Abstract
Objective
Mitochondrial dysfunction, oxidative stress and chondrocyte death are important contributors to the development and pathogenesis of osteoarthritis (OA). In this study, we determined the expression and role of Parkin in the clearance of damaged/dysfunctional mitochondria, regulation of ROS levels and chondrocyte survival under pathological conditions.
Methods
Human chondrocytes were from the unaffected area of knee OA cartilage (n=12) and were stimulated with IL-1β to mimic pathological conditions. Mitochondrial membrane depolarization and ROS levels were determined using specific dyes and flow cytometry. Autophagy was determined by Western blotting for ATG5, Beclin1, immunofluorescence staining and confocal microscopy. Gene expression was determined by qRT-PCR. siRNA, wild-type and mutant Parkin plasmids were transfected using Amaxa system. Apoptosis was determined by PI staining of chondrocytes and TUNEL assay.
Results
IL-1β-stimulated OA chondrocytes showed high levels of ROS generation, mitochondrial membrane damage, accumulation of damaged mitochondria and higher incidence of apoptosis. IL-1β stimulation of chondrocytes with depleted Parkin expression resulted in sustained high levels of ROS, accumulation of damaged/dysfunctional mitochondria and enhanced apoptosis. Parkin translocation to depolarized/damaged mitochondria and recruitment of p62/SQSTM1 was required for the elimination of damaged/dysfunctional mitochondria in IL-1β-stimulated OA chondrocytes. Importantly we demonstrate that Parkin elimination of depolarized/damaged mitochondria required the Parkin ubiquitin ligase activity and resulted in reduced ROS levels and inhibition of apoptosis in OA chondrocytes under pathological conditions.
Conclusions
Our data demonstrates that Parkin functions to eliminate depolarized/damaged mitochondria in chondrocytes which is necessary for mitochondrial quality control, regulation of ROS levels and chondrocyte survival under pathological conditions.
Introduction
Osteoarthritis (OA) is the most common disease of the whole joint and a leading cause of disability and considerable socioeconomic cost worldwide [1]. Age-related development of OA is predominantly characterized by the progressive degradation of articular cartilage, oxidative stress and chondrocytes death [2]. Physiologically reactive oxygen species (ROS) performs a variety of functions including signaling and maintenance of chondrocytes homeostasis but an imbalance in favor of oxidants results in disruption of normal redox signaling, homeostasis and contribute to aging and age-related diseases [3–6]. There is increasing evidence of increased levels of ROS in OA (recently reviewed by Loeser RF et al in [7]). Hypertrophic chondrocytes produce cytokines and other factors that are associated with OA pathogenesis and these also stimulate the production of ROS by chondrocytes [8–10]. Increase in cellular ROS leads to oxidative stress, matrix metalloproteases (MMPs) production and degradation of cartilage extracellular matrix (ECM) [11]. It is believed that high level of ROS induces mitochondrial membrane depolarization leading to a sustained production of ROS which causes oxidative stress and ultimately chondrocyte death. This highlights the importance of mitochondrial quality control in chondrocytes survival and for the normal functioning of cartilage.
To prevent oxidant-induced damage to mitochondria and chondrocytes death, multiple anti-oxidant and chaperone systems function to protect mitochondrial damage/depolarization and prevent oxidant buildup [12, 13]. However, despite the presence of the antioxidant defense system, chronic exposure of chondrocytes to factors stimulating ROS production induce oxidative stress and chondrocyte death in OA [7, 10]. Additionally, evidence of mitochondrial dysfunction in chondrocytes has also been reported [14, 15]. These data highlight the need for a chondrocyte mechanism to remove damaged mitochondria in order to prevent the generation of high levels of ROS and oxidative stress. However, to date no chondrocyte mechanism for removal of damaged mitochondria has been identified and the effect of removing damaged mitochondria on ROS levels or survival of chondrocytes has not been established.
Evidence for the existence of a mechanism that could function to remove damaged mitochondria in chondrocytes was recently provided when it was shown that pharmacological activation of autophagy in chondrocytes significantly protected against mitochondrial dysfunction [16, 17]. These publications and a subsequent report [18] suggests that the selective autophagy process called mitophagy may function to eliminate damaged/dysfunctional mitochondria in chondrocytes and prevent oxidative stress. However, active mitophagy has not yet been studied in human chondrocytes. One protein that functions in the removal of damaged/dysfunctional mitochondria via mitophagy is Parkin (PARK2) [19, 20]. Parkin, an E3 ubiquitin ligase, operates in conjunction with PTEN-induced kinase 1 (PINK1) and responds to the loss of mitochondrial membrane potential (ΔΨM) for initiating the process for the clearance of damaged/dysfunctional mitochondria [21, 22]. A recent study has shown that Parkin mediated elimination of damaged/dysfunctional mitochondria is essential for the regulation of oxidative stress, mitochondrial homeostasis and survival of lens epithelial cells [23].
In this study, we used IL-1β to induce ROS generation and mitochondrial dysfunction in primary human OA chondrocytes. We demonstrate that Parkin levels increased in chondrocytes with IL-1β-induced oxidative stress and establish that Parkin translocated to depolarized mitochondria and recruit p62/SQSTM1 and that Parkin was required for the elimination of damaged/depolarized mitochondria. We further demonstrate that clearance of damaged mitochondria by mitophagy was dependent on active autophagy as inhibition of autophagy augmented IL-1β induced mitochondrial dysfunction and ROS production. Importantly we demonstrate that Parkin elimination of depolarized/damaged mitochondria resulted in reduced ROS levels and increased chondrocyte survival under pathological conditions. Our results for the first time establish that Parkin-mediated elimination of damaged/dysfunctional mitochondria in human OA chondrocytes is important for homeostasis and survival. Our data also provide evidence that mitochondria quality control by Parkin in human OA chondrocytes may be important in the maintenance of cartilage integrity and function.
Materials and Methods
Reagents
For chondrocyte culture DMEM-F-12 was purchased from Lonza (Walkersville, MD, USA, #12-719Q). Fetal calf serum (#10437028) and other cell culture reagents were purchased form Life Technologies (Carlsbad, CA, USA). Pronase (#11459643001) and Collagenase (#11088793001) were from Roche Diagnostics (Indianapolis, IN, USA). Recombinant human IL-1β (#201-LB-025) was purchased from R&D Systems (St Paul, MN, USA). Validated antibodies against Parkin (#ab15954) and p62/SQSTM1 (#ab56416) were from Abcam (Cambridge, MA, USA). Antibody against β-Actin (sc-47778) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and for LC3 (#12741) was from Cell Signaling Technology (Beverly, MA, USA). Horseradish peroxidase conjugated appropriate secondary antibodies were from Cell Signaling Technology (#7074) or Santa Cruz Biotechnology (sc-2020).
Preparation of Human OA Chondrocytes
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Northeast Ohio Medical University, Rootstown, Ohio as a “non-human subject study under 45 CFR”. All the methods used in this study were carried out in accordance with the approved guidelines and all experimental protocols were approved by the IRB of Northeast Ohio Medical University, Rootstown, Ohio. Discarded and de-identified knee joint cartilage samples were from donors who underwent arthroplasty due to OA at Crystal Clinic, Akron, OH. Human OA chondrocytes were isolated from the unaffected area of knee OA cartilage (n=12, n refers to the number of patient’s samples), as described previously [24] and cultured in DMEM-F12 supplemented with 10% serum.
Immunofluorescence Staining and Confocal Imaging
Immunofluorescence staining of OA chondrocytes was done as described earlier [25]. Briefly, OA chondrocytes were seeded in an 8-well chamber slide (0.1 × 106 per well) (Nunc Lab-Tek) for 2 to 3 days after isolation and treated with 5ng/ml of IL-1β. Chondrocytes were fixed in 4% PFA and permeabilized in 1x-PBS containing 0.3% Triton X-100. Blocking was performed in 5% BSA in PBS for 30 minutes at room temperature followed by incubation with primary antibody at 4°C overnight and then chondrocytes were incubated with corresponding fluorescently tagged secondary antibody (Anti-mouse: A-11005, anti-rabbit: A-11034 and anti-goat: A11056; Life Technologies). For lysosomal and mitochondrial staining, live chondrocytes were incubated with Lysotracker Red (25 nM) or Mitotracker Deep Red (100 nM) (#L7528 and M22426 respectively, Life Technologies) for 30 minutes and then fixed with 4% PFA. Nuclei were stained with DAPI and were mounted with anti-fade medium (Vectashield, Vector Laboratories, USA, #H-1000). Images were acquired on an Olympus FV1000 confocal microscope using a 60X oil immersion lens.
Gene Expression Analyses by qRT-PCR
Chondrocytes were cultured in 6 wells plates and total RNA was prepared using RNeasy kit (#74104, Qiagen) and the cDNA was synthesized from 1 μg of total RNA using a cDNA synthesis kit (Life Technologies, #4368813). mRNA expression was quantified using TaqMan or SYBR Green primers (IDT, Coralville, Iowa). Relative expression levels of mRNAs were normalized to β-Actin or GAPDH mRNA expression levels.
Western Immunoblotting
Treated and control OA chondrocytes were harvested, washed with 1x PBS and lysed in RIPA buffer supplemented with protease inhibitor cocktail (Roche, 11697498001) homogenized by passing multiple times through a 22 gauge needle. Lysate was clarified by centrifugation (15,000xg for 10 minutes at 4°C) and either was used immediately or stored at −80°C after protein quantitation. Proteins were resolved on 10–12% SDS-PAGE gels and transferred to PVDF membrane (#1704272, Bio-Rad), blocked with 5% BSA in TBS-T and incubated with primary antibody at 4°C overnight. The membrane was then washed with TBS-T and incubated with HRP-conjugated secondary antibody, washed and developed using the chemiluminescent HRP detection reagent (#WBLUF0500, Millipore) and imaged on PXi-4 gel imaging system (Syngene, Frederick, MD).
Measurement of Mitochondrial ROS and Mitochondrial Membrane Potential (ΔΨM)
Mitochondrial ROS was measured using MitoSOX Red dye (Invitrogen, M36008). Briefly, OA chondrocytes were stained with 5.0 μM of MitoSOX Red for 10 minutes at 37°C followed by IL-1 β treatment (5ng/ml). For autophagy inhibition studies and its effect on ROS levels, OA chondrocytes were first treated with inhibitor of autophagy for 2 hours followed by MitoSOX Red staining and IL-1β treatment as above. Loss of ΔΨM was assessed using the mitochondrial-specific fluorescent probe JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine iodide) (Invitrogen, T3168). JC-1 forms multimers in cells with a high red fluorescence indicating normal ΔΨM. Loss of the ΔΨM results in a reduction in the red fluorescence with a concurrent gain in green fluorescence as the dye shifts from multimeric form to monomeric state. Ratio of red to green fluorescence was used as an indicator of loss of ΔΨM. OA chondrocytes treated as above were loaded with JC-1 (5 μM) for 20 minutes at 37 °C, washed with PBS and analyzed by flow cytometry using BD Accuri C6 Flowcytometer. Flow cytometry data was analyzed by Flowjo software (Tree Star Inc. Ashland, OR, USA).
Estimation of apoptosis by PI staining and TUNEL assay
OA chondrocytes were cultured with or without IL-1β for 48 hours. At the end of the treatment chondrocytes were washed with 1x-PBS and incubated with 1 ml staining solution containing 0.5 μg/ml PI, 0.1% sodium citrate, and 0.1% Triton X-100 overnight at 4°C. A total of 20,000 cells were acquired in BD Accuri C6 Flowcytometer and percent apoptotic cells were determined by analyzing sub-G1 population (<2 n DNA content) using FlowJo software [26]. TUNEL assay was performed using a kit according to the manufacturer’s protocols (Biotool, USA #B31112). Nuclei were stained with DAPI and apoptotic cells were visualized by confocal microscopy or analyzed by Flow cytometer.
siRNA and Plasmid Transfections
Validated siRNAs for indicated genes and control siRNA were from Sigma-Aldrich. YFP-Parkin and YFP-Parkin C431N were a gift from Richard Youle (Addgene plasmid #23955 and #46924 respectively) [19, 27]. For transfection, chondrocyte cultures were digested with Pronase-Collagenase enzyme mix for 3 hours, collected by centrifugation and washed with PBS. Chondrocytes (1×106) were transfected with 100 nM siRNAs or 1 μg of plasmid DNA using P3 Primary Cell 4D Nucleofector X kit and the Amaxa Nucleofection System (Lonza).
Statistical analysis
All the results are reported as Mean±SD of at least three independent experiments from different patient samples, each done in triplicate. Statistical significance was evaluated using one-way analysis of variance (ANOVA using Sigmaplot 12.3 software (Systat Software, Inc.) followed by Tukey’s test for post hoc analyses. P values ≤ 0.05 were considered significant.
Results
IL-1β induces high levels of ROS, mitochondrial dysfunction and apoptosis in chondrocytes
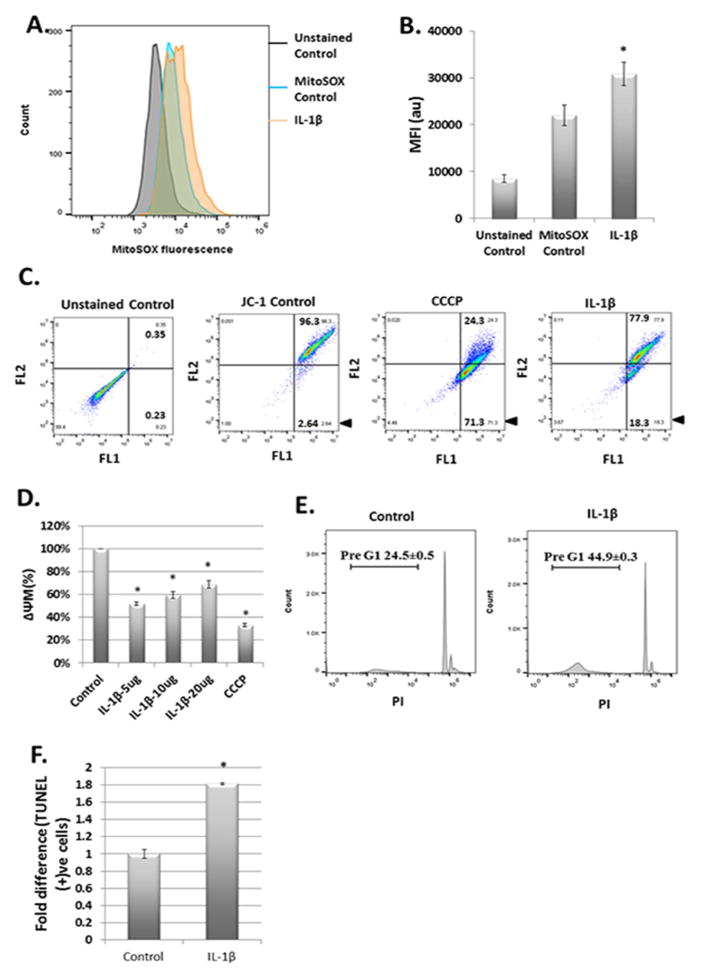
The proinflammatory cytokine IL-1β is believed to be a critical factor in the pathogenesis of OA. We determined whether IL-1β induces the generation of mitochondrial ROS in OA chondrocytes using mitochondrial ROS specific probe MitoSOX Red. We found significantly increased levels of mitochondrial ROS in IL-1β treated chondrocytes (Fig. 1A and 1B). We also analyzed the ROS levels in normal chondrocytes isolated from mouse knee. The total ROS levels were high in normal chondrocytes stimulated with IL-1β (Suppl. Fig. 1A and B) but the mitochondrial ROS levels in normal chondrocytes were similar to OA chondrocytes (Suppl. Fig. 1C). We next determined the mitochondrial membrane depolarization and dysfunction in OA chondrocytes treated with IL-1β. We found that IL-1β was a potent inducer of mitochondrial damage as exposure of chondrocytes for 5 minutes resulted in significant damage to mitochondrial membrane as determined by change in ΔΨM (Fig. 1C). We also found that IL-1β-induced loss of ΔΨM was rapid and sensitive to low doses of IL-1β with the maximum damage inflicted when chondrocytes were exposed to 5ng/ml of IL-1β (Fig. 1D). We also found that the number of apoptotic chondrocytes were double in chondrocyte cultures treated with IL-1β compared to untreated controls as determined by PI staining (Fig. 1E) and TUNEL assay (Fig 1F). Taken together, these results demonstrate that human OA chondrocytes under pathological conditions exhibit increased damage/depolarization of mitochondrial membrane, mitochondrial ROS generation, oxidative stress and enhanced rate of apoptosis.
Figure 1.
IL-1β treatment stimulated ROS production and mitochondrial dysfunction in chondrocytes. (A) and (B) Chondrocytes (1×106 cells/well) were seeded in 6 well plate and treated with MitoSox Red (5μM) for 10 minutes followed by IL-1β for 5 minutes. Chondrocytes were analyzed for mitochondrial ROS by flow cytometer. (C) and (D) Chondrocytes were seeded in 6 well plates as above and treated with IL-1β for 5 minutes followed by incubation with JC-1 dye for 30 minutes. The green (FL1) and red (FL2) fluorescence of JC-1 as marker of ΔΨM loss was analyzed by flow cytometer (BD Accuri C6). CCCP was used as positive control. (E) Chondrocytes were either treated with IL-1β (or left untreated as control) and incubated at 37°C for 48 hours. Chondrocytes were stained with propidium iodide in 01% Triton X-100 and analyzed for pre-G1 population indicating cell death by flow cytometer. (F) Chondrocytes were treated as above and analyzed for cell death by TUNEL staining and analysis by flow cytometer. Data shown are the representative of three independent experiments from different patient’s samples, each done in triplicate. Values are expressed as mean ± SD (*p<0.05).
Expression of Parkin was enhanced in OA chondrocytes under pathological conditions
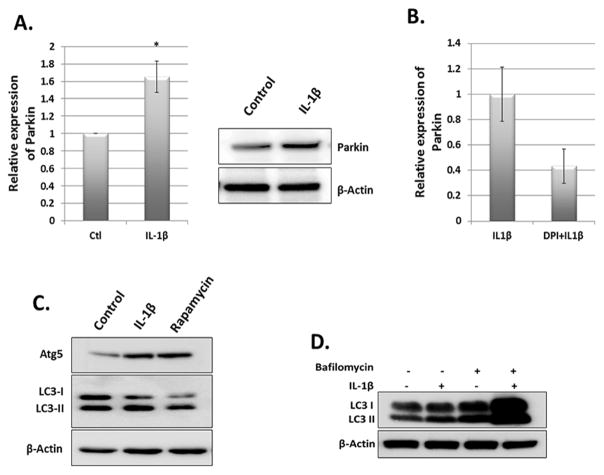
High levels of ROS induce mitochondrial dysfunction that results in further increase in ROS levels eventually leading to DNA damage and cell death [28]. This suggests that removal of dysfunctional mitochondria is necessary for maintaining the redox balance and cell survival. This is accomplished through mitophagy in which Parkin protein has been shown to play a critical role [19]. We found that Parkin mRNA and protein levels were increased in IL-1β stimulated OA chondrocytes compared to untreated controls (Fig. 2A). To determine whether enhanced ROS generation correlates with increased expression of Parkin, we treated OA chondrocytes with IL-1β in the presence or absence of antioxidant Diphenyliodonium (DPI) and performed qPCR analysis of Parkin mRNA expression. We found that Parkin mRNA expression was reduced in the presence of DPI (Fig 2B). We also confirmed whether increased expression of Parkin correlated with increased autophagy in OA chondrocytes, we treated OA chondrocytes with IL-1β for overnight and analyzed for the formation of LC3-II, a marker of autophagy activation and the expression levels of Atg5. We found that IL-1β stimulation of OA chondrocytes resulted in increased formation of LC3-II as well as increased expression of Atg5 protein (Fig. 2C). Rapamycin treated chondrocytes were used as positive control. We also treated the OA chondrocytes with IL-1β in the presence or absence of Bafilomycin and found that LC3-II generation was increased and was further enhanced by IL-1β suggesting that IL-1β treatment of OA chondrocytes enhanced the autophagy flux (Fig 2D). Taken together, this data indicated that mitophagy, in which Parkin is a critical player, may also be active to control the population of dysfunctional mitochondria and prevent oxidant-induced damage in OA chondrocytes under pathological conditions.
Figure 2.
IL-1β induced stress upregulated Parkin expression. Chondrocytes were seeded in 35mm dish (1×106 cells/well) and stimulated with IL-1β for overnight. Cells were harvested for RNA and lysate preparation and expression of Parkin was analyzed by qPCR (right panel) and Western blot (left panel). Data represent mean ± SD of three independent experiments, each done in triplicate (*p<0.05). β-Actin was used as normalization/loading control. (B) Chondrocytes were treated with IL-1β in the presence or absence of DPI for overnight. Total RNA was prepared from cells and analyzed for Parkin mRNA expression. (C) Chondrocytes were treated with IL-1β in the presence of 10% serum for overnight (16 hours) and analyzed for LC3-II and Atg5 levels by immunoblotting. (D) Chondrocytes were treated with IL-1β for overnight in the presence or absence of Bafilomycin to analyze autophagic flux. Cells were lysed in RIPA buffer supplemented with protease inhibitors and analyzed for LC3-II formation. β-Actin was used as loading control.
Parkin accumulates on depolarized mitochondria in chondrocytes under pathological conditions and promotes mitophagy
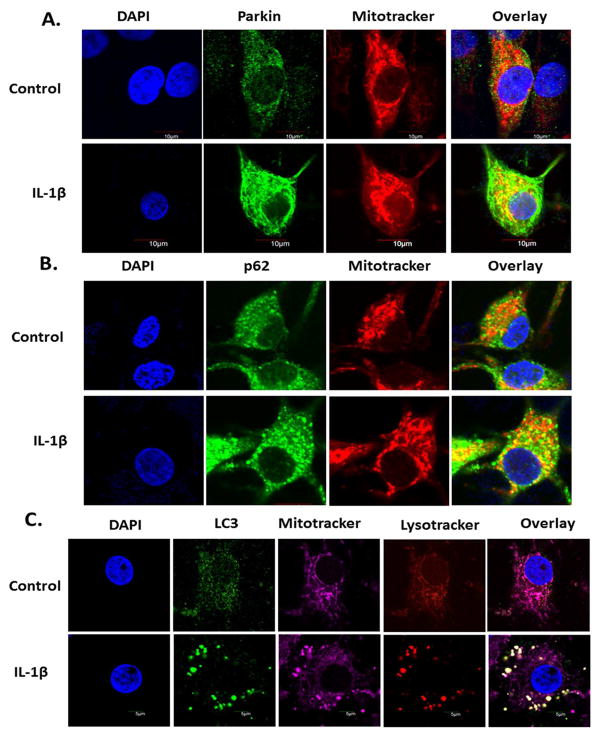
To determine whether increased levels of Parkin in IL1β stimulated OA chondrocytes correlate with active mitophagy, we determined the accumulation of Parkin on the damaged/depolarized mitochondria by immunofluorescence staining followed by confocal microscopy. We found a substantial increase in the translocation of Parkin (green) from cytosol to mitochondrial surface (red) in IL-1β treated chondrocytes (Fig. 3A). Translocation of Parkin is associated with PINK1 dependent increase in its activity that promotes ubiquitination of mitochondrial proteins [29]. Ubiquitination of mitochondrial surface proteins by Parkin recruits p62/SQSTM1 protein that accumulates on damaged mitochondria and has been reported to recruit mitochondria to the autophagosomes by binding to ATG8/LC3 [30, 31]. Consistent with this, we found that Parkin and p62/SQSTM1 were colocalized on damaged mitochondria as shown by increased numbers of distinct yellow puncta indicating colocalization of p62/SQSTM1 to Mitotracker labeled mitochondria in chondrocytes treated with IL-1β (Figure-3B). Mitophagic clearance of depolarized/dysfunctional mitochondria in IL-1β-stimulated OA chondrocytes was determined by colocalization of autophagosomes, immunostained with LC3 specific antibody and mitochondria stained with Mitotracker. We observed increased formation of LC3 positive autophagosomes (green) in IL-1β treated chondrocytes which were colocalized with the mitochondria (magenta) (Figure-3C). In the process of mitophagy, the autophagosomes eventually fuse with the lysosomes where the degradation of dysfunctional mitochondria occurs. Data presented in Figure-3C also demonstrated that the autophagosomes engulfed mitochondria were colocalized with lysosomes (red) thus demonstrating active mitophagy for the clearance of damaged/dysfunctional mitochondria from IL-1β treated OA chondrocytes.
Figure 3.
Autophagy targeted dysfunctional mitochondria in IL-1β stimulated chondrocytes. Chondrocytes were seeded in 8 well chamber slides (0.1×106/well) and treated with IL-1β (or left untreated as control) for 24 hours. (A) Chondrocytes were stained with Mitotracker Deep Red for 30 minutes and fixed with 4% paraformaldehyde. Chondrocytes were permeabilized with 0.3% Triton X-100 and probed with rabbit anti-Parkin antibody followed by anti-rabbit Alexa-Fluor 488. Chondrocytes were stained with DAPI, mounted with anti-fade mounting media and visualized by Olympus FV1000 confocal microscope. (B) Chondrocytes stained with Mitotracker Deep Red as above and probed with mouse anti-p62 antibody followed by anti-mouse Alexa Fluor 488 and visualized as above. (C) Chondrocytes were stained with Mitotracker Deep Red followed by Lysotracker Red and fixed and permeabilized. Chondrocytes were probed with rabbit anti-LC3 antibody and visualized as above.
Inhibition of autophagy blocks the clearance of damaged mitochondria and enhances IL-1β induced oxidative stress
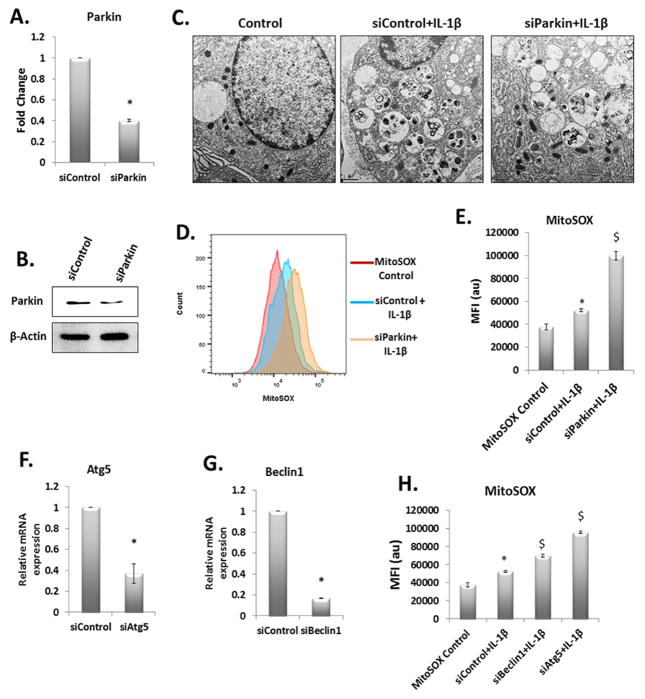
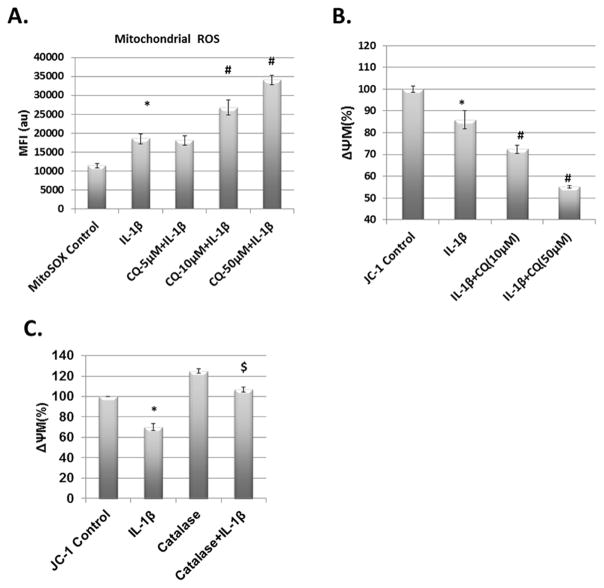
Translocation and activation of Parkin on the surface of damaged mitochondria and active autophagy are considered as an essential mechanism for autophagic clearance of damaged/dysfunctional mitochondria and disruption in any one of these arms may result in accumulation of dysfunctional mitochondria. To test this hypothesis, we used siRNA-mediated depletion of Parkin to first interfere with the mitophagy branch of autophagy. Parkin expression was significantly reduced in OA chondrocytes transfected with siRNAs targeting Parkin in comparison to chondrocytes transfected with scrambled siRNAs (Fig. 4A and 4B). Parkin depleted OA chondrocytes showed significant increase in the accumulation of damaged/dysfunctional mitochondria upon IL-1β-stimulation as determined by electron microscopy (Figure-4C) and production of mitochondrial ROS (Fig. 4D and 4E). We also analyzed the levels of Atg5 and Beclin1 in IL-1β stimulated OA chondrocytes and found increased expression of Atg5 and Beclin1 (Suppl. Fig. 2). We next used siRNAs to knockdown Atg5 or Beclin1 expression, but not Parkin expression, to block the autophagy pathway to prove our hypothesis that Parkin-mediated clearance of damaged/dysfunctional mitochondria in OA chondrocytes requires active autophagy. Transfection of OA chondrocytes with siRNAs targeting ATG5 or Beclin1 (siAtg5 or siBeclin1) significantly reduced the expression of targeted genes (Fig. 4F and 4G respectively). Downregulation of Atg5 or Beclin1 expression significantly increased the mitochondrial ROS production upon IL-1β stimulation (Fig. 4H). These results indicated that Parkin-mediated clearance of dysfunctional mitochondria in OA chondrocytes is dependent on active autophagy and is essential to prevent accumulation of damaged mitochondria and prevention of oxidative stress. This was further confirmed by using pharmacological inhibitors of autophagy and then treating the OA chondrocytes with IL-1β. We measured the mitochondrial ROS levels in OA chondrocytes pretreated with Chloroquine followed by stimulation with IL-1β. Mitochondrial ROS production upon IL-1β treatment was signifcantly higher in chondrocytes with autophagy inhibition than in chondrocytes treated with IL-1β alone (Fig. 5A). Inhibition of autophagy also resulted in enhanced loss of ΔΨM and accumulation of dysfunctional mitochondria in chondrocytes treated with IL-1β (Fig. 5B and Suppl. Fig. 3). To find out whether IL-1β induced mitochondrial dysfunction was through ROS or some other mechanism, we treated chondrocytes with catalase, an antioxidant enzyme before IL-1β stimulation and JC-1 staining. The analysis of JC-1 fluorescence revelaed that addition of Catalase inhibited the generation of high levels of ROS and prevented the loss of ΔΨM induced by IL-1β (Fig. 5C) indicating that cytokine-induced ROS generation plays a role in inducing mitochondrial damage/dysfunction. Overall, these results indicated that mitophagic clearance of dysfunctional mitochondria is important for preventing the accumulation of damaged/dysfunctional mitochondria in OA chondrocytes under pathological conditions.
Figure 4.
Knockdown of Parkin or Atg5 or Beclin-1 hyper sensitized chondrocytes against IL-1β induced oxidative stress and mitochondrial dysfunction. Chondrocytes were transfected with control siRNA or siRNA targeting Pakrin for 48 hours. Total RNA and cell lysate was prepared to analyze Parkin expression by qPCR (A) and immunoblot analysis (B). β-Actin is used as loading or normalization control. (*p<0.05) (C) Chondrocytes were transfected with siControl or siParkin as above and treated with IL-1β followed by transmission electron microscopy. Untreated chondrocytes were taken as control. (D) and (E) Chondrocytes were transfected as above with siControl or siParkin for 48 hours and incubated with MitoSOX Red dye followed by IL-1β and analyzed by flow cytometer for MitoSOX fluorescence. Values are mean±SD (*p<0.05). (F) and (G) Chondrocytes were transfected with siControl or siAtg5 or siBeclin1 as above. Cells were incubated at 37°C for 48 hours fo llowed by RNA isolation and Atg5 and Beclin1 knockdown was analyzed by qPCR (*p<0.05). (H) siControl, siAtg5 and siBeclin1 transfected cells were stained with MitoSOX Red, treated with IL-1β and analyzed the fluorescence with Flow cytometer. Data represent mean ± SD of three independent experiments, each done in triplicate. (* IL-1β vs control, $ target siRNA vs control siRNA, p<0.05).
Figure 5.
Autophagy inhibition exaggerated IL-1β induced oxidative stress and mitochondrial dysfunction. (A) Chondrocytes were seeded in 6 well plates and pretreated with different concentrations of autophagy inhibitor chloroquine (CQ) followed by addition of MitoSOX red dye. Cells were treated with IL-1β for 5 minutes and analyzed by flow cytometer to measure mitochondrial ROS. (B) Chondrocytes were pretreated with chloroquine as above followed by treatment with IL-1β for five minutes and addition of JC-1 dye for 30 minutes. The red and green fluorescence of JC-1 was measured by fluorimeter and the ΔΨM loss was calculated as ratio of red and green fluorescence (C) Chondrocytes were treated with catalase for 1 hour followed by IL-1β treatment and JC-1 staining. ΔΨM loss was calculated as above. Data is shown as mean ± SD of three independent experiments, each done in triplicate (* IL-1β vs control, # CQ+IL-1β vs IL-1β, $ Catalse+IL-1β vs IL-1β, p<0.05).
Parkin clearence of damaged mitochondria results in reduced ROS levels and increased survival of chondrocytes under pathological conditions
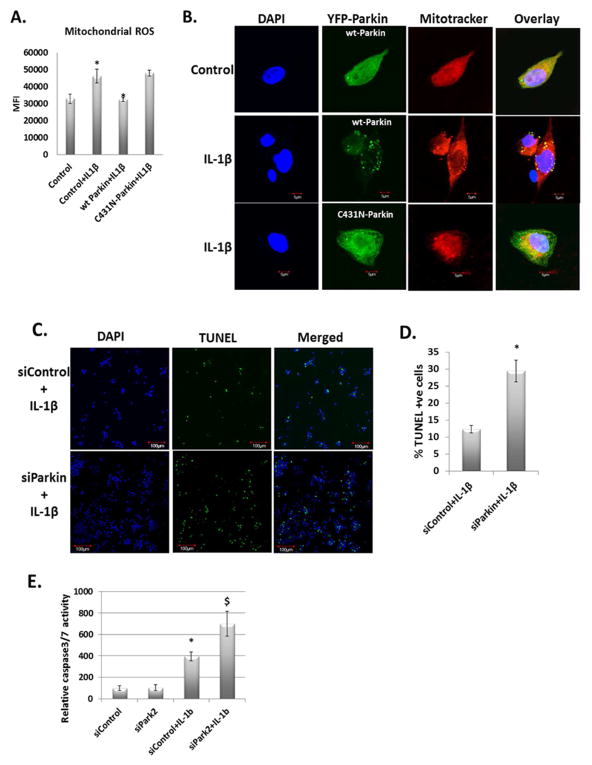
Data presented above indicated that chondrocytes mitochondria were damaged and generated high levels of ROS under pathological conditions. We therefore determined whether clearence of damaged/depolarized mitochondria in OA chondrocytes by Parkin eliminate excessive ROS under pathological conditions. To test this we transfected the OA chondrocytes with wild type Parkin (YFP-wt-Parkin) or the E3 ubiquitin ligase mutant YFP-C431N-Parkin and then stimulated them with IL-1β and measured the ROS production using MitoSOX. Following stimulation with IL-1β, control trasfected OA chondrocytes or OA chondrocytes overexpressing the mutant form of Parkin showed increased ROS production compared to unstimulated OA chondrocytes or OA chondrocytes overexpressing the wt-Parkin (Fig. 6A) as determined by increased MitoSOX fluorescence. In addition we found that wt-Parkin, but not the mutant form of Parkin, colocalized with depolarized/damaged mitochondria in OA chondrocytes stimulated with IL-1β (Fig. 6B). There was also a significant increase in the number of TUNEL positive OA chondrocytes with depleted expression of Parkin compared to controls (Fig. 6C and 6D). We further confirmed the enhanced apoptotic death of chondrocytes using Caspase Glo 3/7 activity assay (Promega, #G8090). As shown in the Figure 6E, Parkin depletion enhanced the apoptotic death of OA chondrocytes compared to wild type OA chondrocytes in response to IL-1β.
Figure 6.
Overexpression of wt-Parkin downregulates mitochondrial ROS by clearing dysfunctional mitochondria. (A) Chondrocytes were transfected with YFP-wt-Parkin and YFP-C431N-Parkin and incubated at 37°C for 48 hours. Chondrocytes were stained with MitoSOX Red followed by IL-1β stimulation. MitoSOX red fluorescence was measured by fluorimeter. (B) Chondrocytes were transfected with YFP-wt-Parkin and YFP-C431N-Parkin as above and treated with IL-1β. Chondrocytes were stained with Mitotracker Deep Red and fixed with 4% paraformaldehyde. Mounted with anti-fade mounting media and visualized as above. (C) and (D) Chondrocytes were transfected with siControl or siParkin for 48 hours and stimulated with IL-1β for 48 hours. Chondrocytes were processed for TUNEL assay as per manufacturer’s instruction. Around 600 cells were counted in both the samples and plotted as TUNEL positive cells. (E) Chondrocytes were transfected with siControl and siParkin and treated with IL-1β as above. Apoptotic cell death was analyzed by measuring caspase 3/7 activities using Caspase Glo 3/7 luminescence assay system. Data is shown as mean ± SD of three independent experiments, each done in triplicate.
Discussion
In the aging population, OA is a leading cause of chronic disability and has been suggested to be due to disruption of chondrocyte homeostasis and oxidative stress which contributes to OA pathogenesis [7, 32]. ROS-induced chondrocyte death [33] indicates that survival of chondrocytes is dependent on the presence of a functional mitochondrial population. IL-1β is upregulated in OA joints and has been shown to induce the expression of cartilage matrix degrading proteases and other inflammatory mediators [24]. Previous studies have shown that dysfunction in the removal of damaged mitochondrial is associated with the pathogenesis of OA [34]. Here we show that stimulation of human OA chondrocytes with IL-1β resulted in loss of ΔΨM, significant increase in mitochondrial ROS levels, and chondrocyte death. Loss of ΔΨM causes reduced synthesis of ATP and increased production of ROS and if the dysfunctional mitochondria are not removed from cells, this may result in release of pro-apoptotic proteins that lead to apoptosis. In this regard, recent findings that respiratory chain activity of complex II and III are compromised in human OA chondrocytes in comparison to normal chondrocytes are important because these implicate directly mitochondrial damage/dysfunction in OA pathogenesis [15]. Additionally, treatment of human normal chondrocytes with respiratory chain dysfunction inducers like Rotenone, Oligomycin, Sodium azide etc. has been reported to alter the expression of MMPs, collagen type II and other cartilage ECM components [35]. Since mitochondria play critical role in several cellular processes, from energy production through oxidative phosphorylation to cell signaling and apoptosis [28, 36], there is a need for mechanism(s) to remove damaged mitochondria to prevent generation of excessive ROS and cell death. However, no such chondrocyte mechanism for the removal of damaged/dysfunctional mitochondria has been identified and neither the effect of their removal on ROS levels and chondrocyte survival under pathological conditions has been reported.
In this study, we sought to establish how mitochondria are damaged, role of Parkin in their elimination and its impact on the ROS levels and OA chondrocytes survival under pathological conditions. We found that IL-1β-induced ROS generation precedes depolarization of mitochondria in OA chondrocytes. This is a novel finding and has not been previously reported. Our data also revealed that autophagy was increased and Parkin expression was significantly upregulated in human OA chondrocytes under pathological conditions. Increased expression of Parkin suggested that it might be involved in the clearance of damaged mitochondria and indeed OA chondrocytes with depleted Parkin expression showed increased production of ROS, accumulation of dysfunctional mitochondria, and apoptosis establishing an important role of Parkin in the clearance of dysfunctional mitochondria in human OA chondrocytes. This gets further strength from our data showing that overexpression of wt-Parkin significantly reduced the production of mitochondrial ROS, and was dependent on the E3 ubiquitin ligase activity of Parkin. Consistent with our data, in a recent study Parkin mediated clearance of dysfunctional mitochondria was found essential to regulate ROS levels and survival of lens epithelial cells [23]. Furthermore, Parkin null mouse have been reported to show compromised mitophagy and increased accumulation of dysfunctional mitochondria in myocytes under stress [37]. A number of reports have documented increased inflammation in human patients with Parkinson’s disease establishing the link between Parkin, ROS levels and mitochondrial dysfunction [38, 39].
Autophagy plays a central role in maintaining cellular homeostasis by clearing intracellular protein aggregates and dysfunctional organelles. Defective autophagy has been linked to several human diseases, including Parkinson’s disease, cancer [40, 41] and OA [42]. Several drugs and inhibitors such as CCCP, acetaminophen etc., which induce mitochondrial dysfunction have been reported to induce mitophagy and clearance of dysfunctional mitochondria [21]. Our data demonstrate that Parkin translocate to damaged mitochondria and recruits p62/SQSTM1 followed by engulfment of dysfunctional mitochondria by autophagosomes and degradation by lysosomes thus providing evidence that Parkin removes damaged mitochondria by recruiting the autophagy machinery to degrade the defective organelle in human OA chondrocytes stimulated with IL-1β. Importantly, overexpression of wild-type Parkin resulted in reduced levels of ROS. Our data thus provide evidence that Parkin-mediated clearance of damaged/depolarized mitochondria limit the generation of ROS and prevent the induction of oxidative stress and possibly the damage caused by oxidative stress in OA chondrocytes. To our knowledge, this is the first report demonstrating that Parkin-mediated elimination of depolarized/damaged mitochondria is an important mechanism to limit ROS production and improve OA chondrocytes survival under pathological conditions. Thus, it is tempting to speculate that loss of Parkin function could contribute directly to the pathogenesis of OA. However, this needs further study including the data on the incidence of OA in Parkinson’s patients with an established mutation preventing its translocation to mitochondria and initiation of mitophagy.
In conclusion, our data provides first detailed study of clearance of dysfunctional mitochondria in human OA chondrocytes. We show for the first time that Parkin protein is required for autophagic clearance of dysfunctional mitochondria under pathological conditions. We show that depletion of Parkin in chondrocytes resulted in increased mitochondrial dysfunction and oxidative stress in response to IL-1β stimulation indicating that Parkin is essential for mitophagy of dysfunctional mitochondria. Thus, the above data indicate that Parkin could be critical for maintaining chondrocyte homeostasis under pathological conditions. Further study is required to understand the detailed mechanism of Parkin mediated mitophagy in chondrocytes under pathological conditions in vitro as well as in whole body models of OA to better understand its role in chondrocyte/cartilage homeostasis and its potential development as a therapeutic target for the management of OA.
Supplementary Material
Acknowledgments
Role of Funding Source
This work was supported in part by USPHS/National Institutes of Health grants (RO1-AT-005520; RO1-AT-007373; RO1-AR- 067056) and funds from the Northeast Ohio Medical University to TMH.
Footnotes
Author Contributions
All authors were involved in drafting the article for important intellectual content, and all authors approved the final version to be published. Dr. Haqqi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Haqqi, Ansari.
Acquisition of data. Ansari, Khan, Ahmad, Haqqi.
Analysis and interpretation of data. Ansari, Khan, Ahmad, Haqqi.
Ethics Approval
This study was conducted with the approval of the Institutional Review Board at the Northeast Ohio Medical University.
Conflict of Interest
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 2.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labunskyy VM, Gladyshev VN. Role of reactive oxygen species-mediated signaling in aging. Antioxid Redox Signal. 2013;19:1362–1372. doi: 10.1089/ars.2012.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones DP. Redox theory of aging. Redox Biol. 2015;5:71–79. doi: 10.1016/j.redox.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 7.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- 9.Del Carlo M, Schwartz D, Erickson EA, Loeser RF. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic Biol Med. 2007;42:1350–1358. doi: 10.1016/j.freeradbiomed.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43:517–521. [PubMed] [Google Scholar]
- 11.Reed KN, Wilson G, Pearsall A, Grishko VI. The role of mitochondrial reactive oxygen species in cartilage matrix destruction. Mol Cell Biochem. 2014;397:195–201. doi: 10.1007/s11010-014-2187-z. [DOI] [PubMed] [Google Scholar]
- 12.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 13.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 14.Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7:161–169. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 15.Maneiro E, Martin MA, de Andres MC, Lopez-Armada MJ, Fernandez-Sueiro JL, del Hoyo P, et al. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:700–708. doi: 10.1002/art.10837. [DOI] [PubMed] [Google Scholar]
- 16.Lopez de Figueroa P, Lotz MK, Blanco FJ, Carames B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 2015;67:966–976. doi: 10.1002/art.39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki H, Takayama K, Matsushita T, Ishida K, Kubo S, Matsumoto T, et al. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64:1920–1928. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 18.Carames B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015;67:1568–1576. doi: 10.1002/art.39073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Springer MZ, Macleod KF. In Brief: Mitophagy: mechanisms and role in human disease. J Pathol. 2016;240:253–255. doi: 10.1002/path.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennan L, Khoury J, Kantorow M. Parkin elimination of mitochondria is important for maintenance of lens epithelial cell ROS levels and survival upon oxidative stress exposure. Biochim Biophys Acta. 2016;1863:21–32. doi: 10.1016/j.bbadis.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ansari MY, Haqqi TM. Interleukin-1beta induced Stress Granules Sequester COX-2 mRNA and Regulates its Stability and Translation in Human OA Chondrocytes. Sci Rep. 2016;6:27611. doi: 10.1038/srep27611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haseeb A, Ansari MY, Haqqi TM. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J Orthop Res. 2016 doi: 10.1002/jor.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan NM, Ansari MY, Haqqi TM. Sucrose, But Not Glucose, Blocks IL1-beta-Induced Inflammatory Response in Human Chondrocytes by Inducing Autophagy via AKT/mTOR Pathway. J Cell Biochem. 2017;118:629–639. doi: 10.1002/jcb.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarou M, Narendra DP, Jin SM, Tekle E, Banerjee S, Youle RJ. PINK1 drives Parkin self442 association and HECT-like E3 activity upstream of mitochondrial binding. J Cell Biol. 2013;200:163–172. doi: 10.1083/jcb.201210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkin449 mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 31.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui W, Young DA, Rowan AD, Xu X, Cawston TE, Proctor CJ. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann Rheum Dis. 2016;75:449–458. doi: 10.1136/annrheumdis-2014-206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jallali N, Ridha H, Thrasivoulou C, Underwood C, Butler PE, Cowen T. Vulnerability to ROS456 induced cell death in ageing articular cartilage: the role of antioxidant enzyme activity. Osteoarthritis Cartilage. 2005;13:614–622. doi: 10.1016/j.joca.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Blanco FJ, Lopez-Armada MJ, Maneiro E. Mitochondrial dysfunction in osteoarthritis. Mitochondrion. 2004;4:715–728. doi: 10.1016/j.mito.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Cillero-Pastor B, Rego-Perez I, Oreiro N, Fernandez-Lopez C, Blanco FJ. Mitochondrial respiratory chain dysfunction modulates metalloproteases -1, -3 and -13 in human normal chondrocytes in culture. BMC Musculoskelet Disord. 2013;14:235. doi: 10.1186/1471-2474-14-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Z, Ristow M. Mitochondria and metabolic homeostasis. Antioxid Redox Signal. 2013;19:240–242. doi: 10.1089/ars.2013.5255. [DOI] [PubMed] [Google Scholar]
- 37.Kubli DA, Quinsay MN, Gustafsson AB. Parkin deficiency results in accumulation of abnormal mitochondria in aging myocytes. Commun Integr Biol. 2013;6:e24511. doi: 10.4161/cib.24511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stojkovska I, Wagner BM, Morrison BE. Parkinson’s disease and enhanced inflammatory response. Exp Biol Med (Maywood) 2015;240:1387–1395. doi: 10.1177/1535370215576313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tufekci KU, Meuwissen R, Genc S, Genc K. Inflammation in Parkinson’s disease. Adv Protein Chem Struct Biol. 2012;88:69–132. doi: 10.1016/B978-0-12-398314-5.00004-0. [DOI] [PubMed] [Google Scholar]
- 40.Jiang X, Overholtzer M, Thompson CB. Autophagy in cellular metabolism and cancer. J Clin Invest. 2015;125:47–54. doi: 10.1172/JCI73942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li YS, Zhang FJ, Zeng C, Luo W, Xiao WF, Gao SG, et al. Autophagy in osteoarthritis. Joint Bone Spine. 2016;83:143–148. doi: 10.1016/j.jbspin.2015.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.