Abstract
Background
Adults over age 65 represent the fastest growing population in the US. Decline in cognitive abilities is a hallmark of advanced age and is associated with loss of independence and dementia risk. There is a pressing need to develop effective interventions for slowing or reversing the cognitive aging process. While certain forms of cognitive training have shown promise in this area, effects only sometimes transfer to neuropsychological tests within or outside the trained domain. This paper describes a NIA-funded Phase III adaptive multisite randomized clinical trial, examining whether transcranial direct current stimulation (tDCS) of frontal cortices enhances neurocognitive outcomes achieved from cognitive training in older adults experiencing age-related cognitive decline: the Augmenting Cognitive Training in Older Adults study (ACT).
Methods
ACT will enroll 360 participants aged 65 to 89 with age-related cognitive decline, but not dementia. Participants will undergo cognitive training intervention or education training-control combined with tDCS or sham tDCS control. Cognitive training employs a suite of eight adaptive training tasks focused on attention/speed of processing and working memory from Posit Science BrainHQ. Training control involves exposure to educational nature/history videos and related content questions of the same interval/duration as the cognitive training. Participants are assessed at baseline, after training (12 weeks), and 12-month follow-up on our primary outcome measure, NIH Toolbox Fluid Cognition Composite Score, as well as a comprehensive neurocognitive, functional, clinical and multimodal neuroimaging battery.
Significance
The findings from this study have the potential to significantly enhance efforts to ameliorate cognitive aging and slow dementia.
Keywords: Transcranial direct current stimulation, tDCS, cognitive training, aging, Phase III, adaptive randomized clinical trial design
1. Introduction
Increased life expectancy has resulted in rapid growth of the older population. The cohort of adults 65 years and older in the United States is expected to double by the year 2050 and represents one of the fastest growing age groups in many countries. Even in the absence of neurodegenerative disease, cognitive abilities can decline significantly with advanced age. Cognitive decline in later life is associated with loss of independence, decrements in financial security and quality of life, and is a predictor of dementia risk.1-8 The increased prevalence of older adults living with cognitive difficulties has given rise to significant clinical and public health concerns.
Current cognitive training approaches have demonstrated some promise in slowing age-related cognitive decline and decreasing dementia risk.9-13 Findings over the past decade (e.g., Advanced Cognitive Training for Independent and Vital Elderly, ACTIVE) suggest that certain cognitive training programs hold promise as an approach to ameliorate cognitive aging in healthy older adults.9,11,13-27 Unfortunately, most training studies have shown intervention benefits mostly restricted to measures of the trained ability. Transfer to untrained cognitive and functional abilities in older adults has been found infrequently and the degree of transfer can be variable in both effectiveness and duration. This paucity of training generalization represents a significant barrier to overall cognitive intervention effectiveness. Methods that could potentially enhance the overall effectiveness of transfer from cognitive training are important to optimizing the overall efficacy of these programs for older adults.
Transcranial direct current stimulation (tDCS) is a non-invasive and safe electrical brain stimulation method that alters the sub-threshold membrane potential of neurons, facilitates neuroplasticity and learning, and provides a novel approach for augmenting cognitive training.28-59 During tDCS, a weak electrical current is applied to the scalp that penetrates to stimulate underlying cortical and subcortical tissue.50,51,60-63 tDCS applied to cortical regions has been shown to improve performance on a variety of cognitive tasks.64-67 Bilateral tDCS to the frontal cortices improves decision-making, attention, and working memory performance in older adults.68-71 Small pilot randomized clinical trials (RCTs) pairing cognitive training with bilateral frontal tDCS show significant and lasting improvements in older adults experiencing declining cognitive function.72-76 Maintenance of these tDCS and cognitive training effects have been shown to last beyond one year.73,76-79 These studies demonstrate that cognitive training combined with tDCS may lead to lasting improvement in cognitive training effectiveness for older adults. Furthermore, augmenting cognitive training with tDCS may have preventative benefits for people likely to develop dementia later in life. Our conceptual model for the effects of cognitive training and tDCS on brain function, cognitive performance, and functional outcomes is depicted in Figure 1.
Figure 1.
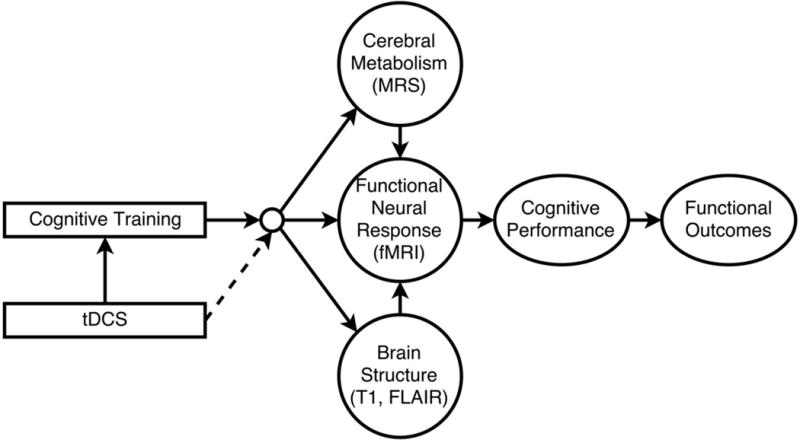
ACT Conceptual Model. Cognitive training holds promise for reducing the adverse effects of cognitive aging, enhancing neuroplasticity, cognitive efficiency, functional capacity, and quality of life. In theory, coupling cognitive training with an intervention that increases neuroplasticity (e.g., tDCS) could augment training outcomes. We hypothesize that CT leads to improvements in neuroplasticity (GABA MRS) and functional brain response (FMRI). In turn this can lead to improved cerebral metabolic health and structural brain preservation. Coupling cognitive training with tDCS will increase neuroplasticity in brain areas important for working memory, focused attention/executive attention, and processing speed, improve effectiveness of cognitive training, and ultimately cognitive health and functional abilities.
At present, large well-controlled clinical trials are needed to determine whether adjunctive tDCS and cognitive training produces clinically meaningful change in cognitive function in older adults. This paper describes the methods and design for the NIA-funded Augmenting Cognitive Training in Older Adults study (ACT). The ACT study will be the first Phase III RCT in the field of tDCS and will provide definitive insight into the adjunctive benefit of tDCS paired with cognitive training.
2. Study design and methods
2.1. Overall design
This National Institute on Aging (NIA) funded study employs a two-phase randomized clinical trial with a planned 360 participants total across three sites (University of Florida, University of Miami, and University of Arizona; 120 at each site). The trial is registered at clinicaltrials.gov as NCT028511. A unique feature of the trial is the study design, which is intended to increase the efficiency of the trial. In Phase 1, an initial cohort of 80 participants collected across all three sites will be assigned to one of four conditions as shown in Figure 2a. Half of the recruited sample in Phase 1 will undergo cognitive training; the other half will undergo education training, which is serving as a control. The first interim analysis, to be performed when the initial cohort of 80 participants completes a 3-month follow-up (Phase 1), will investigate whether cognitive training is significantly better than training control on a composite measure of cognitive training performance on the Posit Science BrainHQ tasks (Posit Composite Score). This will then determine whether we can eliminate the training control condition. Cognitive training in older adults has previously been established to improve proximal cognitive training outcomes in hundreds of published studies (for reviews, see80-83). In addition, our pilot data supported proximal transfer to cognitive training outcomes in the cognitive training condition versus the education control condition. These data are consistent with decades of cognitive training literature. No additional participants will be assigned to the training control groups if 1) cognitive training is found to be significantly superior to training control on proximal training outcome measures or 2) conditional power is calculated to be less than 80% even is sample size were increased by 80 participants. If conditional power from interim analyses is calculated to be greater than 80%, 40 or 80 participants (the smaller sample size that provides at least 80% conditional power) will be assigned to the four arms. Data from Phase 1 will also provide important mechanistic insight regarding neural mechanisms of cognitive training vs. a well-matched education training control, facilitating overall interpretation of Phase 2 data. Neural mechanisms to be compared between those who did and did not receive training (i.e., education control) will include change in: (a) functional connectivity between regions of interest (ROIs) attributed to training, (b) GABA concentrations in frontal cortices, (c) gray matter surface area and cortical thickness in training related ROIs, d) white matter volume in training-related ROIs, e) white matter hyperintensity load within training-related ROIs. In Phase 2, the remaining 280 participants will be randomized to the two cognitive training arms (i.e., eliminating the training control arms; cognitive training with tDCS and cognitive training with the sham, Figure 2b). After the remaining 280 participants have completed follow up in the cognitive training arms (including those in Phase 1, total n=360) analyses will investigate the benefit of adjunctive administration of cognitive training with tDCS on the primary outcome measure: NIH Toolbox Fluid Cognition Composite Score. Participants will be assessed at three primary time points: 1) baseline pre-training; 2) post-12 weeks of cognitive training/training control + stimulation/sham; and 3) one year follow-up after all training (see Figure 3 for timeline). This design will enable longitudinal analyses of cognitive training and tDCS effects individually and in combination. We will examine cognitive training and tDCS effects on secondary measures of cognitive performance, functional and metabolic neuroimaging measures, and everyday functional abilities. At each assessment, we will obtain clinical and medical history, neurocognitive measures (e.g., neuropsychological tests within or outside the trained domain), and neuroimaging [structural magnetic resonance imaging (MRI), functional MRI (FMRI), magnetic resonance spectroscopy (MRS)]. All participants will undergo neuroimaging at baseline, following training (12 weeks), and at one-year follow-up. The ACT study will be conducted as closely as possible to consort standards. It is important to note that the primary outcome differs between Phase 1 and Phase 2. Phase 1 aims to verify that cognitive training improves training targets better than no-training, whether or not tDCS is present, while also collecting important mechanistic data regarding training specific brain changes. Phase 2 then pursues the ultimate goal of the trial, which is to demonstrate that tDCS plus cognitive training can produce greater transfer to untrained neurocognitive outcomes. This goal has been rarely achieved by cognitive training alone. If anticipated results are achieved in Phase 1, per our analysis plan, this will trigger our ability to proceed to Phase 2 without no-training conditions, enabling us to concentrate greater statistical power on the contrast between training with and without tDCS.
Figure 2.
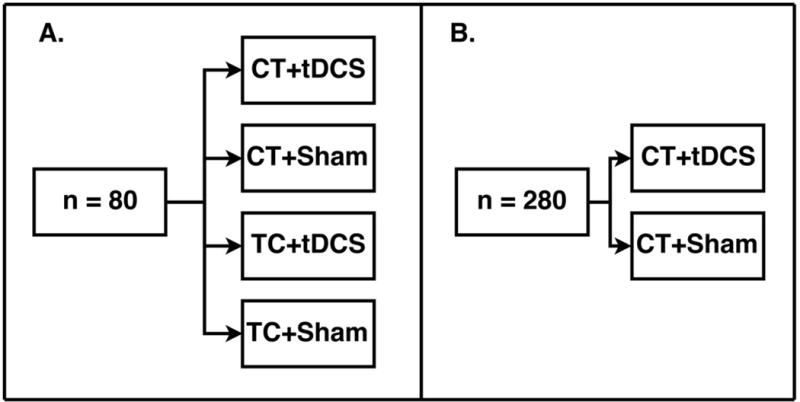
ACT Cell Design. A) Phase 1 four-cell design (n=80). B) Phase 2 two-cell design (n=280). CT = cognitive training, TC = education training control, tDCS = transcranial direct current stimulation, Sham = sham tDCS.
Figure 3.
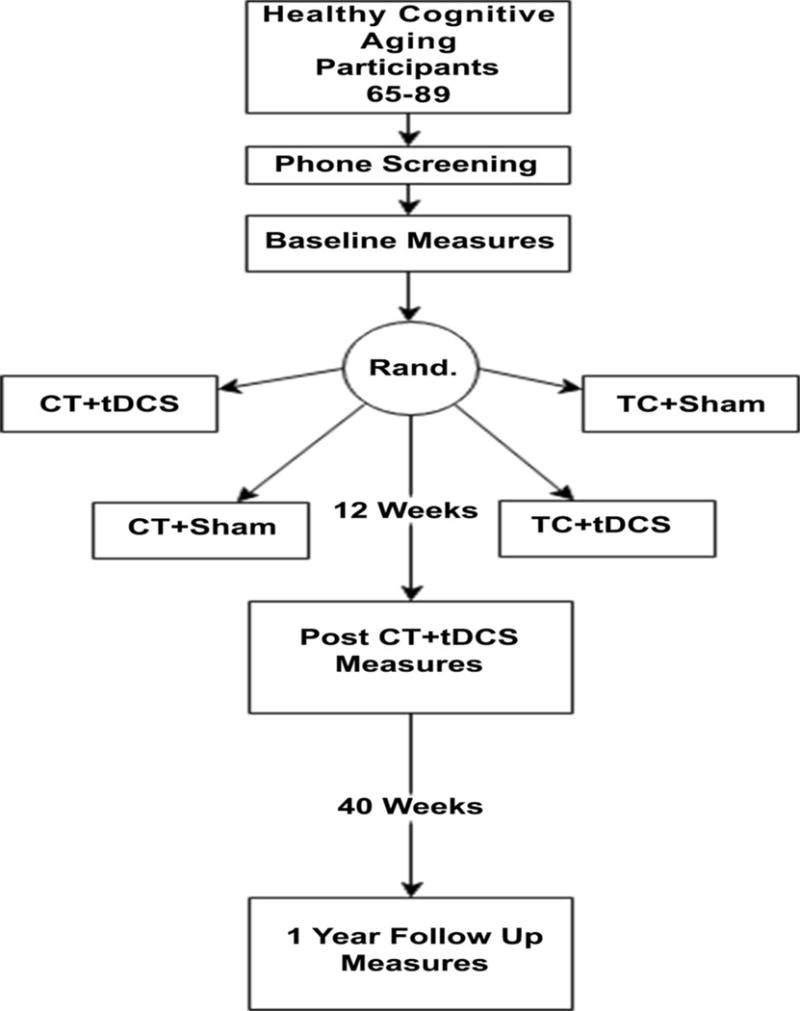
ACT Study Design. Figure depicts time points of contact, randomization, intervention, and assessment for ACT participants.
2.2. Participant eligibility, recruitment, randomization and retention
2.2.1. Participant eligibility criteria
Our inclusion and exclusion criteria are designed to minimize risks to participants.
Inclusion criteria
1) Age 65 to 89 years; this age group was selected because it is at high risk of age-related cognitive decline and has a sufficiently long life expectancy319 to participate in the study. 2) Evidence of age-related cognitive decline in the Posit BrainHQ Cognitive Training assessment defined by performance below the 80th percentile. 3) Ability and willingness to participate in the intervention, attend training sessions, and be randomized to any treatment group.
Exclusion criteria
1) Neurological disorders (e.g., dementia, stroke, seizures, traumatic brain injury). 2) Evidence of cognitive impairment (as defined by National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS-III) performance below 1.5 standard deviations on age/sex/education normative data in at least one cognitive domain).84 3) Past opportunistic brain infection 4) Major psychiatric illness (schizophrenia, intractable affective disorder, current substance dependence diagnosis or severe major depression and/or suicidality. 5) Unstable (e.g., cancer other than basal cell skin) and chronic (e.g. severe diabetes) medical conditions. 6) MRI contraindications (e.g., claustrophobia, metal implants). 7) Physical impairment precluding motor response or lying still for 1 hr and inability to walk two blocks without stopping. 8) Currently on GABA-ergic or glutamatergic medications, or on sodium channel blockers that may alter response to tDCS.85 9) Left-handedness, 10) Prior participation in a tDCS or a repetitive transcranial magnetic stimulation study.
2.2.2. Recruitment, informed consent and enrollment
All study participants will provide written informed consent. Persons will be recruited at each site using research registries, community outreach, community agencies, newspaper advertisement, public service announcements, mailings, and posted flyers. People interested in participating in the study will call the local site recruitment coordinator and be provided with more information about the study. Those who remain interested will be assessed on basic study criteria (e.g., age) via a standardized phone screening script. Participants that meet inclusion criteria will be invited for an in-person screening visit. At the start of the in-person screening visit, participants will indicate their agreement to participate by signing a site university institutional review board (IRB) approved informed consent document. Participants that remain willing to participate and meet the remaining inclusion/exclusion criteria (assessed at the baseline visit) will be enrolled into the study.
2.2.3. Randomization
Randomization will occur at the beginning of the first intervention visit. As noted, an initial cohort of 80 participants will be randomly assigned to one of four conditions: cognitive training+tDCS, cognitive training+Sham, training control+tDCS, training control+Sham. Half of the recruited sample in Phase 1 will undergo cognitive training; the other half will undergo the education training control. In Phase 2, the remaining 280 participants will be randomized to the two cognitive training arms (i.e., eliminating the training control arms), unless interim analysis results suggest that additional 40 or 80 participants are needed for the cognitive training vs. training control comparison. A permuted block randomization will be used with block sizes of 8 and 12 and with site treated as a stratification factor. Specifically, at each site, two participants will be assigned to each one of the 4 conditions among the first eight participants in random order, and three participants will be assigned to each one of the 4 conditions among the next twelve participants in random order. The block sizes are chosen so that there will be enough laptops configured for cognitive training and the training control, respectively.
2.3. Safety considerations
There are minimal risks associated with participation in the study. The potential risks are as follows:
Magnetic resonance imaging (MRI)
MRI is a procedure that is used routinely for medical care and is very safe for most people, but participants will be monitored during the entire MRI scan in case any problems occur. The risks of MRI are: 1) metal contraindicated for proximity to the scanner (e.g., metal in the eye, certain types of heart valves or brain aneurysm clips, etc.), 2) temporary hearing loss due to noise levels in the scanner environment (ear plugs will be used to minimize this risk), 3) claustrophobia.
Transcranial direct current stimulation (tDCS)
tDCS is considered safe but a small number of people do experience some side effects.49,54 The most common side effects are itching and tingling or mild discomfort at the area of stimulation, and headache. Other possible side effects include dizziness and nausea. Whenever an electrical stimulation is applied to the body, it could possibly cause a seizure or abnormal heartbeat, but this has never occurred with the transcranial direct current stimulation parameters used in this study.
Cognitive Training, Education Training, Neurocognitive and Functional tests, Questionnaires
There is a risk participants will find these tasks challenging, fatiguing, and/or boring. Research staff will explain what to do and how to perform the tasks during study visits. Participants will also have access to a 24-hour help line should they have trouble interacting with the training computers.
Other possible risks to participants may include fatigue due to the testing. Should this occur, participants can take a rest-break at any time or may discontinue the testing at any time.
2.3.1. Management of potential safety risks
Protection against risks associated with neuroimaging
MRI is widely regarded as a safe, noninvasive procedure for visualization of brain tissue in both adults and children. Prior to study participation, all participants will be informed of the MRI procedure during the informed consent/assent process. The proposed study will be performed on an FDA approved Siemens 3 Tesla scanners. There are no known long-term effects of MRI procedures on the body. Both study staff and trained MRI staff will check for exclusion criteria. In sum, the MRI neuroimaging procedures pose no radiological or medical risk, given that participants with metal implants susceptible to magnetic heating will be excluded based on standard scanner policies. A small number of people may become anxious in the small space of the scanner. These individuals will have the opportunity to terminate the scan session. Furthermore, all recruits will be screened for phobias prior to enrollment.
Protection of risks related to tDCS
To minimize risk associated with tDCS, participants will be monitored throughout stimulation sessions and asked to report any discomfort. If scalp sensation is uncomfortable, stimulation will be stopped. In the event of a headache, stimulation will be stopped. All tDCS sessions will be administered and continually supervise by a trained experimenter. The above symptoms have only been reported when participants are actively being stimulated.49 However, to assess for any symptoms occurring during the 24-hour interval between stimulation sessions, we will administer a brief symptom screening questionnaire at the beginning (symptoms in the past 24 hours) and end of each session (symptoms during stimulation). tDCS has not been shown to cause seizures nor lower the seizure threshold in animals. There are no reports of seizure induced by tDCS in human participants in the literature. However, this may not be true for epilepsy patients, whose seizure threshold rates are likely abnormal. Prior history of neurological disorders is an exclusionary criterion for our study and thus no participants will have a history of seizure.
Protection against risks associated with Cognitive Training, Education Training, Neurocognitive tests, Functional tests, and Questionnaires
These procedures have minimal risk associated with them. Breaks will be given in those cases where participants experience frustration with these tasks. Research staff that collect data have been trained in the conduct of all tests by senior staff members in the ACT Administrative Coordinating Center. Research staff members will be master certified in the conduct of these tests before they interact with study participants.
Protection against Risk of confidentiality
A study wide data safety monitoring plan (DSMP) has been adopted for protection of all data. Information pertaining to research participants will be obtained from (1) interviews with participants and (2) procedures described above. All data will be considered confidential according to HIPAA guidelines for personal health information. All participants will sign a combined consent to participate in research and HIPAA compliant confidentiality document approved by the IRB overseeing the Field Center recruitment setting.
2.3.2. Data Safety Monitoring Board
A five external member Data Safety Monitoring Board (DSMB) is established, with responsibility to monitor all aspects of the study, including those that require access to any masked data. The DSMB and its chair were named and approved by the NIA. The DSMB will meet by conference call every six months as determined by the DSMB and the NIA. The DSMB has access to all de-identified study data, documents and progress. The Safety Committee, comprised of safety personnel from each site, the Chair, and a representative of the Data Management and Quality Control Center (DMAQC) reports to the DSMB for issues related to participant safety. The DSMB reviewed the study protocol and approved the study for participant enrollment.
2.4. Treatment conditions
2.4.1. Cognitive Training
Cognitive training will involve sixty forty-minute sessions over 12-weeks (40 hours total); this includes ten daily sessions combined with stimulation for two weeks, then one weekly session combined with stimulation for the remaining ten weeks. The remaining 40 sessions will be performed by participants in their home on days they do not receive tDCS stimulation. Cognitive training duration was chosen based on prior cognitive training research, tDCS research and pilot studies carried out by the PI. Training platform. Cognitive training employs an eight component, Posit Science BrainHQ suite accessed via its researcher portal. Four of the tasks train attention/speed of processing, while the remaining four tasks train working memory (see Table 1). These tasks are web-based and multi-platform (i.e., Windows, Mac). Participants are provided with a Dell e5570 4G LTE enabled laptop with a 15.5 inch (diagonal) screen. Laptops are locked into a custom kiosk mode such that powering on the laptop only provides access to the ACT Posit Science Brain HQ training portal and will not allow access to any other features of the laptop. Kiosk laptops were designed for ease of use by older adult participants so that closing the laptop lid powers down the device, while the power button will power on the device and auto login to the training portal. Participants will also be provided with an optical mouse and comfortable headphones with an audio level adjustment dial. In addition, the custom cognitive training portal only allows access to the eight chosen Brain HQ tasks (Table 1). Participants will complete four tasks per day for ten minutes per task. A timer is built into the portal that only allows participants to progress to the next task after ten minutes of training on a given task. The order of presentation of tasks is counterbalanced to present each task equally over the 3-month training period, but is also randomized so that the four tasks presented per day are not the same each day. Study interventionists will provide weekly performance summaries to participants, allowing for consistent tracking of adherence and the formation of training remediation strategies throughout the study to reach the cognitive training target dose. These cognitive training tasks are commercially available (www.positscience.com), with well-documented protocols/manuals and thus not described in detail here. Participants will undergo basic computer training and orientation sessions and will have access to 24/7 telephone support.
Table 1.
Cognitive Training Sub-tests
| Attention/Speed of Processing | |
| Hawk Eye | Trains visual precision, which helps the participant perceive what is seen quickly and accurately so that it can be recalled more accurately. |
| Divided Attention | Requires the participant to focus in on and react to particular details quickly—matching colors, shapes, and/or fill patterns—while at the same time dismissing competing information. |
| Target Tracker | Designed to build divided attention by requiring participants to track several items moving around their screen at the same time |
| Double Decision | Requires speeded visual search and selective attention to peripheral objects among distractors.20 Difficulty increases relative to object similarity, presentation rate, and distractor complexity and eccentricity. Previously referred to as Useful Field of View training in the ACTIVE study. |
| Working Memory | |
| To Do List Training | Participants’ hear a set of instructions, then uses memory of those instructions to follow them in order. The instructions get longer and more complex over time at the task, making greater demands on the working memory system. |
| Memory Grid - | Auditory processing is one of the most important building blocks of memory. Only when participants take in information with crystal clarity can the brain store it accurately and recall it clearly later. In Memory Grid, the task is to match cards representing syllables together. |
| Auditory Aces- | Participants are presented with auditory information about playing cards. The information is presented one card at a time. The task is to decide if the current card information matches the card information presented a specific number of steps back (auditory n-back) in the sequence. |
| Card Shark- | Participants are presented with visual information about playing cards. The information is presented one card at a time. The task is to decide if the current card information matches the card information presented a specific number of steps back (visual n-back) in the sequence. |
2.4.2. Education Training Control
The training control condition will serve as a control for the cognitive training condition. The training control will be administered using the same methods as in the cognitive training condition except that the content loaded onto study laptops will be different. As with cognitive training, training control will involve sixty forty-minute sessions over 12-weeks (40 hours total); this includes ten daily sessions combined with stimulation for two weeks, then one weekly session combined with stimulation for the remaining ten weeks. The remaining 40 sessions will be performed by participants in their home on days they do not receive tDCS stimulation. The duration and frequency of training control will match that of cognitive training. Participants will be provided with the same laptops described above in the cognitive training condition. The training control involves watching 40-minute educational videos produced by the National Geographic Channel, which cover a range of topics such as history, nature, and wildlife. All video content is unique for each day of training. To encourage active engagement and attention, participants will be asked to answer questions regarding the content of the videos. As with cognitive training laptops, training control laptops will be locked in custom kiosk mode and will auto-login upon startup to a local html website (not requiring internet access) that presents participants with the ACT Education Training Video menu (Figure 4). This menu contains a list of links to each daily video (Days 1-60). Participants will click on the day’s training link (e.g., Day 20). This link will take participants to a full screen 40-minute video of the day’s contents. Each video starts with a welcome screen and ends with a reminder to fill out the daily 4-6 questions related to the 40-minute video content. Participants in this condition are provided with a binder containing each day’s questions. At each intervention visit, questions are collected from participants, providing a mechanism for weekly feedback on training adherence and for establishing adherence remediation strategies to reach the target dose of education training.
Figure 4.
The Education Training Control video menu. Participants randomized to the education training condition use this menu to select their daily videos during the intervention phase of the trial.
2.4.3. tDCS
A Soterix Clinical Trials Direct Current Stimulator will apply 20 minutes of 2.0mA (30s ramp up/down) direct current through two biocarbon rubber electrodes encased in saline soaked 5×7cm2 sponges (8cc of 0.9% saline solution per sponge) placed over the frontal cortices at F3 and F4 (10-20 system).86 Stimulation will occur during the first twenty minutes of the 40-minute training session, capitalizing on both acute and after effects of tDCS. Stimulation parameters and frequency were based on pilot data and prior tDCS research. Electrode placement locations are determined using the International 10-20 measurement system. Current inflow will occur on the right (F4), and outflow on the left (F3). Impedance quality will be ≤10kΩ to insure proper stimulation of brain tissue. The Soterix Clinical Trials Direct Current Stimulator device has built in RCT double blinding protocols requiring entry of a six-digit code to initiate stimulation. Six digit codes were pre-programmed into the stimulators used in the study and transmitted to the study statistician to maintain blinding of all study staff. Codes were preset during the manufacturing process to either deliver active tDCS or sham tDCS and are uniquely assigned to participants by the DMAQC. Quality Control: 3D mesh models of participant’s heads with electrodes affixed are taken before and after stimulation. Using a Structure 3D Scanner attached to an iPad using TechMed3D model capture software, these models provide 1mm resolution whole head 3D mesh models in approximately 2 minutes. By placing green non-conductive markers at the nasion, Fz and center of each electrode location, backwards calculation of target vs. actual electrode location can be determined and measured, providing a physical metric of electrode placement quality (pre-stim model) as well as electrode drift during session (post-stim model). This is performed for all 20 stimulation sessions. In addition, participants are administered a pre and post stimulation sensation questionnaire to capture perceived sensation before during and after each stimulation session.
2.4.4. Sham tDCS
Sham stimulation is performed with the same device and all procedures are identical except for the duration of stimulation. Participants in the sham tDCS condition receive 30 seconds of 2 mA of direct current stimulation (30 second ramp up/down) at the beginning of the session. Participants typically habituate to the sensation of tDCS within 30-60 seconds of stimulation. This procedure provides the same sensation of tDCS without the full duration of stimulation, making it a highly effective sham procedure. The same 3D modeling and sensation questionnaire procedures are performed before and after stimulation.
2.5. Measurements
Participants first undergo a brief screening assessment to assess eligibility. As described above, all participants enrolled in the trial undergo intervention assessment measurements at 3 time points (baseline, 12 week, and 12 month follow-up).
2.5.1. Phase 1 Outcome
The outcome measure for Phase 1 (n=80) will be the Posit Science BrainHQ Cognitive Training Composite Performance measure. The goal of phase 1 is to determine if cognitive training is superior to education training in proximal transfer to cognitive training performance metrics. This measure involves performance on the 8 selected cognitive training tasks set to the medium difficulty level and provides a measure of proximal performance on cognitive training tasks central to the cognitive training condition. The Posit BrainHQ Cognitive Training Composite Performance measure will be acquired at three time points (screening, 12 weeks, and 12 months). As the goal of phase 1 is to confirm previously demonstrated differences in efficacy between cognitive training and training control, irrespective of tDCS, the Phase 1 outcome was chosen to reflect standard metrics used in previous research (i.e., proximal outcome on cognitive training task performance).
2.5.2. Primary Outcome (Phase 2)
The primary outcome measure for Phase 2 is a composite index of cognitive abilities previously shown to decline with advanced age (i.e., fluid cognitive abilities). The ACT primary outcome measurement is the Fluid Cognition Composite Score (FCCS) from the NIH Toolbox Cognitive Function Battery. The FCCS comprises five measurement assessing attention (Flanker Inhibitory Control and Attention Test), speed of processing (Pattern Comparison Processing Speed Test), working memory (List Sorting Working Memory Test), episodic memory (Picture Sequence Memory Test), and executive function (Dimensional Change Card Sort Test).87-95 This measure provides a single composite score sensitive to cognitive aging.
2.5.3. Secondary Outcomes
Neuroimaging
We will conduct neuroimaging on a Siemens 3.0 Tesla research dedicated scanner with an existing research agreement. Scanning will take 1 hour to acquire: 1) Structural MRI (T1, FLAIR), 2) FMRI (EPI-BOLD), 3) Proton MRS. FBIRN and GABA phantoms were created from single batch chemicals and divided into three separate MRI/MRS phantoms, one for each site. All phantoms were scanned at each site and each site assigned a single FBIRN (fMRI) and GABA (GABA/proton MRS) phantoms for weekly imaging throughout the study. A human phantom was also recorded at each site prior to study enrollment and in years 3 and 5.
Structural MRI
High-resolution whole brain axial gradient-echo MPRAGE 3-D T1-weighted images will be acquired for volumetric and cortical thickness analyses and FMRI localization.
FLAIR
A high-resolution 3D FLAIR sequence will be collected to quantify white matter hyperintensities using semi-automated analysis tools for determining distribution of pixel values, measuring ROIs, and segmentation of images.96-98
FMRI
We will present two FMRI tasks (2-Back, UFOV/Double Decision) using E-Prime 2 software (Psychology Software Tools, Inc., Pittsburgh, PA), with the video signal on a screen behind the participant’s head. The screen is viewed through a double-mirror attached to the head coil. An MR-compatible piano-key response box attached to the stimulus presentation computer will collect performance data. We will apply a cushioned-pillow head stabilizer to minimize head movement during scanning.
2-Back
This task will measure brain changes due to our N-back training
We will assess verbal working memory on a 2-Back task, as in past studies99-101. Consonants are visually presented briefly with a small rest period between each (Figure 5). Participants determine if each stimulus is the same or different from previously stimuli, responding by binary button press (yes vs. no). Executive control, phonemic buffering, and sub-vocal phonemic rehearsal are required. 0-back and 2-Back conditions are alternated in a block design with two 5-minute runs of eight blocks (consonant lists), with four blocks of the 0-Back and four blocks of the 2-Back. 0-Back: Four blocks of nine consonants of random case and order (33% targets). Yes-no responses are made if targets that match stimuli occurring two earlier. 2-Back: Four blocks of 15 consonants (33% targets) will be pseudorandomly presented across the visual field. Accuracy and reaction time (RT) are recorded.
Figure 5.

N-Back fMRI Task. An example of N-Back working memory stimuli presented to participants in scanner. Participants are presented with a fixation crosshair followed by a consonant on the screen and required to determine whether the letter was either an ‘X’ in the 0-Back paradigm or the same as the letter presented “2-back” in the 2-Back paradigm.
Useful Field of View
This task will measure brain changes due to alterations in attention and decision-making processes due to BrainHQ Double Decision (Previously referred to as Useful Field of View training in the ACTIVE study)
We will assess attentional and decision-making processes on a scanner adapted event related UFOV/Double Decision training task that requires participants to simultaneously apprehend the identification of a centrally located target (car or truck) and the location of a target (car) among a parametrically manipulated array of distractors (0-47 distractors). Following a visual mask, participants then make a two-alternative forced choice (correct or incorrect) decision based on whether both the central target and distal target (without distractors) are identical to what was seen in the prior display (Figure 6). Two five-minute blocks of 56 trials are presented. Accuracy and reaction times are recorded. Jitter prior to stimulus presentation and response probe allows contrasts assessing unique activation associated with attentional and decision-making brain regions, providing mechanistic insight into cognitive training effects.
Figure 6.
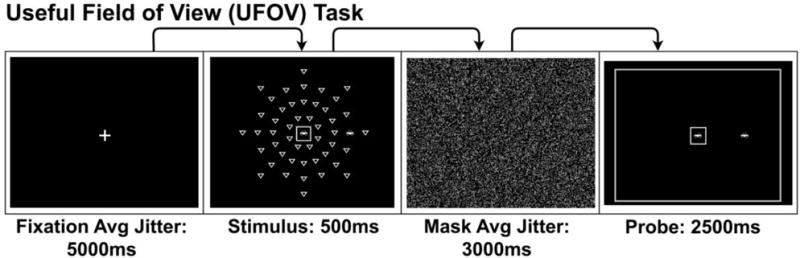
Useful-Field-of-View (UFOV)/Double Decision fMRI Task. An example of a single trial of the UFOV/Double Decision fMRI task. Participants are presented with a fixation crosshair followed by stimulus display where they are required to remember the central presented car or truck and the location of a peripherally presented car amongst a varied level of distractors in the shape of a yield sign. Participants then view a visual mask image to interfere with retinal images created by the prior stimulus and finally response screen that depicts a car or truck in the center box and a car in a location on the periphery. Participants respond yes/no indicating if the response screen matches the stimulus screen.
Resting State fMRI
Participants will also be asked to rest for 6 minutes while functional data is being collected to assess resting state activation. Participants will view a crosshair centrally presented on the screen and will be asked to fix their gaze on the crosshair and let their mind wander.
Proton MRS
GABA-edited spectra will be acquired using the MEGA-PRESS experiment, from a 2.7×3×4 cm3 voxels (medial frontal). Spectra will be analyzed using Gannet and LCModel to assess cerebral metabolites and neurotransmitter concentrations.102-104
Neurocognitive Assessment
In addition to the NIH Toolbox Cognitive Function Battery, assessments will include a neurocognitive battery (see Table 2) to assess broader generalization and specificity of intervention effects. The battery consists of standardized, well-established neurocognitive measures with strong reliability and validity 105. For cognitive measures with functions assessed see Table 2 below. Our goal is to assess global cognitive ability (NIH-Toolbox: cognitive module), and specifically attention-executive functions, working memory, processing speed, and memory. These are domains affected by aging19,26,106-116 and will also assess the domains assessed by FMRI. Additional neurocognitive measures were included as secondary measures to supplement domains assessed by the primary outcome measure and assist in better understand patterns of transfer facilitated by tDCS.
Table 2.
Neurocognitive Assessment
| Measure: | Domain: |
|---|---|
| Hopkins Verbal Learning Test-Revised 117 | Verbal Learning/Memory |
| Stroop 118 | Attention/Executive |
| Trail Making A & B 119 | Executive |
| Controlled Oral Word Association & Animal | Verbal Fluency |
| Naming 120 | |
| Brief Visuospatial Memory Test-Revised 121 | Visual Memory |
| Symbol Digit Coding (WAIS IV) 122 | Processing Speed |
| Letter Number Sequencing (WAIS IV) 122 | Working Memory |
| Paced Auditory Serial Addition Test 123 | Working Memory |
| Digit Span (WAIS IV) 122 | Working Memory |
| NIH Toolbox Cognitive Battery Subtests: 87-95 | |
| Dimensional Card Sort | Executive Function* |
| Flanker | Attention/Executive * |
| Picture Sequence | Episodic Memory* |
| Picture Vocabulary | Language |
| Oral Reading | Language |
| Pattern Comparison | Processing Speed* |
| List Sorting | Working Memory* |
= subtest included in the NIHTB Fluid Cognition Composite Score
Functional Outcomes
The iFunction touchscreen computer-based functional assessment tool will be used to measure performance on everyday tasks such medication management, ATM banking, and refilling a prescription via a voice menu.124,125 Task difficulty can be varied and real-time efficiency and accuracy data are collected; the measure is correlated with component cognitive abilities targeted in this study124,125. This provides an ecologically valid measure of every day function. The iFunction composite performance index will be used to assess change in functional abilities. In addition, we will administer the Driving Habits126 and Independent Activities of Daily Living (IADL)127 questionnaires from the original ACTIVE cognitive training trial. The IADL measure was originally adapted from the Minimum Data Set for Home Care (MDS).128 These measures will allow assessment of change in an objective metric of everyday function, driving cessation, driving difficulty and avoidance, and self-rated functional abilities.
Quality of Life (QOL) and Patient-Reported Outcomes Measurement Information System (PROMIS) self-reported health assessment
We will administer the Medical Outcomes Study Short Form-36 (SF-36: v. 2.0, a widely used QOL measure), and the PROMIS self-report measures at each assessment. The PROMIS measures assess change in self-reported cognitive and physical function.129 Change in self-reported physical and mental health status correlate with QOL and mental and physical health status. 130-132 These two measures will serve as important indicators of the impact of the interventions on everyday life.
Health Events/Adverse Events
An abbreviated Medical History and Events Form will be administered at follow-up assessments to evaluate any new medical events since baseline that would signify adverse events and track changes in medical co-morbidities and medication use over the intervening periods between assessment time points.
Alcohol and Drug Use Questionnaires
We will administer the Alcohol Use Disorders Test (AUDIT-10)133 and Drug Abuse Screening Test (DAST-10).134 These measures will provide valuable information about how drug and alcohol use may alter the overall efficacy to tDCS, cognitive training and education training.
Driving Record Assessment
Driving records will be requested following the completion of the intervention; records will be requested at 5 years post intervention and at 10 years post intervention. These records will allow us to examine real world driving outcomes (e.g., accidents, citations, driving cessation, etc.). The driving record assessment is optional (the participant choses to consent to this portion or not at screening). Participants who do not consent to the driving record assessment can still participate in the study.
Walking assessment
We will administer a 10-meter walk test.135 This test measures the time it takes participants to walk ten meters in a line. Participants are instructed to walk at their normal pace, as if they were walking down the street. Participants are instructed to use any walking aids they normally use (e.g. cane).
Additional questionnaires
We will also administer a series of questionnaires to assess: depression (Beck Depression Inventory-II),136 anxiety (State Trait Anxiety Inventory)137, apathy (Starkstein Apathy Scale),138 social isolation (UCLA Loneliness Scale),139 social engagement (Lubben Social Network Scale),140 sleep quality (Pittsburgh Sleep Quality Index),141 and chronic pain (Graded Chronic Pain Scale).142 Additional questionnaires in ACT will provide measures for analysis of potential secondary effects of intervention (e.g., improvement in social engagement with improved cognitive function, decreased anxiety or depression secondary to intervention, etc.). In addition, participants in ACT will receive study specific questionnaires assessing 1) expectations of cognitive training, 2) expectations of brain stimulation, 3) and blinding efficacy. In addition, interventionists will also complete a questionnaire evaluating whether blinding to stimulation condition was successful. These questionnaires were developed based on recommendations for assessing brain training expectations in Rabipour and Davidson (2015).143 These questionnaires are included as supplemental material.
2.5.4. Other Measures
Physiological Recording
During stimulation sessions, participants will be asked to wear an Empatica E4 wristband to record physiological information such as beat-to-beat heart rate and galvanic skin response. Recording will start 5 minutes prior to stimulation and continue until 5 minutes after completion of the 40 minute training session. Using the built in event logging function, we will place an event marker at the start of stimulation, allowing reconstruction of the time course of the pre-stimulation, during and after stimulation periods of acquisition.
Blood
Participants will be invited to participate in an optional blood draw at baseline with draw of plasma, serum, and whole blood. Blood from participants will be stored in a centrally managed blood bank at the University of Florida for future analyses.
Screening Measures
During in-person screening prior to baseline assessment, participants will be asked to perform a brief battery of tests to assess inclusion/exclusion criteria. The table of measures is presented below (Table 3).
Table 3.
Screening Measures
| Measure: | Purpose: |
|---|---|
| Informed Consent | Voluntary participation |
| Medical History and Prescription Drug List | Medical conditions and drug exclusion criteria |
| MRI Screening Form | MRI/tDCS contraindications |
| UDS-III84 | Cognitive impairment |
| AD-8144 | Self-Reported dementia scale |
| Wechsler Test of Adult Reading 145 | Verbal intelligence |
| Beck Depression Inventory - II 136 | Depression |
| Computer Use Questionnaire | Prior computer experience |
| Ishihara Color Vision Test146 | Color vision |
| NIH Toolbox Visual Acuity 147 | Visual acuity corrected to at least 20/80 |
| NIH Toolbox Words-in-noise 148 | Hearing (absence of severe hearing impairment averaged across both ears) |
| Posit Science BrainHQ Cognitive Training Assessment | Composite performance less than 80%ile on cognitive training tasks |
2.5.4. Training, Supervision, and Adherence of Study Assessors
The study will be highly manualized. The Administrative Coordinating Center (ACC) has developed a detailed manuals of procedures (MOPs), which is organized into 19 chapters to document each component of the ACT study (study organization, blood processing, assessment, etc.). In addition, approximately 30 hours of video MOPs were recorded and edited to provide training in all assessment and intervention tasks. Pre-site visit training is completed over a four-week period, with weekly items due to the ACC. Site visit training involves 4 consecutive days of onsite training to provide hands on training in each component of assessment and intervention. Post-site visit training involves four-weeks of training with weekly items due to the ACC. Post-site visit training is organized around completion of a set number of video recorded “mock” screening (2 mandatory, 3 optional upon performance), assessment (2 mandatory, 3 optional upon performance), and intervention (6 mandatory) sessions submitted to the ACC for scoring and feedback. There are separate training checklists and training goals for intervention vs. assessment coordinators. All personnel performing data entry will also complete Data Entry certification, consisting of entry of 2 full mock screening, assessment and intervention data sets into a “mock” REDCap database that mirrors the actual study database. Data entry accuracy is assessed. Coordinators are given master certification and allowed to see participants in assessment or intervention roles upon completion of pre-site visit training, site visit training, and post-site visit training in their specific role. New study coordinators will be required to complete pre and post-site visit training checklists and receive on-site training from a master-certified coordinator before receiving certification for participant interaction. All coordinators actively interacting with participants will be required to submit video recording of one session (intervention session for interventionists, assessment visit for assessment coordinators) once per month for study assessor and intervention adherence monitoring. In addition, tDCS Quality Assurance metrics, 3D head modeling, MRI quality metrics, and data entry accuracy (assessed by DMAQC cross check of scanned de-identified copies of data) will occur throughout the trial. A project manager and the Field Center PI will oversee day-to-day activities at each site. Field Center PIs will have weekly conference calls for regular updates and study oversight. In addition, separate weekly conference calls will take place for interventionists and assessment coordinators to troubleshoot ongoing issues and provide consistency across sites.
2.6. Power and Sample Size Considerations
We will enroll a total of 360 participants, enabling us to have at least 90% power to reconfirm that cognitive training is better than training control) and at least 90% power to detect the benefits of adjunctive interventions (tDCS) on the primary outcome measure: NIH Toolbox Fluid Cognition Composite Score (NIHTB FCCS). Cognitive training vs. training control effects will be tested based on the first cohort of 80 participants (effective sample size of 60, considering 25% attrition). Based on the strong effect of cognitive training on cognitive outcomes (mean of −3.88 for UFOV trained vs. −.81 for controls and conservative SD estimate of 3.07, Cohen’s d = 1) observed in ACTIVE and other trials9,12,13,16,17,19,81,107,149-151, a linear contrast test at one-sided 0.05 level will have 98% power. tDCS vs. sham superiority with cognitive training will be tested based on the 320 participants who are assigned to the two cognitive training arms in both stages (including the 40 participants assigned to cognitive training arms in phase 1, effective sample size of 240, considering 25% attrition). The planned sample size will enable us to have at least 90% power to detect a difference of effect size 0.42 between cognitive training+tDCS and cognitive training+sham, using a normal inverse combination test at one-sided 0.025 level. We will have similar power for secondary neuroimaging measures of brain change.
2.7. Data analysis plan
Aim 1 of the study is to determine whether neurocognitive improvement and longer-term functional outcome are better when cognitive training is coupled with tDCS. We hypothesize that: H1.1) cognitive training will produce significant improvements on a composite measure of cognitive training performance on the Posit Science BrainHQ tasks (Posit Composite Score) compared to the treatment control condition; H1.2) tDCS combined with cognitive training will produce significant improvements on a composite measure of attention, working memory, processing speed, and executive function (NIH Toolbox Fluid Cognition Composite Score, NIHTB FCCS) compared to the sham control condition; and H1.3) Near and far transfer of cognitive training and tDCS will occur as assessed by improvement in the iFunction Composite Index (far) and comprehensive neurocognitive assessment (near).
H1.1 will be assessed at the end of Phase 1 when the first cohort of 80 completes the Posit Cognitive Training Measure at 3-months. We will investigate whether cognitive training is significantly better than training control in near transfer effects on cognitive training outcomes thereby enabling elimination of the training control condition. To ensure sufficient power to test hypothesis, we will perform data reduction and use a composite measure of cognitive training performance on the Posit Science BrainHQ tasks for this analysis. The superiority of cognitive training over training control will be tested using a linear contrast test in regression analysis where the change of composite outcome from baseline to 3-month follow-up is the dependent variable. To enhance the statistical inference about the training control effect, we will remove between participant variations due to the tDCS effect by including it in the regression analysis along with pre-specified covariates (age, gender, clinical site and baseline composite measure), however the estimate of tDCS effect will not be disclosed to the investigators. The cognitive training superiority hypothesis will be tested at one-sided 0.05 level. However, due to potential sample size re-estimation, the overall significance of the cognitive training effect will be tested based on the inverse-normal combination of two p-values, one from this interim analysis (p1) and the other from the data after interim analysis (p2). More specifically, the overall test statistics will be [sqrt(2)Φ−1(1− p1) + Φ−1(1− p2)]/ sqrt(3), where Φ−1 is the inverse of standard normal distribution function; and early rejection boundary in the interim is chosen to be p1 < 0.04. No additional participants will be assigned to the training control groups if the primary hypothesis H1.1 is rejected in the interim or conditional power is less than 80% even with an increase of 80 participants; otherwise, 40 or 80 participants (the smaller sample size that provides at least 80% conditional power) will be assigned to the four arms. It is worth pointing out, while we wait for the 3-month posit cognitive training outcomes of the first 80 participants, recruitment will not be stopped and participants will be randomized to the two cognitive training arms until interim analysis results suggest a change in allocation ratios. Also, even if cognitive training superiority is rejected in the interim, the study will not be stopped because the remaining participants will be randomized to the two cognitive training arms to study the tDCS effect (primary trial hypothesis).
The primary hypothesis H1.2 will be tested based on NIHTB FCCS at one-sided 0.025 Type-I error level. This outcome measure provides an overall index of performance across the domains of attention, working memory, processing speed, and executive functioning. By using this measure, we provide a single cognitive outcome upon which the success of the RCT can be judged. The analysis plan is similar to the one for H1.1. The overall test will be based on the inverse-normal combination of two p-values, one from the interim analysis and the other from the data after interim analysis. In both analyses, a regression model will be fitted with change of NIHTB FCCS as dependent variable and assignment group as independent variable along with pre-specified covariates (age, gender, clinical site and baseline FCCS), and p-values will be evaluated for the effect of tDCS vs sham. The combination weights will be proportional to square root of preplanned sample sizes (40 and 280). For H1.3, University of Miami Functional Battery Composite Index and comprehensive neurocognitive assessment will be analyzed similar to Posit Composite Score and NIHTB FCCS.
Aim 2 of the study is to determine whether cognitive training combined with tDCS leads to greater functional and metabolic brain changes (fMRI, MRS). Effects will parallel Aim 1. The analysis plan for Aim 2 is similar to the plan for Aim 1, except that the dependent variables will be changed from cognitive and functional outcomes to neuroimaging indices from FMRI and MRS (i.e., activation in working memory, attentional brain systems, and other regions of interest). In addition, a longitudinal multivariate model (LMV) for continuous-valued FMRI indices will be employed. Zero-mean error terms that account for both temporal correlation and cross-correlation among percent signal change values will be used for each region of interest. Cognitive performance indices associated with brain activation will be modeled by generalized linear mixed effects model (GLMix), with fixed effects such as treatment group, time, and their interaction and random effects are subject-specific parameters that model variation between subjects and within-subject correlation of longitudinal data. In both LMV and GLMix models, we will test rates of improvement of the neurocognitive indices, with model checking by residual analysis for the fitted models. Models will be fitted in SAS (SAS Institute, Inc.), estimated by maximum likelihood parameters and SEs (restricted ML approach).
Finally, we will conduct exploratory analyses to examine which baseline factors (e.g., clinical, demographic, neuroimaging, cognitive) best predict individual differences in neurocognitive and functional outcome. Support vector machine (SVM)104 and classification and regression tree (CART)105-110 methods will be used to develop models predicting cognitive and functional outcomes using baseline neuroimaging (MRS, FMRI) and clinical indices, along with treatment condition and demographic characteristics.
Secondary outcomes (see 2.5.3) will be analyzed using the same general approach described for assessing primary outcome measures. Each secondary measure provides the potential for examination of secondary effects of intervention on potentially related cognitive, behavioral and brain systems (e.g., social engagement, pain, functional connectivity, etc.), which may provide important information regarding future intervention targets. In the event that the trial produces negative findings for the primary outcome measure, analyses of secondary outcome measures and exploratory analysis described above to identify individual differences in treatment response and identify subgroups of responders will be important for optimizing intervention approaches and planning future trials.
3. Discussion
Preserving optimal cognitive and functional capacity is essential for successful aging in older adults. This concern has increasingly become a public health issue given that people are living longer, and at increased risk for cognitive and functional decline as they reach advanced age. Besides impacting quality of life, the prospect of functional decline among an increasing proportion of the population has profound social and economic consequences. Accordingly, there is a pressing need for effective interventions remediating the cognitive aging process and slowing or preventing the onset of dementia. While certain cognitive training approaches have shown promise in transfer to cognitive abilities and functional outcomes, effects tend to vary in effectiveness and duration.9,13,17,19,152 tDCS has the potential to improve the effectiveness of cognitive training through its direct neurophysiological effect on the neuroplasticity of brain regions important for cognitive training gains.
We have described the rationale and design of a trial designed to investigate whether adjunctive tDCS and cognitive training in older adults will produce clinically meaningful impact on cognitive function. This study will serve as the first phase III tDCS trial to date and represents a significant step toward clinical translation in the field. Since the pioneering work of Wilder Penfield (1951), it has been recognized that sensory, motor, and cognitive functions could be altered via electrical stimulation of specific brain regions. In laboratory animals, brain stimulation represented an alternative approach to experimental lesions, enabling both the potentiation and inhibition of neural activity depending on where in the brain stimulation was applied. Until recently, most human brain stimulation studies involved neurosurgically implanted electrodes, which has obvious limitations for general clinical use. As tDCS is safe and can be consistently applied with training, it represents a method that has high potential for ready translation into clinical space.
There are several design features worth noting in the ACT study. The ACT intervention will be tested at three sites, providing access to a diverse study population and enhancing the generalizability of the trial. In addition, ACT uses an active control condition that matches the cognitive training condition in duration and interval, while also attempting to match conditions for level of participant engagement in the training tasks (National Geographic content and post-video content questions). Both cognitive training and education training are administered via the same computer hardware with kiosk mode providing access only to study content. This is an important control, as participants that do not have access to computers at home, but are provided an internet enabled computer, could use the computers to become otherwise active computer users – an active intervention in its own right. By locking access to study content only, we avoid introduction of an additional variable of increased general computer use in a subset of participants.
The goal of ACT is to facilitate neuroplasticity in the frontal lobes during cognitive training. Thus, stimulation parameters in ACT were selected based on this goal. 2mA stimulation for 20 minutes was specifically chosen in ACT based on prior work demonstrating net excitatory effects of stimulation under both electrodes, in comparison to prior work showing net excitation under anode and net inhibition under cathode electrodes at 1mA.45,58,59,153 F3-F4 stimulation sites were chosen based on our prior work and others demonstrating both behavioral effects in our targeted training modalities, as well as finite element computational models of this montage demonstrating broad stimulation of frontal lobes from these stimulation sites.48,50,51,53 In addition, 3D scanner modeling of electrode location accuracy provides a heretofore unavailable feature in prior tDCS studies to verify accuracy of electrode location and calculation of an intervention quality variable within and between participants for use as a statistical factor in assessing treatment outcome. In addition, one element that commonly leads to un-blinding of tDCS conditions relates to warming of electrodes and skin and reddening of skin in the active condition. This is avoided in ACT by using a 20 minute stimulation period at the start of a 40 minute training session, allowing ample time for electrodes and skin to return to a pre-stimulation state before electrodes are removed by interventionists.
Assessment of driving records over a ten-year period provides an important real-world metric of IADLs and functional ability. In addition, UFOV cognitive training has previously shown significant treatment effects on driving performance using this same approach. In this same vein, we also adopt two other ACTIVE measures that demonstrated response to UFOV training: Driving Abilities and Independent Activities of Daily Living (IADL) questionnaires. Adoption of these three measures provides important converging measures between our study and prior studies of cognitive training efficacy.21,154 Combined with innovative assessment of ecologically valid measures of functional abilities (iFunction), the ACT battery will provide robust insight into the impact of intervention on older adult’s everyday functional skills.
The adaptive design used in ACT provides the ability to obtain mechanistic data on all four possible conditions within the study, while still obtaining the necessary power to assess the primary trial outcomes. The multimodal neuroimaging approach used in ACT provides an important source of data on the neural mechanisms underlying effects in the ACT study. In addition, the ACT study will provide insight into the neural mechanisms of change between cognitive training and an active training control, as well as mechanisms of additive benefit from tDCS. Furthermore, magnetic resonance spectroscopy methods in ACT will provide insight into change in two neurotransmitter concentrations central to neuroplasticity: GABA and glutamate. In addition, specific fMRI tasks assessing change in functional brain response on UFOV/Double Decision training and N-Back will provide direct insight into training related changes. FLAIR imaging will provide insight into a potentially important predictor of treatment response: white matter hyperintensity load (i.e., white matter integrity). Collection of blood at baseline in ACT will also provide the ability to assess a variety of blood-based markers that may predict treatment response, such as APOE4 and BDNF. Assessment of depression, chronic pain and other clinical variables will also provide important insight into potential predictors, mediators, and moderators of treatment response. Combined with the broad assessment battery in the ACT study, this study will not only answer central questions regarding adjunctive benefit of tDCS and cognitive training, but also shed light on a variety of related processes potentially impacted by frontal tDCS and cognitive training, such as change in depression, apathy, social engagement, and pain.
As the first phase III clinical trial in tDCS, the design and methods used in the ACT trial also forge new ground in the management and administration of rigorous tDCS application in large-scale trials. As a field, a majority of phase II tDCS trials have been limited to 20-40 participants. The ACT study will provide the first population size study on the benefits of adjunctive tDCS administration. The ACT study will have a significant impact on the field of tDCS regarding perceived utility of tDCS as a clinical tool.
While the ACT study represents a variety of significant advances in the field of tDCS and utilizes a state of the art adaptive trial design, there are potential limitations to the study that are worth noting. The ACT trial is presently only funded to follow participants for 1 year. Thus, the effects of intervention on slowing dementia onset are unlikely to be clear with only 1 year of follow-up. At informed consent, participants are asked to provide written consent (if willing) to be re-contacted for a five-year follow-up visit. Future funding will be sought to enable 5-year follow-up of the cohort, providing clearer insight into intervention impact on dementia development and long-term maintenance of training gains beyond 1 year. Research on the overall effectiveness of combined intervention approaches are mixed. While some have shown little additional gain or decreased gain relative to individual intervention approaches,155 others have found amplified effects of combined intervention strategies.156 Our pilot data leading to this trial are consistent with the latter. Interim analyses after the initial cohort of 80 participants will allow for assessment and adjustment of intervention strategy should this be required.
In summary, the ACT study will capitalize on the promise of cognitive training as an intervention for cognitive aging and the ability of tDCS to directly influence neuroplasticity to attempt to remediate age-related cognitive decline and potentially slow the onset of dementia. This adaptive phase III multisite randomized clinical trial will enroll 360 participants and collect a robust set of cognitive, functional, and brain-based metrics of cognitive and brain health to understand both efficacy and mechanism of change from adjunctive tDCS and cognitive training intervention. Depending on the success of the trial, ACT may provide a non-invasive option for addressing cognitive aging in older adults.
Supplementary Material
Acknowledgments
The authors would like to acknowledge support from the National Institute on Aging R01AG054077 (ACT), K01AG050707, AG019610, the state of Arizona and Arizona DHS, the Advanced Research Institute for Biomedical Imaging, the UF Cognitive Aging and Memory Clinical Translational Research Program, and the McKnight Brain Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aubertin-Leheudre M, Woods AJ, Anton S, Cohen R, Pahor M. Frailty Clinical Phenotype: A Physical and Cognitive Point of View. Nestle Nutrition Institute workshop series. 2015;83:55–63. doi: 10.1159/000382061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton SD, Woods AJ, Ashizawa T, et al. Successful aging: Advancing the science of physical independence in older adults. Ageing research reviews. 2015;24(Pt B):304–327. doi: 10.1016/j.arr.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods AJ, Cohen RA, Pahor M. Cognitive frailty: frontiers and challenges. The journal of nutrition, health & aging. 2013;17(9):741–743. doi: 10.1007/s12603-013-0398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas KR, Marsiske M. Age trajectories of everyday cognition in African American and White older adults under prompted and unprompted conditions. Neuropsychological rehabilitation. 2017;27(4):522–539. doi: 10.1080/09602011.2015.1092453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas KR, Marsiske M. Verbal prompting to improve everyday cognition in MCI and unimpaired older adults. Neuropsychology. 2014;28(1):123–134. doi: 10.1037/neu0000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diehl M, Marsiske M, Horgas AL, Rosenberg A, Saczynski JS, Willis SL. The Revised Observed Tasks of Daily Living: A Performance-Based Assessment of Everyday Problem Solving in Older Adults. Journal of applied gerontology : the official journal of the Southern Gerontological Society. 2005;24(3):211–230. doi: 10.1177/0733464804273772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yam A, Gross AL, Prindle JJ, Marsiske M. Ten-year longitudinal trajectories of older adults’ basic and everyday cognitive abilities. Neuropsychology. 2014;28(6):819–828. doi: 10.1037/neu0000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yam A, Marsiske M. Cognitive longitudinal predictors of older adults’ self-reported IADL function. J Aging Health. 2013;25(8 Suppl):163S–185S. doi: 10.1177/0898264313495560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebok GW, Ball K, Guey LT, et al. Ten-Year Effects of the Advanced Cognitive Training for Independent and Vital Elderly Cognitive Training Trial on Cognition and Everyday Functioning in Older Adults. J Am Geriatr Soc. 2014 doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sisco SM, Marsiske M, Gross AL, Rebok GW. The influence of cognitive training on older adults’ recall for short stories. J Aging Health. 2013;25(8 Suppl):230S–248S. doi: 10.1177/0898264313501386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsiske M, Dzierzewski JM, Thomas KR, et al. Race-related disparities in 5-year cognitive level and change in untrained active participants. J Aging Health. 2013;25(8 Suppl):103S–127S. doi: 10.1177/0898264313497794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payne BR, Gross AL, Parisi JM, et al. Modelling longitudinal changes in older adults’ memory for spoken discourse: Findings from the ACTIVE cohort. Memory. 2013 doi: 10.1080/09658211.2013.861916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RN, Marsiske M, Ball K, et al. The ACTIVE cognitive training interventions and trajectories of performance among older adults. J Aging Health. 2013;25(8 Suppl):186S–208S. doi: 10.1177/0898264312461938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ball KK, Beard BL, Roenker DL, Miller RL, Griggs DS. Age and visual search: expanding the useful field of view. Journal of the Optical Society of America. A, Optics and image science. 1988;5(12):2210–2219. doi: 10.1364/josaa.5.002210. [DOI] [PubMed] [Google Scholar]
- 15.Ball K, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. Journal of the American Optometric Association. 1993;64(1):71–79. [PubMed] [Google Scholar]
- 16.Jobe JB, Smith DM, Ball K, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials. 2001;22(4):453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards JD, Wadley VG, Vance DE, Wood K, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging & mental health. 2005;9(3):262–271. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- 19.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball K, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. The journals of gerontology. Series B, Psychological sciences and social sciences. 2007;62(1):19–31. doi: 10.1093/geronb/62.special_issue_1.19. [DOI] [PubMed] [Google Scholar]
- 21.Edwards JD, Ross LA, Ackerman ML, et al. Longitudinal predictors of driving cessation among older adults from the ACTIVE clinical trial. The journals of gerontology. Series B, Psychological sciences and social sciences. 2008;63(1):P6–12. doi: 10.1093/geronb/63.1.p6. [DOI] [PubMed] [Google Scholar]
- 22.Edwards JD, Myers C, Ross LA, et al. The longitudinal impact of cognitive speed of processing training on driving mobility. The Gerontologist. 2009;49(4):485–494. doi: 10.1093/geront/gnp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolinsky FD, Vander Weg MW, Martin R, et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64(4):468–472. doi: 10.1093/gerona/gln044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball K, Edwards JD, Ross LA, McGwin G., Jr Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58(11):2107–2113. doi: 10.1111/j.1532-5415.2010.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolinsky FD, Mahncke H, Vander Weg MW, et al. Speed of processing training protects self-rated health in older adults: enduring effects observed in the multi-site ACTIVE randomized controlled trial. International psychogeriatrics / IPA. 2010;22(3):470–478. doi: 10.1017/S1041610209991281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belchior P, Marsiske M, Sisco SM, et al. Video game training to improve selective visual attention in older adults. Computers in human behavior. 2013;29(4):1318–1324. doi: 10.1016/j.chb.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross L, Edwards JD, Ball K. Mobility Outcomes in ACTIVE Paper presented at: Gerontological Society of America Meetings. New Orleans, LA: 2013. [Google Scholar]
- 28.Antal A, Fischer T, Saiote C, et al. Transcranial electrical stimulation modifies the neuronal response to psychosocial stress exposure. Human brain mapping. 2013 doi: 10.1002/hbm.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arlotti M, Rahman A, Minhas P, Bikson M. Axon terminal polarization induced by weak uniform DC electric fields: a modeling study. Conference proceedings : & Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2012;2012:4575–4578. doi: 10.1109/EMBC.2012.6346985. [DOI] [PubMed] [Google Scholar]
- 30.Chrysikou EG, Hamilton RH. Noninvasive brain stimulation in the treatment of aphasia: exploring interhemispheric relationships and their implications for neurorehabilitation. Restorative neurology and neuroscience. 2011;29(6):375–394. doi: 10.3233/RNN-2011-0610. [DOI] [PubMed] [Google Scholar]
- 31.Clark VP, Coffman BA, Mayer AR, et al. TDCS guided using fMRI significantly accelerates learning to identify concealed objects. Neuroimage. 2012;59(1):117–128. doi: 10.1016/j.neuroimage.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva MC, Conti CL, Klauss J, et al. Journal of physiology. 6. Vol. 107. Paris: 2013. Behavioral effects of transcranial Direct Current Stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence; pp. 493–502. [DOI] [PubMed] [Google Scholar]
- 33.Moreno-Duarte I, Morse LR, Alam M, Bikson M, Zafonte R, Fregni F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage. 2014;85(Pt 3):1003–1013. doi: 10.1016/j.neuroimage.2013.05.097. [DOI] [PubMed] [Google Scholar]
- 34.Nawani H, Kalmady SV, Bose A, et al. Neural Basis of tDCS Effects on Auditory Verbal Hallucinations in Schizophrenia: A Case Report Evidence for Cortical Neuroplasticity Modulation. The journal of ECT. 2014;30(1):e2–4. doi: 10.1097/YCT.0b013e3182a35492. [DOI] [PubMed] [Google Scholar]
- 35.Nitsche MA, Niehaus L, Hoffmann KT, et al. MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2004;115(10):2419–2423. doi: 10.1016/j.clinph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Nord CL, Lally N, Charpentier CJ. Harnessing electric potential: DLPFC tDCS induces widespread brain perfusion changes. Frontiers in systems neuroscience. 2013;7:99. doi: 10.3389/fnsys.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okano AH, Fontes EB, Montenegro RA, et al. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. British journal of sports medicine. 2013 doi: 10.1136/bjsports-2012-091658. [DOI] [PubMed] [Google Scholar]
- 38.Radman T, Datta A, Ramos RL, Brumberg JC, Bikson M. One-dimensional representation of a neuron in a uniform electric field. Conference proceedings : & Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2009;2009:6481–6484. doi: 10.1109/IEMBS.2009.5333586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman A, Reato D, Arlotti M, et al. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. The Journal of physiology. 2013;591(Pt 10):2563–2578. doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reato D, Rahman A, Bikson M, Parra LC. Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Frontiers in human neuroscience. 2013;7:687. doi: 10.3389/fnhum.2013.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sehm B, Schafer A, Kipping J, et al. Dynamic modulation of intrinsic functional connectivity by transcranial direct current stimulation. Journal of neurophysiology. 2012;108(12):3253–3263. doi: 10.1152/jn.00606.2012. [DOI] [PubMed] [Google Scholar]
- 42.Shah PP, Szaflarski JP, Allendorfer J, Hamilton RH. Induction of neuroplasticity and recovery in post-stroke aphasia by non-invasive brain stimulation. Frontiers in human neuroscience. 2013;7:888. doi: 10.3389/fnhum.2013.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Current biology : CB. 2011;21(6):480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagg CJ, Lin RL, Mezue M, et al. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(28):11425–11431. doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2011;17(1):37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 46.Turkeltaub PE, Benson J, Hamilton RH, Datta A, Bikson M, Coslett HB. Left lateralizing transcranial direct current stimulation improves reading efficiency. Brain stimulation. 2012;5(3):201–207. doi: 10.1016/j.brs.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58(1):26–33. doi: 10.1016/j.neuroimage.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahn H, Woods AJ, Kunik ME, et al. Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: An experimenter- and participant-blinded, randomized, sham-controlled pilot clinical study. Brain stimulation. 2017;10(5):902–909. doi: 10.1016/j.brs.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bikson M, Grossman P, Thomas C, et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain stimulation. 2016;9(5):641–661. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PloS one. 2013;8(9):e76112. doi: 10.1371/journal.pone.0076112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minhas P, Bikson M, Woods AJ, Rosen AR, Kessler SK. Transcranial direct current stimulation in pediatric brain: a computational modeling study. Conference proceedings : & Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2012;2012:859–862. doi: 10.1109/EMBC.2012.6346067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szymkowicz SM, McLaren ME, Suryadevara U, Woods AJ. Transcranial Direct Current Stimulation Use in the Treatment of Neuropsychiatric Disorders: A Brief Review. Psychiatric annals. 2016;46(11):642–646. doi: 10.3928/00485713-20161006-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woods AJ, Hamilton RH, Kranjec A, et al. Space, Time, and Causality in the Human Brain. Neuroimage. 2014;15(92):285–297. doi: 10.1016/j.neuroimage.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woods AJ, Antal A, Bikson M, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2016;127(2):1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woods AJ, Bryant V, Sacchetti D, Gervits F, Hamilton R. Effects of Electrode Drift in Transcranial Direct Current Stimulation. Brain stimulation. 2015;8(3):515–519. doi: 10.1016/j.brs.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woods AJ, Martin DM. Clinical Research and Methodological Aspects for tDCS Research. In: Brunoni AR, Nitsche M, Loo C, editors. Transcranial Direct Current Stimulation in Neuropsychiatric Disorders. Springer; 2016. pp. 393–404. [Google Scholar]
- 57.Fazeli P, Woods AJ, Pope CN, Vance DE, Ball K. The effect of transcranial direct current stimulation combined with cognitive training on cognitive functioning in older adults with HIV: A pilot study. Appl Neuropsychol Adult. 2017 doi: 10.1080/23279095.2017.1357037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of physiology. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nitsche MA, Seeber A, Frommann K, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. The Journal of physiology. 2005;568(Pt 1):291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fregni F, Boggio PS, Santos MC, et al. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2006;21(10):1693–1702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- 61.Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Experimental brain research. 2004;156(4):439–443. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- 62.Lang N, Siebner HR, Ward NS, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? The European journal of neuroscience. 2005;22(2):495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain : a journal of neurology. 2002;125(Pt 10):2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 64.Feng WW, Bowden MG, Kautz S. Review of transcranial direct current stimulation in poststroke recovery. Topics in stroke rehabilitation. 2013;20(1):68–77. doi: 10.1310/tsr2001-68. [DOI] [PubMed] [Google Scholar]
- 65.Polanowska K, Seniow J. Influence of transcranial direct current stimulation on cognitive functioning of patients with brain injury. Neurologia i neurochirurgia polska. 2010;44(6):580–590. doi: 10.1016/s0028-3843(14)60156-0. [DOI] [PubMed] [Google Scholar]
- 66.Schlaug G, Renga V. Transcranial direct current stimulation: a noninvasive tool to facilitate stroke recovery. Expert review of medical devices. 2008;5(6):759–768. doi: 10.1586/17434440.5.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Archives of neurology. 2008;65(12):1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neuroscience letters. 2012;521(2):148–151. doi: 10.1016/j.neulet.2012.05.074. [DOI] [PubMed] [Google Scholar]
- 69.Cerruti C, Schlaug G. Anodal transcranial direct current stimulation of the prefrontal cortex enhances complex verbal associative thought. Journal of cognitive neuroscience. 2009;21(10):1980–1987. doi: 10.1162/jocn.2008.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dell’Osso B, Zanoni S, Ferrucci R, et al. Transcranial direct current stimulation for the outpatient treatment of poor-responder depressed patients. European psychiatry : the journal of the Association of European Psychiatrists. 2012;27(7):513–517. doi: 10.1016/j.eurpsy.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 71.Javadi AH, Walsh V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain stimulation. 2012;5(3):231–241. doi: 10.1016/j.brs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Ditye T, Jacobson L, Walsh V, Lavidor M. Modulating behavioral inhibition by tDCS combined with cognitive training. Experimental brain research. 2012;219(3):363–368. doi: 10.1007/s00221-012-3098-4. [DOI] [PubMed] [Google Scholar]
- 73.Lesniak M, Polanowska K, Seniow J, Czlonkowska A. Effects of Repeated Anodal tDCS Coupled With Cognitive Training for Patients With Severe Traumatic Brain Injury: A Pilot Randomized Controlled Trial. The Journal of head trauma rehabilitation. 2013 doi: 10.1097/HTR.0b013e318292a4c2. [DOI] [PubMed] [Google Scholar]
- 74.Li SC, Schmiedek F, Huxhold O, Rocke C, Smith J, Lindenberger U. Working memory plasticity in old age: practice gain, transfer, and maintenance. Psychology and aging. 2008;23(4):731–742. doi: 10.1037/a0014343. [DOI] [PubMed] [Google Scholar]
- 75.Martin DM, Liu R, Alonzo A, et al. Can transcranial direct current stimulation enhance outcomes from cognitive training? A randomized controlled trial in healthy participants. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16(9):1927–1936. doi: 10.1017/S1461145713000539. [DOI] [PubMed] [Google Scholar]
- 76.Park SH, Seo JH, Kim YH, Ko MH. Long-term effects of transcranial direct current stimulation combined with computer-assisted cognitive training in healthy older adults. Neuroreport. 2014;25(2):122–126. doi: 10.1097/WNR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 77.Brunoni AR, Nitsche MA, Bolognini N, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain stimulation. 2012;5(3):175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen Kadosh R, Soskic S, Iuculano T, Kanai R, Walsh V. Modulating neuronal activity produces specific and long-lasting changes in numerical competence. Current biology : CB. 2010;20(22):2016–2020. doi: 10.1016/j.cub.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamilton RH, Chrysikou EG, Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain and language. 2011;118(1–2):40–50. doi: 10.1016/j.bandl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bherer L. Cognitive plasticity in older adults: effects of cognitive training and physical exercise. Annals of the New York Academy of Sciences. 2015;1337:1–6. doi: 10.1111/nyas.12682. [DOI] [PubMed] [Google Scholar]
- 81.Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PloS one. 2012;7(7):e40588. doi: 10.1371/journal.pone.0040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS medicine. 2014;11(11):e1001756. doi: 10.1371/journal.pmed.1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing research reviews. 2013;12(1):263–275. doi: 10.1016/j.arr.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Weintraub S. UDS-III Norms. 2017 https://www.alz.washington.edu/WEB/npsych_means.html. Accessed 11/13/17, 2017.
- 85.McLaren ME, Nissim NR, Woods AJ. The effects of medication use in transcranial direct current stimulation: A brief review. Brain stimulation. 2017 doi: 10.1016/j.brs.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bikson M, Paneri B, Mourdoukoutas A, et al. Limited output transcranial electrical stimulation (LOTES-2017): Engineering principles, regulatory statutes, and industry standards for wellness, over-the-counter, or prescription devices with low risk. Brain stimulation. 2017 doi: 10.1016/j.brs.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 87.Akshoomoff N, Beaumont JL, Bauer PJ, et al. VIII. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monographs of the Society for Research in Child Development. 2013;78(4):119–132. doi: 10.1111/mono.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akshoomoff N, Newman E, Thompson WK, et al. The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING) Neuropsychology. 2014;28(1):1–10. doi: 10.1037/neu0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bauer PJ, Zelazo PD. IX. NIH Toolbox Cognition Battery (CB): summary, conclusions, and implications for cognitive development. Monographs of the Society for Research in Child Development. 2013;78(4):133–146. doi: 10.1111/mono.12039. [DOI] [PubMed] [Google Scholar]
- 90.Carlozzi NE, Tulsky DS, Chiaravalloti ND, et al. NIH Toolbox Cognitive Battery (NIHTB-CB): the NIHTB Pattern Comparison Processing Speed Test. J Int Neuropsychol Soc. 2014;20(6):630–641. doi: 10.1017/S1355617714000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gershon RC, Slotkin J, Manly JJ, et al. IV. NIH Toolbox Cognition Battery (CB): measuring language (vocabulary comprehension and reading decoding) Monographs of the Society for Research in Child Development. 2013;78(4):49–69. doi: 10.1111/mono.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heaton RK, Akshoomoff N, Tulsky D, et al. Reliability and validity of composite scores from the NIH Toolbox Cognition Battery in adults. J Int Neuropsychol Soc. 2014;20(6):588–598. doi: 10.1017/S1355617714000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Shea A, Cohen RA, Porges EC, Nissim NR, Woods AJ. Cognitive Aging and the Hippocampus in Older Adults. Frontiers in aging neuroscience. 2016;8:298. doi: 10.3389/fnagi.2016.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zelazo PD, Anderson JE, Richler J, et al. NIH Toolbox Cognition Battery (CB): validation of executive function measures in adults. J Int Neuropsychol Soc. 2014;20(6):620–629. doi: 10.1017/S1355617714000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, Weintraub S. II. NIH Toolbox Cognition Battery (CB): measuring executive function and attention. Monographs of the Society for Research in Child Development. 2013;78(4):16–33. doi: 10.1111/mono.12032. [DOI] [PubMed] [Google Scholar]
- 96.Cohen RA, Paul RH, Ott BR, et al. The relationship of subcortical MRI hyperintensities and brain volume to cognitive function in vascular dementia. J Int Neuropsychol Soc. 2002;8(6):743–752. doi: 10.1017/s1355617702860027. [DOI] [PubMed] [Google Scholar]
- 97.Paul RH, Cohen RA, Moser D, et al. Performance on the Mattis Dementia Rating Scale in patients with vascular dementia: relationships to neuroimaging findings. J Geriatr Psychiatry Neurol. 2001;14(1):33–36. doi: 10.1177/089198870101400108. [DOI] [PubMed] [Google Scholar]
- 98.Paul RH, Haque O, Gunstad J, et al. Subcortical hyperintensities impact cognitive function among a select subset of healthy elderly. Arch Clin Neuropsychol. 2005;20(6):697–704. doi: 10.1016/j.acn.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haley AP, Sweet LH, Gunstad J, et al. Verbal working memory and atherosclerosis in patients with cardiovascular disease: an fMRI study. J Neuroimaging. 2007;17(3):227–233. doi: 10.1111/j.1552-6569.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- 100.Irani F, Sweet LH, Haley AP, et al. A fMRI Study of Verbal Working Memory, Cardiac Output, and Ejection Fraction in Elderly Patients with Cardiovascular Disease. Brain Imaging Behav. 2009;3(4):350–357. doi: 10.1007/s11682-009-9077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nissim NR, O’Shea AM, Bryant V, Porges EC, Cohen R, Woods AJ. Frontal Structural Neural Correlates of Working Memory Performance in Older Adults. Frontiers in aging neuroscience. 2016;8:328. doi: 10.3389/fnagi.2016.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in biomedicine. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 103.Porges EC, Woods AJ, Edden RA, et al. Frontal Gamma-Aminobutyric Acid Concentrations Are Associated With Cognitive Performance in Older Adults. Biological psychiatry. Cognitive neuroscience and neuroimaging. 2017;2(1):38–44. doi: 10.1016/j.bpsc.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Porges EC, Woods AJ, Lamb DG, et al. Impact of tissue correction strategy on GABA-edited MRS findings. Neuroimage. 2017;162:249–256. doi: 10.1016/j.neuroimage.2017.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lezak MD. Neuropsychological assessment in behavioral toxicology–developing techniques and interpretative issues. Scandinavian journal of work, environment & health. 1984;10(Suppl 1):25–29. [PubMed] [Google Scholar]
- 106.DiGirolamo GJ, Kramer AF, Barad V, et al. General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport. 2001;12(9):2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- 107.Gross AL, Rebok GW, Brandt J, Tommet D, Marsiske M, Jones RN. Modeling learning and memory using verbal learning tests: results from ACTIVE. The journals of gerontology. Series B, Psychological sciences and social sciences. 2013;68(2):153–167. doi: 10.1093/geronb/gbs053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gunstad J, Cohen RA, Paul RH, Luyster FS, Gordon E. Age effects in time estimation: relationship to frontal brain morphometry. Journal of integrative neuroscience. 2006;5(1):75–87. doi: 10.1142/s0219635206001045. [DOI] [PubMed] [Google Scholar]
- 109.Jones RN, Rosenberg AL, Morris JN, et al. A growth curve model of learning acquisition among cognitively normal older adults. Experimental aging research. 2005;31(3):291–312. doi: 10.1080/03610730590948195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okonkwo OC, Cohen RA, Gunstad J, Tremont G, Alosco ML, Poppas A. Longitudinal trajectories of cognitive decline among older adults with cardiovascular disease. Cerebrovasc Dis. 2010;30(4):362–373. doi: 10.1159/000319564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paul RH, Brickman AM, Cohen RA, et al. Cognitive status of young and older cigarette smokers: data from the international brain database. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2006;13(4):457–465. doi: 10.1016/j.jocn.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 112.Roller CA, Cohen HS, Kimball KT, Bloomberg JJ. Effects of normal aging on visuo-motor plasticity. Neurobiology of aging. 2002;23(1):117–123. doi: 10.1016/s0197-4580(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 113.Woods AJ, Mark VW, Pitts AC, Mennemeier M. Pervasive cognitive impairment in acute rehabilitation inpatients without brain injury. PM & R : the journal of injury, function, and rehabilitation. 2011;3(5):426–432. doi: 10.1016/j.pmrj.2011.02.018. quiz 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zimerman M, Nitsch M, Giraux P, Gerloff C, Cohen LG, Hummel FC. Neuroenhancement of the aging brain: restoring skill acquisition in old subjects. Annals of neurology. 2013;73(1):10–15. doi: 10.1002/ana.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zimmerman ME, Brickman AM, Paul RH, et al. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry. 2006;14(10):823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]
- 116.Zlatar ZZ, Towler S, McGregor KM, et al. Functional language networks in sedentary and physically active older adults. J Int Neuropsychol Soc. 2013;19(6):625–634. doi: 10.1017/S1355617713000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. The Clinical neuropsychologist. 1999;13(3):348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 118.Jensen AR. Scoring the Stroop test. Acta psychologica. 1965;24(5):398–408. doi: 10.1016/0001-6918(65)90024-7. [DOI] [PubMed] [Google Scholar]
- 119.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 120.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167–177. [PubMed] [Google Scholar]
- 121.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychological assessment. 1996;8:145–153. [Google Scholar]
- 122.Wechsler Adult Intelligence Scale-Fourth Edition. Pearson; 2008. [Google Scholar]
- 123.Wiens AN, Fuller KH, Crossen JR. Paced Auditory Serial Addition Test: adult norms and moderator variables. Journal of clinical and experimental neuropsychology. 1997;19(4):473–483. doi: 10.1080/01688639708403737. [DOI] [PubMed] [Google Scholar]
- 124.Taha J, Czaja SJ, Sharit J, Morrow DG. Factors affecting usage of a personal health record (PHR) to manage health. Psychology and aging. 2013;28(4):1124–1139. doi: 10.1037/a0033911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sharit J, Czaja SJ, Nair S, Lee CC. Effects of age, speech rate, and environmental support in using telephone voice menu systems. Human factors. 2003;45(2):234–251. doi: 10.1518/hfes.45.2.234.27245. [DOI] [PubMed] [Google Scholar]
- 126.Owsley C, Stalvey B, Wells J, Sloane ME. Older drivers and cataract: driving habits and crash risk. The journals of gerontology. Series A, Biological sciences and medical sciences. 1999;54(4):M203–211. doi: 10.1093/gerona/54.4.m203. [DOI] [PubMed] [Google Scholar]
- 127.Tennstedt SL, Unverzagt FW. The ACTIVE study: study overview and major findings. J Aging Health. 2013;25(8 Suppl):3S–20S. doi: 10.1177/0898264313518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morris JN, Fries BE, Steel K, et al. Comprehensive clinical assessment in community setting: applicability of the MDS-HC. J Am Geriatr Soc. 1997;45(8):1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- 129.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. Journal of clinical epidemiology. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Magasi S, Ryan G, Revicki D, et al. Content validity of patient-reported outcome measures: perspectives from a PROMIS meeting. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2012;21(5):739–746. doi: 10.1007/s11136-011-9990-8. [DOI] [PubMed] [Google Scholar]
- 131.Tucker CA, Cieza A, Riley AW, et al. Concept Analysis of the Patient Reported Outcomes Measurement Information System (PROMIS) and the International Classification of Functioning, Disability and Health (ICF) Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2014 doi: 10.1007/s11136-014-0622-y. [DOI] [PubMed] [Google Scholar]
- 132.Reeve BB, Hays RD, Bjorner JB, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Medical care. 2007;45(5 Suppl 1):S22–31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 133.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 134.Gavin DR, Ross HE, Skinner HA. Diagnostic validity of the drug abuse screening test in the assessment of DSM-III drug disorders. British journal of addiction. 1989;84(3):301–307. doi: 10.1111/j.1360-0443.1989.tb03463.x. [DOI] [PubMed] [Google Scholar]
- 135.Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. Journal of aging and physical activity. 2015;23(2):314–322. doi: 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Beck Depression Inventory - II (BDI-2) Pearson; 1996. [Google Scholar]
- 137.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- 138.Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. The Journal of neuropsychiatry and clinical neurosciences. 1992;4(2):134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- 139.Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. Journal of personality assessment. 1996;66(1):20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- 140.Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. The Gerontologist. 2006;46(4):503–513. doi: 10.1093/geront/46.4.503. [DOI] [PubMed] [Google Scholar]
- 141.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 142.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 143.Rabipour S, Davidson PSR. Do you believe in brain training? A questionnaire about expectations of computerised cognitive training. Behavioural brain research. 2015;295:64–70. doi: 10.1016/j.bbr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 144.Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942–1948. doi: 10.1212/01.wnl.0000247042.15547.eb. [DOI] [PubMed] [Google Scholar]
- 145.Wechsler Test of Adult Reading (WTAR) Pearson; 2001. [Google Scholar]
- 146.Hardy LH, Rand G, Rittler MC. The Ishihara test as a means of detecting and analyzing color vision. The Journal of general psychology. 1947;36:79–106. doi: 10.1080/00221309.1947.9918108. [DOI] [PubMed] [Google Scholar]
- 147.Li C, Beaumont JL, Rine RM, Slotkin J, Schubert MC. Normative Scores for the NIH Toolbox Dynamic Visual Acuity Test from 3 to 85 Years. Frontiers in neurology. 2014;5:223. doi: 10.3389/fneur.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zecker SG, Hoffman HJ, Frisina R, et al. Audition assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S45–48. doi: 10.1212/WNL.0b013e3182872dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gross AL, Parisi JM, Spira AP, et al. Memory training interventions for older adults: a meta-analysis. Aging & mental health. 2012;16(6):722–734. doi: 10.1080/13607863.2012.667783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Unverzagt FW, Guey LT, Jones RN, et al. ACTIVE cognitive training and rates of incident dementia. J Int Neuropsychol Soc. 2012;18(4):669–677. doi: 10.1017/S1355617711001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Unverzagt FW, Kasten L, Johnson KE, et al. Effect of memory impairment on training outcomes in ACTIVE. J Int Neuropsychol Soc. 2007;13(6):953–960. doi: 10.1017/S1355617707071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: six-year longitudinal findings in the Berlin Aging Study (BASE) Psychology and aging. 2003;18(2):318–331. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- 153.Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. The Journal of physiology. 2013;591(7):1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A, Tennstedt SL. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. The journals of gerontology. Series A, Biological sciences and medical sciences. 2006;61(12):1324–1329. doi: 10.1093/gerona/61.12.1324. [DOI] [PubMed] [Google Scholar]
- 155.Barnes DE, Santos-Modesitt W, Poelke G, et al. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA internal medicine. 2013;173(9):797–804. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ng TP, Ling A, Feng L, et al. Cognitive Effects of Multi-domain Interventions among Pre-frail and Frail Community-living Older Persons: Randomized Controlled Trial. The journals of gerontology Series A, Biological sciences and medical sciences. 2017 doi: 10.1093/gerona/glx207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


