Abstract
The high cost of drug development and the narrow spectrum of coverage typically provided by direct-acting antivirals limit the scalability of this antiviral approach. This review summarizes progress and challenges in the repurposing of approved kinase inhibitors as host-targeted broad-spectrum antiviral therapies.
Keywords: : kinase inhibitors, antiviral drugs, repurposing, broad-spectrum antivirals
Introduction
There is an urgent need for antiviral strategies to combat hundreds of human disease-causing viruses. Currently approved antiviral drugs treat fewer than ten viral infections. A majority of these drugs are direct-acting antivirals (DAAs) that target proteins encoded by individual viruses. As such, this approach provides a narrow spectrum of coverage and therefore cannot address the large clinical need. The high average cost (over two billion dollars) and long timeline (8–12 years) to develop a new drug (Tufts center for the Study of Drug Development, 2014), further limit the scalability of the DAA approach to drug development, particularly with respect to emerging viruses. Lastly, the inability to provide adequate global health protection and national security preparedness against newly emerging viruses and the emergence of viral resistance further challenge conventional DAAs.
The host-targeted broad-spectrum antiviral strategy represents an attractive potential solution to overcome these limitations. Viruses are dependent on cellular proteins for each step of their life cycle. Targeting host proteins required by multiple viruses can thus provide a broad-spectrum coverage with a possible added benefit of a high genetic barrier to resistance. Moreover, a broad-spectrum therapeutic could be administered even before a viral threat has been accurately diagnosed, thereby increasing protection.
A particularly cost- and time-effective strategy for the development of broad-spectrum antivirals is to repurpose already approved drugs that target host functions required by multiple viruses. By lowering the development cost, this approach has the potential to increase drug access for patients. Moreover, it shortens the path to clinic by enabling utilization of available data (e.g., toxicity, pharmacokinetics, dosing, etc.). Lastly, it can promote preparedness for future outbreaks of newly emerging pathogens by facilitating off-label use of approved broad-spectrum antivirals against new viral indications.
Host kinase inhibitors represent one category of compounds with a great potential to be repurposed as broad-spectrum antivirals. Viruses hijack a large number of host kinases at distinct steps of their life cycle (Supekova et al., 2008; Li et al., 2009; Keating and Striker, 2012; Jiang et al., 2014). Some of these host kinases are broadly required and thus represent attractive targets for broad-spectrum therapy (Table 1 and Fig. 1). These findings, combined with the development and approval of a large number of kinase inhibitors for the treatment of cancer (Gross et al., 2015) and inflammatory conditions (Ott and Adams, 2011) have sparked efforts aimed to determine the therapeutic potential of such drugs to combat viral infections. In this review, we summarize recent efforts to determine the therapeutic potential and biological rationale of repurposing already approved kinase inhibitors as antivirals.
Table 1.
Classification, Antiviral Activity, and Mechanism of Action of Approved Kinase Inhibitors
| Kinase family | Kinases | Inhibitors | EC50 | In vitro | In vivo | Stages of viral lifecycle | Antiviral target validation (siRNA) | Molecular target validation | Mechanism of action | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| NAK | AAK1 GAK |
Sunitinib Erlotinib |
0.12–12.9 μM 0.12–4.67 μM |
Flaviviridae (HCV. DENV, WNV, ZIKV) Filoviridae (EBOV) Togaviridae (CHIKV) Arenaviridae (JUNV) Retroviridae (HIV) Paramyxoviridae (RSV) |
DENV (mouse) EBOV(Mouse) |
Entry Assembly Release | + | + | Inhibition of AP-mediated intracellular membrane trafficking | Bekerman et al. (2017) Neveu et al. (2012) Neveu et al. (2015) |
| ErbB | EGFR | Erlotinib Lapatinib Gefitinib |
0.276–0.6 μM 0.18 μM 4.93 μM |
Flaviviridae (HCV, DENV, WNV, ZIKV) Herpesviridae (HCMV) Poxviridae (Orthopoxvirus) |
HCV (Mouse) HCMV (Guinea pig) |
Entry | + | + | Inhibition of receptor-mediated endocytosis | Diao et al. (2012) Neveu et al. (2015) Langhammer et al. (2011) Lupberger et al. (2011) Schleiss et al. (2008) |
| MAPK/ERK | MEK1/2 ERK1/2 |
Trametinib Selumetinib |
0.1–1 μM 1–10 μM |
Coronaviridae (MERS, SARS) | ND | ND | ND | ND | ND | Kindrachuk et al. (2015) |
| Src | SRC FYN |
Dasatinib Sarcatanib |
12.2 μM* 4.7 μM* |
Flaviviridae (DENV) | ND | RNA Replication Assembly | + | ND | Inhibition of actin motility | Chu and Yang (2007) de Wispelaere et al. (2013) |
| Abl | ABL1 | Dasatinib Imatinib Nilotinib |
12.2 μM* 9.8–17.7 μM – |
Filoviridae (EBOV) Flaviviridae (DENV) Coronaviridae (MERS, SARS) Poxviridae (Vaccinia) |
Vaccinia (Mouse) | Entry Release | + | ND | Inhibition of actin motility | Clark et al. (2016) Dyall et al. (2014) Garcia et al. (2012) Reeves et al. (2011) |
| CDK | CDK1 CDK2 CDK5 CDK4/6 CDK9 |
Palbociclib Dinaciclib PHA-690509 Alvocidib Seliciclib |
0.016–0.02 μM 0.02–0.355 μM 0.9–1.72 μM 0.01–0.24 μM 0.024–0.04 μM |
Retroviridae (HIV) Orthomyxoviridae (IAV) Flaviviridae (ZIKV) Herpesviridae (HCMV, HSV) |
HSV-1 (Mouse) | ND | + | + | Inhibition of phosphorylation of viral proteins and reduction of host intracellular dNTPs | Badia et al. (2016) Chao et al. (2000) Guendel et al. (2010) Habran et al. (2005) Pauls et al. (2014a) Perwitasari et al. (2015) Schang et al. (2006) Xu et al. (2016) Yamamoto et al. (2014) |
| PI3K/Akt/mTor | mTor Akt |
Everolimus Miltefosine Teriflunamide Leflunomide |
1–10 μM >10 μM – – |
Coronaviridae (MERS) Herpesviridae (HCMV) Polyomaviridae (BKV) |
HCMV(Rat) BKV (Human) |
ND | + | ND | Disabling of viral block of cell stress response and apoptosis | Kindrachuk et al. (2015) Jaw et al. (2017) Jung et al. (2013) Liacini et al. (2010) Waldman et al. (1999) Williams et al. (2005) |
= EC90.
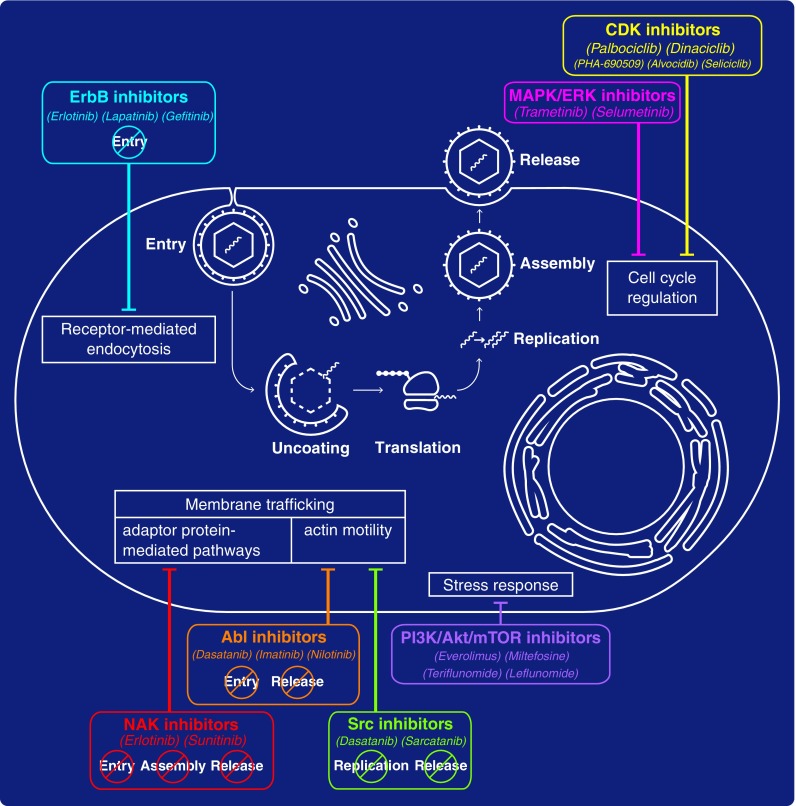
FIG. 1.
Approved kinase inhibitors with broad-spectrum antiviral activity. Examples of classes of kinase inhibitors with broad-spectrum antiviral activity and the specific steps of the viral life cycle they target (if known) are denoted in colored boxes. Blunt arrows connect these compounds to the corresponding targeted cellular process thought to mediate their antiviral effect.
Targeting the NAK Family of Kinases
Targeting the NAK (Numb-associated kinases) family of Ser/Thr kinases by approved kinase inhibitors is one approach showing promise as a broad-spectrum antiviral strategy. We discovered a requirement for the host kinases adaptor protein 2 (AP2)-associated protein kinase 1 (AAK1) and cyclin G-associated kinase (GAK) in the regulation of intracellular viral trafficking during entry, assembly, and release of unrelated viruses (Neveu et al., 2012, 2015; Bekerman et al., 2017).
We recognized that the already approved anticancer drugs sunitinib and erlotinib potently inhibited AAK1 and GAK, respectively, and demonstrated activity against viruses from six viral families, including dengue (DENV) and Ebola viruses (EBOV) in cultured cells (Neveu et al., 2012, 2015; Bekerman et al., 2017). Sunitinib/erlotinib combinations protected against morbidity and mortality in murine models of dengue and Ebola infection (Bekerman et al., 2017). Moreover, we discovered that inhibition of AP-mediated intracellular membrane trafficking regulated by AAK1 and GAK represents an important mechanism by which sunitinib and erlotinib inhibit viral infection in vitro and in vivo (Bekerman et al., 2017). The safety and efficacy of sunitinib/erlotinib combinations will be evaluated in dengue patients in the future and potentially in patients with EBOV disease in future outbreaks (ClinicalTrials.gov NCT02380625).
Targeting the ErbB Family of Kinases
Epidermal growth factor receptor (EGFR), a member of the ErbB family of receptor tyrosine kinases, is another host factor broadly required by viruses (Zheng et al., 2014). EGFR is utilized as an entry coreceptor by human cytomegalovirus (HCMV) (Wang et al., 2003) and adeno-associated virus serotype 6 (AAV6) (Weller et al., 2010). EGFR-mediated signaling is implicated in the endocytosis of hepatitis C virus (HCV) and influenza A virus (IAV) (Eierhoff et al., 2010; Lupberger et al., 2011; Diao et al., 2012; Neveu et al., 2012, 2015; Zona et al., 2013) and in postentry events, as in HCMV nuclear trafficking through regulation of F-actin dynamics (Wang et al., 2005). IAV and other viruses hijack EGFR to evade host immune responses (Ueki et al., 2013).
Altogether, these studies validate EGFR as a broad antiviral target. Indeed, erlotinib and gefitinib, approved anticancer drugs targeting EGFR, and lapatinib, an anticancer drug targeting EGFR and another member of the ERBb kinase family, ERBB2, demonstrated in vitro activity against a number of viruses, including HCV (Lupberger et al., 2011; Diao et al., 2012; Neveu et al., 2015), HCMV (Schleiss et al., 2008), and poxvirus (Langhammer et al., 2011). Notably, erlotinib and gefitinib inhibited HCV and HCMV infections in a mouse and guinea pig model, respectively (Schleiss et al., 2008; Lupberger et al., 2011).
Targeting the MAPK/ERK Pathway
Several kinases in the MAPK/ERK (mitogen-activated protein kinases/extracellular signal-regulated kinases) pathway are essential for viral replication. ERK1 and ERK2 phosphorylate HIV-1 proteins and enhance viral infectivity (Yang and Gabuzda, 1999). IAV activates this pathway and relies on it for effective virus production and export of the viral ribonucleoprotein complexes from the nucleus (Pleschka et al., 2001). Activation of this pathway mediates EBOV entry into target cells and enhances cytokine production and cellular toxicity, thereby possibly contributing to disease pathogenesis (Kolokoltsov et al., 2009) (Zampieri et al., 2007; Johnson et al., 2014).
Moreover, infection with Middle East respiratory syndrome coronavirus (MERS-CoV) activates this pathway and is inhibited by two anticancer compounds, trametinib, an Food and Drug Administration-approved MEK1/2 inhibitor, and selumetinib, an investigational (Phase III) MEK1/ERK1/2 inhibitor (Kindrachuk et al., 2015). The broad-spectrum antiviral potential of these compounds in cultured cells and animal models remains to be established.
Targeting the Src and Abl Families of Kinases
The Src family of kinases (SFKs) is implicated in the life cycle of multiple viruses (Pagano et al., 2013). The replication of viruses from the Flaviviridae family is dependent on the activation of various SFKs, such as Fyn, Lyn, c-Src, and Csk (DENV) (Chu and Yang, 2007; de Wispelaere et al., 2013; Kumar et al., 2016), c-Yes (West Nile Virus, WNV) (Hirsch et al., 2005), and Csk (HCV) (Supekova et al., 2008). The unrelated HIV-1 also activates SFKs to effectively replicate (Narute and Smithgall, 2012).
The approved drug dasatinib and the investigational (Phase III) compound saracatinib, with activity against SFKs, inhibited DENV assembly in vitro (de Wispelaere et al., 2013). This antiviral activity was initially attributed to inhibition of c-Src (Chu and Yang, 2007), yet the effect on assembly was subsequently shown to be non-Src mediated, whereas an independent effect of the drug on DENV RNA replication was found to be Fyn-mediated (de Wispelaere et al., 2013).
Another host kinase targeted by both dasatinib and saracatinib is c-Abl (cellular Abelson tyrosine kinase), a member of the Abl family of nonreceptor tyrosine kinases. C-Abl is implicated in the budding or release of poxviruses (Reeves et al., 2011) and EBOV (Garcia et al., 2012), the entry of coxsackievirus (Coyne and Bergelson, 2006) and polyomavirus (Swimm et al., 2010), and in DENV infection (Clark et al., 2016). Imatinib and/or nilotinib, approved anticancer c-Abl inhibitors lacking anti-SFK activity, inhibited replication of EBOV (Garcia et al., 2012), DENV (Clark et al., 2016), MERS-CoV and/or SARS-CoV (Dyall et al., 2014) in cultured cells. While dasatinib also inhibited MERS-CoV and SARS-CoV, it remained uncertain which molecular target(s) (Abl only or Abl plus Src) mediated its activity against these viruses (Dyall et al., 2014).
In a murine model of vaccinia virus, imatinib effectively reduced viral load, viral spread, and mortality (Reeves et al., 2011). In contrast, dasatinib did not protect mice, but rather induced immunosuppression, raising a concern about its utility in controlling viral infections in vivo. It is thus important to investigate the antiviral activity and safety of drugs targeting either Src and/or Abl kinases in animal models of other viral infections. Moreover, while inhibition of actin motility was proposed as a mechanism of antiviral action of Src and/or Abl inhibitors (Reeves et al., 2011), additional mechanisms remain to be uncovered.
Targeting the Cyclin-Dependent Kinases
Numerous viruses regulate the host cell cycle by modifying the expression level and activity of members of the host cyclin-dependent kinases (CDKs) family (Schang, 2003). To promote its replication, adenovirus increases CDK2 phosphorylation, thereby forcing progression into late-G1/S-phase (Cheng et al., 2013), whereas hepatitis B virus increases activity of the G1-phase kinase CDK4 (Gearhart and Bouchard, 2010). CDK6 and CDK9 have both been validated as potential antiviral targets in HIV-1 infection (Nemeth et al., 2011; Pauls et al., 2014b), and CDK9 in herpes simplex virus type 1 (HSV-1) infection (Durand and Roizman, 2008).
Various CDK inhibitors with variable selectivity have demonstrated antiviral activity (Schang et al., 2006). Palbociclib, an approved CDK4/6 inhibitor suppressed HIV-1 reverse transcription and HSV-1 replication in vitro (Pauls et al., 2014a; Badia et al., 2016). Moreover, four investigational CDK inhibitors, dinaciclib (CDK1/2/5/9 inhibitor, Phase III), seliciclib (CDK2/5 inhibitor, Phase II), alvocidib (CDK9 inhibitor, Phase II), and PHA-690509 (CDK2 inhibitor, Phase I), inhibited multiple viruses, including HIV-1 (Chao et al., 2000; Guendel et al., 2010), IAV (Perwitasari et al., 2015), and Zika in cultured cells (Xu et al., 2016). Alvocidib and a novel CDK9 inhibitor, FIT-039, demonstrated broad in vitro activity against DNA viruses and efficacy in a murine model of HSV-1 (Yamamoto et al., 2014). In addition to suppression of viral replication, targeting CDKs in the context of oncogenic viral infections may also suppress viral persistence and/or transformation (Li et al., 2003).
While grossly undertested, several studies have provided some insight about the potential mechanism of action (MOA) underlying the antiviral effect of CDK inhibitors. Inhibition of phosphorylation of viral proteins plays a role in the case of IAV and varicella zoster virus, (Habran et al., 2005; Perwitasari et al., 2015), whereas reduced intracellular dNTP availability and viral mRNA transcription through inhibition of a cellular protein phosphorylation is one mechanism by which palbociclib exerts its anti-HSV-1 and HIV-1 activities (Pauls et al., 2014a; Badia et al., 2016). A better understanding of the MOA of these compounds in other viral infections remains an important area of investigation.
Targeting the PI3K/Akt/mTOR Pathway
Multiple viruses activate the phosphatidylinositol 3′-kinase–Akt–mammalian target of rapamycin (PI3K/Akt/mTOR) pathway to support their replication and escape cellular stress responses (Buchkovich et al., 2008). HCV (Mannová and Beretta, 2005), WNV (Shives et al., 2014), and IAV (Ehrhardt et al., 2006; Hale et al., 2006) activate this pathway to promote distinct steps in their life cycle and control apoptosis. Although this pathway was shown to be activated in cells infected with MERS-CoV, everolimus, an mTor inhibitor approved to treat cancer and prevent transplant rejection, demonstrated only mild in vitro activity against MERS-CoV, and miltefosine, an approved AKT inhibitor, demonstrated no activity in this infection model (Kindrachuk et al., 2015).
In contrast, leflunomide's active metabolite teriflunomide, which suppresses Akt phosphorylation and is approved for rheumatoid arthritis treatment, has shown potent anti-HCMV activity both in vitro and in a rat model (Waldman et al., 1999). Furthermore, treatment with leflunomide and the two approved immunosuppressant mTOR inhibitors everolimus and sirolimus, either individually or in combination, reduced BK viremia and disease progression in patients with BK virus nephropathy (Williams et al., 2005; Jung et al., 2013; Jaw et al., 2017). Direct suppression of polyomavirus replication was proposed as one underlying MOA of this approach based on in vitro studies (Liacini et al., 2010). These findings underscore the importance of further exploring the broad antiviral activity of inhibitors of this pathway.
Summary and Perspectives
While repurposing of approved drugs as host-targeted broad-spectrum antivirals offers several important advantages over development of new DAAs, it also raises concerns. One obvious concern when targeting host functions is toxicity. Nevertheless, the protective effect of sunitinib/erlotinib combinations in murine models of DENV and EBOV (Bekerman et al., 2017) suggests that for some drugs it may be feasible to find a therapeutic window where the drug level is sufficient to inhibit viral replication with minimal cellular toxicity. Shortening the duration of therapy from months or years required to treat cancer to several days sufficient to treat most acute viral infections should further limit toxicity.
Emergence of viral resistance is another potential challenge of broad-spectrum antiviral drugs (de Wispelaere et al., 2013; Haqqani and Tilton, 2013). Nevertheless, targeting host kinases that are not under the genetic control of viruses is more likely to have a higher barrier to resistance than classical DAAs. Moreover, simultaneous inhibition of several kinases or pathways by the same drug or drug combination could increase the effectiveness while minimizing viral resistance, as previously shown in cancer (Knight et al., 2010). Indeed, while DENV overcame inhibition by a DAA with the emergence of a resistance mutation, it remained susceptible to sunitinib and erlotinib, supporting the higher genetic barrier of the host-targeted antiviral approach (Bekerman et al., 2017).
Lastly, understanding the MOA is challenging since cellular kinase function in a complex network of interactions and their inhibitors are often not selective. For example, erlotinib's effect on HCV entry was first attributed solely to its effect on EGFR, its known cancer target (Lupberger et al., 2011; Diao et al., 2012). We later demonstrated that ectopic expression not only of EGFR, but also GAK, which is inhibited by erlotinib with a comparable potency to EGFR (Kd of 3.4 nM vs. 1 nM) (Karaman et al., 2008), reversed erlotinib's anti-HCV effect (Neveu et al., 2015).
Similarly, the anti-DENV effect of dasatinib was attributed to the inhibition of SFKs, particularly Fyn (Chu and Yang, 2007) (de Wispelaere et al., 2013). Nevertheless, the finding that c-Abl, another dasatinib target, is essential for DENV infection (Clark et al., 2016) suggests that c-Abl inhibition likely represents yet another mechanism through which dasatinib mediates its antiviral activity. These examples underscore the importance of validating not only the antiviral targets but also the molecular targets underlying the antiviral effect of such inhibitors.
In summary, these examples provide a proof-of-concept for the potential feasibility of repurposing of approved kinase inhibitors as host-targeted broad-spectrum antiviral therapies to combat viral infections. While not yet approved, compounds targeting other kinases, such as the phosphatidylinositol 4-kinase family and IκB kinase-α, have already demonstrated antiviral activity (Altan-Bonnet and Balla, 2012; Li et al., 2013), suggesting that the repertoire of kinase inhibitor classes available for repurposing is likely to grow. Such approaches may find utility in combination with other strategies being developed to combat viruses.
Acknowledgments
This research was supported by grants from the NIH (1U19 AI10966201), DoD/CDMRP (PRMRP, PR151090), American Cancer Society (RSG-14-11 0-01-MPC), Stanford Bio-X, and Stanford SPARK program to S.E. The authors acknowledge all the contributions in the field that could not be included in the review.
Disclosure Statement
No competing financial interests exist.
References
- Altan-Bonnet N., and Balla T. (2012). Phosphatidylinositol 4-kinases: hostages harnessed to build panviral replication platforms. Trends Biochem Sci 37, 293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia R., Angulo G., Riveira-Munoz E., Pujantell M., Puig T., Ramirez C., Torres-Torronteras J., Marti R., Pauls E., Clotet B., et al. (2016). Inhibition of herpes simplex virus type 1 by the CDK6 inhibitor PD-0332991 (palbociclib) through the control of SAMHD1. J Antimicrob Chemother 71, 387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekerman E., Neveu G., Shulla A., Brannan J., Pu S.-Y., Wang S., Xiao F., Barouch-Bentov R., Bakken R.R., Mateo R., et al. (2017). Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J Clin Invest 127, 1338–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich N.J., Yu Y., Zampieri C.A., and Alwine J.C. (2008). The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K–Akt–mTOR signalling pathway. Nat Rev Microbiol 6, 266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao S.H., Fujinaga K., Marion J.E., Taube R., Sausville E.A., Senderowicz A.M., Peterlin B.M., and Price D.H. (2000). Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J Biol Chem 275, 28345–28348 [DOI] [PubMed] [Google Scholar]
- Cheng P.-H., Rao X.-M., McMasters K.M., and Zhou H.S. (2013). Molecular basis for viral selective replication in cancer cells: activation of CDK2 by adenovirus-induced Cyclin E. PLoS One 8, e57340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J.J., and Yang P.L. (2007). c-Src protein kinase inhibitors block assembly and maturation of dengue virus. Proc Natl Acad Sci U S A 104, 3520–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M.J., Miduturu C., Schmidt A.G., Zhu X., Pitts J.D., Wang J., Potisopon S., Zhang J., Wojciechowski A., Hann Chu J.J., et al. (2016). GNF-2 inhibits dengue virus by targeting Abl kinases and the viral E protein. Cell Chem Biol 23, 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne C.B., and Bergelson J.M. (2006). Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124, 119–131 [DOI] [PubMed] [Google Scholar]
- de Wispelaere M., LaCroix A.J., and Yang P.L. (2013). The small molecules AZD0530 and dasatinib inhibit dengue virus RNA replication via Fyn kinase. J Virol 87, 7367–7381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J., Pantua H., Ngu H., Komuves L., Diehl L., Schaefer G., and Kapadia S.B. (2012). Hepatitis C virus induces epidermal growth factor receptor activation via CD81 binding for viral internalization and entry. J Virol 86, 10935–10949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand L.O., and Roizman B. (2008). Role of cdk9 in the optimization of expression of the genes regulated by ICP22 of herpes simplex virus 1. J Virol 82, 10591–10599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jr., Jahrling P.B., Laidlaw M., et al. (2014). Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 58, 4885–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt C., Marjuki H., Wolff T., Nurnberg B., Planz O., Pleschka S., and Ludwig S. (2006). Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell Microbiol 8, 1336–1348 [DOI] [PubMed] [Google Scholar]
- Eierhoff T., Hrincius E.R., Rescher U., Ludwig S., and Ehrhardt C. (2010). The Epidermal Growth Factor Receptor (EGFR) Promotes uptake of Influenza A Viruses (IAV) into host cells. PLoS Pathog 6, e1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Cooper A., Shi W., Bornmann W., Carrion R., Kalman D., and Nabel G.J. (2012). Productive replication of Ebola virus is regulated by the c-Abl1 tyrosine kinase. Sci Transl Med 4, 123ra124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart T.L., and Bouchard M.J. (2010). The hepatitis B virus X protein modulates hepatocyte proliferation pathways to stimulate viral replication. J Virol 84, 2675–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S., Rahal R., Stransky N., Lengauer C., and Hoeflich K.P. (2015). Targeting cancer with kinase inhibitors. J Clin Invest 125, 1780–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guendel I., Agbottah E.T., Kehn-Hall K., and Kashanchi F. (2010). Inhibition of human immunodeficiency virus type-1 by cdk inhibitors. AIDS Res Ther 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habran L., Bontems S., Di Valentin E., Sadzot-Delvaux C., and Piette J. (2005). Varicella-zoster virus IE63 protein phosphorylation by roscovitine-sensitive cyclin-dependent kinases modulates its cellular localization and activity. J Biol Chem 280, 29135–29143 [DOI] [PubMed] [Google Scholar]
- Hale B.G., Jackson D., Chen Y.H., Lamb R.A., and Randall R.E. (2006). Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc Natl Acad Sci U S A 103, 14194–14199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqqani A.A., and Tilton J.C. (2013). Entry inhibitors and their use in the treatment of HIV-1 infection. Antiviral Res 98, 158–170 [DOI] [PubMed] [Google Scholar]
- Hirsch A.J., Medigeshi G.R., Meyers H.L., DeFilippis V., Früh K., Briese T., Lipkin W.I., and Nelson J.A. (2005). The Src family kinase c-yes is required for maturation of West Nile virus particles. J Virol 79, 11943–11951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaw J., Hill P., and Goodman D. (2017). Combination of leflunomide and everolimus for treatment of BK virus nephropathy. Nephrology 22, 326–329 [DOI] [PubMed] [Google Scholar]
- Jiang W.-M., Zhang X.-Y., Zhang Y.-Z., Liu L., and Lu H.-Z. (2014). A high throughput RNAi screen reveals determinants of HIV-1 activity in host kinases. Int J Clin Exp Pathol 7, 2229–2237 [PMC free article] [PubMed] [Google Scholar]
- Johnson J.C., Martinez O., Honko A.N., Hensley L.E., Olinger G.G., and Basler C.F. (2014). Pyridinyl imidazole inhibitors of p38 MAP kinase impair viral entry and reduce cytokine induction by Zaire ebolavirus in human dendritic cells. Antiviral Res 107, 102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.H., Moon K.C., Ha J.W., Kim S.-J., Ha I.-S., Cheong H.I., and Kang H.G. (2013). Leflunomide therapy for BK virus allograft nephropathy after pediatric kidney transplantation. Pediatr Transplant 17, E50–E54 [DOI] [PubMed] [Google Scholar]
- Karaman M.W., Herrgard S., Treiber D.K., Gallant P., Atteridge C.E., Campbell B.T., Chan K.W., Ciceri P., Davis M.I., Edeen P.T., et al. (2008). A quantitative analysis of kinase inhibitor selectivity. Nat Biotech 26, 127–132 [DOI] [PubMed] [Google Scholar]
- Keating J.A., and Striker R. (2012). Phosphorylation events during viral infections provide potential therapeutic targets. Rev Med Virol 22, 166–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., Traynor D., Johnson R.F., Dyall J., Kuhn J.H., et al. (2015). Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for middle east respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother 59, 1088–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight Z.A., Lin H., and Shokat K.M. (2010). Targeting the cancer kinome through polypharmacology. Nat Rev Cancer 10, 130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolokoltsov A.A., Saeed M.F., Freiberg A.N., Holbrook M.R., and Davey R.A. (2009). Identification of novel cellular targets for therapeutic intervention against Ebola virus infection by siRNA screening. Drug Dev Res 70, 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Agrawal T., Khan N.A., Nakayama Y., and Medigeshi G.R. (2016). Identification and characterization of the role of c-terminal Src kinase in dengue virus replication. Sci Rep 6, 30490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhammer S., Koban R., Yue C., and Ellerbrok H. (2011). Inhibition of poxvirus spreading by the anti-tumor drug Gefitinib (Iressa). Antiviral Res 89, 64–70 [DOI] [PubMed] [Google Scholar]
- Li J., Li H., and Tsai M.D. (2003). Direct binding of the N-terminus of HTLV-1 tax oncoprotein to cyclin-dependent kinase 4 is a dominant path to stimulate the kinase activity. Biochemistry 42, 6921–6928 [DOI] [PubMed] [Google Scholar]
- Li Q., Brass A., Ng A., Hu Z., Xavier R., Liang T.J., and Elledge S. (2009). A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc Natl Acad Sci U S A 106, 16410–16415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Pene V., Krishnamurthy S., Cha H., and Liang T.J. (2013). Hepatitis C virus infection activates an innate pathway involving IKK-alpha in lipogenesis and viral assembly. Nat Med 19, 722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liacini A., Seamone M.E., Muruve D.A., and Tibbles L.A. (2010). Anti-BK virus mechanisms of sirolimus and leflunomide alone and in combination: toward a new therapy for BK virus infection. Transplantation 90, 1450–1457 [DOI] [PubMed] [Google Scholar]
- Lupberger J., Zeisel M., Xiao F., Thumann C., Fofana I., Zona L., Davis C., Mee C., Turek M., Gorke S., et al. (2011). EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med 17, 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannová P., and Beretta L. (2005). Activation of the N-Ras–PI3K–Akt-mTOR pathway by hepatitis C virus: control of cell survival and viral replication. J Virol 79, 8742–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narute P.S., and Smithgall T.E. (2012). Nef alleles from all major HIV-1 clades activate Src-family kinases and enhance HIV-1 replication in an inhibitor-sensitive manner. PLoS One 7, e32561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth G., Varga Z., Greff Z., Bencze G., Sipos A., Szantai-Kis C., Baska F., Gyuris A., Kelemenics K., Szathmary Z., et al. (2011). Novel, selective CDK9 inhibitors for the treatment of HIV infection. Curr Med Chem 18, 342–358 [DOI] [PubMed] [Google Scholar]
- Neveu G., Barouch-Bentov R., Ziv-Av A., Gerber D., Jacob Y., and Einav S. (2012). Identification and targeting of an interaction between a tyrosine motif within hepatitis C virus core protein and AP2M1 essential for viral assembly. PLoS Pathog 8, e1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu G., Ziv-Av A., Barouch-Bentov R., Berkerman E., Mulholland J., and Einav S. (2015). AP-2-associated protein kinase 1 and cyclin G-associated kinase regulate hepatitis C virus entry and are potential drug targets. J Virol 89, 4387–4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott P.A., and Adams S. (2011). Small-molecule protein kinase inhibitors and their effects on the immune system: implications for cancer treatment. Immunotherapy 3, 213–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M.A., Tibaldi E., Palu G., and Brunati A.M. (2013). Viral proteins and Src family kinases: mechanisms of pathogenicity from a “liaison dangereuse.” World J Virol 2, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls E., Badia R., Torres-Torronteras J., Ruiz A., Permanyer M., Riveira-Munoz E., Clotet B., Marti R., Ballana E., and Este J.A. (2014a). Palbociclib, a selective inhibitor of cyclin-dependent kinase4/6, blocks HIV-1 reverse transcription through the control of sterile alpha motif and HD domain-containing protein-1 (SAMHD1) activity. AIDS (London, England) 28, 2213–2222 [DOI] [PubMed] [Google Scholar]
- Pauls E., Ruiz A., Badia R., Permanyer M., Gubern A., Riveira-Munoz E., Torres-Torronteras J., Alvarez M., Mothe B., Brander C., et al. (2014b). Cell cycle control and HIV-1 susceptibility are linked by CDK6-dependent CDK2 phosphorylation of SAMHD1 in myeloid and lymphoid cells. J Immunol 193, 1988–1997 [DOI] [PubMed] [Google Scholar]
- Perwitasari O., Yan X., O'Donnell J., Johnson S., and Tripp R.A. (2015). Repurposing kinase inhibitors as antiviral agents to control influenza A virus replication. Assay Drug Dev Technol 13, 638–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleschka S., Wolff T., Ehrhardt C., Hobom G., Planz O., Rapp U.R., and Ludwig S. (2001). Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat Cell Biol 3, 301–305 [DOI] [PubMed] [Google Scholar]
- Reeves P.M., Smith S.K., Olson V.A., Thorne S.H., Bornmann W., Damon I.K., and Kalman D. (2011). Variola and monkeypox viruses utilize conserved mechanisms of virion motility and release that depend on abl and SRC family tyrosine kinases. J Virol 85, 21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schang L.M. (2003). The cell cycle, cyclin-dependent kinases, and viral infections: new horizons and unexpected connections. Prog Cell Cycle Res 5, 103–124 [PubMed] [Google Scholar]
- Schang L.M., St. Vincent M.R., and Lacasse J.J. (2006). Five years of progress on cyclin-dependent kinases and other cellular proteins as potential targets for antiviral drugs. Antiviral Chem Chemother 17, 293–320 [DOI] [PubMed] [Google Scholar]
- Schleiss M., Eickhoff J., Auerochs S., Leis M., Abele S., Rechter S., Choi Y., Anderson J., Scott G., Rawlinson W., et al. (2008). Protein kinase inhibitors of the quinazoline class exert anti-cytomegaloviral activity in vitro and in vivo. Antiviral Res 79, 49–61 [DOI] [PubMed] [Google Scholar]
- Shives K.D., Beatman E.L., Chamanian M., O'Brien C., Hobson-Peters J., and Beckham J.D. (2014). West nile virus-induced activation of mammalian target of rapamycin complex 1 supports viral growth and viral protein expression. J Virol 88, 9458–9471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekova L., Supek F., Lee J., Chen S., Gray N., Pezacki J.P., Schlapbach A., and Schultz P.G. (2008). Identification of human kinases involved in hepatitis C virus replication by small interference RNA library screening. J Biol Chem 283, 29–36 [DOI] [PubMed] [Google Scholar]
- Swimm A.I., Bornmann W., Jiang M., Imperiale M.J., Lukacher A.E., and Kalman D. (2010). Abl family tyrosine kinases regulate sialylated ganglioside receptors for polyomavirus. J Virol 84, 4243–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufts Center for the Study of Drug Development. (2014). Cost to develop and win 279 marketing approval for a new drug is $2.6 billion. [online] Available at: http://csdd.tufts.edu/news/complete_story/pr_tufts_csdd_2014_cost_study (accessed October10, 2017)
- Ueki I.F., Min-Oo G., Kalinowski A., Ballon-Landa E., Lanier L.L., Nadel J.A., and Koff J.L. (2013). Respiratory virus–induced EGFR activation suppresses IRF1-dependent interferon λ and antiviral defense in airway epithelium. J Exp Med 210, 1929–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman W.J., Knight D.A., Blinder L., Shen J., Lurain N.S., Miller D.M., Sedmak D.D., Williams J.W., and Chong A.S. (1999). Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology 42, 412–418 [DOI] [PubMed] [Google Scholar]
- Wang X., Huang D.Y., Huong S.-M., and Huang E.-S. (2005). Integrin αvβ3 is a Coreceptor for Human Cytomegalovirus Infection. Nat Med 11, 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Huong S.-M., Chiu M.L., Raab-Traub N., and Huang E.-S. (2003). Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424, 456–461 [DOI] [PubMed] [Google Scholar]
- Weller M.L., Amornphimoltham P., Schmidt M., Wilson P.A., Gutkind J.S., and Chiorini J.A. (2010). Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat Med 16, 662–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.W., Javaid B., Kadambi P.V., Gillen D., Harland R., Thistlewaite J.R., Garfinkel M., Foster P., Atwood W., Millis J.M., et al. (2005). Leflunomide for polyomavirus type BK nephropathy. N Engl J Med 352, 1157–1158 [DOI] [PubMed] [Google Scholar]
- Xu M., Lee E.M., Wen Z., Cheng Y., Huang W.-K., Qian X., Tcw J., Kouznetsova J., Ogden S.C., Hammack C., et al. (2016). Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 22, 1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Onogi H., Kii I., Yoshida S., Iida K., Sakai H., Abe M., Tsubota T., Ito N., Hosoya T., et al. (2014). CDK9 inhibitor FIT-039 prevents replication of multiple DNA viruses. J Clin Invest 124, 3479–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., and Gabuzda D. (1999). Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J Virol 73, 3460–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri C.A., Fortin J.-F., Nolan G.P., and Nabel G.J. (2007). The ERK Mitogen-activated protein kinase pathway contributes to Ebola virus glycoprotein-induced cytotoxicity. J Virol 81, 1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K., Kitazato K., and Wang Y. (2014). Viruses exploit the function of epidermal growth factor receptor. Rev Med Virol 24, 274–286 [DOI] [PubMed] [Google Scholar]
- Zona L., Lupberger J., Sidahmed-Adrar N., Thumann C., Harris, Helen J., Barnes A., Florentin J., Tawar, Rajiv G., Xiao F., Turek M., et al. (2013). HRas signal transduction promotes hepatitis C virus cell entry by triggering assembly of the host tetraspanin receptor complex. Cell Host Microbe 13, 302–313 [DOI] [PubMed] [Google Scholar]