Abstract
Secreted protein, acidic and rich in cysteine (SPARC) is differentially associated with cell proliferation and extracellular matrix (ECM) assembly. We show here the effect of exogenous SPARC inhibition/induction on ECM and mitochondrial proteins expression and on the differentiation of C2C12 cells. The cells were cultured in growth medium (GM) supplemented with different experimental conditions. The differentiation of myoblasts was studied for 5 days, the expressions of ECM and mitochondrial proteins were measured and the formation of the myotubes was quantified after exogenous induction/inhibition of SPARC. The results indicate that the addition of recombinant SPARC protein (rSPARC) in cell culture medium increased the differentiation of C2C12 myoblasts and myogenin expression during the myotube formation. However, the treatment with antibody specific for SPARC (anti-SPARC) prevented the differentiation and decreased myogenin expression. The induction of SPARC in the proliferating and differentiating C2C12 cells increased collagen 1a1 protein expression, whereas the inhibition decreased it. The effects on fibronectin protein expression were opposite. Furthermore, the addition of rSPARC in C2C12 myoblast increased the expression of mitochondrial proteins, ubiquinol-cytochrome c reductase core protein II (UQCRC2) and succinate dehydrogenase iron-sulfur subunit (SDHB), whereas the anti-SPARC decreased them. During the differentiation, only the anti-SPARC had the effects on mitochondrial proteins, NADH dehydrogenase ubiquinone 1 beta subcomplex subunit 8 (NADHB8), SDHB and cytochrome c oxidase 1 (MTCO1). Thus, SPARC plays a crucial role in the proliferation and differentiation of C2C12 and may be involved in the link between the ECM remodeling and mitochondrial function.
Introduction
Adult mammalian skeletal muscle tissue is composed of multinucleated contractile muscle cells and it represents approximately 40% of the total body mass. The muscle fibers are surrounded by a dynamic structure named extracellular matrix (ECM) which contains collagen, glycoproteins and proteoglycans [1]. It is well known that ECM plays a crucial role in muscle cell development, structure maintenance, force transmission, and repair through the modulation of growth factors and ECM molecules interactions as well as cell-matrix signal transduction pathways [2]. Moreover, the myofibril assembly in skeletal muscle cells may be concerned by cell-matrix association. Thus, ECM modulates crucial cellular functions (adhesion, migration, proliferation and differentiation) and itself assembly by integrin-ligand combinations.
Skeletal muscle contains collagens type I and III which are fibrillar in nature. Furthermore, earlier studies have reported the importance of collagen as a substrate in the fusion of myoblasts into myotubes and showed the influence of ECM on myogenesis [3]. Multinucleated myotubes formation is an important step in skeletal muscle development. Myogenesis is a complex process characterized by the expression of myogenic regulatory factors (MRF) including myogenic factor-5 (Myf5), myoblast determination protein (MyoD), myogenin and MRF4 which led to cell division [4]. The analysis of the transcriptional changes during the differentiation of C2C12 myoblasts has shown that myogenin is an early marker for the entry of myoblasts into the differentiation pathway and that this key transcription factor governed the terminal differentiation [5]. However, not only MRF are involved in the regulation of skeletal muscle differentiation, ECM components can also play a critical role in the myogenic process [6]. Additionally, previous study has demonstrated the importance of ECM proteins in the differentiation of skeletal muscle [7]. On the other hand, ECM associated proteins, also termed matricellular proteins, do not play an architectural role in the ECM. Their interactions with cell-surface receptors, as well as with the structural matrix proteins as collagen modulate cell function and can be involved in tissue development, in satellite cell maintenance, activation, proliferation and differentiation during skeletal muscle regeneration [8, 9]. Moreover, the analysis of the skeletal muscle transcriptome after mild-exercise training in elderly has revealed the induction of 3 transcripts related to ECM, namely collagen type III alpha 1, collagen type IV alpha 1 and secreted protein, acidic and rich in cysteine (SPARC), which accounted for 25% (3/12) of modulated transcripts in elderly [10].
SPARC also known as osteonetin or basement membrane-40, is a calcium binding matricellular glycoprotein secreted by several types of cells and is associated with development, tissue remodeling, repair and injury [11]. In skeletal muscle, SPARC is expressed during muscle development and in regenerating muscle as well as in satellite cells/myoblasts and in myotubes and muscle fibers, suggesting a crucial role for SPARC in the skeletal muscle compartment [12]. SPARC is a multifunctional protein implicated in osteogenesis, wound healing, angiogenesis and disease pathogenesis [13]. Further, in vitro studies demonstrated that SPARC concerns cell shape modulation [14], cell cycle inhibition [15], the disruption of cell adhesion [16] and the regulation of cell differentiation [17]. Moreover, it is well known that SPARC binds to collagen type I-V and VIII [14, 18, 19], affects collagen production and assembly resulting in the modification of the ECM. For instance, collagen I deficiency deteriorates SPARC deposition in the ECM of Mov-13 mice [18] and the dermis of SPARC-null mice display a reduced collagen content [20]. Taken together, a functional relationship between SPARC and collagen I has been proposed. Furthermore, a previous study reported the interaction of SPARC with adenosine monophosphate activated protein kinase (AMPK) [21], which is known to induce the master regulator of mitochondrial biogenesis, peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1a) [22]. Thus, SPARC may contribute to the improvement of mitochondrial function and energy metabolism via the regulation of mitochondrial proteins expression.
Therefore, this study aimed to investigate the effect of SPARC on ECM remodeling, muscle differentiation and mitochondrial function. First, we confirmed the previous findings that the treatment with antibody specific for SPARC (anti-SPARC) prevents the differentiation of C2C12 myoblasts [23] and investigate whether the addition of recombinant SPARC protein (rSPARC) to C2C12 myoblasts promotes their differentiation using myogenin as a differentiation marker. To identify the modulator effect of SPARC on the ECM, we measured the levels of collagen 1a1 and fibronectin proteins during the proliferation and the differentiation of C2C12 cells as well as after the formation of mature myotubes. Finally, we also reported that the addition of rSPARC in C2C12 myoblast increased the mitochondrial oxidative phosphorylation (OXPHOS) proteins expression, whereas the anti-SPARC showed the opposite results during proliferation and during the differentiation of C2C12 myoblasts. These results indicate that SPARC plays a key role in the differentiation of C2C12 myoblasts and it may be involved in the link between the ECM remodeling and mitochondrial function.
Materials and methods
http://dx.doi.org/10.17504/protocols.io.jsycnfw [PROTOCOL DOI]
Materials
Mouse skeletal muscle cell lines, C2C12 myoblasts were from American Type Culture Collection (cat#ATCC® CRL1772™, ATCC, Manassas, USA). Cell culture equipment was from VWR international (VWR, Mississauga, Canada). Dulbecco's modified Eagle's medium (DMEM), protein-free T20 (TBS) blocking buffer and phosphate buffered saline (PBS) were obtained from ThermoFisher Scientific (Invitrogen, Waltham, USA). Fetal bovine serum (FBS) and horse serum (HS) were purchased from GE Healthcare Life Sciences (Hyclone, Utah, USA). Antibiotics were from Sigma-Aldrich (Oakville, Canada), and May-Grünwald and Giemsa Stain Solutions were from Wako Pure Chemical Industries (Toronto, Canada). Antibodies for western blot were all purchased from Santa Cruz Biotechnology (Texas, USA) except of MitoProfile Total OXPHOS from Abcam (Toronto, Canada). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transfer buffer (Tris glycine buffer), washing buffer (Tris buffer saline) and western blot chemiluminescent solution (Clarity western ECL substrate solution) were from Bio-Rad Laboratories (Mississauga, Canada). Monoclonal anti-SPARC and rSPARC were obtained from ThermoFisher Scientific (R&D Systems, Ottawa, Canada).
Myoblasts culture and ECM/mitochondrial protein expression levels measurement in proliferating C2C12 cells
C2C12 mouse adherent myoblasts were cultured in grown in DMEM supplemented with 10% FBS, 100 U/ml of penicillin, and 100 mg/ml of streptomycin (growth medium, GM) and they were maintained at 37°C under a 5% CO2 atmosphere [24]. One day before adding rSPARC or anti-SPARC, cells were trypsinezed and plated at density of 4×105/well in 12-well plates (day 1). On day 2, cells were cultured in different conditions and kept in GM supplement with rSPARC and/or anti-SPARC for 48 h. On day 4, proteins extraction was performed as described below (Immunoblot analysis). Collagen 1a1 and fibronectin protein expression levels as well as the expression of five different mitochondrial OXPHOS proteins were measured.
Myoblasts differentiation, ECM/mitochondrial and myogenin protein expression levels measurement
C2C12 myoblasts were trypsinized and plated in 12-well at 4×105 cells/well with GM (day 0). 24 h after plating, cells reached 80–90% confluence and the confluent C2C12 myoblasts were induced to differentiate by being transferred into DMEM with 2% heat-inactivated horse serum and 100 U/ml of penicillin, and 100 mg/ml of streptomycin (differentiation medium, DM) for 5 days. Thereafter medium was changed every other day. On day 6, proteins extraction was performed and myogenin, collagen 1a1, fibronectin and mitochondrial OXPHOS protein levels were measured by western blot.
Measurement of collagen 1a1 expression after induction/inhibition of SPARC in the mature myotubes
The differentiation of C2C12 myoblasts was performed for 5 days without any exogenous induction and/or inhibition of SPARC. On day 6, mature myotubes are generally fully formed, rSPARC and anti-SPARC were added to the DM and kept for 2 days. On day 8, proteins extraction followed by western blot were performed to analyze collagen 1a1 protein level after induction/inhibition of SPARC in mature myotubes.
Exogenous SPARC inhibition/induction
Monoclonal anti-SPARC (Mouse SPARC MAb "Clone 124413", Rat IgG2B, R&D systems, catalogue number: MAB942) (10–40 μg/ml) and rSPARC (R&D systems, catalogue number: 942-SP-050) (2–8 μg/ml) were directly added into GM or DM. For our fourth experimental condition, first, anti-SPARC was added into GM or DM, and then rSPARC was appended at 1 h later.
Measurement of cell fusion
To demonstrate the implication of SPARC in the differentiation of mononucluated myoblasts, a simple effective quantitative method was used to quantify myotube formation using May-Grünwald and Giemsa staining since myotubes are darkly stained, which makes it easy to distinguish nuclei and myotubes [25, 26]. Only slight modifications were brought. C2C12 cells were grown in GM and seeded at 4×104 cells/well in 24-well plates (day 0). On day 1, the differentiation was induced in DM with four different experimental conditions (day 1) for 5 days. DM with all experimental conditions was changed each 48 h. On day 6, cultured cells were fixed for 5 min in methanol, dried for 10 min and incubated for 5 min in May-Grünwald diluted solution (1:3 in sodium phosphate buffer). Cells were washed twice with distilled water (DW), stained with Giemsa diluted solution (1:10 in DW) for 20 min, and then rinsed 3× with DW. All the preparations were observed under light microscopy (Zeiss Axiophot, Germany) and microscopic images were captured at 30× and 100× of magnification. Cells were considered to be fused only if at least three nuclei were present in each myotube. Each value represents the average of at least 10 randomly selected fields. The fusion index was calculated from the ratio of nuclei number in the myotubes versus the total number of nuclei.
lmmunoblot analysis
After the proliferation as well as the differentiation of C2C12 myoblasts, the resulting culture were washed twice with PBS and scraped on ice by using cell lifter. Then, the PBS containing cells were centrifuged at 3000 rpm for 4 min at 4°C. The pellets were resuspended in radio-immunoprecipitation assay (RIPA) buffer supplemented with protease inhibitors cocktail and incubated on ice for 15 min. Lysates were sonicated 3× for 1 sec, centrifuged at 14,000 rpm for 5 min at 4 °C. The whole cell extracts were stored at -80 °C until use. Pooled samples were made and used to decide the amount of the protein to load for each antibody. This will assure that the densitometric data for each target protein will be within the linear dynamic (quantitative) range to give accurate and reproducible results reflecting the true biology between samples in the study set [27]. The most popular loading controls include housekeeping such as beta-actin, tubulin and GAPDH, however these proteins are generally highly expressed in samples and are overloaded in the gel lane with the target protein such that they would not serve to normalize the loading [27]. Thus, we loaded the same pooled samples in each gel to be used as a control to normalize the difference between each membrane. Five to thirty μg of proteins from each cell lysate including pooled samples were separated by electrophoresis through SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membrane. Once the transfer was completed, membranes were incubate with diluted 10× RedAlert for 5 min following by washing with DW as previously described [28] and pictures were taken to normalize the quantity of total protein loaded and the differences between the membranes using the pooled samples or control for mitochondrial proteins (see S1 Fig for RedAlert pictures). The ratio of samples/pooled samples from RedAlert picture (RM) was calculated for each membrane and used to normalize the quantity of total protein loaded. Then, membranes were blocked using suitable blocking buffer and incubated with primary antibodies (see S1 Table for western blot conditions). After washed (3×) with appropriates washing buffers, membranes were incubated with species-appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies, washed (3×) again and finally the visualization of the immune complexes was carried out with an enhanced chemiluminescent reagent. The intensity of bands was measured using Image J software. The density of each lane on the on the film (DF) was expressed as a ratio to each pooled sample on the same film to normalize the difference of each membrane. Then, the quantity of protein loaded was normalized by dividing DF by RM as previously described [29].
Statistical analysis
The data presented are repeated measures from three independent experiments (three different passages of C2C12 cells to measure the effect of SPARC on C2C12 phenotype, not only on one passage of this cell line), except for the myoblasts fusion data. One-way ANOVA with repeated measurements was used to adjust the validations of the different passages and a contrast analysis was performed. P value was set at < 0.05 after the Bonfferoni adjustments. For the myoblasts fusion data, the same passage was used with three repetitions for each condition, thus, one-way ANOVA followed by the Tukey's HSD post-hoc test was used. All results are reported as means ± SEM.
Results
Effect of SPARC on the modulation of structural matrix proteins during the proliferation of C2C12 myoblasts
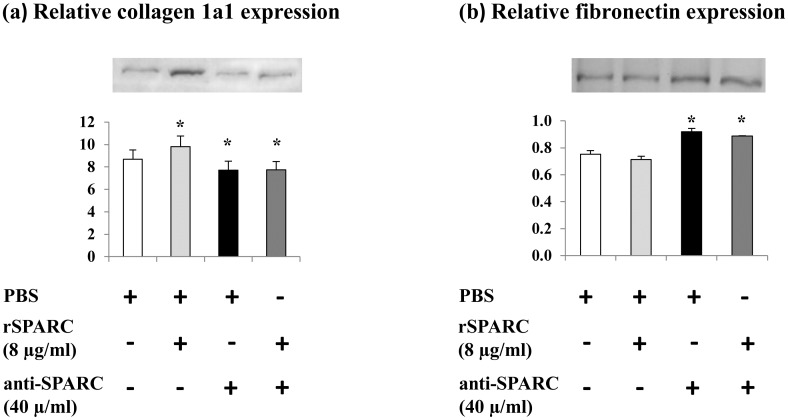
SPARC is reported to bind fibrillar collagens I [30] on its binding [31]. In SPARC-null cells, decreased collagen type I expression [32] and enhanced collagen assembly with SPARC overexpression [33] are observed. Moreover, SPARC is “anti-adhesive” protein and induces focal adhesion disassembly [16]. On the other hand, fibronectin, ECM adhesive glycoprotein, is found to be expressed in all stages of primary myogenesis [34] and may play an important role in muscle development [35]. Thus, to study the implication of SPARC in ECM remodeling, we examined the effect of SPARC on collagen 1a1 and fibronectin expression during the proliferation of C2C12 cells. Our results showed that exogenous SPARC induction (8 μg rSPARC/ml) increased the expression level of collagen 1a1 and trended to decrease fibronectin expression (p < 0.1) in the proliferating C2C12 myoblasts. However, SPARC inhibition (40 μg anti-SPARC/ml) decreased collagen 1a1 expression and increased fibronectin expression (Fig 1). These results provide evidence that SPARC modulates ECM proteins during myoblasts proliferation.
Fig 1. Effect of SPARC on the modulation of ECM proteins in proliferating C2C12 cells.
Representative western blots for collagen 1a1 and fibronectin expressions in C2C12 myoblasts after induction/inhibition of SPARC. C2C12 myoblasts were cultured in GM. One day before adding anti-SPARC (40 μg/ml) or rSPARC (8 μg/ml), cells were trypsinezed and plated at density of 4×105/well in 12-well plates. Cells were cultured in four different conditions for 48 h. Proteins extraction was performed as described above and collagen 1a1 and fibronectin protein expression levels were measured by western blot. The experiments were repeated three times (three different passages of C2C12 cells) and a representative western blot image was shown. Data were expressed as a ratio to the positive control (pooled samples). One-way ANOVA with repeated measurements was used to adjust the validations of the different passages and a contrast analysis was performed. P value was set at < 0.05 after the Bonfferoni adjustments. (a) Thirty μg of whole cell lysate proteins were loaded to measure collagen 1a1 levels in C2C12 proliferating myoblasts. Addition of SPARC induced collagen 1a1 expression, however, SPARC inhibition decreased it. (b) Five μg of the proteins from proliferating cells were loaded to measure fibronectin levels. Fibronectin expression trended to decrease after rSPARC addition and anti-SPARC increased it. Abbreviations: PBS: phosphate buffered saline, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody. *Significant differences between experimental conditions.
Effect of SPARC on the modulation of OXPHOS proteins in proliferating myoblasts
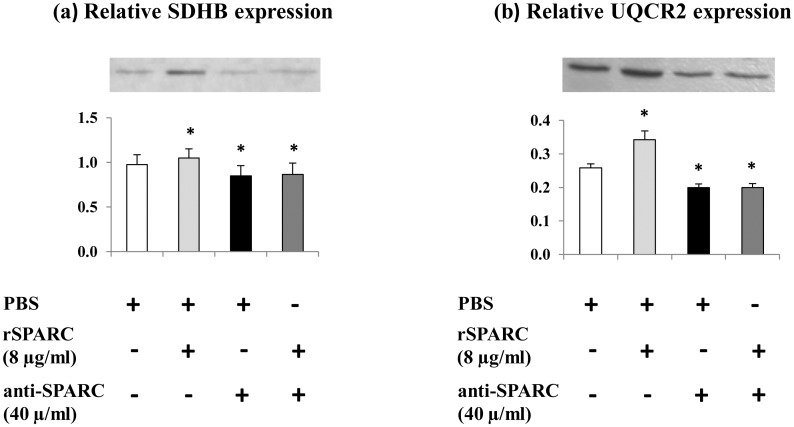
Mitochondrial dysfunction is found to be implicated in the development of many diseases in the skeletal muscle [36, 37]. There is increased evidence that mitochondria may function as detectors for changes in ECM composition and changes in mitochondrial functioning modify the ECM [38, 39]. Furthermore, SPARC which modulated ECM proteins (Fig 1) interacts with AMPK [21] which is known to induce the master regulator of mitochondrial biogenesis [22]. Thus, to examine the effect of exogenous induction/inhibition of SPARC on mitochondrial proteins expression, same cell culture conditions and procedures were followed as above and the expression of mitochondrial OXPHOS proteins were measured using the total OXPHOS antibody Cocktail (1:200). Our data demonstrated that the addition of rSPARC (8 μg/ml) in muscle cell culture medium increased the expression of two mitochondrial proteins (succinate dehydrogenase assembly factor 4 (SDHB) and ubiquinol-cytochrome-C reductase complex core protein 2 (UQCR2)). In contrast, the addition of anti-SPARC (40 μg/ml) decreased the content in these key oxidative energy components (Fig 2). These findings are the first proof that SPARC can modulate expression of mitochondrial proteins during myoblasts proliferation.
Fig 2. Modulation of mitochondrial proteins expression after exogenous induction/inhibition of SPARC in proliferating myoblasts.
C2C12 cells were cultured in four different conditions which were kept in GM supplement with/without rSPARC and/or anti-SPARC for 48 h. Extracted cells lysates were prepared to perform western blot and the expression of mitochondrial OXPHOS proteins were measured using 30 μg of whole cell lysate proteins. The experiments were repeated three times (three different passages of C2C12 cells) and a representative western blot image was shown. Data were expressed as a ratio to the positive control (pooled samples). One-way ANOVA with repeated measurements was used to adjust the validations of the different passages and a contrast analysis was performed. P value was set at < 0.05 after the Bonfferoni adjustments. The addition of rSPARC (8 μg/ml) increased SDHB (a) and UQCR2 (b) proteins levels, whereas, anti-SPARC (40 μg/ml) decreased it. Abbreviations: SDHB: succinate dehydrogenase iron-sulfur subunit beta, UQCR2: ubiquinol-cytochrome c reductase core protein 2, PBS: phosphate buffered saline, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody. *Significant differences between experimental conditions.
Involvement of SPARC in the differentiation of C2C12 myoblasts
C2C12 in vitro cell culture is a great model to study the muscle development and differentiation because these cells have all the characteristics needed for the study of myogenesis: differentiates rapidly, forms contractile skeletal myotubes and produces characteristic muscle proteins [40].
It has been demonstrated that ECM is involved in the control of muscle differentiation [41]. The expression of SPARC is detected both in satellite cells/myoblasts and myotubes and muscle fibers, suggesting a crucial role for SPARC in the skeletal muscle compartment [12]. To demonstrate the implication of SPARC in the differentiation of mononucluated myoblasts, a simple effective method was employed to quantify myotubes formation [25, 26]. The differentiation was induced in DM with four different experimental conditions for 5 days. The DM with/without exogenous induction (2 μg rSPARC/ml) and/or inhibition (10 μg anti-SPARC/ml) of SPARC were changed each 48 h. C2C12 were stained with May-Grünwald and Giemsa solutions and observed under light microscope. As expected, the exogenous induction of SPARC increased the fusion of myotubes in the differentiating C2C12 cells, whereas SPARC inhibition decreased the muscle cell fusion (Fig 3). These results confirm that anti-SPARC prevents the differentiation of C2C12 myoblasts [23] and provides new evidence that SPARC induces differentiation of C2C12 cells.
Fig 3. Involvement of SPARC in myoblasts fusion.
Myoblasts (4×104 cells/well in 24-well plates) were induced to differentiate in DM with/without the induction/inhibition of SPARC for 5 days. The mediums were changed each 48 h. C2C12 were stained with May-Grünwald and Giemsa solutions, and the stained wells were observed under light microscopy. One-way ANOVA followed by the Tukey's HSD post-hoc test was used. All results are reported as means ± SEM. The addition of rSPARC (2 μg/ml) induced myotube formation, whereas, anti-SPARC (10 μg/ml) inhibited it. (a) Captured microscopic images of C2C12 fusion. The images were captured using 30× objectives under light microscopy (see S2 Fig for 100× objectives). Examples of multinucleated myotubes were shown by arrows. (b) Fusion index was calculated from 10 different photos per condition. Abbreviations: PBS: phosphate buffered saline, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody. *Significant differences between experimental conditions (n = 3): p < 0.05.
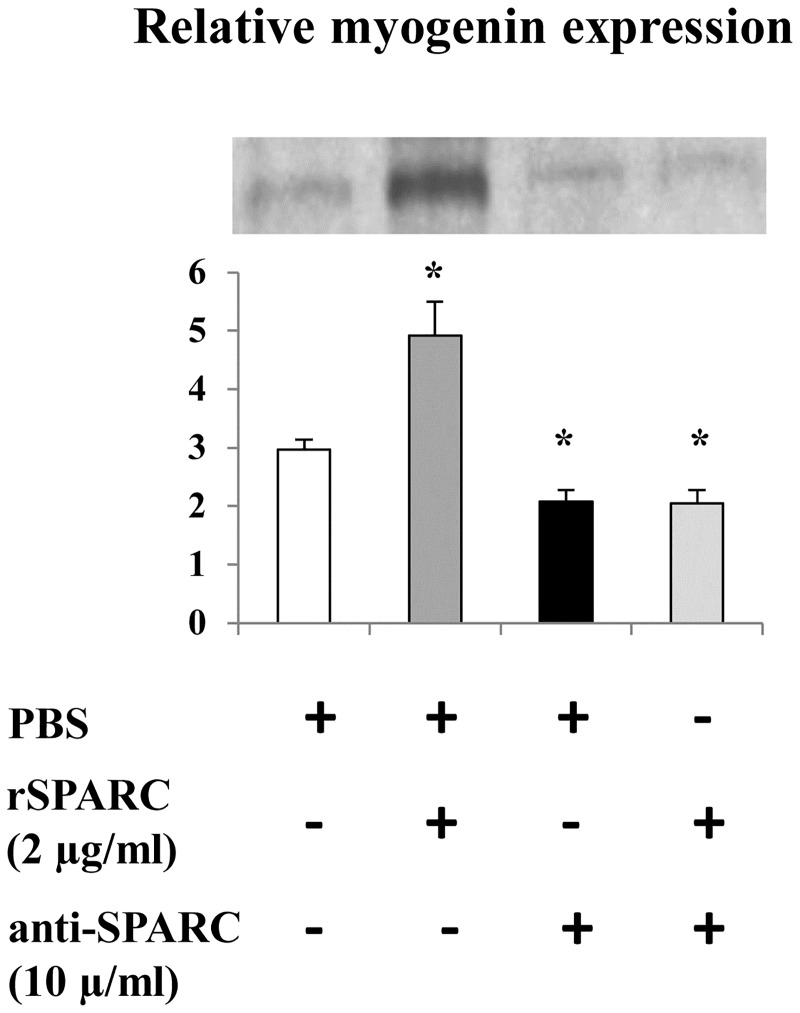
Myogenin is an important regulator of skeletal muscle cell differentiation [42, 43]. To confirm the involvement of SPARC in the differentiation of muscle cells, myogenin expression level was measured by western blot after the induction (2 μg rSPARC/ml)/inhibition (10 μg anti-SPARC/ml) of SPARC during 5 days of C2C12 myoblasts differentiation. Our data showed that during the differentiation of C2C12 cells, SPARC induction increased, whereas the inhibition decreased myogenin expression, respectively (Fig 4). This data demonstrates a key role of SPARC in the differentiation of muscle cells.
Fig 4. Exogenous effect of SPARC on myogenin expression in differentiating myoblasts.
Confluent C2C12 myoblasts were trypsinized and plated in 12-well at 4×105 cells/well with GM. The myogenic differentiation was induced in DM with/without the induction/inhibition of SPARC for 5 days, and the mediums were replaced every 2 days. Proteins extraction was performed and five μg from total cell extracts were analyzed by western blot using anti-myogenin antibody. The experiments were repeated three times (three different passages of C2C12 cells) and a representative western blot image was shown. Data were expressed as a ratio to the positive control (pooled samples). One-way ANOVA with repeated measurements was used to adjust the validations of the different passages and a contrast analysis was performed. P value was set at < 0.05 after the Bonfferoni adjustments. The SPARC induction increased myogenin expression and SPARC inhibition decreased it. Abbreviations: PBS: phosphate buffered saline, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody. *Significant differences between experimental conditions.
SPARC as a modulator of structural matrix proteins during the differentiation of muscle cells
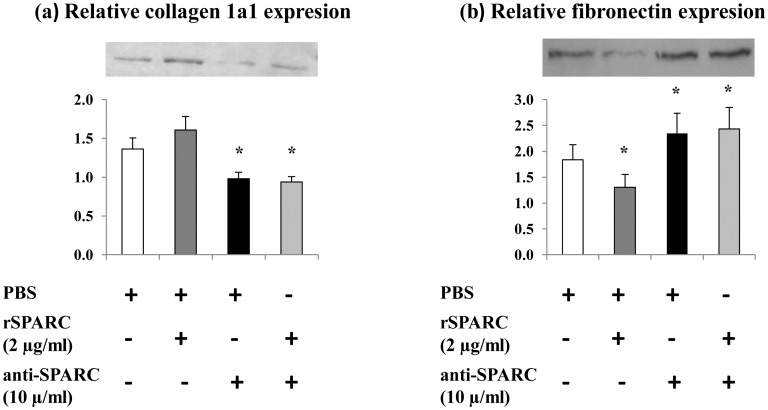
SPARC specifically binds several ECM proteins including collagen and is implicated in the regulation of cell-matrix interactions [44]. SPARC also modulates the production of ECM molecules [45]. Further, In vitro studies have been demonstrated that the inhibition of collagen suppresses the differentiation of myoblasts in vitro, suggesting that collagen is necessary for myogenesis [46, 47]. Thus, collagen 1a1 and fibronectin protein levels were measured to study the effect of SPARC on the modulation of the ECM in differentiating C2C12 cells. After the induction of C2C12 differentiation for 5 days with/without exogenous induction/inhibition of SPARC, whole cell lysates were subjected to western blot using anti-collagen 1a1 and anti-fibronectin antibodies. The addition of rSPARC (2 μg/ml) trended to increase the expression levels of collagen 1a1 (p < 0.1) and decreased fibronectin in the differentiating C2C12 cells. SPARC inhibition (10 μg anti-SPARC/ml) decreases collagen 1a1 and increases fibronectin (Fig 5). The obtained results suggested that SPARC acts as a modulator of muscle ECM during the differentiation.
Fig 5. SPARC effect on ECM proteins in differentiating myoblasts.
C2C12 cells were cultured in DM for 5 days with/without exogenous rSPARC (2 μg/ml) and/or anti-SPARC (10 μg/ml). The mediums were changed each 2 days. Proteins extraction was performed, and western blot analysis was done on RIPA soluble lysates to indicate proteins. The experiments were repeated three times (three different passages of C2C12 cells) and a representative western blot image was shown. Data were expressed as a ratio to the positive control (pooled samples). One-way ANOVA with repeated measurements was used to adjust the validations of the different passages and a contrast analysis was performed. P value was set at < 0.05 after the Bonfferoni adjustments. (a): collagen 1a1 levels were measured using the 30 μg proteins and the results showed that the induction of SPARC increased collagen 1a1 expression, however, an opposite effect of anti-SPARC was observed. (b) Five μg of the proteins were loaded to measure fibronectin levels. A decrease of fibronectin expression with rSPARC and an increase after anti-SPARC were observed. Abbreviations: PBS: phosphate buffered saline, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody. *Significant differences between experimental conditions.
Effect of SPARC on the expression of mitochondrial OXPHOS proteins in the differentiating C2C12 cells
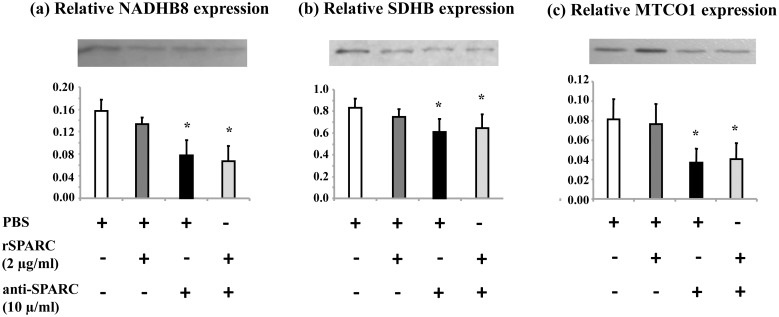
It is well known that mitochondria are potential regulator of myogenesis and skeletal muscle cells devoid of mitochondria cannot differentiate [48]. The presence of ECM is also essential for the formation of myotubes [49]. In the Figs 3 and 4, we demonstrated that SPARC induced myotubes formation and modulated ECM proteins expression during the differentiation of C2C12 cells. Thus, to study the exogenous effect of SPARC on mitochondrial proteins expression during the differentiation of C2C12 cells, mononucleated myoblasts were treated with rSPARC and/or anti-SPARC during 5 days of myoblasts differentiation. The mitochondrial OXPHOS proteins level was measured by western blot. The results showed that only exogenous SPARC inhibition decreased the expression of three mitochondrial proteins (NADH dehydrogenase (ubiquinone) 1 beta sub-complex 8 (NDUFB8), SDHB and cytochrome c oxidase I (MTCO1)) during the differentiation of C2C12 cells (Fig 6). According to this result, a modulator role of SPARC on mitochondrial proteins expression during muscle cells differentiation is suggested.
Fig 6. Modulation of mitochondrial OXPHOS proteins expression after exogenous inhibition of SPARC in differentiating myobalsts.
The myogenic differentiation was induced in DM supplemented with the induction/inhibition of SPARC for 5 days. DM with rSPARC (2 μg/ml) and anti-SPARC (10 μg/ml) was replaced each 2 days. Proteins extraction was performed, and extracted cells were prepared to perform western blot and the expression of mitochondrial OXPHOS proteins were measured using 30 μg of whole cell lysate proteins. The experiments were repeated three times (three different passages of C2C12 cells) and a representative western blot image was shown. Data were expressed as a ratio to the positive control (pooled samples). One-way ANOVA with repeated measurements was used to adjust the validations of the different passages and a contrast analysis was performed. P value was set at < 0.05 after the Bonfferoni adjustments. The expression of three different mitochondrial proteins was shown to be reduced after SPARC inhibition. Abbreviations: SDHB: succinate dehydrogenase iron-sulfur subunit beta, NADHB8: NADH dehydrogenase ubiquinone 1 beta subcomplex subunit 8, MTCO1: cytochrome c oxidase 1, PBS: phosphate buffered saline, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody. *Significant differences between experimental conditions.
Modulation of collagen 1a1 expression after induction/inhibition of SPARC in the mature myotubes
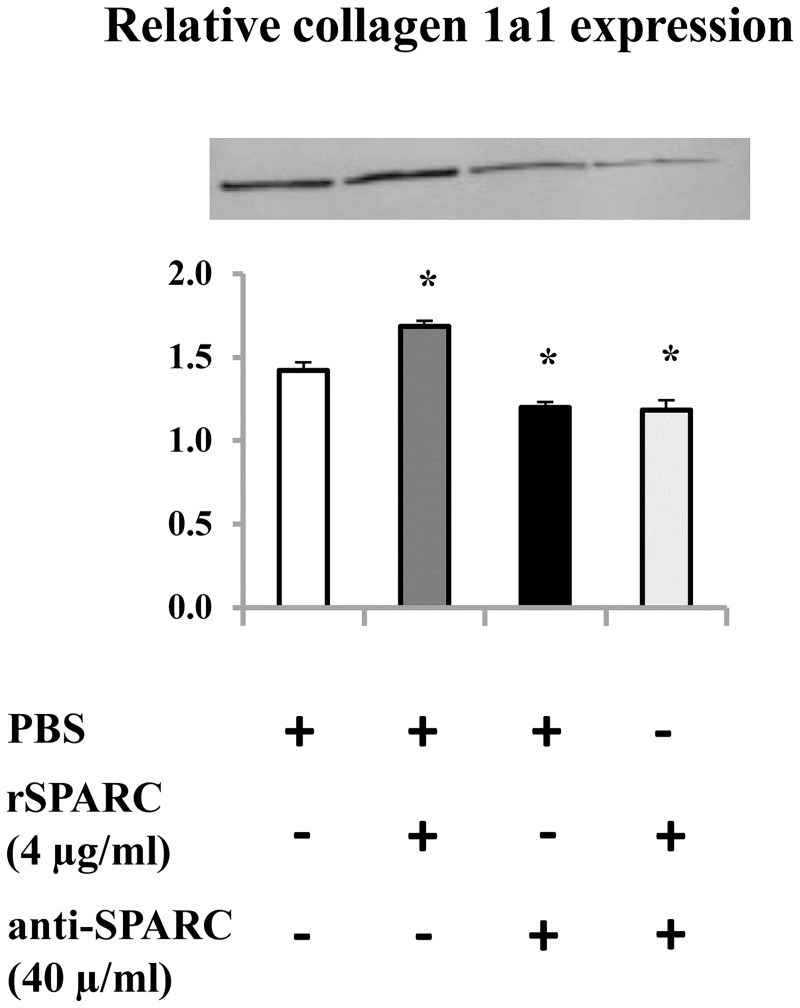
It has been shown that the expression and the organization of collagen are critical to both skeletal muscle function and development [50]. Moreover, the production of collagen I is a requisite for the association of SPARC with ECM [31]. Thus, we studied the effect of exogenous SPARC on collagen 1a1 expression in myotubes. The differentiation of C2C12 myoblasts were performed for 5 days without any exogenous induction and/or inhibition of SPARC. On day 6, mature myotubes are generally formed, rSPARC and anti-SPARC were added to the DM and kept for 2 days. On day 8, proteins extraction followed by western blot was performed to analyze collagen 1a1 protein level after induction/inhibition of SPARC in mature myotubes. SPARC induction increased collagen 1a1 expression in C2C12 myotubes, whereas a decrease was observed by SPARC inhibition (Fig 7). The obtained result confirmed the modulator effect of SPARC on ECM proteins expression in the differentiated muscle cells as seen in proliferating and differentiating C2C12 myoblasts.
Fig 7. Modulation of collagen 1a1 expression after induction/inhibition of SPARC in the mature myotubes.
C2C12 myoblasts were cultured in DM for 5 days. On day 6, exogenous rSPARC (4 μg/ml) and anti-SPARC (40 μg/ml) were added and kept for 48 h. Proteins extraction was performed on day 8. Total cell extracts (30 μg proteins per lane) were subjected to western blot analysis with anti-collagen 1a1 antibody. The experiments were repeated three times (three different passages of C2C12 cells) and a representative western blot image was shown. Data were expressed as a ratio to the positive control (pooled samples). One-way ANOVA with repeated measurements was used to adjust the validations of the different passages and a contrast analysis was performed. P value was set at < 0.05 after the Bonfferoni adjustments. Abbreviations: PBS: phosphate buffered saline, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody. *Significant differences between experimental conditions.
Discussion
In this study, we investigated the modulator effect of SPARC on the ECM and mitochondrial proteins expression in muscle cells. Moreover, we demonstrated the involvement of SPARC in the differentiation of C2C12 myoblasts.
SPARC as a modulator of the ECM in muscle cells
ECM is a crucial multifunctional dynamic structure which modulates different cellular functions. It is well known that skeletal muscle cells interact with the ECM resulting in the mediation of their morphogenesis [2]. Previous studies have demonstrated the requirement for the ECM and its components in the differentiation of myoblasts [43, 51]. Furthermore, it has been reported that collagen is the principal structural protein in ECM skeletal muscle [52] and an early data has highlighted the importance of collagen 1 in muscle development [53]. SPARC is a collagen-binding matricellular glycoprotein secreted into the ECM and may have a fundamental role in muscle compartment [12]. The importance of the production of collagen 1 in the association between SPARC and the ECM as well as the interactions of SPARC with the collagen I precursor and procollagen 1 have been demonstrated [31]. The mediator effect of SPARC on the association of procollagen I with cells, its processing and incorporation into the ECM has been shown in dermal fibroblasts [54]. Moreover, the reduction of collagen 1 content in SPARC-null mice [20] and the deterioration of SPARC deposition in type 1 collagen deficient mice [18] have been elucidated. Taken all these studies, a functional relationship between SPARC and collagen 1 is evident. However, to our knowledge, no study has shown the effect of SPARC on collagen 1 protein expression during the myogenic process. Thus, we described here that exogenous induction of SPARC increased collagen 1 protein expression during the proliferation and after the formation of mature myotubes and trended to increase collagen 1 protein level during the differentiation. On the other hand, SPARC inhibition decreased collagen 1 expression during the three steps of the myogenesis process. One possibility to explain the effect of SPARC on collagen 1 protein expression during the myogenic process is their direct binding [31]. Importantly, our data supports the findings of Francki et al. in kidney mesangial cells where SPARC-null cells display diminished expression of collagen type I mRNA and protein [32]. The same study has reported that the addition of rSPARC to SPARC-null cells restores the expression of collagen type I mRNA [32].
Fibronectin is a multifunctional ECM glycoprotein that plays a structural role in the ECM and binds several ECM molecules [55]. Fibronectin belongs to the components of cell-ECM adhesion complexes and its interaction with integrin receptors mediates cell attachment, migration and signaling [56]. In myoblasts, it has been demonstrated that fibronectin promotes cell attachment for important physiological processes such as proliferation and differentiation [57]. The same study has reported the influence effect of the conformational changes in fibronectin on C2C12 muscle cells [57]. In contrast, SPARC has a counter-adhesive activity by the disruption of cell-ECM interactions [58], although, this activity of SPARC depends on the target cell type and the origin of SPARC protein. The mechanism governed the dissociation of cell adhesion after SPARC addition is not completely clear [16]. Furthermore, in vascular smooth muscle cells, SPARC inhibits cell proliferation and cell cycle progression [15, 59], while fibronectin promotes cell proliferation [60]. Moreover, cell adhesion has been described as a calcium-binding mechanism [61]. Indeed, SPARC is a calcium-binding protein [62] and an interaction of calcium with fibronectin has been also elucidated [63].
Here, we showed that exogenous SPARC induction trended to decrease fibronectin expression in the proliferating C2C12 myoblasts and decreased fibronectin in the differentiating C2C12 cells. However, SPARC inhibition increased fibronectin expression in proliferating and differentiating C2C12 cells. Hence, SPARC can be implicated in the ECM remodeling through the modulation of structural matrix proteins expression in muscle cells.
Involvement of SPARC in the differentiation of C2C12 myoblasts
The organization and the composition of ECM are influenced by exercise-induced changes, fiber maturation and myogenesis-related changes [64, 65]. SPARC is induced after exercise [10, 66] and during recovery from muscle injury [12]. Moreover, ECM components are involved in myoblasts differentiation. For instance, laminin, a structural matrix protein, enhances myotube formation, whereas fibronectin inhibits this process [67, 68]. An important study has demonstrated the implication of SPARC in the myogenesis of skeletal myoblasts in vitro. The authors have reported: 1) an increase of SPARC expression during mouse myoblasts differentiation, 2) SPARC expression may be controlled by calcium-dependent pathway in myogenesis, and 3) the treatment with anti-SPARC almost completely preventes myobalsts differentiations [23].
Therefore, we confirmed the effect of anti-SPARC on the myoblasts differentiation and we investigated the induction of myoblasts fusion after rSPARC addition. Moreover, myoblasts differentiation is governed by myogenin expression [42, 43]. Consequently, we showed here that SPARC induction increased, whereas the inhibition decreased myogenin expression, respectively. Therefore, SPARC is importantly implicated in muscle differentiation, and this process can be regulated in part by MRFs expression, including myogenin, and by ECM remodeling.
Based on the involvement of SPARC in the differentiation of skeletal muscle cells and its key role in this process, we found that the addition of SPARC enhanced myotube formation through, may be in part, by decreasing fibronectin protein level. Our results correlate with other data showing that the alteration or the degradation of fibronectin is required for myoblasts fusion and also high level of fibronectin reduces myotube formation and that decrease and/or loss of fibronectin during myoblast fusion is closely correlated with the fusion of myoblasts [69, 70]. In addition, study of Chen has confirmed that during myoblasts differentiation, a reduction of fibronectin concentration on myoblast surface is observed [71].
Effect of SPARC on the mitochondrial function of muscle cells
It is well known that the main role of mitochondria is energy production. However, evidence is increasing that mitochondria plays also a crucial role in cell regulatory, signaling events and in the response of cells to various stimulus [72]. In addition, mitochondria generate adenosine triphosphate (ATP) via OXPHOS complexes and plays fundamental role in cell proliferation as well as calcium signaling [73]. Further, previous studies have shown that alterations in mitochondria can affect ECM remodeling and vice versa collagen VI deficiency affects mitochondrial function in skeletal muscle [38, 74]. In the support of mitochondria can influence ECM, He et al. have demonstrated that the suppression of mitochondrial complex I influences expressions of ECM molecules and its related proteins [75]. Accordingly, a subtle relationship between the ECM and the mitochondria may be suggested and supported our hypothesis about the possible link between ECM remodeling and mitochondria function. On the other hand, the involvement of AMPK, a key regulator of many metabolic process in skeletal muscle, in the regulation of glucose metabolism in skeletal muscle has been elucidated [76]. In skeletal muscle, AMPK is known to induce mitochondrial function via direct phosphorylation of peroxisome-proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha), a master regulator of mitochondrial biogenesis [22]. Moreover, in vitro study reported the interaction of SPARC with AMPK and suggested the implication of SPARC in glucose metabolism via AMPK activation [21]. Thus, we investigate the effect of SPARC on mitochondrial OXPHOS protein expression during the proliferation and differentiation of C2C12 myoblasts. Our results demonstrated that in proliferating C2C12, SPARC addition increased the expression of mitochondrial proteins. On the other hand, the addition of anti-SPARC decreased their content. In differentiating C2C12 cells, only exogenous SPARC inhibition decreased the expression of mitochondrial proteins. The involvement of SPARC in the modulation of these key oxidative energy components during myogenesis process provides another way whereby ECM may regulate mitochondrial function.
Conclusions
In summary, SPARC is a secreted peptide which has the potential to act similarly to a hormone in order to control important functions, such as extracellular cell growth and proliferation, and energy metabolism. This study is the first to demonstrate the effect of SPARC on the ECM remodeling and mitochondrial proteins expression during myogenesis process. The investigation of SPARC addition on myotube fusion as well as on MRFs (myogenin) expression confirms the involvement of SPARC in myogenic process. The decrease/increase of SPARC expression with ageing/exercise, respectively, and its involvement in the possible link between ECM and mitochondria may allow to understand the role of ECM remodeling in muscle integrity and to give an overview of ECM communications and its influence on muscle mitochondrial function. Finally, the implication of SPARC in the possible link between ECM remodeling and mitochondrial function provides new insight into the biological functions of SPARC and the pathway ECM/mitochondria may serve as a potential target to understand metabolic disorders related to ECM and mitochondria dysfunction which is known to be the major factor contributing to ageing and sarcopenia in muscle.
Supporting information
SM: size marker, 0: Pooled samples, 1: PBS, 2: rSPARC, 3: anti-SPARC, 4: Anti-SPARC+rSPARC. Abbreviations: ECM: extracellular matrix, PBS: phosphate buffered saline, OXPHOS: oxidative phosphorylation, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody.
(PDF)
Abbreviations: PBS: phosphate buffered saline, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody.
(PDF)
Abbreviations: AB: antibody, CAPS: N-cyclohexyl-3-aminopropanesulfonic acid, h: hours, Cat#: catalog number, ON: overnight, OXPHOS: oxidative phosphorylation, SDS: sodium dodecyl sulfate polyacrylamide, V: voltage.
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Canadian Institute of Health Research – 133669 to JS and JP, http://www.cihr-irsc.gc.ca/e/37788.html<https://webmail.chuq.qc.ca/owa/redir.aspx?C=bc30cc15bf6a41a683083b7095652a6f&URL=http%3a%2f%2fwww.cihr-irsc.gc.ca%2fe%2f37788.html>.
References
- 1.Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278(15):12601–4. Epub 2003/01/31. 10.1074/jbc.R200027200 . [DOI] [PubMed] [Google Scholar]
- 2.Katz BZ, Yamada KM. Integrins in morphogenesis and signaling. Biochimie. 1997;79(8):467–76. . [DOI] [PubMed] [Google Scholar]
- 3.Levene CI. Chemistry and Molecular Biology of the Intercellular Matrix. Proc R Soc Med. 1971;64(2):240-. [Google Scholar]
- 4.Emerson CP. Myogenesis and developmental control genes. Curr Opin Cell Biol. 1990;2(6):1065–75. 10.1016/0955-0674(90)90157-A. [DOI] [PubMed] [Google Scholar]
- 5.Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132(4):657–66. Epub 1996/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLennan IS. Localisation of transforming growth factor beta 1 in developing muscles: implications for connective tissue and fiber type pattern formation. Dev Dyn. 1993;197(4):281–90. Epub 1993/08/01. 10.1002/aja.1001970406 . [DOI] [PubMed] [Google Scholar]
- 7.Moon KY, Shin KS, Song WK, Chung CH, Ha DB, Kang MS. A candidate molecule for the matrix assembly receptor to the N-terminal 29-kDa fragment of fibronectin in chick myoblasts. J Biol Chem. 1994;269(10):7651–7. Epub 1994/03/11. . [PubMed] [Google Scholar]
- 8.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal. 2009;3(3–4):163–5. 10.1007/s12079-009-0069-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239(1):79–94. Epub 2002/01/11. 10.1006/dbio.2001.0416 . [DOI] [PubMed] [Google Scholar]
- 10.Riedl I, Yoshioka M, Nishida Y, Tobina T, Paradis R, Shono N, et al. Regulation of skeletal muscle transcriptome in elderly men after 6 weeks of endurance training at lactate threshold intensity. Exp Gerontol. 2010;45(11):896–903. Epub 2010/09/04. 10.1016/j.exger.2010.08.014 . [DOI] [PubMed] [Google Scholar]
- 11.Tai IT, Tang MJ. SPARC in cancer biology: its role in cancer progression and potential for therapy. Drug Resist Updat. 2008;11(6):231–46. Epub 2008/10/14. 10.1016/j.drup.2008.08.005 . [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen LH, Petersson SJ, Sellathurai J, Andersen DC, Thayssen S, Sant DJ, et al. Secreted protein acidic and rich in cysteine (SPARC) in human skeletal muscle. J Histochem Cytochem. 2009;57(1):29–39. Epub 2008/09/18. 10.1369/jhc.2008.951954 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane TF, Iruela-Arispe ML, Johnson RS, Sage EH. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J Cell Biol. 1994;125(4):929–43. Epub 1994/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sage H, Vernon RB, Funk SE, Everitt EA, Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol. 1989;109(1):341–56. Epub 1989/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funk SE, Sage EH. The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88(7):2648–52. Epub 1991/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy-Ullrich JE, Lane TF, Pallero MA, Sage EH. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca(2+)-binding EF-hand. J Cell Biochem. 1995;57(2):341–50. Epub 1995/02/01. 10.1002/jcb.240570218 . [DOI] [PubMed] [Google Scholar]
- 17.Bassuk JA, Birkebak T, Rothmier JD, Clark JM, Bradshaw A, Muchowski PJ, et al. Disruption of the Sparc locus in mice alters the differentiation of lenticular epithelial cells and leads to cataract formation. Exp Eye Res. 1999;68(3):321–31. Epub 1999/03/18. 10.1006/exer.1998.0608 . [DOI] [PubMed] [Google Scholar]
- 18.Iruela-Arispe ML, Vernon RB, Wu H, Jaenisch R, Sage EH. Type I collagen-deficient Mov-13 mice do not retain SPARC in the extracellular matrix: implications for fibroblast function. Dev Dyn. 1996;207(2):171–83. Epub 1996/10/01. . [DOI] [PubMed] [Google Scholar]
- 19.Maurer P, Hohenadl C, Hohenester E, Gohring W, Timpl R, Engel J. The C-terminal portion of BM-40 (SPARC/osteonectin) is an autonomously folding and crystallisable domain that binds calcium and collagen IV. J Mol Biol. 1995;253(2):347–57. Epub 1995/10/20. 10.1006/jmbi.1995.0557 . [DOI] [PubMed] [Google Scholar]
- 20.Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene Sage E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol. 2003;120(6):949–55. Epub 2003/06/06. 10.1046/j.1523-1747.2003.12241.x . [DOI] [PubMed] [Google Scholar]
- 21.Song H, Guan Y, Zhang L, Li K, Dong C. SPARC interacts with AMPK and regulates GLUT4 expression. Biochem Biophys Res Commun. 2010;396(4):961–6. Epub 2010/05/13. 10.1016/j.bbrc.2010.05.033 . [DOI] [PubMed] [Google Scholar]
- 22.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–22. Epub 2007/07/05. 10.1073/pnas.0705070104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho WJ, Kim EJ, Lee SJ, Kim HD, Shin HJ, Lim WK. Involvement of SPARC in in vitro differentiation of skeletal myoblasts. Biochem Biophys Res Commun. 2000;271(3):630–4. Epub 2000/05/18. 10.1006/bbrc.2000.2682 . [DOI] [PubMed] [Google Scholar]
- 24.Yaffe D, Saxel ORA. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270(5639):725–7. [DOI] [PubMed] [Google Scholar]
- 25.Velica P, Bunce CM. A quick, simple and unbiased method to quantify C2C12 myogenic differentiation. Muscle Nerve. 2011;44(3):366–70. Epub 2011/10/15. 10.1002/mus.22056 . [DOI] [PubMed] [Google Scholar]
- 26.Ono Y, Sakamoto K. Lipopolysaccharide inhibits myogenic differentiation of C2C12 myoblasts through the Toll-like receptor 4-nuclear factor-kappaB signaling pathway and myoblast-derived tumor necrosis factor-alpha. PLoS One. 2017;12(7):e0182040 Epub 2017/07/26. 10.1371/journal.pone.0182040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor SC, Berkelman T, Yadav G, Hammond M. A Defined Methodology for Reliable Quantification of Western Blot Data. Mol Biotechnol. 2013;55(3):217–26. 10.1007/s12033-013-9672-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eslami A, Lujan J. Western Blotting: Sample Preparation to Detection. JoVE. 2010; (44):2359 10.3791/2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor SC, Posch A. The Design of a Quantitative Western Blot Experiment. BioMed Res Int. 2014;2014:8 10.1155/2014/361590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki T, Hohenester E, Gohring W, Timpl R. Crystal structure and mapping by site-directed mutagenesis of the collagen-binding epitope of an activated form of BM-40/SPARC/osteonectin. Embo j. 1998;17(6):1625–34. Epub 1998/05/02. 10.1093/emboj/17.6.1625 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Fertala A, Ratner BD, Sage EH, Jiang S. Identifying the SPARC binding sites on collagen I and procollagen I by atomic force microscopy. Anal Chem. 2005;77(21):6765–71. Epub 2005/11/01. 10.1021/ac051349d . [DOI] [PubMed] [Google Scholar]
- 32.Francki A, Bradshaw AD, Bassuk JA, Howe CC, Couser WG, Sage EH. SPARC regulates the expression of collagen type I and transforming growth factor-beta1 in mesangial cells. J Biol Chem. 1999;274(45):32145–52. Epub 1999/11/05. . [DOI] [PubMed] [Google Scholar]
- 33.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206(1):113–23. Epub 2008/12/24. 10.1084/jem.20081244 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cachaco AS, Pereira CS, Pardal RG, Bajanca F, Thorsteinsdottir S. Integrin repertoire on myogenic cells changes during the course of primary myogenesis in the mouse. Dev Dyn. 2005;232(4):1069–78. Epub 2005/03/02. 10.1002/dvdy.20280 . [DOI] [PubMed] [Google Scholar]
- 35.Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell. 2003;14(8):3065–81. Epub 2003/08/20. 10.1091/mbc.E02-08-0537 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumas JF, Simard G, Flamment M, Ducluzeau PH, Ritz P. Is skeletal muscle mitochondrial dysfunction a cause or an indirect consequence of insulin resistance in humans? Diabetes Metab. 2009;35(3):159–67. Epub 2009/04/08. 10.1016/j.diabet.2009.02.002 . [DOI] [PubMed] [Google Scholar]
- 37.Hepple RT. Mitochondrial Involvement and Impact in Aging Skeletal Muscle. Front Aging Neurosci. 2014;6:211 10.3389/fnagi.2014.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35(4):367–71. Epub 2003/11/20. 10.1038/ng1270 . [DOI] [PubMed] [Google Scholar]
- 39.de Cavanagh EM, Ferder M, Inserra F, Ferder L. Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am J Physiol Heart Circ Physiol. 2009;296(3):H550–8. Epub 2009/01/20. 10.1152/ajpheart.01176.2008 . [DOI] [PubMed] [Google Scholar]
- 40.Milasincic DJ, Calera MR, Farmer SR, Pilch PF. Stimulation of C2C12 myoblast growth by basic fibroblast growth factor and insulin-like growth factor 1 can occur via mitogen-activated protein kinase-dependent and -independent pathways. Mol Cell Biol. 1996;16(11):5964–73. Epub 1996/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casar JC, Cabello-Verrugio C, Olguin H, Aldunate R, Inestrosa NC, Brandan E. Heparan sulfate proteoglycans are increased during skeletal muscle regeneration: requirement of syndecan-3 for successful fiber formation. J Cell Sci. 2004;117(Pt 1):73–84. Epub 2003/11/25. 10.1242/jcs.00828 . [DOI] [PubMed] [Google Scholar]
- 42.Brunetti A, Goldfine ID. Role of myogenin in myoblast differentiation and its regulation by fibroblast growth factor. J Biol Chem. 1990;265(11):5960–3. Epub 1990/04/15. . [PubMed] [Google Scholar]
- 43.Osses N, Brandan E. ECM is required for skeletal muscle differentiation independently of muscle regulatory factor expression. Am J Physiol Cell Physiol. 2002;282(2):C383–94. Epub 2002/01/15. 10.1152/ajpcell.00322.2001 . [DOI] [PubMed] [Google Scholar]
- 44.Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. Faseb j. 1994;8(2):163–73. Epub 1994/02/01. . [PubMed] [Google Scholar]
- 45.Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH. Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest. 2003;111(4):487–95. Epub 2003/02/18. 10.1172/JCI16804 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nandan D, Clarke EP, Ball EH, Sanwal BD. Ethyl-3,4-dihydroxybenzoate inhibits myoblast differentiation: evidence for an essential role of collagen. J Cell Biol. 1990;110(5):1673–9. 10.1083/jcb.110.5.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saitoh O, Periasamy M, Kan M, Matsuda R. cis-4-Hydroxy-L-proline and ethyl-3,4-dihydroxybenzoate prevent myogenesis of C2C12 muscle cells and block MyoD1 and myogenin expression. Exp Cell Res. 1992;200(1):70–6. Epub 1992/05/01. [DOI] [PubMed] [Google Scholar]
- 48.Rochard P, Rodier A, Casas F, Cassar-Malek I, Marchal-Victorion S, Daury L, et al. Mitochondrial activity is involved in the regulation of myoblast differentiation through myogenin expression and activity of myogenic factors. J Biol Chem. 2000;275(4):2733–44. Epub 2000/01/25. . [DOI] [PubMed] [Google Scholar]
- 49.Melo F, Carey DJ, Brandan E. Extracellular matrix is required for skeletal muscle differentiation but not myogenin expression. J Cell Biochem. 1996;62(2):227–39. Epub 1996/08/01. . [DOI] [PubMed] [Google Scholar]
- 50.Velleman SG, McFarland DC. Myotube morphology, and expression and distribution of collagen type I during normal and low score normal avian satellite cell myogenesis. Dev Growth Differ. 1999;41(2):153–61. Epub 1999/05/01. . [DOI] [PubMed] [Google Scholar]
- 51.Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, et al. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30(12):2393–9. Epub 2009/01/27. 10.1016/j.biomaterials.2008.12.069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44(3):318–31. Epub 2011/09/29. 10.1002/mus.22094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hauschka SD, Konigsberg IR. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966;55(1):119–26. Epub 1966/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem. 2007;282(30):22062–71. Epub 2007/05/25. 10.1074/jbc.M700167200 . [DOI] [PubMed] [Google Scholar]
- 55.McDonald JA. Extracellular matrix assembly. Annu Rev Cell Biol. 1988;4:183–207. Epub 1988/01/01. 10.1146/annurev.cb.04.110188.001151 . [DOI] [PubMed] [Google Scholar]
- 56.Brown KE, Yamada KM. The role of integrins during vertebrate development. Semin Dev Biol. 1995;6(2):69–77. 10.1016/S1044-5781(06)80016-2. [DOI] [Google Scholar]
- 57.Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10(3):785–98. Epub 1999/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huynh MH, Sage EH, Ringuette M. A calcium-binding motif in SPARC/osteonectin inhibits chordomesoderm cell migration during Xenopus laevis gastrulation: evidence of counter-adhesive activity in vivo. Dev Growth Differ. 1999;41(4):407–18. Epub 1999/08/31. . [DOI] [PubMed] [Google Scholar]
- 59.Motamed K, Funk SE, Koyama H, Ross R, Raines EW, Sage EH. Inhibition of PDGF-stimulated and matrix-mediated proliferation of human vascular smooth muscle cells by SPARC is independent of changes in cell shape or cyclin-dependent kinase inhibitors. J Cell Biochem. 2002;84(4):759–71. Epub 2002/02/09. . [DOI] [PubMed] [Google Scholar]
- 60.Mercurius KO, Morla AO. Inhibition of vascular smooth muscle cell growth by inhibition of fibronectin matrix assembly. Circ Res. 1998;82(5):548–56. Epub 1998/04/07. . [DOI] [PubMed] [Google Scholar]
- 61.Pokutta S, Herrenknecht K, Kemler R, Engel J. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem. 1994;223(3):1019–26. Epub 1994/08/01. . [DOI] [PubMed] [Google Scholar]
- 62.Engel J, Taylor W, Paulsson M, Sage H, Hogan B. Calcium binding domains and calcium-induced conformational transition of SPARC/BM-40/osteonectin, an extracellular glycoprotein expressed in mineralized and nonmineralized tissues. Biochemistry. 1987;26(22):6958–65. 10.1021/bi00396a015 [DOI] [PubMed] [Google Scholar]
- 63.Amphlett GW, Hrinda ME. The binding of calcium to human fibronectin. Biochemical and Biophysical Research Communications. 1983;111(3):1045–53. 10.1016/0006-291X(83)91405-5. [DOI] [PubMed] [Google Scholar]
- 64.Lund DK, Cornelison DD. Enter the matrix: shape, signal and superhighway. Febs j. 2013;280(17):4089–99. Epub 2013/02/05. 10.1111/febs.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hjorth M, Norheim F, Meen AJ, Pourteymour S, Lee S, Holen T, et al. The effect of acute and long-term physical activity on extracellular matrix and serglycin in human skeletal muscle. Physiol Rep. 2015;3(8):e12473 10.14814/phy2.12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y-P, Hsiao M. Exercise-induced SPARC prevents tumorigenesis of colon cancer. Gut. 2013;62(6):810–1. 10.1136/gutjnl-2012-303235 [DOI] [PubMed] [Google Scholar]
- 67.Foster RF, Thompson JM, Kaufman SJ. A laminin substrate promotes myogenesis in rat skeletal muscle cultures: analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Dev Biol. 1987;122(1):11–20. Epub 1987/07/01. . [DOI] [PubMed] [Google Scholar]
- 68.Podleski TR, Greenberg I, Schlessinger J, Yamada KM. Fibronectin delays the fusion of L6 myoblasts. Exp Cell Res. 1979;122(2):317–26. Epub 1979/09/01. . [DOI] [PubMed] [Google Scholar]
- 69.Dourdin N, Brustis JJ, Balcerzak D, Elamrani N, Poussard S, Cottin P, et al. Myoblast fusion requires fibronectin degradation by exteriorized m-calpain. Exp Cell Res. 1997;235(2):385–94. Epub 1997/09/23. 10.1006/excr.1997.3684 . [DOI] [PubMed] [Google Scholar]
- 70.Chung CY, Kang MS. Correlation between fibronectin and its receptor in chick myoblast differentiation. J Cell Physiol. 1990;142(2):392–400. Epub 1990/02/01. 10.1002/jcp.1041420224 . [DOI] [PubMed] [Google Scholar]
- 71.Chen LB. Alteration in cell surface LETS protein during myogenesis. Cell. 1977;10(3):393–400. Epub 1977/03/01. . [DOI] [PubMed] [Google Scholar]
- 72.Goldenthal MJ, Marin-Garcia J. Mitochondrial signaling pathways: a receiver/integrator organelle. Mol Cell Biochem. 2004;262(1–2):1–16. Epub 2004/11/10. . [DOI] [PubMed] [Google Scholar]
- 73.Sharma LK, Lu J, Bai Y. Mitochondrial Respiratory Complex I: Structure, Function and Implication in Human Diseases. Curr Med Chem. 2009;16(10):1266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zamurs LK, Idoate MA, Hanssen E, Gomez-Ibanez A, Pastor P, Lamande SR. Aberrant mitochondria in a Bethlem myopathy patient with a homozygous amino acid substitution that destabilizes the collagen VI alpha2(VI) chain. J Biol Chem. 2015;290(7):4272–81. Epub 2014/12/24. 10.1074/jbc.M114.632208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He X, Zhou A, Lu H, Chen Y, Huang G, Yue X, et al. Suppression of mitochondrial complex I influences cell metastatic properties. PLoS One. 2013;8(4):e61677 Epub 2013/05/01. 10.1371/journal.pone.0061677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jørgensen SB, Richter EA, Wojtaszewski JFP. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J Physiol. 2006;574(Pt 1):17–31. 10.1113/jphysiol.2006.109942 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SM: size marker, 0: Pooled samples, 1: PBS, 2: rSPARC, 3: anti-SPARC, 4: Anti-SPARC+rSPARC. Abbreviations: ECM: extracellular matrix, PBS: phosphate buffered saline, OXPHOS: oxidative phosphorylation, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody.
(PDF)
Abbreviations: PBS: phosphate buffered saline, rSPARC: recombinant SPARC protein, and anti-SPARC: anti-SPARC antibody.
(PDF)
Abbreviations: AB: antibody, CAPS: N-cyclohexyl-3-aminopropanesulfonic acid, h: hours, Cat#: catalog number, ON: overnight, OXPHOS: oxidative phosphorylation, SDS: sodium dodecyl sulfate polyacrylamide, V: voltage.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.