Abstract
Carbon monoxide-fermenting microorganisms can be used for the production of a wide range of commodity chemicals and fuels from syngas (generated by gasification of, e.g., wastes or biomass) or industrial off-gases (e.g., from steel industry). Microorganisms are normally more resistant to contaminants in the gas (e.g., hydrogen sulfide) than chemical catalysts, less expensive and self-regenerating. However, some carboxydotrophs are sensitive to high concentrations of CO, resulting in low growth rates and productivities. We hypothesize that cultivation of synthetic cocultures can be used to improve overall rates of CO bioconversion. As a case study, a thermophilic microbial coculture, consisting of Carboxydothermus hydrogenoformans and Methanothermobacter thermoautotrophicus was constructed to study the effect of cocultivation on conversion of CO-rich gases to methane. In contrast to the methanogenic monoculture, the coculture was able to efficiently utilize CO or mixtures of H2/CO/CO2 to produce methane at high efficiency and high rates. In CSTR-bioreactors operated in continuous mode, the coculture converted artificial syngas (66.6% H2:33.3% CO) to an outflow gas with a methane content of 72%, approaching the 75% theoretical maximum. CO conversion efficiencies of 93% and volumetric production rates of 4 m3methane/m3liquid/day were achieved. This case shows that microbial cocultivation can result in a significant improvement of gas-fermentation of CO-rich gases.
Keywords: Methanogenesis, Hydrogenogenesis, Syngas fermentation, Carboxydothermus hydrogenoformans, Methanothermobacter thermoautotrophicus, Sabatier process
Short abstract
Utilization of biomass/carbon waste to generate fuel via biomethanation of syngas using a cocultivation approach.
Introduction
Biobased technologies are quickly upcoming to take part in the closing of carbon- and other waste streams in our society. However, many of these biobased technologies cannot deal with recalcitrant substrates, such as lignified biomass or municipal waste. Conversion of such carbon-wastes via gasification technology yields synthesis gas (syngas), a mixture of mainly CO, H2 and CO2, giving opportunity to access the full carbon spectrum of the initial material via gas fermentation. Alternative sources interesting for gas fermentation are off-gases from industry (e.g., steel mills) and syngas generated via high temperature coelectrolysis (HTCE), reforming steam and CO2 into syngas.1 HTCE can also be operated with solely sunlight as energy source, deriving syngas from inorganic sources.2 Making use of microbial gas fermentation processes, waste streams can be converted via a uniform substrate into fuels and commodity chemicals. Companies, such as Lanzatech, establish large scale production systems to generate biobased products from CO-rich, steel mill waste gases, showing the application potential of gas fermentation technology.3,4
Carbon monoxide is one of the main components in untreated syngas, and is known as an odorless, colorless and toxic gas. Despite its toxicity, it can act as a natural substrate for anaerobic microorganisms, driving acetogenic, hydrogenogenic and methanogenic metabolisms.5 Its low reduction potential (E0′ = −520 mV) makes it a strong electron donor, and theoretically allows for higher energy conservation compared to hydrogen oxidation (E0′ = −414 mV). However, generally methanogens grow poorly on CO. This can also be deduced from the fact that only four methanogens have been shown to grow on CO as a sole substrate: Methanosarcina acetivorans,6Methanothermobacter thermoautotrophicus,7Methanothermobacter marburgensis8 and Methanosarcina barkeri.7 The hydrogenotrophic methanogens M. thermoautotrophicus and M. marburgensis both showed CO conversion to methane but preferred H2/CO2 over CO.8 These poor CO utilization capabilities are likely related to the excess reducing equivalents in the cytoplasm of the cell.8
Cocultivation of microbes can be used to improve the growth of microorganisms and generate a different product spectrum. This can either be via product removal, generating a thermodynamic advantage for one or both strains, or via excretion of useful secondary metabolites to support growth. Coculture engineering can be used to tune and optimize the production system for specific products, significantly improving the production potential.9 For CO fermentation, cocultivation was shown as one of the approaches to expand the product spectrum toward chain elongated products and alcohols.10,11 In these cocultures the partner strain accepted products from the CO-fermenter, allowing for production of caproate and hexanol. Such products are rarely formed in mono- or undefined mixed cultures grown on syngas and, when formed, their production rates and final product concentrations are low.10
This study aimed to overcome the poor methanogenic potential of CO containing gases by cocultivating M. thermoautotrophicus, a relatively well studied hydrogenotrophic methanogen, with Carboxydothermus hydrogenoformans,12 a carboxydotrophic hydrogenogen. Hydrogenogens are highly efficient CO utilizers, rapidly converting the CO to H2 via the water–gas shift reaction. At standard conditions the energy yield of this reaction is rather low (ΔG0′ = −20 kJ), but this gets more negative with increasing temperature. Doubling times of hydrogenogens in general are rather short (1–2 h) indicating a high rate, low energy yielding metabolism.5,13 The organisms selected for this study are thermophiles with a growth optimum around 65 °C, allowing for increased reaction kinetics and increase in the transfer rate of gas during operation. A downside of the thermophilic conditions is the lower saturation level of gases at higher temperatures. But, as gas transfer rate is usually the limiting factor, the system is likely not much affected by the maximal gas saturation levels.5
We hypothesize that methanogenesis from CO-rich gases is more efficient by the coculture when compared to the monoculture, due to the removal of CO by the hydrogenogen, lifting the toxic effects on the methanogen while simultaneously providing substrate in the form of H2/CO2. In contrast to an open mixed culture approach, the defined coculture is expected to have better product specificities, higher production rates and have a more robust production profile.
Experimental Section
Strains and Cultivation
Strains M. thermoautotrophicus ΔH (DSM-1053) and C. hydrogenoformans Z-2901 (DSM-6008) were ordered from the DSMZ strain collection (Braunschweig, Germany). Strains were initially cultivated at 65 °C in medium recommended by the provider, using anaerobic cultivation procedures. After growth was confirmed, the strains were transferred to a carbonate-phosphate buffered medium with the following composition: 0.4 g/L KH2PO4, 0.53 g/L K2HPO2·2H2O, 0.3 g/L NH4Cl, 0.3 g/L NaCl, 0.1 g/L MgCl2·6H2O, 0.5 g/L yeast extract and 0.5 mg/L resazurin. Medium was supplemented, per liter, with 61.8 μg H3BO3, 61.25 μg MnCl2, 943.5 μg FeCl2, 64.5 μg CoCl2, 12.86 μg NiCl2, 67.7 μg ZnCl2, 13.35 μg CuCl2, 17.3 μg Na2SeO3, 29.4 μg Na2WO4 and 20.5 μg Na2MoO4. Medium was prepared, boiled and subsequently cooled under a continuous nitrogen flow. Bottles (120 mL total volume) were filled with 50 mL medium and instantly capped with rubber stopper and aluminum cap. The gas phase was exchanged with 80:20 H2:CO2 in the case of M. thermoautotrophicus and 80:20 N2/CO2 in the case of C. hydrogenoformans, resulting in a final pressure of 170 kPa. The headspace was further tuned by partial removal of gas and introduction of additional CO, H2 or CO2. The bottles were autoclaved and stored at room temperature until further use. Before inoculation, medium was augmented with the following volumes of stock solutions: 1% of 11 g/L CaCl2·2H2O, 1% of a vitamin solution containing per liter: biotin 20 mg, nicotinamide 200 mg, p-aminobenzoic acid 100 mg, thiamin 200 mg, panthotenic acid 100 mg, pyridoxamine 500 mg, cyanocobalamine 100 mg, riboflavine 100 mg. The medium was reduced by introducing a 5% volume of a stock solution containing 4.8 g/L Na2S·7–9 H2O and 80 g/L NaHCO3. Unless stated otherwise, bottles were inoculated with an exponentially growing culture in a 1:50 ratio (v/v).
Coculture Establishment and Characterization
Pure cultures of M. thermoautotrophicus and C. hydrogenoformans were pregrown on H2/CO2 and CO as substrate, respectively. During exponential growth phase of both cultures, cross inoculation was performed, establishing coculture conditions. CO was added at 60 kPa partial pressure after cross-inoculation. Cocultures were transferred at least every 5 days to keep them active. Subsequent cocultures were kept under a headspace of approximately 47/40/13 H2/CO/CO2 ratio. The effect of headspace composition on coculture performance was assessed by varying the H2:CO composition between 1:0/2:1/1:2. Cocultures were regularly inspected by microscopy to verify the presence of the two microorganisms.
Bioreactor Operation
Cultivation in both batch and continuous, was performed in a 1.5 L bioreactor (Applikon, Delft, The Netherlands). Hydrogen and CO were supplied using mass flow controllers (Brooks Instruments BV, Ede, The Netherlands). Medium used in the bioreactors was similar as that described above, except the addition of carbonate or CO2 was omitted. The liquid volume in the reactor was set to 750 mL for both batch and continuous experiments. Stirring was performed by two rushton stirrers on a single shaft, stirrers were placed at 33% and 66% of the liquid height. The pH was controlled using 3 M KOH and 33% acetic acid solutions. Gas outflow rates were determined using a bubble counter. After sterilization, reactors were connected to the control tower, initiating temperature (65 °C) and pH (7.2) control. Reactors were flushed for 3 h with N2 at a rate of 20 mL/min, to create anaerobic conditions. Right before inoculation the N2 flow was changed for a CO/H2 flow. Additionally, salts, vitamins, yeast extract, and H2S were introduced in the reactor in the same ratio as described above. When the redox potential of the medium was lowered to a value below −300 mV, the reactor was inoculated with the coculture (5% inoculum, v/v); establishment of the coculture in the bioreactor was visually monitored by microscopy. For continuous operation a peristaltic pump (Masterflex, Gelsenkirchen, Germany) was used, and a HRT of 1.5 days was applied. Sterile medium was supplied from 10 L medium vessels continuously sparged with nitrogen (5 L/h) during the experiment to ensure anaerobic conditions of the inflow medium. All mentions of gas-volumes in supply or production rates throughout the text are considered to be at 1 atm pressure and 298 K.
Analytical Procedures
For gas analysis, gas samples of 0.2 mL were taken by syringe and analyzed in a Compact GC 4.0 (Global Analyzer Solutions, Breda, The Netherlands). CO, CH4 and H2 were measured using a molsieve 5A column operated at 100 °C coupled to a Carboxen 1010 precolumn. CO2 was measured using a Rt-Q-BOND column operated at 80 °C. Detection was in all cases done via a thermal conductivity detector.
Liquid phase composition was analyzed with a high pressure liquid chromatograph equipped with a MetaCarb 67H column (Agilent Technologies, Santa Clara, CA). The column was operated at a temperature of 45 °C with a flow rate of 0.8 mL/min. Detection was done via a RI and UV detector. H2SO4 (0.01 N) was used as eluent. In all cases, samples of 0.5 mL were taken and immediately centrifuged at 13000g. Subsequently, 0.4 mL supernatant was added to 0.6 mL 10 mM DMSO in 0.1 N H2SO4 solution. Concentrations below 0.3 mM could not accurately be quantified and are referred to as trace amounts.
Dry weight of the biomass was determined from 4 mL fresh sample. Samples were centrifuged at 13000 rpm for 2 min. In order to remove salts and other medium components, the pellet was washed with deionized water, centrifuged and subsequently resuspended in 1 mL deionized water. The suspended biomass was dried on a preweighed aluminum container at 120 °C for at least 3h, after which the dry weight was determined.
Results and Discussion
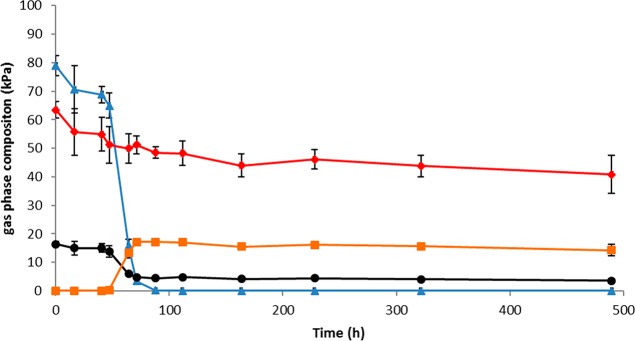
Pure cultures of C. hydrogenoformans and M. thermoautotrophicus were tested for growth on CO. C. hydrogenoformans utilized the provided 60 kPa CO substrate within 30 h, producing H2 and CO2 as the main end products. M. thermoautotrophicus could be grown on H2/CO2 in presence and absence of CO, but CO was utilized only slowly. It becomes clear that hydrogen is relatively quickly utilized in the presence of CO (within 100 h), but only a fraction of the CO itself is consumed over 500 h (Figure 1). This is in accordance with what has been reported earlier for this strain.7,8 Growth on CO as a sole substrate was not tested here for M. thermoautotrophicus, but has been reported to be almost 100 times slower than growth on H2/CO2.7
Figure 1.
Substrate product spectrum of M. thermoautotrophicus grown on a mixture containing CO, H2 and CO2. Standard deviations are shown over triplicate bottle experiments. CO, red diamonds; H2, blue triangles, CO2 black circles, CH4, orange squares.
Cocultivation Significantly Increases Methane Production Rate and Efficiency from CO
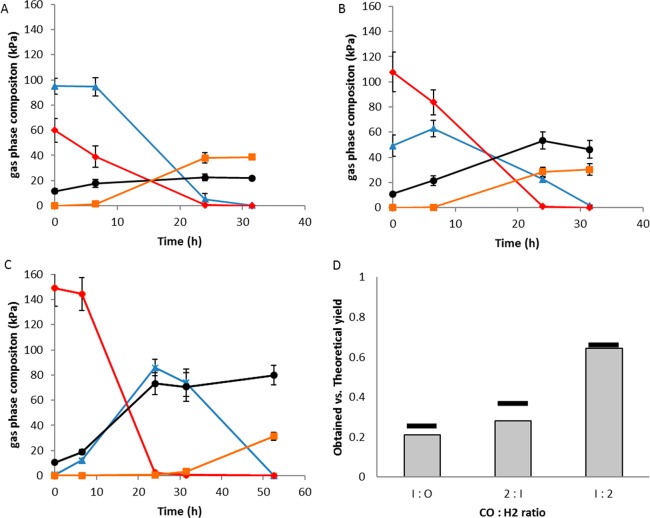
Cocultivation of C. hydrogenoformans and M. thermoautotrophicus resulted in rapid conversion of a H2/CO2/CO mixture (Figure 2A,B) or pure CO (Figure 2C) to methane. The coculture was able to utilize 60 kPa CO in approximately 24 h, whereas M. thermoautotrophicus monocultures needed over 500 h to utilize less than this amount of CO (Figure 1).
Figure 2.
Methanogenic coculture converting different headspace compositions to methane. (A) 1:2 CO:H2 mixture, (B) 2:1 CO:H2, (C) 1:0 CO:H2 mixture. Standard deviations are shown over triplicate bottle experiments. CO, red diamonds; H2, blue triangles; CO2, black circles; CH4, orange squares. (D). Methane yield per CO consumed under different initial CO:H2 compositions. Horizontal bars above the graphs display the theoretical yield based on initial gas content in the bottles.
After 20 h of incubation, traces of acetate, in the range of 0.5 to 1.5 mM, were found in the coculture incubations as additional end-products. This is likely due to the acetogenic potential of C. hydrogenoformans.14 From the tested conditions, it becomes clear that relatively high CO pressures, up to the maximum tested pressure of 150 kPa, can be utilized by this coculture to produce methane and CO2. When grown solely on CO as a substrate, a clear distinction can be made between the phases where C. hydrogenoformans and M. thermoautotrophicus are metabolically active. However, within 48 h all substrate was converted to methane and CO2. Shifting the initial headspace composition to contain relatively more hydrogen (1:2 CO/H2) decreases the time required for conversion to 24 h. Additionally, the higher H2 content decreases the amount of CO2 released. It is hypothesized that both strains profit from the cocultivation: C. hydrogenoformans removes toxic CO and is capable of producing H2, CO2 and acetate, supporting the growth of the methanogen. The methanogen is capable of rapidly removing hydrogen and CO2 from the environment, creating thermodynamically more favorable conditions for C. hydrogenoformans to grow. Such interactions are also expected to occur in the natural habitat of these organisms, where hydrogenogens are suggested to cleanse the environment of CO for other organisms to grow.15
Several reactions take place in the coculture (eqs 1–3). As M. thermoautotrophicus utilizes CO inefficiently compared to C. hydrogenoformans, we assume a neglectable amount of CO is directly converted to methane. As can be seen from eq 3, in the ideal situation (CO:H2 = 1:3), the overall stoichiometry is CO2 neutral and can yield solely methane and water as end-product. Additionally, this poses an interesting scenario as no protons are produced or consumed in the overall reaction, requiring minimal addition of acid or base during the process.
| 1 |
| 2 |
| 3 |
The methane production yields per CO consumed (Figure 2D) approximate the theoretical values in each of the conditions, showing that most CO is eventually converted to methane and that there is minor conversion to other side products. When using open mixed cultures for biomethanation of syngas, side products such as acetate, propionate and ethanol are produced, lowering the overall efficiency of the system.16,17 In studies with immobilized methanogenic mixed cultures on charcoal, CO was largely converted to methane (∼50%); however, methanogenesis was partly inhibited by CO and formation of acetate/formate as byproducts was observed.18
High Methane Content Gas Production in Batch Bioreactors
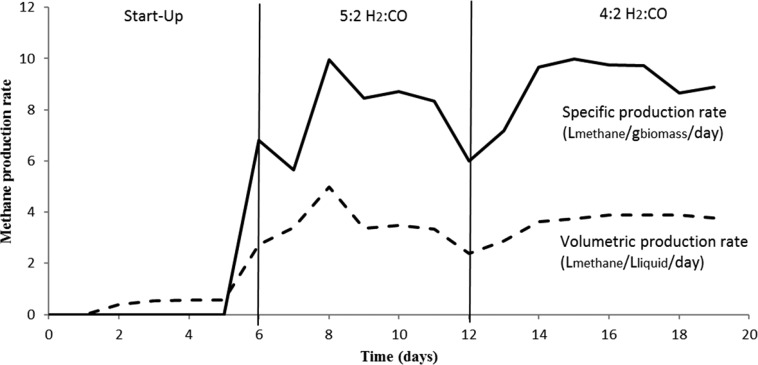
The methane production capacity of the coculture was further tested by cultivation in a gas fed CSTR. Different reactor parameters were tested to assess their effect on coculture functioning. Initial reactor runs were performed with 100 rpm stirring, resulting only in a methane content of 2% in the outflow gas at a rate of 0.150 m3methane/m3liquid/day. Increasing the stirring speed from 100 to 400 rpm resulted in an increase of average H2 consumption efficiency from ∼20% to ∼80%, whereas average CO consumption efficiency increased from ∼20% to ∼60% (Supporting Information S1.A) and average relative methane production efficiency increased from ∼3% to ∼40% (Supporting Information S1.B). The increase in stirring rate increases the gas mass transfer, making it more accessible to the microbes. Runs with stirring speeds up to 400 rpm resulted in a system generating a headspace with peak concentrations up to 77% CH4, with 13% hydrogen and 5% CO and CO2. Increasing the stirring above 400 rpm resulted in a drop in methanogenic activity, potentially due to high shear stress, or CO accumulation in the liquid due to a high transfer rate. This inhibition could not be reversed by lowering the stirring speed. When applying batch conditions with 400 rpm stirring speed, the culture could be maintained for about 10 days without any addition of new medium while continuously converting the inflow gas to mainly methane. After ∼10 days, production rates declined which was likely due to depletion of nutrients.
Continuous Production of Methane Enriched Gas
During continuous operation the coculture maintained biomass concentrations of approximately 0.5 g L–1. Consumption of CO at high rates, indicating activity of C. hydrogenoformans, and methane production, indicating activity of M. thermoautotrophicus, showed that none of the two organisms washed out during the whole run. This was confirmed by visual inspection of the coculture: two distinct phenotypes, corresponding to C. hydrogenoformans and M. thermoautotrophicus, could be observed throughout bioreactor operation.
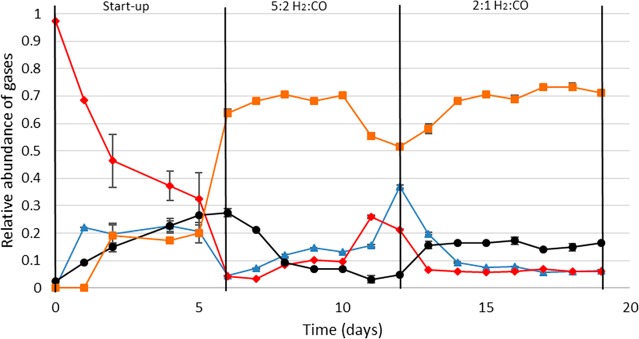
During the first phase of continuous operation, hydrogen inflow was set to 5 mL min–1 while CO inflow was set to 2 mL min–1. Under these conditions, average outflow gas was composed of 70% methane and a fraction of 14% H2, 8% CO2 and 8% CO (Figure 3, day 6–12). As not all hydrogen was used under these conditions, hydrogen gas flow was not further increased. Based on mass balance calculations, introduced CO was removed with 90% efficiency whereas introduced hydrogen was removed with 93% efficiency. Hydrogen was almost completely converted to methane (∼90%). Volumetric methane production rates reached an average value of 3.5 Lmethane/Lliquid/day (Figure 4, day 6–12). A steady state could however not be obtained with these operation conditions as can be seen from the instability in production rates during this phase (Figure 4). The increased CO content in the gas phase of the reactor, together with a drop in methane production, during the period from day 6 to day 12 suggests that the culture cannot deal properly with the provided conditions. During this phase cyclic patterns appeared for the redox potential, alternating between −500 and −600 mV. The issue was solved by lowering the hydrogen inflow rate to 4 mL min–1, and covering the reactor from light. The instability might be related to the sensitivity of M. thermoautotrophicus to light, which has been observed earlier.19
Figure 3.
Relative outflow gas composition of the coculture in a continuous bioreactor.
Relative gas composition in the reactor is shown. Total pressure in the system was 1 atm. Average values and standard deviations shown are calculated from triplicate measurements. Day 0–6: start-up phase in which CO and H2 flow and stirring were ramped up. Day 6–12: operation was performed with 2 mL/min CO and 5 mL/min H2. Day 12–19: operation was performed with 2 mL/min CO and 4 mL/min H2. CO, red diamonds; H2, blue triangles; CO2, black circles; CH4, orange squares.
Figure 4.
Volumetric and specific production rates of methane by the coculture in a continuous bioreactor.
Solid line represent specific methane production rates in Lmethane/gbiomass/day. Dashed line represent volumetric methane production rates in Lmethane/Lliquid/day.
The reactor was able to reach a more stable state (day 12–19) after covering the reactor from light, and lowering the hydrogen flow to 4 mL min–1, resulting in a CO:H2 ratio of 1:2. Redox was no longer going down to −600 mV and stabilized around −500 mV. CO levels in the outflow were decreased further to 4%, and the overall production rates increased to 4 m3methane/m3liquid/day (Figure 4). For reverse membrane reactors, rates of about 0.2 m3methane/m3liquid/day were reported,20,21 which is significantly lower than rates reported here for the nonbiomass retaining coculture system. Average methane outflow content in the coculture reactor system during steady operation was around 72% (Figure 3, day 14–19), close to the theoretical maximum of 75% at this inflow gas composition. A CO conversion efficiency of 93% was achieved under these conditions and 97% of the inflowing hydrogen was converted. Similarly as in the bottle experiments, small amounts of acetate were produced in the reactor. After correction for the acetate externally added to the system for pH control, average production rates of 13 ± 1.4 mmol/L/day (day 6–12) and 7 ± 0.99 mmol/L/day (day 13–19) were observed, which is 8 and 4% of the total product spectrum in those conditions, respectively. This amount of acetate produced is relatively low compared to mixed culture fermentations for biomethanation of syngas, where CO is for a larger fraction (up to 50%) converted into other products.17,18
The observed gas composition could accurately be modeled by calculating the metabolic fluxes through the system using the obtained efficiencies and gas inflow ratios (Figure 5). Despite the high efficiency of the coculture, other gases (mainly CO2) are still in the outflow gas. This can potentially be explained by either of the following two factors, or both: (i) not enough electron donor is available to convert all CO2, (ii) a four times decrease in gas phase volume due to formation of methane from hydrogen and CO, causing the gases to be retained in the system for a longer time. Despite the high methane content in the outflow gas, the gas is still not optimal for introduction into the gas grid. Mainly the leftover percentage of CO is a problem and further removal is desired. Batch bottle experiments show no traces of CO after incubation and suggest the gas can be completely purified from CO by the coculture. Additionally, the system needs to be further adapted to be able to deal with 3:1 inflow ratios of H2:CO, and might be achieved by increasing the HRT of the system to retain more biomass, coculture optimization, medium improvement or using a different reactor setup, such as gas-lift. Overall, the work performed here shows that a continuous system can maintain the methanogenic coculture, efficiently producing methane at a high rate from a CO rich gas.
Figure 5.
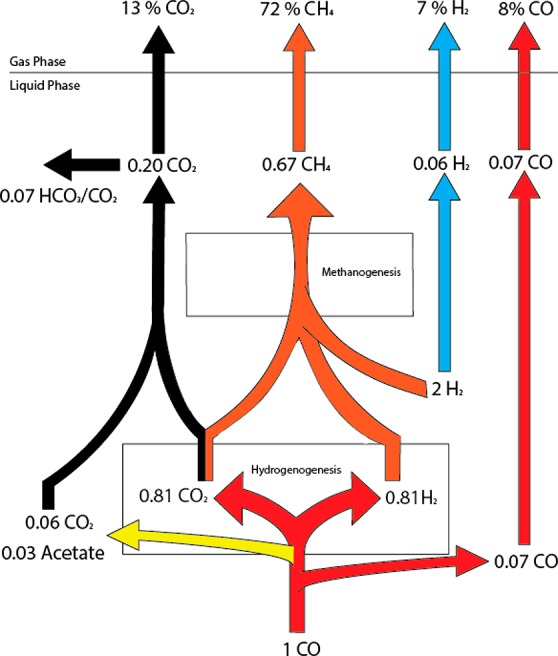
Schematic representation of the metabolite flow in a continuous bioreactor operated with a methanogenic coculture system. Acetate production is assumed to take place directly from CO only, by the hydrogenogen. In contrast to CO, H2 and CH4, CO2 is also removed in significant amounts via the liquid outflow (HCO3/CO2). Calculations were performed using pH 7.2.
Application Perspectives of Syngas Biomethanation
One can discuss the feasibility of utilization of methane versus biohydrogen, or alcohols as fuels. Hydrogen is considered a clean fuel, as solely water is produced when combusted. Additionally, hydrogen (142 MJ/kg) has a higher energy density per mass as methane (55.5 MJ/kg). However, as with other biofuels, biohydrogen production from renewable biomass or waste indirectly generates CO2, and is at best CO2 neutral. Therefore, hydrogen production from noncarbon generated electricity, such as solar power, is “cleaner” from a CO2 emission perspective. Additionally, a pure-hydrogen mixture is a difficult fuel to store and to transport,22 and cannot be used in the current natural gas infrastructure. Methane gas on the other hand can be transported, stored and used in the current infrastructure, and can be blended with natural gas.23 Liquid fuels, such as ethanol and butanol, do not have to be compressed before use, which is an advantage over gaseous fuels. However, in contrast to gaseous fuels, alcohols need to be extracted from the aqueous broth, reducing the overall efficiency of the process. Also, the heating value of methane per mass is almost two times higher than that of ethanol (29.7 MJ/kg) and 1.5 times that of butanol (36.7 MJ/kg). Therefore, methane-containing fuels still have the potential to be widely applied in industry in the future, and are expected to be good replacements for fossil transportation and jet fuels.24,25
Conventional processes for biogas production from biomass are often limited by the poor degradation potential of its lignocellulosic fraction (Table 1), losing a large fraction of the initial energy stored and resulting in an overall chemical efficiency between 20 and 40%.26 Extensive pretreatment methods, such as thermal pressure hydrolysis or enzyme addition, have to be applied to access the bulk part of the biomass efficiently.27 The final biogas composition can contain large fractions of CO2, thus requiring the gas to be cleaned or upgraded before injection into the gas grid is possible. Cleaning and upgrading of biogas can be done in various ways, such as CO2 fixation by hydrogenotrophic methanogens or using CO2-fixing phototrophs.28 However, upgrading of biogas can often not be carried out in the anaerobic digester, and thus requires additional process steps to obtain an applicable gas. Despite biogas upgrading being possible, conversion of tough substrates such as lignin or the utilization of more recalcitrant wastes via anaerobic digestion remains difficult.
Table 1. Overview of Different Processes Capable of Converting Renewable Biomass to Methane Gas.
| Anaerobic digestion | Syngas biomethanation | Syngas methanation | |
|---|---|---|---|
| Pretreatment | Mechanical, Chemical, Biological | Gasification | Gasification |
| Conversion mechanism | Saccharolysis, acidogenesis, methanogenesis | Hydrogenogenesis, methanogenesis | Chemical methanation |
| Catalyst | Biological (undefined mixed culture) | Biological (defined (co)culture) | Metal catalysts (e.g., nickel) |
| Disadvantages | Low substrate conversion efficiency | Low production rates compared to chemical conversion | Sensitive to different syngas compositions |
| High CO2 content in outlet (up to 50%) | Sensitive to syngas impurities | ||
| Relatively low production rates | Outlet gas not completely free of CO2, CO and H2 | Relatively expensive catalysts | |
| Outlet gas not completely free of CO2, CO and H2 | |||
| Advantages | Robust and cheap process | Cheap, self-replicating catalysts | High production rates |
| Can convert dilute organic wastes (e.g., wastewater) | Good production rate for a biological system | High methane content in a single step | |
| High methane content in a single step | |||
| Robust for different syngas compositions | |||
| Tolerance to syngas pollutants | |||
| Gas composition | CH4, CO2 | CH4, H2, CO2, CO | CH4, H2, CO2, CO |
| Chemical efficiencya | 20–40%26 | N.D. | 50–70% (wood to SNG)35,36 |
Energy of the product compared to the energy content of the original feedstock.
The biological conversion of syngas as described here requires gasification as the main pretreatment step (Table 1). Interestingly, not only biomass can be fed to gasifiers, but other poor-quality carbon-containing streams, e.g., municipal waste, excess sludge, can also be supplied as a starting source. The feasibility of production of methane from renewable syngas is debatable as methane is a relatively low value product, and other products such as commodity chemicals can also be generated from this substrate. However, methane remains an interesting end-product when considering fuel production, as using an optimal methanation gas composition (CO:H2 = 1:3) conserves more energy (73%) than the fermentation of syngas (CO:H2 = 2:4) to ethanol (69%). Additionally, downstream processing of methane is relatively easy as it is almost completely present in the gas phase under standard conditions. Gas composition of syngas can vary widely, depending on process operation. A 3:1 ratio of H2:CO is optimal for biomethanation, but is not strictly required, as a lower ratio will still result in methane gas, but with an increased CO2 content. Additionally, depending on the purity of the feed gas, other contaminants might still be present in the outflow gas, similarly to contaminants in biogas (e.g., hydrogen sulfide). However, this also depends on the operation conditions of the reactor (e.g., pH, HRT), potentially allowing contaminants to be removed via the liquid phase of the reactor.
Methane production via chemical catalysis of syngas (Sabatier process) is currently already applied, obtaining SNG.29 The syngas is converted to methane via a methanation reaction using mainly nickel catalysts (Table 1). SNG can be formed in two ways: (i) Production of high methane content producer gas directly from biomass, formed during gasification at “lower” temperatures of around 600 °C. (ii) Syngas is formed at higher temperatures of >700 °C, requiring a methanation step to form SNG.30 Production rates of this process are relatively high, reaching gas retention times in the reactors of 50 ms.31 These retention times are significantly lower than those applied in biological reactor systems. Therefore, a relatively small chemical reactor can be used to produce large amounts of SNG. However, the chemical process makes use of relatively expensive catalyst materials that can be quickly inactivated by reactive molecules such as sulfur species32,33 and chlorine,29 often present in the biomass derived syngas. Additionally, carbon deposits on the metal catalyst material can cause reduction of surface area, making the catalyst less efficient.30 In contrast to biomethanation, the chemical methanation process requires an obligate gas composition with a H2:CO ratio of 3 or higher in order to produce methane efficiently.29 Renewable syngas composition can vary widely based on the starting material used, and additionally often contain high amounts of impurities. Strong gas purification and fine-tuning are thus required to operate the chemical process. The strong exergonic nature of the methanation reaction generates heat, which can be reused in the chemical process to generate steam. The biological methanation does not generate high potential heat streams, but does also not require steam in order to perform the methanation process. Additionally, heat originating from the gasification process might be used in biomass drying, closing part of the energy loop. Also, depending on the heat generation of the microbial system, heating of the reactor might be required as it is operated at thermophilic temperatures. Total energy requirement of the bioreactor on large scale depends on its type and exact size, making it difficult to currently state anything on this or its economic feasibility compared to the chemical process.
The biological process described here uses a self-replicating catalyst, making growth medium the main requirement to sustain the biocatalysts. Additionally, the biological catalyst is expected to be less prone to inhibition by sulfur compounds, and might even use those as growth supplements, and thus is expected to require less stringent syngas purification before application.34 The chemical process has a higher production rate compared to the biological process, but the robustness of the biological system is advantageous. Additionally, the study here is a proof of concept and does not yet involve major process optimizations. The biomethanation of syngas can likely be improved further in terms of production rates and efficiency, by factors such as medium optimization, reactor configuration and biomass retention. Also tests with different coculture combinations or defined mixed cultures might improve the overall culture performance.
Conclusion
This study shows that by using a defined coculture of C. hydrogenoformans and M. thermoautotrophicus it is possible to convert CO-rich gases to methane at high efficiency and high rate in a conventional CSTR bioreactor without gas recycling. In contrast to anaerobic digestion of biomass, this syngas route utilizes the full carbon spectrum of the initial material, can process recalcitrant carbon wastes and can generate a gas with a high content of methane in a single process step. Compared to chemical methanation of syngas, the biological processes are relatively slow. However, the robustness of the biological catalysts compared to the relatively expensive metal catalysts is an advantage. Further research is required to assess the (economic) feasibility of a biological syngas methanation system, which in the future might be used as an addition to or alternative for current methane production processes.
Acknowledgments
The authors thank Ton van Gelder for laboratory support. This research is supported by a Gravitation grant (project 024.002.002) of The Netherlands Ministry of Education, Culture and Science and The Netherlands Science Foundation (NWO). Research of A.J.M.S. and D.Z.S. is supported by an ERC grant (project 323009).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssuschemeng.7b03601.
Efficiency of methane production in CSTR batch reactors, (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Stoots C. M.; O’Brien J. E.; Herring J. S.; Hartvigsen J. J. Syngas production via high-temperature coelectrolysis of steam and carbon dioxide. J. Fuel Cell Sci. Technol. 2009, 6 (1), 011014. 10.1115/1.2971061. [DOI] [Google Scholar]
- Frost L. J.; Hartvigsen J.; Elangovan S.. Formation of synthesis gas using solar concentrator photovoltaics (SCPV) and high temperature co-electrolysis (HTCE) of CO2 and H2O. Offshore Technology Conference, 2010.
- Köpke M.; Mihalcea C.; Bromley J. C.; Simpson S. D. Fermentative production of ethanol from carbon monoxide. Curr. Opin. Biotechnol. 2011, 22 (3), 320–325. 10.1016/j.copbio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Lanzatech. Arcelor Mittal, LanzaTech and Primetals Technologies announce partnership to construct breakthrough €87m biofuel production facility (2015). http://www.lanzatech.com/arcelormittal-lanzatech-primetals-technologies-announce-partnership-construct-breakthrough-e87m-biofuel-production-facility/ (accessed November 20, 2017).
- Diender M.; Stams A. J. M.; Sousa D. Z. Pathways and Bioenergetics of Anaerobic Carbon Monoxide Fermentation. Front. Microbiol. 2015, 6, 1275. 10.3389/fmicb.2015.01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother M.; Metcalf W. W. Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: an unusual way of life for a methanogenic archaeon. Proc. Natl. Acad. Sci. U. S. A. 2004, 101 (48), 16929–34. 10.1073/pnas.0407486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L.; Fuchs G.; Thauer R.; Zeikus J. Carbon monoxide oxidation by methanogenic bacteria. J. Bacteriol. 1977, 132 (1), 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diender M.; Pereira R.; Wessels H. J.; Stams A. J. M.; Sousa D. Z.. Proteomic Analysis of the Hydrogen and Carbon Monoxide Metabolism of Methanothermobacter marburgensis. Front. Microbiol. 2016, 7, DOI: 10.3389/fmicb.2016.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santala S.; Karp M.; Santala V. Rationally engineered synthetic coculture for improved biomass and product formation. PLoS One 2014, 9 (12), e113786. 10.1371/journal.pone.0113786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diender M.; Stams A. J. M.; Sousa D. Z. Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnol. Biofuels 2016, 9 (1), 1. 10.1186/s13068-016-0495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H.; Molitor B.; Diender M.; Sousa D. Z.; Angenent L. T.. A narrow pH Range Supports Butanol, Hexanol, and Octanol Production From Syngas in a Continuous Co-culture of Clostridium ljungdahlii and Clostridium kluyveri with in-line Product Extraction. Front. Microbiol. 2016, 7, DOI: 10.3389/fmicb.2016.01773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlichny V.; Sokolova T.; Gerhardt M.; Ringpfeil M.; Kostrikina N.; Zavarzin G. Carboxydothermus hydrogenoformans gen. nov., sp. nov., a CO-utilizing thermophilic anaerobic bacterium from hydrothermal environments of Kunashir Island. Syst. Appl. Microbiol. 1991, 14 (3), 254–260. 10.1016/S0723-2020(11)80377-2. [DOI] [Google Scholar]
- Oelgeschlager E.; Rother M. Carbon monoxide-dependent energy metabolism in anaerobic bacteria and archaea. Arch. Microbiol. 2008, 190 (3), 257–69. 10.1007/s00203-008-0382-6. [DOI] [PubMed] [Google Scholar]
- Henstra A. M.; Stams A. J. M.. Deep conversion of carbon monoxide to hydrogen and formation of acetate by the anaerobic thermophile Carboxydothermus hydrogenoformans. Int. J. Microbiol. 2011, 2011, DOI: 10.1155/2011/641582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Techtmann S. M.; Colman A. S.; Robb F. T. That which does not kill us only makes us stronger’: the role of carbon monoxide in thermophilic microbial consortia. Environ. Microbiol. 2009, 11 (5), 1027–1037. 10.1111/j.1462-2920.2009.01865.x. [DOI] [PubMed] [Google Scholar]
- Guiot S. R.; Cimpoia R.; Carayon G. Potential of wastewater-treating anaerobic granules for biomethanation of synthesis gas. Environ. Sci. Technol. 2011, 45 (5), 2006–12. 10.1021/es102728m. [DOI] [PubMed] [Google Scholar]
- Navarro S. S.; Cimpoia R.; Bruant G.; Guiot S. R.. Biomethanation of Syngas Using Anaerobic Sludge: Shift in the Catabolic Routes with the CO Partial Pressure Increase. Front. Microbiol. 2016, 7, DOI: 10.3389/fmicb.2016.01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede S.; Bruchmann F.; Thorin E.; Gerber M.. Biological syngas methanation via immobilized methanogenic archaea on biochar. The 8th International Conference on Applied Energy–ICAE2016, 2016.
- Olson K. D.; McMahon C. W.; Wolfe R. Light sensitivity of methanogenic archaebacteria. Appl. Environ. Microbiol. 1991, 57 (9), 2683–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman S. Y.; Chandolias K.; Taherzadeh M. J. Syngas Biomethanation in a Semi-Continuous Reverse Membrane Bioreactor (RMBR). Fermentation 2016, 2 (2), 8. 10.3390/fermentation2020008. [DOI] [Google Scholar]
- Youngsukkasem S.; Chandolias K.; Taherzadeh M. J. Rapid bio-methanation of syngas in a reverse membrane bioreactor: Membrane encased microorganisms. Bioresour. Technol. 2015, 178, 334–340. 10.1016/j.biortech.2014.07.071. [DOI] [PubMed] [Google Scholar]
- Dunn S. Hydrogen futures: toward a sustainable energy system. Int. J. Hydrogen Energy 2002, 27 (3), 235–264. 10.1016/S0360-3199(01)00131-8. [DOI] [Google Scholar]
- Thrän D.; Billig E.; Persson T.; Svensson M.; Daniel-Gromke J.; Ponitka J.; Seiffert M.; Baldwin J.; Kranzl L.; Schipfer F.. Biomethane–status and factors affecting market development and trade. In IEA Task, 2014; Vol. 40. [Google Scholar]
- Adlunger K.; Dziekan K.; Lange M.; Mönch L.. Climate Neutral Mobility: Natural Gas and Methane as Part of the Solution. Natural Gas and Renewable Methane for Powertrains; Springer, 2016; pp 7–25. [Google Scholar]
- Edwards R.; Mahieu V.; Griesemann J.-C.; Larivé J.-F.; Rickeard D. J.. Well-to-wheels analysis of future automotive fuels and powertrains in the European context; SAE Technical Paper 0148-7191; SAE, 2004.
- McKendry P. Energy production from biomass (part 2): conversion technologies. Bioresour. Technol. 2002, 83 (1), 47–54. 10.1016/S0960-8524(01)00119-5. [DOI] [PubMed] [Google Scholar]
- Weiland P. Biogas production: current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85 (4), 849–860. 10.1007/s00253-009-2246-7. [DOI] [PubMed] [Google Scholar]
- Muñoz R.; Meier L.; Diaz I.; Jeison D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Bio/Technol. 2015, 14 (4), 727–759. 10.1007/s11157-015-9379-1. [DOI] [Google Scholar]
- Kopyscinski J.; Schildhauer T. J.; Biollaz S. M. Production of synthetic natural gas (SNG) from coal and dry biomass–A technology review from 1950 to 2009. Fuel 2010, 89 (8), 1763–1783. 10.1016/j.fuel.2010.01.027. [DOI] [Google Scholar]
- Sutton D.; Kelleher B.; Ross J. R. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73 (3), 155–173. 10.1016/S0378-3820(01)00208-9. [DOI] [Google Scholar]
- Liu Z.; Chu B.; Zhai X.; Jin Y.; Cheng Y. Total methanation of syngas to synthetic natural gas over Ni catalyst in a micro-channel reactor. Fuel 2012, 95, 599–605. 10.1016/j.fuel.2011.12.045. [DOI] [Google Scholar]
- Czekaj I.; Struis R.; Wambach J.; Biollaz S. Sulphur poisoning of Ni catalysts used in the SNG production from biomass: Computational studies. Catal. Today 2011, 176 (1), 429–432. 10.1016/j.cattod.2010.10.078. [DOI] [Google Scholar]
- Struis R. P.; Schildhauer T. J.; Czekaj I.; Janousch M.; Biollaz S. M.; Ludwig C. Sulphur poisoning of Ni catalysts in the SNG production from biomass: A TPO/XPS/XAS study. Appl. Catal., A 2009, 362 (1), 121–128. 10.1016/j.apcata.2009.04.030. [DOI] [Google Scholar]
- Daniell J.; Köpke M.; Simpson S. D.. Commercial biomass syngas fermentation. Energies 2012, 5, DOI: 10.3390/en5125372. [DOI] [Google Scholar]
- Duret A.; Friedli C.; Maréchal F. Process design of Synthetic Natural Gas (SNG) production using wood gasification. J. Cleaner Prod. 2005, 13 (15), 1434–1446. 10.1016/j.jclepro.2005.04.009. [DOI] [Google Scholar]
- van der Meijden C. M.; Veringa H. J.; Rabou L. P. The production of synthetic natural gas (SNG): A comparison of three wood gasification systems for energy balance and overall efficiency. Biomass Bioenergy 2010, 34 (3), 302–311. 10.1016/j.biombioe.2009.11.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.