Highlights
-
•
Ghrelin exerts broad pharmacological effects on systems metabolism.
-
•
Ghrelin regulates glucose metabolism and insulin secretion in a variety of species.
-
•
Ghrelin inhibition has pharmacological potential for the treatment of T2DM.
Keywords: Ghrelin, Glucose metabolism, Insulin sensitivity, Diabetes, Diet-induced obesity
Abstract
The a 28-amino acid peptide ghrelin was discovered in 1999 as a growth hormone (GH) releasing peptide. Soon after its discovery, ghrelin was found to increase body weight and adiposity by acting on the hypothalamic melanocortinergic system. Subsequently, ghrelin was found to exert a series of metabolic effects, overall testifying ghrelin a pleiotropic nature of broad pharmacological interest. Ghrelin acts through the growth hormone secretagogue-receptor (GHS-R), a seven transmembrane G protein-coupled receptor with high expression in the anterior pituitary, pancreatic islets, thyroid gland, heart and various regions of the brain. Among ghrelins numerous metabolic effects are the most prominent the stimulation of appetite via activation of orexigenic hypothalamic neurocircuits and the food-intake independent stimulation of lipogenesis, which both together lead to an increase in body weight and adiposity. Ghrelin effects beyond the regulation of appetite and GH secretion include the regulation of gut motility, sleep-wake rhythm, taste sensation, reward seeking behaviour, and the regulation of glucose metabolism. The latter received recently increasing recognition because pharmacological inhibition of ghrelin signaling might be of therapeutic value to improve insuin resistance and type 2 diabetes. In this review we highlight the multifaceted nature of ghrelin and summarize its glucoregulatory action and discuss the pharmacological value of ghrelin pathway inhibition for the treatment of glucose intolerance and type 2 diabetes.
1. Introduction
Obesity and diabetes are major health threats of our society, leading annually to more than 1.5 million casualties [1]. The obesity pandemic affects nowadays almost every culture and ethnic civilization, placing an enormous burden on modern health care systems. From the numerous co-morbidities associated with excess body fat are the most prominent type 2 diabetes, cardiovasclar diseases and certain types of, predominantly gastrointestinal, cancer [2,3]. Underscoring the relevance of adequate glucose buffering, type 2 diabetes represents as of today the most frequent cause of overweight-related death [4]. In line with obesity being the major risk factor for the development of type 2 diabetes, weight loss achieved by either dieting [5] or through pharmacology [6] or bariatric surgery [7,8] improves glucose handling and numerous clinical studies have demonstrated that placebo-subtracted weight loss in the magnitute of even 5% is sufficient to show meaningful improvements in systemic glucose metabolism and of other obesity linked co-morbidities [[9], [10], [11], [12]]. Further underlining the direct relation between body weight and glucose control, weight loss induced by bariatric surgery most often results in complete resolution of type 2 diabetes, an observation that prompted the American Diabetes Association (ADA) to even recommend such surgical intervention under certain circumstances for the treatment of type 2 diabetes [[13], [14], [15]]. Since the correlation between body weight and glucose control is solidly confirmed by numerous preclinical and clinical studies [16,17], drugs to control body weight appear intuitively promising to also improve glucose metabolism. In line with this notion, prominent examples of such strategy is e.g. the administration of GLP-1 mimetics, which not only improve glycemic control via their insulinotropic action but that also indirectly improve glucose metabolism via their ability to decrease body weight through central regulation of food intake [[18], [19], [20], [21]]. While a plethora of weight lowering drugs have been shown to offer beneficial effects on glycemia, including GLP-1 mimetics [22], thyroid hormone [23,24], amphetamines [25], serotonergics [26] or lipase inhibitors [27], hormones with the ability to increase body weight are commonly known to rather impair glucose metabolism. A prominent example of the latter is the gut-derived peptide hormone ghrelin, which increases body weight and body fat mass via activation of orexigenic hypothalamic neurocircuits and via food-intake independent stimulation of lipogenesis [[28], [29], [30], [31]]. In this manuscript we will summarize the multifaceted nature of ghrelin with a special focus on its role to regulate glucose metabolism. A key central aspect is thereby be the question of whether blocking of ghrelin signaling might be of therapeutic value to improve glucose metabolism?
2. Ghrelin production, activation and degradation
Ghrelin is derived from preproghrelin, a 117 amino-acid precursor that is produced by X/A-like cells within gastric oxyntic glands of the stomach [32]. Preproghrelin is cleaved into a small signal peptide, ghrelin and obestatin. Obestatin has previously been thought to play a role in food intake via acting on the G protein-coupled receptor 39 (GPR39) but this was not supported by all studies [33,34]. Cleaved from preproghrelin, the 28 amino acid peptide ghrelin is highly conserved among species with only two amino acids differing between the rat and human peptide [35].
Ghrelin promotes its biological action via binding to the growth hormone secretagogue receptor 1a (GHSR1a), a seven transmembrane G protein-coupled receptor with highest expression in the pituitary, pancreatic islets, adrenals, thyroid gland, the myocardium, the hypothalamic arcuate nucleus (ARC), hippocampus, the substantia nigra pars compacta (SNpc), the ventral tegmental area (VTA), and raphe nuclei [36,37]. In the feeding center of the hypothalamus, GHSR1a is localized in neurons that express neuropeptide Y (Npy) and Agouti related peptide (Agrp), well known neuropeptides stimulating food intake [38]. GHSR1 is present in two forms, the long form (GHSR1a), which is mediating most, if not all, of acyl-ghrelins metabolic effects and a truncated form, GHSR1b [36].
To activate its only known receptor, ghrelin needs to be post-translationally modified (acylated) to carry a fatty acid, preferably C:8 or C:10, on its third N-terminal amino acid position, which is a serine [35]. This rare post-translational modification is achieved by the ghrelin O-acyl-transferase (GOAT), a member of the membrane bound O acyltransferase (MBOAT) family [39,40]. GOAT is essential to acylate ghrelin in vivo, as demonstrated by the absence of acyl-ghrelin in plasma of mice deficient for GOAT [39,[41], [42], [43], [44]].
The reported half-life of acyl-ghrelin varies between 30 min in rats to 240 min in humans [45]. Reflecting the species-related differences in ghrelin degradation, butyrylcholinesterase is the main enzyme inactivating ghrelin in humans whereas in rodents carboxylesterases allow for an 8-times faster des-octanoylation of ghrelin [45].
3. Physiological effects of unacylated ghrelin
While substantial evidence indicates that most metabolic effects of ghrelin require acylation of the peptide, there is accumulating evidence suggesting that also des-acyl ghrelin has physiologically relevant effects on systems metabolism, potentially via a receptor that yet still needs to be identified. In line with this notion, desacyl ghrelin affects differentiation of C2C12 skeletal muscle cells [46], prevents muscle atrophy [47], has protective effects on the heart [48,49] and affects glucose metabolism via pathways that are independent of GHSR1 [[50], [51], [52]]. When injected directly into the third ventricle of the hypothalamus, des-acyl ghrelin seems to acutely stimulate food intake through mechanisms that are independent of GHSR1a and Npy signaling [51]. When injected into the periphery, des-acyl ghrelin is either reported to not affect food intake [51] or to even decrease food intake [53]. Nevertheless, mice overexpressing des-acyl ghrelin under control of the FABP4 promoter seem to be protected from diet-induced obesity and show reduced body fat mass when fed with a standard chow diet [52]. These data align with a growing body of evidence testifying des-acyl ghrelin a certain potential to prevent diet-induced obesity and to improve HFD-induced derangements in glucose and lipid metabolism [54,55]. Interestingly, the glycemic effects of ghrelin to increase blood glucose through inhibition of insulin secretion seems to be antagonized by co-administration of des-acyl ghrelin [56]. Despite not supported by all studies [57], also several human studies report positive effects of des-acyl ghrelin on insulin sensitivity [58,59]. In line with this notion, there is recent evidence suggesting that des-acyl ghrelin promotes survival of pancreatic β-cells and protects from streptozotocin-induced β-cell damage [[60], [61], [62], [63]].
4. Ghrelins effects beyond the stimulation of food intake
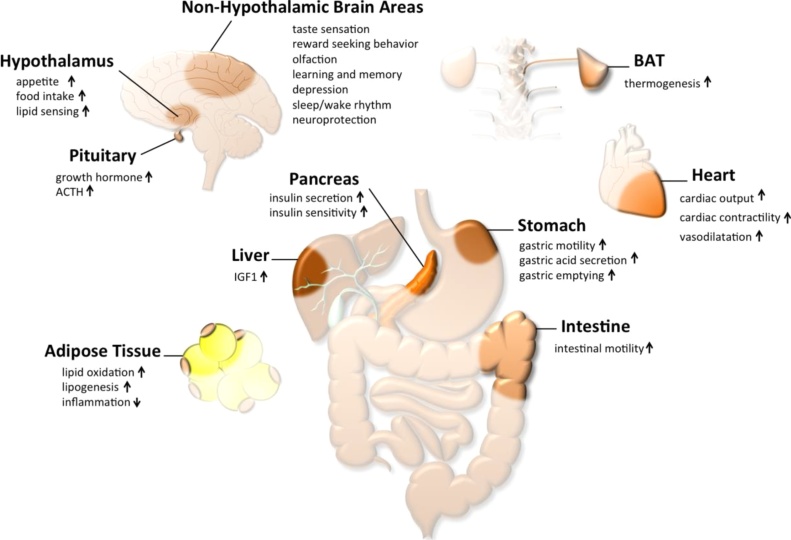
The most prominent effect of ghrelin is its ability to stimulate food intake via activation of hypothalamic neurocircuits [28]. In line with this notion, in the hypothalamic arcuate nucleus (ARC), ghrelin increases the activity of neurons expressing neuropeptide y (Npy) and the agouti-related protein (Agrp) while at the same time inhibiting neurons that express proopiomelanocortin (Pomc) [29,38]. Ghrelin signaling via these neurons is essential for ghrelins orexigenic effect since ghrelin fails to increase food intake in mice lacking Npy and Agrp [64]. Intracerebroventricular (icv) injection of ghrelin further increases food intake in rats, but fails to do so when NPY and AgRP neurons were blocked [65], further underlining the importance of the hypothalamic melanocortinergic system. In line with its effect on the melanocortinergic system, a ying yang balance between ghrelin and leptin has been suggested and ghrelin accordingly seems to counteract food intake inhibition by leptin [66]. Beside its ability to stimulate food intake, ghrelin activates gastric emptying and motility, as well as gastric acid secretion (Fig. 1) [67,68]. Ghrelin further modulates food reward and taste sensation, increases locomotor activity, motivation towards food reward, and enhances olfactory sensitivity [[69], [70], [71], [72], [73], [74]]. As a pulsatile hormone, ghrelin is also involved in sleep regulation as suggested by different studies [[75], [76], [77]].
Fig. 1.
Schematic on physiological effects of acyl-ghrelin.
Acutely, ghrelin seems to induce anxiolytic and anti-depressant like effects in mice, most likely via stimulating the activity of the HPA axis [78,79]. Under stress, also the preference for HFD seems to be affected by ghrelin signaling [80]. Collectively, these data suggest a role for ghrelin in sleep regulation, stress and depression. Ghrelin also enhances differentiation and fusion of skeletal muscles cells in vitro and impairs skeletal muscle atrophy in mice [46,47]. Ghrelin further increases myocardial contractility, has a protective effect on the heart, and plays a role in atherogenesis [81]. Acute or chronic administration of ghrelin improves left ventricular (LV) dysfunction, and limits LV abnormal development in patients with chronic heart failure. Ghrelin also increases exercise capacity in both rats and humans [82,83]. In healthy humans, forearm blood flow is further increased by ghrelin, suggesting also a role in vasodilatation [84].
Effects on energy expenditure are frequently reported upon administration of ghrelin. Single peripheral or central (icv) injection of ghrelin suppresses BAT sympathetic nerve activity, thereby decreasing BAT temperature via CNS-dependent mechanisms [85,86]. Chronic ghrelin treatment further decreases Ucp1 mRNA expression in the BAT [87]. Corroborating a role of ghrelin in regulating BAT function, mice lacking ghrelin or administration of GHSR antisense mRNA increases BAT activity [88,89].
5. Preclinical studies on ghrelins role in glucose metabolism
Numerous studies have evaluated ghrelins effects on glucose metabolism (as reviewed in [30]). Ghrelin inhibition of insulin secretion has been shown in a variety of species including mice [90], rats [91], pigs [92] monkeys [93,94] and humans [95]. In line with ghrelins ability to decrease insulin release in vivo, levels of blood glucose are typically decreased in mice lacking either ghrelin or GHSR relative to wildtype controls [96]. When exposed to a HFD, mice deficient for ghrelin or its receptor show a better glucose tolerance and insulin sensitivity when compared to wildtype controls [97,98]. Underling ghrelins role in glucose metabolism, ghrelin deletion in ob/ob mice decreases hyperglycemia and enhances glucose-induced insulin secretion, thereby improving insulin sensitivity in peripheral tissues relative to ob/ob controls [99].
The endocrine pancreas comprises four main cell types, the glucagon-producing α-cells, the insulin-prducing β-cells, the somatostatin producing δ-cells and the pancreatic polypeptide producing PP-cells [100,101]. Notably, a fifth endocrine cell type, the ghrelin-producing ε-cells have also been described [[102], [103], [104]] but their presence in mature adult islets remains subject of investigation [101]. Rats carrying a loss-of-function mutation in the cyclin-dependent kinase inhibitor p27 show an elevated number of ghrelin producing ε-cells, which coincides with increased food intake, higher fat mass and decreased glucose stimulation of insulin secretion [105]. In the pancreas, ghrelin is also produced in pancreatic α-cells [90,[106], [107], [108]] and blockade of pancreatic ghrelin enhances insulin secretion and prevents high-fat diet (HFD) induced glucose intolerance in mice [108,109]. Apart from ghrelin itself, also its receptor is expressed in the pancreatic α-cells and several lines of evidence suggests a role of ghrelin in affecting glucose metabolism not only by directly inhibiting glucose stimulation of insulin secretion but also via stimulation of α-cell glucagon secretion [110]. Supporting the glucoregulatory role of acyl-ghrelin, pharmacological inhibition of GOAT improves glycemic control and stimulates the release of insulin [41]. Despite not confirmed by all studies [111], the GOAT-ghrelin systems further seems to be essential for the prevention of hypoglycemia during extreme episodes of calorie restriction [43].
Ghrelins negative insulinotropic action is mediated by Gαi-dependent GHSR1a signaling in the beta-cells [112] and involves interaction with the somatostatin receptor subtype-5 (SST5) [113]. Counter-intuitively, beyond its role as a negative regulator of insulin secretion, ghrelin seems to also have protective effects on the β-cells under conditions of type 1 diabetes [114,115] despite evidence showing that levels of ghrelin decline with the onset of type 1 diabetes [116,117].
Ghrelins well confirmed glycemic effects suggest that pharmacological inhibition of ghrelin action might offer beneficial effects in the treatment of type 2 diabetes. In line with this notion, GHSR1a antagonism induces weight loss and improves glucose tolerance in rats, potentially via stimulation of glucose-dependent insulin secretion [118]. Similar results are reported from mice showing a MODY-type diabetes due to lack of the hepatocyte nuclear factor-1α (HNF1α). In these mice, pharmacological inhibition of ghrelin signaling by administration of the GHSR antagonist GHRP-6 improves glycemic control via restoration of insulin sensitivity [119]. Notably, GHSR1a shows a certain degree of intrinsic constitutive activity, potentially resulting in a certain degree of ligand-independent GHSR effects on glycemia [120,121]. Ghrelin receptor inverse agonists might thus be of pharmacological value to improve systems metabolism and administration of such GHSR inverse agonist has recently been shown to decrease body weight and adiposity and to improve glucose metabolism in zucker diabetic fatty (ZDF) rats [122]. Central icv administration of the GHSR1a inverse agonist [d-Arg1, d-Phe5, d-Trp7,9, Leu11]-substance P, was further shown to decrease food intake and body weight gain, supposedly via modulation of Npy expression [123].
Vaccination has been traditionally used to prevent infectious diseases, but the concept has over the last years been refined to also allow the pharmacological regulation of body weight. In line with this notion, rats vaccinated with ghrelin immunoconjugates display decreased body weight and adiposity due to lower food efficiency [124]. Another anti-obesity vaccine targeting the ghrelin system has recently been developed in mice. Ghrelin was here combined with a carrier protein (Pspa), which is normally used in pneumococcal vaccine. The vaccine was developed in a nanogel to allow intranasal administration and upon administration in mice, it decreases HFD-induced body weight gain both in wildtype mice and in ob/ob mice, thereby improving glucose tolerance and insulin sensitivity [125].
6. Clinical studies on ghrelins role in glucose metabolism
In line with a series of preclinical studies all testifying ghrelin a hyperglycemic nature due to inhibition of insulin secretion [90,[106], [107], [108]], 65 min of continuous ghrelin infusion in healthy human volunteers suppresses glucose-stimulated insulin secretion and impairs glucose tolerance [95]. These data are supported by a series of other studies overall demonstrating that plasma levels of glucose increase while insulin levels decrease following ghrelin administration [91,[126], [127], [128], [129]]. Notably, a link between ghrelin and insulin is also suggested by the fact that both hormones exhibit a reciprocal correlation over the day with insulin levels being high when ghrelin levels are low and vice versa [130,131]. Also epidemiological studies support the inverse relationship between ghrelin and indexes of impaired glucose tolerance and insulin resistance [132]. Single intravenous administration of ghrelin increases plasma glucose levels followed by drop in fasting insulin levels in lean [126] and obese subjects with or without polycystic ovarian syndrome [133], further supporting an inhibitory role of the ghrelin pathway in insulin secretion.
The Prader Willi syndrome (PWS) is a genetic disorder associated with the development of obesity. Patients with PWS are typically hyperphagic and show increased plasma levels of ghrelin [134,135], notably also relative to weight-matched non-PWS and lean subjects and both after fasting and post-prandially [134]. These data might indicate that hyperghrelinemia might underly the hyperphagia and obesity of PWS patients, suggesting that blocking of ghrelin action might be beneficial to decrease body weight and to improve glycemic control in these patients. In line with this notion AZP-531 (Alizé Pharmaceuticals), a stabilized peptide analog of unacylated ghrelin is in phase I clinical trials for the treatment of obesity in PWS patients and 14-day treatment of healthy and type 2 diabetic overweight/obese individuals with AZP-531 has recently been shown to decrease body weight and to improve glycemic control as indicated by decreased levels of Hba1c [136].
Pfizer recently developed a GHSR1a receptor antagonist, PF-05190457, that is currently in clinical evaluation for the treatment of T2D. This drug shows beneficial effect on glucose-dependent insulin secretion in vitro, and increases insulin secretion in isolated human islet [137,138]. Interestingly, PF-05190457 was stopped after the phase I clinical trials but nor for safety reasons [139] and it remains in clinical evaluation for the treatment of insomnia [140]. In summary, there is accumulating preclinical and clinical evidence overall supporting a beneficial effect of ghrelin pathway inhibition for the treatment of type 2 diabetes. Beyond ghrelin’s direct glucoregulatory role, it has to be noted that also body weight loss due to ghrelin pathway inhibition might offer a certain potential to secondarily further improve glucose handling.
7. Conclusion
The endogenous ghrelin system has over the last decade emerged as being implicated in a myriad of metabolic effects that go well beyond it’s initial classification as a hormone affecting food intake and GH secretion (Fig. 1). Along with ghrelins role in systemic metabolism, a variety of studies evaluated the therapeutic impact of ghrelin pathway modulation. While ghrelin agonism might offer potential to treat diabetic gastroparesis and anorexia associated with pathological underweight and cachexia [32], ghrelin receptor antagonism might be of therapeutic value to decrease body weight under certain conditions of obesity (as in patients with PWS) and also to improve glucose metabolism and type 2 diabetes. Interestingly, while ghrelins orexigenic effect is known for more than 1.5 decades, the peptide is always good for a surprise and it is not unlikely that other physiological effects of ghrelin are yet to be discovered.
Declaration of interest
The authors declare that there is no conflict of interest.
Acknowledgements
This work was supported by the Alexander von Humboldt Foundation, the Helmholtz Alliance ICEMED & the Helmholtz Initiative on Personalized Medicine iMed by Helmholtz Association, and the Helmholtz cross-program topic “Metabolic Dysfunction.” This work was further supported by grants from the German Research Foundation DFG-TS226/1-1, DFG-TS226/3-1, the European Research Council ERC AdG HypoFlam no. 695054 and the German Center for Diabetes Research (DZD e.V.).
References
- 1.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 3.Ebbeling C.B., Pawlak D.B., Ludwig D.S. Childhood obesity: public-health crisis common sense cure. Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 4.Collaborators G.B.D.O., Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raynor H.A., Davidson P.G., Burns H., Nadelson M.D.H., Mesznik S., Uhley V. Medical nutrition therapy and weight loss questions for the evidence analysis library prevention of type 2 diabetes project: systematic reviews. J. Acad. Nutr. Diet. 2017;117:1578–1611. doi: 10.1016/j.jand.2017.06.361. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava G., Apovian C.M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 2018;14:12–24. doi: 10.1038/nrendo.2017.122. [DOI] [PubMed] [Google Scholar]
- 7.Ardestani A., Rhoads D., Tavakkoli A. Insulin cessation and diabetes remission after bariatric surgery in adults with insulin-treated type 2 diabetes. Diabetes Care. 2015;38:659–664. doi: 10.2337/dc14-1751. [DOI] [PubMed] [Google Scholar]
- 8.Landau Z., Karplus G., Hanukoglu A., Abiri S., Levy A., Serour F. Laparoscopic sleeve gastrectomy (LSG) in adolescents with morbid obesity. Harefuah. 2011;150:765–768. 816, 815. [PubMed] [Google Scholar]
- 9.Heymsfield S.B., Wadden T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017;376:254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 10.Magkos F., Fraterrigo G., Yoshino J., Luecking C., Kirbach K., Kelly S.C. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing R.R., Lang W., Wadden T.A., Safford M., Knowler W.C., Bertoni A.G. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindstrom J., Louheranta A., Mannelin M., Rastas M., Salminen V., Eriksson J. The Finnish diabetes prevention study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230–3236. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 13.Chakradhar S. All in one: researchers create combination drugs for diabetes and obesity. Nat. Med. 2016;22:694–696. doi: 10.1038/nm0716-694. [DOI] [PubMed] [Google Scholar]
- 14.Rubino F., Nathan D.M., Eckel R.H., Schauer P.R., Alberti K.G., Zimmet P.Z. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861–877. doi: 10.2337/dc16-0236. [DOI] [PubMed] [Google Scholar]
- 15.Brito J.P., Montori V.M., Davis A.M. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. JAMA. 2017;317:635–636. doi: 10.1001/jama.2016.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulson Q.X., Hong J., Holcomb V.B., Nunez N.P. Effects of body weight and alcohol consumption on insulin sensitivity. Nutr. J. 2010;9:14. doi: 10.1186/1475-2891-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadde K.M., Pritham Raj Y. Pharmacotherapy of obesity: clinical trials to clinical practice. Curr. Diab. Rep. 2017;17:34. doi: 10.1007/s11892-017-0859-2. [DOI] [PubMed] [Google Scholar]
- 18.Clemmensen C., Finan B., Fischer K., Tom R.Z., Legutko B., Sehrer L. Dual melanocortin-4 receptor and GLP-1 receptor agonism amplifies metabolic benefits in diet-induced obese mice. EMBO Mol. Med. 2015;7:288–298. doi: 10.15252/emmm.201404508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finan B., Ma T., Ottaway N., Muller T.D., Habegger K.M., Heppner K.M. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013;5:209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 20.Sisley S., Gutierrez-Aguilar R., Scott M., D'Alessio D.A., Sandoval D.A., Seeley R.J. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J. Clin. Invest. 2014;124:2456–2463. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finan B., Clemmensen C., Muller T.D. Emerging opportunities for the treatment of metabolic diseases: glucagon-like peptide-1 based multi-agonists. Mol. Cell. Endocrinol. 2015;418(Pt 1):42–54. doi: 10.1016/j.mce.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Finan B., Yang B., Ottaway N., Smiley D.L., Ma T., Clemmensen C. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015;21:27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 23.Baxter J.D., Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat. Rev. Drug Discov. 2009;8:308–320. doi: 10.1038/nrd2830. [DOI] [PubMed] [Google Scholar]
- 24.Fliers E., Klieverik L.P., Kalsbeek A. Novel neural pathways for metabolic effects of thyroid hormone. Trends Endocrinol. Metab. 2010;21:230–236. doi: 10.1016/j.tem.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Haddock C.K., Poston W.S., Dill P.L., Foreyt J.P., Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int. J. Obes. Relat. Metab. Disord. 2002;26:262–273. doi: 10.1038/sj.ijo.0801889. [DOI] [PubMed] [Google Scholar]
- 26.Levine L.R., Rosenblatt S., Bosomworth J. Use of a serotonin re-uptake inhibitor, fluoxetine, in the treatment of obesity. Int. J. Obes. 1987;11(Suppl. 3):185–190. [PubMed] [Google Scholar]
- 27.Drent M.L., Larsson I., William-Olsson T., Quaade F., Czubayko F., von Bergmann K. Orlistat (Ro 18-0647), a lipase inhibitor, in the treatment of human obesity: a multiple dose study. Int. J. Obes. Relat. Metab. Disord. 1995;19:221–226. [PubMed] [Google Scholar]
- 28.Tschop M., Smiley D.L., Heiman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 29.Cowley M.A., Smith R.G., Diano S., Tschop M., Pronchuk N., Grove K.L. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 30.Muller T.D., Nogueiras R., Andermann M.L., Andrews Z.B., Anker S.D., Argente J. Ghrelin. Mol. Metab. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varela L., Vazquez M.J., Cordido F., Nogueiras R., Vidal-Puig A., Dieguez C. Ghrelin and lipid metabolism: key partners in energy balance. J. Mol. Endocrinol. 2011;46:R43–63. doi: 10.1677/JME-10-0068. [DOI] [PubMed] [Google Scholar]
- 32.Collden G., Tschop M.H., Muller T.D. Therapeutic potential of targeting the ghrelin pathway. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J.V., Ren P.G., Avsian-Kretchmer O., Luo C.W., Rauch R., Klein C. Obestatin a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 34.Depoortere I. GI functions of GPR39: novel biology. Curr. Opin. Pharmacol. 2012;12:647–652. doi: 10.1016/j.coph.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 36.Gnanapavan S., Kola B., Bustin S.A., Morris D.G., McGee P., Fairclough P. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 37.Guan X.M., Yu H., Palyha O.C., McKee K.K., Feighner S.D., Sirinathsinghji D.J. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 38.Willesen M.G., Kristensen P., Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez J.A., Solenberg P.J., Perkins D.R., Willency J.A., Knierman M.D., Jin Z. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J., Brown M.S., Liang G., Grishin N.V., Goldstein J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Barnett B.P., Hwang Y., Taylor M.S., Kirchner H., Pfluger P.T., Bernard V. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330:1689–1692. doi: 10.1126/science.1196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirchner H., Gutierrez J.A., Solenberg P.J., Pfluger P.T., Czyzyk T.A., Willency J.A. GOAT links dietary lipids with the endocrine control of energy balance. Nat. Med. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao T.J., Liang G., Li R.L., Xie X., Sleeman M.W., Murphy A.J. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J., Zhao T.J., Goldstein J.L., Brown M.S. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10750–10755. doi: 10.1073/pnas.0805353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Vriese C., Gregoire F., Lema-Kisoka R., Waelbroeck M., Robberecht P., Delporte C. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology. 2004;145:4997–5005. doi: 10.1210/en.2004-0569. [DOI] [PubMed] [Google Scholar]
- 46.Filigheddu N., Gnocchi V.F., Coscia M., Cappelli M., Porporato P.E., Taulli R. Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol. Biol. Cell. 2007;18:986–994. doi: 10.1091/mbc.E06-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porporato P.E., Filigheddu N., Reano S., Ferrara M., Angelino E., Gnocchi V.F. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J. Clin. Invest. 2013;123:611–622. doi: 10.1172/JCI39920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L., Zhang L.K., Pang Y.Z., Pan C.S., Qi Y.F., Chen L. Cardioprotective effects of ghrelin and des-octanoyl ghrelin on myocardial injury induced by isoproterenol in rats. Acta Pharmacol. Sin. 2006;27:527–535. doi: 10.1111/j.1745-7254.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 49.Baldanzi G., Filigheddu N., Cutrupi S., Catapano F., Bonissoni S., Fubini A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J. Cell Biol. 2002;159:1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson N.M., Gill D.A., Davies R., Loveridge N., Houston P.A., Robinson I.C. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145:234–242. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- 51.Toshinai K., Yamaguchi H., Sun Y., Smith R.G., Yamanaka A., Sakurai T. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147:2306–2314. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W., Chai B., Li J.Y., Wang H., Mulholland M.W. Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology. 2008;149:4710–4716. doi: 10.1210/en.2008-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C.Y., Inui A., Asakawa A., Fujino K., Kato I., Chen C.C. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129:8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 54.Delhanty P.J., Huisman M., Baldeon-Rojas L.Y., van den Berge I., Grefhorst A., Abribat T. Des-acyl ghrelin analogs prevent high-fat-diet-induced dysregulation of glucose homeostasis. FASEB J. 2013;27:1690–1700. doi: 10.1096/fj.12-221143. [DOI] [PubMed] [Google Scholar]
- 55.Delhanty P.J., Neggers S.J., van der Lely A.J. Des-acyl ghrelin: a metabolically active peptide. Endocr. Dev. 2013;25:112–121. doi: 10.1159/000346059. [DOI] [PubMed] [Google Scholar]
- 56.Broglio F., Gottero C., Prodam F., Gauna C., Muccioli G., Papotti M. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J. Clin. Endocrinol. Metab. 2004;89:3062–3065. doi: 10.1210/jc.2003-031964. [DOI] [PubMed] [Google Scholar]
- 57.Tong J., Davis H.W., Summer S., Benoit S.C., Haque A., Bidlingmaier M. Acute administration of unacylated ghrelin has no effect on basal or stimulated insulin secretion in healthy humans. Diabetes. 2014;63:2309–2319. doi: 10.2337/db13-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barazzoni R., Zanetti M., Ferreira C., Vinci P., Pirulli A., Mucci M. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J. Clin. Endocrinol. Metab. 2007;92:3935–3940. doi: 10.1210/jc.2006-2527. [DOI] [PubMed] [Google Scholar]
- 59.Cederberg H., Koivisto V.M., Jokelainen J., Surcel H.M., Keinanen-Kiukaanniemi S., Rajala U. Unacylated ghrelin is associated with changes in insulin sensitivity and lipid profile during an exercise intervention. Clin. Endocrinol. (Oxf.) 2012;76:39–45. doi: 10.1111/j.1365-2265.2011.04135.x. [DOI] [PubMed] [Google Scholar]
- 60.Granata R., Settanni F., Julien M., Nano R., Togliatto G., Trombetta A. Des-acyl ghrelin fragments and analogues promote survival of pancreatic beta-cells and human pancreatic islets and prevent diabetes in streptozotocin-treated rats. J. Med. Chem. 2012;55:2585–2596. doi: 10.1021/jm201223m. [DOI] [PubMed] [Google Scholar]
- 61.Granata R., Settanni F., Biancone L., Trovato L., Nano R., Bertuzzi F. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3',5'-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2 and phosphatidyl inositol 3-kinase/Akt signaling. Endocrinology. 2007;148:512–529. doi: 10.1210/en.2006-0266. [DOI] [PubMed] [Google Scholar]
- 62.Granata R., Settanni F., Trovato L., Destefanis S., Gallo D., Martinetti M. Unacylated as well as acylated ghrelin promotes cell survival and inhibit apoptosis in HIT-T15 pancreatic beta-cells. J. Endocrinol. Invest. 2006;29:RC19–22. doi: 10.1007/BF03347367. [DOI] [PubMed] [Google Scholar]
- 63.Granata R., Volante M., Settanni F., Gauna C., Ghe C., Annunziata M. Unacylated ghrelin and obestatin increase islet cell mass and prevent diabetes in streptozotocin-treated newborn rats. J. Mol. Endocrinol. 2010;45:9–17. doi: 10.1677/JME-09-0141. [DOI] [PubMed] [Google Scholar]
- 64.Chen H.Y., Trumbauer M.E., Chen A.S., Weingarth D.T., Adams J.R., Frazier E.G. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 65.Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 66.Kalra S.P., Ueno N., Kalra P.S. Stimulation of appetite by ghrelin is regulated by leptin restraint: peripheral and central sites of action. J. Nutr. 2005;135:1331–1335. doi: 10.1093/jn/135.5.1331. [DOI] [PubMed] [Google Scholar]
- 67.Masuda Y., Tanaka T., Inomata N., Ohnuma N., Tanaka S., Itoh Z. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem. Biophys. Res. Commun. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 68.Asakawa A., Inui A., Kaga T., Yuzuriha H., Nagata T., Ueno N. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 69.Cai H., Cong W.N., Daimon C.M., Wang R., Tschop M.H., Sevigny J. Altered lipid and salt taste responsivity in ghrelin and GOAT null mice. PLoS One. 2013;8:e76553. doi: 10.1371/journal.pone.0076553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Druce M.R., Wren A.M., Park A.J., Milton J.E., Patterson M., Frost G. Ghrelin increases food intake in obese as well as lean subjects. Int. J. Obes. (Lond.) 2005;29:1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 71.Jerlhag E., Egecioglu E., Dickson S.L., Douhan A., Svensson L., Engel J.A. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict. Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 72.Overduin J., Figlewicz D.P., Bennett-Jay J., Kittleson S., Cummings D.E. Ghrelin increases the motivation to eat, but does not alter food palatability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R259–69. doi: 10.1152/ajpregu.00488.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skibicka K.P., Hansson C., Egecioglu E., Dickson S.L. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict. Biol. 2012;17:95–107. doi: 10.1111/j.1369-1600.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tong J., Mannea E., Aime P., Pfluger P.T., Yi C.X., Castaneda T.R. Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J. Neurosci. 2011;31:5841–5846. doi: 10.1523/JNEUROSCI.5680-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tolle V., Bassant M.H., Zizzari P., Poindessous-Jazat F., Tomasetto C., Epelbaum J. Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology. 2002;143:1353–1361. doi: 10.1210/endo.143.4.8712. [DOI] [PubMed] [Google Scholar]
- 76.Szentirmai E., Hajdu I., Obal F., Jr., Krueger J.M. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res. 2006;1088:131–140. doi: 10.1016/j.brainres.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 77.Weikel J.C., Wichniak A., Ising M., Brunner H., Friess E., Held K. Ghrelin promotes slow-wave sleep in humans. Am. J. Physiol. Endocrinol. Metab. 2003;284:E407–15. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]
- 78.Lutter M., Sakata I., Osborne-Lawrence S., Rovinsky S.A., Anderson J.G., Jung S. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spencer S.J., Xu L., Clarke M.A., Lemus M., Reichenbach A., Geenen B. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol. Psychiatry. 2012;72:457–465. doi: 10.1016/j.biopsych.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 80.Chuang J.C., Perello M., Sakata I., Osborne-Lawrence S., Savitt J.M., Lutter M. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Invest. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rizzo M., Rizvi A.A., Sudar E., Soskic S., Obradovic M., Montalto G. A review of the cardiovascular and anti-atherogenic effects of ghrelin. Curr. Pharm. Des. 2013;19:4953–4963. doi: 10.2174/1381612811319270018. [DOI] [PubMed] [Google Scholar]
- 82.Nagaya N., Uematsu M., Kojima M., Ikeda Y., Yoshihara F., Shimizu W. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–1435. doi: 10.1161/hc3601.095575. [DOI] [PubMed] [Google Scholar]
- 83.Nagaya N., Moriya J., Yasumura Y., Uematsu M., Ono F., Shimizu W. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–3679. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- 84.Okumura H., Nagaya N., Enomoto M., Nakagawa E., Oya H., Kangawa K. Vasodilatory effect of ghrelin, an endogenous peptide from the stomach. J. Cardiovasc. Pharmacol. 2002;39:779–783. doi: 10.1097/00005344-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 85.Yasuda T., Masaki T., Kakuma T., Yoshimatsu H. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neurosci. Lett. 2003;349:75–78. doi: 10.1016/s0304-3940(03)00789-4. [DOI] [PubMed] [Google Scholar]
- 86.Mano-Otagiri A., Ohata H., Iwasaki-Sekino A., Nemoto T., Shibasaki T. Ghrelin suppresses noradrenaline release in the brown adipose tissue of rats. J. Endocrinol. 2009;201:341–349. doi: 10.1677/JOE-08-0374. [DOI] [PubMed] [Google Scholar]
- 87.Tsubone T., Masaki T., Katsuragi I., Tanaka K., Kakuma T., Yoshimatsu H. Ghrelin regulates adiposity in white adipose tissue and UCP1 mRNA expression in brown adipose tissue in mice. Regul. Pept. 2005;130:97–103. doi: 10.1016/j.regpep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Mano-Otagiri A., Iwasaki-Sekino A., Nemoto T., Ohata H., Shuto Y., Nakabayashi H. Genetic suppression of ghrelin receptors activates brown adipocyte function and decreases fat storage in rats. Regul. Pept. 2010;160:81–90. doi: 10.1016/j.regpep.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 89.Lin L., Saha P.K., Ma X., Henshaw I.O., Shao L., Chang B.H. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell. 2011;10:996–1010. doi: 10.1111/j.1474-9726.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reimer M.K., Pacini G., Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144:916–921. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- 91.Egido E.M., Rodriguez-Gallardo J., Silvestre R.A., Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur. J. Endocrinol. 2002;146:241–244. doi: 10.1530/eje.0.1460241. [DOI] [PubMed] [Google Scholar]
- 92.Reynolds C.B., Elias A.N., Whisnant C.S. Effects of feeding pattern on ghrelin and insulin secretion in pigs. Domest. Anim. Endocrinol. 2010;39:90–96. doi: 10.1016/j.domaniend.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 93.Angeloni S.V., Glynn N., Ambrosini G., Garant M.J., Higley J.D., Suomi S. Characterization of the rhesus monkey ghrelin gene and factors influencing ghrelin gene expression and fasting plasma levels. Endocrinology. 2004;145:2197–2205. doi: 10.1210/en.2003-1103. [DOI] [PubMed] [Google Scholar]
- 94.Voruganti V.S., Tejero M.E., Proffitt J.M., Cole S.A., Cox L.A., Mahaney M.C. Characterization of ghrelin in pedigreed baboons: evidence for heritability and pleiotropy. Obesity (Silver Spring) 2008;16:804–810. doi: 10.1038/oby.2007.107. [DOI] [PubMed] [Google Scholar]
- 95.Tong J., Prigeon R.L., Davis H.W., Bidlingmaier M., Kahn S.E., Cummings D.E. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun Y., Butte N.F., Garcia J.M., Smith R.G. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149:843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun Y., Wang P., Zheng H., Smith R.G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zigman J.M., Nakano Y., Coppari R., Balthasar N., Marcus J.N., Lee C.E. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun Y., Asnicar M., Saha P.K., Chan L., Smith R.G. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3:379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 100.Bastidas-Ponce A., Scheibner K., Lickert H., Bakhti M. Cellular and molecular mechanisms coordinating pancreas development. Development. 2017;144:2873–2888. doi: 10.1242/dev.140756. [DOI] [PubMed] [Google Scholar]
- 101.Benitez C.M., Goodyer W.R., Kim S.K. Deconstructing pancreas developmental biology. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andralojc K.M., Mercalli A., Nowak K.W., Albarello L., Calcagno R., Luzi L. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia. 2009;52:486–493. doi: 10.1007/s00125-008-1238-y. [DOI] [PubMed] [Google Scholar]
- 103.Prado C.L., Pugh-Bernard A.E., Elghazi L., Sosa-Pineda B., Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2924–2929. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wierup N., Svensson H., Mulder H., Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul. Pept. 2002;107:63–69. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 105.Wiedemann T., Bielohuby M., Muller T.D., Bidlingmaier M., Pellegata N.S. Obesity in MENX rats is accompanied by high circulating levels of ghrelin and improved insulin sensitivity. Diabetes. 2016;65:406–420. doi: 10.2337/db15-0374. [DOI] [PubMed] [Google Scholar]
- 106.Salehi A., de la Cour C. Dornonville, Hakanson R., Lundquist I. Effects of ghrelin on insulin and glucagon secretion: a study of isolated pancreatic islets and intact mice. Regul. Pept. 2004;118:143–150. doi: 10.1016/j.regpep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 107.Qader S.S., Lundquist I., Ekelund M., Hakanson R., Salehi A. Ghrelin activates neuronal constitutive nitric oxide synthase in pancreatic islet cells while inhibiting insulin release and stimulating glucagon release. Regul. Pept. 2005;128:51–56. doi: 10.1016/j.regpep.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 108.Dezaki K., Hosoda H., Kakei M., Hashiguchi S., Watanabe M., Kangawa K. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes. 2004;53:3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 109.Dezaki K., Sone H., Koizumi M., Nakata M., Kakei M., Nagai H. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006;55:3486–3493. doi: 10.2337/db06-0878. [DOI] [PubMed] [Google Scholar]
- 110.Chuang J.C., Sakata I., Kohno D., Perello M., Osborne-Lawrence S., Repa J.J. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol. Endocrinol. 2011;25:1600–1611. doi: 10.1210/me.2011-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yi C.X., Heppner K.M., Kirchner H., Tong J., Bielohuby M., Gaylinn B.D. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS One. 2012;7:e32100. doi: 10.1371/journal.pone.0032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dezaki K., Kakei M., Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes. 2007;56:2319–2327. doi: 10.2337/db07-0345. [DOI] [PubMed] [Google Scholar]
- 113.Park S., Jiang H., Zhang H., Smith R.G. Modification of ghrelin receptor signaling by somatostatin receptor-5 regulates insulin release. Proc. Natl. Acad. Sci. U. S. A. 2012;109:19003–19008. doi: 10.1073/pnas.1209590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adeghate E., Ponery A.S. Ghrelin stimulates insulin secretion from the pancreas of normal and diabetic rats. J. Neuroendocrinol. 2002;14:555–560. doi: 10.1046/j.1365-2826.2002.00811.x. [DOI] [PubMed] [Google Scholar]
- 115.Irako T., Akamizu T., Hosoda H., Iwakura H., Ariyasu H., Tojo K. Ghrelin prevents development of diabetes at adult age in streptozotocin-treated newborn rats. Diabetologia. 2006;49:1264–1273. doi: 10.1007/s00125-006-0226-3. [DOI] [PubMed] [Google Scholar]
- 116.Soriano-Guillen L., Barrios V., Martos G., Chowen J.A., Campos-Barros A., Argente J. Effect of oral glucose administration on ghrelin levels in obese children. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2004;151:119–121. doi: 10.1530/eje.0.1510119. [DOI] [PubMed] [Google Scholar]
- 117.Martos-Moreno G.A., Barrios V., Soriano-Guillen L., Argente J. Relationship between adiponectin levels, acylated ghrelin levels, and short-term body mass index changes in children with diabetes mellitus type 1 at diagnosis and after insulin therapy. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2006;155:757–761. doi: 10.1530/eje.1.02273. [DOI] [PubMed] [Google Scholar]
- 118.Esler W.P., Rudolph J., Claus T.H., Tang W., Barucci N., Brown S.E. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148:5175–5185. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- 119.Brial F., Lussier C.R., Belleville K., Sarret P., Boudreau F. Ghrelin inhibition restores glucose homeostasis in hepatocyte nuclear factor-1alpha (MODY3)-Deficient mice. Diabetes. 2015;64:3314–3320. doi: 10.2337/db15-0124. [DOI] [PubMed] [Google Scholar]
- 120.Damian M., Marie J., Leyris J.P., Fehrentz J.A., Verdie P., Martinez J. High constitutive activity is an intrinsic feature of ghrelin receptor protein: a study with a functional monomeric GHS-R1a receptor reconstituted in lipid discs. J. Biol. Chem. 2012;287:3630–3641. doi: 10.1074/jbc.M111.288324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holst B., Cygankiewicz A., Jensen T.H., Ankersen M., Schwartz T.W. High constitutive signaling of the ghrelin receptor–identification of a potent inverse agonist. Mol. Endocrinol. 2003;17:2201–2210. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- 122.Abegg K., Bernasconi L., Hutter M., Whiting L., Pietra C., Giuliano C. Ghrelin receptor inverse agonists as a novel therapeutic approach against obesity-related metabolic disease. Diabetes Obes. Metab. 2017;19:1740–1750. doi: 10.1111/dom.13020. [DOI] [PubMed] [Google Scholar]
- 123.Petersen P.S., Woldbye D.P., Madsen A.N., Egerod K.L., Jin C., Lang M. In vivo characterization of high Basal signaling from the ghrelin receptor. Endocrinology. 2009;150:4920–4930. doi: 10.1210/en.2008-1638. [DOI] [PubMed] [Google Scholar]
- 124.Zorrilla E.P., Iwasaki S., Moss J.A., Chang J., Otsuji J., Inoue K. Vaccination against weight gain. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13226–13231. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Azegami T., Yuki Y., Sawada S., Mejima M., Ishige K., Akiyoshi K. Nanogel-based nasal ghrelin vaccine prevents obesity. Mucosal Immunol. 2017;10:1351–1360. doi: 10.1038/mi.2016.137. [DOI] [PubMed] [Google Scholar]
- 126.Broglio F., Arvat E., Benso A., Gottero C., Muccioli G., Papotti M. Ghrelin a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J. Clin. Endocrinol. Metab. 2001;86:5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 127.Broglio F., Gottero C., Benso A., Prodam F., Destefanis S., Gauna C. Effects of ghrelin on the insulin and glycemic responses to glucose arginine, or free fatty acids load in humans. J. Clin. Endocrinol. Metab. 2003;88:4268–4272. doi: 10.1210/jc.2002-021940. [DOI] [PubMed] [Google Scholar]
- 128.Guido M., Romualdi D., De Marinis L., Porcelli T., Giuliani M., Costantini B. Administration of exogenous ghrelin in obese patients with polycystic ovary syndrome: effects on plasma levels of growth hormone, glucose, and insulin. Fertil. Steril. 2007;88:125–130. doi: 10.1016/j.fertnstert.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 129.Tassone F., Broglio F., Destefanis S., Rovere S., Benso A., Gottero C. Neuroendocrine and metabolic effects of acute ghrelin administration in human obesity. J. Clin. Endocrinol. Metab. 2003;88:5478–5483. doi: 10.1210/jc.2003-030564. [DOI] [PubMed] [Google Scholar]
- 130.Cummings D.E., Purnell J.Q., Frayo R.S., Schmidova K., Wisse B.E., Weigle D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 131.Flanagan D.E., Evans M.L., Monsod T.P., Rife F., Heptulla R.A., Tamborlane W.V. The influence of insulin on circulating ghrelin. Am. J. Physiol. Endocrinol. Metab. 2003;284:E313–6. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- 132.Tschop M., Wawarta R., Riepl R.L., Friedrich S., Bidlingmaier M., Landgraf R. Post-prandial decrease of circulating human ghrelin levels. J. Endocrinol. Invest. 2001;24:RC19–21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 133.Fusco A., Bianchi A., Mancini A., Milardi D., Giampietro A., Cimino V. Effects of ghrelin administration on endocrine and metabolic parameters in obese women with polycystic ovary syndrome. J. Endocrinol. Invest. 2007;30:948–956. doi: 10.1007/BF03349243. [DOI] [PubMed] [Google Scholar]
- 134.DelParigi A., Tschop M., Heiman M.L., Salbe A.D., Vozarova B., Sell S.M. High circulating ghrelin: a potential cause for hyperphagia and obesity in prader-willi syndrome. J. Clin. Endocrinol. Metab. 2002;87:5461–5464. doi: 10.1210/jc.2002-020871. [DOI] [PubMed] [Google Scholar]
- 135.Cummings D.E., Clement K., Purnell J.Q., Vaisse C., Foster K.E., Frayo R.S. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat. Med. 2002;8:643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 136.Allas S., Delale T., Ngo N., Julien M., Sahakian P., Ritter J. Safety tolerability, pharmacokinetics and pharmacodynamics of AZP-531, a first-in-class analogue of unacylated ghrelin, in healthy and overweight/obese subjects and subjects with type 2 diabetes. Diabetes Obes. Metab. 2016;18:868–874. doi: 10.1111/dom.12675. [DOI] [PubMed] [Google Scholar]
- 137.Bhattacharya S.K., Andrews K., Beveridge R., Cameron K.O., Chen C., Dunn M. Discovery of PF-5190457, a potent, selective, and orally bioavailable ghrelin receptor inverse agonist clinical candidate. ACS Med. Chem. Lett. 2014;5:474–479. doi: 10.1021/ml400473x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kong J., Chuddy J., Stock I.A., Loria P.M., Straub S.V., Vage C. Pharmacological characterization of the first in class clinical candidate PF-05190457: a selective ghrelin receptor competitive antagonist with inverse agonism that increases vagal afferent firing and glucose-dependent insulin secretion ex vivo. Br. J. Pharmacol. 2016;173:1452–1464. doi: 10.1111/bph.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.A Study Of PF-05190457 In Healthy Volunteers And Type-2 Diabetic Patients ClinicalTrials. gov NIH https://clinicaltrials.gov/ct2/show/NCT01372163?term=PF-05190457&rank=3 (2012).
- 140.Denney W.S., Sonnenberg G.E., Carvajal-Gonzalez S., Tuthill T., Jackson V.M. Pharmacokinetics and pharmacodynamics of PF-05190457: the first oral ghrelin receptor inverse agonist to be profiled in healthy subjects. Br. J. Clin. Pharmacol. 2017;83:326–338. doi: 10.1111/bcp.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]