Abstract
A 10-plex urine-based bladder cancer (BCa) diagnostic signature has the potential to non-invasively predict the presence of BCa in at-risk patients, as reported in various case-control studies. The present meta-analysis was performed to re-evaluate and demonstrate the robustness and consistency of the diagnostic utility of the 10-plex urine-based diagnostic assay. We re-analyzed primary data collected in five previously published case-control studies on the 10-plex diagnostic assay. Studies reported the sensitivity and specificity of ten urinary protein biomarkers for the detection of BCa, including interleukin 8, matrix metalloproteinases 9 and 10, angiogenin, apolipoprotein E, syndecan 1, alpha-1 antitrypsin, plasminogen activator inhibitor-1, carbonic anhydrase 9, and vascular endothelial growth factor A. Data were extracted and reviewed independently by two investigators. Log odds ratios (ORs) were calculated to determine how strongly the 10-plex biomarker panel and individual biomarkers are associated with the presence of BCa. Data pooled from 1,173 patients were analyzed. The log OR for each biomarker was improved by 1.5 or greater with smaller 95% CI in our meta-analysis of the overall cohort compared with each analysis of an individual cohort. The combination of the ten biomarkers showed a higher log OR (log OR: 3.46, 95% CI: 2.60–4.31) than did any single biomarker irrespective of histological grade or disease stage of tumors. We concluded that the 10-plex BCa-associated diagnostic signature demonstrated a higher potential to identify BCa when compared to any single biomarker. Our results justify further advancement of the 10-plex protein-based diagnostic signature toward clinical application.
Keywords: urine biomarkers, meta-analysis, urinary bladder, urothelial carcinoma, diagnosis
INTRODUCTION
Bladder cancer (BCa) is the second most common genitourinary malignancy in the United States, with 79,030 new cases and 16,870 deaths estimated to occur in 2017 [1]. It is also among the five most common malignancies worldwide [2]. The most common form of BCa in Western countries is urothelial carcinoma, accounting for approximately 90% of all cases [3]. The majority of BCa cases present as non-muscle invasive bladder cancer (NMIBC), which has a 5-year survival rate of >90%. However, once BCa progresses to muscle-invasive bladder cancer (MIBC), the 5-year survival rates do not exceed 50%, and distant metastasis frequently occurs. Metastatic BCa is highly lethal, with a 5-year survival rate of <15% and an estimated median survival of 12 to 14 months [4]. Therefore, early identification, both at the initial diagnosis and at recurrence, is crucial [5].
BCa detection and diagnosis require cystoscopy and bladder biopsy, which are unpleasant and costly procedures. Although NMIBC can be treated with transurethral resection (TUR) with an excellent survival outcome, this method is associated with an intravesical recurrence rate of approximately 70% within two years after TUR [6]. This is the highest recurrence rate among any type of tumor [7]. Therefore, NMIBC patients must be monitored for recurrence, which requires repeat cystoscopies. The high recurrence rates as well as lengthy treatment regimens have caused BCa to be one of the most costly malignancies to manage on a per-patient basis [8]. With an accurate urine biomarker, the number of cystoscopy would be reduced. Thus, there is an urgent need to develop novel diagnostics that are less invasive and less expensive without compromising accuracy for both initial detection and surveillance for BCa.
Recent advancements in proteomics technology have promoted discovery of novel protein markers and the number of published reports on urine-based biomarkers has dramatically increased with reported sensitivity ranges from 52% to 97%, and specificity from 43% to 100% for individual biomarkers (Table 1) (modified from D’Costa and colleagues [9]). Despite these efforts, single use of existing urinary biomarkers is not accurate enough to replace the most widely used urine-based assay, voided urinary cytology (VUC), which has a low sensitivity (range: 13–75%, median 35%) [10].
Table 1. Reported sensitivity and specificity of urine-based single protein biomarkers for the detection of bladder cancer.
| Protein name | Sensitivity (%) | Specificity (%) | Cancer (n) | Control (n) | Ref. |
|---|---|---|---|---|---|
| Alpha-1-anti-trypsin | 74 | 80 | 54 | 46 | [32] |
| Alpha-1-anti-trypsin | 71 | 72 | 102 | 206 | [40] |
| Angiogenin | 66 | 75 | 50 | 40 | [41] |
| Apolipoprotein A1 | 95 | 92 | 49 | 37 | [42] |
| Apolipoprotein A4 | 79 | 100 | 110 | 66 | [18] |
| AMFR | 84 | 75 | 45 | 62 | [43] |
| BIGH3 | 93 | 80 | 30 | 15 | [44] |
| Calprotectin | 80 | 93 | 46 | 40 | [45] |
| Cathepsin B | 56 | 56 | 122 | 107 | [46] |
| Cathepsin L | 71 | 75 | 122 | 107 | [46] |
| CCL18 | 70 | 68 | 102 | 206 | [40] |
| CD147 | 97 | 100 | 30 | 15 | [44] |
| CEACAM1 | 74 | 95 | 95 | 82 | [47] |
| Clusterin | 68 | 61 | 68 | 61 | [48] |
| Clusterin | 70 | 83 | 50 | 40 | [41] |
| Coronin-1A | 67 | 100 | 110 | 66 | [18] |
| CXCL1 | 72 | 95 | 95 | 30 | [49] |
| CXCL1 | 56 | 84 | 43 | 43 | [50] |
| CYFRA21-1 | 79 | 89 | 82 | 70 | [51] |
| CYFRA21-1 | 81 | 97 | 86 | 76 | [52] |
| CYFRA21-1 | 70 | 43 | 125 | 321 | [53] |
| CYFRA21-1 | 97 | 67 | 48 | 80 | [54] |
| DJ1 | 83 | 100 | 110 | 66 | [18] |
| EN2 | 82 | 75 | 466 | 55 | [55] |
| FDP | 52 | 91 | 57 | 139 | [56] |
| Fibronectin | 91 | 88 | 75 | 55 | [57] |
| Fibronectin | 72 | 82 | 126 | 41 | [58] |
| Prothrombin | 71 | 75 | 76 | 80 | [17] |
| Reg-1 | 81 | 81 | 23 | 48 | [59] |
| Semenogelin-2 | 67 | 80 | 110 | 66 | [18] |
| Stathmin-1 | 90 | 87 | 30 | 15 | [44] |
| Telomerase | 70 | 99 | 57 | 139 | [56] |
| Telomerase | 83 | 89 | 73 | 37 | [60] |
| g-synuclein | 88 | 90 | 110 | 66 | [18] |
Recent publications have proposed panels of protein biomarkers for the detection of BCa [11–19]. Chen and colleagues conducted a case-control study to test diagnostic performance of 63 urinary proteins found in their earlier iTRAQ study [17]. They developed a 6-peptide panel that yielded an AUC of 0.814, with a 76.3% positive predictive value, and a 77.5% negative predictive value. Kumar and colleagues developed a panel of five urinary proteins [18]. Both ELISA and Western blot (WB) assays yielded an AUC of 0.9 or more. Particularly, their WB-based assay showed more than 90% sensitivity with an almost 100% specificity. In another study, Theodorescu et. al. obtained polypeptide patterns in urine samples using capillary-electrophoresis-coupled mass spectrometry. From signatures of polypeptide mass, they established a model for predicting the presence of BCa at any stage [20] or muscle-invasive disease [21].
In a more recent report, Frantzi and colleagues developed two biomarker panels: one that contained 116 peptides and one that contained 106 peptides [19]. The authors validated the diagnostic performance of the panels using independent cohorts, showing area under the curve (AUC) values of 0.87 and 0.75 for detecting primary and recurrent BCa, respectively. They also demonstrated that the combination of their model with VUC exhibited superior diagnostic accuracy compared with the performance of either test alone. These findings further support the results demonstrating that the multiplex urine-based biomarker panel has superior diagnostic performance compared with single protein markers. Further analyses incorporating these other promising multiplex assays as well as VUC and UroVysion® will be warranted in the future studies.
While the concept that a panel of biomarkers is preferable to single biomarkers is well supported, such marker panels have not widely been developed and implemented in the clinic. In previous studies designed to establish and validate a multiplex urinary immunoassay for BCa detection [11–16, 22], we have examined approximately 1,300 urine samples. This series of studies identified a promising multivariate combination of ten urine-based biomarkers: interleukin 8 (IL8), matrix metalloproteinases 9 and 10 (MMP9 and MMP10), angiogenin (ANG), apolipoprotein E (APOE), syndecan 1 (SDC1), alpha-1 antitrypsin (A1AT), plasminogen activator inhibitor-1 (PAI1), carbonic anhydrase 9 (CA9), and vascular endothelial growth factor A (VEGFA) [23]. In the present study, we conducted a meta-analysis to re-evaluate and demonstrate the diagnostic performance of our 10-biomarker panel.
RESULTS
Study selection
We initially selected five studies that our group previously published on the diagnostic abilities for BCa detection of the following urinary biomarkers: ANG, APOE, A1AT, CA9, IL8, MMP9, MMP10, PAI1, SDC1, and VEGF [11–15]. We made an additional systematic search (see Materials and Methods section) but found no other study that met our criteria for the purpose of evaluating diagnostic ability of the 10-plex urinary biomarker panel. Adequacy of the study quality was confirmed using The Newcastle-Ottawa Scale (NOS) [24, 25], while the reporting of each study was evaluated according to Standards for Reporting of Diagnostic Accuracy (STARD) criteria [26, 27].
Data extraction and categorization
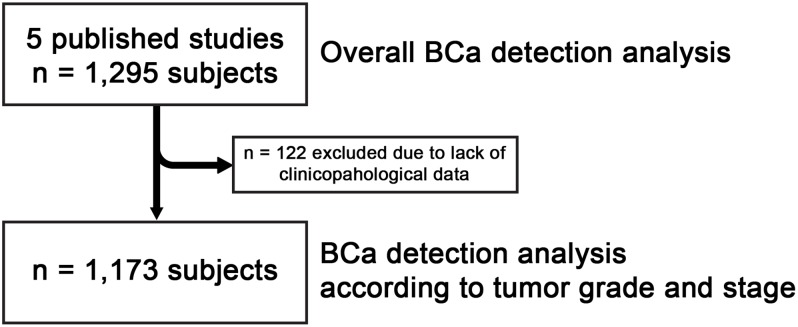
Data extraction from primary data of the five studies [11–15] was conducted independently by 2 investigators (N.M. and T.K.), and categorization was validated in the presence of the moderator (O.O.). Data pooled from the five reports [11–15] consisted of 1,295 patients (Table 2), including 247 females and 1048 males that consisted of clinicopathological and normalized molecular data. The study cohorts were mutually exclusive and there was no overlap in study subjects between the study. Data from these 1,295 patients were analyzed for overall BCa detection. Then 122 patients from Goodison 2012 [11] were excluded due to lack of histological grade or disease stage and data from the remaining 1,173 patients were analyzed for BCa detection according to tumor grade or stage, as depicted in Figure 1. This was accomplished by review of the original data.
Table 2. Summary of bladder cancer cases and controls in each cohort analyzed in the present study.
| Cohort | n | Male (%) | Median age (Years) | HG tumor (%) | MIBC (%) | Assay method | |
|---|---|---|---|---|---|---|---|
| Goodison 2012 [11] | Case Control |
64 63 |
86 87 |
69.5 60 |
86.0 | 58.7 | ELISA |
| Rosser 2013 [12] | Case Control |
102 206 |
82 74 |
69 56 |
62.7 | 40.2 | ELISA |
| Chen 2014 [13] | Case Control |
183 137 |
84 72 |
69 65 |
55.7 | 16.4 | ELISA |
| Shimizu 2016 [14] Cohort 1 |
Case Control |
29 33 |
86 82 |
68 50 |
86.2 | 44.8 | Multi-Array |
| Cohort 2 | Case Control |
100 100 |
82 81 |
70 50.5 |
79.0 | 42.0 | Multi-Array |
| Goodison 2016 [15] | Case Control |
211 67 |
87 79 |
75 70 |
58.8 | 19.4 | Multi-Array |
ELISA, enzyme-linked immunosorbent assay; HG, high-grade; MIBC, muscle-invasive bladder cancer.
Figure 1. Study subjects for the present analyses.
Meta-analysis
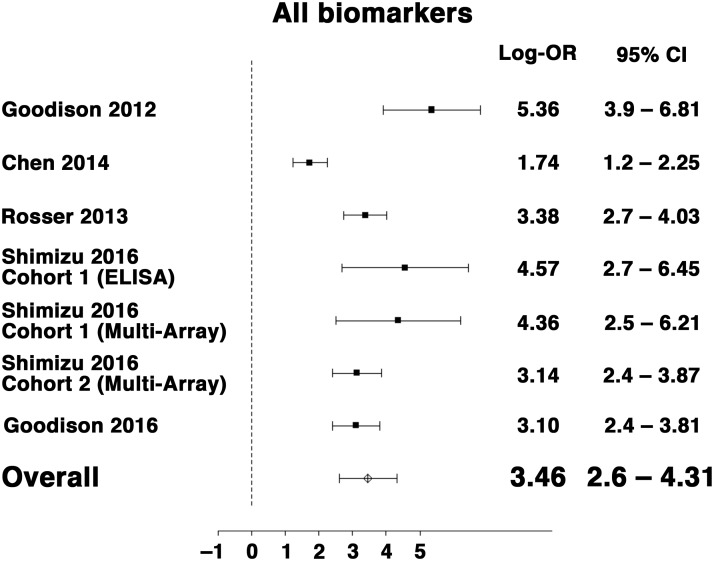
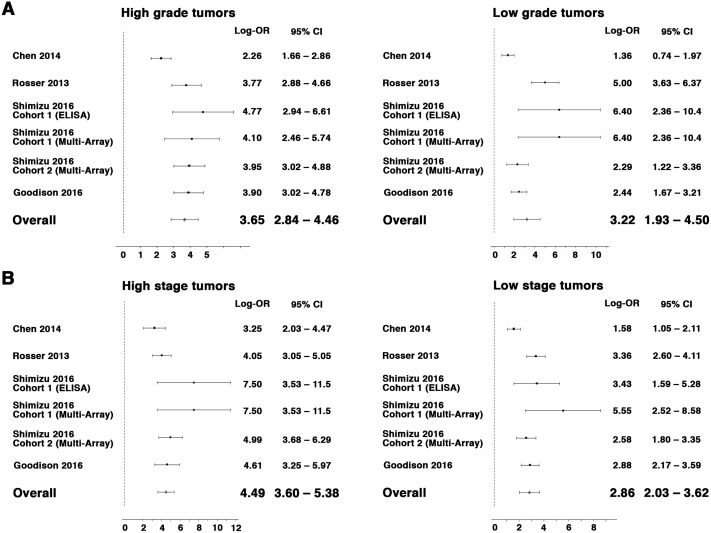
As shown in Figure 2, the log OR for the combination of the ten urinary protein biomarkers (n = 1,295, log OR: 3.46, 95% CI: 2.60–4.31), ranged from 1.74 to 5.36 depending on the report confirming the utility of the ten protein biomarkers in detecting BCa from a urine sample. Furthermore, advantage of the combination of the ten urinary protein biomarkers was robust when it was analyzed with regard to high-grade (log OR: 3.65, 95% CI: 2.84–4.46) and low-grade (log OR: 3.22, 95% CI: 1.93–4.50) disease as well as with regard to high stage (T2 or greater, log OR: 4.49, 95% CI: 3.60–5.38) and low stage (Ta/T1, log OR: 2.86, 95% CI: 2.03–3.62) disease (Figure 3).
Figure 2. Forest plot for random-effects meta-analysis of the association between multiplex BCa biomarkers and the outcome of detecting BCa from voided urines (any stage or grade, n = 1,295).
Effect sizes are expressed as odds ratios. Studies are represented by symbols whose area is proportional to the weight of the study in the analysis.
Figure 3.
Forest plot for random-effects meta-analysis of the association between multiplex BCa biomarkers and tumor grade (A, high-grade, left panel, low-grade, right panel) and tumor stage (B, T2 or greater stage, left panel and Ta/T1 stage, right panel) (n = 1,173). Effect sizes are expressed as odds ratios. Studies are represented by symbols whose area is proportional to the weight of the study in the analysis.
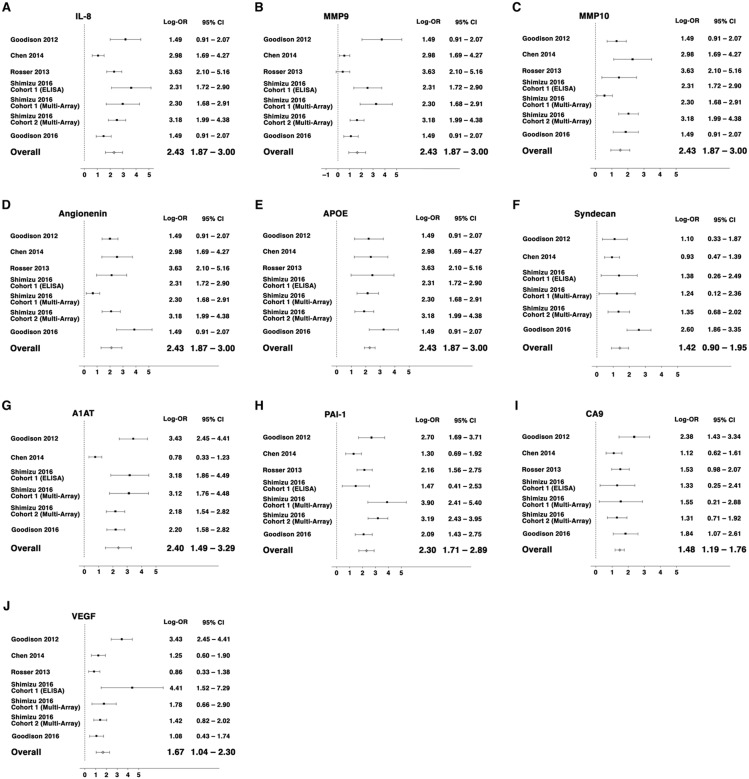
The log OR for each biomarker was improved by 1.5 or greater with smaller 95% CI in our meta-analysis of the overall cohort compared with each analysis of an individual cohort. A1AT (log OR: 2.40, 95% CI: 1.49–3.29), PAI1 (log OR: 2.30, 95% CI: 1.71–2.89) and IL-8 (log OR: 2.29, 95% CI: 1.63–2.96) showed the highest log OR, while MMP10 (log OR: 1.36, 95% CI: 0.87–1.85) showed the lowest (Figure 4).
Figure 4. Forest plots for random-effects meta-analysis of the association between individual BCa biomarkers and the outcome of detecting BCa from voided urines (any stage or grade, n = 1,295).
Effect sizes are expressed as odds ratios. Studies are represented by symbols whose area is proportional to the weight of the study in the analysis.
DISCUSSION
A successful meta-analysis allows compiling data from previous studies, thus elevating the robustness and the level of evidence from the single studies. In the present study, indeed, we demonstrated that the combination of 10 urine-based biomarkers was more strongly associated with BCa than was any single biomarker. The finding is in agreement with other studies. For example, other investigators have employed capillary electrophoresis coupled with mass spectrometry (CE-MS), followed by support vector machine algorithms [28], to develop diagnostic models for BCa [19–21] and other diseases [29, 30]. In these previous reports, panels of multiple protein biomarkers exhibited diagnostic accuracy superior to any single protein biomarker.
The urine-based protein biomarkers analyzed in the present study were originally established as a panel of 14 protein biomarkers [11] using a bioinformatics approach integrating information from genomics [31] and proteomics [32, 33] analyses. Subsequent studies streamlined this into a panel of 10 protein biomarkers on the ELISA platform [12, 13, 16]. More recently, a custom electrochemiluminescent multiplex platform was developed [14] and validated [15] to facilitate quick and high-throughput analysis of all 10 protein biomarkers simultaneously in a single assay without loss of performance.
Currently, published guidelines recommend that patients presenting with hematuria undergo VUC and examination using cystoscopy [5, 34–36], an invasive, uncomfortable and expensive procedure associated with possible adverse effects. We believe that the employment of a multiplex, proteomic urinary assay can reduce the need to subject large numbers of patients who do not have BCa to uncomfortable and expensive cystoscopic examinations and thus ‘rule-in’ patients who require a more thorough evaluation. The 10-plex proteomic assay evaluated in this study is currently being tested in a phase III study in the US for both detection and surveillance.
As for influence of other diseases, the urine-based protein biomarkers have been already assessed in patients with other genitourinary malignancies and renal disorders, e.g., prostate cancer, kidney cancer and chronic kidney disease. There was limited overlap of the biomarkers in prostate cancer (only IL-8 was elevated) and kidney cancer (only CA9 and VEGF were elevated). In chronic kidney disease, i.e., GFR < 45 mL/min, significant amounts of proteins were evident in the urine and thus the assay is unable to accurately discriminate if a patient has cancer (data not shown). Urinary tract infection (UTI) is another coincidence that can negatively affect the diagnostic performance of urine-based biomarkers. The present study included 96 subjects with UTI and the 10-plex panel as well as most of the single markers showed better performance with the subjects excluded from the analysis (data not shown). These findings suggest that the 10-plex panel is anticipated to yield an excellent performance in a cohort including those with UTI although it should be applied to those subjects with caution.
Several limitations of this study must be acknowledged. Although targets in all included studies were quantitatively measured, the antibodies used to monitor each urine-based biomarker were not identical among the included studies. The present study did not incorporate detailed data such as race, gender, age, and smoking history, which has been reported to influence diagnostic performance of the multiplex urinary protein panel [22]. Since all included studies were case-control designs, it is unclear whether the diagnostic accuracy will be reproducible in clinically relevant cohorts such as consecutive individuals who are referred with hematuria, or those on post-TUR surveillance for intravesical recurrence of BCa, in which the prevalence of BCa may be different from those in the included studies. It is not clear whether the replacement of cystoscopy by the 10-plex assay is cost-effective or not, since the cost of the 10-plex assay is yet to be determined. Despite these limitations, this study emphasizes the potential of a multiplex urinary protein assay and justifies the advancement of the assay to the next phase of the developmental stages of urinary biomarkers for BCa detection, proposed by the International Bladder Cancer Network [37, 38].
In conclusion, our meta-analysis confirmed significant association between urinary levels of the protein biomarkers and BCa detection. In particular, the combination of the ten biomarkers demonstrated a higher potential for detection of BCa than did any single biomarker. The study has justified further advancement of the multiplex urinary protein biomarker assay toward clinical application as a noninvasive method of detecting BCa in our daily practice. However, further validation steps including analyses of consecutive patients are needed before clinical adoption [39].
MATERIALS AND METHODS
Database search
An additional search was conducted using Medline and Embase using the following urinary biomarkers for BCa in the search bar: ANG, APOE, A1AT, CA9, IL8, MMP9, MMP10, PAI1, SDC1, and VEGF. The following additional filters were selected: “Publication dates from January 1, 2012 to December 31, 2016” and studies in “Humans.” Studies assessing the biomarker panel in subjects for the purpose of tumor surveillance were excluded. Similarly, studies not describing the 10 biomarkers in a multiplex format for the diagnosis of BCa were excluded. Eventually no article was found in addition to the five studies that we initially selected.
Meta-analysis
We performed a meta-analysis using a random-effect model followed by multivariable-pooled analysis of the molecular data using the weighted least-squares method to account for size effects. Random-effect meta-regression models (linear mixed models) were used to assess the relationship between the estimates and the outcome (BCa vs. no BCa), adjusted for other potential confounders and/or mediators, as appropriate. Note that the weighted least-squares method under the multivariable-pooled analysis can better overcome small-sample-size bias, whereas the random-effect meta-regression model can better overcome between- and within-study heterogeneity. Both methods were applied to generate the most robust results. Statistical analyses were performed using R version 3.2.3 and reviewed by Y.D.
ACKNOWLEDGMENTS AND FUNDING
T. Kobayashi received Grant-in-aid for Young Scientist (A) (Japanese Society for the Promotion of Science, # 25713055). C.J. Rosser received research grants from Weinman Foundation Fund, 5P30CA0717890-6071 (Investigator) and 1R01CA198887-01A1. S. Goodison received NIH/NCI R44CA173921.
Footnotes
CONFLICTS OF INTEREST
C.J. Rosser and S. Goodison are officers for Nonagen BioScience Corp. Norihiko Masuda, Osamu Ogawa, Meyeon Park, Alvin Y Liu, Yunfeng Dai, Landon Kozai, Hideki Furuya, Yair Lotan, and Takashi Kobayashi declare that they have no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.IARC GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2012 http://globocan.iarc.fr/Default.aspx.
- 3.Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, Guo CC, Lotan Y, Kassouf W. Bladder cancer. Lancet. 2016;388:2796–2810. doi: 10.1016/S0140-6736(16)30512-8. [DOI] [PubMed] [Google Scholar]
- 4.Calabro F, Sternberg CN. Metastatic bladder cancer: anything new? Curr Opin Support Palliat Care. 2012;6:304–309. doi: 10.1097/SPC.0b013e3283552d19. [DOI] [PubMed] [Google Scholar]
- 5.Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD, Skinner EC, Smith ND, McKiernan JM. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196:1021–1029. doi: 10.1016/j.juro.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 6.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Algaba F, Vicente-Rodriguez J. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164:680–684. doi: 10.1016/s0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- 7.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 8.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 9.D’Costa JJ, Goldsmith JC, Wilson JS, Bryan RT, Ward DG. A systematic review of the diagnostic and prognostic value of urinary protein biomarkers in urothelial bladder cancer. Bladder Cancer. 2016;2:301–317. doi: 10.3233/BLC-160054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lokeshwar VB, Habuchi T, Grossman HB, Murphy WM, Hautmann SH, Hemstreet GP, 3rd, Bono AV, Getzenberg RH, Goebell P, Schmitz-Drager BJ, Schalken JA, Fradet Y, Marberger M, et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology. 2005;66:35–63. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 11.Goodison S, Chang M, Dai Y, Urquidi V, Rosser CJ. A multi-analyte assay for the non-invasive detection of bladder cancer. PLoS One. 2012;7:e47469. doi: 10.1371/journal.pone.0047469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosser CJ, Ross S, Chang M, Dai Y, Mengual L, Zhang G, Kim J, Urquidi V, Alcaraz A, Goodison S. Multiplex protein signature for the detection of bladder cancer in voided urine samples. J Urol. 2013;190:2257–2262. doi: 10.1016/j.juro.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LM, Chang M, Dai Y, Chai KX, Dyrskjot L, Sanchez-Carbayo M, Szarvas T, Zwarthoff EC, Lokeshwar V, Jeronimo C, Parker AS, Ross S, Borre M, et al. External validation of a multiplex urinary protein panel for the detection of bladder cancer in a multicenter cohort. Cancer Epidemiol Biomarkers Prev. 2014;23:1804–1812. doi: 10.1158/1055-9965.EPI-14-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu Y, Furuya H, Bryant Greenwood P, Chan O, Dai Y, Thornquist MD, Goodison S, Rosser CJ. A multiplex immunoassay for the non-invasive detection of bladder cancer. J Transl Med. 2016;14:31. doi: 10.1186/s12967-016-0783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodison S, Ogawa O, Matsui Y, Kobayashi T, Miyake M, Ohnishi S, Fujimoto K, Dai Y, Shimizu Y, Tsukikawa K, Furuya H, Rosser CJ. A multiplex urinary immunoassay for bladder cancer detection: analysis of a Japanese cohort. J Transl Med. 2016;14:287. doi: 10.1186/s12967-016-1043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosser CJ, Chang M, Dai Y, Ross S, Mengual L, Alcaraz A, Goodison S. Urinary protein biomarker panel for the detection of recurrent bladder cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:1340–1345. doi: 10.1158/1055-9965.EPI-14-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YT, Chen HW, Domanski D, Smith DS, Liang KH, Wu CC, Chen CL, Chung T, Chen MC, Chang YS, Parker CE, Borchers CH, Yu JS. Multiplexed quantification of 63 proteins in human urine by multiple reaction monitoring-based mass spectrometry for discovery of potential bladder cancer biomarkers. J Proteomics. 2012;75:3529–3545. doi: 10.1016/j.jprot.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Kumar P, Nandi S, Tan TZ, Ler SG, Chia KS, Lim WY, Butow Z, Vordos D, De la Taille A, Al-Haddawi M, Raida M, Beyer B, Ricci E, et al. Highly sensitive and specific novel biomarkers for the diagnosis of transitional bladder carcinoma. Oncotarget. 2015;6:13539–13549. doi: 10.18632/oncotarget.3841. https://doi.org/10.18632/oncotarget.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frantzi M, van Kessel KE, Zwarthoff EC, Marquez M, Rava M, Malats N, Merseburger AS, Katafigiotis I, Stravodimos K, Mullen W, Zoidakis J, Makridakis M, Pejchinovski M, et al. Development and validation of urine-based peptide biomarker panels for detecting bladder cancer in a multi-center study. Clin Cancer Res. 2016;22:4077–4086. doi: 10.1158/1078-0432.CCR-15-2715. [DOI] [PubMed] [Google Scholar]
- 20.Theodorescu D, Wittke S, Ross MM, Walden M, Conaway M, Just I, Mischak H, Frierson HF. Discovery and validation of new protein biomarkers for urothelial cancer: a prospective analysis. Lancet Oncol. 2006;7:230–240. doi: 10.1016/S1470-2045(06)70584-8. [DOI] [PubMed] [Google Scholar]
- 21.Schiffer E, Vlahou A, Petrolekas A, Stravodimos K, Tauber R, Geschwend JE, Neuhaus J, Stolzenburg JU, Conaway MR, Mischak H, Theodorescu D. Prediction of muscle-invasive bladder cancer using urinary proteomics. Clin Cancer Res. 2009;15:4935–4943. doi: 10.1158/1078-0432.CCR-09-0226. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Kou L, Furuya H, Yu C, Goodison S, Kattan MW, Garmire L, Rosser CJ. A nomogram derived by combination of demographic and biomarker data improves the noninvasive evaluation of patients at risk for bladder cancer. Cancer Epidemiol Biomarkers Prev. 2016;25:1361–1366. doi: 10.1158/1055-9965.EPI-16-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosser CJ, Urquidi V, Goodison S. Urinary biomarkers of bladder cancer: an update and future perspectives. Biomark Med. 2013;7:779–790. doi: 10.2217/bmm.13.73. [DOI] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 25.Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch M, Losos M, Tugnell P. Newcastle-Otawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. 2014 http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 26.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG. Standards for Reporting of Diagnostic Accuracy. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem. 2003;49:7–18. doi: 10.1373/49.1.7. [DOI] [PubMed] [Google Scholar]
- 27.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC, STARD Group Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Fam Pract. 2004;21:4–10. doi: 10.1093/fampra/cmh103. [DOI] [PubMed] [Google Scholar]
- 28.Dakna M, He Z, Yu WC, Mischak H, Kolch W. Technical, bioinformatical and statistical aspects of liquid chromatography-mass spectrometry (LC-MS) and capillary electrophoresis-mass spectrometry (CE-MS) based clinical proteomics: a critical assessment. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1250–1258. doi: 10.1016/j.jchromb.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 29.Frantzi M, Metzger J, Banks RE, Husi H, Klein J, Dakna M, Mullen W, Cartledge JJ, Schanstra JP, Brand K, Kuczyk MA, Mischak H, Vlahou A, et al. Discovery and validation of urinary biomarkers for detection of renal cell carcinoma. J Proteomics. 2014;98:44–58. doi: 10.1016/j.jprot.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Jantos-Siwy J, Schiffer E, Brand K, Schumann G, Rossing K, Delles C, Mischak H, Metzger J. Quantitative urinary proteome analysis for biomarker evaluation in chronic kidney disease. J Proteome Res. 2009;8:268–281. doi: 10.1021/pr800401m. [DOI] [PubMed] [Google Scholar]
- 31.Urquidi V, Goodison S, Cai Y, Sun Y, Rosser CJ. A candidate molecular biomarker panel for the detection of bladder cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:2149–2158. doi: 10.1158/1055-9965.EPI-12-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang N, Feng S, Shedden K, Xie X, Liu Y, Rosser CJ, Lubman DM, Goodison S. Urinary glycoprotein biomarker discovery for bladder cancer detection using LC/MS-MS and label-free quantification. Clin Cancer Res. 2011;17:3349–3359. doi: 10.1158/1078-0432.CCR-10-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreunin P, Zhao J, Rosser C, Urquidi V, Lubman DM, Goodison S. Bladder cancer associated glycoprotein signatures revealed by urinary proteomic profiling. J Proteome Res. 2007;6:2631–2639. doi: 10.1021/pr0700807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamat AM, Hegarty PK, Gee JR, Clark PE, Svatek RS, Hegarty N, Shariat SF, Xylinas E, Schmitz-Drager BJ, Lotan Y, Jenkins LC, Droller M, van Rhijn BW, et al. screening, diagnosis, and molecular markers. ICUD-EAU International Consultation on Bladder Cancer. Eur Urol. 2012;2013;63:4–15. doi: 10.1016/j.eururo.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 35.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, Sylvester RJ, Kaasinen E, Bohle A, Palou Redorta J, Roupret M. European Association of Urology. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, Inman BA, Kuban DA, Kuzel TM, Lele SM, Michalski J, Pagliaro LC, Pal SK, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11:446–475. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 37.Goebell PJ, Groshen S, Schmitz-Drager BJ, Sylvester R, Kogevinas M, Malats N, Sauter G, Barton Grossman H, Waldman F, Cote RJ. The International Bladder Cancer Bank: proposal for a new study concept. Urol Oncol. 2004;22:277–284. doi: 10.1016/S1078-1439(03)00175-3. [DOI] [PubMed] [Google Scholar]
- 38.Goebell PJ, Groshen SL, Schmitz-Drager BJ. Guidelines for development of diagnostic markers in bladder cancer. World J Urol. 2008;26:5–11. doi: 10.1007/s00345-008-0240-9. [DOI] [PubMed] [Google Scholar]
- 39.D’Costa JJ, Ward DG, Bryan RT. Urinary biomarkers for the diagnosis of urothelial bladder cancer. New Horiz Transl Med. 2017;3:221–223. [Google Scholar]
- 40.Miyake M, Ross S, Lawton A, Chang M, Dai Y, Mengual L, Alcaraz A, Giacoia EG, Goodison S, Rosser CJ. Investigation of CCL18 and A1AT as potential urinary biomarkers for bladder cancer detection. BMC Urol. 2013;13:42. doi: 10.1186/1471-2490-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shabayek MI, Sayed OM, Attaia HA, Awida HA, Abozeed H. Diagnostic evaluation of urinary angiogenin (ANG) and clusterin (CLU) as biomarker for bladder cancer. Pathol Oncol Res. 2014;20:859–866. doi: 10.1007/s12253-014-9765-y. [DOI] [PubMed] [Google Scholar]
- 42.Chen YT, Chen CL, Chen HW, Chung T, Wu CC, Chen CD, Hsu CW, Chen MC, Tsui KH, Chang PL, Chang YS, Yu JS. Discovery of novel bladder cancer biomarkers by comparative urine proteomics using iTRAQ technology. J Proteome Res. 2010;9:5803–5815. doi: 10.1021/pr100576x. [DOI] [PubMed] [Google Scholar]
- 43.Korman HJ, Peabody JO, Cerny JC, Farah RN, Yao J, Raz A. Autocrine motility factor receptor as a possible urine marker for transitional cell carcinoma of the bladder. J Urol. 1996;155:347–349. [PubMed] [Google Scholar]
- 44.Bhagirath D, Abrol N, Khan R, Sharma M, Seth A, Sharma A. Expression of CD147, BIGH3 and Stathmin and their potential role as diagnostic marker in patients with urothelial carcinoma of the bladder. Clin Chim Acta. 2012;413:1641–1646. doi: 10.1016/j.cca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Ebbing J, Mathia S, Seibert FS, Pagonas N, Bauer F, Erber B, Gunzel K, Kilic E, Kempkensteffen C, Miller K, Bachmann A, Rosenberger C, Zidek W, Westhoff TH. Urinary calprotectin: a new diagnostic marker in urothelial carcinoma of the bladder. World J Urol. 2014;32:1485–1492. doi: 10.1007/s00345-013-1227-8. [DOI] [PubMed] [Google Scholar]
- 46.Svatek RS, Karam J, Karakiewicz PI, Gallina A, Casella R, Roehrborn CG, Shariat SF. Role of urinary cathepsin B and L in the detection of bladder urothelial cell carcinoma. J Urol. 2008;179:478–484. doi: 10.1016/j.juro.2007.09.037. discussion 484. [DOI] [PubMed] [Google Scholar]
- 47.Tilki D, Singer BB, Shariat SF, Behrend A, Fernando M, Irmak S, Buchner A, Hooper AT, Stief CG, Reich O, Ergun S. CEACAM1: a novel urinary marker for bladder cancer detection. Eur Urol. 2010;57:648–654. doi: 10.1016/j.eururo.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 48.Hazzaa SM, Elashry OM, Afifi IK. Clusterin as a diagnostic and prognostic marker for transitional cell carcinoma of the bladder. Pathol Oncol Res. 2010;16:101–109. doi: 10.1007/s12253-009-9196-3. [DOI] [PubMed] [Google Scholar]
- 49.Nakashima M, Matsui Y, Kobayashi T, Saito R, Hatahira S, Kawakami K, Nakamura E, Nishiyama H, Ogawa O. Urine CXCL1 as a biomarker for tumor detection and outcome prediction in bladder cancer. Cancer Biomark. 2015;15:357–364. doi: 10.3233/CBM-150472. [DOI] [PubMed] [Google Scholar]
- 50.Burnier A, Shimizu Y, Dai Y, Nakashima M, Matsui Y, Ogawa O, Rosser CJ, Furuya H. CXCL1 is elevated in the urine of bladder cancer patients. Springerplus. 2015;4:610. doi: 10.1186/s40064-015-1393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nisman B, Barak V, Shapiro A, Golijanin D, Peretz T, Pode D. Evaluation of urine CYFRA 21-1 for the detection of primary and recurrent bladder carcinoma. Cancer. 2002;94:2914–2922. doi: 10.1002/cncr.10565. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez-Carbayo M, Espasa A, Chinchilla V, Herrero E, Megias J, Mira A, Soria F. New electrochemiluminescent immunoassay for the determination of CYFRA 21-1: analytical evaluation and clinical diagnostic performance in urine samples of patients with bladder cancer. Clin Chem. 1999;45:1944–1953. [PubMed] [Google Scholar]
- 53.Fernandez-Gomez J, Rodriguez-Martinez JJ, Barmadah SE, Garcia Rodriguez J, Allende DM, Jalon A, Gonzalez R, Alvarez-Mugica M. Urinary CYFRA 21.1 is not a useful marker for the detection of recurrences in the follow-up of superficial bladder cancer. Eur Urol. 2007;51:1267–1274. doi: 10.1016/j.eururo.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 54.Pariente JL, Bordenave L, Michel P, Latapie MJ, Ducassou D, Le Guillou M. Initial evaluation of CYFRA 21-1 diagnostic performances as a urinary marker in bladder transitional cell carcinoma. J Urol. 1997;158:338–341. [PubMed] [Google Scholar]
- 55.Morgan R, Bryan RT, Javed S, Launchbury F, Zeegers MP, Cheng KK, James ND, Wallace DM, Hurst CD, Ward DG, Knowles MA, Pandha H. Expression of Engrailed-2 (EN2) protein in bladder cancer and its potential utility as a urinary diagnostic biomarker. Eur J Cancer. 2013;49:2214–2222. doi: 10.1016/j.ejca.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 56.Ramakumar S, Bhuiyan J, Besse JA, Roberts SG, Wollan PC, Blute ML, O’Kane DJ. Comparison of screening methods in the detection of bladder cancer. J Urol. 1999;161:388–394. [PubMed] [Google Scholar]
- 57.Mutlu N, Turkeri L, Emerk K. Analytical and clinical evaluation of a new urinary tumor marker: bladder tumor fibronectin in diagnosis and follow-up of bladder cancer. Clin Chem Lab Med. 2003;41:1069–1074. doi: 10.1515/CCLM.2003.165. [DOI] [PubMed] [Google Scholar]
- 58.Li LY, Yang M, Zhang HB, Su XK, Xu WF, Chen Y, Shen ZJ, Gao X. Urinary fibronectin as a predictor of a residual tumour load after transurethral resection of bladder transitional cell carcinoma. BJU Int. 2008;102:566–571. doi: 10.1111/j.1464-410X.2008.07637.x. [DOI] [PubMed] [Google Scholar]
- 59.Orenes-Pinero E, Corton M, Gonzalez-Peramato P, Algaba F, Casal I, Serrano A, Sanchez-Carbayo M. Searching urinary tumor markers for bladder cancer using a two-dimensional differential gel electrophoresis (2D-DIGE) approach. J Proteome Res. 2007;6:4440–4448. doi: 10.1021/pr070368w. [DOI] [PubMed] [Google Scholar]
- 60.Eissa S, Labib RA, Mourad MS, Kamel K, El-Ahmady O. Comparison of telomerase activity and matrix metalloproteinase-9 in voided urine and bladder wash samples as a useful diagnostic tool for bladder cancer. Eur Urol. 2003;44:687–694. doi: 10.1016/s0302-2838(03)00417-2. [DOI] [PubMed] [Google Scholar]