Abstract
Objective
To assess clinical characteristics and cytokine levels in children with severe pneumonia who required ventilatory support.
Methods
In this prospective, descriptive, cross-sectional study, blood and endotracheal fluid samples were obtained from patients with severe pneumonia, aged <5 years, within 24 h following intubation. Blood samples were also obtained from age-matched healthy controls. Cytokine levels were investigated using flow cytometry-assisted immunoassay.
Results
Forty-five patients with severe pneumonia requiring mechanical ventilation (aged 10 ± 5 months) and 35 healthy age-matched controls were included. Patients with severe pneumonia had significantly increased serum interleukin (IL)-6, IL-8, and granulocyte/macrophage colony-stimulating factor concentrations compared with controls (80.84 pg/ml versus 2.06 pg/ml, 90.03 pg/ml versus 6.62 pg/ml, and 115.58 pg/ml versus 11.47 pg/ml, respectively). In the severe pneumonia group, serum IL-10 levels were significantly higher in patients aged <6 months versus those aged 6–12 months. Age-group differences in serum cytokine levels did not correspond to age-group differences in endotracheal-fluid cytokine levels. Serum IL-6 levels were significantly higher in patients who subsequently died versus those who survived (267.12 pg/ml versus 20.75 pg/ml, respectively).
Conclusion
High IL-6 concentrations were associated with mortality in patients <5 years of age with severe pneumonia requiring mechanical ventilation.
Keywords: Cytokines, pneumonia, mechanical ventilation, endotracheal fluid
Introduction
Pneumonia is one of the leading causes of death in children, particularly in those under 5 years of age,1 and can be caused by different types of agents, such as bacteria, viruses, exposure to toxic substances, pollutants, irritants, and allergens.2 Under normal circumstances, microorganisms do not penetrate into the alveoli, due to protective mechanisms and the anatomy of the bronchioles and alveoli. When microorganisms do enter the bronchioles and alveoli, inflammation occurs, and many cells are activated to release cytokines and mediators to launch and maintain the inflammatory response.2 A balance between inflammatory response and anti-inflammatory processes is necessary to maintain lung homeostasis.
Macrophage cells transit into the lungs from the pulmonary capillaries and reside in the respiratory tract, alveoli and interstitial space around the alveoli. Macrophages play a role in regulating the acute and chronic inflammatory response, and although they can proliferate in the lungs, their numbers are often not high enough to fight infection.3,4 Macrophages have phagocytic ability and are a source of cytokines, chemokines, and other inflammatory mediators.5,6 Other cell types including neutrophils, lymphocytes, epithelial cells and mast cells also participate in inflammation, producing cytokines and helping to modulate the inflammatory response.2
An imbalance in the two types of cytokines, proinflammatory and anti-inflammatory, may influence the prognosis of sepsis and other infectious and inflammatory diseases.7,8 The proinflammatory cytokines include tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, and interferon (IFN)-γ, all of which activate the immune system and participate in the acute inflammatory response. The anti-inflammatory cytokines, such as IL-10, transforming growth factor (TGF)-α and IL-1 receptor antagonist (RA) play a major role in regulating the activities of cells and other cytokines.9 Alveolar macrophages secrete anti-inflammatory cytokines to reduce inflammation in the lungs,10,11 for example, TGF-α receptor is present on most cells and TGF is known to promote wound healing and fibrosis.10 Proinflammatory cytokines encourage transition of T helper (Th) type 0 cells towards Th1, which can further amplify the inflammatory cascade by secretion of INF-γ, TNF-α, IL-1, IL-12, and granulocyte/macrophage colony-stimulating factor (GM-CSF).12–14 In turn, IL-10 inhibits the production of proinflammatory cytokines by T cells, natural killer cells, and monocytes, thus reducing inflammation.12 Dysregulation of cytokines and the cells that procure them may predispose to severe pneumonia.13,14
Macrophages alone may not be able to contain and destroy bacteria during infection, and require the influx of polymorphonuclear leukocytes (PMNs) to assist in containment.15 Cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, are secreted by alveolar macrophages and act as chemotactic agents for PMNs, enhancing the phagocytic activity occurring in the lungs. Over production of these cytokines, however, can be destructive, damaging normal lung structure, and increase morbidity and mortality.15
Several life-threatening bacterial lung diseases are demonstrated to be caused by excessive neutrophil-mediated inflammatory signals, including TNF-α, IL-1β, IL-6; IL-8 and other components.16 In a study of 201 hospitalized patients with pneumonia, blood levels of cytokines such as IL-1RA, IL-6, IL-8, IL-10 were shown to increase in the acute phase, and rapidly decrease when patients were discharged from hospital.17 In bacterial pneumonia the cytokine response is mostly confined to the affected lung,18–21 but systemic concentrations of cytokines are also increased.18,19,22 Higher concentrations of cytokines in blood are reflected by, and have been shown to predict, the severity of pneumonia.19,23,24
Prior studies of infectious diseases have mainly focused on the systemic inflammatory response, investigating markers such as the number of white blood cells, the number of neutrophils, and C-reactive protein (CRP) levels, that also demonstrate the severity of inflammation.25 Studying cytokine transition in patients with severe pneumonia may help in determining prognostic factors of disease severity and outcomes, and may help to determine interventions. In Vietnam, minimal data are available on peripheral blood and endotracheal fluid cytokine levels in children with pneumonia who are intubated. Thus, the objective of the present research was to study clinical characteristics and cytokine levels in young patients with severe pneumonia requiring mechanical ventilation compared with healthy age-matched controls, in order to determine if cytokine levels were associated with outcome in paediatric cases of severe pneumonia.
Patients and methods
Study population
This prospective, descriptive, cross-sectional study sequentially enrolled patients <5 years of age, who were admitted to the Intensive Care Unit, National Hospital of Paediatrics, Hanoi, Vietnam diagnosed with severe pneumonia and respiratory failure requiring mechanical respiratory support, and healthy age-matched controls who received routine care also at the National Hospital of Paediatrics, between February 2014 and August 2014. Inclusion criteria comprised: severe pneumonia requiring mechanical respiratory support; endotracheal intubation within 24 h following hospital admission; aged between 1 month and 5 years; and legal proxy consent to participate in the study. Exclusion criteria comprised: severe pneumonia with other chronic diseases, such as congenital heart disease, encephalitis, stroke, and chronic lung diseases; or endotracheal intubation conducted >24 h following hospital admission.
Pneumonia was diagnosed according to clinical symptoms, signs and laboratory tests:26 Fever or low temperature, a dry cough or cough with phlegm, fast breathing, chest indrawing, crackles upon auscultation of both lungs, and chest X-ray with typical findings consistent with pneumonia.
Severe pneumonia was diagnosed based on signs and symptoms of pneumonia, chest indrawing, respiratory failure without cyanosis, SpO2 <95%, PaO2 <60 mmHg, and normal or slightly decreased PaCO2.26
Seriously severe pneumonia was diagnosed based on signs and symptoms of pneumonia, respiratory failure with cyanosis of varying degrees, respiratory rate (slow or fast breathing, episodes of apnea, and with chest indrawing), SpO2 <95%, PaO2 <60 mmHg, and increased PaCO2 >50 mmHg.26
This study was approved by the Medical Ethics Committee of the National Hospital of Paediatrics, Hanoi, Vietnam (No. 954B/BV.NTW- VNCSKTE), and written informed consent was obtained from the legal proxies of all study participants.
Data and sample collection
Relevant data for the present study were gathered from the patient’s hospital database records, and a complete history and exam was obtained for all participants. Venous blood samples (2 ml) were drawn into tubes without anticoagulant from patients meeting the inclusion criteria within the first 24 h following endotracheal intubation, and from control participants. Immediately following collection and transfer to the processing laboratory, blood samples were placed at 37℃ for 30 min to allow clotting. Samples were then centrifuged at 1 000 g for 5 min at 4℃. The subsequent fibrin plug was removed with glass chopsticks followed by further sample centrifugation at 10 000 g for 15 min at 4℃. Endotracheal fluid samples (2 ml) were collected by aspiration via catheter following injection of 5 ml sterile 0.9% NaCl into the endotracheal tube from patients meeting the inclusion criteria within the first 24 h following intubation. Serum and endotracheal fluid samples were stored at –80℃ prior to analysis.
Quantification of peripheral blood and endotracheal fluid cytokine levels
Serum and endotracheal fluid cytokine quantification was performed at the Immunology Laboratory of Military Medical Academy 103, Hanoi, Vietnam, via flow cytometry-assisted immunoassay using the Bio-Plex® system of reagents and equipment (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions.
First, 96-well assay plates were pre-wetted by adding 100 µl of assay buffer (Bio-Plex Pro™; Bio-Rad) and removing the liquid using vacuum filtration. Magnetic bead sets coloured with two fluorescent dyes (red and infrared) at distinct ratios, and conjugated to capture monoclonal antibodies specific to target cytokine epitopes (Bio-Plex®, Bio-Rad), were diluted to a 1 × working suspension, vortexed for 30 s at medium speed, then 50 µl was transferred to each well of the assay plate. The plate was washed twice with 100 µl of wash buffer using a Bio-Plex Pro™ Wash Station with the MAG × 2 programme setting (Bio-Rad). Diluted standards, blanks, samples, and controls were gently vortexed for 5 s, and 50 µl was transferred to each well of the assay plate, which was then covered with sealing tape, protected from light with aluminium foil and incubated on shaker at room temperature. Following incubation, the sealing tape was slowly removed and discarded, and the plate washed three times with 100 µl of wash buffer as before. Secondary biotinylated monoclonal detection antibodies, specific to a different epitope on the target cytokines (1 × dilution; Bio-Plex® human cytokines; Bio-Rad), were gently vortexed for 5 s, poured into a reagent reservoir, and 25 µl was then transferred to each well. Plates were covered with new sealing tape, protected from light with aluminium foil, and incubated on a shaker for 30 min at room temperature before washing three times with 100 µl of wash buffer. Finally, 50 µl streptavidin-phycoerythrin (SA-PE) reporter conjugate (diluted 1 ×) was vortexed and transferred to each well, and the covered plate incubated at room temperature to form streptavidin-biotin complexes. Following the streptavidin-phycoerythrin incubation step, the plate was washed three times with 100 µl of wash buffer, then 125 µl of assay buffer (Bio-Plex Pro™; Bio-Rad) was added to each well, and the covered plate incubated on a shaker for 30 s at room temperature. The plate was ready to read following removal of the sealing tape. The identity and quantity of the cytokines was assessed using a flow cytometry/laser excitation–based Bio-Plex 200 system (Bio-Rad) with two different sources of laser (532–635 nm) and detectors to generate and record two types of independent fluorescent signal emitted by the beads (qualitative signals) and from the specific reaction emitted by the particle surface (quantitative signals).
Based on the density of the fluorescence emitted from the particles incubated with known concentrations of cytokines, the target cytokines were quantified. The normal range of studied cytokines with age-matched values for healthy subjects under 6 years of age in the Military Medical Academy 103 Immunology Laboratory were: IL-6, 1.73–9.26 pg/ml; IL-8, 2.32–9.58 pg/ml; IL-10, 0.52–5.56 pg/ml; IL-12, 0.01–1.05 pg/ml; GM-CSF, 7.64–19.34 pg/ml; INF-γ, 5.54–98.2 pg/ml; and TNF-α, 1.94–3.76 pg/ml.
Statistical analyses
The recorded parameters were analysed using IBM-SPSS software, version 20.0 (SPSS Inc., Chicago, IL, USA). Values are presented as mean ± SD or median (min–max) for quantitative variables, and n (%) prevalence for qualitative variables. Normally distributed quantitative parameters were compared using analysis of variance, or by non-parametric methods (Mann–Whitney U-test and Kruskal–Wallis test) if not normally distributed. Z-test was used for proportion comparisons. A P value < 0.05 was considered to be statistically significant.
Results
A total of 45 patients with severe pneumonia and respiratory failure requiring ventilatory support (mean age, 10 ± 5 months; range, 2–31 months) and 35 healthy age-matched controls (mean age, 13 ± 4 months; range, 2–36 months) were included in the present study. There were no statistically significant differences between the patient group and age-matched controls, and demographic and clinical characteristics are shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients <5 years of age with severe pneumonia and respiratory failure requiring mechanical ventilation, and healthy age-matched controls.
| Study group |
||
|---|---|---|
| Parameter | Patients with severe pneumonia (n = 45) | Control (n = 35) |
| Age, months | 10 ± 5 | 13 ± 4 |
| <6 months | 4 (8.9) | 3 (8.6) |
| 6–12 months | 36 (80.0) | 28 (80.0) |
| >12 months | 5 (11.1) | 4 (11.4) |
| Sex, male | 30 (66.7) | 24 (68.6) |
| Fever | 29 (64.4) | – |
| Respiratory failure | 45 (100) | – |
| Cyanosis | 20 (44.4) | – |
| SpO2 <90% | 40 (88.9) | – |
| Crackles, % | 41 (91.1) | – |
| Malnutrition, % | 24 (53.3) | – |
| Diarrhoea, % | 25 (55.6) | – |
| Duration of mechanical ventilation, days | ||
| All patients | 8 ± 2 | – |
| Aged <6 months | 6 ± 2 | – |
| Aged 6–12 months | 8 ± 2 | – |
| Aged >12 months | 12 ± 3 | – |
| Duration of treatment, days | ||
| All patients | 19 (3–60) | – |
| Aged <6 months | 28 ± 8 | – |
| Aged 6–12 months | 16 ± 9 | – |
| >12 months | 29 ± 22 | – |
Data presented as mean ± SD, n (%) prevalence, or median (min–max).
There were no statistically significant between-group differences in terms of age or sex (Z-test).
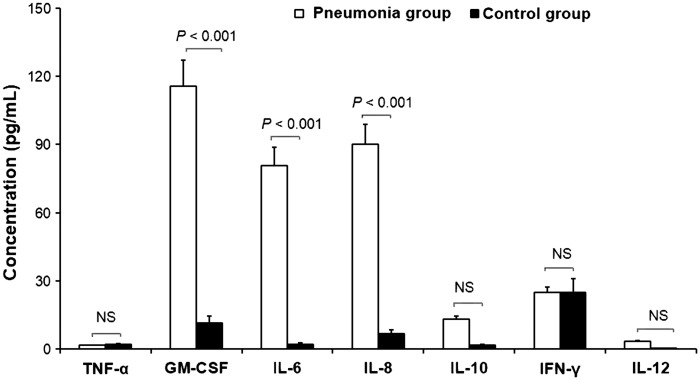
Serum IL-6, IL-8 and GM-CSF levels were significantly higher in patients with severe pneumonia on mechanical support than in healthy age-matched controls (P < 0.001). There were no statistically significant differences in serum concentrations of IL-10, IL-12, TNF-α and INF-γ between patients with pneumonia and healthy age-matched controls (Table 2 and Figure 1).
Table 2.
Serum cytokine levels obtained within 24 h of endotracheal intubation in patients <5 years of age with severe pneumonia and respiratory failure requiring mechanical ventilation compared with serum cytokine levels in healthy age-matched controls.
| Study group |
|||
|---|---|---|---|
| Cytokine | Patients with severe pneumonia (n = 45) | Control (n = 35) | Statistical significance |
| TNF-α, pg/ml | 1.57 (0.003–24.27) | 1.93 (0.27–44.46) | NS |
| GM-CSF, pg/ml | 115.58 (0.43–409.46) | 11.47 (1.82–198.3) | P < 0.001 |
| IL-6, pg/ml | 80.84 (15.38–680.62) | 2.06 (2.06–36.63) | P < 0.001 |
| IL-8, pg/ml | 90.03 (23.04–280.22) | 6.62 (1.5–29.62) | P < 0.001 |
| IL-10, pg/ml | 13.11 (0.07–386.07) | 1.55 (0.07–43) | NS |
| IFN-γ, pg/ml | 24.82 (4.86–635.14) | 24.82 (5.53–1477.2) | NS |
| IL-12, pg/ml | 3.41 (0.04–52.39) | 0.02 (0.02–1.83) | NS |
Data presented as median (min–max).
TNF, tumour necrosis factor; GM-CSF, granulocyte/macrophage colony-stimulating factor; IL, interleukin; IFN, interferon.
NS, no statistically significant between-group difference (P > 0.05; Mann–Whitney U-test).
Figure 1.
Serum cytokine levels obtained within 24 h of endotracheal intubation in patients <5 years of age with severe pneumonia and respiratory failure requiring mechanical ventilation (study group) compared with serum cytokine levels in healthy age-matched controls. TNF, tumour necrosis factor; GM-CSF, granulocyte/macrophage colony-stimulating factor; IL, interleukin; IFN, interferon; P < 0.001, patients with pneumonia versus controls; NS, no statistically significant difference (Mann–Whitney U-test).
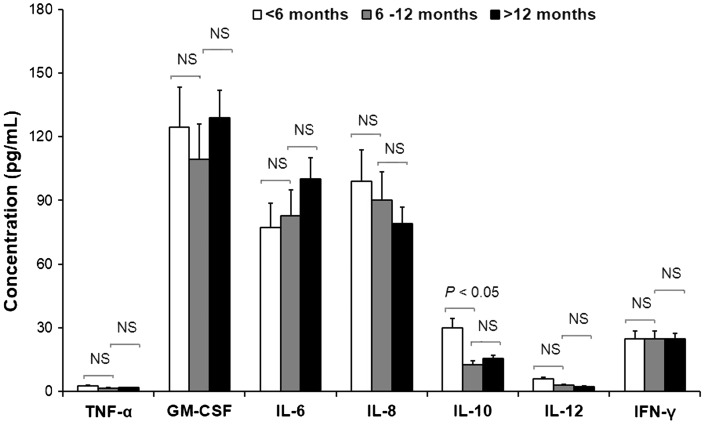
Serum cytokine levels in patients with severe pneumonia classified by age are presented in Figure 2 and Table 3. Serum IL-10 levels in patients with severe pneumonia aged <6 months were significantly higher than those aged 6–12 months (P < 0.05). There were no statistically significant differences in other serum cytokine concentrations between the different age groups (Figure 2 and Table 3). Endotracheal fluid levels of TNF-α, IL-6, and IL-8 were significantly higher in patients aged 6–12 months versus the <6 months or >12 months patient groups (P < 0.001; Table 3). Endotracheal fluid INF-γ levels were significantly higher in patients aged 6–12 months and >12 months compared with those aged <6 months (P < 0.01; Table 3).
Figure 2.
Serum cytokine levels obtained within 24 h of endotracheal intubation in patients <5 years of age with severe pneumonia and respiratory failure requiring mechanical ventilation and classified by age. TNF, tumour necrosis factor; GM-CSF, granulocyte/macrophage colony-stimulating factor; IL, interleukin; IFN, interferon; Statistically significant differences were observed in IL-10 levels only between the <6 months and 6–12 months groups (P < 0.05; Mann–Whitney U-test).
Table 3.
Serum and endotracheal fluid cytokine levels obtained within 24 h of endotracheal intubation in patients <5 years of age with severe pneumonia and respiratory failure requiring mechanical ventilation.
| Age group |
|||||
|---|---|---|---|---|---|
| Cytokine | Sample type | <6 months (n = 4) | 6–12 months (n = 36) | >12 months (n = 5) | All patients (n = 45) |
| TNF-α, pg/ml | Serum | 2.59 (0.57–24.27) | 1.57 (0.003–8.16) | 1.83 (0.86–5.07) | 1.57 (0.003–24.27) |
| ETF | 17.44 (10.22–128.84) | 414.7 (40.24–2021.25)*** | 29.34 (6.76–535.6) | 153.48 (6.76–2021.25)b | |
| GM-CSF, pg/ml | Serum | 124.63 (30.41–165.14) | 109.51 (1.82–189.12) | 129.08 (1.82–179.25) | 115.58 (0.43–409.46) |
| ETF | 106.96 (12.13–332.21) | 122.28 (7.12–844.15) | 96.88 (28. 21–134.24) | 118.09 (12.13–844.15) | |
| IL-6, pg/ml | Serum | 77.19 (20.75–267.12) | 82.65 (15.38–2502.32) | 100.24 (56.2–680.62) | 80.84 (15.38–680.62) |
| ETF | 35.97 (8.78–143.51) | 258.51 (9.16–1610.53)*** | 190.30 (55.49–862.88) | 236.97 (8.78–1610.51)a | |
| IL-8, pg/ml | Serum | 99.03 (25.27–280.22) | 90.03 (23.04–203.18) | 79.12 (43.85–114.16) | 90.03 (23.04–280.22) |
| ETF | 1492.69 (186.48–6732.98) | 6047.82 (25.89–18967.25)*** | 2139.66 (461.24–9435.87) | 5444.71 (25.89–18967.25)c | |
| IL-10, pg/ml | Serum | 30.01 (6.58–52.48)* | 12.51 (0.07–386.07) | 15.53 (0.07–159.41) | 13.11 (0.07–386.07)d |
| ETF | 1.30 (0.78–1.98) | 1.59 (0.39–5.56) | 2.91 (0.78–3.56) | 1.62 (0.78–5.56) | |
| INF-γ, pg/ml | Serum | 24.20 (4.86–198.88) | 24.80 (4.86–635.14) | 24.22 (4.86–110.67) | 24.82 (4.86–635.14)d |
| ETF | 0.04 (0.04–0.98) | 0.23 (0.04–1.78)** | 0.22 (0.04–0.27)** | 0.21 (0.04–1.78) | |
| IL-12, pg/ml | Serum | 5.82 (0.04–52.39) | 3.07 (0.04–34.57) | 2.4 (0.04–23.23) | 3.41 (0.04–52.39) |
| ETF | 6.80 (0.56–9.4) | 9.17 (0.04–32.98) | 6.72 (1.23–15.86) | 7.27 (0.04–32.98) | |
Data presented as median (min–max).
TNF, tumour necrosis factor; ETF, endotracheal fluid; GM-CSF, granulocyte/macrophage colony-stimulating factor; IL, interleukin; IFN, interferon.
P < 0.05 versus patients aged 6–12 months; **P < 0.01 versus patients aged <6 months; ***P < 0.001 versus patients aged <6 months and >12 months; aP < 0.01 versus serum; bP < 0.001 versus serum; and cP < 0.0001 versus serum; dP < 0.01 versus ETF (Kruskal–Wallis test).
Serum and endotracheal fluid cytokine levels were compared in patients with severe pneumonia requiring mechanical ventilation, and showed that levels of TNF-α, IL-6, and IL-8 were significantly higher in endotracheal fluid than in blood serum (P < 0.01; Table 3). In contrast, levels of IL-10 and INF-γ were significantly higher in serum than in endotracheal fluid (P < 0.01; Table 3). There were no statistically significant differences in GM-CSF and IL-12 levels between serum and endotracheal fluid.
Serum cytokine levels were compared between patients with severe pneumonia who survived and patients who died. Serum IL-6 levels obtained within 24 h of endotracheal intubation and mechanical ventilation were significantly higher in patients who subsequently died versus those who survived (267.12 pg/ml versus 20.75 pg/ml; respectively; P < 0.05; Table 4).
Table 4.
Serum cytokine levels obtained within 24 h of endotracheal intubation in patients <5 years of age with severe pneumonia and respiratory failure requiring mechanical ventilation who either survived or died during treatment.
| Cytokine | Patient subgroup |
||
|---|---|---|---|
| Survived (n = 21) | Died (n = 24) | Statistical significance | |
| Proinflammatory cytokine, pg/ml | |||
| TNF-α | 1.46 (0.003–17.4) | 1.93 (0.86–24.27) | NS |
| GM-CSF | 131.47 (0.43–374.79) | 91.84 (0.43–409.46) | NS |
| IL-6 | 20.75 (15.38–160.9) | 267.12 (54.87–689.62) | P < 0.05 |
| IL-8 | 90.03 (23.04–203.18) | 99.03 (25.27–280.22) | NS |
| INF-γ | 24.80 (5.53–635.14) | 24.20 (4.86–111.43) | NS |
| Anti-inflammatory cytokine, pg/ml | |||
| IL-10 | 11.02 (0.07–28.74) | 15.01 (5.47–386.07) | NS |
| IL-12 | 5.82 (0.04–23.23) | 2.74 (0.45–52.39) | NS |
Data presented as median (min–max).
TNF, tumour necrosis factor; GM-CSF, granulocyte/macrophage colony-stimulating factor; IL, interleukin; IFN, interferon.
NS, no statistically significant between-group difference (P > 0.05; Mann–Whitney U-test).
Discussion
The present study focused on cytokine concentrations in peripheral blood from patients <5 years of age, with severe pneumonia requiring ventilatory support, compared with healthy age-matched controls. Cytokine levels in endotracheal fluid from the patients with severe pneumonia were also investigated.
Fever is a host self-defence mechanism against pathogens during infection, stimulated by IL-1, IL- 6 and TNF, and is a common symptom of pneumonia.27 Although all patients in the present study had severe pneumonia, only 62% of patients were found to have fever, which may be explained by the fact that the patients had been previously treated with antibiotics, and also because malnutrition, seen in 53% of patients, may have blunted the immune response and led to atypical clinical signs of infection.28,29 Malnutrition is the leading risk factor for death in children with pneumonia, particularly in infants under 1 year of age.30,31
The present results showed that serum IL-6, IL-8, and GM-CSF concentrations were significantly higher in patients with severe pneumonia than in age-matched healthy controls (Table 2 and Figure 1). Cytokines are soluble proteins of low molecular weight, that function as signalling molecules between cells. The immune response in patients with pneumonia varies, depending on cause and severity of the disease, but the most important physiological reaction is the release of various cytokines by inflammatory cells, to magnify the inflammatory reaction in the lungs.8 The inflammatory response depends on the types and concentrations of cytokines produced, and research into the inflammatory response in pneumonia has shown an imbalance between proinflammatory and anti-inflammatory factors, which is often related to mortality in children.17 Cytokines, such as GM-CSF and IL-8, are known to cause an increase in the inflammatory response and influx of neutrophils into the airways. During the same time, there is a transition from Th0 lymphocytes to Th1 and Th17, both of which further enhance inflammation and the influx of other infammatory cells. The cytokines produced by Th1 cells include TNF-α, INF-γ, and IL-6. Th-2 cytokines are key players in the development of allergic disease, while Th17 cells cause a neutrophil response, and are associated with IL-17, IL-21, IL-22, and IL-26.17,32,33 The patients in the present study showed increased IL-6, IL-8 and GM-CSF levels, as expected for an acute bacterial pneumonia. Levels of IL-10, which downregulate inflammation,34 were not significantly elevated in the present patients, and reflected the lack of downregulation of the immune response. In addition, IL-12, TNF-α, and INF-γ are more commonly elevated in response to intracellular-infecting microorganisms35 and a more pure Th1 response, and for this reason were not expected to be elevated.
Published research suggests that cytokines are time-dependent following the initiation of infection. TNF-α and IL-6 normally act as proinflammatory cytokines, with the early response cytokine, TNF-α, appearing early in patients with pneumonia.36 During acute respiratory distress syndrome (ARDS), proinflammatory cytokines increase quickly,37 for example, in bronchoalveolar lavage samples from patients with early stage ARDS, the concentration of TNF-α is increased.20 The present study failed to demonstrate an increase in TNF-α and IFN-γ, possibly because the latter is more important in viral and intracellular organism infections,38 and the present patient population differed from those expected to have viral infections. Most importantly, the present findings suggest an association between significantly elevated serum IL-6 levels and mortality. Other published studies have shown that the severity of pneumonia is reflected by levels and types of cytokines in the blood.18
In a prospective study of 1886 patients admitted to the emergency department for pneumonia,19 in which blood concentrations of TNF-α, IL-6, and IL-10 were measured daily during the first week of pneumonia, concentrations of all cytokines were shown to be increased in 82% of patients. Cytokine concentrations were highest at hospital admission and decreased quickly following the first day, but remained high in the first week. High concentrations of IL-6 and IL-10 reflected serious disease and a higher risk of mortality.19 In patients with pneumonia in the early days and the 30th day of the disease,17 IL-6 concentrations were found to be increased by 1 695%, CRP increased by 1 088%, IL-10 increased by 332%, and IL-8 increased by 96%. In addition, the concentration of IL-12 increased by 6%, and IFN-γ by 6%, in the acute phase,17 which was similar to the present data in patients with severe pneumonia versus healthy age-matched controls. The present results showed that, compared with healthy controls, IL-6 concentration increased 39 fold (80.84 pg/ml versus 2.06 pg/ml), IL-8 concentration increased 14 fold (90.03 pg/ml versus 6.62 pg/ml), and GM-CSF concentration increased 10 fold (115.58 pg/ml versus 11.47 pg/ml).
In a study of bronchoalveolar lavage from 74 patients with pneumonia on mechanical ventilation and 17 control patients without pneumonia on ventilators,20 concentrations of IL-6 and IL-8 were significantly increased compared with controls, while TNF-α was not elevated. In a further study of 31 patients with pneumonia aged 13 years, and 6 healthy age-matched controls,23 bronchoalveolar lavage IL-8 concentration was significantly increased in patients with pneumonia versus controls, and there was no correlation between bronchoalveolar lavage IL-8 concentration and polymorphonuclear leukocyte quantities. IL-8 is a cytokine produced by macrophages and epithelial cells, amongst other cell types, and airway smooth muscle cells and endothelial cells are the first to secrete IL-8 to attract polymorphonuclear leukocytes to the inflammatory area.39 Similar to other studies, in which blood IL-8 concentration was shown to be increased in children with pneumonia,40 and the concentration of IL-6 and IL-8 was shown to be increased in the first 48 hours of pneumonia,18 the present results showed that IL-8 concentration was highly increased in the blood serum of very young children with pneumonia under 5 years of age, compared with healthy age-matched children. These results demonstrate that even in early life, increases in pro-inflammatory cytokines are detected in response to infection.
In the present study, serum TNF-α levels were not increased within 24 h of intubation, but levels in bronchoalveolar lavage were high compared with serum. These data suggest that the inflammatory response occurs in the airways early, before the systemic reaction. TNF-α concentration was shown to be increased in the alveolar and lung parenchyma of mice following stimulation with different respiratory allergens.21 Inhibition of TNF-α reduces neutrophil concentration in the lungs, and the process of destroying bacteria, resulting in rapid increase in the severity of disease, and mortality rate was shown to increase from 10% in healthy mice to 80% in mice with complete inhibition of TNF-α following injection of 102 units of Klebsiella pneumoniae.21
A published study in children with pneumonia15 showed that blood IL-6 concentration in children with pneumonia on mechanical ventilation who survived was 464 pg/ml, compared with 1209 pg/ml in children with pneumonia who did not survive. In this study,15 IL-6 and TNF-α concentration in endotracheal fluids was high, corresponding to the scale of lung injury, and blood IL-6 concentration was considered to be a prognostic factor for mortality in children. Similarly, the present study found that serum IL-6 concentrations were high in patients with pneumonia who did not survive versus patients who survived (267.12 pg/ml versus 20.75 pg/ml, respectively), suggesting an association between high blood IL-6 concentration and mortality. Analyses of serum cytokine concentrations between three age groups in the present study revealed statistically significant differences in IL-10 levels only. Endotracheal fluid concentrations of IL-6, IL-8 and TNF-α, however, were significantly higher in patients aged 6–12 months than in patients aged under 6 months or over 12 months, and IFN-γ was significantly higher in patients aged 6–12 months and >12 months versus those aged <6 months.
In conclusion, cytokines such as IL-6, IL-8, IL-10, and GM-CSF appear to play an important role in acute inflammation in young patients, under 5 years of age, with severe pneumonia. The inflammatory response was shown to occur at the infection site (in the lungs) and systemically (in blood serum). High IL-6 concentration was associated with mortality.
Acknowledgements
We would like to thank the doctors and nurses in the Intensive Care Unit, National Hospital of Pediatrics (Hanoi, Vietnam) for helping us to implement this study. We also thank the technicians in the Faculty of Biochemistry, National Hospital of Pediatrics and the staff at the Immunology Lab, Military Medicine Academy103 for helping us to store samples, and quantify blood and endotracheal fluid cytokines.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Foundation for Science & Technology Development (NAFOSTED) grant No. 106.99-2012.12.
References
- 1.Wang H, Liddell CA, Coates MM, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 957–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moldoveanu B, Otmishi P, Jani P, et al. Inflammatory mechanisms in the lung. J Inflamm Res 2009; 2: 1–11. [PMC free article] [PubMed] [Google Scholar]
- 3.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakata K, Gotoh H, Watanabe J, et al. Augmented proliferation of human alveolar macrophages after allogeneic bone marrow transplantation. Blood 1999; 93: 667–673. [PubMed] [Google Scholar]
- 5.Bender AT, Ostenson CL, Wang EH, et al. Selective up-regulation of PDE1B2 upon monocyte-to-macrophage differentiation. Proc Natl Acad Sci USA 2005; 102: 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma L, Wu W, Dholakiya SL, et al. Assessment of phagocytic activity of cultured macrophages using fluorescence microscopy and flow cytometry. Methods Mol Biol 2014; 1172: 137–145. [DOI] [PubMed] [Google Scholar]
- 7.Anderson MR, Blumer JL. Advances in the therapy for sepsis in children. Pediatr Clin North Am 1997; 44: 179–205. [DOI] [PubMed] [Google Scholar]
- 8.Lipscomb MF, Bice DE, Lyons CR, et al. The regulation of pulmonary immunity. Adv Immunol 1995; 59: 369–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim EY, Moudgil KD. Regulation of autoimmune inflammation by pro-inflammatory cytokines. Immunol Lett 2008; 120: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toossi Z, Hirsch CS, Hamilton BD, et al. Decreased production of TGF-beta 1 by human alveolar macrophages compared with blood monocytes. J Immunol 1996; 156: 3461–3468. [PubMed] [Google Scholar]
- 11.Moore SA, Strieter RM, Rolfe MW, et al. Expression and regulation of human alveolar macrophage-derived interleukin-1 receptor antagonist. Am J Respir Cell Mol Biol 1992; 6: 569–575. [DOI] [PubMed] [Google Scholar]
- 12.Ding L, Linsley PS, Huang LY, et al. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol 1993; 151: 1224–1234. [PubMed] [Google Scholar]
- 13.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev 2003; 83: 309–336. [DOI] [PubMed] [Google Scholar]
- 14.Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets – an updated view. Mediators Inflamm 2013; 2013: 165974–165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montón C, Torres A. Lung inflammatory response in pneumonia. Monaldi Arch Chest Dis 1998; 53: 56–63. [PubMed] [Google Scholar]
- 16.Craig A, Mai J, Cai S, et al. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun 2009; 77: 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endeman H, Meijvis SC, Rijkers GT, et al. Systemic cytokine response in patients with community-acquired pneumonia. Eur Respir J 2011; 37: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 18.Wick YJ. Proinflammatory cytokines: A guide to antibiotic therapy for pneumococcal pneumonia. Pharmacy Timeshttp://www.pharmacytimes.com/news/Proinflammatory-Cytokines-A-Guide-to-Antibiotic-Therapy-for-Pneumococcal-Pneumonia (accessed 15 January 2012).
- 19.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the genetic and inflammatory markers of sepsis (GenIMS) study. Arch Intern Med 2007; 167: 1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schütte H, Lohmeyer J, Rosseau S, et al. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur Respir J 1996; 9: 1858–1867. [DOI] [PubMed] [Google Scholar]
- 21.Standiford TJ, Mehrad B, Tsai WC, et al. Pulmonary host defenses: The role of cytokines in mediating lung inflammation. Medscape Pulmonary Medicine eJournal 1999; 3, http://www.medscape.com/viewarticle/717400_6 (accessed 11 January 2016).
- 22.Kolsuz M, Erginel S, Alataş O, et al. Acute phase reactants and cytokine levels in unilateral community-acquired pneumonia. Respiration 2003; 70: 615–622. [DOI] [PubMed] [Google Scholar]
- 23.Bohnet S, Kötschau U, Braun J, et al. Role of interleukin-8 in community acquired pneumonia: relation to microbial load and pulmonary function. Infection 1997; 25: 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Dossow V, Rotard K, Redlich U, et al. Circulating immune parameters predicting the progression from hospital-acquired pneumonia to septic shock in surgical patients. Crit Care 2005; 9: R662–R669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nargis W, Ibrahim M, Ahamed BU. Procalcitonin versus C-reactive protein: usefulness as biomarker of sepsis in ICU patient. Int J Crit Illn Inj Sci 2014; 4: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. The management of acute respiratory infection in children: Practical guideline for outpatient care, Geneva: World Health Organization, 1994, pp. 26–26. [Google Scholar]
- 27.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009; 22: 240–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caulfield LE, de Onis M, Blössner M, et al. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 2004; 80: 193–198. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzo MJ, Moret I, Sarria B, et al. Lung inflammatory pattern and antibiotic treatment in pneumonia. Respir Res 2015; 16: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chisti MJ, Tebruegge M, La Vincente S, et al. Pneumonia in severely malnourished children in developing countries - mortality risk, aetiology and validity of WHO clinical signs: a systematic review. Trop Med Int Health 2009; 14: 1173–1189. [DOI] [PubMed] [Google Scholar]
- 31.Ginsburg AS, Izadnegahdar R, Berkley JA, et al. Undernutrition and pneumonia mortality. Lancet Glob Health 2015; 3: e735–e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Shao B, Wang R, et al. Role of interleukin-17 in defense against pseudomonas aeruginosa infection in lungs. Int J Clin Exp Med 2014; 7: 809–816. [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai HC, Velichko S, Hung LY, et al. IL-17A and Th17 cells in lung inflammation: an update on the role of Th17 cell differentiation and IL-17R signaling in host defense against infection. Clin Dev Immunol 2013; 2013: 267971–267971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol 2008; 180: 5771–5777. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Wakeham J, Harkness R, et al. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J Clin Invest 1999; 103: 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bordon J, Aliberti S, Fernandez-Botran R, et al. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis 2013; 17: e76–83. [DOI] [PubMed] [Google Scholar]
- 37.Suter PM, Suter S, Girardin E, et al. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock or sepsis. Am Rev Respir Dis 1992; 145: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 38.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and Immunology. Clin Microbiol Rev 2010; 23: 74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol 1993; 64: 456–460. [PubMed] [Google Scholar]
- 40.Li MC, Lee NY, Lee CC, et al. Pneumocystis jiroveci pneumonia in immunocompromised patients: delayed diagnosis and poor outcomes in non-HIV-infected individuals. J Microbiol Immunol Infect 2014; 47: 42–47. [DOI] [PubMed] [Google Scholar]