Synopsis
Prediabetes is a state characterized by impaired fasting glucose or impaired glucose tolerance. This review discusses the pathophysiology and macrovascular complications of prediabetes. The pathophysiologic defects underlying prediabetes include insulin resistance, alpha- and beta-cell dysfunction, increased lipolysis, inflammation, and suboptimal incretin effect. Recent studies have revealed that the long-term complications of diabetes manifest in some people with prediabetes; these complications include microvascular and macrovascular disorders. Finally, we present an overview of randomized control trials aimed at preventing progression from prediabetes to type 2 diabetes and discuss their implications for macrovascular risk reduction.
Keywords: Impaired fasting glucose, impaired glucose tolerance, prediabetes complications, cardiovascular disease, macrovascular
Introduction
Type 2 diabetes mellitus (T2DM) is one of the major causes of premature morbidity and mortality worldwide with the World Health Organization (WHO) reporting that one in ten adults worldwide had T2DM in 2014.1 In the United States, one out of every five health care dollars is spent on diabetes related healthcare.2 Diabetes Mellitus also imposes a huge drain in developing countries on national health budgets comprising on average at least 5% of their total health expenditures on diabetes in 2010.3 Of these, macrovascular complications are the largest contributor to the direct and indirect costs of diabetes.4 The development of T2DM is punctuated by an interlude of prediabetes, itself a toxic state that is associated with the development of macrovascular complications.
Diagnosis and Burden of Prediabetes
The prelude to diabetes is prediabetes in what can be described as a continuum from normoglycemia through worsening dysglycemia. Prediabetes is defined specifically as impaired glucose tolerance and/or impaired fasting glucose.5 According to the American Diabetes Association (ADA) impaired glucose tolerance (IGT) is defined as a 2-hour plasma glucose value in the 75-gram oral glucose tolerance test (OGTT) of 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L).6 Impaired fasting glucose (IFG) is defined as a fasting plasma glucose of 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L).6 Finally prediabetes can also be defined as a hemoglobin A1c (HbA1c) of 5.7% –6.4% (39–46 mmol/mol).6,7 It bears stressing that the ADA criteria stipulate normal glucose tolerance (NGT) as a fasting glucose level less of than 100mg/dl and a 2-hr post-load OGTT plasma glucose level of less than 140mg/dl. In regards to using HbA1c as a diagnosis of prediabetes, it must be stressed that there are many well characterized “pitfalls” such as anemia, chronic kidney disease, and other systemic illness and hematological disorders that disrupt the reliability of HbA1c as an integrated measure of mean plasma glucose.8–12 In particular, racial and ethnic differences in the relationship between blood glucose values and HbA1c call for caution when utilizing HbA1c levels for the diagnosis of prediabetes. 8–14 It is always prudent to confirm diagnosis with actual blood glucose measurement before instituting therapeutic measures.8 Estimates by the Centers for Disease Control and Prevention (CDC) in the United States indicated that there were ~29 million adults with diabetes and 86 million with prediabetes in 2014.15,16 Worldwide, there are more than 400 million people with prediabetes and projections indicate that more than 470 million people will have prediabetes by 2030.17 In addition, many studies from across the globe have pointed out that the risk of many co-morbidities are the same in diabetes and prediabetes and affect all age groups.18–23
Pathophysiological Defects in Prediabetes
The known pathophysiological defects that underlie T2DM are being increasingly recognized in the prediabetic state.24–28 The natural progression of dysglycemia involves increasing insulin resistance and loss of pancreatic β-cell function.29 Significant defects in insulin action and secretion are consistently demonstrable in the prediabetic state of IGT.30–32 Several cross-sectional studies and a few longitudinal studies have carefully documented the various defects leading to prediabetes and T2DM.33–35
Findings from Longitudinal Assessment of Insulin Action and Secretion
A landmark longitudinal study that tracked high risk subjects from the stage of NGT to prediabetes reported that the transition to prediabetes was associated with an increase in body weight, increase in insulin resistance, and a decline in the endogenous insulin secretion (beta-cell dysfunction).29 The study further demonstrated that progression from prediabetes to T2DM was accompanied by a worsening of weight gain, insulin resistance, and beta-cell dysfunction.29 Thus the salient finding from the longitudinal observation was that insulin resistance and beta-cell failure co-evolve simultaneously rather than sequentially, as was previously believed. Individuals who maintained NGT status, despite weight gain and associated insulin resistance, were those who mounted a robust endogenous insulin secretory response.29 Thus, if beta-cells cannot overcome insulin resistance, dysglycemia ensues. A supportive post-mortem study reported ~40% deficit in relative β-cell volume among individuals with prediabetes compared to those with normal fasting glucose concentrations.35
Lipolysis, Incretin, Alpha Cell, and Inflammation in Prediabetes
Further defects in the prediabetic state include increased lipolysis, decreased endogenous levels of glucagon-like peptide 1 (GLP-1), and impaired postprandial suppression of glucagon secretion by the alpha-cells of the pancreas.36–40 Additionally, as listed in Table 1, aberrant expression of proinflammatory cytokines adds to the toxic milieu of prediabetes. For instance, low adiponectin levels have been demonstrated to be predictive of progression from NGT to prediabetes, and from prediabetes to T2DM.39,41 Elevated levels of molecular markers such as intercellular adhesion molecule-1 (ICAM-1) and tumor necrosis factor-α (TNF-α) have been reported.42 Figure 1 shows the recognized pathophysiological defects in T2DM, highlighting those that have also been described in prediabetes.43–44
Table 1.
Pathophysiological Defects in Prediabetes
| Defect | References |
|---|---|
| Loss of beta-cell volume | 35 |
| Defects in insulin action and secretion | 24,26,29–31,33,51,52 |
| Endothelial dysfunction | 26,52 |
| Arterial Stiffness | 72–74 |
| Increased lipolysis | 25,36 |
| Reduced incretin levels | 36,38 |
| Increased hepatic glucose production | 26, 32,33 |
| Impaired glucagon levels | 33 |
| Dysregulated cytokines | 41,42,99 |
Figure 1.
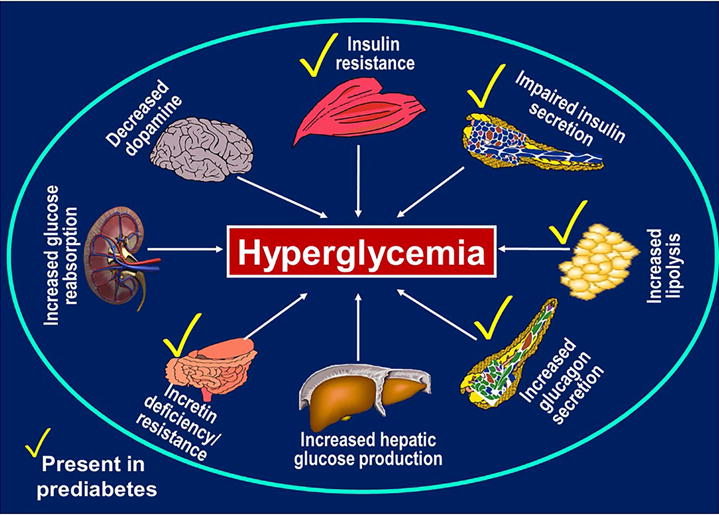
Recognized pathophysiological defects in type 2 diabetes mellitus and prediabetes. From Dagogo-Jack, S. Diabetes risks from prescription and nonprescription drugs. ADA press, Alexandria VA, 2016, with permission.
Emerging insights regarding the gut microbiome and its association with cardiometabolic disorders, such as obesity, diabetes, dyslipidemia, etc., have relevance to prediabetes. Recently, a disturbed gut microbiota expressed as gut dysbiosis (an intestinal physical barrier abnormality) has been associated with the progression and maintenance of obesity, T2DM, cardiovascular disease (CVD) and the metabolic syndrome.45–50 Future research into this area will help shed light onto the proximal chronology of the associated between gut dysbiosis and early dysglycemia/prediabetes.
Predictors of Glycemic Progression to Prediabetes/T2DM
Conversion from Prediabetes to T2DM
An analysis of six prospective studies on progression from prediabetes to T2DM revealed the following features: (1) baseline fasting plasma glucose (FPG) and the 2-h OGTT glucose values were positively associated with diabetes risk; (2) the rate of progression from prediabetes to T2DM was exponential among subjects in the top quartile of baseline FPG but increased linearly with increasing 2-h OGTT glucose levels; (3) incident diabetes occurred at higher rates in Hispanic, Mexican-Americans, Pima, and Nauruan populations than among other ethnicities such as Caucasians; (4) increased BMI predicted T2DM risk in low risk populations but not in populations with the highest incidence of T2DM.51,52 Of note, weight gain significantly predicted the risk of incident T2DM in African-Americans in the Atherosclerosis Risk in Communities study.53
Normoglycemia to Prediabetes Transition
Longitudinal studies in subjects from a high-risk population (PIMA Indians) with baseline NGT indicated that weight gain, insulin resistance, and progressive loss of insulin secretory response to glucose predicted transition from NGT to IGT (prediabetes).29,53 The longitudinal mean weight change in NGT → NGT subjects (nonprogressors) vs. NGT → IGT progressors was 2.6 kg vs. 5.2 kg during a 6-year follow-up period.24
In the Baltimore Longitudinal Study of Aging (BLSA), 62% of the initially NGT participants progressed to prediabetes during 10 years of follow-up, yielding an annualized rate of prediabetes 6.2% in the BLSA.54
In the Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) study, initially normoglycemic African American and European American offspring of parents with T2DM were followed longitudinally for the primary outcome of incident prediabetes (IFG or IGT). During a mean 2.62 years of follow-up 101 of 343 POP-ABC participants developed incident prediabetes, yielding an annualized rate of ~11%.55 Compared to nonprogressors, POP-ABC participants who developed incident prediabetes were older, more likely to be male, had higher baseline BMI and fat mass, lower levels of physical activity (adjusted for food habits), lower measures of insulin sensitivity, disposition index; serum adiponectin and HDL cholesterol levels, and higher triglyceride levels.41,55–57
Macrovascular Complications of Prediabetes
The macrovascular disorders associated with prediabetes include CVD, stroke, and peripheral vascular disease. As figure 2 depicts, these disorders are established in patients with T2DM, but their initiation and progression are well recognized to occur during the prediabetes stage.58–61 In fact, the traditional CVD risk factors (dyslipidemia, obesity, hypertension) are quite prevalent among individuals with prediabetes.62–67
Figure 2.
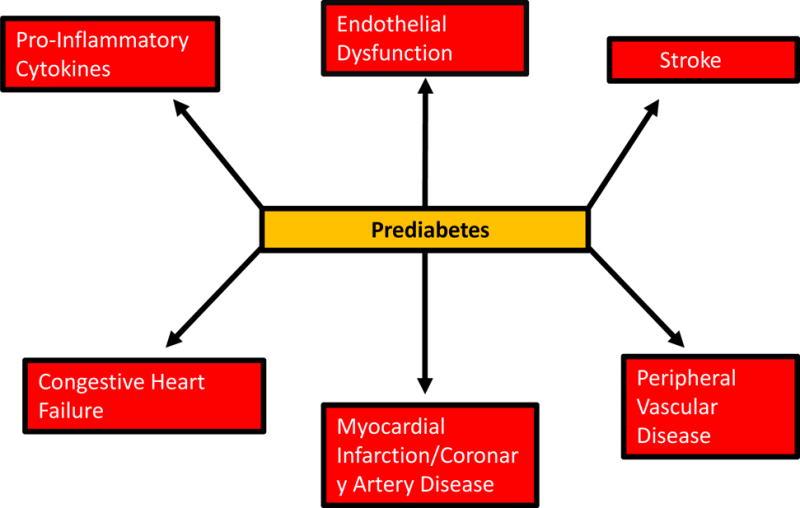
Recognized macrovascular complications associated with prediabetes.
Cardiovascular Disease
A recent meta-analysis based on 35 studies reported data for the association between myocardial infarction and congestive heart failure as well as coronary artery disease and atherosclerosis have all been reported in individuals with prediabetes.67–69 In the EPIC-Norfolk study, a 1% increase in 27 HbA1c within the normal range was associated with increased 10-year cardiovascular mortality. The EPIC-Norfolk findings are in accord with data from the Paris Prospective Study cohort which 70 showed a doubling of CVD mortality in IGT subjects compared with NGT subjects. The finding of increased mortality is underscored by the fact that most patients with prediabetes harbor features of insulin resistance (metabolic) syndrome, including upper-body obesity, hypertriglyceridemia, decreased HDL cholesterol levels and hypertension, among others. Components of the metabolic syndrome often can be identified in prediabetic subjects several years before the diagnosis of 71,72 T2DM. These features translate into advanced atherosclerotic vascular changes which are often preceded by impairment of endothelium-dependent vasodilation, vascular smooth muscle dysfunction and increased arterial stiffness.73
A recent cross-sectional study reported a positive association between prediabetes and the prevalence of arterial stiffness, suggesting that early intervention on prediabetes control might prevent arterial stiffness.74 Remarkably, the prediabetes state is associated a nearly 3-fold higher prevalence of unrecognized myocardial infarction compared with NGT status, as was demonstrated in the Multi-Ethnic Study of Atherosclerosis (MESA) study.62
More recently, a randomized controlled trial of 6522 patients with coronary artery disease and prediabetes found that while acarbose did not reduce the risk of major adverse cardiovascular events, it did reduce the incidence of diabetes.75
Stroke
Compared to NGT subjects, individuals with prediabetes have an increased risk of cerebrovascular diseases, including transient ischemic attack, stroke, and recurrent stroke.65,76–79 A recent study by Tanaka and colleagues demonstrated that both diabetes and prediabetes were associated with poor early prognosis 30 days after acute ischemic stroke.80 A study by Qiao et al. indicates that the 2-hr postload OGTT glucose level is a strong predictor of stroke and future cardiovascular disease.81 In the recently published KORA-MRI study, among 400 subjects who underwent MRI, 103 subjects had prediabetes and 54 had established diabetes. Subjects with prediabetes had an increased risk for carotid plaque and adverse functional cardiac parameters.82 In the IRIS trial involving patients without diabetes who had insulin resistance along with a recent history of ischemic stroke or transient ischemic attack, treatment with pioglitazone significantly decreased the risks of stroke, myocardial infarction or development of T2DM as compared with placebo treament.83,84
Peripheral Vascular Disease
Prediabetes is common in patients with peripheral vascular disease; however, the exact mechanisms remain to be fully elucidated. The development of diabetes is independently associated with mortality in PVD patients in some but not all studies.85,86
Interactions among Prediabetes and CVD Risk Factors
Epidemiologic studies have shown that prediabetes is a strong predictor of CVD.84–89 The studies include DECODE and Funagata Diabetes Study, among others.88–91 In the San Antonio Heart Study, there was evidence that the risk for CVD starts to increase long before the onset of clinical diabetes.92–94 Obesity and overweight, known risk factors for T2DM and prediabetes, have also been associated with CVD risk.95–98 Overweight and obesity have been frequently associated with low-grade, chronic, systemic inflammation characterized by increased levels of pro-inflammatory markers including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-8 (IL-8), and C-reactive protein.99 Also, overweight people with prediabetes often present with dyslipidemia (higher triglycerides and lower HDL cholesterol)99–104 Hypertension is another CVD risk factor that has been examined in prediabetes.105–109 The DREAM105 and NAVIGATOR106 studies assessed the effect of blood pressure control on the progression from prediabetes to T2DM. Similarly, the Diabetes Prevention Program investigators reported lower rates of incident hypertension among prediabetic individuals randomized to the intensive lifestyle arm as compared to the placebo arm.107
Intervention Studies to Prevent Progression from Prediabetes to T2DM and Development of CVD
Prevention of T2DM
Table 2 lists lifestyle and pharmacological intervention studies to prevent T2DM among individuals enrolled with prediabetes. Lifestyle intervention has clearly been demonstrated to decrease progression to T2DM.106–111 The results of the DPP, Finnish Diabetes Prevention Study (FDPS), and other pertinent studies showed approximately 60% risk reduction for incident T2DM in the lifestyle arm compared to placebo.112–116 In both the DPP and FDPS, every 1 kg decrease in weight in the lifestyle arm was associated with 15%–16% in future T2DM risk.115,117–122
Table 2.
Pertinent Prediabetes Intervention Studies
| Study | Intervention | Number of subjects | Study population | Risk reduction | Years |
|---|---|---|---|---|---|
| Da Qing121 | Diet and exercise | 577 | Chinese IGT adults, mean age 46, BMI 26 | 31–46% after 6 years | 1986–1992 |
| Finnish DPS119 | Diet and exercise | 522 | IGT adults, mean age 55, BMI 31 | 58% after 3.2 years | 1993–1998 |
| STOP-NIDDM124 | Acarbose | 1428 | IGT adults, mean age 55, BMI 31 | 25% after 3.3 years | 1995–1998 |
| DPP120 | Diet and exercise | 3234 | IGT adults, mean age 54 years, BMI 34 | Metformin 31%, lifestyle 58% after 2 years | 1996–1999 |
| Xendos125 | Orlistat and diet and exercise | 3305 | Swedish, BMI >30, mean age 43, 21% with IGT | Entire group 37%, IGT 45% after 4 years | 1997–2002 |
| DREAM126 | Rosiglitazone | 5269 | IGT and/or IGF subjects mean age 54.7 years, BMI 30.9 | 62% after approximately 3 years | 2001–2003 |
| IDDP-1129 | Lifestyle modifications and metformin or lifestyle modifications | 531 | Indian, IGT mean age 46 years, BMI 25.8 | Diet and exercise 28.5%, Metformin 26.4%, Diet and exercise and metformin 28.2% after 30 months | 2001–2004 |
| ACT-NOW132 | Pioglitazone | 602 | IGT, mean age 53, BMI 33 | 72% with pioglitazone over 2.4 years | 2004–2006 |
| CANOE127 | Combination rosiglitazone and metformin vs placebo | 207 | IGT, mean age 50, BMI 31.3 | 26% in the combination group after 3.9 years | 2004–2006 |
| IDDP-2130 | Lifestyle modifications or pioglitazone and lifestyle modifications | 407 | Indian IGT, mean age 45.3, BMI 25.9 | 28% though pioglitazone not additive to lifestyle modification | 2006–2009 |
| Navigator128 | Nataglinide and lifestyle modifications or Valsartan and lifestyle modifications | 9306 | IGT, mean age 63.7, BMI 30.5 | Nataglinide none, Valsartan 14% | 2005–2010 |
There are also studies showing potential benefits from a variety of different pharmacotherapies.122 Pioglitazone was found to decrease the risk of diabetes by ~70% in obese subjects with prediabetes in the ACT NOW study.123 Table 2 lists pertinent randomized controlled trials from around the world that demonstrated significant decrease in progression of prediabetes to diabetes. These include the STOP-NIDDM (25% risk reduction), Xendos (45% risk reduction), DREAM (62% risk reduction), CANOE (26% risk reduction) and the Valsartan arm of the NAVIGATOR trial (14% risk reduction).124–128 It must be noted, however, that in both the IDPP1 and the follow-up study IDPP2, pharmacologic intervention provided no additional benefit beyond lifestyle modification in subjects randomized to Lifestyle + Metformin or Lifestyle + Pioglitazone arms.129,130 Furthermore, all the medications tested for diabetes prevention have had untoward adverse effects (sometimes severe), and attempts to withdraw the medications have resulted in glycemic rebound.131–132 Given these limitations of pharmacotherapy, the current guidelines from the ADA recommend lifestyle modifications as first line approach for diabetes prevention.6 Indications for possible use of metformin include women with a history of gestational diabetes and high-risk individuals unresponsive to optimal lifestyle modification.6
Prevention of CVD in Prediabetes
Long term follow-up of the Da Qing study demonstrated that diabetes prevention through lifestyle modification was associated with decreased cardiovascular and all-cause mortality after 23 years.133 In the DPP, intensive lifestyle intervention that significantly decreased the risk of T2DM also reduced the need for antihypertensive medications.107 The prevalence of hypertension in the DPP cohort at baseline was approximately 30% in the three comparison groups (placebo, metformin, lifestyle).107 After 3 years of follow-up, the prevalence of hypertension was observed to have increased to approximately 40% in the placebo and metformin arms.107 Surprisingly, the hypertension prevalence remained at the baseline rate of 30% in the intensive lifestyle group 3 years later.107 Thus, lifestyle intervention designed to prevent T2DM also seems to have prevented incident hypertension in this initially prediabetic cohort.107 The DPP investigators also reported that subjects assigned to intensive lifestyle intervention showed decreased blood pressure, increased HDL cholesterol levels, and lower triglyceride levels and a reduction in the more atherogenic small, dense LDL particles during approximately 3 years of follow-up.107 Consonant with these findings, there was a reduced need for lipid lowering medications in the DPP,107, 109,110 as has been observed by others.111–113,134,135
The Mediterranean diet is appealing as a specific nutritional recommendation, based on convincing reports of its benefits on cardiometabolic endpoints. For example, results from the PREDIMED (PREvención con DIeta MEDiterránea) randomized nutrition intervention trial for the primary prevention of CVD showed a 40% reduction in the incidence of T2DM in participants assigned to a Mediterranean diet supplemented with extra-virgin olive oil compared with those assigned to a low-fat control diet.136,137 Other reports on the Mediterranean diet showed concordant findings on cardiometabolic profile. Mediterranean-style diet has been shown to result in greater weight loss along with improvement in inflammatory markers compared with general lifestyle counseling (14kg vs. 3kg, P< 0.001).138–141
Besides the impact of lifestyle intervention on CVD risk factors, there is considerable interest in knowing whether prevention of diabetes also prevents related CVD. This has been a question under investigation by the Diabetes Prevention Program Outcome Study (DPPOS) research group. Analysis of regression patterns in the DPPOS showed that individuals whose blood glucose returned to normal experienced a 56% long-term reduction in diabetes incidence compared to those who remained dysglycemic.109 Although CVD outcomes data collection is still in progress, additional inference from the DPPOS study would suggest that regression from prediabetes to normal may also be associated with decreased risk for CVD.111,142
Clinical Translation and Conclusion
Prediabetes is a toxic cardiometabolic state associated with increased risk for microvascular and macrovascular complications.143 Physicians and healthcare providers should screen patients routinely for prediabetes and refer those with the condition for intensive lifestyle counseling. The goal is to achieve and maintain > 5% weight loss through caloric restriction and increased physical activity, similar to the DPP and kindred studies (Table 2).6,34,63–64 Healthcare providers should endeavor to build strong ties within healthcare systems, communities, and payers, to increase the availability of evidence-based structured lifestyle programs.
In a recent survey 33.6% of outpatients (out of 1.16 million outpatient visits analyzed) had prediabetes, based on HbA1c results. Amazingly, < 1% of those patients whose HbA1c tests showed prediabetes were recognized and diagnosed as such by clinicians. Of the abysmally low numbers whose prediabetes status was properly captured in the clinical records, only 23% had documentation of treatment (lifestyle modification and/or metformin) in the medical record.144 The prediabetes period presents an opportunity to intervene during the disease process. Primary care physicians, specialists, health systems and patients themselves all have an obligation to ensure that the opportunity for prevention is not missed.145–149
While it has long been known that diabetes confers significant cardiovascular risks, it is now becoming established that CVD risks precede diabetes and are evident in people with prediabetes. Given the millions of people with prediabetes around the globe, the impact on cardiovascular health is staggering. However, identifying and intervening in the at-risk prediabetic populations requires education, increased awareness, care coordination, organization and novel reimbursement mechanisms at multiple levels (health systems, society and individual).
Key Points.
Prediabetes carries an increased risk in cardiovascular disease
Significant physiological, metabolic, and biochemical features are dysregulated in prediabetes
Extensive Randomized Controlled Trials have demonstrated that lifestyle modification can decrease the rate of progression from prediabetes to diabetes.
Early detection and intervention is vitally important for prevention of prediabetes progression to diabetes
Acknowledgments
SD-J is supported, in part, by Grant R01 DK067269 from the National Institutes of Health.
Disclosure Statement
Dr. Brannick has nothing to disclose. Dr. Dagogo-Jack is supported, in part, by Grant R01 DK067269 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions: SD-J, as senior author, created the design and content of this manuscript. Both SD-J and BB drafted, revised and produced the final version of the manuscript.
References
- 1.World Health Organization. World Health Organization. Geneva (Switzerland): 2016. Global status report on noncommunicable diseases 2016. [Google Scholar]
- 2.Dall TM, Yang W, Halder P, et al. The Economic Burden of Elevated Blood Glucose Levels in 2012. Diabetes Care. 2014;37:3172–3179. doi: 10.2337/dc14-1036. [DOI] [PubMed] [Google Scholar]
- 3.Zhang P, Zhang X, Brown J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013 Apr;36(4):1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Abdul-Ghani M. Assessment treatment of cardiovascular risk in prediabetes: impaired glucose tolerance impaired fasting glucose. Am J Cardiol. 2011;108:3B–24B. doi: 10.1016/j.amjcard.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of Medical Care in Diabetes-2017. Diabetes Care. 40:S11–24. [Google Scholar]
- 7.World Health Organization (WHO) Consultation. Definition and diagnosis of diabetes and intermediate hyperglycaemia. World Health Organization Geneva; Switzerland: 2006. (Available at: http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf.) Accessed March 10, 2017. [Google Scholar]
- 8.Dagogo-Jack S. Pitfalls in the use of HbA1(c) as a diagnostic test: the ethnic conundrum. Nat Rev Endocrinol. 2010;6:589–593. doi: 10.1038/nrendo.2010.126. [DOI] [PubMed] [Google Scholar]
- 9.Chapp-Jumbo E, Edeoga C, Wan J, Dagogo-Jack S. Ethnic disparity in hemoglobin A1c levels among normoglycemic offspring of parents with type 2 diabetes. Endocr Pract. 2012;18:356–362. doi: 10.4158/EP11245.OR. [DOI] [PubMed] [Google Scholar]
- 10.Ebenibo S, Edeoga C, Wan J, Dagogo-Jack S. Glucoregulatory function among African Americans and European Americans with normal or pre-diabetic hemoglobin A1c levels. Metabolism. 2014;63:767–72. doi: 10.1016/j.metabol.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman WH, Dungan KM, Wolffenbuttel BH, Buse JB, Fahrbach JL, Jiang H, Martin S. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 15-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab. 2009 May;94(5):1689–94. doi: 10.1210/jc.2008-1940. [DOI] [PubMed] [Google Scholar]
- 12.Soranzo N. Genetic determinants of variability in glycated hemoglobin (HbA(1c)) in humans: review of recentprogress and prospects for use in diabetes care. Curr Diab Rep. 2011 Dec;11(6):562–9. doi: 10.1007/s11892-011-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman WH. Are There Clinical Implications of Racial Differences in HbA1c? Yes, to Not Consider Can Do Great Harm! Diabetes Care. 2016 Aug;39(8):1458–61. doi: 10.2337/dc15-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolffenbuttel BH, Herman WH, Gross JL, Dharmalingam M, Jiang HH, Hardin DS. Ethnic differences in glycemic markers in patients with type 2 diabetes. Diabetes Care. 2013 Oct;36(10):2931–6. doi: 10.2337/dc12-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta: US Department of Health and Human Services; 2014. [Google Scholar]
- 16.International Diabetes Federation IDF diabetes atlas 2015. 7th. Brussels: International Diabetes Federation; 2015. [Google Scholar]
- 17.Middelbeek RJW, Abrahamson MJ. Diabetes, prediabetes, and glycemic control in the United States: challenges and opportunities. Ann Intern Med. 2014;160:572–573. doi: 10.7326/M14-0539. [DOI] [PubMed] [Google Scholar]
- 18.Hu D, Fu P, Xie J, et al. MS for the InterASIA Collaborative Group Increasing prevalence and low awareness, treatment and control of diabetes mellitus among Chinese adults: the InterASIA study. Diabetes Res Clin Pract. 2008;81:250–257. doi: 10.1016/j.diabres.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Faeh D, William J, Tappy L, Ravussian E, Bovet P. Prevalence, awareness and control of diabetes in the Seychelles and relationship with excess body weight. BMC Public Health. 2007;7:163. doi: 10.1186/1471-2458-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saadi H, Carruthers SG, Nagelkerke N, et al. Prevalence of diabetes mellitus and its complications in a population-based sample in Al Ain, United Arab Emirates. Diabetes Res Clin Pract. 2007:369–377. doi: 10.1016/j.diabres.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Echouffo-Tcheugui JB, Dagogo-Jack S. Preventing diabetes mellitus in developing countries. Nat Rev Endocrinol. 2012;8:557–562. doi: 10.1038/nrendo.2012.46. [DOI] [PubMed] [Google Scholar]
- 22.Dagogo-Jack S. Predicting diabetes: our relentless quest for genomic nuggets. Diabetes Care. 2012;35:193–195. doi: 10.2337/dc11-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Defronzo RA, Banting Lecture From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerasi E, Luft R. The prediabetic state, its nature and consequences–a look toward the future. Diabetes. 1972;21:685–694. doi: 10.2337/diab.21.2.s685. [DOI] [PubMed] [Google Scholar]
- 26.Halban PA, Polonsky KS, Bowden DW, et al. Beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37:1751–1758. doi: 10.2337/dc14-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease mortality in adults: the European Prospective Investigation. Ann Intern Med. 2004;141:413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Zhao G, Li C. Pre-diabetes the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–1317. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 29.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitabchi AE, Temprosa M, Knowler WC, et al. The Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eschwege E, Richard JL, Thibult N, et al. Coronary heart disease mortality in relation with diabetes, blood glucose and plasma insulin levels. The Paris Prospective Study, ten years later. Horm Metab Res Suppl. 1985;15:41–46. [PubMed] [Google Scholar]
- 32.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 33.Mitrakou A, Kelley D, Mokan M, et al. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med. 1992;326:22–29. doi: 10.1056/NEJM199201023260104. [DOI] [PubMed] [Google Scholar]
- 34.Meigs JB, D’Agostino RB, Sr, Nathan DM, Rifai N, Wilson PW. Longitudinal association of glycemia and microalbuminuria: the Framingham Offspring Study. Diabetes Care. 2002;25:977–983. doi: 10.2337/diacare.25.6.977. [DOI] [PubMed] [Google Scholar]
- 35.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 36.Dagogo-Jack S, Askari H, Tykodi G. Glucoregulatory physiology in subjects with low-normal, high-normal, or impaired fasting glucose. J Clin Endocrinol Metab. 2009;94:2031–2036. doi: 10.1210/jc.2008-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeFronzo R, Kanat M, Abdul-Ghani M. Treatment of prediabetes. World J Diabetes. 2015;6:1207–1222. doi: 10.4239/wjd.v6.i12.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 39.Mather KJ, Funahashi T, Matsuzawa Y, et al. Diabetes Prevention Program. Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes. 2008;57:980–986. doi: 10.2337/db07-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitrakou A, Kelley D, Mokan M, et al. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med. 1992;326:22–29. doi: 10.1056/NEJM199201023260104. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y, Owei I, Wan J, Ebenibo S, Dagogo-Jack S. Adiponectin levels predict prediabetes risk: The Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study. BMJ Open Diabetes Res Care. 2016;4(1):e000194. doi: 10.1136/bmjdrc-2016-000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Z, Chen C, Li S, Kong F, Shan P, Huang W. Serum Markers of Endothelial Dysfunction and Inflammation Increase in Hypertension with Prediabetes Mellitus. Genet Test Mol Biomarkers. 2016;20:322–327. doi: 10.1089/gtmb.2015.0255. [DOI] [PubMed] [Google Scholar]
- 43.Dagogo-Jack S. Diabetes risks from prescription and nonprescription drugs. ADA press; Alexandria VA: 2016. [Google Scholar]
- 44.Lefèbvre PJ, Scheen AJ. The postprandial state risk of cardiovascular disease. Diabet Med. 1998;4:S63–8. doi: 10.1002/(sici)1096-9136(1998120)15:4+<s63::aid-dia737>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 45.von Toerne C, Huth C, de Las Heras Gala T, et al. MASP1, THBS1, GPLD1 and ApoA-IV are novel biomarkers associated with prediabetes: the KORA F4 study. Diabetologia. 2016;9:1882–1892. doi: 10.1007/s00125-016-4024-2. [DOI] [PubMed] [Google Scholar]
- 46.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 47.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 48.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 51.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46:701–710. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence prevalence in Pima Indians: a 19-fold greater incidence than in RochesterMinnesota. Am J Epidemiol. 1978;108:497–505. doi: 10.1093/oxfordjournals.aje.a112648. [DOI] [PubMed] [Google Scholar]
- 53.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American white adults: The Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 54.Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. Baltimore Longitudinal Study of Aging. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–1484. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- 55.Dagogo-Jack S, Edeoga C, Ebenibo S, Nyenwe E, Wan J, Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) Research Group Lack of racial disparity in incident prediabetes and glycemic progression among black and white offspring of parents with type 2 diabetes: the pathobiology of prediabetes in a biracial cohort (POP-ABC) study. J Clin Endocrinol Metab. 2014;99:E1078–1087. doi: 10.1210/jc.2014-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boucher AB, Adesanya EA, Owei I, et al. Dietary habits and leisure-time physical activity in relation to adiposity dyslipidemia and incident dysglycemia in the pathobiology of prediabetes in a biracial cohort study. Metabolism. 2015;64:1060–1067. doi: 10.1016/j.metabol.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Owei I, Umekwe N, Wan J, Dagogo-Jack S. Plasma lipid levels predict dysglycemia in a biracial cohort of nondiabetic subjects: potential mechanisms. Exp Biol Med (Maywood) 2016;241:1961–1967. doi: 10.1177/1535370216659946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Selvin E, Lazo M, Chen Y, et al. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation. 2014;130:1374–1382. doi: 10.1161/CIRCULATIONAHA.114.010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balkau B, Eschwège E, Papoz L, et al. Risk factors for early death in non-insulin dependent diabetes and men with known glucose tolerance status. BMJ. 1993;307:295–299. doi: 10.1136/bmj.307.6899.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dagogo-Jack S, Egbuonu N, Edeoga C. Principles and practice on non-pharmacological interventions to reduce cardiometabolic risk. Med Princ Pract. 2010;19:167–175. doi: 10.1159/000285280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nyenwe EA, Dagogo-Jack S. Metabolic syndrome prediabetes and the science of primary prevention. Minerva Endocrinol. 2011;36:129–145. [PubMed] [Google Scholar]
- 62.Stacey RB, Leaverton PE, Schocken DD, Peregoy JA, Bertoni AG. Prediabetes the association with unrecognized myocardial infarction in the multi-ethnic study of atherosclerosis. Am Heart J. 2015;170:923–928. doi: 10.1016/j.ahj.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002;25:1129–1134. doi: 10.2337/diacare.25.7.1129. [DOI] [PubMed] [Google Scholar]
- 64.Coutinho M, Gerstein HC, Wang Y, et al. Analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 65.Smith NL, Barzilay JI, Shaffer D, et al. Fasting and 2-hour post challenge serum glucose measures and risk of incident cardiovascular events in the elderly: The Cardiovascular Health Study. Arch Intern Med. 2002;162:209–216. doi: 10.1001/archinte.162.2.209. [DOI] [PubMed] [Google Scholar]
- 66.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all-cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. doi: 10.1136/bmj.i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonora E, Kiechl S, Willeit J, et al. Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck study. Diabetes Care. 2003;26:1251–1257. doi: 10.2337/diacare.26.4.1251. [DOI] [PubMed] [Google Scholar]
- 68.Tai ES, Goh SY, Lee JJ, et al. Lowering the criterion for impaired fasting glucose: impact on disease prevalence and associated risk of diabetes and ischemic heart disease. Diabetes Care. 2004;27:1728–1734. doi: 10.2337/diacare.27.7.1728. [DOI] [PubMed] [Google Scholar]
- 69.Rijkelijkhuizen J, Nijpels J, Heine R, Bouter L, Stehouwer C, Dekker J. High risk of cardiovascular mortality in individuals with impaired fasting glucose is explained by conversion to diabetes: the Hoorn study. Diabetes Care. 2007;30:332–336. doi: 10.2337/dc06-1238. [DOI] [PubMed] [Google Scholar]
- 70.Balkau B, Eschwège E, Papoz L, et al. Risk factors for early death in non-insulin dependent diabetes and men with known glucose tolerance status. BMJ. 1993;307:295–299. doi: 10.1136/bmj.307.6899.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dagogo-Jack S, Egbuonu N, Edeoga C. Principles and practice on non-pharmacological interventions to reduce cardiometabolic risk. Med Princ Pract. 2010;19:167–175. doi: 10.1159/000285280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dagogo-Jack S. Endocrinology & metabolism: complications of diabetes mellitus. In: Singh AK, editor. Scientific American medicine. Hamilton, ON: Decker Intellectual Properties; 2015. [Google Scholar]
- 73.Papa G, Degano C, Iurato MP, Licciardello C, Maiorana R, Finocchiaro C. Macrovascular complication phenotypes in type 2 diabetic patients. Cardiovasc Diabetol. 2013;12:20. doi: 10.1186/1475-2840-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Liu L, Zhou Y, et al. Increased fasting glucose and the prevalence of arterial stiffness: a cross-sectional study in Chinese adults. Neurol Res. 2014;5:427–433. doi: 10.1179/1743132814Y.0000000345. [DOI] [PubMed] [Google Scholar]
- 75.Holman RR, Coleman RL, Chan JCN, et al. ACE Study Group Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017 Sep 12;:pii. doi: 10.1016/S2213-8587(17)30309-1. S2213-8587(17)30309-1. [DOI] [PubMed] [Google Scholar]
- 76.Roquer J, Rodríguez-Campello A, Cuadrado-Godia E, et al. Ischemic stroke in prediabetic patients. J Neurol. 2014;261:1866–1870. doi: 10.1007/s00415-014-7431-7. [DOI] [PubMed] [Google Scholar]
- 77.Urabe T, Watada H, Okuma Y, et al. Prevalence of abnormal glucose metabolism and insulin resistance among subtypes of ischemic stroke in Japanese patients. Stroke. 2009;40:1289–1295. doi: 10.1161/STROKEAHA.108.522557. [DOI] [PubMed] [Google Scholar]
- 78.Kernan WN, Viscoli CM, Inzucchi SE, et al. Prevalence of abnormal glucose tolerance following a transient ischemic attack or ischemic stroke. Arch Intern Med. 2005;165:227–233. doi: 10.1001/archinte.165.2.227. [DOI] [PubMed] [Google Scholar]
- 79.Matz K, Keresztes K, Tatschl C, et al. Disorders of glucose metabolism in acute stroke patients: an underrecognized problem. Diabetes Care. 2006;29:792–797. doi: 10.2337/diacare.29.04.06.dc05-1818. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka R, Ueno Y, Miyamoto N, et al. Impact of diabetes and prediabetes on the short-term prognosis in patients with acute ischemic stroke. J Neurol Sci. 2013;332:45–50. doi: 10.1016/j.jns.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 81.Qiao Q, Pyörälä K, Pyörälä M, et al. Two hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J. 2002;23:1267–1275. doi: 10.1053/euhj.2001.3113. [DOI] [PubMed] [Google Scholar]
- 82.Bamberg F, Hetterich H, Rospleszcz S, et al. Subclinical Disease Burden as Assessed by Whole-Body MRI in Subjects with Prediabetes, Subjects with Diabetes, and Normal Control Subjects from the General Population: The KORA-MRI Study. Diabetes. 2017;1:158–169. doi: 10.2337/db16-0630. [DOI] [PubMed] [Google Scholar]
- 83.Holman RR, Coleman RL, Chan JCN, et al. IRIS Trial Investigators Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016 Apr 7;374(14):1321–31. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Inzucchi SE, Viscoli CM, Young LH, Furie KL, Gorman M, Lovejoy AM, Dagogo-Jack S, Ismail-Beigi F, Korytkowski MT, Pratley RE, Schwartz GG, Kernan WN, IRIS Trial Investigators Pioglitazone prevents diabetes in insulin-resistant patients with cerebrovascular disease. Diabetes Care. 2016;39:1684–92. doi: 10.2337/dc16-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Golledge J, Quigley F, Velu R, Walker PJ, Moxon JV. Association of impaired fasting glucosediabetes and their management with the presentation outcome of peripheral artery disease: a cohort study. Cardiovasc Diabetol. 2014;13:147. doi: 10.1186/s12933-014-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kamalesh M, Shen J. Diabetes and peripheral arterial disease in men: trends in prevalence, mortality, and effect of concomitant coronary disease. Clin Cardiol. 2009;32:442–446. doi: 10.1002/clc.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DeFronzo RA, Abdul-Ghani M. Assessment treatment of cardiovascular risk in prediabetes: impaired glucose tolerance impairedfasting glucose. Am J Cardiol. 2011;108:3B–24B. doi: 10.1016/j.amjcard.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 88.The DECODE Study Group. Consequence of the new diagnostic criteria for diabetes in older men and women. Diabetes Care. 1999;22:1667–1671. doi: 10.2337/diacare.22.10.1667. [DOI] [PubMed] [Google Scholar]
- 89.Zhang L, Qiao Q, Tuomilehto J, et al. DECODE Study Group The impact of dyslipidaemia on cardiovascular mortality in individuals without a prior history of diabetes in the DECODE Study. Atherosclerosis. 2009;206:298–302. doi: 10.1016/j.atherosclerosis.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 90.Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- 91.Balkau B, Bertrais S, Ducimetière P, Eschwege E. Is there a glycemic threshold for mortality risk? Diabetes Care. 1999;22:696–699. doi: 10.2337/diacare.22.5.696. [DOI] [PubMed] [Google Scholar]
- 92.Resnick HE, Harris MI, Brock DB, Harris TB. American Diabetes Association diabetes diagnostic criteria advancing age and cardiovascular disease risk profiles: results from the Third National Health Nutrition Examination Survey. Diabetes Care. 2000;23:176–80. doi: 10.2337/diacare.23.2.176. [DOI] [PubMed] [Google Scholar]
- 93.De Marco M, de Simone G, Roman MJ, et al. Cardiac geometry and function in diabetic or prediabetic adolescents young adults: The Strong Heart Study. Diabetes Care. 2011;34:2300–2305. doi: 10.2337/dc11-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 95.Yan F, Cha E, Lee ET, Mayberry RM, Wang W, Umpierrez G. A Self-Assessment Tool for Screening Young Adults at Risk of Type 2 Diabetes Using Strong Heart Family Study Data. Diabetes Educ. 2016;42:607–617. doi: 10.1177/0145721716658709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care. 2012;35:2613–2617. doi: 10.2337/dc12-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Franssen R, Monajemi H, Stroes ES, Kastelein JJ. Obesity and dyslipidemia. The Medical Clinics of North America. 2011;95:893–902. doi: 10.1016/j.mcna.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 98.Liu T. A Comparison of Biological and Physical Risk Factors for Cardiovascular Disease in Overweight/Obese Individuals with and Without Prediabetes. Clin Nurs Res. 2016 doi: 10.1177/1054773816658644. [DOI] [PubMed] [Google Scholar]
- 99.Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. International Journal of Obesity. 2006;30:1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- 100.Li C, Ford E, Zhao G, et al. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care. 2009;32:342–347. doi: 10.2337/dc08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Festa A, Williams K, Hanley AJ, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 102.Zheng S, Zhou H, Han T, et al. Clinical characteristics and beta cell function in Chinese patients with newly diagnosed type 2 diabetes mellitus with different levels of serum triglyceride. BMC Endocr Disord. 2015;15:21. doi: 10.1186/s12902-015-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ren X, Chen ZA, Zheng S, et al. Association between triglyceride to HDL-C ratio (TG/HDL-C) and insulinresistance in Chinese patients with newly diagnosed type 2 diabetes mellitus. PLoS One. 2016;11:e0154345. doi: 10.1371/journal.pone.0154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance VLDL overproduction and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32:2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- 105.Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355:1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 106.Navigator Study Group. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 107.The Diabetes Prevention Program Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care. 2005;28:888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moebus S, Hanisch JU, Aidelsburger P, Bramlage P, Wasem J, Jöckel K-H. Impact of four different definitions used for assessment of the prevalence of metabolic syndrome in a primary health care setting. The German Metabolic Cardiovascular Risk Project (GEMCAS) Cardiovasc Diabetol. 2007;6:22. doi: 10.1186/1475-2840-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 110.Kahn R, Alperin P, Eddy D. Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet. 2010;375:1365–1374. doi: 10.1016/S0140-6736(09)62162-0. [DOI] [PubMed] [Google Scholar]
- 111.Gæde P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomized trial. Diabetologia. 2016;59:2298–2307. doi: 10.1007/s00125-016-4065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Urbanski P, Wolf A, Herman W. Cost-Effectiveness of Diabetes Education. J Am Diet Assoc. 2008;108:S6–11. doi: 10.1016/j.jada.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 114.Stefan N, Staiger H, Wagner R, et al. A high-risk phenotype associates with reduced improvement in glycaemia during a lifestyle intervention in prediabetes. Diabetologia. 2015;58:2877–2884. doi: 10.1007/s00125-015-3760-z. [DOI] [PubMed] [Google Scholar]
- 115.The Look AHEAD Research Group. Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DPP Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sprague R, Ellsworth M. Vascular Disease in Pre-Diabetes: New Insights Derived from Systems Biology. Mo Med. 2010;107:265–269. [PMC free article] [PubMed] [Google Scholar]
- 118.Hostalek U, Gwilt M, Hildemann S. Therapeutic Use of Metformin in Prediabetes and Diabetes Prevention. Drugs. 2015;75:1071–1094. doi: 10.1007/s40265-015-0416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 120.Anderson J. Achievable Cost Saving and Cost-Effective Thresholds for Diabetes Prevention Lifestyle Interventions in People Aged 65 Years and Older: A Single-Payer Perspective. Journal of the Academy of Nutrition and Dietetics. 2012;112:1747–1754. doi: 10.1016/j.jand.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 121.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 122.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeFronzo RA, Tripathy D, Schwenke DC, et al. ACT NOW Study Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–1115. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 124.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. STOP-NIDDM Trial Research Group: Acarbose treatment the risk of cardiovascular disease hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 125.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 126.DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 127.Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet. 2010;376:103–111. doi: 10.1016/S0140-6736(10)60746-5. [DOI] [PubMed] [Google Scholar]
- 128.NAVIGATOR Study Group. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463–1476. doi: 10.1056/NEJMoa1001122. [DOI] [PubMed] [Google Scholar]
- 129.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 130.Ramachandran A, Snehalatha C, Mary S, et al. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2) Diabetologia. 2009;52:1019–1026. doi: 10.1007/s00125-009-1315-x. [DOI] [PubMed] [Google Scholar]
- 131.The Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care. 2003;26:977–980. doi: 10.2337/diacare.26.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tripathy D, Schwenke DC, Banerji M, et al. Diabetes Incidence and Glucose Tolerance after Termination of Pioglitazone Therapy: Results from ACT NOW. J Clin Endocrinol Metab. 2016;101:2056–2062. doi: 10.1210/jc.2015-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2:474–480. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 134.Xiang AH, Hodis HN, Kawakubo M, et al. Effect of pioglitazone on progression of subclinical atherosclerosis in non-diabetic premenopausal Hispanic women with prior gestational diabetes. Atherosclerosis. 2008;199:207–214. doi: 10.1016/j.atherosclerosis.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.DeFronzo RA, Tripathy D, Schwenke DC, et al. ACT NOW Study Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–1115. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 136.Salas-Salvadó J, Guasch-Ferré M, Lee CH, Estruch R, Clish CB, Ros E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J Nutr. 2016 Mar 9;:pii. doi: 10.3945/jn.115.218487. jn218487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mozaffarian D, Marfisi R, Levantesi G, Silletta MG, Tavazzi L, Tognoni G, Valagussa F, Marchioli R. Incidence of new-onset diabetes impaired fasting glucose in patients with recent myocardial infarctionthe effect of clinical lifestyle risk factors. Lancet. 2007;370:667–75. doi: 10.1016/S0140-6736(07)61343-9. [DOI] [PubMed] [Google Scholar]
- 138.Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015 Aug 10;5(8):e008222. doi: 10.1136/bmjopen-2015-008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Monlezun DJ, Kasprowicz E, Tosh KW, Nix J, Urday P, Tice D, Sarris L, Harlan TS. Medical school-based teaching kitchen improves HbA1c, blood pressure, and cholesterol for patients with type 2 diabetes: Results from a novel randomized controlled trial. Diabetes Res Clin Pract. 2015 Aug;109(2):420–6. doi: 10.1016/j.diabres.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 140.Salas-Salvadó J, Bulló M, Estruch R, Ros E, Covas MI, Ibarrola-Jurado N, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med. 2014 Jan 7;160(1):1–10. doi: 10.7326/M13-1725. [DOI] [PubMed] [Google Scholar]
- 141.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 142.Perreault L, Temprosa M, Mather KJ, et al. The Diabetes Prevention Program Research Group Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk: results from the Diabetes Prevention Program outcomes study. Diabetes Care. 2014;37:2622–2631. doi: 10.2337/dc14-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med (Maywood) 2016;241:1323–1331. doi: 10.1177/1535370216654227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mainous AG, 3rd, Tanner RJ, Scuderi CB, Porter M, Carek PJ. Prediabetes screening treatment in diabetes prevention: The impact of physician attitudes. J Am Board Fam Med. 2016;29:663–671. doi: 10.3122/jabfm.2016.06.160138. [DOI] [PubMed] [Google Scholar]
- 145.Gopalan A, Lorincz IS, Wirtalla C, Marcus SC, Long JA. Awareness of Prediabetes Engagement in Diabetes Risk-Reducing Behaviors. Am J Prev Med. 2015;49:512–519. doi: 10.1016/j.amepre.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 146.Dagogo-Jack S. Preventing diabetes-related morbidity and mortality in the primary care setting. J Natl Med Assoc. 2002;94:549–560. [PMC free article] [PubMed] [Google Scholar]
- 147.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: recommendation from the Centers for Disease Control Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 148.Kimm SY, Glynn NW, Kriska AM, et al. Decline in physical activity in black and white girls during adolescence. N Engl J Med. 2002;347:709–715. doi: 10.1056/NEJMoa003277. [DOI] [PubMed] [Google Scholar]
- 149.Dagogo-Jack S. Primary prevention of cardiovascular disease: the glass is half full and half empty. Diabetes Care. 2005;28:971–972. doi: 10.2337/diacare.28.4.971. [DOI] [PubMed] [Google Scholar]


