Abstract
Study Objective
To test the effectiveness of a 4-week behavioral Sleep Intervention Program (SIP: sleep compression, modified stimulus control, and sleep hygiene) compared to a 4-week information-only control (IC) among older adults attending a VA Adult Day Health Care (ADHC) program in a double-blind, randomized, clinical trial.
Methods
Forty-two individuals (mean age: 77 years, 93% male) enrolled in a VA ADHC program were randomized to receive SIP or IC. All completed in-person sleep and health assessments at baseline, post-treatment and 4-months follow-up that included 3 days/nights of wrist actigraphy, the Pittsburgh Sleep Quality Index (PSQI), and the Insomnia Severity Index (ISI). Mixed repeated measures analysis was used to compare sleep outcomes at post-treatment and 4-months follow-up, with baseline values as covariates.
Results
SIP participants (n = 21) showed significant improvement on actigraphy sleep efficiency (p = .007), number of nighttime awakenings (p = .016), and minutes awake at night (p = .001) at post-treatment, compared to IC participants (n = 21). Benefits were slightly attenuated but remained significant at 4-month follow-up (all p’s < .05). There were no differences in total sleep time between groups. There was significant improvement on PSQI factor 3 (daily disturbances) at 4-month follow-up (p = .016), but no differences were observed between SIP and IC on other PSQI components or ISI scores at post-treatment or 4-month follow-up.
Conclusions
A short behavioral sleep intervention may have important benefits in improving objectively measured sleep in older adults participating in ADHC. Future studies are needed to study implementation of this intervention into routine clinical care within ADHC.
Keywords: adult day health care, aging, veterans, sleep, behavioral interventions
Statement of Significance
This work demonstrates the positive impact of a brief behavioral sleep intervention for older adults participating in adult day health care. Improved sleep may be associated with improvement in other symptoms and ultimately may prolong independence and prevent functional decline.
INTRODUCTION
Sleep problems are common in older adults, particularly among those with functional limitations, and functional impairments in older adults are associated with poor sleep quality.1,2 Sleep difficulties have been studied extensively in institutional long-term care settings, including nursing homes and other settings;3–5 however, little is known about how best to improve sleep quality among older adults living in the community who require noninstitutional support, such as Adult Day Health Care (ADHC).
Older adults with functional limitations are increasingly using ADHC services to maintain independence.6 More than 4600 ADHC centers exist across the United States with a 35% increase since 2002. More than 260,000 individuals and their family caregivers use ADHC services, which can include care planning, assistance with activities of daily living, chronic health condition oversight and management, nursing care, physical, occupational, and speech therapy, meals, transportation, social services, and personal care activities.6,7 ADHC participants are often cognitively impaired, physically disabled, and typically have multiple chronic medical conditions (eg, hypertension, diabetes) and mental health issues (eg, depression). Given that veterans are more medically complex than the population at large,8 their need for ADHC services may be greater than the general population of older adults, and VA considers ADHC one component of its overall plan for noninstitutional long-term care for aging veterans.9
Numerous studies have shown that sleep problems are associated with depression, low quality of life, functional decline, nursing home placement, and mortality among older adults4,10–13 including older adults attending ADHC programs.14 We previously found that over two-thirds of VA ADHC participants have sleep-related complaints, and over one-third meet basic criteria for insomnia disorder.15 Available studies also show that sleep problem is typically not addressed within routine clinical care of older patients,16 and treatment of sleep issues is often limited to medications (eg, hypnotics, sedating antidepressants), which are not recommended for older adults.17 Both untreated insomnia18 and pharmacological treatment of insomnia can be associated with increased risk of falls and other adverse health events among older persons.19 On the other hand, nonpharmacological interventions, such as cognitive-behavioral therapy for insomnia (CBT-I) do not show these adverse effects. CBT-I has been shown to be as effective for older as for younger adults;17 however, studies of older adults have typically been conducted in outpatient settings with participants who do not have significant functional impairments.20–23 It also is not clear whether adapting CBT-I for patients with limited physical abilities will reduce potency. ADHC participants may not be able to adhere to all of the traditional recommendations of CBT-I, such as getting out of bed at night (because of high fall risk) or completing complex sleep diaries (due to visual or cognitive difficulties). Studies have not been done to evaluate whether sleep improvements can be achieved with behavioral interventions delivered within ADHC programs.
The goal of this study was to test the effectiveness of a four-session manual-based Sleep Intervention Program (SIP), which was based on CBT-I, adapted for older adults with sleep difficulties who attend an ADHC program. The basic components of the SIP were: individualized sleep education, sleep compression, modified stimulus control, and targeted sleep hygiene recommendations. The intervention was designed to facilitate translation into routine care and application in other similar ADHC programs. The main outcomes were patient-reported sleep quality and objectively measured sleep parameters (based on actigraphy). The aims of the study were to evaluate whether the nonpharmacological SIP, delivered in the context of ADHC, lead to significant improvements in self-reported and objectively measured (by wrist actigraphy) sleep quality, and whether treatment-related improvements were maintained at 4-month follow-up. The main hypothesis was that greater improvements would be shown in the SIP group compared to the information-only control (IC) group in patient-reported sleep quality, insomnia symptoms and in actigraphy-measured total time awake, number of nighttime awakenings, total sleep time, and sleep efficiency from baseline to post-treatment. We also hypothesized that these improvements would be maintained at 4-month follow-up.
METHODS
Study Design and Participants
This 3-year randomized controlled trial was conducted among older adults in an ADHC program at the VA Greater Los Angeles Healthcare System (Clinical Trials Identifier: NCT01259401). All veterans aged 60 years or older who had been enrolled in the ADHC program for at least 1 month were invited to complete a screening questionnaire to assess basic study eligibility (ie, the ability to understand screening items and to communicate verbally during the screening process). Individuals who met these basic criteria and were interested in participating were asked to provide written informed consent. Study data were collected between November 2010 and June 2012.
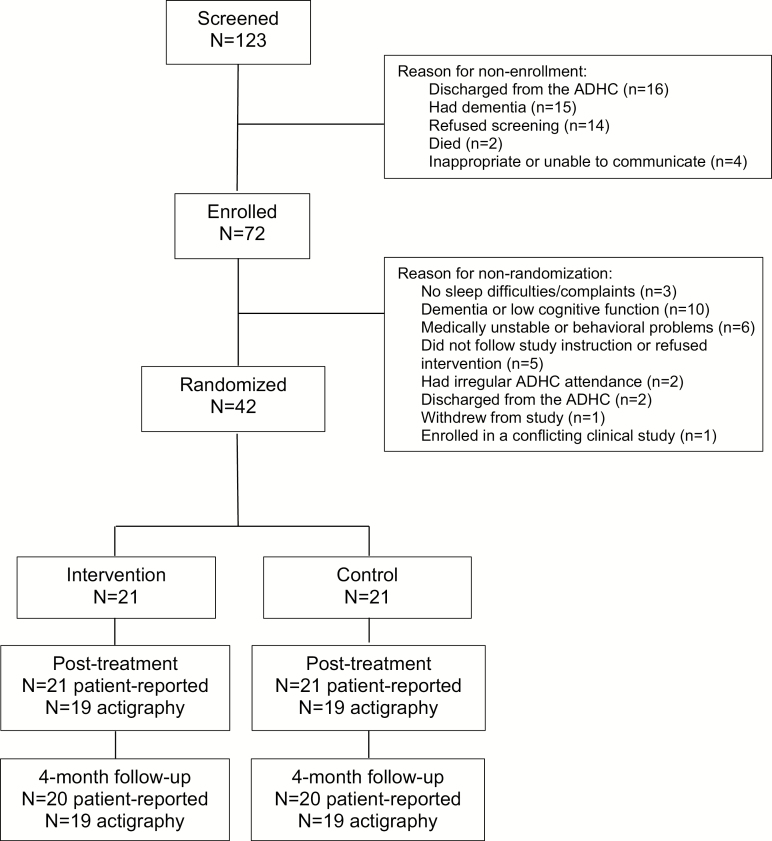
A total of 123 veterans were screened for the study, 72 of whom were enrolled. Among the 51 individuals who were not enrolled, the most common reasons were discharged from the ADHC, significant dementia based on reports from ADHC nursing or social work staff or research staff observation during the screening process suggesting the participant could not provide informed consent and refusal to complete the study screening. The remaining 72 individuals consented to participate, of whom, 30 did not meet eligibility criteria for randomization, 10 due to dementia or low cognitive function (based on Mini-Mental State Examination [MMSE] scores below 20; described below), six due to unstable medical or psychiatric conditions (based on medical record review by the PI and a study physician), five refused, three did not have any sleep difficulties or complaints (based on baseline sleep questionnaires, described below), two were discharged from the ADHC, two had irregular attendance, one withdrew, and one was enrolled in a conflicting clinical trial. A total of 42 individuals were randomized to intervention or control (described below). Figure 1 shows the flow of participants through the study. This study was reviewed and approved by the Institutional Review Board of the VA Greater Los Angeles Healthcare System.
Figure 1.
Study participant recruitment, screening, and enrollment. ADHC = Adult Day Health Care.
Assessment Procedures
Enrolled participants completed a baseline assessment interview, conducted by a trained research staff member. To minimize participant burden, we used brief and abbreviated measures whenever possible. The assessment included administration of self-report sleep questionnaires (ie, Pittsburgh Sleep Quality Index [PSQI]24 and Insomnia Severity Index [ISI])25 and other clinical metrics. These metrics included assessment of depression (Patient Health Questionnaire 9; PHQ-9),26 post-traumatic stress disorder (primary care–post traumatic stress disorder; PC-PTSD),27 cognitive function (MMSE),28 physical function (activities of daily living and instrumental activities of daily living; ADL/IADL),29 fatigue (Flinders Fatigue Scale; FFS),30 and health-related quality of life (Short Form-12 Health Survey [SF-12] physical and mental health component scores).31 Participants also wore a wrist actigraph for 3 days and nights to objectively estimate sleep (Phillips Respironics Actiwatch Spectrum with default settings; 1-minute epochs and medium threshold for sleep scoring). Initially, participants were asked to complete a 12-item sleep diary while wearing the actigraph; however, this was overly burdensome, and completion rates were low. We therefore simplified the sleep diary and asked participants to maintain a four-item sleep diary documenting only bedtime, rise time, sleep quality, and actigraph removal for each recorded day. A review of the patient’s electronic health record was completed to obtain health history information and medications prescribed.
Determination of Randomization Eligibility and Randomization Procedure
The complete baseline assessment measures were reviewed by the study coordinator and PI (a clinical sleep psychologist), and participants who were excluded from randomization if they had dementia or low cognitive function (based on MMSE < 20 or documentation of moderate to severe dementia in the electronic health records), were medically unstable or exhibited behavioral problems in the ADHC program, did not follow study instructions, withdrew during baseline or refused the intervention, had irregular ADHC attendance or were discharged from the ADHC program prior to randomization, were enrolled in a conflicting VA study, or did not have sleep complaints. In terms of sleep complaints, multiple data sources were included. Participants had to indicate sleep disturbances on screening items (described below) or on baseline questionnaires (reporting poor sleep quality, total sleep time <6 hours per night, sleep efficiency <85%, or have a PSQI score >5 or an ISI score >7) or show evidence of poor sleep quality on actigraphy (sleep efficiency <85%, total sleep time <6 hours per night).
Eligible participants were randomly assigned, using random allocation concealment, to receive the manualized SIP or an IC. Participants were randomized in three strata to help insure that SIP and IC groups were balanced in baseline severity of sleep problems. The strata were based on the number of sleep disturbance items endorsed during preconsent screening (0/1, 2, or 3 items). The three items were based on the PSQI and assessed: (1) taking more than 30 minutes to fall asleep, (2) sleeping less than 6 hours a night, and (3) fairly bad or very bad self-rated sleep quality. A total of 42 participants were randomized to the SIP (N = 21) and IC (N = 21) groups.
Intervention
The SIP and IC treatments were provided by Master’s-level trained Health Educators (HEs) under the supervision of the PI (JLM), a licensed clinical psychologist who is certified by the American Board of Sleep Medicine as a Behavioral Sleep Medicine Specialist. Staff members involved in outcome assessment were blinded to group assignment. The HE could not be blinded to treatment condition, so multiple steps were taken to insure that the fidelity of both the SIP and IC were maintained. First, interventionists were carefully trained to avoid overlap between the SIP and IC conditions. Second, structured patient materials were provided to guide each session and reduce risk of contamination of the control condition with content from the active treatment. Third, interventionists maintained checklists and notes during each session to document use of the study materials in that particular session. Fourth, intervention fidelity was monitored throughout the study via direct observation by the PI and ongoing feedback during weekly supervision. We were not able to obtain permission to record intervention sessions within the ADHC program.
Table 1 outlines the content of each session of the SIP. The SIP involved four weekly sessions, each lasting approximately 45 minutes with the HE. Sessions focused on: (1) individualized education about sleep, (2) sleep compression therapy, (3) targeted sleep hygiene education, (4) modified stimulus control, and (5) maintenance of sleep improvements over time, and (6) coping with future bouts of insomnia. The SIP approach was based on CBT-I, which is an empirically supported treatment for insomnia disorder.32 The most significant modifications to traditional CBT-I included (1) substitution of sleep compression33 in place of sleep restriction therapy and (2) modifications of standard stimulus control instructions (eg, no instruction to get out of bed at night due to high fall risk among ADHC participants).33 To implement sleep compression (rather than sleep restriction), the initial time in bed window was set to equal the number of hours the patient was spending in bed (rather than the number of hours of sleep). That was then gradually reduced by 15–30 minutes per week until sleep quality improved without increasing daytime sleepiness. Stimulus control principles were followed and informed recommendations to move all nonsleep activities out of the bed (eg, read or watch TV in another part of the house) before bedtime and after morning rise times. Participants were not specifically instructed to get out of bed during the night if they had awakenings with difficulty returning to sleep; however, they were encouraged to do other relaxing activities (eg, listen to music, read) in or near the bed if they had trouble sleeping during the night. Because of high fall risk and mobility limitations in many ADHC participants, they were not instructed to get out of bed and go to another room if they had trouble sleeping during the night. In addition, this program was similar to CBT-I in terms of the number of sessions, but each session was shorter than many CBT-I intervention programs, which are typically 4–8 sessions lasting 60 minutes per session.20,22,23 Some studies of brief interventions are also effective with older patients.21,34 Consistent with traditional CBT-I interventions, all recommendations were tailored to the individual patient, addressing their specific circumstances, sleep patterns, and level of motivation to engage in the intervention. At the conclusion of each session, a written outline of what was discussed was provided to the participant with the specific, individualized recommendations written down in clear language. Participants maintained the simple four-item daily sleep diary throughout the intervention period.
Table 1.
The Four-Session Sleep Intervention Program: Session by Session Content and At-Home Activities.
| Session and topics covered | At-home Activities |
|---|---|
| Session 1: How sleep works | |
|
|
| Session 2: Steps to getting sleep | |
|
|
| Session 3: Healthy habits for healthy sleep | |
|
|
| Session 4: Preventing the return of chronic insomnia | |
|
|
The IC group was structured to closely resemble the “observable” aspects of the SIP to enhance credibility and to assist with blinding of the research assessment staff members and ADHC providers. The IC involved four meetings with the HE, lasting up to 45 minutes each. During these meetings, two educational brochures (published by the American Academy of Sleep Medicine, Darien, IL) were reviewed and discussed. One brochure focused on changes in sleep with age, and the other focused on sleep hygiene education.
Measures
Sleep Outcomes
Primary outcomes for the study included patient-reported and objective sleep measures (actigraphy). Sleep outcomes were assessed at each time point (ie, baseline, post-treatment, and 4-month follow-up).
Patient-reported sleep quality was assessed with the PSQI (total and three-factor subscale scores)35 and the ISI total score. The PSQI is a 19-item questionnaire that assesses self-reported sleep quality and disturbances over the past month. The items include hours of sleep, ratings for frequency of sleep concerns, general sleep quality, and daytime factors related to poor sleep. A total score greater than 5 indicates poor quality sleep.18 We modified the PSQI by asking about participants’ sleep over the past week (rather than the past month) because the duration of the intervention itself was fairly short (ie, about 4 weeks). We also elected to use a three-factor scoring system, which has superior psychometric properties compared to the originally developed seven-factor PSQI scoring system.35 In addition to the single total score, the three dimensional assessments (sleep efficiency, perceived sleep quality, and daily disturbances) from the PSQI were used to obtain more nuanced information regarding the nature of sleep problems. We used a total of four main outcome variables from the PSQI: (1) PSQI total score, (2) PSQI Factor 1 (sleep efficiency), (3) PSQI Factor 2 (perceived sleep quality), and (4) PSQI Factor 3 (daily disturbances).35 The ISI was used to measure severity of insomnia symptoms over the past week.19,20 The ISI is a seven-item questionnaire assessing the nature, severity, and impact of insomnia. A five-point Likert-like scale is used to rate each item, ranging from 0 (no problem) to 4 (very severe problem). Total scores range from 0 to 28 and are interpreted as follows: no insomnia (0–7), subthreshold insomnia (8–14), moderate insomnia (15–21), and severe insomnia (22–28).
Objective sleep was measured by actigraphy (Actiwatch Spectrum, Philips Respironics) on the dominant wrist for three consecutive days (ie, 72 hours). Actigraphs are small, watch-sized devices useful in longitudinal, naturalistic (ie, not in a sleep laboratory) assessment of sleep-wake patterns.36 Actigraphy devices used for estimating sleep parameters contain subminiature solid-state accelerometers, and in general, wrist activity below an established threshold is interpreted as sleep, whereas high-wrist activity is interpreted as wakefulness, using mathematical algorithms within commercially available software accompanying the devices used. In this study, sleep was scoring using medium threshold settings and default parameters for sleep scoring based on 1-minute epochs. Participants were also asked to complete a sleep diary to record bedtime and rise time for each night while they were wearing the actigraph. Information from this diary was used to identify the “in bed” period for actigraphy scoring. Four outcomes from the actigraphy were used for our main analyses: nighttime sleep efficiency (sleep time divided by total time in bed), total sleep time, number of nighttime awakenings, and total nighttime wake time.
Other Measures
Participant demographic information was collected at baseline and included age, gender, race/ethnicity, marital status, living arrangement, employment status, level of education, and duration of ADHC enrollment. Comorbidity was assessed by the number of medical conditions on the problem list within the participants’ electronic health records that were defined by the International Classification of Diseases, Ninth Revision (ICD-9).37
We also collected health information at each of the three time points. Information on medication use was collected using electronic health record review and a structured interview with the participant based on the patient’s medication list in the medical record. Pain was measured using one subscale (seven items of pain intensity) from the Geriatric Pain Measure.38 PTSD was screened using the four-item Primary Care PTSD (PC-PTSD).39 The PC-PTSD is considered positive if an individual answers yes to three or more items. Cognitive function was assessed using the MMSE.28 This 19-item scale assesses orientation, registration, attention/calculation, recall, language, and construction and has standard instructions. MMSE scores range from 0 to 30, and higher scores indicate better functioning. A score below 24 is consistent with at least mild cognitive impairment; scores below 20 are consistent with moderate-to-severe cognitive impairment.
Physical function was assessed using components of the Older Americans Resources and Services (OARS) multidimensional functional assessment questionnaire.29 These components were comprised of seven items of ADL and seven items on IADL. ADLs assessed included eating, dressing, grooming, walking, getting in and out of bed, taking a bath or shower, and continence. IADLs included telephone use, going places beyond walking distance, shopping, preparing meals, doing housework, handling money, and taking medications. Each item is scored as 0 = completely dependent, 1 = can do with some help, or 2 = completely independent. Total scores range from 0 to 28 with higher scores indicating greater independence. Depression was assessed using the PHQ-9.40 The PHQ-9 is the nine-item depression module from the PHQ (a self-administered diagnostic instrument for common mental disorders). The PHQ-9 total score ranges from 0 to 27 with each item ranging from 0 (not at all) to 3 (nearly every day). Self-rated health data were obtained from the SF-12.31 The 12 items are divided into eight subscales: (1) physical functioning, (2) role limitations due to physical problems and (3) emotional problems, (4) general health perceptions, (5) vitality, (6) social functioning, (7) general mental health, and (8) bodily pain. It also produces two summary component scores: physical and mental health component summary score, with scores ranging from 0 to 100. The FFS was used to screen for the presence and severity of daytime fatigue associated with insomnia over the past 7 days.30 It is a seven-item questionnaire in which six items are presented in Likert-like format with responses ranging from 0 (not at all) to 4 (extremely) and one item presented as the sum of all times of day when fatigue is experienced. Total scores range from 0 to 31.
Finally, we examined patient-reported total sleep time and sleep efficiency using items within the PSQI. Sleep efficiency was computed by dividing total sleep time by time in bed (ie, time from reported bedtime to reported rise time), converted to a percent.
Statistical Analysis
All analyses were performed using Stata version 13.1, and the mixed repeated measures analysis was performed using the Stata mixed command. In preparation for our main analysis, we examined scatterplots of each outcome at baseline versus post-treatment and baseline versus 4-month follow-up to identify potentially influential observations for sensitivity analyses.
A two (group) by three (time) mixed repeated measures analysis was performed using an unstructured residual covariance to account for the covariance of the residuals across the three time points. There were no missing data at the post-treatment time point. All available data were analyzed at the 4-month follow-up, following intention-to-treat principles; that is, all participants’ data were included regardless of treatment completion. We computed the marginal means for each outcome as a function of group membership (SIP and control) and time (baseline, post-treatment, and 4-month follow-up) as obtained from the mixed repeated measures analysis. The treatment effect at post-treatment was assessed by computing an interaction contrast that compared the change (post-treatment vs. baseline) for the treatment versus control groups. Likewise, the treatment effect at 4 months was estimated via interaction contrasts that compared the change (4-month follow-up vs. baseline) for the treatment versus control groups.
Based on identification of six potentially influential observations, we conducted sensitivity analyses by repeating our original analysis omitting observations that appeared influential.
RESULTS
Baseline Patient Characteristics
Table 2 shows demographic and health characteristics of the overall sample and the two experimental conditions. Participants included 39 men and three women with a mean (standard deviation [SD]) age of 77.1 (9.9) years (71.4% white). Participants typically had multiple comorbid conditions, high levels of pain, and poor health. Sleep characteristics are shown in Table 3. Sleep characteristics are shown in Table 3. Self-reported sleep quality was poor as measured by the PSQI total score (mean = 6.8; SD = 4.2) and subthreshold insomnia on the ISI total score (mean = 8.5; SD = 6.6). However, objective (actigraphy-assessed) sleep efficiency was relative high, with a mean of 84.9% (SD = 7.9%) but with multiple nighttime awakenings (mean = 23.6; SD = 7.9). As expected due to randomization, there were no significant differences between the SIP and IC groups in terms of demographic variables, with the exception of current living arrangement, and there were no significant differences between either objective or patient-reported sleep quality between the two groups at baseline.
Table 2.
Demographic and Health Characteristics of Randomized Participants and Differences Between Treatment Groups at Baseline.
| Variable | Overall (N = 42) | SIP (N = 21) | IC (N = 21) | p-value |
|---|---|---|---|---|
| Age in years [M (SD)] | 77.1 (9.9) | 77.7 (10.2) | 76.4 (9.9) | .680 |
| Gender [n (%) male] | 39 (92.9%) | 18 (85.7%) | 21 (100%) | .232 |
| Race [n (%) non-Hispanic white] | 30 (71.4%) | 13 (61.9%) | 17 (81%) | .306 |
| Years of education [M (SD)] | 14.5 (2.5) | 14.7 (2.9) | 14.2 (2.0) | .463 |
| Marital status [n (%) married] | 21 (50%) | 10 (47.6%) | 11 (52.4%) | 1.00 |
| Employment status | .509 | |||
| Unable to work [n (%)] | 6 (14.3%) | 4 (19%) | 2 (9.5%) | |
| Volunteer [n (%)] | 4 (9.5%) | 1 (4.8%) | 3 (14.3%) | |
| Retired [n (%)] | 32 (76.2%) | 16 (76.2%) | 16 (76.2%) | |
| Current living arrangement | .012 | |||
| Own home [n (%)] | 26 (63.4%) | 12 (57%) | 14 (70%) | |
| Relative or friend’s home [n (%)] | 4 (9.8%) | 0 (0%) | 4 (20%) | |
| Board and care home/ assisted living facility [n (%)] | 11 (26.8%) | 9 (43%) | 2 (10%) | |
| Years since ADHC enrollment [M (SD)] | 2.0 (2.7) | 2.3 (2.9) | 1.7 (2.6) | .473 |
| Number of diagnosed conditions in the electronic health record [M (SD)] | 24.3 (15.9) | 23.8 (16.9) | 24.8 (15.2) | .841 |
| Geriatric Pain Measure Score [M (SD)] | 16.9 (12.4) | 16.1 (12.0) | 17.7 (13.1) | .674 |
| Primary Care PTSD score [n (%) with score ≥3] | 5 (13.2%) | 3 (15.0%) | 2 (11.1%) | .723 |
| MMSE score [M (SD)] | 25.9 (2.8) | 26.0 (2.7) | 25.8 (2.9) | .870 |
| OARS ADL/IAD total score [M (SD)] | 19.7 (4.2) | 19.3 (3.1) | 20.1 (5.2) | .547 |
| PHQ-9 score [M (SD)] | 6.3 (5.5) | 5.4 (4.1) | 7.2 (6.6) | .298 |
| SF-12 Physical health component score [M (SD)] | 36.3 (8.5) | 35.1 (6.1) | 37.6 (10.4) | .353 |
| SF-12 Mental health component score [M (SD)] | 50.1 (11.9) | 53.3 (10.3) | 46.9 (12.7) | .081 |
| Flinders Fatigue scale score [M (SD)] | 8.7 (7.9) | 8.0 (6.9) | 9.5 (9.0) | .555 |
M = mean; SD = standard deviation; ADHC = Adult Day Health Care; MMSE = Mini-Mental State Examination; PTSD = post-traumatic stress disorder; OARS = the Older Americans Resources and Services; ADL = activities of daily living; IADL = instrumental activities of daily living; SF-12 = Short-Form v12.
Table 3.
Sleep Characteristics of Randomized Study Participants at Baseline.
| Variable | Overall (N=42) | SIP (N=21) | IC (N = 21) | p-value |
|---|---|---|---|---|
| Objective sleep (actigraphy) | ||||
| Sleep efficiency [M (SD)] | 84.9% (7.9%) | 83.1% (9.3%) | 86.6% (5.9%) | .152 |
| Total sleep time [M (SD)] minutes | 467.6 (86.2) | 459.7 (86.8) | 475.4 (86.9) | .563 |
| Number of nighttime awakenings [M (SD)] | 23.6 (7.9) | 23.3 (8.0) | 23.9 (8.0) | .793 |
| Total nighttime wake time [M (SD)] minutes | 82.6 (43.6) | 93.5 (52.2) | 71.6 (30.3) | .104 |
| Patient-reported sleep | ||||
| PSQI total score [M (SD)] | 6.8 (4.2) | 6.6 (4.2) | 7.0 (4.4) | .721 |
| PSQI factor 1 (sleep efficiency) [M (SD)] | 2.5 (2.3) | 2.7 (2.5) | 2.3 (2.2) | .647 |
| PSQI factor 2 (perceived sleep quality) [M (SD)] | 2.6 (2.1) | 2.1 (1.8) | 3.2 (2.2) | .088 |
| PSQI factor 3 (daily disturbances) [M (SD)] | 1.7 (0.9) | 1.8 (0.9) | 1.5 (0.9) | .310 |
| ISI total score [M (SD)] | 8.5 (6.6) | 8.1 (6.5) | 8.9 (7.0) | .715 |
| PSQI Total sleep time [M (SD)] | 6.6 (2.0) | 6.6 (1.9) | 6.6 (2.0) | .950 |
| PSQI Sleep efficiency [M (SD)] | 73.6 (18.0) | 73.7 (17.4) | 73.6 (19.1) | .981 |
M = mean; SD = standard deviation; PSQI = Pittsburgh Sleep Quality Index; ISI = Insomnia Severity Index.
Treatment Adherence
Given the health status of ADHC patients, treatment adherence was thoroughly measured. All 42 randomized participants attended all four intervention sessions. One participant in the SIP group did not complete the third intervention session because he had another appointment to attend (missed 13% of the content). Interventionists’ ratings indicated that participants had “good” or “excellent” participation and comprehension during all sessions except one (ie, 167 out of 168 sessions).
Nineteen out of 21 individuals (90.4%) assigned to the SIP program completed at least one weekly sleep diary during the intervention. Based on these diaries, the interventionist noted whether the participant went to bed and got out of bed within 15 minutes of their scheduled times, and based on that definition (ie, no more than 15 minutes deviation from recommended time), the percentage of nights on which each participant adhered to their assigned schedule was calculated. On average, participants went to bed more than 15 minutes earlier than their assigned bedtime on only 16% of nights (ie, the adhered to their schedule bedtime on 84% of nights). Similarly, they got out of bed more than 15 minutes later than their scheduled rise time 19% of nights (ie, they adhered to their scheduled rise time on 81% of nights). These metrics were not available for IC participants because they were not assigned a specific sleep schedule and did not monitor their sleep schedule during the intervention period.
Outcomes
Supplemental Table S1 includes marginal means for each outcome as a function of group membership (SIP, IC) and time (baseline, post-treatment, and 4 months) as obtained from the mixed model estimation.
Objective Sleep Outcomes
Three out of four actigraphy-measured sleep outcomes showed statistically significant differences between the treatment and control groups when assessing (1) the average change from baseline to post-treatment and (2) the average change from baseline to 4 months. Those outcomes were: sleep efficiency, number of nighttime awakenings, and total nighttime wake time, described in more detail below (also see Table 4).
Table 4.
Study Outcomes at Post-Treatment in SIP group Versus IC Group, Controlling for Baseline.
| Variable | Sleep Intervention Program (SIP) vs. information-only control (IC) | |||
|---|---|---|---|---|
| Difference at post-treatment vs. baseline [mean (95% CI] | p-value | Difference at 4-month follow-up vs. baseline [mean (95% CI] | p-value | |
| Objective sleep (actigraphy) | ||||
| Sleep efficiency [M (SD)]1 | 4.2% (1.1%, 7.2%) | .007 | 4.1% (0.5%, 7.7%) | .025 |
| Total sleep time [M (SD)] minutes1 | −2.0 (−38.1, 34.1) | .913 | 17.6 (−36.1, 71.3) | .521 |
| Number of nighttime awakenings [M (SD)]2 | −5.3 (−9.6, −1.0) | .016 | −4.5 (−8.6, −0.3) | .035 |
| Total nighttime wake time [M (SD)] minutes2 | −30.8 (−49.2, −12.4) | .001 | −25.3 (−47.0, −3.5) | .023 |
| Patient-reported sleep quality | ||||
| PSQI total score [M (SD)]2 | 0.5 (−1.2, 2.1) | .571 | −1.5 (−3.5, 0.4) | .129 |
| PSQI factor 1 (sleep efficiency) [M (SD)]2 | 0.4 (−0.8, 1.7) | .508 | −0.7 (−1.8, 0.5) | .237 |
| PSQI factor 2 (perceived sleep quality) [M (SD)]2 | 0.3 (−0.5, 1.1) | .492 | 0.0 (−1.0, 1.0) | .992 |
| PSQI factor 3 (daily disturbances) [M (SD)]2 | −0.3 (−0.9, 0.2) | .242 | −0.7 (−1.3, −0.1) | .016 |
| ISI total score [M (SD)]2 | −1.7 (−4.3, 1.0) | .217 | −0.2 (−3.0, 2.5) | .862 |
| Secondary outcomes | ||||
| PSQI hours of sleep [M (SD)] | 0.3 (−0.5, 1.2) | .458 | 0.3 (−0.6, 1.3) | .517 |
| PSQI sleep efficiency [M (SD)] | −9.5% (−26.0%, 6.9%) | .256 | 2.8% (−7.3%, 13.0%) | .582 |
| Flinders fatigue scale [M (SD)]3 | −3.8 (−7.6, −0.0) | .048 | −1.1 (−4.5, 2.3) | .537 |
| PHQ-9 score [M (SD)]3 | −1.1 (−3.9, 1.8) | .459 | −1.6 (−3.6, 0.5) | .128 |
| SF-12 PCS subscale [M (SD)]4 | −0.5 (−6.7, 5.6) | .864 | −2.1 (−8.4, 4.2) | .510 |
| SF-12 MCS subscale [M (SD)]4 | 0.4 (−4.8, 5.6) | .883 | −0.6 (−6.2, 4.9) | .822 |
Significant differences are shown in bold typeface.
1 Greater scores imply better sleep quality and positive differences represent improvements in sleep quality from baseline.
2 Lower scores imply better sleep quality, and negative differences represent improvements in sleep quality from baseline.
3 Higher scores indicate more depression/fatigue.
4 Higher scores indicate better quality of life.
SIP = Sleep Intervention Program; IC = information-only control; CI = confidence interval; M = mean; SD = standard deviation; PSQI = Pittsburgh Sleep Quality Index; ISI = Insomnia Severity Index; PHQ = Patient Health Questionnaire; PCS = Physical Component Score; MCS = Mental Component Score.
Sleep efficiency
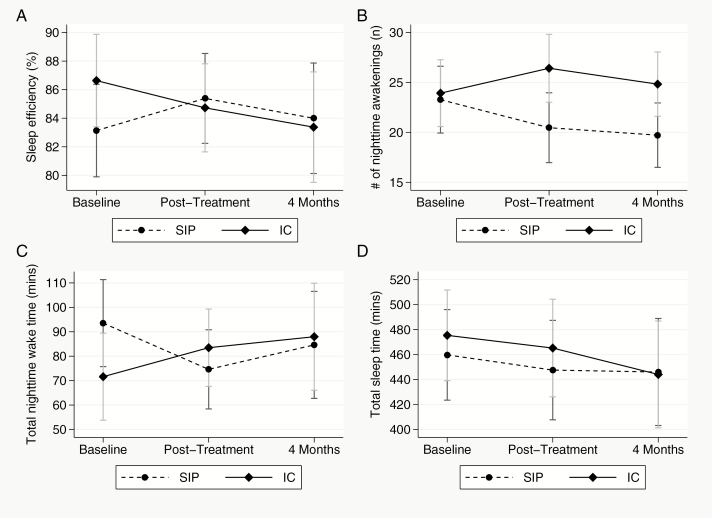
Relative to baseline values, the average change in sleep efficiency was greater for the SIP versus IC group at post-treatment (p = .007) and greater for the SIP versus IC group at 4-month follow-up (p = .025). The average improvement in sleep efficiency (relative to baseline) for the SIP group (vs. IC) at post-treatment was 4.2%. Similarly, relative to baseline, sleep efficiency improved in the SIP group by an average of 4.1% more than in the IC group at 4-month follow-up (see Table 4 and Figure 2, Panel A).
Figure 2.
Mean values for actigraphy (ACTI) outcome variables for the Sleep Intervention Program (SIP) and control groups at baseline, post-treatment and 4-month follow-up for sleep efficiency (panel A), number of nighttime awakenings (panel B), total nighttime wake time (panel C) and Total sleep time (panel D). Data presented here includes all available observations for actigraphy at each time point (N = 42 at baseline, N = 38 post-treatment and, N = 38 at 4-month follow-up; also see Supplementary Figures S27–S30 for evaluation of potentially influential data points).
Number of Awakenings
Compared to baseline values, the change in number of nighttime awakenings was greater for the SIP versus IC group at post-treatment (p = .016) and greater for the SIP versus IC group at 4-months follow-up (p = .035). From baseline to post-treatment, the SIP group averaged 5.3 fewer nighttime awakenings compared to the IC group. Likewise, from baseline to 4-month follow-up, the SIP group averaged 4.5 fewer nighttime awakenings (see Table 4 and Figure 2, Panel B).
Total Nighttime Wake Time
As compared to the baseline values, the average number of nighttime awakenings decreased more for the SIP than the IC group at post-treatment (p = .001) and at 4-month follow-up (p = .023). The average reduction in total nighttime wake time from baseline to post-treatment was 30.8 minutes more for the SIP compared to the IC group. Similarly, from post-treatment to 4-month follow-up, the average reduction in total nighttime awake time was 25.3 minutes more for the SIP (vs. IC) group (see Table 4 and Figure 2; Panel C).
Total Nighttime Sleep Time
No significant differences between two groups were observed for total sleep time at either post-treatment or 4-month follow-up (see Table 4 and Figure 2; Panel D).
Patient-Reported Sleep Outcomes
There were no significant differences between the SIP and IC groups at either post-treatment or at 4-month follow-up for the PSQI total score, PSQI Factor 1 (sleep efficiency) or PSQI Factor 2 (perceived sleep quality see Table 4 and Supplementary Figures S1–S3). The PSQI scores on Factor 3 (daytime disturbances) showed greater reductions (improvement) from baseline to 4-month follow-up for the SIP versus IC group (p = .016 see Table 4 and Supplementary Figure S4). The average change in Factor 3 of the PSQI for the SIP (vs. IC) group was −.7 (−0.18, 0.6). The difference for this outcome at post-treatment was not significant. The treatment effects on ISI at either post-treatment or 4-month follow-up were not significant (see Table 4 and Supplementary Figure S5).
There also were no significant treatment effects in terms of patient-reported hours of sleep or sleep efficiency (based on PSQI items; see Table 4 and Supplementary Figures S6–S7).
Secondary Outcomes
We also tested whether the SIP improved health (ie, fatigue, depression, health-related quality of life) at post-treatment and 4-month follow-ups, compared to the IC (see Supplementary Figures S8–S11). Only fatigue measured by FFS showed significant improvement at post-treatment in SIP group compared to the IC group (4.5 vs. 9.9, p = .048). No other differences were observed in these secondary outcome measures.
Sensitivity Analyses
Sensitivity analyses are presented in Supplementary Table S2 and Supplementary Figures S12–S26. At post-treatment, findings for sleep efficiency, number of nighttime awakenings, and total nighttime time awake were robust to omission of potentially influential data points with one exception. If two data points were excluded from the analysis of number of nighttime awakenings, the p-value increased from .016 to .053. At 4 months, the impact of exclusion of potentially influential data points was still minimal but had some impact on statistical significance, with p-values ranging from .020 to .083 depending on the outcome variable and the number of observations excluded (1–3). Total sleep time remained nonsignificant after omitting a potentially influential observation.
DISCUSSION
The overall pattern of results suggests that the SIP, delivered by a trained HE under the supervision of a sleep psychologist was feasible, with high levels of attendance and engagement by ADHC participants. The SIP resulted in relative improvements in objectively measured sleep based on wrist actigraphy compared to the IC group. Importantly, these differences in sleep were largely maintained at 4-month follow-up. The pattern of results suggest that the control group may have experienced gradually worsening sleep (based on actigraphy) over the study period, whereas the intervention group either improved slightly or declined at a slower rate. This is a common phenomenon in research on older adults, and a recent study found that older adults, particularly if they were using benzodiazepines, showed deterioration in sleep quality over a 1-year period.42
Improvements in patient-reported outcomes were modest, with only one PSQI component (daily disturbances) showing significantly better (lower) scores in the SIP condition at the 4-month follow-up but not at post-treatment. It is possible that, given the duration of sleep difficulties for many of these older patients, sustained improvements in sleep were needed before patients began to feel better during the daytime hours. Another possible explanation is that we did not use a specific cutoff score on either the ISI or PSQI to determine eligibility for randomization, and these two commonly used questionnaires did not appear to reflect the sleep experience of these patients. Older veterans who attend ADHC may not report sleep disturbances in the same way as healthier older adult populations and in fact, may tolerate significantly more sleep disturbance before noting poor sleep quality. Some participants, for example, reporting taking longer than 1 hour to fall asleep, but then reported that their sleep quality was “very good.” Furthermore, in our recent insomnia treatment study of older veterans in the same health care system who were not participating in the ADHC program,23 the mean baseline PSQI score was 9.1 and the mean baseline ISI score was 11.1, while their baseline sleep efficiency based on actigraphy was 83%. ADHC Patients in the current study had similar actigraphically assessed sleep efficiency (85%); however, their questionnaires reflected substantially less “complaint.” The mean baseline PSQI was only 6.8 (slightly above the clinical cutoff of 6 for sleep disturbance), and the mean baseline ISI was only 8.5 (indicating only mild insomnia, on average). It is possible that measures like the PSQI and ISI do not capture the way in which these older veterans describe their difficulties with sleep, subjectively. This may account for our discrepant findings. We also were not able to obtain completed sleep diaries, which are typically used in studies of behavioral treatments for insomnia. We asked the first 11 enrolled participants to complete a daily sleep diary based on the American Academy of Sleep Medicine consensus sleep diary42 during their baseline assessment, and none of the patients fully completed the diary during the 3-day baseline. As a result, we used a very simple four-item diary (bedtime, rise time, sleep quality, and daytime sleepiness), which did not allow for computation of traditional sleep diary measures such as sleep efficiency or total sleep time from daily diaries but could be used for actigraphy scoring and to establish adherence to the assigned sleep schedule in the SIP program. One study of older adults recruited from primary care found improvements in both patient-reported (daily sleep dairy, questionnaires) and objective (actigraphy) outcomes,21 although baseline sleep complaints were more severe (baseline PSQI = 11) in that study compared to our study as well.
We did find improvements in fatigue, which was one of our secondary patient-reported outcomes, at post-treatment. This finding is consistent with the reduced impact of sleep disturbance at 4-month follow-up observed on the PSQI; however, we did not find improvements in depression or quality of life at either post-treatment or 4-month follow-up. It is important to note, however, that this study was not powered to detect the impact of the sleep intervention on these outcomes and additional research is needed. This may be important to emphasize because improvements in daytime symptoms may be seen as a significant benefit to patients (in terms of self-reported outcomes) even if sleep quality itself is not perceived to change.
This study has several strengths, including the implementation of the intervention within the ADHC program; however, despite attempts to minimize costs of participation, it was difficult to identify patients interested in participating in the intervention program. Many individuals did not feel their poor sleep was worthy of clinical attention. Interestingly, improvements in patient-reported sleep quality on the PSQI or insomnia symptoms on the ISI were not seen. Only improvements in objectively measured sleep were observed and maintained over time.
One consideration in the design of the intervention was the tolerability of sleep restriction therapy, which is a common, evidence-based component of cognitive-behavioral interventions. Because we anticipated that many of the study participants would find it difficult to dramatically and quickly reduce their time in bed at the first intervention session, we instead elected to use sleep compression therapy, in which time in bed is slowly reduced, rather than contracted, and then slowly expanded. We found that participants were receptive to this approach and had success adhering to the assigned sleep schedule. In fact, they stayed up until their assigned bedtimes on 84% of nights and got out of bed at or before their assigned rise time on 81% of nights. This level of adherence is similar to what has been seen in studies of healthy, younger individuals in receiving CBT-I.43,44 We also significantly modified the standard stimulus control instructions.45 The main reason for this modification was concern about nighttime fall risk in older adults with functional limitations. Despite this modification, the intervention remained effective in reducing total time awake at night. Rather than instructing participants to get of bed if awake at night, we focused on confining sleep to the bed and bedroom and eliminating nonsleep activities from the sleep environment outside of the nighttime sleep period (eg, watch TV in the family room rather than in bed in the afternoon).
While this study had multiple strength, including a high participant retention rate and implementation of the intervention in the context of an ongoing clinical program, there are also several limitations. One key limitation is that we were not able to screen participants for sleep-disordered breathing, despite data to suggest this is very common in older adults with functional and/or cognitive impairments.46,47 Participants found completion of a home sleep apnea test overly burdensome and were unwilling to complete an overnight study in the sleep laboratory; therefore, while we had planned to identify and exclude participants with severe sleep apnea, this was not possible. In addition, findings from this study may not directly generalize to ADHC programs outside of VA. Veterans are predominantly male and have more complex comorbidities than nonveterans, which may impact the delivery and benefits of the SIP.8 Although the definition of ADHC is similar regardless of where the programs are located,6 there are likely to be differences in ADHC programming and resources outside of VA, and those differences might make it challenging to implement our SIP within community ADHC programs.
In summary, a brief, structured sleep improvement program may improve objectively assessed nighttime sleep in older Veterans participating in an ADHC program, and these improvements were maintained at 4-month follow-up. Modest improvements in daytime functioning, including reduced fatigue and reduced impact of sleep on daytime functioning may also be achieved. Additional research is needed to better understand how to assess patient-reported outcomes and to confirm our findings of relatively improvements in objectively measured sleep. Future research should also evaluate how best to implement this intervention program into routine care at ADHCs and to consider whether it can be delivered in group formats because therapeutic interventions are often delivered to patients in groups in ADHC settings.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
FUNDING
This study was funded by the Veterans Affairs (VA) Rehabilitation Research and Development Service 1RX000135-01 (PI: Martin). Additional funding support for authors includes: VA Advanced Geriatrics Fellowship Program, VA Greater Los Angeles Healthcare System Geriatric Research, Education and Clinical Center (Dzierzewski, Song); UCLA Claude Pepper Older Americans Independence Center (5P30AG028748, PI: Dzierzewski); National Center for Advancing Translational Sciences UCLA CTSI (UL1TR000124, PI: Dzierzewski); National Institute on Aging, National Institutes of Health (K23AG045937, PI: Fung); National Institute on Aging, National Institutes of Health (K23AG049955, PI: Dzierzewski).
ADDRESS WHERE WORK WAS CONDUCTED
VA Greater Los Angeles Healthcare System, Geriatric Research, Education and Clinical Center, 16111 Plummer St. (11E), North Hills, CA 91343.
CLINICAL TRIAL
“Treating Sleep Problems in VA Adult Day Health Care.” NCT01259401.
DISCLOSURE STATEMENT
None declared. The content is solely the responsibility of the authors and does not necessarily represent the official views of Department of Veterans Affairs, National Institutes of Health, or the U.S. Government.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank ADHC program director Jo Ellen Baur, MSW, Maureen Burruel, MSW and all members of the ADHC staff for their support of this project. The authors also wish to acknowledge research staff members Sergio Martinez, Simone Vukelich, Sandra Fontal, MPA, and Diane Lee, MSW. Most of all, we wish to posthumously recognize the many contributions of Terry Z. Vandenberg, MA, who served as the lead health educator for the study.
REFERENCES
- 1. Dam TT, Ewing S, Ancoli-Israel S, Ensrud K, Redline S, Stone K; Osteoporotic Fractures in Men Research Group Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008; 56(9): 1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nóbrega PV, Maciel AC, de Almeida Holanda CM, Oliveira Guerra R, Araújo JF. Sleep and frailty syndrome in elderly residents of long-stay institutions: a cross-sectional study. Geriatr Gerontol Int. 2014; 14(3): 605–612. [DOI] [PubMed] [Google Scholar]
- 3. Neikrug AB, Ancoli-Israel S. Sleep disturbances in nursing homes. J Nutr Health Aging. 2010; 14(3): 207–211. [DOI] [PubMed] [Google Scholar]
- 4. Martin JL, Webber AP, Alam T, Harker JO, Josephson KR, Alessi CA. Daytime sleeping, sleep disturbance, and circadian rhythms in the nursing home. Am J Geriatr Psychiatry. 2006; 14(2): 121–129. [DOI] [PubMed] [Google Scholar]
- 5. Martin JL, Fiorentino L, Jouldjian S, Josephson KR, Alessi CA. Sleep quality in residents of assisted living facilities: effect on quality of life, functional status, and depression. J Am Geriatr Soc. 2010; 58(5): 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The MetLife National Study of Adult Day Services. MMI00156(1010). 2013. New York, NY: Mature Market Institute. [Google Scholar]
- 7. Office of Public Affairs and Media Relations. VA Long-term care. 2005. Washington, DC: Department of Veterans Affairs. [Google Scholar]
- 8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000; 160(21): 3252–3257. [DOI] [PubMed] [Google Scholar]
- 9. Chapko M, Ehreth J, Hedrick SC, Rothman ML. Effects of adult day health care on utilization and cost of care for subgroups of patients. Med Care. 1993; 31(9 Suppl): SS62–SS74. [PubMed] [Google Scholar]
- 10. Martin JL, Alam T, Harker JO, Josephson KR, Alessi CA. Sleep patterns in assisted living facilities: a comparison to home-dwelling elders. J Gerontol A Biol Sci Med Sci. 2008; 163A: 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Webber AP, Martin JL, Harker JO, Josephson KR, Rubenstein LZ, Alessi CA. Depression in older patients admitted for postacute nursing home rehabilitation. J Am Geriatr Soc. 2005; 53(6): 1017–1022. [DOI] [PubMed] [Google Scholar]
- 12. Alessi CA, Webber AP, Josephson KR, Rubenstein L, Harker JO, Martin JL. Sleep and functional improvement in the nursing home setting [abstract]. Gerontologist. 2003; 43: 490 [Google Scholar]
- 13. Stone KL, Blackwell TL, Ancoli-Israel S et al. . Sleep disturbances and risk of falls in older community-dwelling men: the outcomes of sleep disorders in older men (MrOS Sleep) study. J Am Geriatr Soc 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song Y, Dzierzewski JM, Fung CH et al. . Association between sleep and physical function in older veterans in an adult day healthcare program. J Am Geriatr Soc. 2015; 63(8): 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hughes J, Martin JL. Sleep characteristics of veterans affairs adult day health care participants. Behavioral Sleep Medicine. 2013; 11: 258–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meissner HH, Riemer A, Santiago SM, Stein M, Goldman MD, Williams AJ. Failure of physician documentation of sleep complaints in hospitalized patients. West J Med. 1998; 169(3): 146–149. [PMC free article] [PubMed] [Google Scholar]
- 17. Bloom HG, Ahmed I, Alessi CA et al. . Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009; 57(5): 761–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avidan AY, Fries BE, James ML, Szafara KL, Wright GT, Chervin RD. Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J Am Geriatr Soc. 2005; 53(6): 955–962. [DOI] [PubMed] [Google Scholar]
- 19. Le Couteur DG, Latimer Hill E, Cumming RG, Lewis R, Carrington S. Sleep disturbances and falls in older people. J Gerontol A Biol Sci Med Sci. 2007; 62A: 62–66. [DOI] [PubMed] [Google Scholar]
- 20. Sivertsen B, Omvik S, Pallesen S et al. . Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults. JAMA. 2006; 295: 2851–2858. [DOI] [PubMed] [Google Scholar]
- 21. Buysse DJ, Germain A, Moul DE et al. . Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011; 171(10): 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. 2004; 164(17): 1888–1896. [DOI] [PubMed] [Google Scholar]
- 23. Alessi CA, Martin JL, Fiorentino L et al. . Cognitive behavioral therapy for insomnia in older veterans using non-clinician sleep coaches: a randomized controlled trial. J Am Geriatr Soc 2016; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 25. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001; 2(4): 297–307. [DOI] [PubMed] [Google Scholar]
- 26. Kroenke K, Spitzer RL, Williams JBW.. Validity of a brief depression severity measure. J Gen Intern Med. 2001; 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ouimette P, Wade M, Prins A, Schohn M. Identifying PTSD in primary care: comparison of the Primary Care-PTSD screen (PC-PTSD) and the General Health Questionnaire-12 (GHQ). J Anxiety Disord. 2008; 22(2): 337–343. [DOI] [PubMed] [Google Scholar]
- 28. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12(3): 189–198. [DOI] [PubMed] [Google Scholar]
- 29. Duke University Center for the Study of Aging and Human Development. Duke Older Americans Resources and Services Program: An Information System for Functional Assessment, Program Evaluation and Resource Allocation. Center Reports on Advances in Research; 1985; 9:whole issue. [Google Scholar]
- 30. Gradisar M, Lack L, Richards H et al. . The Flinders Fatigue Scale: preliminary psychometric properties and clinical sensitivity of a new scale for measuring daytime fatigue associated with insomnia. J Clin Sleep Med. 2007; 3(7): 722–728. [PMC free article] [PubMed] [Google Scholar]
- 31. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996; 34(3): 220–233. [DOI] [PubMed] [Google Scholar]
- 32. National Institutes of Health. Manifestations and management of chronic insomnia in adults. Sleep. 2005; 28: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 33. Lichstein KL, Riedel BW, Wilson NM, Lester KW, Aguillard RN. Relaxation and sleep compression for late-life insomnia: a placebo-controlled trial. J Consult Clin Psychol. 2001; 69(2): 227–239. [DOI] [PubMed] [Google Scholar]
- 34. McCrae CS, McGovern R, Lukefahr R, Stripling AM. Research Evaluating Brief Behavioral Sleep Treatments for Rural Elderly (RESTORE): a preliminary examination of effectiveness. Am J Geriatr Psychiatry. 2007; 15(11): 979–982. [DOI] [PubMed] [Google Scholar]
- 35. Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006; 29(1): 112–116. [DOI] [PubMed] [Google Scholar]
- 36. Standards of Practice Committee , Morgenthaler T, Alessi CA et al. . Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007; 30: 519–529. [DOI] [PubMed] [Google Scholar]
- 37. Centers for Disease Control. International Classification of Diseases, Ninth Revision (ICD-9). 1979. CDC. [Google Scholar]
- 38. Ferrell BA, Stein WM, Beck JC. The Geriatric Pain Measure: validity, reliability and factor analysis. J Am Geriatr Soc. 2000; 48(12): 1669–1673. [DOI] [PubMed] [Google Scholar]
- 39. Ouimette P, Wade M, Prins A, Schohn M. Identifying PTSD in primary care: comparison of the Primary Care-PTSD screen (PC-PTSD) and the General Health Questionnaire-12 (GHQ). J Anxiety Disord. 2008; 22(2): 337–343. [DOI] [PubMed] [Google Scholar]
- 40. Kroenke K, Spitzer RL, Williams JBW. Validity of a brief depression severity measure. J Gen Intern Med. 2001; 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bourgeois J, Elseviers MM, Van Bortel L, Petrovic M, Vander Stichele RH. One-year evolution of sleep quality in older users of benzodiazepines: a longitudinal cohort study in belgian nursing home residents. Drugs Aging. 2014; 31(9): 677–682. [DOI] [PubMed] [Google Scholar]
- 42. Carney CE, Buysse DJ, Ancoli-Israel S et al. . The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012; 35(2): 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matthews EE, Schmiege SJ, Cook PF, Berger AM, Aloia MS. Adherence to cognitive behavioral therapy for insomnia (CBTI) among women following primary breast cancer treatment: a pilot study. Behav Sleep Med. 2012; 10(3): 217–229. [DOI] [PubMed] [Google Scholar]
- 44. Bouchard S, Bastien C, Morin CM. Self-efficacy and adherence to cognitive-behavioral treatment of insomnia. Behav Sleep Med. 2003; 1(4): 187–199. [DOI] [PubMed] [Google Scholar]
- 45. Bootzin RR, Epstein D. Stimulus control. In: Lichstein KL, Morin CM, eds. Treatment of late-life insomnia. Thousand Oaks, California: Sage Publications, Inc; 2000; 167–184. [Google Scholar]
- 46. Song Y, Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Stone KL; Osteoporotic Fractures in Men (MrOS) Study Group Relationships between sleep stages and changes in cognitive function in older men: the MrOS Sleep Study. Sleep. 2015; 38(3): 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spira AP, Blackwell T, Stone KL et al. . Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008; 56(1): 45–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.