Abstract
Background
Forkhead Transcription Factor L2 (FOXL2) is a member of the forkhead family with important roles in reproduction. Recent studies showed that FOXL2 is expressed in human and bovine endometrium and that its levels fluctuate during pregnancy. In this study, we aimed at evaluating the expression and function of FOXL2 in embryo implantation.
Methods
Mouse uteri at different days of pregnancy were isolated and analyzed for the expression and localization of FOXL2. A lentiviral strategy was further employed to either knockdown or overexpress FOXL2 in non-receptive human endometrial AN3-CA cells and in receptive Ishikawa cells, respectively. These genetically modified cells were compared to cells infected with a control lentivirus to determine the function of FOXL2 in trophectoderm cells adherence to Endometrial Epithelium was associated with the expression of genes known to be involved in acquisition of uterine receptivity.
Results
We report that FOXL2 is expressed in both, the luminal epithelium and the myometrium of the mouse uterus and that its expression declines prior to implantation. We found that endometrial cells expressing low FOXL2 levels, either endogenous or genetically manipulated, were associated with a higher attachment rate of mouse blastocysts or human Jeg3 spheroids and mouse blastocysts. In accordance, low-FOXL2 levels were associated with changes in the expression level of components of the Wnt/Fzd and apoptotic pathways, both of which are involved in uterine receptivity. Furthermore, FOXL2 expression was inversely correlated with G-protein signaling protein 2 (Rgs2) and cytokine expression.
Conclusions
These results suggest that FOXL2 interferes with embryo attachment. Better understanding of the function of FOXL2 in the uterus would possibly suggest novel strategies for treatment of infertility attributed to repeated implantation failure.
Electronic supplementary material
The online version of this article (10.1186/s12958-018-0329-y) contains supplementary material, which is available to authorized users.
Keywords: FOXL2, Uterine receptivity, FOXL2 depletion, FOXL2 overexpression, Embryo attachment, Gene expression, Cell adherence
Background
Forkhead transcription factor L2 (FOXL2) is a member of the forkhead family of transcription factors which plays an indispensable role in differentiation of the embryonic gonads into ovaries [1]. Haplo insufficiency of FOXL2 function, resulting from mutations of the FOXL2 gene has been shown to cause the blepharophimosis–ptosis–epicanthus inversus syndrome (BPES), a genetic disorder characterized by eyelid and mild craniofacial abnormalities, associated with premature ovarian failure in a subset of affected women [2].
The use of DNA chip and quantitative RT-PCR (qRT-PCR) to detect its potential transcriptional targets in granulosa-like cells, revealed that Foxl2 affects the expression of genes involved in reactive oxygen species (ROS) detoxification, inflammation and apoptosis [3, 4].
Later studies suggest that Foxl2 regulates granulosa cell proliferation [5] and ovarian G-protein signaling protein 2 (RGS2) [3, 6]. These multi-functional GTPase-accelerating proteins inactivate the alpha-subunit of G proteins and rapidly switch off the G protein-coupled receptor signaling pathways by promoting GTP hydrolysis [7, 8]. Another recent study found that FOXL2 directly modulates estrogen receptor beta (ESR2) expression through a newly identified intronic element [4]. FOXL2 has also been shown to regulate the expression of follistatin and thereby alters activin and SMAD3 signaling, which are key players in the regulation of reproductive functions by their actions in the ovary and the pituitary [9, 10]. In many species activities of the TGFβ super-family members, including activin-like molecules, play a pivotal role in endometrial remodeling, which is essential for placentogenesis during the peri-implantation period [11]. Interestingly, FOXL2 in the murine pregnant uterus, is exclusively expressed in the implantation sites [7].
The expression of FOXL2 has been shown in human myometrium at term [8]. More recently its expression in human endometrium was reported [12] and its downregulation during the pre-receptive to receptive transition has been described [13]. Another paper demonstrated that FOXL2 expression is downregulated in human endometrial cells upon their co-culture with trophoblast cells [14]. A recent study demonstrated that FOXL2 is expressed in the mouse neonatal mesenchyme and that expression persists in the stroma and the deep inner myometrial layer during uterine maturation [15]. In the adult mouse, FOXL2 is expressed in the differentiated stromal layer [15]. This study further showed that conditional deletion of Foxl2 in the postnatal uterus results in infertility, reduced thickness of the stroma layer and a hypertrophic, disorganized inner myometrial layer [15]. Furthermore, the supplementary muscular layer fails to form a coherent layer around uterine arteries in mice with postnatal targeted deletion of Foxl2 [15].
In the present study, we hypothesized that Foxl2 might play a role in uterus remodeling, preparing the uterine wall for implantation. To challenge this hypothesis we aimed at evaluating the expression and exploring its specific function in regulating critical processes associated with embryo implantation, such as uterine cell proliferation, genes that are involved in apoptosis, and genes that are involved in embryo-maternal recognition, such as Rgs2 transcript. Our experiments demonstrated that that FOXL2 expression in the mouse uterus is modified along pregnancy. Its significant decline towards implantation is consistent with our findings that endometrial FOXL2 levels inversely correlate with the rate of embryo attachment. These findings go along with the effects of FOXL2 on the expression of genes implicated in uterine maturation and embryo attachment.
Methods
Animals
To examine FOXL2 expression during pregnancy, sexually mature, cycling female C57BL/6 mice (7–9 wk. old) were purchased from Harlan (Harlan Laboratories, Rehovot, Israel). The females were mated with C57BL/6 male. The next morning, the females were monitored for vaginal plug (indicating day 0.5 of pregnancy). Uteri were isolated at days 3, 4, 6, 12 and 18 of pregnancy and further analyzed.
In all experiments, three independent repeats were performed as follows: for each time point, uteri, implantation sites and placentas from 3 different random animals were collected. Then, the uteri, placenta or implantation sites collected from the 3 animals at each time point were pooled together and the pool was subjected to further analysis. Thus, a total of 9 uteri/implantation sites/placenta from 9 different random animals were used for each lane.
Cells
The AN3-CA cells, non-receptive human endometrial cells (cell line obtained from CLS Cell Line Services GmbH, Eppelheim, Germany), were grown in MEM (Biological Industries, Israel) with 10% fetal bovine serum (FBS, Hyclone, Biological Industries, Israel), Ishikawa cells, receptive human endometrial cells (cell line obtained from ATCC, Manassas, VA), were grown in DMEM (Biological Industries, Israel) with 10% FBS. Both cell lines were grown at 37 °C under 5% CO2.
shRNA-mediated knockdown of FOXL2
The strategy used to achieve siRNA-mediated Foxl2 knockdown was essentially as described previously [9, 16]. Briefly, lentiviral particles harboring Foxl2-targeted shRNA cassettes or scrambled control shRNA in tandem with IRES-controlled GFP cDNA were prepared using HEK293T as described previously [16] and used to infect AN3-CA cells. The cells were expanded under normal growth conditions and monitored for GFP expression. To achieve uniformity of knockdown for functional studies, approximately 107 cells expressing either GFP alone, scrambled shRNA as control or Foxl2 shRNA were subjected to fluorescence-activated cell sorting (FACS) on a FACSVantage SE DiVa (BD Biosciences, San Jose, CA) equipped with a 488-nm argon laser. Initial gating was based on forward scatter and side scatter to maximize recovery of live single cells. According to the fluorescence intensity histogram of each population of infected AN3-CA cells, the top 5% of GFP+ cells were sorted and collected and expanded for further analysis.
Lentivirus mediated method of FOXL2 overexpression
Viral particules harboring Foxl2 cDNA for overexpression or control vector were prepared using HEK293T as described previously [9]. Briefly, Ishikawa cells were infected with control or FOXL2 overexpressing lentivirus that incorporates IRES-driven GFP as a marker. To achieve uniformity of overexpression for functional studies, the transduced Ishikawa cells were FACS sorted as described above and the top 5% of GFP+ cells were collected and expanded for further analysis.
Establishing a spheroids-endometrial cell attachment assay
The in vitro model for implantation was performed as described previously [17]. Briefly, Jeg3 cells, a human trophoblast cell line, were cultured in a humid atmosphere containing 5% CO2 at 37 °C on a shaker for 24 h. The resulting spheres were stained using calcein-AM (BD Bioscience, San Jose, CA), and were monitored using G-BOX gel-imager (syngene, Cambridge, UK), in order to test their viability and to count them. Then, labeled Jeg3 spheroids of 50–200 μm in diameter, similar in size to an implanting blastocyst, were transferred to the upper surface of the confluent monolayer of Ishikawa or AN3-CA endometrial cells, with or without Foxl2 overexpression or knockdown (approximately 50 spheroids/well), and the co-cultures were maintained for 6 h at 37 °C. At the end of incubation, non-adherent spheroids were removed by washing the culture plates and the plates were examined using a G-BOX gel-imager (syngene, Cambridge, UK). The number of tightly attached spheroids in each well was counted using the GeneTools software. The percentage of attached spheroids relative to the total number of spheroids used to initiate the co-incubation experiments (adhesion percent) was calculated.
Embryo attachment assay
Embryo attachment assay was performed as described previously [18]. Briefly, Wild-type ICR females were purchased from Harlan and super-ovulated by sub-cutaneous injections of 5 units of pregnant mare’s serum gonadotropin (PMSG, Chrono-gest Intervest, The Netherlands) followed by 5 units of intraperitoneal injection of human chorionic gonadotropin (hCG, Chrono-gest Intervest, The Netherlands) 48 h later and then mated with wild-type ICR males. Morula and blastocyst stage embryos were collected from the females at 3.5 d after copulation and then incubated in KSOM medium to obtain expanded blastocysts. Embryos were labeled with Vybrant Cell-Labeling Solution (ThermoFisher Scientific, Waltham, MS) before transferring unselectively to confluent Ishikawa (infected with control or Foxl2 overexpression virus or AN3-CA cells infected with control or Foxl2 siRNA virus) cell monolayers in a 96-well plate coated with Matrigel (In vitro technologies, Victoria, Australia). Between 3 and 4 blastocysts were transferred per well depending upon the total number recovered. Co-cultures were incubated undisturbed at 37 °C in a 5% CO2 atmosphere for 48 h. The stability of embryo attachment was measured by washing the culture plates and shaking them three times from side to side. Each measurement was performed manually under a microscope (Nikon, Tokyo, Japan), by examining the stability of each mouse embryo upon tapping the stage. The percentage of attached embryos relative to the total number of embryos used to initiate the co-incubation experiments (adhesion percent) was calculated (Additional file 1: Figure S1).
Protein extraction and western blot analysis
Proteins from uteri were extracted at the indicated time points in RIPA buffer using homogenizer, and suspended in Laemmli loading buffer (125 mM Tris, pH 6.8, 4% SDS, 10% glycerol, 0.006% bromphenol blue, and 2% β-mercaptoethanol). The proteins were then separated on a 12% acrylamide gel, followed by their transfer to a nitrocellulose membrane. After blocking with 5% skimmed milk, the membranes were incubated with primary antibodies over-night at 4 °C (rabbit anti-FOXL2 1:1000, Bioss, Woburn, MA; rabbit anti-β-ACTIN 1:2000, Thermo Scientific, Waltham, MS), then with the secondary antibodies for 1 h at room temperature (anti-rabbit 1:5000, Jackson laboratory, Bar Harbor, MI). The immunoreactive bands were detected by ECL (Amersham, England).
RNA extraction and analysis by PCR
Total RNA from uteri and endometrial cell lines was extracted using RNeasy mini columns (Qiagen, Hilden, Germany), according to the manufacturer’s guidelines. RNA was converted into cDNA with the High-Capacity cDNA Reverse transcription kit (Applied Biosystems), according to the manufacturer’s guidelines using oligo (dT) and Moloney murine leukemia virus reverse transcriptase. The cDNAs were used for PCR amplification with primer sets for Foxl2 and Hprt (Additional file 2: Table S1) in a 25 μl reaction volume, with 10× buffer, 400 μM of each d-NTP and 0.625 units of Taq DNA Polymerase (Fisher Scientific, Waltham, MA). PCR was performed for the indicated number of cycles (initial denaturation at 94 °C for 3 min, then 35 cycles at 94 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min, and a final incubation at 72 °C for 7 min). The reaction mix (10 μl) was run on 1.5% agarose gels, stained with Ethidium Bromide and quantified using UV imaging (Gel Doc 1000, Bio-Rad) and Molecular Analyst software (Bio-Rad, Hercules, Ca.). Experimental replication of each time point was performed in triplicate.
Quantitive RT-PCR
All real-time PCRs were carried out on a step one plus (ThermoFisher Scientific, Waltham, MS), using the Absolute Blue QPCR Master Mix (ThermoFisher Scientific, Waltham, MS) with SYBR Green. The following is the reaction protocol: 15 min at 95 °C for enzyme activation, followed by 40 cycles of: 15 s at 95 °C, 30 s at 60 °C, and 15 s at 72 °C, at the end of which fluorescence was measured with the Rotor-Gene. SYBR Green-I assays also included a melt curve at the end of the cycling protocol, with continuous fluorescence measurement from 65 to 99 °C. All reactions contained the same amount of cDNA, 10 μl Absolute Blue QPCR Master Mix, primers for the indicated genes (Additional file 2: Table S1) and UltraPure PCR-grade water (Biological Industries, Israel) to a final volume of 20 μl. Each real-time PCR included a no-template control, in duplicate. Relative expression levels (ΔΔCt) were calculated by normalizing to hypoxanthine guanine phosphoribosyl transferase (HPRT). Primers were designed using the primer3 website (http://bioinfo.ut.ee/primer3-0.4.0/).
Immunofluorescence staining
FOXL2 immunofluorescence was performed on deparaffinized uterine sections isolated from non-pregnant C57/BL6 females. The sections were washed in PBS (Biological Industries, Israel), followed by antigen retrieval by standard sodium citrate method. Non-specific binding sites were blocked by incubating the sections for 30 min in 20% fetal bovine serum (Biological Industries, Israel), 0.2% Triton X100 (Sigma, Rehovot, Israel) in PBS. Sections were then incubated overnight at 4 °C with anti-FOXL2 antibody (anti-FOXL2 rabbit polyclonal antibody, 1:50, Biosis, Woburn, MA). Sections were washed with PBS and immunoreacted with Cy3-conjugated anti-rabbit IgG in 2% normal horse serum and PBS for 60 min (dilution 1:150, Jackson Laboratories, Bar Harbor, MI). The sections were subsequently washed with PBS and visualized, using fluorescence microscope (Nikon, Tokyo, Japan). All images were taken in identical conditions.
Statistical analysis
For statistical comparisons, replicate experiments were averaged and using Student’s 2 tailed unpaired t-test and considered statistically different when P < 0.05. These statistical analyses were conducted with JMP software (SAS Institute, 2005). All numerical data shown in the figures are from representative experiments expressed as the means −/+S.E.M of replicates.
Results
FOXL2 expression and its localization in the mouse uterus
Our initial experiments were directed at the analysis of the spatio-temporal expression of FOXL2 in the mouse uterus during pregnancy. We found that the FOXL2 protein, which is high upon establishment of pregnancy (days 3 and 4, vaginal plug is day 0.5), significantly decreases prior to implantation (occurring on pregnancy day 4.5 (implantation occurs at day 4.5, vaginal plug is day 0.5, Fig. 1a-b).
Fig. 1.
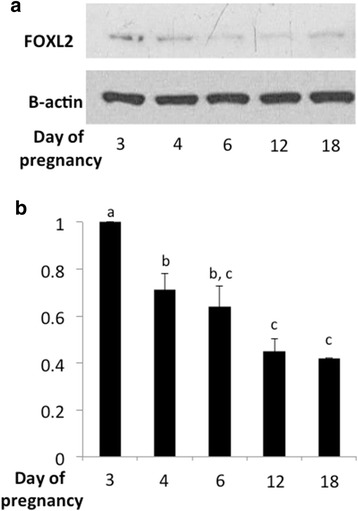
FOXL2 expression in the murine uterus during pregnancy. a FOXL2 protein levels decrease prior to implantation (implantation occurs at pregnancy day 4.5). Western blot analysis of FOXL2 protein using β-actin as a loading control. The results represent one out of 3 independent experiments with similar results. b Quantitation of three independent experiments with similar results. Different letters represent significant differences (p < 0.05)
In the bovine, FOXL2 has been localized to uterine endometrial, stromal and glandular cells [19]. In agreement, using anti-FOXL2, our immunofluorescence analysis of non-pregnant murine uterine sections showed that FOXL2 localizes to the myometrium, glandular epithelium and the luminal epithelium of (Fig. 2a-b). Analysis of pregnant mouse uterine tissue at embryonic day 4.5 revealed that FOXL2 is expressed by the luminal epithelium as well as by the embryo (Fig. 2c). Interestingly, this experiment showed that FOXL2 could also be detected at the attachment area of the embryo with the luminal epithelium. However, the expression of this protein in luminal epithelial cells surrounding the attached blastocysts is substantially lower than that in the luminal epithelial cells away from the implantation site (Fig. 2c). The specificity of the FOXL2 antibody was confirmed by the absence of signal in sections incubated without the primary anti-FOXL2 antibody (Fig. 2d) The decrease in FOXL2 expression in granulosa cells as folliculogenesis progresses (Additional file 3: Figure S2) confirmed the findings in a previous study [20] and was used as a complementary experiment for specificity demonstration.
Fig. 2.
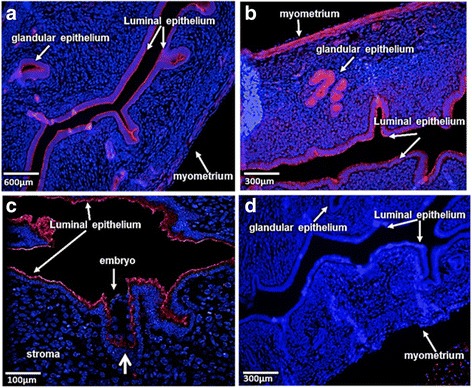
FOXL2 Localization in the Uterus. a FOXL2 is localized to the luminal epithelium, myometrium and glandular epithelium in the non-pregnant uterus of a female mouse. b A closer view of FOXL2 localization in the non-pregnant uterus. c FOXL2 is localized to the luminal epithelium, and to the embryo on day E4.5 of pregnancy. The thick arrow indicates reduced expression of FOXL2 in uterine cells surrounding the attached embryo. d No staining is detected in sections incubated without the primary antibody, anti-FOXL2. Blue = DNA staining (DAPI), RED = FOXL2
Analysis of uterine tissue from healthy women showed that FOXL2 is present in human endometrial tissues [12]. We analyzed two human endometrial cell lines, AN3-CA and Ishikawa cells, and confirmed by RT-PCR that both lines express FOXL2 mRNA (Additional file 4: Figure S3A).
Establishment of FOXL2 depleted or FOXL2 overexpressing cells
Our next experiments were directed at the validation of human endometrial cell lines models to be employed for further evaluation of the function of FOXL2 in embryo implantation. We used two human endometrial cell lines, AN3-CA and Ishikawa cells that are considered to be representative of non-receptive and receptive endometrium, respectively ([21, 22] and our unpublished data). Interestingly, the two cell lines differ by the level of endogenous FOXL2, with significantly higher levels of Foxl2 mRNA in AN3-CA non-receptive endometrial cells (Fig. 3 and Additional file 4: Figure S3b). In order to assess the effect of FOXL2 on endometrial functions, we manipulated Foxl2 levels in the two cell lines by knocking down Foxl2 in AN3-CA cells by shRNA expression using lentiviral particles harboring Foxl2 -targeted shRNA (shFOXL2) cassettes and, conversely, overexpressing Foxl2 in Ishikawa cells using lentiviral particles harboring the FOXL2 cDNA. These strategies resulted in a 60–70% reduction of Foxl2 mRNA levels in AN3-CA cells infected with sh Foxl2 -encoding lentivirus (Additional file 1: Figure S1A) and as much as a 25-fold increase in Foxl2 mRNA levels in Ishikawa cells infected with Foxl2 overexpressing virus, compared to cells infected with a control virus (Additional file 1: Figure S1B). We used these modified and the parent cell lines to examine the effect of Foxl2 on embryo attachment.
Fig. 3.
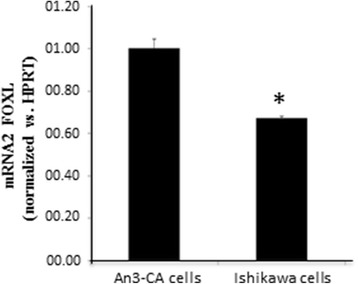
Foxl2 expression in uterine cell lines. qRT-PCR of Foxl2 mRNA expression in AN3-CA and Ishikawa endometrial cell lines. Endogenous expression in Ishikawa cells is significantly lower
Attachment of murine embryos and Jeg3 spheroids to human endometrial cells with FOXL2 either depletion or overexpression
The effect of changes in Foxl2 abundance on embryo attachment was assessed by an in vitro adhesion assay (Additional file 5: Figure S4A-C, [18]). We found that the rate of embryo attachment to Foxl2 -depleted AN3-CA was higher than that to control AN3-CA cells (Fig. 4a). By contrast, overexpression of Foxl2 in Ishikawa cells led to a reduction in embryo attachment compared to control Ishikawa cells (Fig. 4b). In a complementary experiment we used the in vitro implantation assay to examine Jeg3 human spheroid attachment to the same endometrial cell lines with either high or low FOXL2 protein expression. Similar to our results with mouse embryo attachment, we observed that, Jeg3 spheroid attachment to Foxl2 -depleted AN3-CA cells was higher than that to control AN3-CA cells (Fig. 4c). Conversely, overexpression of Foxl2 in Ishikawa cells led to inhibition of spheroid attachment compared to control Ishikawa cells with lower FOXL2 levels (Fig. 4d). These observations support the possibility that the reduced FOXL2 protein expression seen around the time of implantation (Fig. 1b) contribute to the process of implantation by allowing the attachment of the embryo to the endometrial epithelium.
Fig. 4.
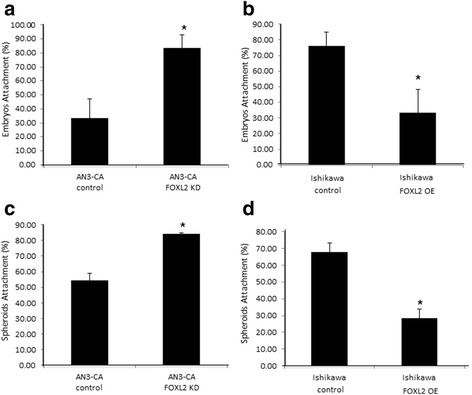
Foxl2 effects in an in vitro model of attachment. a Mouse embryo attachment is higher after Foxl2 knockdown in AN3-CA cells. b Overexpression of Foxl2 in Ishikawa cells reduces mouse embryo attachment. c Jeg3 spheroid attachment is higher after Foxl2 knockdown in AN3-CA. d Overexpression of Foxl2 in Ishikawa cells decreases Jeg3 spheroid attachment. * p < 0.05
FOXL2 effects on the expression of members of the wnt/fzd family
The Wnt/Fzd signaling pathway has been shown to play a role in embryo implantation and stromal cell differentiation [23]. We therefore evaluated the influence of FOXL2 on the expression levels of representative members of the Wnt/Fzd family, Wnt11 and Fzd6, shown to be expressed in the mouse uterus before and around the time of implantation [24]. Using the cellular models described above (Fig. 3 and Additional file 1: Figure S1), we show that Foxl2 levels are inversely correlated with the expression levels of Fzd6 and Wnt11 mRNA. We found that both Fzd6 and Wnt11 mRNA are expressed in significantly lower levels in AN3-CA, which contain higher endogenous Foxl2, than in Ishikawa cells, which express lower Foxl2 (Fig. 5a and d). Knockdown of Foxl2 by siRNAs in AN3-CA cells led to an increase in both, Fzd6 mRNA (Fig. 5b) and Wnt11 mRNA (Fig. 5e), while overexpression of Foxl2 in Ishikawa cells led to a reduction in Fzd6 mRNA (Fig. 5c) as well as Wnt11mRNA (Fig. 5f) expression. We also determined the effect of Foxl2 levels on the expression of antagonists of this pathway. Indeed, these experiments revealed an opposite effect of FOXL2 on the expression Kremen2 mRNA, a coreceptor for Dickkopf 1 and 2, which are Wnt/Fzd signaling antagonists. Cells with lower Foxl2 expression, either endogenous (Fig. 5g) or genetically manipulated (Fig. 5h), had exhibited lower Kremen2 mRNA levels (Fig. 5g) whereas FOXL2 overexpression led to induction of Kremen2 mRNA (Fig. 5i).
Fig. 5.
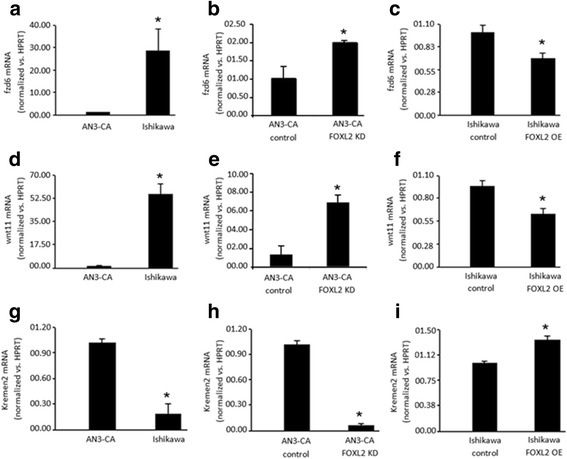
Foxl2 effect on the expression of Wnt/Fzd family members. a, d, g The expression of Fzd6 and Wnt11 is higher, while the expression of Kremen2, a Wnt/Fzd family inhibitor, is lower in receptive endometrial Ishikawa cells, which express low Foxl2 levels, compared to AN3-CA endometrial non-receptive cells, which express higher levels of Foxl2. b, e, h Knockdown of Foxl2 in AN3-CA leads to an increase in Fzd6 and Wnt11 expression and a reduction in Kremen2 levels. c, f, i Overexpression of Foxl2 in Ishikawa cells leads to a decrease in Fzd6 and Wnt11 levels but elevates Kremen2 levels. * p < 0.05
FOXL2 regulation of genes involved in cellular stress responses and apoptosis
Uterine cell apoptosis is an important process for normal uterine function [25]. FOXL2 has been implicated in the regulation of processes such as apoptosis, by affecting the expression of relevant genes, including IER3, TNFAIP3 and ATF3 [3]. We evaluated if FOXL2 is also involved in the regulation of these genes in the endometrium. We found that TNFAIP3 mRNA (Fig. 6a and b) and ATF3 mRNA (Fig. 6d and e) are expressed at higher levels in cells with lower levels FOXL2, namely Ishikawa cells or AN3-CA cells following FOXL2 knockdown. Overexpression of FOXL2 in Ishikawa cells, on the other hand, was associated with a reduction in TNFAIP3 and ATF3 mRNA levels (Fig. 6c and f). By contrast, IER3 mRNA levels were lower in cells with low endogenous FOXL2 (Ishikawa cells) and induced upon FOXL2 overexpression (Fig. 6g and i). AN3-CA cells with FOXL2 depletion, exhibit tendency to express lower levels of IER3 (Fig. 6h).
Fig. 6.
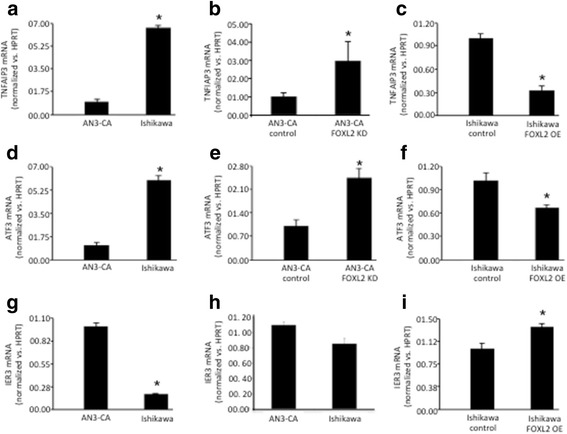
Foxl2 effect on the expression of genes involved in apoptosis. a, d, g The expression of Tnfip3 and Atf3 is elevated, while the expression of Ier3 is decreased, in receptive endometrial Ishikawa cells, expressing low Foxl2 levels as compared to AN3-CA, endometrial non-receptive cells, expressing high levels of Foxl2. b, e, h Tnfip3 and Atf3 expression is reduced, while the expression of Ier3 is elevated in AN3-CA after Foxl2 knockdown. c, f, i Overexpression of Foxl2 in Ishikawa cells is associated with a decrease in Tnfip3 and Atf3 levels and increase in Ier3 levels. * p < 0.05
FOXL2 effects on genes involved in embryo implantation and uterus receptivity
Uterus receptivity, embryo-maternal recognition and embryo implantation are processes regulated by multiple embryonic and maternal uterine genes. Of these, one of the most intriguing is RGS2, the expression of which in murine pregnant uterus is exclusive to the implantation sites [7]. Another gene that has been demonstrated to play a role in the crosstalk between the embryo and the uterus is the chemokine, CXCl1 [26]. Interestingly, both RGS2 and CXCl1 were reported to be targets of FOXL2 in the ovary [3]. We examined the mRNA expression of both Rgs2 and Cxcl1 in our low- and high-FOXL2 expressing cell lines. We found that Rgs2 mRNA levels are higher in the Ishikawa cells, which express lower levels of FOXL2 and are considered to be representative of a receptive endometrium, as compared to AN3-CA cells, a non-receptive endometrial cell type with higher levels of endogenous FOXL2 (Fig. 7a). Consistent with this trend, Rgs2 mRNA levels were increased or reduced following FOXL2 knockdown in AN3-CA cells or FOXL2 overexpression in Ishikawa cells, respectively (Fig. 7b and c). Similar to the pattern seen for Rgs2, high or low FOXL2 protein expression was inversely associated with low or high Cxcl1 mRNA expression, respectively (Fig. 7d-f).
Fig. 7.
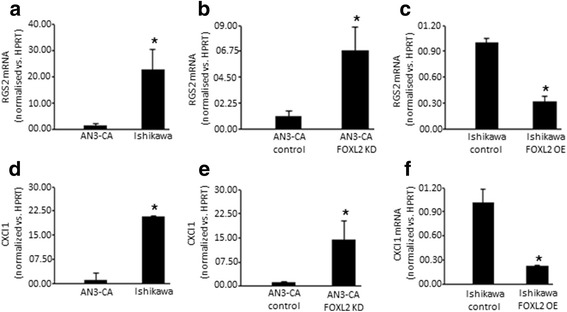
Foxl2 effect on the expression of genes involved in embryo implantation. The expression of Rgs2 and Cxcl1 is elevated in receptive endometrial Ishikawa cells, expressing low Foxl2 levels as compared to AN3-CA, endometrial non-receptive cells, expressing high levels of Foxl2, (a, d) as well as in AN3-CA Foxl2 depleted cells (b, e). Overexpression of Foxl2 in Ishikawa cells resulted in a decrease in Rgs2 and Cxcl1 levels (c, f). * p < 0.05
Discussion
We demonstrate in this study that FOXL2 is expressed by both human endometrial cells and mouse uteri, and that FOXL2 protein expression in the mouse uterus is pregnancy-stage dependent. We found that FOXL2 is localized to the luminal and glandular epithelium as well as the myometrium of both, non-pregnant and pregnant female mice. FOXL2 expression has been only recently discovered in mouse, human and bovine uteri [12, 15, 27], thus little is known about its role in this tissue. To unveil the functions of FOXL2 in the uterus, we evaluated its influence on the attachment of mouse embryos and spheroids generated from the human trophoblast cell line JEG3 to endometrial cells expressing either low or high FOXL2 levels. Employing complementary experimental models we show an inverse correlation between the abundance of FOXL2 in the endometrial cells and the success rate of trophectoderm cells adherence to Endometrial Epithelium. Our experiments also reveal negative effects of Foxl2 on the expression of genes known to play crucial roles in uterine function. Altogether our data suggest that, by controlling the expression profile of endometrial genes, Foxl2 might have an important role in regulating uterus receptivity and embryo implantation. In support to our findings it has been previously reported that Foxl2 expression decrease during the pre-receptive to receptive transition as well as after co-culturing of human endometrial cells with trophoblast cells [14].
In the current study, we show that FOXL2 localizes to the luminal epithelium and the myometrium. A recent study of neonatal and adult mouse uteri also found FOXL2 protein in the myometrium, but not in the endometrium [15]. The discrepancy between this study and our results might be due to the different methods used. Bellessort et al. used FOXL2Lacz mice while we used immunoflorsence staining with FOXL2 specific antibody. Taken altogether, these studies combined with our data suggest that FOXL2 has a key role in uterus remodeling and towards its preparation for implantation. A study of the expression pattern of Foxl2 in bovine uterus confirmed its presence in the endometrium and showed that Foxl2 levels are regulated in a hormonal dependent manner [27]. This study showed that both Foxl2 transcript and FOXL2 protein were expressed from day 5 to day 20 of the estrous cycle, with significant increases during the luteolytic phase followed by a gradual decline corresponding to increased progesterone levels. Consistent with the latter, progesterone supplementation was found to suppress FOXL2 protein levels [27]. These observations are in line with our findings in mouse uteri and human endometrial cells, suggesting that the roles of Foxl2 in the uterus might be conserved across species, including humans, mice and farm animals. Further studies are needed to examine this possibility. With respect to this, some differences between mice and goats have been found in Foxl2 effects on gonadal differentiation and function [6, 20, 28–31].
As mentioned above, Foxl2 expression has been only recently discovered in human, bovine and murine uteri [12, 15, 19, 27]. Thus, little is known about its role in this tissue. In a recent study [15], conditional deletion of Foxl2 in the postnatal uterus resulted in infertility, severely reduced thickness of the stroma layer and a hypertrophic, disorganized appearance of the myometrium [15]. Moreover, uterine-specific deletion of Foxl2 in the adult animal resulted in the appearance of a supplementary muscular layer at the stroma/myometrium border and failure of vascularization of smooth muscle around uterine arteries [15]. In order to expand our understanding of the role of FOXL2 in the human uterus, we utilized two human endometrial cell types to examine the effects of Foxl2, either knockdown or overexpression.
In agreement with the findings of Bellassort et al. [15], we observed a reduction in the successful attachment of either mouse blastocysts or Jeg3 trophoblast cells to human Ishikawa receptive endometrial cells with overexpression of Foxl2, compared to control Ishikawa cells. Conversely, we found that knockdown of Foxl2 in AN3-CA non-receptive cells, which express higher levels of endogenous Foxl2, improved the attachment of mouse blastocysts and Jeg3 spheroids. Our findings from experiments with human endometrial cells, combined with data from mouse uteri reported by Bellassort et al. [15] support the notion that Foxl2 plays a negative role in the acquisition uterine receptivity and processes involved in its preparation towards embryo attachment. This notion is further supported by our finding that Foxl2 is expressed in a lesser extend in uterine epithelial cells surrounding the attached embryo than cells distant from the implantation site.
Additionally, Wnt genes were found to be deregulated in this conditional deletion model [15]. Both FOXL2 and the Wnt/Fzd family are necessary for the development of the ovary [32]. In addition, many members of the Wnt/Fzd family are expressed in the uterus [33]. Different Fzd receptors including Fzd6 and Wnt ligands such as Wnt11 were shown to be expressed in the mouse uterus before and around the time of implantation, as well as during stromal cell differentiation [24]. Wnt11 was detected in the uterus endometrium and epithelial glands in the non-pregnant uterus and its expression was shown to be unregulated by progesterone [24, 34, 35]. Wnt11 was shown to regulate endometrial gland development [35]. During pregnancy, WNT11 is adjacent to the embryo and FZD6 is localized in the endometrium and stroma during implantation [35].
Our results show an inverse relationship between Wnt/Fzd and Foxl2 levels. Low Foxl2 levels are associated with an elevation in Wnt11 and Fzd6 and reduction in the levels of Wnt/Fzd inhibitor, Kremen2, while the opposite is seen in cells with high Foxl2 expression levels. Consistent with these findings, conditional deletion of Foxl2 in the uterus was also found to disrupt Wnt gene expression [15]. These results suggest that Foxl2 has a role in the regulation of normal uterine function, such as uterine glandular generation. Indeed, the decrease in Foxl2 expression on day 3 of pregnancy might be a crucial mechanism for Wnt/Fzd pathway activation and embryo implantation. Consistent with this notion, the aberrant expression of Dkk1, a KREMEN2 receptor, was demonstrated to cause impairment in embryo attachment and implantation [21]. Altogether, these findings provide support for the possibility that conditions that elevate Foxl2 expression or activity would compromise embryo implantation.
Apoptosis plays an important role in the uterus and is necessary for embryo implantation. Foxl2 has been implicated in the regulation of both anti- and pro-apoptotic processes in the ovary, based upon microarray data obtained from an ovarian cell line transfected with Foxl2 [3]. This study reported that Foxl2 overexpression activated the transcription of several anti-apoptotic genes, such as immediate-early response 3 (Ier3), BCL2-related protein A1 (Bcl2A1) and tumor necrosis factor alpha-induced protein 3 (Tnfaip3) [3], but also increased transcription of pro-apoptotic factors, including activating transcription factor 3 (Atf3) [3]. Our results from endometrial cells suggest that, in parallel to the ovary, FOXL2 affects the expression of both, anti-apoptotic and pro-apoptotic genes. This apparently ambivalent behavior of Foxl2 on apoptosis is likely to reflect the complexity of the pathways that directly, or indirectly regulate apoptosis and the manner in which differential interactions of Foxl2 might influence or contribute to processes of uterine cell differentiation, proliferation or programmed cell death. The transcriptional regulation of apoptotic proteins is altered in the endometrium of infertile women with chronic endometriosis as compared to healthy women [36]. Estrogen promotes uterine cell apoptosis [37] and estrogen production and actions are influenced by Foxl2 [4]. Based upon these observations, we predict that altered Foxl2 expression or activity would compromise uterine cell apoptosis and ultimately lead to impairment of uterine function and the progression of uterine pathology. This prediction requires further investigations although it is consistent with a recent study showing that Foxl2 levels are elevated in the endometrium of patients with endometriosis [12].
A variety of factors, including Rgs2 and chemokines, such as Cxcl1, are implicated in mediating the crosstalk between the embryo and the uterus [7, 26]. Both factors were reported to be FOXL2 targets in the ovary [3]. Of these, Rgs2, exclusively expressed in the implantation sites of the murine pregnant uterus, is of particular interest [3, 7]. RGS’s are multi-functional, GTPase-accelerating proteins that promote GTP hydrolysis by the alpha-subunit of G proteins, thereby inactivating G proteins and rapidly switching off G protein-coupled receptor signaling pathways. It is thought that RGS2 regulates implantation and pregnancy by influencing intracellular calcium flux or, alternatively, that it participates in the local immunoregulation of uterine tissues during implantation by activating T cells and interleukin-2 production [38] and attenuating the bioactivity of Mnsfβ, a molecule implicated in uterine immune tolerance during pregnancy in mice [39]. Similarly, chemokines, such as Cxcl1, are implicated in immunoregulatory and inflammatory processes that play a pivotal role in embryo implantation [26]. Here we show that the expression of both Rgs2 and Cxcl1 is sensitive to the level of Foxl2, suggesting that Foxl2 might indirectly regulate processes that are controlled by Rgs2 and Cxcl1. Our results suggest that Foxl2 depletion just prior to embryo implantation might be necessary for preparing the uterus for successful attachment of the embryo to the uterine wall.
Conclusions
Our data showing a decrease in Foxl2 levels just before implantation are consistent with the notion that Foxl2 levels must decline to allow successful embryo attachment, which is an essential prelude for pregnancy progression. This is supported by our observations that Foxl2 overexpression in endometrial cells reduces embryo and spheroid attachment to receptive cells while attachment is improved by Foxl2 knockdown in non-receptive cells. Our results further suggest that these effects Foxl2 are mediated by regulating genes involved in those processes. Further investigations into the contributions of Foxl2 function to uterus remodeling toward embryo implantation will lead to better understanding of these complex processes. Finding Foxl2 target genes could also unveil new players in the uterus, both under normal and pathological conditions, and lead to development of new means to improve fertility when implantation fails.
Additional files
Figure S1. Embryos attachment to endometrial cells. Mouse blastocysts co-incubated with endometrial cell lines for 48 h (A). Attached embryos that stayed on the plate after the plates were washed and shaken (B). Thin arrows indicate attached embryos, thick arrow indicate unattached embryos. (C) The localization of osteopentin, an adhesion molecule, between the embryo and the endometrial cells. Osteopentin-green, DAPI-blue. (TIFF 2025 kb)
Table S1. PCR primers list. (DOCX 15 kb)
Figure S2. FOXL2 localization in the ovary. FOXL2 expressed by granulosa cells of early follicles declines at later stages of folliculogenesis. Blue = DNA staining (DAPI), RED = FOXL2. EA-Early antral, GF-Graffian Folicle. (TIFF 2025 kb)
Figure S3. Foxl2 expression in human endometrial cell lines. A) Human endometrial cell lines express Foxl2 mRNA. Foxl2 mRNA expression in AN3-CA and Ishikawa endometrial cell lines was analyzed by RT-PCR, using Hprt mRNA as an internal control. B) Foxl2 levels are shown as fold mRNA expression, normalized to Hprt. (TIFF 2025 kb)
Figure S4. Manipulating Foxl2 expression in human endometrial cell lines. A) Foxl2 expression is decreased in endometrial non-receptive AN3-CA cells infected with lentivirus expressing Foxl2 siRNA cassette. B) Ishikawa cells infected with lentivirus overexpress Foxl2 exhibit higher FOXL2 levels as compared to control. The results of one representative out of a total of 3 independent experiments with similar results is presented. + * p < 0.05. (TIFF 2025 kb)
Acknowledgements
We thank Alissa Blackler for her technical contributions to the project.
Ethical approval and consent to participate
All protocols for animal breeding, handling and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Agricultural Research Organization (approval no. IL 467/13).
Funding
This work and L.M.B. were supported in part by Grant 2R01HD046941 awarded by the NICHD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- BPES
Blepharophimosis–ptosis–epicanthus inversus syndrome
- FOXL2
Forkhead Transcription Factor L2
- hCG
Human chorionic gonadotropin
- KD
Knockdown
- OE
Overexpression
- PMSG
Pregnant mare serum gonadotropin
- RGS2
G-protein signaling protein 2
Authors’ contributions
ME, RH and EG preformed the experiments, LMB and EG designed the experiments and wrote the manuscript. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12958-018-0329-y) contains supplementary material, which is available to authorized users.
Contributor Information
Michal Elbaz, Email: elbazonit@gmail.com.
Ron Hadas, Email: ron.hadas@weizmann.ac.il.
Louise M. Bilezikjian, Email: bilezikjian@salk.edu
Eran Gershon, Email: eran.gershon1@mail.huji.ac.il.
References
- 1.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 2.Zlotogora J, Sagi M, Cohen T. The blepharophimosis, ptosis, and epicanthus inversus syndrome: delineation of two types. Am J Hum Genet. 1983;35:1020–1027. [PMC free article] [PubMed] [Google Scholar]
- 3.Batista F, Vaiman D, Dausset J, Fellous M, Veitia RA. Potential targets of FOXL2, a transcription factor involved in craniofacial and follicular development, identified by transcriptomics. Proc Natl Acad Sci U S A. 2007;104:3330–3335. doi: 10.1073/pnas.0611326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georges A, L’Hote D, Todeschini AL, Auguste A, Legois B, Zider A, Veitia RA. The transcription factor FOXL2 mobilizes estrogen signaling to maintain the identity of ovarian granulosa cells. elife. 2014;3 [DOI] [PMC free article] [PubMed]
- 5.Caburet S, Georges A, L'Hote D, Todeschini AL, Benayoun BA, Veitia RA. The transcription factor FOXL2: at the crossroads of ovarian physiology and pathology. Mol Cell Endocrinol. 2012;356:55–64. doi: 10.1016/j.mce.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Elzaiat M, Jouneau L, Thepot D, Klopp C, Allais-Bonnet A, Cabau C, Andre M, Chaffaux S, Cribiu EP, Pailhoux E, Pannetier M. High-throughput sequencing analyses of XX genital ridges lacking FOXL2 reveal DMRT1 up-regulation before SOX9 expression during the sex-reversal process in goats. Biol Reprod. 2014;91:153. doi: 10.1095/biolreprod.114.122796. [DOI] [PubMed] [Google Scholar]
- 7.Huang ZP, Ni H, Yang ZM, Wang J, Tso JK, Shen QX. Expression of regulator of G-protein signalling protein 2 (RGS2) in the mouse uterus at implantation sites. Reproduction. 2003;126:309–316. doi: 10.1530/rep.0.1260309. [DOI] [PubMed] [Google Scholar]
- 8.Ladds G, Zervou S, Vatish M, Thornton S, Davey J. Regulators of G protein signalling proteins in the human myometrium. Eur J Pharmacol. 2009;610:23–28. doi: 10.1016/j.ejphar.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Blount AL, Schmidt K, Justice NJ, Vale WW, Fischer WH, Bilezikjian LM. FoxL2 and Smad3 coordinately regulate follistatin gene transcription. J Biol Chem. 2009;284:7631–7645. doi: 10.1074/jbc.M806676200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McTavish KJ, Nonis D, Hoang YD, Shimasaki S. Granulosa cell tumor mutant FOXL2C134W suppresses GDF-9 and activin A-induced follistatin transcription in primary granulosa cells. Mol Cell Endocrinol. 2013;372:57–64. doi: 10.1016/j.mce.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugawara K, Kizaki K, Herath CB, Hasegawa Y, Hashizume K. Transforming growth factor beta family expression at the bovine feto-maternal interface. Reprod Biol Endocrinol. 2010;8:120. doi: 10.1186/1477-7827-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Governini L, Carrarelli P, Rocha AL, Leo VD, Luddi A, Arcuri F, Piomboni P, Chapron C, Bilezikjian LM, Petraglia F. FOXL2 in human endometrium: hyperexpressed in endometriosis. Reprod Sci. 2014;21:1249–1255. doi: 10.1177/1933719114522549. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Yao G, Wang Y, Xu H, Ji X, He Y, Zhu Q, Chen Z, Sun Y. Transcriptomic changes during the pre-receptive to receptive transition in human endometrium detected by RNA-Seq. J Clin Endocrinol Metab. 2014;99(12):E2744–E2753. doi: 10.1210/jc.2014-2155. [DOI] [PubMed] [Google Scholar]
- 14.Popovici RM, Betzler NK, Krause MS, Luo M, Jauckus J, Germeyer A, Bloethner S, Schlotterer A, Kumar R, Strowitzki T, von Wolff M. Gene expression profiling of human endometrial-trophoblast interaction in a coculture model. Endocrinology. 2006;147:5662–5675. doi: 10.1210/en.2006-0916. [DOI] [PubMed] [Google Scholar]
- 15.Bellessort B, Bachelot A, Heude E, Alfama G, Fontaine A, Le Cardinal M, Treier M, Levi G. Role of Foxl2 in uterine maturation and function. Hum Mol Genet. 2015; [DOI] [PubMed]
- 16.Blount AL, Vaughan JM, Vale WW, Bilezikjian LM. A Smad-binding element in intron 1 participates in activin-dependent regulation of the follistatin gene. J Biol Chem. 2008;283:7016–7026. doi: 10.1074/jbc.M709502200. [DOI] [PubMed] [Google Scholar]
- 17.Aboussahoud W, Bruce C, Elliott S, Fazeli A. Activation of toll-like receptor 5 decreases the attachment of human trophoblast cells to endometrial cells in vitro. Hum Reprod. 2010;25:2217–2228. doi: 10.1093/humrep/deq185. [DOI] [PubMed] [Google Scholar]
- 18.Kang YJ, Forbes K, Carver J, Aplin JD. The role of the osteopontin-integrin alphavbeta3 interaction at implantation: functional analysis using three different in vitro models. Hum Reprod. 2014;29:739–749. doi: 10.1093/humrep/det433. [DOI] [PubMed] [Google Scholar]
- 19.Eozenou C, Mansouri-Attia N, Aubert J, Reinaud P, Giraud-Delville C, Taghouti G, Galio L, Everts RE, Degrelle S, Richard C, et al. Gene expression profiles of bovine caruncular and intercaruncular endometrium at implantation. Physiol Genomics. 2009;39:14–27. doi: 10.1152/physiolgenomics.90404.2008. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Kodithuwakku SP, Ng PY, Chai J, Ng EH, Yeung WS, Ho PC, Lee KF. Excessive ovarian stimulation up-regulates the Wnt-signaling molecule DKK1 in human endometrium and may affect implantation: an in vitro co-culture study. Hum Reprod. 2010;25:479–490. doi: 10.1093/humrep/dep429. [DOI] [PubMed] [Google Scholar]
- 22.Rahnama F, Thompson B, Steiner M, Shafiei F, Lobie PE, Mitchell MD. Epigenetic regulation of E-cadherin controls endometrial receptivity. Endocrinology. 2009;150:1466–1472. doi: 10.1210/en.2008-1142. [DOI] [PubMed] [Google Scholar]
- 23.Sonderegger S, Pollheimer J, Knofler M. Wnt signalling in implantation, decidualisation and placental differentiation--review. Placenta. 2010;31:839–847. doi: 10.1016/j.placenta.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi K, Erikson DW, Tilford SA, Bany BM, Maclean JA, 2nd, Rucker EB, 3rd, Johnson GA, Spencer TE. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod. 2009;80:989–1000. doi: 10.1095/biolreprod.108.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moumne L, Batista F, Benayoun BA, Nallathambi J, Fellous M, Sundaresan P, Veitia RA. The mutations and potential targets of the forkhead transcription factor FOXL2. Mol Cell Endocrinol. 2008;282:2–11. doi: 10.1016/j.mce.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod. 2007;76:102–117. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- 27.Eozenou C, Vitorino Carvalho A, Forde N, Giraud-Delville C, Gall L, Lonergan P, Auguste A, Charpigny G, Richard C, Pannetier M, Sandra O. FOXL2 is regulated during the bovine estrous cycle and its expression in the endometrium is independent of conceptus-derived interferon tau. Biol Reprod. 2012;87:32. doi: 10.1095/biolreprod.112.101584. [DOI] [PubMed] [Google Scholar]
- 28.Boulanger L, Pannetier M, Gall L, Allais-Bonnet A, Elzaiat M, Le Bourhis D, Daniel N, Richard C, Cotinot C, Ghyselinck NB, Pailhoux E. FOXL2 is a female sex-determining gene in the goat. Curr Biol. 2014;24:404–408. doi: 10.1016/j.cub.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 29.Pailhoux E, Vigier B, Chaffaux S, Servel N, Taourit S, Furet JP, Fellous M, Grosclaude F, Cribiu EP, Cotinot C, Vaiman D. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat Genet. 2001;29:453–458. doi: 10.1038/ng769. [DOI] [PubMed] [Google Scholar]
- 30.Pailhoux E, Vigier B, Vaiman D, Servel N, Chaffaux S, Cribiu EP, Cotinot C. Ontogenesis of female-to-male sex-reversal in XX polled goats. Dev Dyn. 2002;224:39–50. doi: 10.1002/dvdy.10083. [DOI] [PubMed] [Google Scholar]
- 31.Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- 32.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A. 2005;102:8579–8584. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi K, Burghardt RC, Bazer FW, Spencer TE. WNTs in the ovine uterus: potential regulation of periimplantation ovine conceptus development. Endocrinology. 2007;148:3496–3506. doi: 10.1210/en.2007-0283. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi K, Yoshioka S, Reardon SN, Rucker EB, 3rd, Spencer TE, DeMayo FJ, Lydon JP, MacLean JA, 2nd: WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development. Biol Reprod 2011, 84:308–319. [DOI] [PMC free article] [PubMed]
- 36.Di Pietro C, Cicinelli E, Guglielmino MR, Ragusa M, Farina M, Palumbo MA, Cianci A. Altered transcriptional regulation of cytokines, growth factors, and apoptotic proteins in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol. 2013;69:509–517. doi: 10.1111/aji.12076. [DOI] [PubMed] [Google Scholar]
- 37.Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Berges H, Laurell H, Gourdy P, et al. The AF-1 activation function of estrogen receptor alpha is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology. 2013;154:2222–2233. doi: 10.1210/en.2012-2059. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira-Dos-Santos AJ, Matsumoto G, Snow BE, Bai D, Houston FP, Whishaw IQ, Mariathasan S, Sasaki T, Wakeham A, Ohashi PS, et al. Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc Natl Acad Sci U S A. 2000;97:12272–12277. doi: 10.1073/pnas.220414397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nie GY, Li Y, Hampton AL, Salamonsen LA, Clements JA, Findlay JK. Identification of monoclonal nonspecific suppressor factor beta (mNSFbeta) as one of the genes differentially expressed at implantation sites compared to interimplantation sites in the mouse uterus. Mol Reprod Dev. 2000;55:351–363. doi: 10.1002/(SICI)1098-2795(200004)55:4<351::AID-MRD1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Embryos attachment to endometrial cells. Mouse blastocysts co-incubated with endometrial cell lines for 48 h (A). Attached embryos that stayed on the plate after the plates were washed and shaken (B). Thin arrows indicate attached embryos, thick arrow indicate unattached embryos. (C) The localization of osteopentin, an adhesion molecule, between the embryo and the endometrial cells. Osteopentin-green, DAPI-blue. (TIFF 2025 kb)
Table S1. PCR primers list. (DOCX 15 kb)
Figure S2. FOXL2 localization in the ovary. FOXL2 expressed by granulosa cells of early follicles declines at later stages of folliculogenesis. Blue = DNA staining (DAPI), RED = FOXL2. EA-Early antral, GF-Graffian Folicle. (TIFF 2025 kb)
Figure S3. Foxl2 expression in human endometrial cell lines. A) Human endometrial cell lines express Foxl2 mRNA. Foxl2 mRNA expression in AN3-CA and Ishikawa endometrial cell lines was analyzed by RT-PCR, using Hprt mRNA as an internal control. B) Foxl2 levels are shown as fold mRNA expression, normalized to Hprt. (TIFF 2025 kb)
Figure S4. Manipulating Foxl2 expression in human endometrial cell lines. A) Foxl2 expression is decreased in endometrial non-receptive AN3-CA cells infected with lentivirus expressing Foxl2 siRNA cassette. B) Ishikawa cells infected with lentivirus overexpress Foxl2 exhibit higher FOXL2 levels as compared to control. The results of one representative out of a total of 3 independent experiments with similar results is presented. + * p < 0.05. (TIFF 2025 kb)
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.


