Abstract
Background
Inadequate intake of micronutrients with antioxidant properties is common among older adults and has been associated with higher risk of frailty, adverse functional outcome, and impaired muscle health. However, a causal relationship is less well known. The aim was to determine in old mice the impact of reduced dietary intake of vitamins A/E/B6/B12/folate, selenium, and zinc on muscle mass, oxidative capacity, strength, and physical activity (PA) over time.
Methods
Twenty‐one‐month‐old male mice were fed either AIN‐93‐M (control) or a diet low in micronutrients with antioxidant properties (=LOWOX‐B: 50% of mouse recommended daily intake of vitamins A, E, B6, and B12, folate, selenium, and zinc) for 4 months. Muscle mass, grip strength, physical activity (PA), and general oxidative status were assessed. Moreover, muscle fatigue was measured of m. extensor digitorum longus (EDL) during an ex vivo moderate exercise protocol. Effects on oxidative capacity [succinate dehydrogenase (SDH) activity], muscle fibre type, number, and fibre cross‐sectional area (fCSA) were assessed on m. plantaris (PL) using histochemistry.
Results
After 2 months on the diet, bodyweight of LOWOX‐B mice was lower compared with control (P < 0.0001), mainly due to lower fat mass (P < 0.0001), without significant differences in food intake. After 4 months, oxidative status of LOWOX‐B mice was lower, demonstrated by decreased vitamin E plasma levels (P < 0.05) and increased liver malondialdehyde levels (P = 0.018). PA was lower in LOWOX‐B mice (P < 0.001 vs. control). Muscle mass was not affected, although PL‐fCSA was decreased (~16%; P = 0.028 vs. control). SDH activity and muscle fibre type distribution remained unaffected. In LOWOX‐B mice, EDL force production was decreased by 49.7% at lower stimulation frequencies (P = 0.038), and fatigue resistance was diminished (P = 0.023) compared with control.
Conclusions
Reduced dietary intake of vitamins A, E, B6, and B12, folate, selenium, and zinc resulted in a lower oxidative capacity and has major impact on muscle health as shown by decreased force production and PA, without effects on muscle mass. The reduced fCSA in combination with similar SDH activity per fibre might explain the reduced oxidative capacity resulting in the increased fatigue after exercise in LOWOX‐B mice.
Keywords: Nutrition, Antioxidants, Muscle quality, Function, Strength
Introduction
Inadequate intake of micronutrients has been associated with a higher risk of frailty and impaired mobility in older adults.1, 2 While the most compelling evidence exists for a role of vitamin D deficiency (reviewed in Bischoff‐Ferrari3), inadequate intake of other micronutrients has also directly or indirectly been associated with reduced physical activity and impaired muscle strength with advanced age.4, 5 Several of these micronutrients contribute to the antioxidant capacity, which is impaired during ageing.6 The increased production of reactive oxygen species with age that exceeds the antioxidant defence capacity7 may contribute to lower physical function and a reduced potential to adapt to muscle exercise and mechanical loading.8, 9, 10 An age‐related reduction in muscle mitochondrial DNA and an increased mtDNA oxidation could play a role in this phenomenon.11, 12 Moreover, an impaired muscle protein synthesis response to anabolic stimuli in ageing13 may be related to oxidative stress.14 For example, low serum levels of vitamins B6 and B12 predict an inability to perform the activities of daily living in community‐dwelling older women,15 and vitamin B12 deficiency has been suggested to contribute to the frailty syndrome.16 Inadequate intake of vitamins B6 and B12 may contribute to hyperhomocysteinemia, which is an indicator of oxidative stress.17 High serum homocysteine has been associated with reduced physical performance in older adults.18 Vitamin B12 deficiency is more severe in older adults due to food‐bound cobalamin malabsorption.19 An elevated plasma homocysteine level in 30–50% of adults over 60 years20 may also point towards suboptimal intake or metabolism of vitamins B6 and B12 and folate. The relevance of B vitamins is likely due to their action as cofactors in enzymatic reactions that are essential for cellular and mitochondrial functions.21 Hyperhomocysteinemia may, therefore, also be an indicator of oxidative stress.22 A deficiency of other micronutrients with antioxidant properties, such as carotenoids, selenium, and vitamin E, has also been negatively associated with sarcopenia (i.e. loss of muscle mass and function with age), frailty, and disability.15, 23, 24 A decreased blood value of selenium has been associated with low skeletal muscle mass and strength.25, 26
While association studies suggest that deficiencies in micronutrients with antioxidant properties play a role in frailty and sarcopenia, a causal relationship is less well studied. We hypothesized that with this specific diet intervention, low in antioxidants, natural ageing is accelerated by a further decrease of muscle mass, strength, and physical function. Therefore, the aim of this study was to determine in aged mice the impact of a reduced dietary intake of vitamins A, E, B6, and B12, folate, selenium, and zinc on muscle mass, strength, and physical function over time. Vitamin C, a well‐described antioxidant, was not in scope of the diet intervention because mice can endogenously synthesize vitamin C.27 In addition, underlying determinants of these physical traits were explored, focusing on muscle force production, muscle mitochondrial density and muscle fibre characterization (i.e. number, size, and type), immune response, and muscle protein synthesis and breakdown. By creating a deficiency of these specific micronutrients in ageing mice, we were able to mimic the human situation and gain further insight into the relevance of such deficiencies for muscle health with age.
Methods
Animals
Male C57/BL6J mice of 21 months of age were obtained from Janvier Labs (Saint Berthevin, France). Animals were individually housed in a climate‐controlled room (12:12 dark–light cycle with a constant room temperature of 21 ± 1°C). Mice were ad libitum fed with a standard diet (AIN‐93‐M) and had free access to tap water. All diets were provided by Research Diets Services (Wijk bij Duurstede, the Netherlands). All experimental procedures were approved by the Animal Ethical Committee (DEC consult, Bilthoven, the Netherlands). After 2 weeks of acclimatization, mice were randomized in three different groups: (1) baseline control group at 21 months of age, (2) mice ageing until 25 months on a normal diet, and (3) mice ageing until 25 months on a diet low in antioxidants. The control group received AIN‐93‐M for 4 months (control diet, ageing control group, n = 11), and the other group (n = 11) received a diet lowered in vitamin A, E, B6, B12, folate, selenium, and zinc (=LOWOX‐B) to levels representing 50% of the daily recommended intake of lab rodents (Table 1).28 Because the non‐essential amino acid cysteine is a strong antioxidant,30 the LOWOX‐B diet was not fortified with cysteine as in AIN‐93‐M. Bodyweight and food intake were determined twice a week. For a schematic overview of the experimental setup, refer to Figure 1. During the total duration of the experiment, mice were carefully observed for symptoms of general malaise or signs of moribund conditions. After an overnight fast, mice were sacrificed by block randomization at 21 and 25 months of age; skeletal muscles from the hind limb (tibialis anterior, extensor digitorum longus, soleus, plantaris, and gastrocnemius muscle) and liver were dissected, weighted, and stored at −80°C until further use. The plantaris (PL) muscles were (control n = 8; LOWOX‐B n = 7) pinned on a piece of Sylguard (Dow Corning, the Netherlands) and frozen in liquid nitrogen as in van der Zwaard et al.31 Within a month after the excision, serial cross sections (10 μm) were cut from the mid‐belly of PL using a cryostat at −20°C. Sections were mounted on glass slides (Menzel‐Gläser, Superfrost® plus, GER), air‐dried, and stored at −80°C until further use. The incubation of the sections for determination of succinate dehydrogenase activity (SDH) was performed32 immediately after sectioning and drying of the sections for 15 min at room temperature.
Table 1.
Composition of the intervention diets
| Diet name | Control diet | LOWOX‐B | RDA mousec |
|---|---|---|---|
| Ingredients | g/kg dry matter | ||
| Cornstarch | 466 | 466 | Unknown |
| Dextrinized cornstarch | 155 | 155 | Unknown |
| Casein | 140 | 141.8 | 180 |
| l‐Cystine | 1.8 | 0 | 7.6d |
| Sucrose | 100 | 100 | Unknown |
| Soybean oil | 40 | 40 | 50 |
| Fibera | 50 | 50 | Unknown |
| Mineral mixb | 35 | 35 | |
| Selenium (mg/kg) | 0.170 | 0.075 | 0.150 |
| Zinc (mg/kg) | 35 | 5 | 10 |
| Vitamin mixb | 10 | 10 | |
| Vitamin A (IU/kg) | 4000 | 1200 | 2400 |
| Vitamin B6 (mg/kg) | 6 | 4 | 8 |
| Vitamin B12 (μg/kg) | 25 | 5 | 10 |
| Folate (mg/kg) | 2 | 0.25 | 0.50 |
| Vitamin E (IU/kg) | 75 | 16 | 32 |
| Choline bitrate chloride | 1.4 | 1.4 | Unknown |
| tert‐Butylhydroquinone | 0.008 | 0.008 | Unknown |
The LOWOX‐B diet was based on the control diet, except for the vitamin and mineral mixes. These mixes were prepared by leaving out vitamins A/E/B6/B12 and folate in the mixes and subsequently add these micronutrients in amounts as indicated in table. All diets were equal in amounts of protein (137.5 g), carbohydrates (674.6 g), fat (41.1 g), and kilocalorie (3718) per kilogram dry matter.
Fibre source is cellulose.
As basis for the nutritional preparation is the AIN‐93‐M mineral and vitamin mix28 used without addition of selenium, zinc, vitamins A, B6, B12, and E, and folate. Subsequently for the control mice, the amounts listed in the table were added and the lower amounts for the LOWOX‐B mice.
Known recommended minimal daily requirement for growing mice based on the intake of 5 g food per day per mouse.29
7.6 g/kg is for cysteine plus phenylalanine,29 both included in the casein protein.
Figure 1.
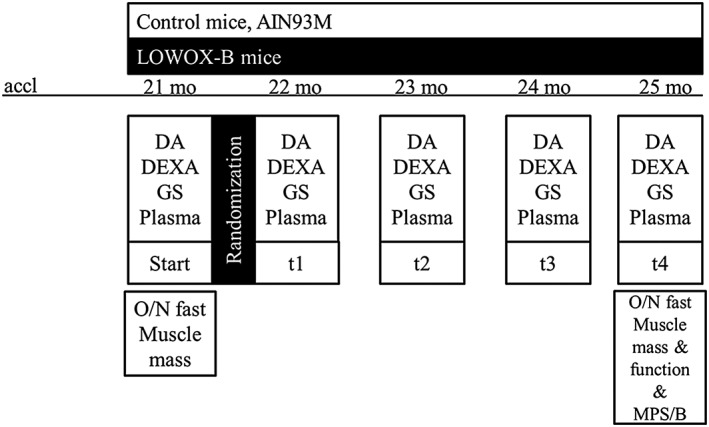
Experimental set‐up. accl, acclimatization period; mo, months; LOWOX‐B, group of mice with diet low in specific micronutrients with antioxidant properties; DA, daily activity measurements; GS, in vivo muscle grip strength measurements; DEXA, body composition analyses; MPS, muscle protein synthesis analysis; MPB, muscle protein breakdown; O/N, overnight.
Daily activity
During the acclimatization period, daily activity was measured for 1 week as normalization. During the experiment, daily activity was measured at the start of the experiment and every subsequent month, each time for 1 week as described before.33
Body composition
Body composition, i.e. lean mass, fat mass, and bone mineral density/content, was measured by dual‐energy X‐ray absorptiometry scan under general anaesthesia (isoflurane) using a PIXImus imager (GE Lunar, Madison, WI, USA).
In vivo muscle strength
In vivo muscle strength was measured as forelimb grip strength with a calibrated grip strength tester (Panlab, Cornella, Spain) by prompting the mouse to grip the trapeze bar with both forelimbs and pulling the mouse by the tail (proximal to the body) parallel to the orientation of the strain gauge and the trapeze bar.34 For mean grip strength analyses, a set included five repetitions, results were calculated as follows: (a) absolute mean grip strength and (b) absolute maximum grip strength, defined as the maximum tension recorded over five repetitions.
Ex vivo muscle function
Contractile characteristics of the extensor digitorum longus (EDL) muscle were assessed ex vivo, as described previously.33, 35 Briefly, muscles were allowed to stabilize at their slack length in the organ bath (Hugo Sachs Elektronik, March‐Hugstetten, Germany), for 30 min in Kreb's Heinselet buffer (mM: NaCl 118, KCl 4.75, MgSO4 1.18, CaCl2 2.5, KH2PO4 1.17, NaHCO3 24.9, and glucose 10) at 30°C and continuously perfused with 95% O2 and 5% CO2, after which optimal stimulation current and tension were determined. Subsequently, force–frequency characteristics (10–167 Hz, 250 ms) were determined. Isometric force signals of the force–frequency curve were analysed for maximal and total force production.33 After 5 min of rest and refreshing of the buffer, muscles were subjected to a moderate exercise protocol. Muscles were stimulated (143 Hz, 250 ms) 100 times, and the force production and the fatigue index were calculated. The single maximal force production during the first 40 contractions of the exercise protocol was summed up, and the ratio between the mean of the first three contractions and of the last three contractions was used as fatigue index.36, 37
Ex vivo muscle protein synthesis and breakdown
Gastrocnemius (GM) muscles were incubated ex vivo in Kreb's Heinselet buffer (refer to the previous section) with the addition of 0.1% BSA and 2 mM sodium pyruvate (=neutral buffer) (Sigma) at 36°C and continuously perfused with 95% O2 and 5% CO2. After 30 min of calibration, muscles were switched to either new neutral buffer or to neutral buffer with 100 μM leucine added (=anabolic buffer) and both containing 5 μM puromycin (Calbiochem). After additional 30 min, muscles were washed twice with PBS, and muscle protein synthesis was assessed by western blotting as described before.38, 39 Subsequently, for protein breakdown analysis, the neutral or anabolic buffer containing puromycin was collected and analysed for 3‐methylhistidine content by HPLC.40
Gene expression analyses
Total RNA was extracted using Trizol in combination with Ambion RNAquous micro kit (Fischer Scientific, Amsterdam, the Netherlands) from approximately 500 ng of EDL muscle samples. The quantity and purity of RNA were determined by NanoDrop® spectrophotometer (Isogen Life Science, Belgium). cDNA was synthesized from 1 mg of RNA using the Superscript Vilo cDNA synthesis kit (Fischer Scientific, Amsterdam, the Netherlands) according to the manufacturer's instructions. Real‐time quantitative PCR reactions were performed using a StepOne real‐time PCR system (Applied Biosystems). Mouse‐specific primers for atrogin/ MAFbx and MuRF1 were designed (Table 2). Samples were analysed in duplicate, and the values normalized to 18S rRNA. Melting curves were systematically analysed to ensure the specificity of the amplification process. Relative quantities were calculated with the standard curve method.
Table 2.
Primer sequence (5′‐3′)
| Target gene | Forward | Reverse |
|---|---|---|
| Atrogin/MuRF1 (Trim63) | TGCCCCCTTACAAAGCATCTT | CAGCATGGAGATGCAATTGC |
| Mafbx (Fbxo32) | TGAAGACCGGCTACTGTGGAA | CGGATCTGCCGCTCTGA |
| 18S rRNA | GTAACCCGTTGAACCCCATT | GTAACCCGTTGAACCCCATT |
Biochemical nutrient status measurements
Blood samples were taken via cardiac puncture and collected in tubes coated with heparin. Plasma was obtained by centrifugation at 1300g for 10 min at 4°C and stored at −80°C. Plasma and freeze‐dried liver samples for analyses of vitamins E and A were mixed with ethanol and centrifuged. The content of retinol and α‐tocopherol was determined in supernatant by HPLC, using UV absorbance for detection of retinol and fluorometric properties for detection of α‐tocopherol, by comparing with standard solutions. Serum samples were used for immunological parameters: Bio‐Plex Pro Mouse kits (Th17 panel A 6‐plex, IL‐15, MIP‐2) were used according to the manufacturer's description (Bio‐Rad). Small n is due to limitation of serum samples.
Liver malondialdehyde
After cryo‐desiccation of total liver, 10 mg of the liver was used to perform malondialdehyde (MDA) analysis, a marker for lipid peroxidation.41 In short, thiobarbituric reacts with MDA at pH 3.5. Thiobarbituric–MDA adduct is separated in a reversed‐phase column and quantified by fluorescence detection (ex 515 nm; em 553 nm).
Liver glutathione
To determine total glutathione (tGSH) content as a marker for whole‐body oxidative stress, another 10 mg of liver was used. For tGSH analyses, 0.4 M perchloric acid was added. The samples were placed in an ultrasonic bath for 30 min, vortexed, and centrifuged for 10 min at 13 000 rpm. The supernatant was diluted 100 times in phosphate‐EDTA buffer (0.1 M, pH 7.5). Subsequently, 40 μL sample and 20 μL DTNB (2.38 g/L in phosphate‐EDTA buffer) reagent, 40 μL glutathione reductase (10 μL/mL in phosphate‐EDTA buffer), and 100 μL NADPH (0.333 g/L in phosphate‐EDTA buffer) were added, and the plate was read immediately. Measurements were performed every 21 s at 405 nm during 4 min. Maximal velocity was calculated over at least seven measurements. Sample concentrations were calculated as micromole per milligram dry tissue.
Muscle fibre typing
For myosin heavy chain typing, serial sections were immunohistochemically stained against types I, IIA, IIX, and IIB myosin heavy chains as described before31 using monoclonal antibodies BAD5 (1 μg/mL), SC‐71 (1 μg/mL), 6H1 (10 μg/mL), and BF‐F3 (1–10 μg/mL) (Developmental Studies Hybridoma Bank, USA), respectively. Briefly, sections were fixated with acetone for 10 min at 4°C and washed in phosphate‐buffered saline with 0.05% Tween‐20 (PBST, Sigma‐Aldrich, the Netherlands) three times for 5 min at room temperature. After blocking with 10% normal swine serum for 30 min, sections were co‐incubated for 90 min with primary antibodies against type I and type IIB or against type IIA and type IIX. Subsequently, sections were washed in PBST three times for 3 min and incubated for 30 min in the dark with secondary antibodies against mouse IgG2b (Alexa Fluor 488, 1:200), IgG1 (Alexa Fluor 488, 1:200), and IgM (Alexa Fluor 647, 1:200) (Invitrogen, USA, Life Technologies, the Netherlands); sections were incubated with wheat germ agglutinin (1:50, Life Technologies, the Netherlands) for 20 min, washed in PBST, and subsequently washed once more in PBS, all in the dark. Then, sections were enclosed with Vectashield® hardset mounting medium with DAPI (1.5 μg/mL; Vector Laboratories, USA). Images were captured using a CCD camera (PCO; Sensicam, Kelheim, Germany) at 20× objective connected to a fluorescent microscope (Axiovert 200M; Zeiss, Göttingen, Germany) across the entire cross section using image processing software (Slidebook 4.1; Intelligent Image Innovations, Denver, CO) and were assembled into a composite image.
Analyses of succinate dehydrogenase activity
Succinate dehydrogenase activity, an indicator of mitochondrial oxidative capacity (VO2max),42 was determined according to the protocol described by Pool et al.32 Sections were incubated for SDH activity in a medium consisting of 37.5 mM sodium phosphate buffer, pH 7.6, 75 mM sodium succinate, 5 mM sodium azide, and 0.4 mM tetranitro blue tetrazolium, in the dark at 37°C for 20 min. The reaction was stopped in 10 mM HCl. The staining intensity of SDH was determined by measuring the absorbance of the final reaction product using a Leica DMRB microscope (Wetzlar, Germany) and an interference filter at 660 nm (A 660). This absorbance was converted to the rate of staining and expressed as absorbance units (A 660) per micrometre section thickness per second of incubation time (ΔA 660·μm−1 section thickness s−1 incubation time), where ΔA 660 is the change in absorbance at 660 nm. To assess the oxidative capacity of the muscle fibres, SDH activity was multiplied by the muscle fibre cross‐sectional area (refer in the succeeding texts) which yielded the spatially integrated SDH activity (ΔA 660·μm section thickness s−1 incubation time). The spatially integrated SDH activity is proportional to the VO2max per millimetre of fibre length (in nmol μm−1 s −1) of the cell.42
Assessment of muscle fibre cross‐sectional area
The cross‐sectional area of muscle fibres and absorbance values of the final SDH reaction product in muscle fibre sections were determined as follows. Sections were imaged using a Leica DMRB microscope (Wetzlar, Germany) fitted with calibrated grey filters using an appropriate interference filter (refer to the previous texts). Images were recorded with a 20× objective and a Sony XC‐77CE camera (Towada, Japan) connected to an LG‐3 frame grabber (Scion, USA) in an Apple Power Macintosh computer and analysed with NIH Image V1.61 (US National Institutes of Health). Grey values were converted to absorbance values per pixel using the grey filters and a third‐degree polynomial fit. Morphometry was calibrated using a slide micrometre and the set scale option in NIH Image, taking the pixel‐to‐aspect ratio into account. For each muscle cross section, images were taken from the high and low oxidative region, and for each fibre type, at least 20 muscle fibres were selected and measured. The public domain software ImageJ 1.45s (Rasband, W.S., ImageJ US National Institutes of Health, Bethesda, MD, USA) was used to measure the cross‐sectional area (CSA) and absorbances.
Statistical analyses
All data are expressed as means ± standard error of the mean (SEM). Statistical analyses were performed using SPSS 19.0 (SPSS Benelux, Gorinchem, the Netherlands). For randomization of experimental groups based on body weight and all other experimental outcomes, statistical analyses were performed by the use of a mixed model ANOVA with post hoc SIDAK testing, including mouse numbers, diet, and age as covariates. Bodyweight results were analysed by GLM analyses with post hoc SIDAK testing. Ex vivo skeletal muscle function data were analysed by mixed model ANOVA corrected for growth curve analyses with post hoc LSD. Muscle immunohistochemical analyses were tested with ANOVA and post hoc Bonferronni corrections. Differences were considered significant at a P value below 0.05.
Results
Mortality
Mortality at the start of the experiment, i.e. after 2 weeks of acclimatization, was none. Mortality over 16 weeks was 45.6% (5 of 11 mice) in the control group and 9.1% (1 of 11 mice) in the LOWOX‐B group. Cause of death in the control group was as follows: Two mice were taken out of the experiment due to preliminary death; during autopsy, no visible abnormalities were found, pointing towards ageing as being the cause of death. Another two mice were excluded due to liver tumours discovered during sections. One mouse was excluded due to a general decrease in well‐being and decrease in body weight with an enlarged heart and spleen observed at autopsy. The mouse in the LOWOX‐B group was taken out of the experiment due to preliminary death; autopsy revealed no visible cause.
Biomarkers of nutritional and oxidative status
As shown in Figures 2A and 2C, plasma levels of vitamin A and homocysteine (a biomarker for vitamins B6 and B12 and folate status) showed a significant age effect (P < 0.013), but no significant diet effect. In contrast, plasma levels of vitamin E were significantly (65%) lowered by the LOWOX‐B diet already after 1 month (data not shown) and continued to be significantly lower throughout the total duration of the experiment (P < 0.0001, Figure 2B). The presence of elevated oxidative stress levels was assessed by analyses of liver GSH and MDA. Figures 2D and 2E show that there was no significant age effect from 21 to 25 months of age [only a tendency for an age‐related decline in GSH (P = 0.055)]; however, MDA levels were significantly increased after 4 months of LOWOX‐B diet compared with those in control mice (P = 0.018).
Figure 2.
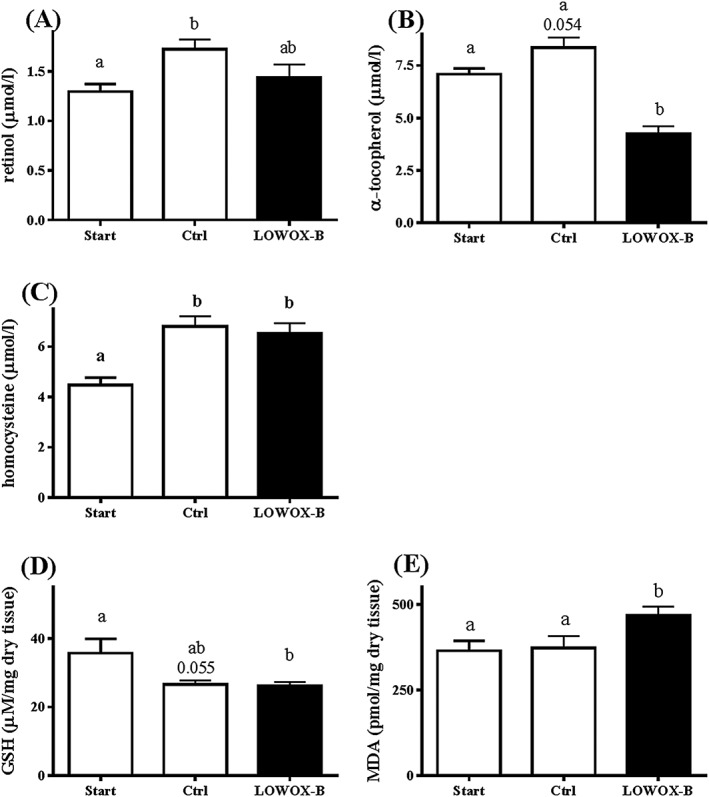
Biomarkers of nutritional and oxidative status at start (t = 0) and after 4 months of LOWOX‐B diet. (A) Vitamin A plasma levels, (B) homocysteine plasma levels, (C) vitamin E plasma levels, (D) hepatic total GSH levels, and (E) hepatic MDA levels. At start n = 11, ctrl n = 6, and LOWOX‐B n = 10. Different characters indicate statistically significant differences (mixed model with post hoc SIDAK, P < 0.05).
Bodyweight, food intake, and body composition
As shown in Figure 3A, bodyweight of the LOWOX‐B mice significantly deviated from control mice from 2 months after start of the diet (P < 0.0001). Food intake did not significantly differ between the diet groups (ctrl: 0.105 ± 0.003 g/g BW/day, LOWOX‐B: 0.110 ± 0.003 g/g BW/day). Lean mass (Figure 3B) and fat mass (Figure 3C) were already significantly decreased in the LOWOX‐B mice after 1 month compared with control mice (resp., P = 0.036 and P < 0.0001). In contrast to the LOWOX‐B mice, control mice significantly increased their fat mass from 21 to 25 months of age (P < 0.002). This was confirmed by the weight of absolute fat storages as shown in Table 2. The sum of hind limb muscles as well as individual muscle weights (Table 3) was not affected by the LOWOX‐B diet.
Figure 3.
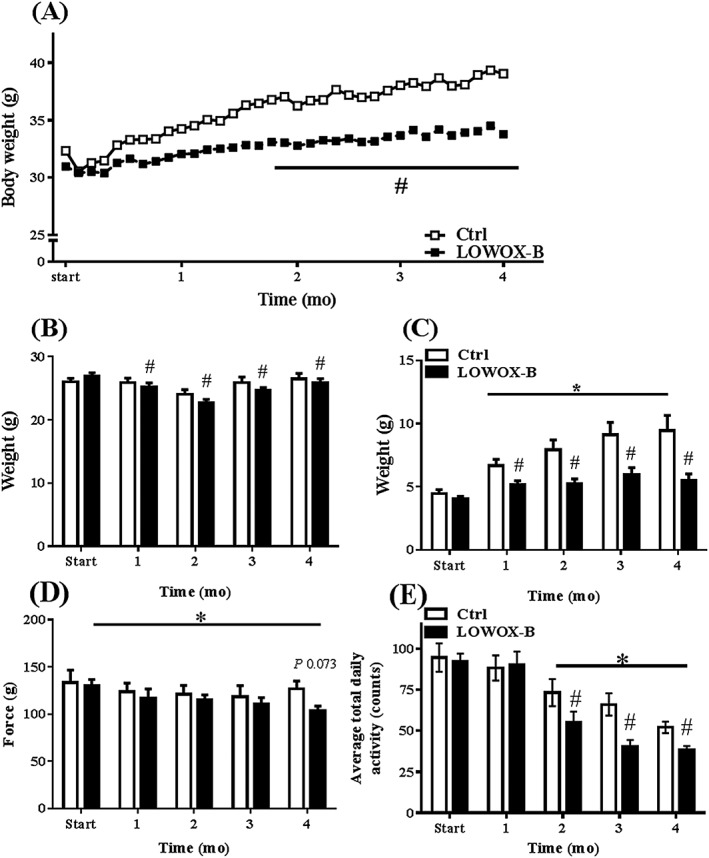
Effects of age and LOWOX‐B diet on body composition and physical strength. (A) Bodyweight development, (B) lean mass, (C) fat mass, (D) absolute maximal forelimb strength, with a trend (P = 0.073) to decreased strength after 4 months of the LOWOX‐B diet, and (E) mean total daily physical activity. At start n = 11, ctrl n = 6 and LOWOX‐B n = 10. *Statistically significant age effect, #statistically significant diet effect at indicated time point (mixed model with post hoc SIDAK, P < 0.05).
Table 3.
Individual muscle weights (mg) and absolute fat mass at different storages (mg)
| Baseline | Control | LOWOX‐B | |
|---|---|---|---|
| Age (months) | 21 | 25 | 25 |
| n | 11 | 6 | 10 |
| Tibialis anterior | 53.13 ± 0.98 | 56.28 ± 1.62* | 58.23 ± 1.83* |
| EDL | 10.97 ± 0.37 | 12.96 ± 0.41* | 12.76 ± 0.54* |
| Soleus | 11.52 ± 0.28 | 10.83 ± 0.42 | 10.08 ± 0.22 |
| Plantaris | 15.95 ± 0.40 | 19.75 ± 0.60* | 19.93 ± 0.52* |
| SUM | 91.57 ± 1.57 | 99.82 ± 2.83* | 100.99 ± 2.55* |
| Epididymis fat | n/m | 1125.82 ± 236.34 | 516.51 ± 78.21** |
| Dorsal fat | n/m | 52.78 ± 7.62 | 31.89 ± 3.40** |
| Brown fat | n/m | 225.43 ± 43.95 | 122.69 ± 10.12** |
| Liver | 1144.72 ± 0.03 | 1355.28 ± 0.16 | 1133.80 ± 0.03 |
| Heart | 149.56 ± 4.13 | 192.18 ± 13.36 | 176.54 ± 6.27** |
| Myeloid lymph nodes | 42.47 ± 6.57 | 49.22 ± 10.74 | 27.73 ± 4.82 |
n/m, not measured.
Statistically significant age effect on muscle mass (no diet effect) from 21 to 25 months of age.
Statistically significant diet effect at 25 months of age (mixed model analysis with post hoc SIDAK, P < 0.05).
Muscle strength and physical activity
Maximal grip strength declined with age (P = 0.04). During 4 months on the LOWOX‐B diet, mice showed significantly lower maximal grip strength compared with control mice (P = 0.013) (Figure 3D). Total daily activity was significantly decreased with ageing, and LOWOX‐B mice showed an even further decrease compared with control mice during ageing (P < 0.001) (Figure 3E). The decrease in total daily activity was due to less activity of the LOWOX‐B mice during the dark (active) period; there were no significant changes in activity levels during the light (inactive) period (control: 114.42 ± 36.18 counts; LOWOX‐B: 102.89 ± 32.54 counts).
Ex vivo muscle function
Force–frequency relationship analysis showed that maximal force production (Figure 4A) was not significantly affected by the LOWOX‐B diet for the total curve (P = 0.089). However, significant differences were found between the diet groups in the lower frequency range at 67 and 83 Hz (resp., P = 0.015 and P = 0.038). The LOWOX‐B mice showed a significantly lower contraction velocity over the full frequency range (P = 0.048, Figure 4B) with no effect on relaxation velocity (P = 0.208, Figure 4C). However, a significantly lower relaxation velocity value in the LOWOX‐B group was observed at individual frequencies of 40, 67, and 167 Hz (resp., P = 0.030, P = 0.025, and P = 0.05, Figure 4C).
Figure 4.
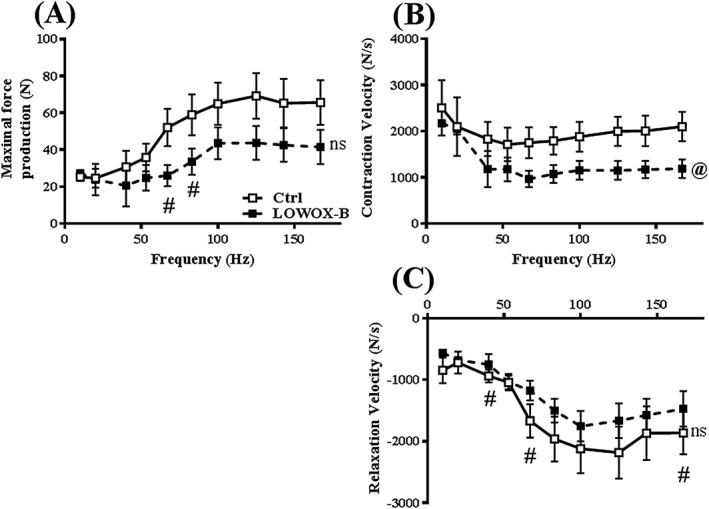
Ex vivo muscle function: effects of 4 month LOWOX‐B diet on force–frequency of EDL muscle. (A) Maximal force production, (B) contraction velocity, and (C) relaxation velocity. Ctrl n = 6 and LOWOX‐B n = 10. @Overall statistically significant diet effect, #statistically significant diet effect at indicated time point (mixed model adjusted for growth curve analysis with post hoc SIDAK, P < 0.05) and ns not significant.
After a moderate exercise protocol, force development and endurance were compromised in LOWOX‐B mice compared with control mice (P = 0.01) (Figure 5A). Moreover, LOWOX‐B mice showed a significantly lower maximal force production during the first 25 contractions of the exercise protocol (P = 0.010, Figure 5B) and lower contraction velocity (P = 0.028, Figure 5C). Relaxation velocity was not affected by the diet (P = 0.278, Figure 5D). LOWOX‐B mice were less resistant to fatigue after the exercise protocol, as indicated by the lower fatigue index (P = 0.023) (Figure 5E).
Figure 5.
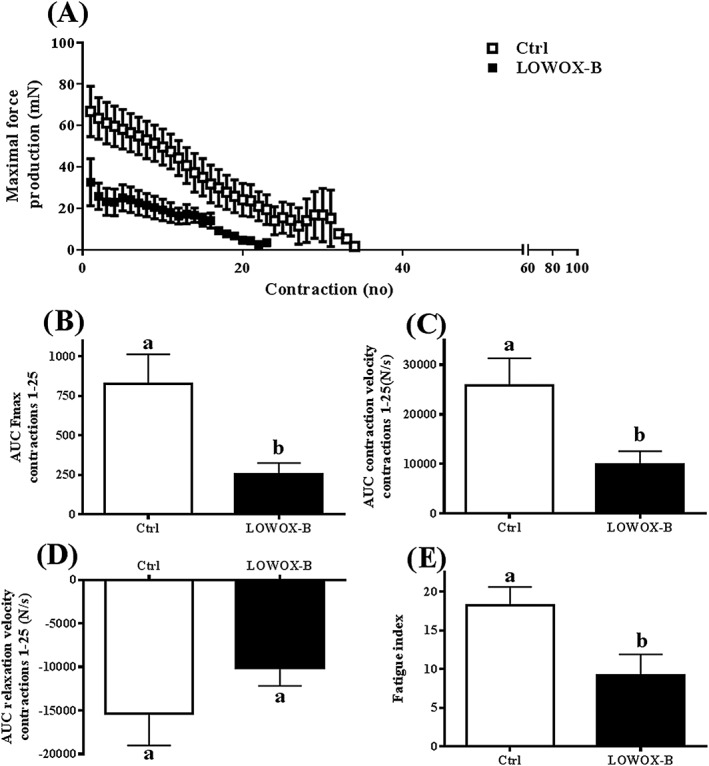
Ex vivo muscle function: effects of 4 month LOWOX‐B diet on exercise performance. (A) Maximal rate of force production, (B) maximal force production during first 25 contractions of exercise protocol, (C) contraction velocity development, (D) relaxation velocity development, and (E) fatigue index. Ctrl n = 6 and LOWOX‐B n = 10. Different characters indicate a statistically significant diet effect (mixed model with post hoc SIDAK, P < 0.05).
Ex vivo muscle protein synthesis and breakdown
As shown in Figure 6A, GM muscle of LOWOX‐B mice did not show an increase in muscle protein synthesis after incubation with leucine when compared with neutral buffer (basal state). This was in contrast to control mice (fasted compared with leucine stimulated, P = 0.039; leucine‐stimulated control vs. LOWOX‐B mice, P = 0.036). Muscle protein breakdown was significantly increased in LOWOX‐B mice compared with control (Figure 6B). In addition to the ex vivo 3‐methylhistidine measurements, MAFbx/atrogin gene expression of the EDL muscle was significantly increased (P = 0.01), and MuRF1 showed a similar trend (Figures 6C and 6D).
Figure 6.
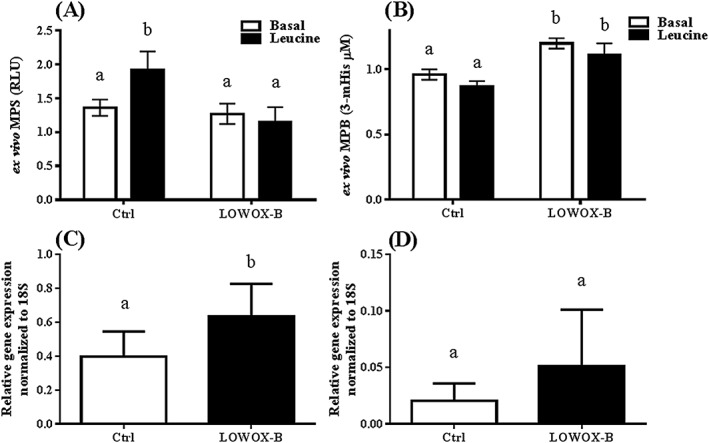
Ex vivo muscle protein synthesis (MPS) and breakdown (MPB). (A) MPS in GM muscles and (B) MPB in buffer of GM muscle, (C) atrogin/MAFbx EDL gene expression and (D) MuRF1 EDL gene expression at 25 months of age after 4 month diet. Ctrl n = 6 and LOWOX‐B n = 10. MPS was measured during incubation with neutral buffer (basal state) and after addition of leucine (anabolic buffer). Different characters indicate a statistically significant difference (mixed model with post hoc SIDAK, P < 0.05).
Plantaris fibre typing, muscle fibre cross‐sectional area, and succinate dehydrogenase activity
Within the PL muscle, a distinction was made between a more oxidative part and a more glycolytic part (Figures 7C and 7F). Close to the proximal aponeurosis, there were more oxidative fibres. Type IIA was hardly found in the glycolytic part of the muscle (Figures 7A and 7D). Besides that, muscle fibre type I was barely present in the PL muscle (Figures 7B and 7E). Therefore, type I muscle fibres were not included in fibre CSA (fCSA) and SDH activity analysis.
Figure 7.
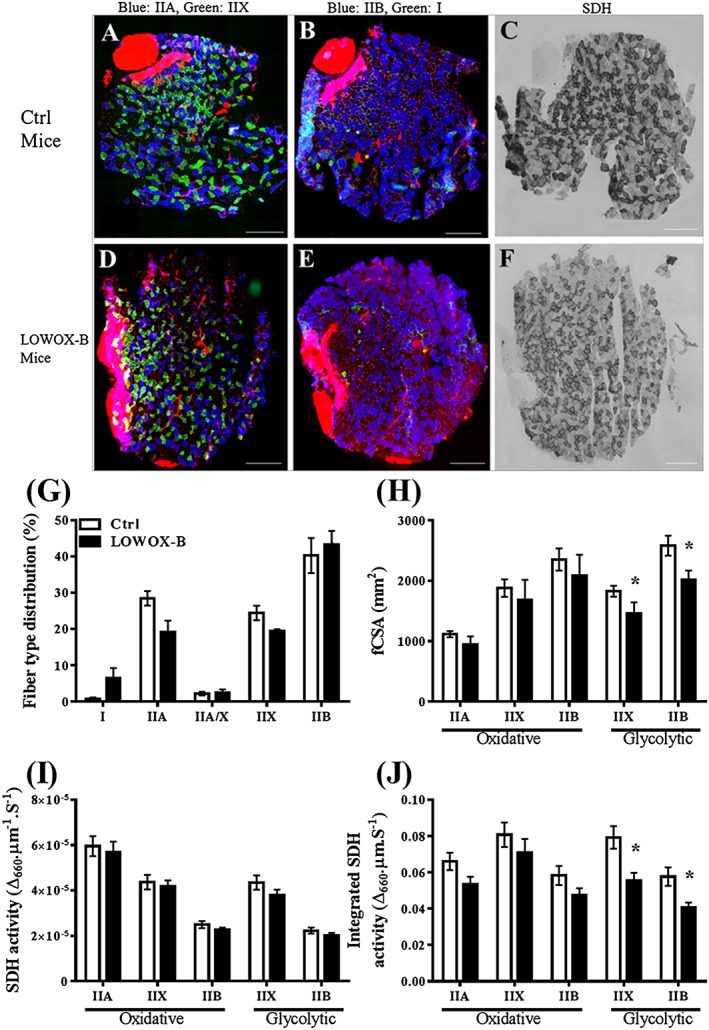
Effects of 4 month LOWOX‐B diet on PL muscle fibre‐type distribution, fibre CSA, and SDH activity. (A–F) Typical examples of PL muscle cross sections with double immunofluorescent labelled muscle fibres for either myosin heavy chain types IIA and IIX (A and D; green type IIA and blue type IIX) or types I and IIB (B and E, green type I and blue type IIB) or stained for SDH activity (C and F). The basal lamina, intramuscular connective tissue, and aponeuroses were stained red by wheat germ agglutinin. Note that around the distal, aponeurosis is the high oxidative region with predominantly type IIA, IIAX, IIX, and IIB muscle fibres. On the outer side, is the low oxidative (glycolytic) region with predominantly type IIX and IIB muscle fibres. (G) Muscle fibre‐type distributions. (H) Mean CSA for the different muscle fibre types. (I) SDH activity in the different muscle fibres types. (J) Spatially integrated SDH activity (i.e. product of muscle fibre CSA and SDH activity). Ctrl: n = 8 and LOWOX‐B: n = 7. Scale bar indicates 300 μm. *Statistically significant difference (P < 0.05).
For both glycolytic and oxidative regions, mean fCSA and SDH activity of each muscle fibre type is presented in Figures 7G–7J. Muscle fibre type showed a significant interaction effect of diet and region (P < 0.003) (Figure 7G). Post hoc analysis showed that types IIX and IIB muscle fibres of the glycolytic region of LOWOX‐B mice were significantly smaller than those of control mice (P < 0.01), whereas fCSA in the oxidative region did not differ between the two diets (Figure 7H). SDH activity in both oxidative and glycolytic region of the PL muscle differed per fibre type (P < 0.001), but was not affected by diet nor region. As Figure 7J shows, the spatially integrated SDH activity differed significantly for muscle fibre type (P < 0.001) as well as for diet (P < 0.028) between LOWOX‐B and control mice. Post hoc analysis showed that integrated SDH activity in the glycolytic region was significantly lower in the LOWOX‐B diet group than in the control group.
Immunological parameters
As shown in Table 4, the immune serum parameters measured did not show any significant difference between control and LOWOX‐B mice. Serum levels of IL‐10 and IL‐1b were below detection levels.
Table 4.
Serum immune parameters (pg/mL)
| Control | LOWOX‐B | |
|---|---|---|
| Age (months) | 25 | 25 |
| n | 6 | 9 |
| TNFα | 135.8 ± 34.6 | 98.5 ± 15.5 |
| IFNγ | 0.632 ± 0.1 | 0.922 ± 0.2 |
| IL‐6 | 7.6 ± 2.0 | 7.2 ± 1.3 |
| IL‐17a | 5.7 ± 0.8 | 5.0 ± 0.6 |
| IL‐15 | 968.6 ± 41.3 | 1152 ± 100 |
| MIP‐2 | 19.21 ± 5.7 | 18.29 ± 1.0 |
| IL‐1b | n.d. | n.d. |
| IL‐10 | n.d. | n.d. |
n.d., not detectable.
Discussion
The current study shows that reduced dietary intake of a combination of vitamins A, E, B6, and B12, folate, selenium, and zinc in aged mice resulted in a lower oxidative capacity and has a major impact on muscle health, by reducing muscle function and physical activity of the mice. The effect on m. EDL function was particularly evident after moderate exercise with decreased maximal force production and increased muscle fatigue. The decreased CSA of the glycolytic fibres of the m. plantaris, in combination with unaltered SDH activity per fibre between the groups, suggests muscle fibre atrophy (i.e. smaller fibres) and reduced muscle mitochondrial capacity, without an effect on total muscle mass. In addition, the rate of muscle protein synthesis in response to leucine was impaired in muscles of the LOWOX‐B mice, while the rate of muscle protein breakdown was increased.
Deficiencies of micronutrients with antioxidant properties (e.g. vitamins A and E, selenium and zinc, B vitamins B6, and B12, and folate) have been associated with frailty and sarcopenia in humans.17, 43, 44, 45 Our study shows that a decrease of these specific micronutrients in the diet of aged mice causes a decrease of serum vitamin E and an increase in liver MDA. These effects indicate increased oxidative stress in the mice kept on the LOWOX‐B diet. The absence of a decrease in plasma vitamin A levels in the LOWOX‐B group is not surprising, because previous studies in mice showed that serum vitamin A concentrations remain normal, even after 20 weeks of vitamin A deprivation.46, 47 Moreover, we observed no effect on plasma homocystein levels after reduced nutritional intake of vitamins B6/B12/folate, indicating that mice were not deficient. However, an age‐related increase in plasma homocystein was observed, which indicates that vitamin B metabolism is already affected by ageing per se.6, 20, 48 Similarly, GSH levels decreased with advanced age, indicative of increased systemic oxidative stress with ageing. The absence of a further decrease in the LOWOX‐B group could be due to a compensatory mechanism. These data show that in these aged mice, oxidative stress was further enhanced due to the micronutrient‐deficient diet.
Our study showed further that physical activity and muscle function were substantially compromised. Grip strength decreased gradually with age and was lowest in the LOWOX‐B group. Because for both groups the mass of all muscles of the lower leg was slightly increased compared with the values of the baseline group at 21 months of age, this indicates that the decrease in force generating capacity from the age of 21 months was mainly due to a reduction in specific force and/or a reduction in the number of muscle fibres. The initial reduction in muscle quality in ageing mice has been reported before.49 Maximal ex vivo EDL tetanic force of the LOWOX‐B group was substantially lower compared with that of the control group (~50%). In addition, we observed atrophy in fast glycolytic fibres from LOWOX‐B PL muscle (~20% compared with control). Due to lack of additional tissue, we have not been able to determine the percentage of atrophy in the EDL muscle. However, we did observe increased atrogin/MAFbx gene expression, suggesting that proteolysis was increased. Combined observations in these two different muscles, we hypothesize that the loss in force generating capacity due to low antioxidants caused both a further reduction in specific force and muscle fibre atrophy.
Moreover, the compromised muscle function was particularly apparent during a moderate exercise protocol, showing lowered maximal force production, lowered contraction velocity, and decreased resistance to fatigue. There have been only a few animal studies reporting the impact of deficiencies of individual or combined micronutrients with antioxidant properties on muscle health.46, 47, 50, 51 This study tested the effect of a combination of deficiencies. Vitamin E and/ or selenium deficiency has been associated with muscle dystrophy or white muscle disease in ruminant livestock,52 with symptoms that include muscle weakness and impaired gait. Vitamin E‐deficient young mice showed ataxia and neuromuscular deficits,51 which could have a negative impact on muscle function. Labazi et al.53 showed that rats deficient of vitamin E displayed increased fatigue and a disabled membrane repair function after being challenged with eccentric contraction‐induced membrane disruptions. The authors, therefore, suggested that vitamin E plays an important role in muscle force generating capacity as a requisite component for plasma membrane repair mechanisms. Our results in the LOWOX‐B group are in line with these findings. Our observation that fatigue resistance was affected as well suggests that mitochondrial membrane may also be compromised by the reduced vitamin E. Note that lowering of daily physical activity and grip strength was already observed in our control mice at advanced age. This indicates that ageing per se has an impact on physical activity and is worsened by increased oxidative stress.
While muscle function was clearly compromised by the LOWOX‐B diet, muscle mass was not affected in the LOWOX‐B group. However, we observed a decrease in CSA of glycolytic fibres with similar SDH activity levels, suggesting that LOWOX‐B mice had smaller and less and/or shorter muscle fibres. The combination of effects on muscle fibre size and oxidative capacity is reflected in the spatially integrated SDH activity, which is representative for the oxidative capacity determining the maximal steady‐state power of a muscle in vivo. The reduction in integrated SDH activity could mean that either the mitochondrial volume density was lower,54 mitochondrial function was compromised55, or both. Of course, mitochondrial functioning depends not only on its density but also on its dynamics.56 Mitochondrial fusion and fission processes were not investigated in this recent study. Nutritional vitamin B6/B12/folate deficiency is likely relevant in this context due to their action as cofactors in enzymatic reactions that are essential for cellular and mitochondrial functions.21
The impaired ex vivo muscle protein synthesis response of GM muscle to leucine on the LOWOX‐B diet and increased muscle protein breakdown as confirmed by increased atrogin/MAFbx gene expression in the EDL muscle point towards a change in muscle (protein) metabolism and reduced anabolic sensitivity. Our findings on impaired muscle protein synthesis after an anabolic stimulus with leucine fit those of the study by Dardevet et al.14, 57 who showed that antioxidant supplementation in combination with leucine has a synergistic effect on muscle protein anabolism in aged rats. Another hypothesis that warrants further research is that endoplasmic reticulum stress may contribute to the impaired anabolic response in these deficient mice.58, 59 In addition, the unfolded protein response may result in non‐functional protein and also impair muscle quality with respect to the force‐generating capacity as well as fatigue resistance.58 Surprisingly, LOWOX‐B mice showed lower body weight, mainly due to limited increase in fat mass as compared with control mice. Because LOWOX‐B mice also showed less activity compared with control mice, it is possible that these LOWOX‐B mice shift their metabolism by burning more fat. However, this needs further investigation, for instance by using metabolic cages and body temperature measurements in future studies.
This study has several strengths and limitations. Because the present study includes a mixture of components with antioxidant capacities and does not take individual components in account, it is difficult to distinguish which component was responsible for which outcome. Nevertheless, the elderly population develops deficiencies for multiple micronutrients, associated with sarcopenia, frailty, and disability,15, 23, 24 which makes this design more applicable to the clinical setting. Indeed, our observations on impaired muscle health are clinically relevant for the ageing human population. Selenium deficiency also plays a role in the immune pathway.52 While the effect of the LOWOX‐B diet apparently is not mediated by an inflammatory response, as observed with antioxidant supplementation,57 local immune responses cannot be excluded. In addition, another mechanism we did not touch upon in this study is the neuronal process affected by vitamin E deficiency.51 To study the impact of diets low in antioxidants on neurological factors requires a new designed experiment, including behavioural tests and/or in situ muscle force measurements. Finally, mice have limited yield of blood to determine parameters of antioxidant deficiencies. Although serum levels did not reflect the changes in diet concentrations, we cannot make conclusions for selenium, zinc, or vitamin B6, because analyses were practically not feasible. To address this limitation, for instance, we chose to analyse homocysteine, a more indirect measure for defects of vitamin B metabolism.20 In addition, the cages in the study were not equipped to minimize coprophagia, which is not optimal for developing deficiencies. The cages were also galvanized, which for the mice may have resulted in exposure to extra minerals. Also, the drinking water was normal tap water and was not demineralized. Finally, subsequent intervention by nutritional supplementation of micronutrients in future studies could further prove the importance of micronutrients in maintaining muscle function at old age. It is noteworthy that the highest dropout was in the normal aged mice. This could be a coincidence, but one could also speculate that the AIN‐93‐M diet provides some vitamin(s) and mineral(s) in excessive amounts, exceeding the daily recommended requirements of lab animals (Table 3).29 For example, too much vitamin A can lead to birth defects, liver abnormalities, central nervous system disorders, and lower bone mineral density.60 This aspect requires further research.
In conclusion, this study shows that reduced dietary intake of vitamins A, E, B6, and B12, folate, selenium, and zinc has a major impact on muscle health as shown by decreased force‐generating capacity and fatigue resistance as well as impaired physical activity, without an effect on muscle mass. The reduced fCSA in combination with a lack of effect on SDH activity suggests that such dietary reductions cause muscle fibre atrophy and reduced muscle oxidative capacity, which explains the observed changes in muscle contractile properties. The results support the hypothesis that micronutrient deficiencies in elderly contribute to impaired muscle health, leading to accelerated ageing.
Conflict of interest
The authors declare that this work has not been previously published elsewhere. Miriam van Dijk, Francina Dijk, Sjors Verlaan, Klaske van Norren, Ardy van Helvoort, and Yvette Luiking are employers of Nutricia Research. Richard Jaspers has no conflict of interest.
Acknowledgements
The authors thank Jolanda Nagel for her assistance with animal care, and Sam Ballak and Guus Baan for their help in the tissue collection. Frank van 't Hoff and Joshua Dunnik are acknowledged for the Immunohistochemistry data analyses and Carla Offringa for her assistance in qPCR. The immunological parameters analyses were performed by Reinilde Loonstra. The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015.61
van Dijk, M. , Dijk, F. J. , Hartog, A. , van Norren, K. , Verlaan, S. , van Helvoort, A. , Jaspers, R. T. , and Luiking, Y. (2018) Reduced dietary intake of micronutrients with antioxidant properties negatively impacts muscle health in aged mice. Journal of Cachexia, Sarcopenia and Muscle, 9: 146–159. doi: 10.1002/jcsm.12237.
References
- 1. Semba RD, et al. Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci 2006;61:594–599. [DOI] [PubMed] [Google Scholar]
- 2. Inzitari M, et al. Nutrition in the age‐related disablement process. J Nutr Health Aging 2011;15:599–604. [DOI] [PubMed] [Google Scholar]
- 3. Bischoff‐Ferrari HA. Relevance of vitamin D in muscle health. Rev Endocr Metab Disord 2012;13:71–77. [DOI] [PubMed] [Google Scholar]
- 4. Marshall TA, et al. Inadequate nutrient intakes are common and are associated with low diet variety in rural, community‐dwelling elderly. J Nutr 2001;131:2192–2196. [DOI] [PubMed] [Google Scholar]
- 5. Ter Borg S, et al. Micronutrient intakes and potential inadequacies of community‐dwelling older adults: a systematic review. Br J Nutr 2015;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ter Borg S, et al. Micronutrient intakes and potential inadequacies of community‐dwelling older adults: a systematic review. Br J Nutr 2015;113:1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harman D. Free radical theory of aging: dietary implications. Am J Clin Nutr 1972;25:839–843. [DOI] [PubMed] [Google Scholar]
- 8. Javadov S, et al. Mitochondria‐targeted antioxidant preserves contractile properties and mitochondrial function of skeletal muscle in aged rats. Oncotarget 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ballak SB, et al. Blunted angiogenesis and hypertrophy are associated with increased fatigue resistance and unchanged aerobic capacity in old overloaded mouse muscle. Age (Dordr) 2016;38:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballak SB, et al. Blunted hypertrophic response in old mouse muscle is associated with a lower satellite cell density and is not alleviated by resveratrol. Exp Gerontol 2015;62:23–31. [DOI] [PubMed] [Google Scholar]
- 11. Short KR, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 2005;102:5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson MJ, McArdle A. Age‐related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol 2011;589:2139–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuthbertson D, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–424. [DOI] [PubMed] [Google Scholar]
- 14. Marzani B, et al. Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats. J Nutr 2008;138:2205–2211. [DOI] [PubMed] [Google Scholar]
- 15. Bartali B et al. Low micronutrient levels as a predictor of incident disability in older women. Arch Intern Med 2006;166:2335–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matteini AM et al. Markers of B‐vitamin deficiency and frailty in older women. J Nutr Health Aging 2008;12:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baik HW, Russell RM. Vitamin B12 deficiency in the elderly. Annu Rev Nutr 1999;19:357–377. [DOI] [PubMed] [Google Scholar]
- 18. van Schoor NM, et al. Cross‐sectional and longitudinal association between homocysteine, vitamin B12 and physical performance in older persons. Eur J Clin Nutr 2012;66:174–181. [DOI] [PubMed] [Google Scholar]
- 19. Allen LH. How common is vitamin B‐12 deficiency? Am J Clin Nutr 2009;89:693S–696S. [DOI] [PubMed] [Google Scholar]
- 20. van Wijngaarden JP, et al. Vitamin B12, folate, homocysteine, and bone health in adults and elderly people: a systematic review with meta‐analyses. J Nutr Metab 2013;2013:486186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Depeint F, et al. Mitochondrial function and toxicity: role of B vitamins on the one‐carbon transfer pathways. Chem Biol Interact 2006;163:113–132. [DOI] [PubMed] [Google Scholar]
- 22. Hoffman M. Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses 2011;77:1088–1093. [DOI] [PubMed] [Google Scholar]
- 23. Ble A, et al. Lower plasma vitamin E levels are associated with the frailty syndrome: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2006;61:278–283. [DOI] [PubMed] [Google Scholar]
- 24. Khor SC, et al. Vitamin E in sarcopenia: current evidences on its role in prevention and treatment. Oxid Med Cell Longev 2014;2014:914853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen YL, et al. Low serum selenium level is associated with low muscle mass in the community‐dwelling elderly. J Am Med Dir Assoc 2014;15:807–811. [DOI] [PubMed] [Google Scholar]
- 26. Lauretani F, et al. Association of low plasma selenium concentrations with poor muscle strength in older community‐dwelling adults: the InCHIANTI Study. Am J Clin Nutr 2007;86:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsao CS, Miyashita K, Leung PY. Effect of ascorbic acid on calcium elimination in humans. J Nutr Sci Vitaminol (Tokyo) 1986;32:437–446. [DOI] [PubMed] [Google Scholar]
- 28. Reeves PG, Nielsen FH, Fahey GC Jr. AIN‐93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN‐76A rodent diet. J Nutr 1993;123:1939–1951. [DOI] [PubMed] [Google Scholar]
- 29. van Zutphen LFM, Baumans V., Beynen AC. Handboek Proefdierkunde. 2001. [Google Scholar]
- 30. Kerksick C, Willoughby D. The antioxidant role of glutathione and N‐acetyl‐cysteine supplements and exercise‐induced oxidative stress. J Int Soc Sports Nutr 2005;2:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Zwaard S, et al. Maximal oxygen uptake is proportional to muscle fiber oxidative capacity, from chronic heart failure patients to professional cyclists. J Appl Physiol (1985), 2016. 121(3): p. 636–45. [DOI] [PubMed] [Google Scholar]
- 32. Pool CW, Diegenbach PC, Scholten G. Quantitative succinate‐dehydrogenase histochemistry. I. A Methodological study on mammalian and fish muscle. Histochemistry 1979;64:251–262. [DOI] [PubMed] [Google Scholar]
- 33. van Norren K, et al. Dietary supplementation with a specific combination of high protein, leucine, and fish oil improves muscle function and daily activity in tumour‐bearing cachectic mice. Br J Cancer 2009;100:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leiter JR, Peeler J, Anderson JE. Exercise‐induced muscle growth is muscle‐specific and age‐dependent. Muscle Nerve 2011;43:828–838. [DOI] [PubMed] [Google Scholar]
- 35. Gorselink M, et al. Mass‐dependent decline of skeletal muscle function in cancer cachexia. Muscle Nerve 2006;33:691–693. [DOI] [PubMed] [Google Scholar]
- 36. Peters SJ, et al. Dose‐dependent effects of leucine supplementation on preservation of muscle mass in cancer cachectic mice. Oncol Rep 2011. [DOI] [PubMed] [Google Scholar]
- 37. Gineste C, et al. Combined MRI and (3)(1)P‐MRS investigations of the ACTA1(H40Y) mouse model of nemaline myopathy show impaired muscle function and altered energy metabolism. PLoS One 2013;8: e61517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Dijk M, et al. Improved muscle function and quality after diet intervention with leucine‐enriched whey and antioxidants in antioxidant deficient aged mice. Oncotarget 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goodman CA, Hornberger TA. Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc Sport Sci Rev 2013;41:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van den Hoven R, et al. A preliminary study on the changes in some potential markers of muscle‐cell degradation in sub‐maximally exercised horses supplemented with a protein and amino acid mixture. J Anim Physiol Anim Nutr (Berl) 2011;95:664–675. [DOI] [PubMed] [Google Scholar]
- 41. Jackson JR, Ryan MJ, Alway SE. Long‐term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. The journals of gerontology. Series A, Biologic Sci Med Sci 2011;66:751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van der Laarse WJ, Diegenbach PC, Elzinga G. Maximum rate of oxygen consumption and quantitative histochemistry of succinate dehydrogenase in single muscle fibres of Xenopus laevis. J Muscle Res Cell Motil 1989;10:221–228. [DOI] [PubMed] [Google Scholar]
- 43. Blount BC, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A 1997;94:3290–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clarke R et al. Screening for vitamin B‐12 and folate deficiency in older persons. Am J Clin Nutr 2003;77:1241–1247. [DOI] [PubMed] [Google Scholar]
- 45. Damms‐Machado A, Weser G, Bischoff SC. Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutr J 2012;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Etchamendy N, et al. Vitamin A deficiency and relational memory deficit in adult mice: relationships with changes in brain retinoid signalling. Behav Brain Res 2003;145:37–49. [DOI] [PubMed] [Google Scholar]
- 47. Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem 2005;280:40226–40234. [DOI] [PubMed] [Google Scholar]
- 48. Verlaan S, et al. Nutritional status, body composition, and quality of life in community‐dwelling sarcopenic and non‐sarcopenic older adults: a case‐control study. Clin Nutr 2015. [DOI] [PubMed] [Google Scholar]
- 49. Ballak SB, et al. Plantaris muscle weakness in old mice: relative contributions of changes in specific force, muscle mass, myofiber cross‐sectional area, and number. Age (Dordr) 2014;36:9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jinno N, Nagata M, Takahashi T. Marginal zinc deficiency negatively affects recovery from muscle injury in mice. Biol Trace Elem Res 2014;158:65–72. [DOI] [PubMed] [Google Scholar]
- 51. Ouahchi K, et al. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha‐tocopherol transfer protein. Nat Genet 1995;9:141–145. [DOI] [PubMed] [Google Scholar]
- 52. Hooven LA et al. Microarray analysis of selenium‐depleted and selenium‐supplemented mice. Biol Trace Elem Res 2006;109:173–179. [DOI] [PubMed] [Google Scholar]
- 53. Labazi M, et al. The antioxidant requirement for plasma membrane repair in skeletal muscle. Free Radic Biol Med 2015;84:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scheffler TL, et al. Fiber hypertrophy and increased oxidative capacity can occur simultaneously in pig glycolytic skeletal muscle. Am J Physiol Cell Physiol 2014;306:C354–C363. [DOI] [PubMed] [Google Scholar]
- 55. Pietrangelo L, et al. Age‐dependent uncoupling of mitochondria from Ca2(+) release units in skeletal muscle. Oncotarget 2015;6:35358–35371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol 2015;4:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mosoni L, et al. Antioxidant supplementation had positive effects in old rat muscle, but through better oxidative status in other organs. Nutrition 2010;26:1157–1162. [DOI] [PubMed] [Google Scholar]
- 58. Chalil S, et al. Increased endoplasmic reticulum stress in mouse osteocytes with aging alters Cox‐2 response to mechanical stimuli. Calcif Tissue Int 2015;96:123–128. [DOI] [PubMed] [Google Scholar]
- 59. Deldicque L, et al. ER stress induces anabolic resistance in muscle cells through PKB‐induced blockade of mTORC1. PLoS One 2011;6: e20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Beste LA, et al. Clinical problem‐solving. Too much of a good thing. N Engl J Med 2016;374:873–878. [DOI] [PubMed] [Google Scholar]
- 61. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


