Abstract
Epigenetic perturbations induced by environmental exposures at susceptible lifestages contribute to disease development. Even so, the influence of early life and ongoing exposures on the adolescent epigenome is rarely examined. We examined the association of exposure biomarkers for lead (Pb), bisphenol A (BPA), and nine phthalates metabolites with blood leukocyte DNA methylation at LINE-1 repetitive elements and environmentally responsive genes ( IGF2 , H19 , and HSD11B2 ) in peri-adolescents. Participants ( n = 247) from the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) birth cohorts were followed-up once between the ages of 8 and 14 years, and concurrent exposures were measured in biospecimen collected at that time (blood Pb, urinary BPA, and phthalate metabolites). Prenatal and childhood exposures to Pb were previously approximated using maternal and child samples. BPA and phthalate metabolites were measured in third trimester maternal urine samples. Significant associations ( P < 0.05) were observed between DNA methylation and exposure biomarkers that were gene and biomarker specific. For example, Pb was only associated with LINE-1 hypomethylation during pregnancy ( P = 0.04), while early childhood Pb was instead associated with H19 hypermethylation ( P = 0.04). Concurrent urinary mono (2-ethylhexyl) phthalate (MEHP) was associated with HSD11B2 hypermethylation ( P = 0.005). Sex-specific associations, particularly among males, were also observed. In addition to single exposure models, principal component analysis was employed to examine exposure mixtures. This method largely corroborated the findings of the single exposure models. This study along with others in the field suggests that environment-epigenetic relationships vary by chemical, exposure timing, and sex.
Keywords: prenatal exposures, childhood exposures, DNA methylation, lead, phthalates, bisphenol A
Introduction
Exposures to heavy metals such as lead (Pb) and other endocrine disrupting chemicals (EDCs) including bisphenol A (BPA) and phthalates pose a public health burden across the globe. A major source of Pb exposure, tetraethyl Pb as a gasoline additive, has been banned in most countries and exposure is decreasing worldwide [ 1 ]. Even so, exposure sources such as Pb-glazed ceramics (in Mexico) [ 2 , 3 ], electronics, historic leaded paint, and Pb batteries [ 4 ] remain problematic around the globe. BPA, a high-production chemical used in the manufacture of epoxy resins, thermal paper, and polycarbonate plastics is encountered in various aspects of everyday life including handling receipts and consuming canned foods [ 5 , 6 ]. BPA levels are detectable in many populations including pregnant women and children in Mexico and the US [ 7–9 ]. Phthalate plasticizer exposures result from a variety of products including personal care products and are also common among adults and children [ 7 , 10 ].
Pregnant women and developing children are particularly susceptible to the effects of EDCs. In Mexico, leaded gasoline was in use until the 1990s and exposure through Pb-glazed ceramics is ongoing [ 2 , 3 ]. These early life exposures may have health implications for today’s adolescents and adults who were exposed in utero and beyond. Furthermore, BPA levels and various phthalate metabolites were detectable in a majority of pregnant Mexican women from the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) cohort [ 11 ]. Their children also had a range of detectable urinary BPA and phthalate levels in urine samples collected during peri-adolescence [ 7 , 11 ].
Evidence for the implication of early life exposures to Pb, BPA, and phthalates on growth trajectories and adiposity at various stages through adolescence and sexual maturation is growing [ 11–19 ]. Impacts on early childhood growth from in utero and childhood Pb exposure include lower birth weight [ 20 ], lower birth length and smaller head circumference [ 21 ], less weight gain in the first month of life among breastfed infants [ 14 ], decreased weight among girls at age five [ 16 ], and decreased height by four years of age [ 15 ]. Prenatal BPA exposure is associated with lower BMI and percent body fat among girls [ 12 ], while childhood exposure is associated with accelerated growth from 2 to 5 years [ 13 ] and higher obesity-related characteristics among girls and boys at age 9 [ 12 ]. In utero phthalate exposure has been linked to preterm birth [ 22 ] which is a risk factor for latent cardiometabolic and respiratory health problems [ 23 , 24 ]. Phthalate exposures during childhood through adolescence have also been linked to altered growth, hormone levels, puberty onset, and fat distributions among children and peri-adolescents, many relationships of which were sex-specific [ 11 , 17–19 , 22 , 25 ].
A common theme apparent among these EDCs (Pb, BPA, and phthalates) is the ability of early exposures to impact growth and body composition – both temporally close to the timing of exposure and also with a degree of latency. A growing body of evidence suggests that epigenetic perturbations set the landscape for development of disease and latent phenotypic changes, and that windows of susceptibility exist in which the epigenome is especially malleable. Gestation represents a sensitive window during which epigenetic perturbations can persist throughout life and influence susceptibility to latent disease as posited by the Developmental Origins of Health and Disease (DOHaD) hypothesis [ 26 , 27 ]. Perinatal exposures to Pb, BPA, and phthalates have been linked to epigenetic changes in animal models [ 28–31 ] and in human samples [ 32–35 ]. DNA methylation patterns at specific genes can impact growth and body composition of adolescents. Both persistent epigenetic perturbations induced by in utero exposures and epigenetic changes accumulated from continued EDC exposures may contribute to growth and metabolic phenotypes in adolescents. For example, hypermethylation in peripheral blood DNA at the imprint control region of IGF2/H19 was associated with greater skin fold thickness and subcutaneous adiposity among 17 year olds [ 36 ]. Even so, the adolescent epigenome is rarely examined with regards to concurrent or previous toxicant exposures. Exceptions include a study of prenatal cigarette smoke exposure that revealed persistent DNA methylation alterations measured in cord blood and in samples from children and adolescents [ 37 ]. In a cross-sectional study of prepubescent Egyptian girls, urinary BPA concentrations were associated with altered DNA methylation at specific sites identified by a genome-wide array enriched for gene promoters [ 38 ].
Using a sample ( n = 247) from the rich set of longitudinal birth cohorts collectively called ELEMENT, we examine associations of Pb, BPA, and phthalate exposure biomarkers from multiple developmental stages, including prenatally, with DNA methylation of repetitive elements (LINE-1) and environmentally responsive genes ( IGF2 , H19 , and HSD11B2 ) in peri-adolescents (PA, ages 8–14 years). Specifically, we aim to: (1) determine associations between early life exposures (to Pb, BPA, and phthalates in utero and Pb in early childhood) and methylation at these regions in PA blood leukocyte DNA, (2) determine associations between concurrent exposures to Pb, BPA, and phthalates and PA blood leukocyte DNA methylation in a cross-sectional analysis, and (3) explore whether there are groups of exposure biomarkers that jointly predict DNA methylation levels.
Results
Descriptive Statistics
Characteristics of the study population including exposure biomarker levels from all follow up visits can be found in Table 1 . Children were between the ages of 8 and 14 years at the time of the PA study visit with a mean of 10.3 years of age. 53% of the participants are female. Only 2% of the mothers reported any smoking during pregnancy.
Table 1:
Characteristics of the study population including biomarker levels of in utero , childhood, and peri-adolescent (PA) exposures and DNA methylation at LINE-1 and three environmentally responsive genes.
| N | % | Mean (SD) | Geometric Mean (95% CI) | Min a | Max | ||
|---|---|---|---|---|---|---|---|
| Sex | Males | 117 | 47.4 | ||||
| Females | 130 | 52.6 | |||||
| Age at PA Study Visit (yr) | 247 | 10.3 (1.7) | 8 | 14 | |||
| Maternal Smoking | Smoked During Pregnancy | 5 | 2.0 | ||||
| Did Not Smoke During Pregnancy | 242 | 98.0 | |||||
| Specific Gravity | Maternal Trimester 3 Urine (T3) | 220 | 1.013 (0.006) | 1.002 | 1.042 | ||
| Children's PA Urine | 239 | 1.017 (0.007) | 1.002 | 1.034 | |||
| Pb Continuous | Maternal Tibia (µg/g) | 138 | 7.44 (9.71) | −15.08 | 34.51 | ||
| Maternal Patella (µg/g) | 219 | 8.92 (10.24) | −11.94 | 47.07 | |||
| Pregnancy, Average Maternal Blood Pb (µg/dL) | 231 | 4.51 (4.15–4.90) | 0.43 | 19.80 | |||
| Early Childhood Average Blood Pb (µg/dL) | 246 | 4.53 (4.27–4.79) | 1.25 | 15.27 | |||
| Late Childhood Blood (µg/dL) | 167 | 2.89 (2.65–3.16) | 0.79 | 25.09 | |||
| PA Blood Pb (µg/dL) | 247 | 2.75 (2.57–2.95) | 0.98 | 20.00 | |||
| Pb Categorical b | All Low (LLL) | 52 | 22.6 | ||||
| Low in pregnancy (LHH, LLH, LHL) | 62 | 27.0 | |||||
| High in pregnancy (HLL, HHL, HLH) | 66 | 28.7 | |||||
| All High (HHH) | 50 | 21.7 | |||||
| Bisphenol A (BPA) (µg/L) | T3 Urine | 220 | 0.70 (0.63–0.79) | <LOQ | 18.70 | ||
| PA Urine | 239 | 1.21 (1.08–1.37) | <LOQ | 33.20 | |||
| Phthalates (µg/L) | Monoethyl phthalate (MEP), T3 | 220 | 112.8 (93.19–136.5) | <LOQ | 9810.00 | ||
| Monoethyl phthalate (MEP), PA | 239 | 82.04 (68.86–97.75) | 3.77 | 4970.00 | |||
| Mono n-butyl phthalate (MBP), T3 | 220 | 53.73 (45.86–62.96) | 1.18 | 1190.00 | |||
| Mono n-butyl phthalate (MBP), PA | 238 | 101.3 (89.90–114.1) | 6.05 | 1760.00 | |||
| Mono-isobutyl phthalate (MIBP), T3 | 220 | 1.90 (1.65–2.18) | <LOQ | 40.10 | |||
| Mono-isobutyl phthalate (MIBP), PA | 239 | 10.17 (9.15–11.31) | 0.97 | 121.00 | |||
| Mono(3-carboxypropyl) phthalate (MCPP), T3 | 220 | 1.09 (0.95–1.24) | <LOQ | 11.10 | |||
| Mono(3-carboxypropyl) phthalate (MCPP), PA | 239 | 2.15 (1.91–2.41) | <LOQ | 140.00 | |||
| Monobenzyl phthalate (MBzP), T3 | 220 | 4.26 (3.75–4.84) | <LOQ | 109.00 | |||
| Monobenzyl phthalate (MBzP), PA | 239 | 5.69 (5.08–6.36) | 0.23 | 177.00 | |||
| Mono(2-ethylhexyl) phthalate (MEHP), T3 | 220 | 5.00 (4.37–5.72) | <LOQ | 62.00 | |||
| Mono(2-ethylhexyl) phthalate (MEHP), PA | 238 | 5.76 (5.12–6.48) | <LOQ | 203.00 | |||
| Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), T3 | 220 | 19.23 (16.74–22.10) | 0.19 | 167.00 | |||
| Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), PA | 238 | 45.55 (40.83–50.81) | 2.62 | 2100.00 | |||
| Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), T3 | 220 | 11.65 (10.16–13.35) | 0.11 | 133.00 | |||
| Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), PA | 238 | 20.14 (18.07–22.45) | 1.09 | 751.00 | |||
| Mono(2-ethyl-5-carboxypentyl) phthalate, (MECPP), T3 | 220 | 31.32 (27.52–35.64) | 0.29 | 251.00 | |||
| Mono(2-ethyl-5-carboxypentyl) phthalate, (MECPP), PA | 238 | 62.32 (56.21–69.09) | 4.15 | 1620.00 | |||
| ∑ DEHP Metabolites c , T3 | 220 | 68.15 (60.06–77.33) | 1.32 | 539.2 | |||
| ∑ DEHP Metabolites c , PA | 238 | 132.6 (119.4–147.2) | 9.85 | 4597 | |||
| DNA Methylation (%) | LINE-1 d | 243 | 78.5 (2.3) | 69.3 | 83.2 | ||
| H19 d | 244 | 58.4 (5.1) | 49.3 | 81.5 | |||
| IGF2 | 229 | 45.2 (4.2) | 31.0 | 58.2 | |||
| HSD11B2 d | 246 | 8.89 (1.08) | 6.02 | 13.4 |
LOQ = limit of quantitation.
Pb broken down in low/high (<median, ≥median) for: (1) average maternal blood Pb during pregnancy (median = 4.7 µg/dL), (2) child blood Pb at 1–4 years (up to four measures; median = 4.5 µg/dL), (3) PA blood Pb (median = 2.6 µg/dL).
DEHP = di-2-ethylhexyl phthalate, metabolites are MEHP, MEHHP, MEOHP, and MECPP.
Standardized to controls within each batch due to experimental batch effects.
As shown in Table 1 , a wide range of Pb levels were observed representing exposures during pregnancy, early childhood, late childhood, and PA, including blood Pb levels above reference levels for children (5 µg/dL according to the Centers for Disease Control and Prevention). Biomarkers of in utero Pb exposure were significantly correlated with one another (Spearman’s ρ 0.39–0.44, P < 0.0001). Likewise, blood Pb levels in children measured in early and late childhood and at PA were correlated (ρ 0.39–0.68, P < 0.0001). Pb levels among children did not differ by sex (data not shown).
Urinary BPA concentrations varied widely among mothers in T3 (<LOQ – 18.7 µg/L) and children at PA, and a substantial portion of T3 and PA samples fell below the LOQ (31 and 15%, respectively). BPA concentrations from T3 and PA were mildly correlated (ρ 0.18, P = 0.007). BPA did not significantly differ by sex.
Maternal (from T3) and child urinary (PA) concentrations of nine phthalate metabolites are displayed in Table 1 . Within the same sample type, phthalate metabolites were significantly correlated with one another (ρ 0.23–0.99, P < 0.0001) and with BPA (ρ 0.30–0.59, P < 0.0001). Except for MEP (higher concentrations among girls, ANOVA P = 0.03), sex-differences in PA phthalate metabolites or BPA were not apparent.
Table 1 also reports average percent methylation, standardized to batch when necessary, at LINE-1 and three genes ( H19 , IGF2 , and HSD11B2 ) quantified via Sequenom EpiTYPER ( IGF2 ) or pyrosequencing (all others) using bisulfite converted DNA from PA blood leukocytes. Average methylation at LINE-1 was significantly higher among boys (ANOVA P -value 0.005), and IGF2 methylation was higher among girls (ANOVA P -value 0.03).
Pb and DNA Methylation
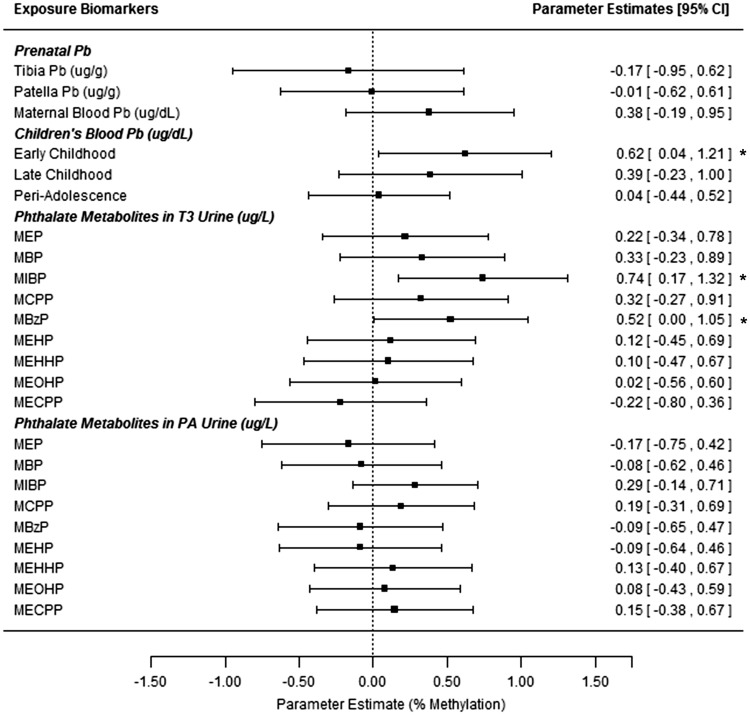
Pb biomarkers from specific developmental stages were associated with PA DNA methylation ( P < 0.05). Average maternal blood Pb during pregnancy was associated with hypomethylation of LINE-1 ( Supplemental Figure 1 and Supplemental Table 3 ). One IQR increase in maternal blood Pb (natural-log transformed) was associated with a 0.29% decrease in LINE-1 methylation ( P = 0.04). Early childhood Pb exposure was associated with H19 hypermethylation ( Supplemental Table 4, Figure 2 ). An IQR increase in early childhood blood Pb (natural-log transformed) was associated with 0.62% higher H19 methylation ( P = 0.037). Marginally significant interaction terms ( P < 0.1) between sex and Pb were observed for LINE-1 (tibia and early childhood Pb), H19 (tibia Pb), and HSD11B2 (patella Pb). Sex-stratified models revealed several significant ( P < 0.05) relationships between Pb and DNA methylation primarily among boys ( Supplemental Table 5 ). LINE-1 methylation decreased with higher maternal tibia Pb ( P = 0.03) but increased with early childhood blood Pb ( P = 0.006). HSD11B2 hypermethylation was also associated with maternal patella Pb among males ( P = 0.016).
Biomarkers of Pb exposure at three potential windows of susceptibility (the in utero period, early childhood, and PA) were available for the majority of subjects ( n = 230). In the categorical Pb analysis ( Supplemental Figure 3 ), H19 methylation was hypermethylated (1.32%) among individuals with high Pb at all three windows of exposure compared with those with low Pb at the three windows ( P = 0.073, Supplemental Table 4 ). In a post hoc analysis examining seven individual exposure combinations (with LLL as the reference), four groups were associated with H19 methylation: HHH, HHL, LHH, and LHL (parameter estimates 1.37–2.55, P -values 0.019–0.088). This suggests that the association is driven by early childhood Pb exposure, regardless of exposure at other developmental stages. Sex differences were observed with LINE-1 methylation and the three windows of Pb exposure ( Supplemental Table 5 ). Females with high Pb exposure during pregnancy (HHL, HLH, or HLL) exhibited LINE-1 hypomethylation ( P = 0.029). Males in the low pregnancy Pb category (LHH, LHL, or LLH) and the high category (HHH) had LINE-1 hypermethylation ( P = 0.026 and 0.042) compared with LLL males.
BPA and DNA Methylation
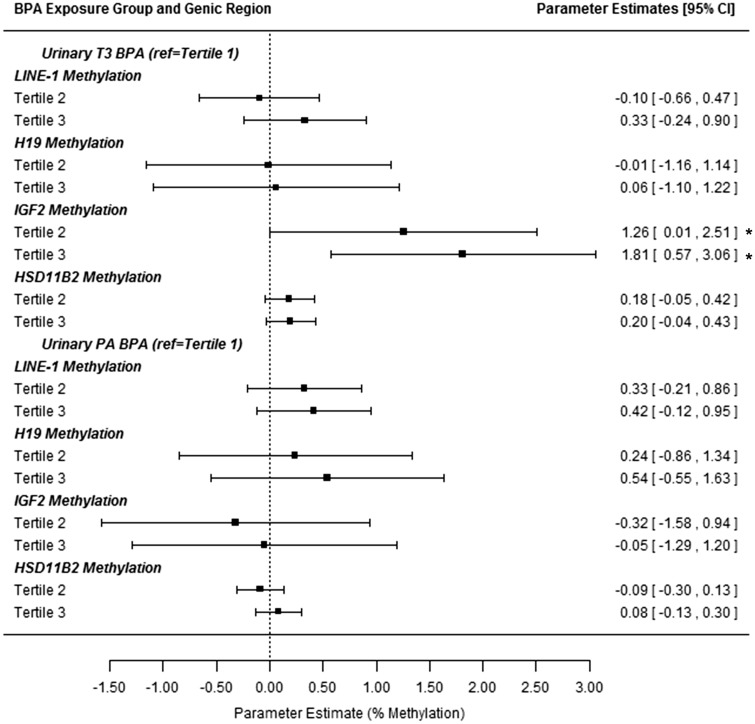
Since 31 and 15% of maternal and child urinary BPA concentrations fell below the LOQ, associations of tertiles of BPA exposure with DNA methylation were examined ( Figure 4 ). Compared with the lowest tertile of maternal T3 urinary BPA, children in the second and highest tertiles had 1.26 and 1.81% higher IGF2 methylation ( P = 0.005 for trend). Concurrent BPA tertiles were not significantly associated with methylation ( Supplemental Table 4 ).
Figure 4:
Associations between tertiles of BPA exposure and peri-adolescent DNA methylation at four regions. BPA was measured in maternal urine samples from trimester 3 (T3) and children’s urine samples at the PA study visit. All estimates are from repeated measures models of methylation at LINE-1, H19 , HSD11B2 , and IGF2 (at least 4 CpG sites for each). Models adjust for children’s age and sex. Estimates are interpreted as the change in % DNA methylation for tertile 2 or 3 compared with the lowest tertile. *Denotes significant associations at the P ≤ 0.05 level.
Phthalate Metabolites and DNA Methylation
After correcting for specific gravity, maternal T3 urinary MBzP and MIBP were positively associated with H19 methylation ( P ≤ 0.05, Supplemental Table 3, Figure 2 ). An IQR-increase in ln-transformed and specific gravity adjusted MIBP or MBzP was associated with a 0.74% or 0.52% increase in H19 methylation, respectively.
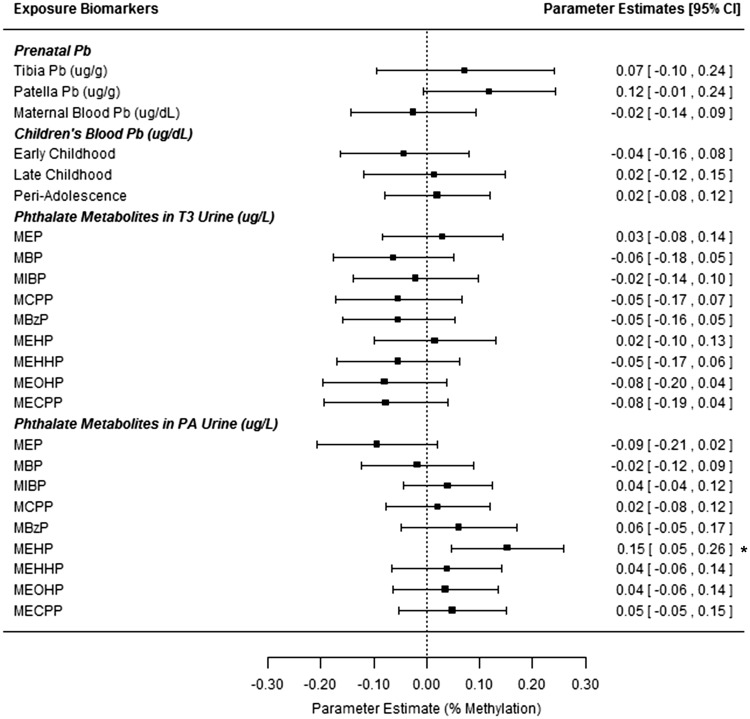
Concurrent MEHP exposure was associated with hypermethylation at HSD11B2 following correction for urinary specific gravity ( Figure 3 , Supplemental Table 4 ). An IQR increase in ln-transformed MEHP concentrations was associated with 0.15% higher methylation at HSD11B2 promoter ( P = 0.005). Interactions between concurrent phthalates and sex ( P < 0.1) were observed. Sex stratified models ( Supplemental Table 5 ) revealed opposite associations of concurrent MCPP on H19 methylation by sex ( P = 0.03 for each in specific gravity corrected models). Hypermethylation of HSD11B2 with increasing MEHP concentrations was stronger among males ( P = 0.002).
Figure 3:
associations between peri-adolescent HSD11B2 methylation and exposure biomarkers from multiple developmental stages. All estimates are from repeated measures models for HSD11B2 methylation at 4 CpG sites (from blood leukocyte DNA, PA study visit). Models adjust for children’s age and sex. All estimates give the change in % DNA methylation for an IQR range increase in the exposure biomarker (based on ln-transformed values for all biomarkers except for tibia and patella Pb). *Denotes significant association at the P < 0.05 level. T3= trimester 3 (maternal samples).
Exposure Mixtures and DNA Methylation
Four principal components were created representing 80% of the variability of urinary T3 BPA and nine phthalate metabolites ( Supplemental Table 2 ). T3 component 1 primarily represents DEHP metabolites, component 2 represents MBP, MBzP, MIBP, and MCPP, component 3 is driven by MEP, and component 4 by BPA. When modeled with these components, maternal blood Pb was still significantly associated with LINE-1 hypomethylation ( P = 0.03), and component 2 predicted H19 hypermethylation ( P = 0.006; Supplemental Table 6 ).
In the concurrent exposures analysis, four components contained 82% of the variability of PA BPA and phthalate metabolites with PA component 1 primarily representing DEHP metabolites, component 2 representing MBP and MCPP, component 3 representing BPA and to a lesser extent MIBP and MBzP, and component 4 driven by MEP ( Supplemental Table 2 ). None of the exposures were significantly associated when these components were modeled along with children’s blood Pb at the PA visit. In the third PCA-based analysis with Pb from three developmental periods and components from both T3 and PA, associations observed in the first and second PCA models remained ( P < 0.05), and T3 component 4 was associated with IGF2 hypermethylation ( Supplemental Table 6 ).
Overall, exposure biomarkers significantly associated with DNA methylation in the single exposure models were also identified as important in the principal component mixture models with the exception of early childhood Pb and H19 hypermethylation ( P = 0.1, model 3) and PA MEHP and HSD11B2 hypermethylation ( P = 0.07 for the component representing DEHP metabolites, model 3). Additional exposures of interest were not identified with the PCA method.
Discussion
In a longitudinal birth cohort, we observed associations ( P < 0.05) between exposures to Pb, BPA, and phthalates at multiple developmental stages and blood leukocyte DNA methylation in children followed up through ages 8–14 years. Among all children, maternal blood Pb during pregnancy was associated with LINE-1 hypomethylation. Prenatal BPA exposure was associated with IGF2 hypermethylation. Maternal T3 urinary phthalate metabolite levels (MBzP and MIBP) and early childhood blood Pb were associated with H19 hypermethylation. In the cross-sectional analysis, concurrent levels of the phthalate metabolite MEHP were associated with HSD11B2 hypermethylation. Sex-specific associations were also observed ( Supplemental Table 5 ) with males exhibiting more significant exposure-methylation relationships than females. While these findings were significant with nominal P < 0.05, they would not remain significant at a P -value corrected for multiple hypothesis testing ( P ≤ 0.0018).
Even though results only attained nominal significance, effect sizes were on par with those observed in other epidemiological studies focused on environmental exposures, and evidence from previous studies suggests that small epigenetic effect sizes may be important biologically. For example, in a cross-sectional study of 3-year-old Taiwanese children, hypomethylation of tumor necrosis factor α ( TNFα ) was associated with higher urinary MEHP, and methylation was inversely associated with TNFα protein levels in plasma and mediated 20% of the effect of MEHP on asthma risk [ 39 ]. The effect size observed in the Taiwanese study (0.14% decrease in methylation per ln-transformed unit of MEHP in adjusted models) was similar to effect sizes observed in the present study (e.g., 0.14% increase in HSD11B2 promoter methylation per ln-transformed unit of concurrent urinary MEHP, Supplemental Table 4 ). Though marginally significant ( P = 0.06), the relationship we observed between maternal patella Pb levels and HSD11B2 methylation in PA was similar to that of our previous study examining prenatal Pb exposure and cord blood leukocyte DNA methylation. In the previous study, 10 µg/g maternal patella Pb increases were associated with 0.14% higher methylation of HSD11B2 at birth [ 32 ], and we observed a similar persistent effect (equivalent of 0.09% increase per 10 µg/g patella Pb, Supplemental Table 3 ) among 8–14 year olds.
The results and those of other studies suggest the epigenome may have multiple windows of vulnerability to environmental perturbations. In line with the DOHaD hypothesis, evidence for epigenetic change associated with in utero exposures has been observed in many studies at birth [ 32 , 33 , 35 , 37 ]. Persistent changes associated with prenatal exposures have also been observed in children, adolescents [ 37 ], and adults [ 40 ]. In this study, we observe several associations ( P < 0.05) between biomarkers of in utero exposure and DNA methylation in PA, notably at DMRs of imprinted genes (MIBP and MBzP with H19 ; BPA exposure with IGF2 ) and in LINE-1 repetitive elements (with maternal blood Pb). Additional windows of susceptibility were observed as early childhood Pb exposure (ages 1–4 years) was significantly associated with H19 hypermethylation. Concurrent MEHP concentrations were associated with HSD11B2 hypermethylation.
Our results corroborate the need to examine sex-specific effects in epigenetics and toxicology. Similar to other epigenetic studies, we found males to have higher LINE-1 methylation compared with females [ 32 , 41 ] and noted sex-specific associations between exposure biomarkers and DNA methylation at LINE-1, H19 , and HSD11B2 ( Supplemental Table 5 ). Epigenome-wide studies have reported sex-specific associations between cord blood leukocyte DNA methylation and Pb [ 35 ], cadmium [ 42 ], and arsenic [ 43 ] exposures. Sex-specific epigenetic patterns may underlie differences in gene expression and function, as suggested by an epigenome-wide study using DNA from human prefrontal cortex [ 44 ]. These differences may also contribute to sex differences in response to chemical exposures (e.g., toxicity) as seen in animal models [ 29 , 45 ]. As more evidence arises for the sexually dimorphic impact of exposures to toxicants such as Pb, BPA, and phthalates on growth and body composition [ 11 , 12 , 16 , 18 ], the potential role of epigenetic change in this process will need to be elucidated.
Results from the exposure mixtures analysis via PCA were similar to that of the single-exposure models. For example, in the multi-developmental stage model, maternal blood Pb remained associated with LINE-1 hypomethylation while adjusting for additional exposures. The biomarkers analyzed in this study approximate exposures at two (BPA and phthalate metabolites) or four (Pb) windows in the life course and encompass a small portion of environmental exposures that may influence the epigenome and subsequently phenotype. As better tools emerge to characterize the full exposome – the totality of environmental exposures from conception onwards [ 46 , 47 ] – complex statistical methods beyond PCA will be necessary to analyze the impact of multiple correlated exposures and development of these methods is well underway [ 48 , 49 ].
This study had many advantages including recruitment during pregnancy, follow up through a maximum of 14 years of age, and the assessment of multiple EDC exposures from several windows in development and their impact on DNA methylation. Despite the moderate sample size, we were able to observe nominally significant associations between exposure and DNA methylation, including in sex-stratified analyses. This study had several limitations. There is a potential for selection bias because only a subset of original ELEMENT participants were followed up through PA. Since participants did not know their exposure or DNA methylation levels when deciding whether to participate, we expect selection bias to be minimal. Only four hypothesis-driven regions were selected for DNA methylation analysis, and these loci may not be the most susceptible to perturbation by the chemicals studied here. Epigenome-wide analysis would enable better detection of susceptible sites including those that may be more sensitive to exposure at certain time periods (e.g., metastable epialleles and peri-conceptual exposures [ 50 ]). The biomarker used for in utero BPA and phthalate exposures (maternal T3 urine) may only reflect exposures late in pregnancy, and we lack data on exposures within the first few weeks of life when the epigenome is particularly vulnerable to environmental perturbation.
In conclusion, we observed nominally significant associations ( P < 0.05) between Pb, BPA, and phthalate metabolite levels representing in utero , early childhood, and concurrent exposures and DNA methylation at LINE-1, IGF2 , H19 , and HSD11B2 in blood leukocyte DNA from PA of the longitudinal birth cohort, ELEMENT. This field will benefit from future work modeling multiple exposures at several windows of vulnerability in larger populations of children or young adults to assess the combined burden of the developmental exposome on the epigenome. Ultimately, research in this area should examine epigenetic change as a mediator between exposures at key windows in development, phenotypic change, and risk for disease.
Materials and Methods
Sample Population
This study used data from participants of the longitudinal birth cohort study ELEMENT. The ELEMENT project is a group of sequentially enrolled mother-child cohorts from three maternity hospitals in Mexico City. Families from Cohorts 2 and 3 were included in this study [ 16 , 51 , 52 ]. Initially, 1459 women were recruited from 1997 to 2004 during their first trimester of pregnancy (or, for a subset of Cohort 2, at delivery). Children were subsequently followed up at multiple visits from birth through 5 years of age ( n = 1079). From 2007 to 2009, 622 children attended an additional study visit when they were 6–12 years of age. At the follow up visits, demographic and dietary data were collected via questionnaires, anthropometry measures were taken, and biomarkers (venous blood, spot urine samples) were collected from the children. During pregnancy, biomarkers were also collected from the mothers.
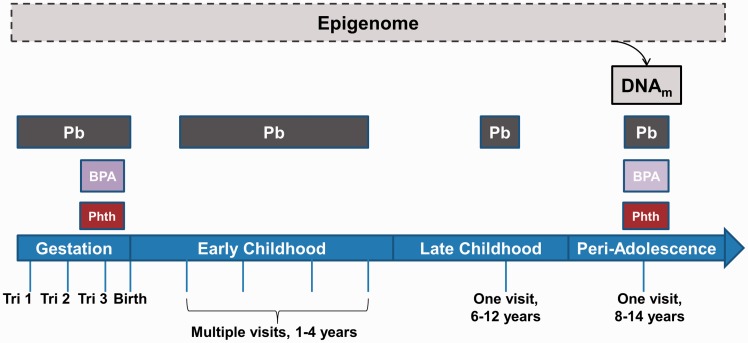
This study includes children who attended an additional follow up visit during “peri-adolescence” (PA) between 2011 and 2012. Children from ELEMENT Cohorts 2 and 3 were re-recruited with priority given to families that had provided at least some of the following biospecimen or exposure measures in the past: maternal urine during pregnancy (highest priority for third trimester samples), cord blood, maternal blood Pb measured during pregnancy, and maternal postpartum bone Pb. Based on statistical power to achieve the overall aims of the larger follow up study and feasibility, 250 children were re-enrolled and all were between the ages of 8 and 14 years old when attending the “PA” study visit. Children’s biospecimen were collected for DNA isolation (blood) and exposure assessment of Pb (blood), BPA and phthalate metabolites (urine). Three children did not provide enough blood for DNA isolation, and therefore 247 are included in the analyses described herein (see Figure 1 for timing of sample collection).
Figure 1:
exposures at multiple developmental stages throughout early life and DNA methylation in peri-adolescents. To examine the impact of both early life and concurrent EDC exposures on the epigenome, DNA methylation was quantified at LINE-1 and three environmentally-responsive genes ( H19 , IGF2 , and HSD11B2 ) in blood leukocyte DNA from 8- to 14-year-old children of the ELEMENT study. The windows of exposure to Pb, BPA, and phthalates estimated by maternal (during gestation) or child (birth and after) biomarkers are depicted. Abbreviations used in figure: Phth = phthalates (9 metabolites), Tri = trimester, DNA m = DNA methylation.
Figure 2:
associations between peri-adolescent H19 methylation and exposure biomarkers from multiple developmental stages. All estimates are from repeated measures models for H19 methylation at 4 CpG sites (from blood leukocyte DNA, PA study visit). Models adjust for children’s age and sex. All estimates give the change in % DNA methylation for an IQR range increase in the exposure biomarker (based on ln-transformed values for all biomarkers except tibia and patella Pb). *Denotes significant associations at the P ≤ 0.05 level. T3 = Trimester 3 (maternal samples).
Prior to participation, study procedures were explained to mothers and children. Mothers provided written consent upon enrollment in the study, and children also provided assent at late childhood and PA study visits. The research protocol was approved by the Human Subjects Committee of the National Institute of Public Health of Mexico, participant hospitals, and the Internal Review Board at all participating institutions including the University of Michigan.
Lead (Pb) Measurements
Lead (Pb) was measured in three biomarkers of in utero exposure: (1) average of maternal venous blood Pb from at least two trimesters, (2) maternal tibia at one month postpartum, and (3) maternal patella at one month postpartum using methods described previously [ 14 ]. Briefly, graphite-furnace atomic absorption spectroscopy via a Perkin-Elmer 3000 (Chelmsford, MA) was used to quantify blood Pb at the American British Cowdray Hospital in Mexico City. Precision and accuracy were validated by comparing measurements with blinded replicates at the Wisconsin State Laboratory of Hygiene as previously detailed [ 20 ]. At 1-month postpartum, maternal cortical and trabecular bone Pb measurements were obtained from the left mid-tibia and left patella, respectively, using a spot-source 109 Cd K-XRF instrument. Details and validation of this procedure are discussed elsewhere [ 53–55 ]. Negative estimates for bone Pb result due to normalization to bone density when the true value is near zero, and these values are often retained in epidemiological studies [ 53 , 56 ].
Venous blood was collected from the children for Pb analysis as a marker of exposure at three developmental stages: (1) early childhood (one to four samples collected at 1, 2, 3, and/or 4 years of age), (2) late childhood (one sample collected between ages 6 and 12 years), and (3) PA (one sample collected 2–3 years later, between ages 8 and 14 years). All blood samples were collected in trace-metals free tubes (Becton-Dickinson, Franklin Lakes, NJ). Early childhood Pb was measured with graphite-furnace atomic absorption spectroscopy (same method as maternal samples). Late childhood and PA samples were analyzed via inductively coupled plasma mass spectrometry (ICP-MS) at the Michigan Department of Community Health Trace Metals Laboratory, a nationally accredited facility for Pb analysis. Quality control included periodically running the blood standard reference material, QMEQAS09B-02 (Institut National de Santé Publique Québec) which averaged 106 ± 2.2% recovery. Sample duplicates reflected good precision (average 2.1 ± 2.4% relative SD).
BPA and Phthalate Metabolite Measurements
Total BPA and phthalate metabolite concentrations were quantified in archived maternal urine samples from the third trimester (T3) and PA children’s urine samples via isotope dilution liquid chromatography-tandem mass spectrometry (ID LC-MS/MS) as previously described by us [ 7 , 19 ]. Phthalate metabolites measured were: mono n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), mono (3-carboxypropyl) phthalate (MCPP), mono (2-ethyl-5-carboxylpentyl) phthalate (MECPP), mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono (2-ethylhexyl) phthalate (MEHP), mono (2-ethyl-5-oxohexyl) phthalate (MEOHP), monoethyl phthalate (MEP), and mono-isobutyl phthalate (MIBP). The analytical methods were modified from the Centers for Disease Control and Prevention Laboratory Procedure Manuals for phthalates and BPA (method no. 6306.03 and 6301.01, respectively) as previously described [ 7 , 19 ]. Calibration curves ranged from correlation coefficients of 0.985 to 1.000. Intra- and inter-day variability was assessed through six replicates spiked at four different concentrations run on three separate days. Accuracy was 85–119% nominal concentration across all analytes, and precision ranged from 1.3% to 10.8% relative SD. Specific gravity of all maternal and child urine was measured with a digital refractometer (ATAGO Company, Ltd., Tokyo, Japan).
Concentrations below the limit of quantitation (LOQ) were assigned a value of LOQ/√2. Specific gravity corrected values were calculated by multiplying the urinary BPA or phthalate metabolite concentration by (SG p − 1)/(SG i − 1), where SG p = average specific gravity of the maternal or children’s study population and SG i = specific gravity of the individual. Specific gravity correction was used when indicated to account for variability due to urinary dilution [ 57 ].
Epigenetic Analyses
Whole blood was collected at the PA study visit in PAXgene tubes, and DNA was isolated from blood leukocytes via the PAXgene Blood DNA kit (PreAnalytiX, Switzerland). DNA was bisulfite converted with Epitect (Qiagen, Valencia, CA) or EZ DNA Methylation kits (Zymo Research, Irvine, CA) according to standard methods which convert unmethylated cytosine to uracil leaving methylated cytosine unchanged [ 58 ].
Methylation (percent of methylated cells) was quantified at global repetitive elements (long interspersed element 1, LINE-1) and three genes (H19 paternally imprinted, maternally expressed transcript (non-coding), H19 ; hydroxysteroid (11-beta) dehydrogenase 2 HSD11B2 ; insulin-like growth factor 2, maternally imprinted, paternally expressed, IGF2 ) via pyrosequencing (LINE-1, H19 , HSD11B2 at 4 CpG sites each) or Sequenom EpiTYPER ( IGF2 at 5 cleaved units representing a total of 7 CpG sites due to the resolution capabilities of EpiTYPER) using assays previously developed by us [ 32 , 59 ] and others [ 60 , 61 ]. LINE-1 was selected as a global marker of repetitive element methylation that has been previously associated with Pb [ 62 , 63 ]. H19 , IGF2 , and HSD11B2 were selected due to previous evidence that methylation levels at these genes are associated with early life exposures to Pb and/or phthalates in humans [ 32 , 33 , 33 , 64 ]. For all regions, sequences were amplified by primers specified in Supplemental Table 1 with HotStarTaq Master Mix (Qiagen) from approximately 50 ng bisulfite-converted DNA. All PCR plates (batches) contained at least two positive controls of known methylation status (0% and 100%). For pyrosequencing assays, reverse primers were biotinylated, and percent methylated cells was quantified by a PyroMark MD Pyrosequencer (Qiagen). With this platform, Pyro Q-CpG Software computes percent methylation and incorporates internal quality control checks (e.g., completed bisulfite conversion, signal vs. background). Samples were run in duplicate, and duplicate reads were averaged. For IGF2 , the Sequenom EpiTYPER was used to quantify methylation. This method involves base-specific cleavage of bisulfite converted DNA followed by analysis with matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) [ 65 ]. A subset of samples was run in duplicate, and values were averaged.
Statistical Analyses
Statistical analyses were performed with SAS v. 9.4 (SAS Institute, Cary, NC). Univariate statistics and distributions of all variables were examined, and non-normally distributed variables were natural log-transformed for downstream analyses. ANOVA tests comparing mean methylation values at each gene across experimental plates (e.g., batches, 5–6 per gene) revealed batch effects in LINE-1, H19 , and HSD11B2 data. Methylation data for these three regions were standardized to reduce batch effects using measures of controls with known methylation from each experimental batch [ 32 ]. Unmethylated (0%) control values from the appropriate batch were subtracted from LINE-1 and HSD11B2 data (and 10 were added to HSD11B2 to avoid negative values after correction). H19 values were multiplied by the average of methylated (100%) controls from all plates divided by the 100% controls for a given batch. Batch standardization was not necessary for IGF2 methylation data.
ANOVA tests were used to compare percent DNA methylation and exposure biomarker levels (natural log transformed when necessary) by sex. Spearman’s rank correlations coefficients were calculated to examine relationships between (1) methylation at CpG sites within the same gene, (2) Pb biomarker levels, and (3) BPA and phthalate metabolites.
Multivariable analyses were employed to examine relationships between methylation at four regions (LINE-1, H19 , IGF2 , and HSD11B2 ) and exposure biomarkers for: (1) Pb ( in utero exposure: maternal tibia and patella Pb, average maternal blood Pb during pregnancy; childhood exposure: early and late; concurrent exposure), (2) BPA ( in utero : T3; concurrent); and (3) nine phthalate metabolites ( in utero : T3; concurrent). Concurrent refers to the exposure biomarkers collected at the same time as the blood sample used for DNA isolation and epigenetic analysis (from the PA study visit). Exposure variables were natural-log transformed when necessary. Due to the number of samples below the LOQ, BPA from the T3 and PA samples was analyzed as tertiles. A general linear model (GLM) for repeated measures using an unstructured variance-covariance matrix was employed to model DNA methylation at each region. DNA methylation at a given gene is the dependent variable, treated as a repeated measure of the methylation values at each CpG site analyzed in that region. The utility of this modeling strategy for analysis of candidate gene methylation data was detailed previously [ 32 ]. Model covariates (age of child at PA visit and sex) were pre-selected due to their biological relevance to methylation data, and exposure variables were included in the model one at a time. Maternal smoking during pregnancy was not included in final models as only 2% of mothers smoked during pregnancy, and maternal smoking was not associated with DNA methylation at any of the four regions studied. Results were considered statistically significant at a nominal P -value <0.05. Due to testing 28 exposure-gene relationships, we also discuss results in the context of a stringent P -value corrected for multiple hypothesis testing ( P ≤ 0.0018). One highly influential subject was removed from all analyses of di-2-ethylhexyl phthalate (DEHP) metabolites (MEHP, MEHHP, MEOHP, and MECPP) at PA, one for MBP at PA, two for maternal blood Pb during pregnancy, and one from H19 analyses.
To explore the combined impact of Pb exposure at multiple time points, a categorical variable was added to the DNA methylation models containing information about exposure levels at three developmental stages: in utero (average maternal blood Pb during pregnancy), early childhood (child blood Pb from 1 to 4 years of age), and PA (child blood Pb at PA visit). Subjects were placed into one of four groups: (1) all low Pb exposures (e.g., LLL; L = below median), (2) low during pregnancy (LLH, LHL, and LHH; H = greater than or equal to the median), (3) high during pregnancy (HLL, HHL, and HLH), and (4) all high Pb exposures (HHH). The four groups were selected because exposure during pregnancy specifically is hypothesized to impact methylation patterns irrespective of later exposures which may also influence methylation separately. To explore the combined impact of phthalate metabolites from the same parent compound DEHP, we added the molar equivalents of MEHP, MEHHP, MEOHP, and MECPP for each individual (∑DEHP metabolites). This value was multiplied by the average molecular weight of the four metabolites (294.3 µg/µmol) to express the data in µg/L [ 66 ]. All urinary exposure levels (BPA and phthalates) were tested with and without specific gravity correction. To examine sex-specific effects, interaction terms between sex and exposure were added into all main models. Sex-stratified models were implemented when P < 0.1 for the interaction term.
Since subjects were exposed to multiple EDCs simultaneously, we sought to assess the impact on DNA methylation of the exposure mixture by modeling Pb, BPA, and phthalates together (at T3, PA, or both) using principal component analysis (PCA). Due to the high correlations between urinary BPA and nine phthalate metabolite concentrations, PCA with varimax rotation was used to reduce urinary T3 and PA measures into uncorrelated principal components representing the variability of the 10 urinary measures from each time point [ 48 ]. The first four principal components were selected to represent >80% of the variability of BPA and phthalate concentrations from T3 or PA. Components were calculated for each subject by summing the standardized score ( Supplemental Table 2 ) multiplied by the z -score of each urinary metabolite (from ln-transformed specific gravity adjusted data). One repeated-measures GLM was used per genic region and per time point (T3 or PA) that adjusted for sex and age (standardized to mean 0, SD 1) and assessed the contribution of BPA/phthalates represented by the first four components and Pb (represented by average maternal blood Pb during pregnancy or children’s blood Pb at the PA visit). A multi-developmental stage model for each gene was also run including the four components for T3, four for PA, maternal blood Pb, early childhood blood Pb, and child’s blood Pb at the PA visit. Pb variables were standardized (mean 0, SD 1) prior to analysis.
Supplementary Material
Supplementary Data
Acknowledgements
The authors acknowledge the research staff at participating hospitals and the American British Cowdray Hospital in Mexico City for providing research facilities. We thank the mothers and children for participating in the study. This study was made possible by U.S. Environmental Protection Agency (US EPA) grants RD834800 and RD83543601 and National Institute for Environmental Health Sciences (NIEHS) grants P20 ES018171, P01 ES02284401, R01 ES007821, R01 ES014930, R01 ES013744, and P30 ES017885. This study was also supported and partially funded by the National Institute of Public Health/Ministry of Health of Mexico. The contents of this publication are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA or the NIH. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. No conflict of interest is declared.
Conflict of interest statement . None declared.
Supplementary data
Supplementary data is available at EnvEpig online.
References
- 1. Laidlaw M, Filippelli G. Resuspension of urban soils as a persistent source of lead poisoning in children: a review and new directions . Appl Geochem 2008. ; 23 : 2021 – 39 . [Google Scholar]
- 2. Hernandez Avila M, Romieu I, Rios C , et al. . Lead-glazed ceramics as major determinants of blood lead levels in Mexican women . Environ Health Perspect 1991. ; 94 : 117 – 20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romieu I, Palazuelos E, Hernandez Avila M , et al. . Sources of lead exposure in Mexico City . Environ Health Perspect 1994. ; 102 : 384 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meyer P, Brown M, Falk H. Global approach to reducing lead exposure and poisoning . Mutat Res 2008. ; 659 : 166 – 75 . [DOI] [PubMed] [Google Scholar]
- 5. Ehrlich S, Calafat A, Humblet O , et al. . Handling of thermal receipts as a source of exposure to bisphenol A . JAMA 2014. ; 311 : 859 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carwile J, Ye X, Zhou X , et al. . Canned soup consumption and urinary bisphenol A: a randomized crossover trial . JAMA 2011. ; 306 : 2218 – 20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis R, Meeker J, Peterson K , et al. . Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children . Chemosphere 2013. ; 93 : 2390 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calafat A, Ye X, Wong L , et al. . Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004 . Environ Health Perspect 2008. ; 116 : 39 – 44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodruff T, Zota A, Schwartz J. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004 . Environ Health Perspect 2011. ; 119 : 878 – 85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silva M, Barr D, Reidy J , et al. . Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000 . Environ Health Perspect 2004. ; 112 : 331 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watkins D, Tellez-Rojo M, Ferguson K , et al. . In utero and peripubertal exposure to phthalates and BPA in relation to female sexual maturation . Environ Res 2014. ; 134 : 233 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harley K, Aguilar Schall R, Chevrier J , et al. . Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort . Environ Health Perspect 2013. ; 121 : 514 – 20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braun J, Lanphear B, Calafat A , et al. . Early-life bisphenol a exposure and child body mass index: a prospective cohort study . Environ Health Perspect 2014. ; 122 : 1239 – 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanin L, Gonzalez-Cossio T, Romieu I , et al. . Effect of maternal lead burden on infant weight and weight gain at one month of age among breastfed infants . Pediatrics 2001. ; 107 : 1016 – 23 . [DOI] [PubMed] [Google Scholar]
- 15. Afeiche M, Peterson K, Sanchez B , et al. . Windows of lead exposure sensitivity, attained height, and body mass index at 48 months . J Pediatr 2012. ; 160 : 1044 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Afeiche M, Peterson K, Sanchez B , et al. . Prenatal lead exposure and weight of 0- to 5-year-old children in Mexico city . Environ Health Perspect 2011. ; 119 : 1436 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xie C, Zhao Y, Gao L , et al. . Elevated phthalates' exposure in children with constitutional delay of growth and puberty . Mol Cell Endocrinol 2015. ; 407 : 67 – 73 . [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Meng X, Chen L , et al. . Age and sex-specific relationships between phthalate exposures and obesity in Chinese children at puberty . PLoS ONE 2014. ; 9 : e104852.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watkins D, Peterson K, Ferguson K , et al. . Relating phthalate and BPA exposure to metabolism in peripubescence: the role of exposure timing, sex, and puberty . J Clin Endocrinol Metab 2015. ; 101 : 79 – 88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez-Cossio T, Peterson K, Sanin L , et al. . Decrease in birth weight in relation to maternal bone-lead burden . Pediatrics 1997. ; 100 : 856 – 62 . [DOI] [PubMed] [Google Scholar]
- 21. Hernandez-Avila M, Peterson K, Gonzalez-Cossio T , et al. . Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants . Arch Environ Health 2002. ; 57 : 482 – 8 . [DOI] [PubMed] [Google Scholar]
- 22. Ferguson K, McElrath T, Meeker J. Environmental phthalate exposure and preterm birth . JAMA Pediatr 2014. ; 168 : 61 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bolton C, Bush A, Hurst J , et al. . Lung consequences in adults born prematurely . Thorax 2015. ; 70 : 574 – 80 . [DOI] [PubMed] [Google Scholar]
- 24. Sipola-Leppanen M, Vaarasmaki M, Tikanmaki M , et al. . Cardiometabolic risk factors in young adults who were born preterm . Am J Epidemiol 2015. ; 181 : 861 – 73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boas M, Frederiksen H, Feldt-Rasmussen U , et al. . Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth . Environ Health Perspect 2010. ; 118 : 1458 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waterland R, Kellermayer R, Laritsky E , et al. . Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles . PLoS Genet 2010. ; 6 : e1001252.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Waterland R, Michels K. Epigenetic epidemiology of the developmental origins hypothesis . Annu Rev Nutr 2007. ; 27 : 363 – 88 . [DOI] [PubMed] [Google Scholar]
- 28. Kim J, Sartor M, Rozek L , et al. . Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome . BMC Genomics 2014. ; 15 : 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faulk C, Barks A, Liu K , et al. . Early-life lead exposure results in dose- and sex-specific effects on weight and epigenetic gene regulation in weanling mice . Epigenomics 2013. ; 5 : 487 – 500 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dolinoy D, Huang D, Jirtle R. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development . Proc Natl Acad Sci USA 2007. ; 104 : 13056 – 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yaoi T, Itoh K, Nakamura K , et al. . Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A . Biochem Biophys Res Commun 2008. ; 376 : 563 – 7 . [DOI] [PubMed] [Google Scholar]
- 32. Goodrich J, Sanchez B, Dolinoy D , et al. . Quality control and statistical modeling for environmental epigenetics: a study on in utero lead exposure and DNA methylation at birth . Epigenetics 2015. ; 10 : 19 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. LaRocca J, Binder AM, McElrath TF , et al. . The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes . Environ Res 2014. ; 133 : 396 – 406 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nahar M, Kim J, Sartor M , et al. . Bisphenol A-associated alterations in the expression and epigenetic regulation of genes encoding xenobiotic metabolizing enzymes in human fetal liver . Environ Mol Mutagen 2014. ; 55 : 184 – 95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sen A, Heredia N, Senut M , et al. . Early life lead exposure causes gender-specific changes in the DNA methylation profile of DNA extracted from dried blood spots . Epigenomics 2015. ; 7 : 379 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang R, Galati J, Burrows S , et al. . DNA methylation of the IGF2/H19 imprinting control region and adiposity distribution in young adults . Clin Epigenetics 2012. ; 4 : 21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee K, Richmond R, Hu P , et al. . Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age . Environ Health Perspect 2015. ; 123 : 193 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim J, Rozek L, Soliman A , et al. . Bisphenol A-associated epigenomic changes in prepubescent girls: a cross-sectional study in Gharbiah, Egypt . Environ Health 2013. ; 12 : 33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang I, Karmaus W, Chen S , et al. . Effects of phthalate exposure on asthma may be mediated through alterations in DNA methylation . Clin Epigenetics 2015. ; 7 : 27.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heijmans B, Tobi E, Stein A , et al. . Persistent epigenetic differences associated with prenatal exposure to famine in humans . Proc Natl Acad Sci USA 2008. ; 105 : 17046 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huen K, Yousefi P, Bradman A , et al. . Effects of age, sex, and persistent organic pollutants on DNA methylation in children . Environ Mol Mutagen 2014. ; 55 : 209 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kippler M, Engstrom K, Mlakar S , et al. . Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight . Epigenetics 2013. ; 8 : 494 – 503 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Broberg K, Ahmed S, Engstrom K , et al. . Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys . J Dev Orig Health Dis 2014. ; 5 : 288 – 98 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu H, Wang F, Liu Y , et al. . Sex-biased methylome and transcriptome in human prefrontal cortex . Hum Mol Genet 2014. ; 23 : 1260 – 70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kundakovic M, Gudsnuk K, Franks B , et al. . Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure . Proc Natl Acad Sci USA 2013. ; 110 : 9956 – 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wild C, Scalbert A, Herceg Z. Measuring the exposome: a powerful basis for evaluating environmental exposures and cancer risk . Environ Mol Mutagen 2013. ; 54 : 480 – 99 . [DOI] [PubMed] [Google Scholar]
- 47. Rappaport S, Barupal D, Wishart D , et al. . The blood exposome and its role in discovering causes of disease . Environ Health Perspect 2014. ; 122 : 769 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun Z, Tao Y, Li S , et al. . Statistical strategies for constructing health risk models with multiple pollutants and their interactions: possible choices and comparisons . Environ Health 2013. ; 12 : 85.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park S, Tao Y, Meeker J , et al. . Environmental risk score as a new tool to examine multi-pollutants in epidemiologic research: an example from the NHANES study using serum lipid levels . PLoS One 2014. ; 9 : e98632.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dominguez-Salas P, Moore S, Baker M , et al. . Maternal nutrition at conception modulates DNA methylation of human metastable epialleles . Nat Commun 2014. ; 5 : 3746 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tellez-Rojo M, Hernandez-Avila M, Lamadrid-Figueroa H , et al. . Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy . Am J Epidemiol 2004. ; 160 : 668 – 78 . [DOI] [PubMed] [Google Scholar]
- 52. Ettinger A, Lamadrid-Figueroa H, Tellez-Rojo M , et al. . Effect of calcium supplementation on blood lead levels in pregnancy: a randomized placebo-controlled trial . Environ Health Perspect 2009. ; 117 : 26 – 31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hu H, Aro A, Rotnitzky A. Bone lead measured by X-ray fluorescence: epidemiologic methods . Environ Health Perspect 1995. ; 103 Suppl 1 : 105 – 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aro A, Todd A, Amarasiriwardena C , et al. . Improvements in the calibration of 109Cd K x-ray fluorescence systems for measuring bone lead in vivo . Phys Med Biol 1994. ; 39 : 2263 – 71 . [DOI] [PubMed] [Google Scholar]
- 55. Hu H, Milder F, Burger DE. The use of K X-ray fluorescence for measuring lead burden in epidemiological studies: high and low lead burdens and measurement uncertainty . Environ Health Perspect 1991. ; 94 : 107 – 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lamadrid-Figueroa H, Tellez-Rojo M, Angeles G , et al. . Bias correction by use of errors-in-variables regression models in studies with K-X-ray fluorescence bone lead measurements . Environ Res 2011. ; 111 : 17 – 20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pearson M, Lu C, Schmotzer B , et al. . Evaluation of physiological measures for correcting variation in urinary output: implications for assessing environmental chemical exposure in children . J Expos Sci Environ Epidemiol 2008. ; 19 : 336 – 42 . [DOI] [PubMed] [Google Scholar]
- 58. Grunau C, Clark S, Rosenthal A. Bisulfite genomic sequencing: systematic investigation of critical experimental parameters . Nucleic Acids Res 2001. ; 29 : E65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Virani S, Dolinoy D, Halubai S , et al. . Delivery type not associated with global methylation at birth . Clin Epigenet 2012. ; 4 : 8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heijmans B, Kremer D, Tobi E , et al. . Heritable rather than age-related environmental and stochastic factors dominate variation in DNA methylation of the human IGF2/H19 locus . Hum Mol Genet 2007. ; 16 : 547 – 54 . [DOI] [PubMed] [Google Scholar]
- 61. Hoyo C, Murtha A, Schildkraut J , et al. . Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy . Epigenetics 2011. ; 6 : 928 – 36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wright R, Schwartz J, Wright R , et al. . Biomarkers of lead exposure and DNA methylation within retrotransposons . Environ Health Perspect 2010. ; 118 : 790 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li C, Yang X, Xu M , et al. . Epigenetic marker (LINE-1 promoter) methylation level was associated with occupational lead exposure . Clin Toxicol (Phila) 2013. ; 51 : 225 – 9 . [DOI] [PubMed] [Google Scholar]
- 64. Li Y, Xie C, Murphy S , et al. . Lead exposure during early human development and DNA methylation of imprinted gene regulatory elements in adulthood . Environ Health Perspect 2016. ; 124 : 666 – 73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ehrich M, Nelson M, Stanssens P , et al. . Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry . Proc Natl Acad Sci USA 2005. ; 102 : 15785 – 90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zota A, Calafat A, Woodruff T. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010 . Environ Health Perspect 2014. ; 122 : 235 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data