (C) Depdc5cc+ mice (n=8) have a shorter latency to GTC seizures after 40mg/kg PTZ administration compared to controls (n=11). p<0.05, one-tailed Mann-Whitney test. (D) Increased mortality of Depdc5cc+ mice (n=8) compared to controls after 40mg/kg PTZ, p<0.05 by Chi-Square analysis.
We then tested whether Depdc5cc+ mice have a lowered seizure threshold when challenged with the proconvulsant PTZ while being recorded with video-EEG. After PTZ injection, mice exhibited various clinical epileptic stages from behavioral arrest to myoclonic jerking to GTC seizures. The representative traces show the gradual progression of control (Fig. 6B, top) and Depdc5cc+ (Fig. 6B, bottom) mice EEG into a GTC (Fig. 6B, insets) after PTZ injection. Depdc5cc+ mice have a significantly shorter latency to GTC seizures after PTZ administration (p<0.05) (Fig. 6C). There was no difference in total GTC seizure duration between the genotypes. After a GTC seizure, Depdc5cc+ mice display shorter duration of postictal baseline suppression before myoclonic seizures (Fig. 6B). Sixty-three percent (n=5/8) of Depdc5cc+ mice died due to PTZ-induced seizures compared to nine percent (n=1/11) of controls (p<0.05) (Fig. 6D). Despite having a relatively normal interictal EEG pattern, these data indicate Depdc5cc+ mice have lowered seizure threshold and increased rate of seizure-related death.
Discussion
We present the first evidence of an animal model of DEPDC5-related epilepsy that recapitulates many features of the human conditions associated with this important epilepsy- and malformation-related gene. Humans with heterozygous loss-of-function DEPDC5 variants exhibit epilepsy with or without developmental cortical malformations. Histopathology from resected tissue from an individual with a pathogenic DEPDC5 variant has been reported to show increased mTORC1 activity by pS6 staining in cytomegalic neurons (Scerri et al., 2015). Familial DEPDC5-related epilepsy penetrance is variable and incomplete (Dibbens et al., 2013; Ishida et al., 2013; Scheffer et al., 2014), raising the question of whether a 2nd somatic variant may be required to create loss of function in a localized region and thus focal epilepsy. Given published evidence that a “two-hit” functional knockout of DEPDC5 may underlie the human condition (Baulac et al., 2015), we created a neuron-specific Depdc5 knockout mouse model to analyze in vivo the effects of loss of function of DEPDC5 in the brain.
We utilized a Depdc5 floxed allele combined with a Synapsin I promotor-driven Cre allele to eliminate Depdc5 in the majority of neurons beginning at E13. This resulted in excision of exon 5 from the Depdc5 gene exclusively in the brain. Despite the lack of availability of reliable DEPDC5 antibodies for mouse tissue, we demonstrated brain-specific loss of full-length Depdc5 mRNA by RT-PCR. Taken together, our data provide convincing evidence of Cre-mediated excision of Depdc5 exon 5, reduced full-length Depdc5 transcript, and an expected phenotype similar to humans with DEPDC5 variants and other mTORopathies (Scheffer et al., 2014).
Depdc5cc+ mice exhibit many features consistent with mTORopathies (Lipton and Sahin, 2014). Depdc5cc+ mice have late onset poor weight gain similar to the hypomorphic Tsc2cc+ (Tsc2cc-SynCre+) mice, but less severe than Tsc1cc+ (Tsc1cc-SynCre+) that have marked weight loss and wasting syndrome. Weight reduction was only noted in male Depdc5cc+ mice. The reason for the sex difference is unclear, but prior studies have noted sex-dependent effects of mTORC1 on lifespan and longevity (Selman et al., 2009). The neurological phenotype, however, was seen in both sexes, and no differences among subsequent analysis were seen. Depdc5cc+ mice displayed mild hind limb clasping behavior in aged mice that correlates with neurological dysfunction. These findings are similar to but milder than the TSC models (Meikle et al., 2007; Yuan et al., 2012). The brain pathology of Depdc5cc+ mice demonstrated increased brain weight and larger neuronal size, which are typical of mTORopathies (Winden et al., 2015). Neuronal density was decreased in the deeper cortical layers of Depdc5cc+ mice, consistent with Tsc1-NestinCre+ mice (Anderl et al., 2011). Importantly, we found evidence of dysplastic cortical neurons of Depdc5cc+ mice. Similar dysplastic neurons are also found in developmental brain malformations of patients with DEPDC5 variants (Scerri et al., 2015).
We demonstrate that neuronal Depdc5 loss results in mTOR hyperactivation restricted to neurons as measured by downstream pS6 in the Depdc5cc+ mice. Our results are consistent with those of previous studies, with increased pS6 with DEPDC5 loss both in vivo (Marsan et al., 2016) and in vitro (Bar-Peled et al., 2013), which confirm that DEPDC5 loss results in increased mTOR1 activity. Interestingly, Depdc5cc+ cortical lysates show a reduction in AKT activity as measured by decreased AKT Ser473 phosphorylation. TSC knockout models also exhibit reduction in pAKT levels (Yuan et al., 2012). Decreased pAKT after TSC2 loss is thought to be mediated by either feedback mechanisms or direct effects on mTOR2 (Um et al., 2004). Our data indicate common downstream effects between TSC and GATOR mediated mTOR regulation, yet further studies are needed to delineate similarities and differences between these pathways. Taken together, our results demonstrating DEPDC5 regulation of mTOR corroborates prior in vivo and in vitro data, but, importantly, add the first surviving model to study the role of DEPDC5 in vivo.
Neuronal Depdc5 loss results in astrogliosis in our neuron-specific model. Astrogliosis is common in epilepsy and is implicated in promoting epileptogenesis (Coulter and Steinhauser, 2015). mTORC1 may play a role in reactive astrogliosis formation after seizures, and inhibition of mTOR by rapamycin attenuates seizure-induced astrocyte injury (Guo et al., 2017). Depdc5cc+ mice did not show constitutively elevated mTOR activation in astrocytes as measured by pS6 staining. These results suggest that the astrogliosis may be a result of dysfunctional neural activity or seizures related to loss of Depdc5 in neurons, which has been demonstrated in other mTORopathy models (Switon et al., 2017). It is also possible that neuronal mTOR activity interacts with neighboring astrocytes via a secreted/extracellular molecular as we have shown to occur between neurons and oligodendrocytes (Ercan et al., 2017). Our identification of astrogliosis in Depdc5cc+ mice provide a foundation for future studies to uncover the mechanism of astrogliosis after DEPDC5 loss.
The background EEG pattern in Depdc5cc+ mice was relatively normal, which is not inconsistent with the observations that many individuals with focal epilepsy may have a normal EEG (Pillai and Sperling, 2006). Although we did not capture seizures on EEG, we witnessed three mice have clinical seizures, one immediately precipitating death of the animal and mimicking the human phenomenon of seizure-related death that falls under the category of SUDEP. It is possible that EEG recording even closer to the time of death may exhibit interictal abnormalities. Other mTOR-related conditional knockout mice models such as Tsc1cc+ and Tsc2cc+ mice exhibit terminal seizures (Meikle et al., 2007; Yuan et al., 2012), therefore we suspect that terminal seizure events were a major cause of death of Depdc5cc+ mice as well.
Interestingly, the witnessed seizures in Depdc5cc+ mice that did not result in death were not GTC seizures but rather prolonged periods (up to 1 minute) of unresponsive tonic freezing. The low rate of spontaneous seizures in our model (3 of 43 Depdc5cc+ mice), with 0/7 mice recorded on video-EEG displaying seizures, led us to evaluate propensity to seizure using a PTZ-induced seizure paradigm that clearly demonstrated lowered seizure threshold in our model. These results are comparable to those reported in a mouse model deficient in SZT2, part of the KICSTOR complex upstream of DEPDC5 (Peng et al., 2017; Wolfson et al., 2017), which also have a lowered seizure threshold to PTZ but do not have spontaneous or stress-induced seizures (Frankel et al., 2009).
TSC models generally have a more severe phenotype with frequent unprovoked seizures (see review(Switon et al., 2017)) compared to our Depdc5 model or the Szt2 model (Frankel et al., 2009). DEPDC5 and SZT2 are implicated in mTOR mediated amino acid sensing, which is distinct from the TSC pathway (Saxton and Sabatini, 2017). These data support the hypothesis that these two pathways upstream of mTORC1 have different effects, also supported by the observation that the human phenotypes where DEPDC5-related epilepsy is often less severe and more responsive to medication as compared to TSC (Scheffer et al., 2014). We hypothesize that the less severe phenotype with DEPDC5 loss is a result of the intact feedback inhibition of mTORC1 through mTORC2, AKT, and TSC (Um et al., 2004). Further studies directly comparing Tsc and Depdc5 animal models may elucidate the phenotypic differences across the mTORopathies.
We also demonstrated increased death related to seizures in Depdc5cc+ mice compared to controls, in one of the two animals with spontaneous seizures and in the animals with PTZ-induced seizures. SUDEP is an important cause of mortality related in particular to patients with epilepsy who have pathogenic DEPDC5 variants (Bagnall et al., 2016; Nascimento et al., 2015; Weckhuysen et al., 2016) and to TSC patients (Amin et al., 2017). The pathophysiology and molecular underpinnings of SUDEP are unknown, including the specific mechanisms related to these genes. Given the mortality of otherwise healthy-appearing Depdc5cc+ mice, further characterization of the cardiopulmonary features and electrophysiological characteristics preceding the death of Depdc5cc+ mice will provide a potential model to understand the pathophysiology of SUDEP.
Conclusions
We demonstrate in a neuron-specific conditional knockout mouse model that Depdc5 loss of function plays a critical role in epileptogenesis and brain development. Depdc5cc+ mice display a propensity to proconvulsant-induced and rare spontaneous behavioral seizures, sometimes associated with increased mortality. We thus report the first animal model of DEPDC5-related epilepsy and present a model that may be studied in future to elucidate the currently elusive mechanisms of SUDEP. Thus, our Depdc5cc+ model recapitulates many of the features of the human patients with DEPDC5 pathogenic variants as well as other models of mTORopathies. Further, Depdc5cc+ mice have increased brain size, increased cortical thickness, and neuropathological abnormalities, including larger neurons and dysplastic neurons similar to those seen in focal cortical dysplasia tissue in humans. We demonstrate that these findings are due to increased mTORC1 activation specifically in neurons by isolated neuronal pS6 staining. Despite restriction of mTORC1 activation to neurons, Depdc5cc+ brains have evidence of reactive astrocytes, suggesting non-cell-autonomous effects of mTORC1 hyperactivation in neurons. The precise mechanisms by which this neuron-specific mTORC1-related pathology leads to epilepsy require additional study. The Depdc5cc+ mouse will provide an essential model to develop and test mTORC1 inhibitors and targeted novel therapeutics.
Supplementary Material
Highlights.
Conditional neuron-specific Depdc5 knockout mice (Depdc5cc+) have dysplastic cortical neurons.
Similar to other mTORopathy models, Depdc5cc+ mice have larger brains and increased neuron size.
Neuronal Depdc5 loss results in neuronal mTORC1 hyperactivation and evidence of gliosis.
Depdc5cc+ mice demonstrate a decreased seizure threshold, and some display seizure.
Depdc5cc+ mice have increased mortality, likely due in part to terminal seizures.
Acknowledgments
We would like to thank the IDDRC Cellular Imaging Core at Boston Children’s Hospital and Dr. Anthony Hill for imaging assistance, Samantha Murphy for aid in generating mice, Samantha Schaeffer for animal breeding, Dr. Alessia Di Nardo for antibodies, Allison Mazzella for technical assistance, and the Experimental Neurophysiology Core at Boston Children’s Hospital.
Funding
This work was supported by the NIH 2R25NS070682-07 (CJY) and U54HD090255 (MS), and the Translational Research Program at Boston Children’s Hospital (AP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin S, et al. Causes of mortality in individuals with tuberous sclerosis complex. Dev Med Child Neurol. 2017;59:612–617. doi: 10.1111/dmcn.13352. [DOI] [PubMed] [Google Scholar]
- Anderl S, et al. Therapeutic value of prenatal rapamycin treatment in a mouse brain model of tuberous sclerosis complex. Hum Mol Genet. 2011;20:4597–604. doi: 10.1093/hmg/ddr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall RD, et al. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol. 2016;79:522–34. doi: 10.1002/ana.24596. [DOI] [PubMed] [Google Scholar]
- Bar-Peled L, et al. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–6. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, et al. Malformations of cortical development and epilepsy. Cold Spring Harb Perspect Med. 2015;5:a022392. doi: 10.1101/cshperspect.a022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulac S, et al. Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann Neurol. 2015;77:675–83. doi: 10.1002/ana.24368. [DOI] [PubMed] [Google Scholar]
- Carvill GL, et al. Epileptic spasms are a feature of DEPDC5 mTORopathy. Neurol Genet. 2015;1:e17. doi: 10.1212/NXG.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Steinhauser C. Role of astrocytes in epilepsy. Cold Spring Harb Perspect Med. 2015;5:a022434. doi: 10.1101/cshperspect.a022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Gama AM, et al. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Annals of neurology. 2015a;77:720–5. doi: 10.1002/ana.24357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Gama AM, et al. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol. 2015b;77:720–5. doi: 10.1002/ana.24357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamne SC, et al. Acute seizure suppression by transcranial direct current stimulation in rats. Ann Clin Transl Neurol. 2015;2:843–56. doi: 10.1002/acn3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamne SC, et al. Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism. Mol Autism. 2017;8:26. doi: 10.1186/s13229-017-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, et al. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Dibbens LM, et al. Mutations in DEPDC5 cause familial focal epilepsy with variable foci. Nat Genet. 2013;45:546–51. doi: 10.1038/ng.2599. [DOI] [PubMed] [Google Scholar]
- Dickinson ME, et al. High-throughput discovery of novel developmental phenotypes. Nature. 2016;537:508–514. doi: 10.1038/nature19356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epi4K c, Epilepsy Phenome/Genome P. Ultra-rare genetic variation in common epilepsies: a case-control sequencing study. Lancet Neurol. 2017;16:135–143. doi: 10.1016/S1474-4422(16)30359-3. [DOI] [PubMed] [Google Scholar]
- Ercan E, et al. Neuronal CTGF/CCN2 negatively regulates myelination in a mouse model of tuberous sclerosis complex. J Exp Med. 2017;214:681–697. doi: 10.1084/jem.20160446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel WN, et al. Szt2, a novel gene for seizure threshold in mice. Genes Brain Behav. 2009;8:568–76. doi: 10.1111/j.1601-183X.2009.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, et al. Rapamycin Attenuates Acute Seizure-induced Astrocyte Injury in Mice in Vivo. Sci Rep. 2017;7:2867. doi: 10.1038/s41598-017-03032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Sahin M. TSC1/TSC2 signaling in the CNS. FEBS Lett. 2011;585:973–80. doi: 10.1016/j.febslet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, et al. Mutations of DEPDC5 cause autosomal dominant focal epilepsies. Nat Genet. 2013;45:552–5. doi: 10.1038/ng.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal D, et al. DEPDC5 mutations in genetic focal epilepsies of childhood. Ann Neurol. 2014;75:788–92. doi: 10.1002/ana.24127. [DOI] [PubMed] [Google Scholar]
- Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;84:275–91. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsan E, et al. Depdc5 knockout rat: A novel model of mTORopathy. Neurobiol Dis. 2016;89:180–9. doi: 10.1016/j.nbd.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Meikle L, et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–32. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–58. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento FA, et al. Two definite cases of sudden unexpected death in epilepsy in a family with a DEPDC5 mutation. Neurol Genet. 2015;1:e28. doi: 10.1212/NXG.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, et al. SZT2 dictates GATOR control of mTORC1 signalling. Nature. 2017;543:433–437. doi: 10.1038/nature21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, et al. DEPDC5 mutations in families presenting as autosomal dominant nocturnal frontal lobe epilepsy. Neurology. 2014;82:2101–6. doi: 10.1212/WNL.0000000000000488. [DOI] [PubMed] [Google Scholar]
- Pillai J, Sperling MR. Interictal EEG and the diagnosis of epilepsy. Epilepsia. 2006;47(Suppl 1):14–22. doi: 10.1111/j.1528-1167.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- Rensing NR, et al. Video-EEG monitoring methods for characterizing rodent models of tuberous sclerosis and epilepsy. Methods Mol Biol. 2012;821:373–91. doi: 10.1007/978-1-61779-430-8_24. [DOI] [PubMed] [Google Scholar]
- Ricos MG, et al. Mutations in the mammalian target of rapamycin pathway regulators NPRL2 and NPRL3 cause focal epilepsy. Ann Neurol. 2016;79:120–31. doi: 10.1002/ana.24547. [DOI] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri T, et al. Familial cortical dysplasia type IIA caused by a germline mutation in DEPDC5. Ann Clin Transl Neurol. 2015;2:575–80. doi: 10.1002/acn3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE, et al. Mutations in mammalian target of rapamycin regulator DEPDC5 cause focal epilepsy with brain malformations. Ann Neurol. 2014;75:782–7. doi: 10.1002/ana.24126. [DOI] [PubMed] [Google Scholar]
- Selman C, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–4. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimobayashi M, Hall MN. Multiple amino acid sensing inputs to mTORC1. Cell Res. 2016;26:7–20. doi: 10.1038/cr.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switon K, et al. Molecular neurobiology of mTOR. Neuroscience. 2017;341:112–153. doi: 10.1016/j.neuroscience.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Tassi L, et al. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain. 2002;125:1719–32. doi: 10.1093/brain/awf175. [DOI] [PubMed] [Google Scholar]
- Ulfig N, et al. Monoclonal antibodies SMI 311 and SMI 312 as tools to investigate the maturation of nerve cells and axonal patterns in human fetal brain. Cell Tissue Res. 1998;291:433–43. doi: 10.1007/s004410051013. [DOI] [PubMed] [Google Scholar]
- Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–5. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Weckhuysen S, et al. Involvement of GATOR complex genes in familial focal epilepsies and focal cortical dysplasia. Epilepsia. 2016;57:994–1003. doi: 10.1111/epi.13391. [DOI] [PubMed] [Google Scholar]
- Winden KD, et al. Megalencephaly and Macrocephaly. Semin Neurol. 2015;35:277–87. doi: 10.1055/s-0035-1552622. [DOI] [PubMed] [Google Scholar]
- Wolfson RL, et al. KICSTOR recruits GATOR1 to the lysosome and is necessary for nutrients to regulate mTORC1. Nature. 2017;543:438–442. doi: 10.1038/nature21423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, et al. Age-dependent motor dysfunction due to neuron-specific disruption of stress-activated protein kinase MKK7. Sci Rep. 2017;7:7348. doi: 10.1038/s41598-017-07845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan E, et al. Graded loss of tuberin in an allelic series of brain models of TSC correlates with survival, and biochemical, histological and behavioral features. Hum Mol Genet. 2012;21:4286–300. doi: 10.1093/hmg/dds262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–76. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.